
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 12 November 2021
Sec. Cardiovascular Therapeutics
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.769138
Background: Current treatment guidelines for arrhythmogenic right ventricular cardiomyopathy (ARVC) mainly emphasize on prevention of ventricular arrhythmic events. Despite the progressive nature of ARVC, therapeutic options focusing on decelerating disease progression are scarce.
Methods and Results: This retrospective observational cohort study included 311 patients [age, 39.1 ± 14.4 years; male, 233 (74.9%)] with a definite diagnosis of ARVC as determined by the 2010 Task Force Diagnostic Criteria. Among them, 113 patients (36.3%) received ACEI/ARB treatment. Disease progression was evaluated according to repeat transthoracic echocardiograms with a linear mixed model. Patients receiving ACEI/ARB treatment were associated with slower disease progression reflected by a gradual decrease in tricuspid annular plane systolic excursion than those not receiving ACEI/ARB treatment (0.37 vs. 0.61 mm per year decrease, P < 0.001) and slower dilation of right ventricular outflow tract (0.57 vs. 1.06 mm per year increased, P = 0.003). Cox proportional hazard regression models were used to evaluate the association between life-threatening ventricular tachycardia events and ACEI/ARB treatment. A reduced risk of life-threatening ventricular arrhythmia was associated with ACEI/ARB treatment compared to that without ACEI/ARB treatment (adjusted HR: 0.71, 95% CI: 0.52–0.96, P = 0.031).
Conclusions: ACEI/ARB treatment is associated with slower disease progression and lower risk of life-threatening ventricular arrhythmia in patients with ARVC. Delaying disease progression may pave way for reducing life-threatening ventricular arrhythmia risk.
ARVC is a leading cause of sudden cardiac death/arrest (SCD/A) in young people and athletes (1). It is characterized by progressive replacement of the myocardium with fibrofatty tissue in the right ventricle (RV) and left ventricle (LV) in some cases (2). Continuous deterioration of the ventricles leads to ventricular arrhythmias (VA) and impaired ventricular function.
The primary goal of treatment is prevention of sudden cardiac death. Although implantable cardioverter defibrillators (ICDs) have been proven to be effective, repeated shock therapy, nevertheless, affects patients' quality of life (3). Attempts have been made to reduce the occurrence of VA by using classic anti-arrhythmic drugs and catheter ablation, but conflicting results have been reported (4–8). Some evidence suggests that the progressive nature of ARVC and persistent deterioration of the RV is associated with an increased risk of VA events (9–11). These findings indicate that therapies focused on delaying disease progression may offer a new approach for reducing the VA risk; however, no validated drug therapy has been established to decelerate disease progression.
Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB) are the standard treatment options for patients with systolic left ventricular dysfunction (12), and accumulating evidence points to their effectiveness in RV dysfunction (13–15). Additionally, ACEI/ARB treatment is related to a lower incidence of VA in patients with congestive heart failure (16). Therefore, in this study, we aimed to determine whether ACEI/ARB might slow disease progression in patients with ARVC and reduce the occurrence of VA events.
Three hundred and fifty-four consecutive patients with a definite diagnosis of ARVC were enrolled, as determined by the revised 2010 Task Force Diagnostic Criteria (17), from October 2001 to November 2017 at a single tertiary national cardiac center in China. Patients who were lost to follow-up (n = 43) were excluded. In total, 311 patients were included for the final analysis. The baseline characteristic of patients with and without follow-up data were compared to confirm random loss; the results are shown in Supplementary Table S1. All medical records of the patients were systemically reviewed; written informed consent was obtained from all patients. The study was approved by the Institutional Ethics committee of Fuwai hospital.
Medication status such as drug type, dosage, and compliance were recorded during routine follow-up, which was carried out every 6 months. For patients refilling prescriptions at our hospital, medication compliance was reconfirmed via the hospital's prescription web system. For follow-ups at patient homes, photographs of prescriptions were requested to confirm medication compliance. Patients were grouped based on ACEI/ARB use. Patients prescribed ACEI/ARB at some point during follow-up before the first episode of life-threatening VA (to those with recurrence of life-threatening VA) or until the end of follow-up (to those without recurrence of life-threatening VA) were included in the ACEI/ARB group. To increase the reliability of the investigation, sensitivity analyses with additional grouping strategy were conducted: (1) the duration of ACEI/ARB treatment exceeding 50% of the whole time until the first episode of life-threatening VA or until the end of follow-up; and (2) ACEI/ARB treatment continued until the first episode of life-threatening VA or until the end of follow-up. The results of sensitivity analyses are shown in Supplementary Table S2.
Disease progression was assessed by long-term surveillance of transthoracic echocardiograms (TTEs). To eliminate interobserver variability, TTE data were re-interpreted by a qualified observer blinded to the study and patient information. For intraobserver variability, TTE data of 50 random patients were re-interpreted by the same observer at least 1 month apart. The right ventricular outflow tract (RVOT) in the parasternal long-axis and tricuspid annular plane systolic excursion (TAPSE) were used to determine the right ventricular structure and function; left ventricular end-diastolic fraction and left ventricular ejection fraction (LVEF) were used to determine the left ventricular structure and function. All parameters were evaluated mainly based on two-dimensional echocardiography using standard processes as previously described (18).
Follow-ups were routinely carried out every 6 months via clinical visit or telephone consultation. The primary outcome was life-threatening VA events, including (1) SCD/A or aborted SCD/A; (2) sustained ventricular tachycardia (VT), lasting over 30 s and exceeding 100 bpm, with hemodynamic compromise or ventricular flutter/fibrillation; or (3) appropriate ICD shock. VA events were also detected via reports from a clinical network of initiatives consisting of dedicated patient phone lines, community primary care physician or specialist nurse reporting, and interval secondary/tertiary care outpatient reviews.
Statistical analyses were performed using R statistical software, version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria). Normally distributed continuous variables, non-normally distributed variables, and categorical variables are expressed as mean ± standard deviation (SD), median [interquartile range (IQR)], and number (percentage), respectively. Student's t-test, χ2 test, or the Mann-Whitney U-test was used for comparisons of baseline characteristics between the ACEI/ARB group and non-ACEI/ARB group. To maximize the statistical power of the study, we used a multivariate multiple imputation method to input missing data. Five imputed datasets were obtained, and a combined coefficient was calculated (see Supplementary Table S3). Sensitivity analyses were also performed to confirm missing randomized data. To evaluate disease progression, we used linear mixed models to analyze repeated measurements of TTE during follow-up. The model was adjusted for variables such as age sex, baseline values, and cardiac function. Cox proportional hazard regression models were used to analyze the association between life-threatening VA events and the use of ACEI/ARB. Model I was adjusted for confounders such as age, sex, RVEF, RV.LGE %, N-terminal pro-brain natriuretic peptide (NT-proBNP), ICD, and catheter ablation. Model II was additionally adjusted for LVEF, LV-LGE %, hypertension, and use of beta-blockers, spironolactone, and digoxin.
Three hundred and eleven patients (average age: 39.1 ± 14.4 years; male: 233, 74.9%) with a definite diagnosis of ARVC were enrolled. The baseline characteristics are shown in Table 1. Of the 311 patients, 113 were prescribed ACEI/ARB after their first discharge from our hospital (ACEI: n = 68; ARB: n = 45) with a median treatment duration of 4.9 (IQR: 3.0–7.5) years from first clinical visit. Fifty-four patients had received ACEI/ARB treatment before they were first admitted to our hospital. Patients with hypertension and LV heart failure tended to be treated with ACEI/ARB, and hence, bi-ventricular dysfunction was more frequently observed in the ACEI/ARB group than in the non-ACEI/ARB group (P < 0.001). Sixteen patients discontinued ACEI/ARB usage during follow-up (10 for poor compliance, 4 for symptomatic hypotension at minimum dose, 1 for pregnancy, and 1 for end-stage renal dysfunction). The most prescribed ACEIs/ARBs were perindopril (n = 32) and losartan (n = 28). Four patients switched their ACEI medication to an ARB within 1 month because of cough, and they were considered a part of the ARB group for this analysis. Six patients started ACEI/ARB treatment with new onset symptomatic left ventricular dysfunction, and all of them had experienced the first episodes of life-threatening VA when the drug was taken, and all of them were considered a part of the non-ACEI/ARB group.
Six hundred and thirty-seven TTEs were completed for 188 patients. According to the baseline characteristics shown in Supplementary Table S4, patients with recurrent life-threatening VA tended to undergo echocardiograms again. The median time between the baseline and the last TTE was 4.1 (IQR: 2.4–6.0) years. TAPSE tended to decline and RVOT tended to dilate over the years; however, ACEI/ARB decelerated this disease progression (Figure 1). In the non-ACEI/ARB group, TAPSE decreased by 0.61 mm per year [95% confidence interval (CI): 0.77–1.03]; this trend was slower in the ACEI/ARB group, in which TAPSE decreased to 0.24 mm per year (95% CI: 0.31–0.42), representing a 60.7% (95% CI: 58.9–64.5%, P < 0.001) relative reduction. Based on the test of power, 77.7% of patients might benefit from ACEI/ARB in terms of RV function restoration (α = 0.05; see Supplementary Table S4). Compared with ARB, ACEI elicited a strong effect and further delayed the declination of TAPSE by 70% (95% CI: 63.1–79.2%, P for interaction = 0.026). In patients with mild RV dysfunction, a slower progression was observed with ACEI/ARB treatment than without treatment (TAPSE: 0.37 vs. 0.61 mm per year decrease, P < 0.001; RVOT: 0.37 vs. 0.61 mm per year decrease, P < 0.001). For intraobserver variability, the ICC values were 0.91 (95% CI: 0.87–0.95) for RVOT-PLAX and 0.85 (95% CI: 0.76–0.94) for TAPSE.
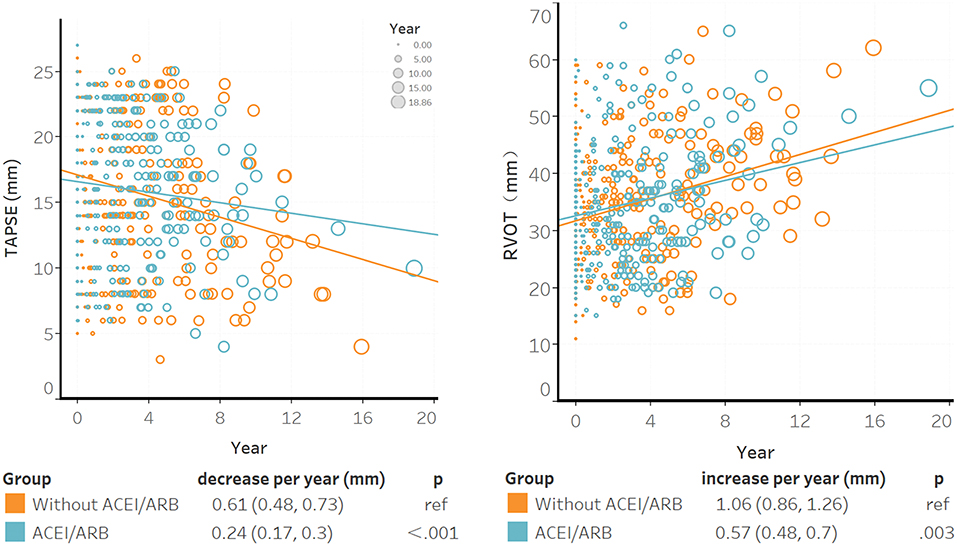
Figure 1. The association between ACEI/ARB treatment and right ventricular progression based on results of the transthoracic echocardiogram. Individual points were the unadjusted time to baseline TAPSE or RVOT during follow-up; larger circles represented longer time intervals from baseline. The blue trendline represents the progression speed in the ACEI/ARB group, and the orange trendline represents the progression in the non-ACEI/ARB group. Both trendlines were calculated using linear mixed models. ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; RVOT, right ventricular outflow tract; TAPSE, tricuspid annular plane systolic excursion.
During the median follow-up of 5.96 (IQR: 3.74–8.61) years, 65.3% (n = 203) of the patients experienced life-threatening VA events, with an annual event rate of 19.8% (95% CI: 17.2–22.8%). The most common life-threatening VA events were sustained VT (n = 128), followed by ICD shock (n = 71) and SCD/A (n = 16). Twelve patients experienced various outcomes. In the multivariable analyses, relatively young age, male sex, a higher burden of 24-h premature ventricular contractions, recorded non-sustained VT, lower TAPSE, lower RVEF, and higher burden of RV-LGE% were associated with an increased risk of life-threatening VA in patients with ARVC (Table 2). The results of the univariate analyses are presented in Supplementary Table S6.
Patients with ACEI/ARB had a lower occurrence of life-threatening VA than those not receiving ACEI/ARB [55.8 vs. 71.2%, crude hazard ratio (HR): 0.69, 95% CI: 0.51–0.93, P = 0.013]. This effect remained after adjustments in Model I (adjusted HR: 0.71, 95% CI: 0.52–0.96, P = 0.031) and Model II (adjusted HR: 0.68, 95% CI: 0.50–0.94, P = 0.018). Patients taking ACEI/ARB also showed a reduced risk of sustained VT (53.5 vs. 63.8%, adjusted HR: 0.63, 95% CI: 0.43–0.92, P = 0.017). Although HR favored a reduction in appropriated ICD shock (adjusted HR: 0.66, 95% CI: 0.40–1.07, P = 0.092) and SCD/A (2.7 vs. 6.6%, adjusted HR: 0.33, 95% CI: 0.09–1.17, P = 0.083), this did not reach statistical significance as shown in Figure 2. Results of the subgroup analyses are shown in Figure 3, with none of the variables showing significant association with ACEI/ARB.
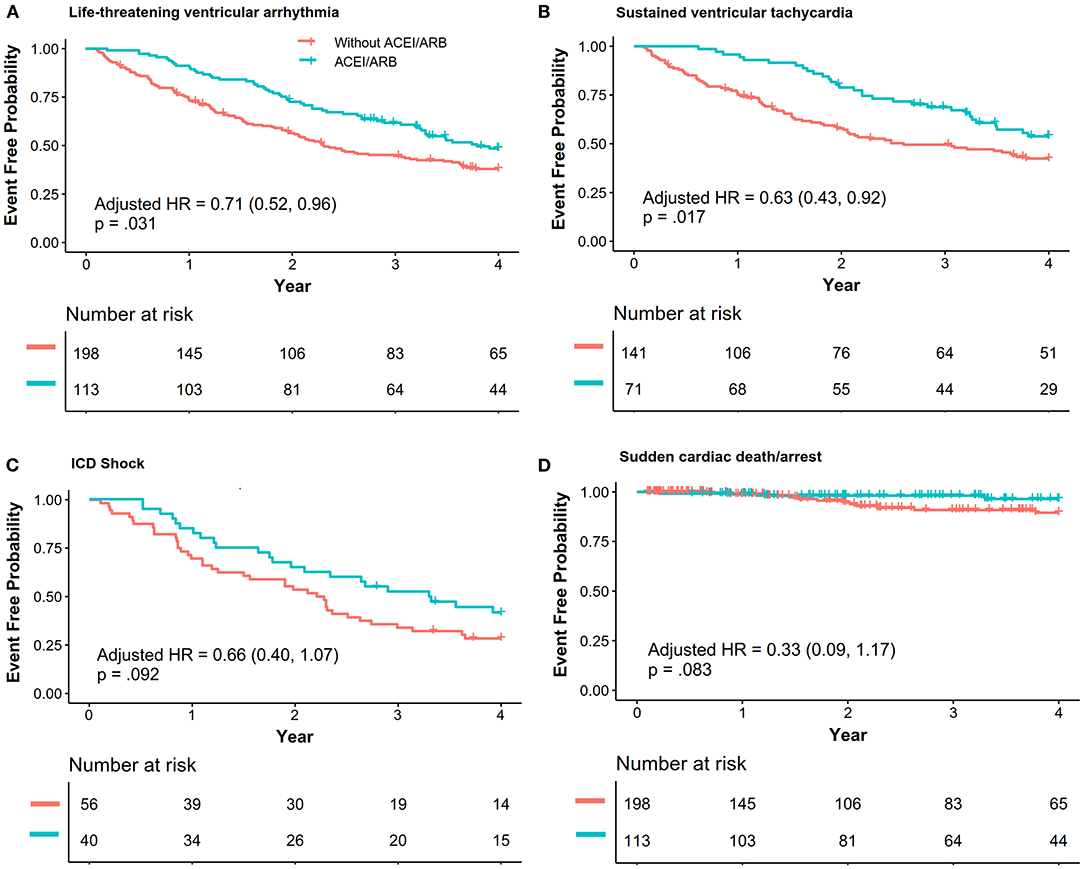
Figure 2. The association between ACEI/ARB treatment and recurrence of life-threatening ventricular VAs. Data were analyzed with composite outcomes as shown in (A), and each outcome is individually shown in (B–D). Hazard ratio (HR) and p-values were calculated via a Cox proportional hazard model, and the results were adjusted with Model I [right ventricular ejection fraction (baseline), percentage of late gadolinium enhancement in the right ventricle, N-terminal pro-brain natriuretic peptide, ICD, catheter ablation]. ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; VA, ventricular arrhythmia; VT, ventricular tachycardia; ICD, implanted cardiac defibrillator; SCD/A, sudden cardiac death/arrest.
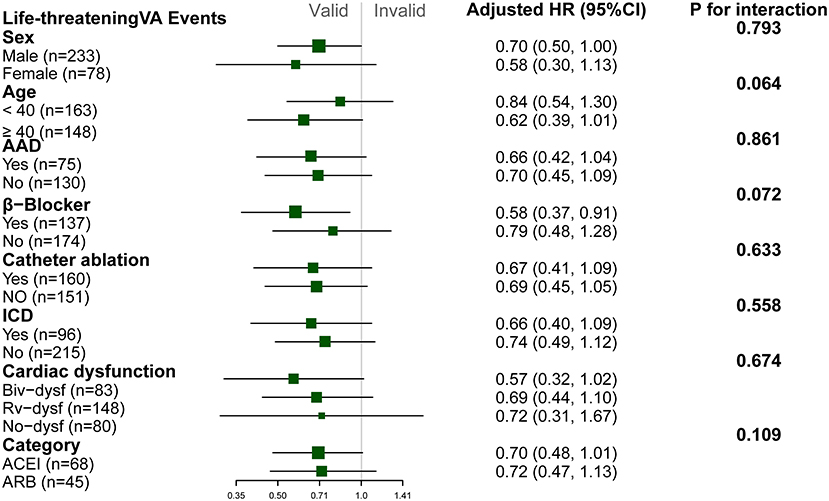
Figure 3. Subgroup analyses of the association between ACEI/ARB and recurrence of life threatening ventricular arrhythmia. Hazard ratio (HR) and p-values were calculated using a Cox proportional hazard model. All subgroup analyses were adjusted for Model I (right ventricular ejection fraction [baseline], N-terminal pro-brain natriuretic peptide, percentage of late gadolinium enhancement in right ventricle, catheter ablation, implanted cardiac defibrillator). No variable interacted with ACEI/ARB. NSVT, non-sustained ventricular tachycardia; PVC, premature ventricular complex; Biv-dysf, bi-ventricular dysfunction; Rv-dysf, right ventricular dysfunction; No-dysfunction, no ventricular dysfunction; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
To the best of our knowledge, this is the first single-center observational study to demonstrate the effects of ACEI/ARB in patients with ARVC during a long-term follow-up. The main findings were as follows. First, in addition to previously reported risk factors (9), a higher level of RV-LGE% was found to be associated with an increased risk of life-threatening VA in patients with ARVC. Second, ACEI/ARB treatment was associated with slower disease progression, which was reflected by a delay in the progress of RV dysfunction and dilation. Third, patients treated with ACEI/ARB had nearly one-third reduced risk of life-threatening VA compared to those without ACEI/ARB treatment.
The overall number of life-threatening VA events was higher in our study than those reported in previous studies (19). This might be due to the high-risk population enrolled in the present study; male sex, relatively young age, and extent of RV dysfunction facilitate arrhythmogenesis as determined both in previous and our studies (9, 10). The abnormal wall stretch caused by cardiac dysfunction may contribute to arrhythmogenesis through a process known as mechano-electrical feedback (20), which corroborates our findings that lower levels of TAPSE and RVEF and higher levels of NT-proBNP are associated with increased risk of life-threatening VA events. We found that higher RV-LGE% was also associated with an increased risk of life-threatening VA events, which correlated with the fact that fibrosis causes electric inhomogeneity and slow conduction, thereby facilitating arrhythmogenesis.
ACEI/ARB exhibit anti-remodeling effects in various types of cardiomyopathies (21, 22). This study revealed that ACEI also showed such an effect in patients with ARVC, which is compatible with the findings of Morel et al. (23), who showed that ACEI had an anti-fibrotic effect in patients with ARVC. Although the detailed pathogenesis of ARVC remains unclear, the major pathophysiological features include cardiomyocyte loss, fibrogenesis, and adipogenesis, which lead to the progression of ventricular dilation and dysfunction (24–26). According to the findings of previous studies, inhibition of apoptosis of cardiomyocytes through the P53 pathway and of fibrogenesis through the canonical and non-canonical transforming growth factor-β signaling pathways might explain this anti-remodeling (27, 28). ACEI showed a stronger anti-remodeling effect than ARB. Additional influence on the Kallikrein-Kinin/bradykinin system, angiotensin1–7, and ACE2 of ACEI likely contributed to this superior anti-remodeling effect (29–31).
Although ACEI/ARB are not classic anti-arrhythmic drugs, we found that patients with ARVC under ACEI/ARB treatment had a reduced risk of life-threatening VA events. This anti-arrhythmic effect has also been observed in other studies concerning left heart failure (16, 32). Additionally, ACEI also prevented newly onset or recurrence of atrial fibrillation due to substrate improvement (33). As mentioned above, inhibition of fibrogenesis might contribute to the anti-arrhythmic effect. Furthermore, reducing abnormal wall stretch of the ventricle by decreasing the preload and afterload of the RV might also lessen the risk of VA events (34–36). Stabilization of electrolyte concentration and prolonged action potential might also promote the anti-arrhythmic effect as suggested by other study (37).
ARVC is characterized by its arrhythmogenic property, but the underlying action is cardiomyopathy. Therefore, based on our study, anti-remodeling might be a potential therapeutic target for reducing VA risk; further randomized clinical studies are warranted to demonstrate this effect of ACEI/ARB.
This study has a few limitations. First, our study was limited by the inherent nature of retrospective cohort studies. Patients with hypertension and LV dysfunction tended to be treated with ACEI/ARB, and residual treatment bias inevitably affected our findings. Although we adjusted the LV dysfunction to generate more reliable results, another study showed that LV dysfunction also facilitated arrhythmogenesis (9), and indicated that patients on ACEI/ARB were at an increased risk for recurrence of life-threatening VA owing to a higher LV dysfunction, which emphasized the effectiveness of ACEI/ARB in reducing the risk of life-threatening VA. Second, imaging data for re-evaluation of the change in RV fraction area were available only up to 2010, when the revised Task Force Criteria were published; hence, this parameter was not considered, which resulted in the loss of a large amount of data. Third, RV size and systolic function were evaluated by two-dimensional echocardiography; even though there is some degree of association with three-dimensional results (18), there is also the potential for inaccuracy considering the complex geometry of the RV chamber.
ARVC is a progressive disease characterized by continuous ventricular deterioration. A higher degree of RV dysfunction and fibrosis is associated with an increased risk of life-threatening VA. By reducing myocardial fibrosis and restoring RV function, ACEI/ARB might provide an anti-arrhythmic benefit to patients with ARVC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of Fuwai Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
YY conceived the study and formulated the analysis plan. LZ supervised the data collection. LL, SL, and LS collected and organized the clinical data and carried out the routine follow-up. LW performed the statistical analyses and edited the manuscript. ZZ re-interpreted the transthoracic echocardiogram results. BT wrote the manuscript. All authors contributed to the interpretation of the data, critical revision of the manuscript, and approval of the final version of the manuscript.
This study was supported by the National Key R&D Program of China (Grant number 2017YFC1307800) and National Natural Science Foundation of China (Grant number 81800300). The funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Editage for writing assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.769138/full#supplementary-material
1. Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. (1988) 318:129-33. doi: 10.1056/NEJM198801213180301
2. Miles C, Finocchiaro G, Papadakis M, Gray B, Westaby J, Ensam B, et al. Sudden death and left ventricular involvement in arrhythmogenic cardiomyopathy. Circulation. (2019) 139:1786-97. doi: 10.1161/CIRCULATIONAHA.118.037230
3. Yin K, Ding L, Li Y, Hua W. Long-term follow-up of arrhythmogenic right ventricular cardiomyopathy patients with an implantable cardioverter-defibrillator for prevention of sudden cardiac death. Clin Cardiol. (2017) 40:216-21. doi: 10.1002/clc.22648
4. Rigato I, Corrado D, Basso C, Zorzi A, Pilichou K, Bauce B, et al. Pharmacotherapy and other therapeutic modalities for managing arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Drugs Ther. (2015) 29:171-7. doi: 10.1007/s10557-015-6583-8
5. Marcus GM, Glidden DV, Polonsky B, Zareba W, Smith LM, Cannom DS, et al. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: a report from the North American ARVC Registry. J Am Coll Cardiol. (2009) 54:609-15. doi: 10.1016/j.jacc.2009.04.052
6. Wichter T, Borggrefe M, Haverkamp W, Chen X, Breithardt G. Efficacy of antiarrhythmic drugs in patients with arrhythmogenic right ventricular disease. Results in patients with inducible and noninducible ventricular tachycardia. Circulation. (1992) 86:29-37. doi: 10.1161/01.CIR.86.1.29
7. Corrado D, Wichter T, Link MS, Hauer RN, Marchlinski FE, Anastasakis A, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation. (2015) 132:441-53. doi: 10.1161/CIRCULATIONAHA.115.017944
8. Bala R, Hutchinson MD. Recurrent ventricular tachycardia after catheter ablation in arrhythmogenic right ventricular cardiomyopathy: scar progression or ineffective ablation? J Cardiovasc Electrophysiol. (2019) 30:593-5. doi: 10.1111/jce.13861
9. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. (2019) 40:1850-8. doi: 10.1093/eurheartj/ehz103
10. Bosman LP, Sammani A, James CA, Cadrin-Tourigny J, Calkins H, van Tintelen JP, et al. Predicting arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy: a systematic review and meta-analysis. Heart Rhythm. (2018) 15:1097-107. doi: 10.1016/j.hrthm.2018.01.031
11. Mast TP, James CA, Calkins H, Teske AJ, Tichnell C, Murray B, et al. Evaluation of structural progression in arrhythmogenic right ventricular dysplasia/cardiomyopathy. JAMA Cardiol. (2017) 2:293-302. doi: 10.1001/jamacardio.2016.5034
12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129-200. doi: 10.1093/eurheartj/ehw128
13. Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. (2010) 122:333-40. doi: 10.1161/CIRCULATIONAHA.109.927988
14. Henein MY, O'Sullivan CA, Coats AJ, Gibson DG. Angiotensin-converting enzyme (ACE) inhibitors revert abnormal right ventricular filling in patients with restrictive left ventricular disease. J Am Coll Cardiol. (1998) 32:1187-93. doi: 10.1016/S0735-1097(98)00412-4
15. Rouleau JL, Kapuku G, Pelletier S, Gosselin H, Adam A, Gagnon C, et al. Cardioprotective effects of ramipril and losartan in right ventricular pressure overload in the rabbit: importance of kinins and influence on angiotensin II type 1 receptor signaling pathway. Circulation. (2001) 104:939-44. doi: 10.1161/hc3401.093149
16. Fonarow GC, Chelimsky-Fallick C, Stevenson LW, Luu M, Hamilton MA, Moriguchi JD, et al. Effect of direct vasodilation with hydralazine versus angiotensin-converting enzyme inhibition with captopril on mortality in advanced heart failure: the Hy-C trial. J Am Coll Cardiol. (1992) 19:842-50. doi: 10.1016/0735-1097(92)90529-V
17. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J. (2010) 31:806-14. doi: 10.1093/eurheartj/ehq025
18. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. (2005) 18:1440-63. doi: 10.1016/j.echo.2005.10.005
19. Bhonsale A, James CA, Tichnell C, Murray B, Gagarin D, Philips B, et al. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter-defibrillator implantation for primary prevention. J Am Coll Cardiol. (2011) 58:1485-96. doi: 10.1016/j.jacc.2011.06.043
20. Taggart P, Sutton PM, Treasure T, Lab M, O'Brien W, Runnalls M, et al. Monophasic action potentials at discontinuation of cardiopulmonary bypass: evidence for contraction-excitation feedback in man. Circulation. (1988) 77:1266-75. doi: 10.1161/01.CIR.77.6.1266
21. Yusuf S, Pitt B, Davis CE, Hood WB Jr., Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. (1992) 327:685–91. doi: 10.1056/NEJM199209033271003
22. Greenberg B, Quinones MA, Koilpillai C, Limacher M, Shindler D, Benedict C, et al. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. (1995) 91:2573-81. doi: 10.1161/01.CIR.91.10.2573
23. Morel E, Manati AW, Nony P, Maucort-Boulch D, Bessiere F, Cai X, et al. Blockade of the renin-angiotensin-aldosterone system in patients with arrhythmogenic right ventricular dysplasia: A double-blind, multicenter, prospective, randomized, genotype-driven study (BRAVE study). Clin Cardiol. (2018) 41:300-6. doi: 10.1002/clc.22884
24. Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med. (1996) 335:1190-6. doi: 10.1056/NEJM199610173351604
25. Li D, Liu Y, Maruyama M, Zhu W, Chen H, Zhang W, et al. Restrictive loss of plakoglobin in cardiomyocytes leads to arrhythmogenic cardiomyopathy. Hum Mol Genet. (2011) 20:4582-96. doi: 10.1093/hmg/ddr392
26. Dubash AD, Kam CY, Aguado BA, Patel DM, Delmar M, Shea LD, et al. Plakophilin-2 loss promotes TGF-β1/p38 MAPK-dependent fibrotic gene expression in cardiomyocytes. J Cell Biol. (2016) 212:425-38. doi: 10.1083/jcb.201507018
27. Ihrie RA, Reczek E, Horner JS, Khachatrian L, Sage J, Jacks T, et al. Perp is a mediator of p53-dependent apoptosis in diverse cell types. Curr Biol. (2003) 13:1985-90. doi: 10.1016/j.cub.2003.10.055
28. Fang QQ, Wang XF, Zhao WY, Ding SL, Shi BH, Xia Y, et al. Angiotensin-converting enzyme inhibitor reduces scar formation by inhibiting both canonical and noncanonical TGF-beta1 pathways. Sci Rep. (2018) 8:3332. doi: 10.1038/s41598-018-21600-w
29. Agata J, Chao L, Chao J. Kallikrein gene delivery improves cardiac reserve and attenuates remodeling after myocardial infarction. Hypertension. (2002) 40:653-9. doi: 10.1161/01.HYP.0000036035.41122.99
30. Ocaranza MP, Godoy I, Jalil JE, Varas M, Collantes P, Pinto M, et al. Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. (2006) 48:572-8. doi: 10.1161/01.HYP.0000237862.94083.45
31. Yamada K, Iyer SN, Chappell MC, Ganten D, Ferrario CM. Converting enzyme determines plasma clearance of angiotensin-(1-7). Hypertension. (1998) 32:496-502. doi: 10.1161/01.HYP.32.3.496
32. Sarrias A, Bayes-Genis A. Is sacubitril/valsartan (Also) an antiarrhythmic drug? Circulation. (2018) 138:551-3. doi: 10.1161/CIRCULATIONAHA.118.034755
33. Makkar KM, Sanoski CA, Spinler SA. Role of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and aldosterone antagonists in the prevention of atrial and ventricular arrhythmias. Pharmacotherapy. (2009) 29:31-48. doi: 10.1592/phco.29.1.31
34. Katragadda S, Arora RR. Role of angiotensin-converting enzyme inhibitors in vascular modulation: beyond the hypertensive effects. Am J Ther. (2010) 17:e11-23. doi: 10.1097/MJT.0b013e31815addd9
35. Correale M, Zicchino S, Monaco I, Di Biase M, Brunetti ND. Angiotensin-converting enzyme inhibitors, angiotensin II receptors antagonists, beta-blockers and ivabradine as supportive therapy in pulmonary hypertension: Drug safety and tolerability. Eur J Intern Med. (2017) 44:e24–e27. doi: 10.1016/j.ejim.2017.07.016
36. Dean JW, Lab MJ. Arrhythmia in heart failure: role of mechanically induced changes in electrophysiology. Lancet. (1989) 1:1309-12. doi: 10.1016/S0140-6736(89)92697-4
Keywords: angiotensin-converting enzyme inhibitors, arrhythmogenic right ventricular cardiomyopathy, anti-arrhythmic drug, ventricular arrhythmia, disease progression
Citation: Tu B, Wu L, Zheng L, Liu S, Sheng L, Liu L, Zhu Z and Yao Y (2021) Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers: Anti-arrhythmic Drug for Arrhythmogenic Right Ventricular Cardiomyopathy. Front. Cardiovasc. Med. 8:769138. doi: 10.3389/fcvm.2021.769138
Received: 01 September 2021; Accepted: 25 October 2021;
Published: 12 November 2021.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Giuseppe Coppola, Policlinico “P. Giaccone”, ItalyCopyright © 2021 Tu, Wu, Zheng, Liu, Sheng, Liu, Zhu and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Yao, aWFueWFvQDI2My5uZXQuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.