- 1Division of Cardiology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 2Faculty of Medicine Siriraj Hospital, Her Majesty's Cardiac Center, Mahidol University, Bangkok, Thailand
Objectives: This study aimed to investigate the incidence of 1-year major adverse cardiac events (MACE) compared between intravascular imaging guidance and angiographic guidance in patients undergoing rotablator atherectomy (RA)-assisted percutaneous coronary intervention (PCI) with drug-eluting stent (DES) implantation.
Methods: This retrospective analysis included 265 consecutive patients with heavy calcified lesion who underwent RA-assisted PCI with DES implantation at our institution during the January 2016-December 2018 study period. This study was approved by the Siriraj Institutional Review Board. Patients were divided into either the angiographic guidance PCI group or the imaging guidance PCI group, which was defined as intravascular ultrasound or optical coherence tomography. The primary endpoint was 1-year MACE.
Results: Two hundred and sixty-five patients were enrolled, including 188 patients in the intravascular imaging guidance group, and 77 patients in the angiographic guidance group. One-year MACE was significantly lower in the imaging guidance group compared to the angiographic guidance group (4.3 vs. 28.9%, respectively; odds ratio (OR): 9.06, 95% CI: 3.82–21.52; p < 0.001). The 1-year rates of all-cause death (OR: 8.19, 95% CI: 2.15–31.18; p = 0.002), myocardial infarction (MI) (OR: 6.13, 95% CI: 2.05–18.3; p = 0.001), and target vessel revascularization (TVR) (OR: 3.67, 95% CI: 1.13–11.96; p = 0.031) were also significantly lower in the imaging guidance group compared with the angiographic guidance group. The rate of stroke was non-significantly different between groups.
Conclusion: In patients with heavy calcified lesion undergoing RA-assisted DES implantation, the intravascular imaging guidance significantly reduced the incidence of 1-year MACE, all-cause death, MI, and TVR compared to the angiographic guidance.
Introduction
Superficial coronary calcified atherosclerotic plaque is a predictor of worse prognosis (1) after percutaneous coronary intervention (PCI), and is associated with a greater risk of complications (2, 3). Plaque modification procedures, such as rotational atherectomy (RA) and orbital atherectomy, improve vessel compliance and enhance stent optimization and expansion. Intravascular imaging demonstrates both plaque morphology and the outcome of stent optimization. The characteristics of coronary calcification based on intravascular imaging predict suboptimal stent expansion (4, 5) and determine the need for plaque modification. The role and benefit of routine use of intravascular imaging is not yet fully understood since the intravascular imaging catheter may not be able to pass through a cross severely calcified lesion. The cost of PCI is also significantly increased when adding both coronary imaging and RA. Conflicting results have been reported regarding the effect of the routine use of intravascular imaging guidance on major adverse cardiovascular events (MACE) compared to angiographic guidance. The results of some randomized trials in drug-eluting stent (DES) implantation reported that routine imaging guidance (intravascular ultrasound [IVUS], optical coherence tomography [OCT]) may not be recommended for all procedures (6, 7). Other studies reported that the use of intravascular imaging guidance facilitated larger postinterventional minimal luminal diameter and lower MACE, especially in complex lesion, such as chronic total occlusion, diffuse long lesion, bifurcation lesion and left main disease (8–20). Data supporting the benefit of intravascular imaging guidance compared to angiographic guidance in the patients with heavily calcified lesion undergoing RA-assisted DES implantation remain scarce. Accordingly, the aim of this study was to investigate the incidence of 1-year MACE compared between intravascular imaging guidance and angiographic guidance in patients undergoing RA-assisted DES implantation.
Materials and Methods
Study Design and Patients
This single-center retrospective cohort study enrolled consecutive patients who underwent RA with or without intracoronary imaging at the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. The protocol for this study was approved by the Siriraj Institutional Review Board (SIRB) (COA number Si 349/2020). This study complied with all of the principles set forth in the Declaration of Helsinki (1964) and all of its later amendments. All consecutive patients aged >18 years who underwent rotational atherectomy (RA) and DES implantation during January 2016 to December 2018. Patients who underwent plain balloon angioplasty (POBA) or who received a bare metal stent were excluded. Operators who perform <100 PCI procedures per year were not permitted to participate in this study. Patients were divided in two groups based on RA with and without intracoronary imaging. The use of intracoronary imaging and the type of intracoronary imaging (IVUS or OCT) was decided based on operator discretion. In the angiographic guidance group, stent diameter and length were determined by visual estimation.
All patients received a bolus injection of heparin 100 unit/kilogram to maintain activated clotting time >250 s. Dual antiplatelet therapy with 81 mg/day aspirin with either 75 mg/day clopidogrel or 10 mg/day prasugrel or 90 mg twice a day ticagrelor was continued for at least a year after the procedure. The conventional 0.014-inch guidewire was replaced with a 0.009-inch ROTAWireTM floppy guidewire (Boston Scientific, Marlborough, MA, USA) or a 0.009-inch ROTAWireTM extra support guidewire (Boston Scientific). The initial burr size was chosen based on angiographically determined vessel size. A step-up approach starting with a 1.25, 1.5, and 1.75 burr was recommended according to European expert consensus on rotational atherectomy (21). In the intravascular imaging guidance group, burr size selection was based on preprocedural intravascular imaging if the lesion could be crossed with imaging. The selected burr speed was 140,000–180,000 revolutions per minute (RPM) with a run duration of 10–15 s. The final burr to artery ratio was targeted within 0.4–0.6. In cases where imaging could not cross the lesion before the procedure, preprocedural imaging was performed after RA. Non-compliance balloon or cutting balloon was routinely used for pre-dilatation before stent implantation. In the intravascular imaging guidance group, the stent diameter was determined by measuring the external elastic lamina diameter at the proximal and distal reference sites. In the angiographic guidance group, the stent diameter was determined by visual estimation. In the intravascular imaging guidance group, post procedure imaging was performed after stent implantation to evaluate for stent apposition, stent optimization, and procedural complication, such as dissection and tissue protrusion. A frequency-domain OCT system (ILUMIENTM OPTISTM; Abbott Vascular, Santa Clara, CA, USA) was used for OCT-guided RA. For IVUS-guided RA, either an OptiCrossTM system (Boston Scientific) or an Eagle Eye PlatinumTM system (Philips Healthcare, Amsterdam, Netherlands) was used based upon operator discretion.
Baseline demographic and clinical characteristics were collected and recorded. Procedural characteristics, such as target vessel, stenosis diameter, type and size of stent, and RA details, such as number and burr size, and procedure duration were also recorded. We also collected procedural complications (angiographic dissection, perforation, or acute closure) and additional required interventions (prolonged balloon inflations, additional stent implantation, intra-aortic balloon pump [IABP], pericardiocentesis). Follow-up data were obtained from our center's database and from telephone calls made to patients or their family.
Quantitative coronary angiography (QCA) analysis was performed using a cardiovascular measurement system (QAngio XA 7.2; MEDIS, Leiden, Netherlands) by an experienced operator who was blinded to the patient's group. Optimal views of lesions were obtained at baseline and after the procedure at the same angle projection.
Study Endpoints
We defined myocardial infarction (MI) according to the consensus definition of the Society of Cardiovascular Angiography and Interventions (22), and stent thrombosis according to the definite or probable criteria of the Academic Research Consortium (23).
The primary endpoint was 1-year MACE, which was defined as all-cause death, myocardial infarction (MI), stroke, or target vessel revascularization (TVR). The secondary endpoint was in-hospital MACE, which was defined as all-cause death, MI, stroke, or TVR before hospital discharge.
Statistical Analysis
The sample size was calculated based on the following data from Gorol (24) and Sakakura (25): event rate of 15% in the angiographic guidance group (24), and an event rate of 1.33% in the intravascular imaging guidance group (25). Using this information, our power analysis and sample size calculation revealed that a sample size of 260 patients would yield 80% statistical power to detect the effect size between groups (alpha = 0.05, beta = 0.20).
Comparisons of continuous data with normal distribution were made using Student's t-test, and using Mann–Whitney U test for non-normally distributed data. Continuous variables are expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical variables were compared using chi-square test or Fisher's exact test, and those results are presented as number and percentage. Outcomes are expressed as proportions, and their variability as 95% confidence intervals. Logistic regression analysis was used to determine the independent effect of variables on outcome. The following variables were included in the analysis: history of previous coronary artery bypass graft (CABG), stent length, maximum stent size, maximum balloon, left anterior descending artery lesion, number of RA used, maximum RA size, and imaging-guided RA DES placement. A p ≤ 0.05 was defined as indicating statistical significance. All statistical analyses were performed using SPSS statistics version 18 (SPSS, Inc., Chicago, IL, USA).
Results
Two-hundred and eighty-eight patients underwent RA-assisted PCI during the study period. Twenty-three patients were excluded because drug eluting stent was not deployed. Two hundred and sixty-five patients were enrolled, including 188 patients in the intravascular imaging guidance group, and 77 patients in the angiographic guidance group. A flow diagram showing the patient enrollment process is shown in Figure 1. One-year clinical follow-up was 98% in both groups, as follows: 186 of 188 patients in the intravascular imaging guidance group, and 76 of 77 patients in the angiographic guidance group.
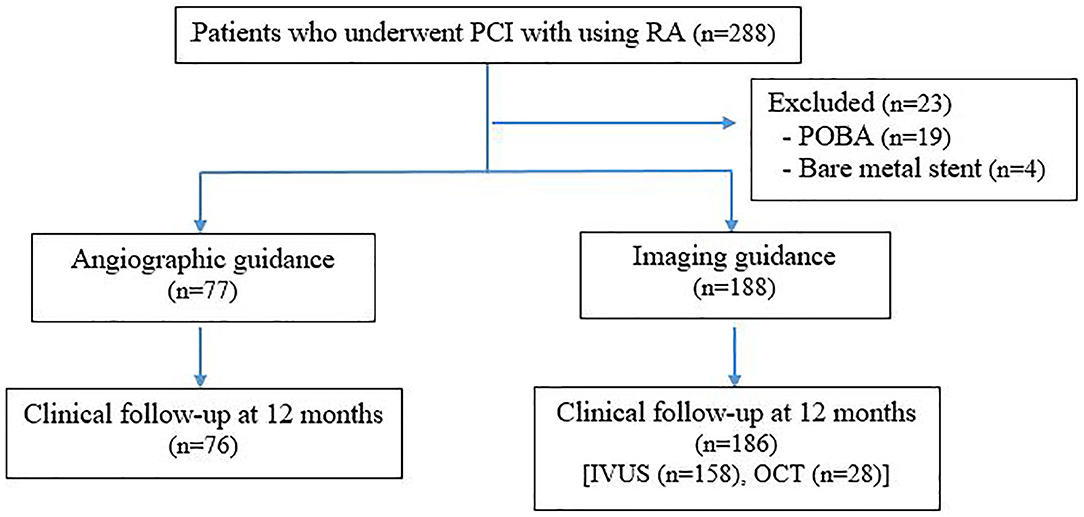
Figure 1. Flowchart of the patient enrollment process. PCI, percutaneous coronary intervention; RA, rotational atherectomy; POBA, percutaneous old balloon angioplasty; IVUS, intravascular ultrasound; OCT, optical coherence tomography.
Baseline demographic and clinical characteristics of patients are shown in Table 1. History of prior coronary artery bypass was significantly higher in the angiographic guidance group. Procedural characteristics are shown in Table 2. Reference vessel diameter, minimal lumen diameter and diameter stenosis by QCA before and after stenting were not significant between group. Patients in the intravascular imaging guidance group had a larger acute gain (1.91 vs 1.77, p < 0.039), larger stent diameter (3.00 vs. 2.75 mm, p < 0.001) and longer stent length (38.0 vs. 33.0 mm, p = 0.004) compared to patients in the angiographic guidance group. Patients in the intravascular imaging guidance group also had significantly more RA burrs (1.210 ± 0.42 vs. 1.070 ± 0.31, p = 0.005) and larger RA size compared to the angiographic guidance group. Radiation dose was lower in the intravascular imaging guidance group compared to the angiographic guidance group (2,727 vs. 3,335 mGy, p = 0.025). The success rates were similar between groups. Intravascular imaging findings are shown in Table 3.
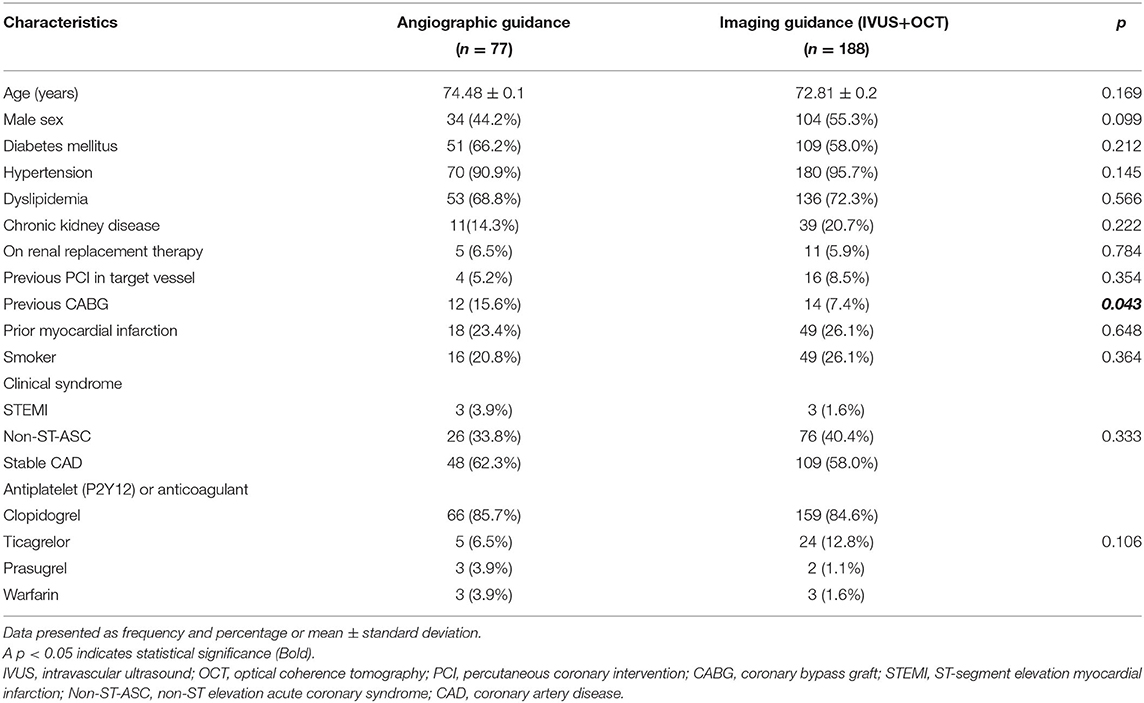
Table 1. Baseline demographic and clinical characteristics compared between the angiographic and intravascular imaging guidance groups.
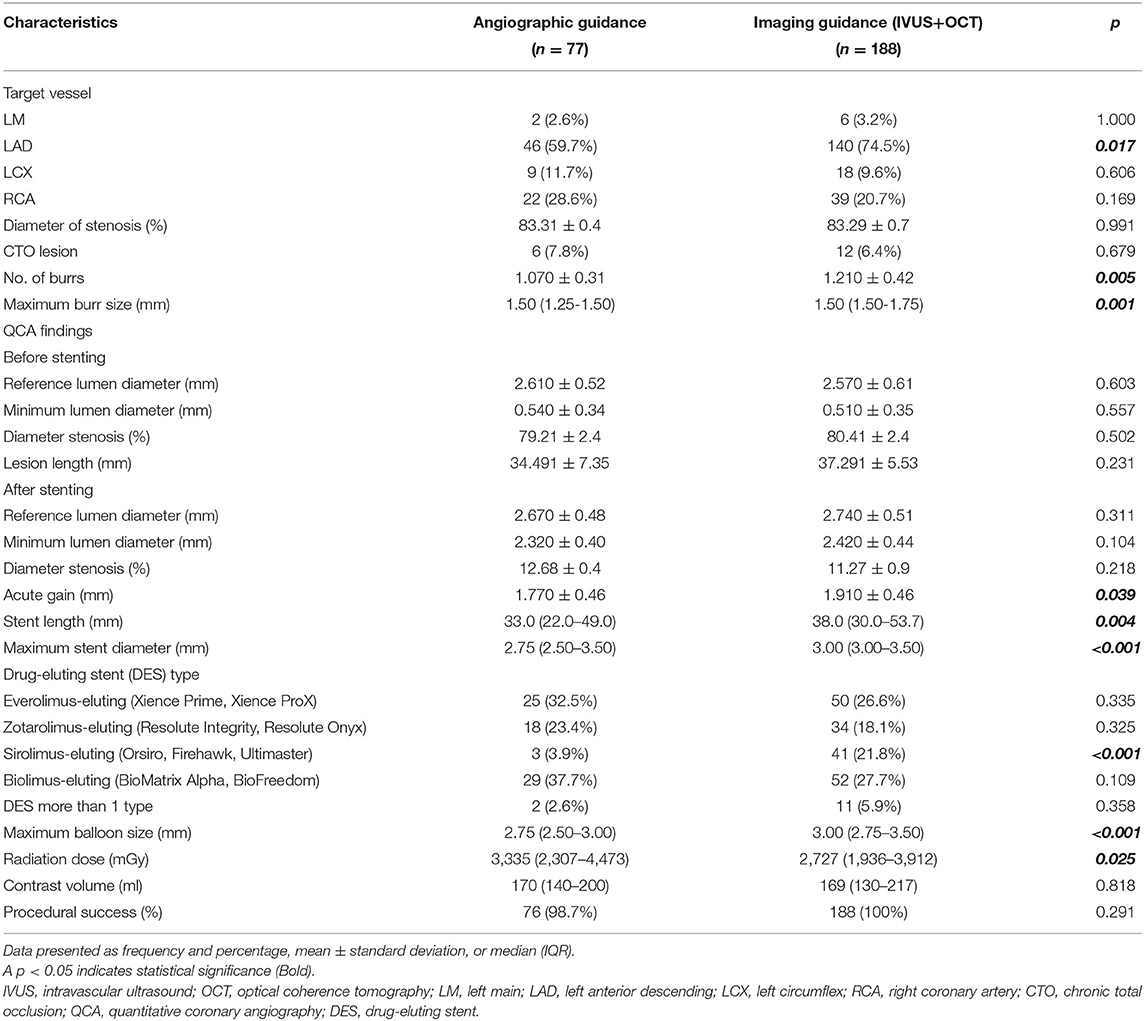
Table 2. Procedural characteristics compared between the angiographic and intravascular imaging guidance groups.
1-Year Clinical Outcomes
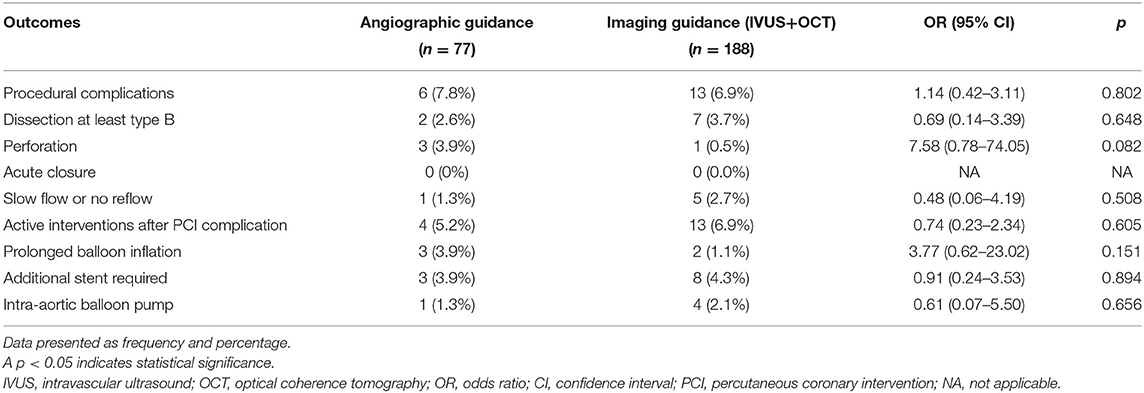
As shown in Figure 2 (Central illustration), the unadjusted 1-year rate of MACE was significantly lower in the intravascular imaging guidance group compared with the angiographic guidance group (4.3% [8 events] vs. 28.9% [22 events]; odds ratio (OR): 9.06, 95% confidence interval (CI): 3.82–21.52; p < 0.001). The 1-year unadjusted rates for all-cause death (OR: 8.19, 95% CI: 2.15–31.18; p = 0.002), MI (OR: 6.13, 95% CI: 2.05–18.30; p = 0.001), and TVR (OR: 3.67, 95% CI: 1.13–11.96; p = 0.031) were also significantly lower in the intravascular imaging guidance group than in the angiographic guidance group. The incidence of stroke was similar between groups. The overall 1-year rate of adjusted MACE was also significantly lower in the intravascular imaging guidance group compared with the angiographic guidance group (adjusted OR: 9.90, 95% CI: 3.78–25.93; p < 0.001). The observed significant decrease in MACE incidence was driven by reductions in all-cause death, MI, and TVR. Procedural complications, and active interventions after complications occurred were not significantly different between the angiographic and intravascular imaging guidance groups (Table 4).
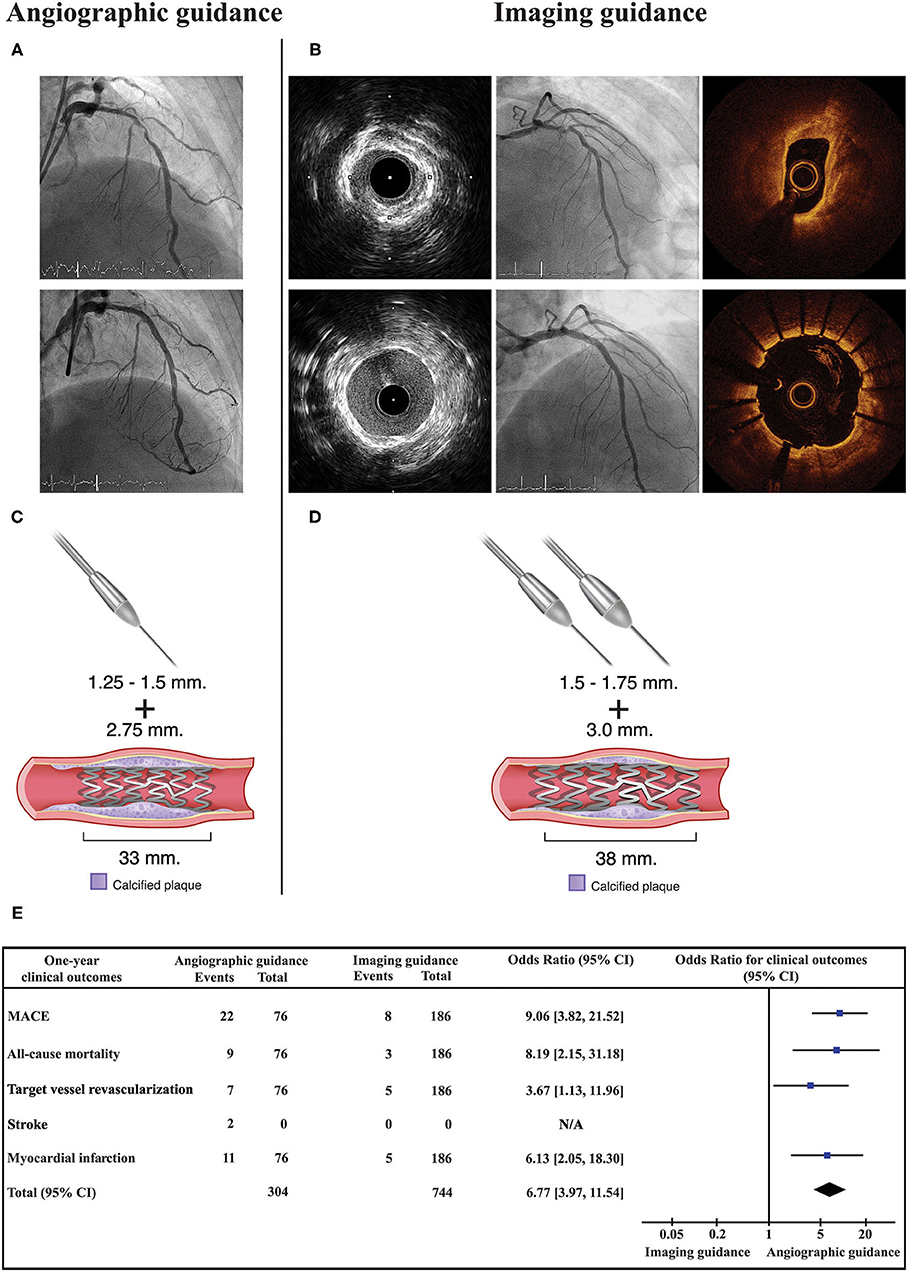
Figure 2. Central illustration. Compared strategy between the angiographic guidance and the intravascular imaging guidance in patients with heavy calcified lesion undergoing RA-assisted PCI. Angiographic guidance, coronary angiogram pre and post stent implantation is shown in (A). Imaging guidance, coronary angiogram and intravascular imaging (IVUS or OCT) pre and post stent implantation is shown in (B). (C,D) illustrated that the intravascular imaging guidance resulted in a greater number of RA burrs and larger RA burrs used, larger stent diameter and longer stent length. Forest plot presented odds ratio for one-year clinical outcomes compared between the angiographic guidance and the intravascular imaging guidance (E). Intravascular imaging guidance had a significantly lower incidence of 1-year MACE. RA, rotational atherectomy; PCI, percutaneous coronary intervention; OCT, optical coherence tomography.
Discussion
The present study investigated the benefit of intravascular imaging guidance compared to angiographic guidance based on the incidence of 1-year MACE in patients with heavy calcified lesion who underwent RA-assisted DES implantation. The main findings of the study were, as follows: (1) Intravascular imaging guidance resulted in more appropriate final RA burr used according to target burr-to-artery ratio, with additional RA burrs used for adequate plaque modification; (2) Intravascular imaging guidance had larger acute gain, larger stent diameter and longer stent length; and, (3) Intravascular imaging guidance had a significantly lower incidence of 1-year MACE, driven by reduction of all-cause death, MI and TVR.
Intravascular imaging guidance facilitated an appropriate burr to artery ratio. An aggressive burr to artery ratio of >0.7 yields no advantage on clinical success or target vessel revascularization, but is associated with higher procedural complications (26, 27). European expert consensus (21) advice recommends a burr to artery ratio of 0.6. North American expert consensus (28) and Japanese expert consensus (29) recommend a burr to artery ratio of 0.4–0.6. Japanese expert consensus recommended the use of intravascular imaging if the operator aimed to achieve a burr to artery ratio of >0.6. Aggressive burr to artery ratio when the size of the vessel is overestimated can cause vessel perforation, dissection, and acute vessel closure. Angiographic guidance alone may underestimate true vessel size, especially in diffuse heavily calcified lesion. For pre-procedural assessment, intravascular imaging guidance helps in estimate true vessel size. Intravascular imaging provided not only proximal and distal reference vessel diameter, but also plaque characteristics, such as calcium distribution, thickness, and length. It is suggested that the operator opt for a larger RA burr without exceeding a burr to artery ratio of 0.6, and that the operator be more aggressive in the size of the device in more complex lesions. Even though a single burr strategy for lesion modification can be accomplished in the majority of cases. An additional RA burr will be required if calcified nodule, residual calcium thickness, and/or inadequate calcium fracture are detected by intravascular imaging. Intravascular imaging also gives an information of post-procedural assessment such as stent expansion, stent apposition, and complication detection. All these information from intravascular imaging guidance would lead to additional procedures such as post stent high pressure balloon dilatation in order to achieve adequate stent expansion and better stent apposition. This was supported by larger acute lumen gain with the minimum stent area (MSA) of 6.82 mm2 were achieved in the intravascular image guidance. Because of the complexity of the calcified lesions, stent malapposition still occurred in 16.8% of patients in the intravascular imaging guidance. The rate of stent malapposition could even higher in the angiographic guidance because of lacking information in preprocedural and post-procedural assessment from intravascular imaging. The observed benefit of intravascular imaging guidance PCI when using larger diameter stents and/or longer stents is similar to the result reported from the ADAPT DES trial (10), which compared between IVUS guidance PCI and angiographic guidance PCI in all-comers. It should be noted that radiation dose was significant lower in the intravascular imaging guidance.
Ali et al. (16) found a similar minimum stent area between the OCT guidance PCI and the IVUS guidance PCI. MACE was not significantly different between the intravascular imaging guidance PCI and the angiographic guidance PCI; however, that study recruited all-comers, and moderate to severe calcification was found in 16–26% of patients in each group. In the calcified lesions, calcium angle, calcium length and calcium thickness predicted stent expansion (5). IVUS has difficulty penetrating calcium depth but OCT has advantage in providing calcium thickness. Wang et al. (30) demonstrated that IVUS detected smooth surface with reverberation pattern was associated with the thinner calcium by OCT compared to IVUS-detected irregular surface without reverberation. Norihiro et al. (31) compared the OCT guidance PCI and the IVUS guidance PCI in patients with heavily calcified lesion who underwent RA. Percent stent expansion was larger in the OCT guidance RA group. They observed a non-significant trend toward lower TVR in the OCT guidance RA group. There were only 11 patients without intravascular imaging from 247 patients who underwent RA, and the clinical outcomes of the patients in the angiographic guidance PCI group were not provided. TVR was 6.8 and 11.6% in the OCT guidance and IVUS guidance PCI groups, respectively. In the present study, TVR was 2.7% in the intravascular imaging guidance PCI group. This observed difference in TVR between studies is likely due to differences in patients baseline characteristic. Twenty percent of patients were on hemodialysis in the Kobayashi study compared to 6% in our study.
The 1-year incidence of MACE in the intravascular imaging guidance group was significantly lower than in the angiographic guidance group. This was driven not only by TVR, but by all-cause death and MI. Shin et al. (32) conducted a meta-analysis of 2,345 patients with complex lesion who underwent DES implantation, and they found benefit of the IVUS guidance PCI over the angiographic guidance PCI for reducing MACE. They found a significant decrease in MI, but not in all-cause death. However, that meta-analysis included three randomized controlled trials that defined complex lesion as chronic total occlusion and long coronary lesion. Elgendy et al. (11) conducted a meta-analysis of seven randomized controlled trials that enrolled 3,192 patients. They also reported the benefit of the IVUS guidance PCI over the angiographic guidance PCI for reducing the incidence of MACE. In that study, TVR was significantly reduced, but only a non-significant trend was observed for mortality and stent thrombosis. Similar to the study by Shin, patients recruited in the Elgendy study was mainly chronic total occlusion and long lesion. Jang et al. (9) conducted a meta-analysis that included three randomized controlled trials and 12 observation studies with a total of 24,849 patients. Broader lesion characteristic, such as simple lesion, multivessel, chronic total occlusion, and left main (LM) disease, were included. In that study, the IVUS guidance PCI had lower MACE, all-cause mortality, MI, and TVR compared to the angiographic guidance PCI. That study reported no information regarding the percentage patients with heavily calcified lesion who underwent RA. Alsidawi et al. (33) conducted a meta-analysis of 10 randomized controlled trials that included 14,197 patients that underwent the intravascular imaging guidance PCI with drug eluting stent or bare metal stent placement. They found that the intravascular imaging guidance PCI significantly lowered the rates of death, MI, stent thrombosis, and MACE. However, the intravascular image guidance PCI had no effect on TVR in patients with DES, likely due to the effect of DES. The main findings of our study are consistent with those reported from previously published meta-analysis that found that the intravascular imaging guidance PCI reduced all-cause death, MI, TVR, and MACE. However, the results of our study provide additional benefit because we focused on patients with heavily calcified lesion who underwent RA-assisted DES placement. Superficial calcification is a predictor of worse prognosis. Fujino et al. (5) demonstrated OCT based calcium score identify lesion that need plaque modification. It is important process in decision making when dealing with heavy calcified lesion. Theoretically, either OCT or IVUS should be incorporated in a clinical algorithm to guide PCI strategy in calcified lesion to improve clinical outcomes. Intravascular imaging guidance PCI provides information specific to the necessity of plaque modification, true vessel sizing leads to use of appropriate burr-to-artery ratio, larger stent diameter and longer stent length, detection of procedural complications, and improving stent expansion and optimization.
Study Limitations
This study has some mentionable limitations. First, our study was an observational, retrospective, single-center study, so our results should be interpreted with caution. The two study groups were non randomized, we cannot exclude the selection bias between patients with and without intravascular imaging guidance RA that could be influencing the study results. Second, pre-angiographic data based on QCA were similar between groups. Even investigators were blinded to patient's group. We could not exclude inter-operator variability in QCA measurement. Third, we included both IVUS guidance RA and OCT guidance RA so we could obtain as large a sample size as possible in the intravascular image guidance group. However, the number of patients was too small for subgroup analysis to detect significant difference between these two imaging techniques. Fourth, we do not have intravascular data in angiographic guidance patients to explain the cause of worse 1-year outcome compared to the intravascular imaging guidance group. There was no significant difference between groups for immediate procedural complications or in-hospital events. A possible cause of worse 1-year outcome could be stent malapposition and stent under expansion in angiographic guidance. Fifth, this study also lacked of a follow up imaging study that would explain the different in long-term procedural outcomes between groups. Further study in a randomized controlled trial is needed to confirm the benefit of intravascular imaging guidance in patients with heavily calcified lesion undergoing RA before recommended the routine use of intravascular imaging guidance in RA-assisted DES implantation.
Conclusion
In patients with heavy calcified lesion undergoing RA-assisted DES implantation, intravascular imaging guidance significantly reduced the incidence of 1-year MACE, driven by reduction of all-cause death, MI, and TVR compared to angiographic guidance.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Siriraj Institutional Review Board (SIRB) of the Faculty of Medicine Siriraj Hospital, Mahidol University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
NatW contributed to conception and design of the study and completed the writing review and editing. PB wrote the first draft of the manuscript. PB and KA collected the data. NatW, DT, CC, RPh, WT, NC, KT, AP, PaP, and PrP participated in the study. NatW, NamW, and KA contributed to the review of quantitative coronary angiography data and intravascular imaging data. NatW, PB, and RPo performed statistical data analysis and interpretation. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to thank Suthipol Udompunturak of the division of Clinical Epidemiology, Department of Research and Development, Faculty of Medicine Siriraj Hospital for statistical analysis, Yanisa Krimouy of Medical Education Technology Center, Faculty of Medicine Siriraj Hospital for drawing the image of central illustration.
Abbreviations
CABG, coronary artery bypass graft; DES, drug-eluting stent; IVUS, intravascular ultrasound; MACE, major adverse cardiovascular event; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; POBA, plain open balloon angioplasty; QCA, quantitative coronary angiography; RA, rotational atherectomy; TVR, target vessel revascularization.
References
1. Bourantas CV, Zhang YJ, Garg S, Iqbal J, Valgimigli M, Windecker S, et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart. (2014) 100:1158–64. doi: 10.1136/heartjnl-2013-305180
2. Gilutz H, Weinstein JM, Ilia R. Repeated balloon rupture during coronary stenting due to a calcified lesion: an intravascular ultrasound study. Catheter Cardiovasc Interv. (2000) 50:212–4. doi: 10.1002/(sici)1522-726x(200006)50:2<212:aid-ccd15>3.0.co;2-t
3. Pershad A, Buchbinder M. Management of calcified lesions in 2004. Int J Cardiovasc Intervent. (2005) 7:199–204. doi: 10.1080/14628840500339512
4. Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging. (2015) 8:461–71. doi: 10.1016/j.jcmg.2015.02.003
5. Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. (2018) 13:e2182–9. doi: 10.4244/eij-d-17-00962
6. Schiele F, Meneveau N, Vuillemenot A, Zhang DD, Gupta S, Mercier M, et al. Impact of intravascular ultrasound guidance in stent deployment on 6-month restenosis rate: a multicenter, randomized study comparing two strategies with and without intravascular ultrasound guidance. RESIST Study Group. REStenosis after IVUS guided STenting. J Am Coll Cardiol. (1998) 32:320–8. doi: 10.1016/s0735-1097(98)00249-6
7. Mudra H, di Mario C, de Jaegere P, Figulla HR, Macaya C, Zahn R, et al. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent re-stenosis (OPTICUS Study). Circulation. (2001) 104:1343–9. doi: 10.1161/hc3701.096064
8. Zhang YJ, Pang S, Chen XY, Bourantas CV, Pan DR, Dong SJ, et al. Comparison of intravascular ultrasound guided versus angiography guided drug eluting stent implantation: a systematic review and meta-analysis. BMC Cardiovasc Disord. (2015) 15:153. doi: 10.1186/s12872-015-0144-8
9. Jang JS, Song YJ, Kang W, Jin HY, Seo JS, Yang TH, et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. (2014) 7:233–43. doi: 10.1016/j.jcin.2013.09.013
10. Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. (2014) 129:463–70. doi: 10.1161/circulationaha.113.003942
11. Elgendy IY, Mahmoud AN, Elgendy AY, Bavry AA. Outcomes with intravascular ultrasound- guided stent implantation: a meta-analysis of randomized trials in the era of drug-eluting stents. Circ Cardiovasc Interv. (2016) 9:e003700. doi: 10.1161/circinterventions.116.003700
12. Ahn JM, Kang SJ, Yoon SH, Park HW, Kang SM, Lee JY, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. (2014) 113:1338–47. doi: 10.1016/j.amjcard.2013.12.043
13. Hong SJ, Kim BK, Shin DH, Nam CM, Kim JS, Ko YG, et al. Effect of intravascular ultrasound- guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA. (2015) 314:2155–63. doi: 10.1001/jama.2015.15454
14. Song HG, Kang SJ, Ahn JM, Kim WJ, Lee JY, Park DW, et al. Intravascular ultrasound assessment of optimal stent area to prevent in-stent restenosis after zotarolimus-, everolimus-, and sirolimus- eluting stent implantation. Catheter Cardiovasc Interv. (2014) 83:873–8. doi: 10.1002/ccd.24560
15. Lotfi A, Jeremias A, Fearon WF, Feldman MD, Mehran R, Messenger JC, et al. Expert consensus statement on the use of fractional flow reserve, intravascular ultrasound, and optical coherence tomography: a consensus statement of the Society of Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. [2014] 83:509–18. doi: 10.1002/ccd.25222
16. Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, et al. Optical[[sb]] [[/s]]coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. (2016) 388:2618–28. doi: 10.1016/s0140-6736(16)31922-5
17. Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one- year angiographic and clinical results. Eur Heart J. (2017) 38:3139–47. doi: 10.1093/eurheartj/ehx351
18. Buccheri S, Franchina G, Romano S, Puglisi S, Venuti G, D'Arrigo P, et al. Clinical outcomes following intravascular imaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation: a systematic review and Bayesian network meta- analysis of 31 studies and 17,882 patients. JACC Cardiovasc Interv. (2017) 10:2488–98. doi: 10.1016/j.jcin.2017.08.051
19. Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, et al. Intravascular ultrasound versus angiography- guided drug-eluting stent implantation: The ULTIMATE Trial. J Am Coll Cardiol. (2018) 72:3126–37. doi: 10.1016/j.jacc.2018.09.013
20. de la Torre Hernandez JM, Baz Alonso JA, Gómez Hospital JA, Alfonso Manterola F, Garcia Camarero T, Gimeno de Carlos F, et al. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease: pooled analysis at the patient-level of 4 registries. JACC Cardiovasc Interv. (2014) 7:244–54. doi: 10.1016/j.jcin.2013.09.014
21. Barbato E, Carrié D, Dardas P, Fajadet J, Gaul G, Haude M, et al. European expert consensus on rotational atherectomy. EuroIntervention. (2015) 11:30–6. doi: 10.4244/eijv11i1a6
22. Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. (2013) 62:1563–70. doi: 10.1016/j.jacc.2013.08.720
23. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115:2344–51.doi: 10.1161/circulationaha.106.68531324.
24. Gorol J, Tajstra M, Hudzik B, Lekston A, Gasior M. Comparison of outcomes in patients undergoing rotational atherectomy after unsuccessful coronary angioplasty versus elective rotational atherectomy. Postepy Kardiol Interwencyjnej. (2018) 14:128–34. doi: 10.5114/aic.2018.76403
25. Sakakura K, Inohara T, Kohsaka S, Amano T, Uemura S, Ishii H, et al. Incidence and Determinants of Complications in Rotational Atherectomy: Insights From the National Clinical Data (J-PCI Registry). Circ Cardiovasc Interv. (2016) 9:e004278. doi: 10.1161/circinterventions.116.004278
26. Whitlow PL, Bass TA, Kipperman RM, Sharaf BL, Ho KK, Cutlip DE, et al. Results of the study to determine rotablator and transluminal angioplasty strategy (STRATAS). Am J Cardiol. (2001) 87:699–705. doi: 10.1016/s0002-9149(00)01486-7
27. Safian RD, Feldman T, Muller DW, Mason D, Schreiber T, Haik B, et al. Coronary angioplasty and Rotablator atherectomy trial (CARAT): immediate and late results of a prospective multicenter randomized trial. Catheter Cardiovasc Interv. (2001) 53:213–20. doi: 10.1002/ccd.1151
28. Sharma SK, Tomey MI, Teirstein PS, Kini AS, Reitman AB, Lee AC, et al. North American expert review of rotational atherectomy. Circ Cardiovasc Interv. (2019) 12:e007448. doi: 10.1161/circinterventions.118.007448
29. Sakakura K, Ito Y, Shibata Y, Okamura A, Kashima Y, Nakamura S, et al. Clinical expert consensus document on rotational atherectomy from the Japanese association of cardiovascular intervention and therapeutics. Cardiovasc Interv Ther. (2021) 36:1–18. doi: 10.1007/s12928-020-00715-w
30. Wang X, Matsumura M, Mintz GS, Lee T, Zhang W, Cao Y, et al. In vivo calcium detection by comparing optical coherence tomography, intravascular ultrasound, and angiography. JACC Cardiovasc Imaging. (2017) 10:869–79. doi: 10.1016/j.jcmg.2017.05.014
31. Norihiro K, Yoshiaki I, Masahiro Y, Motoharu A, Masaru O, Yasunari S, et al. Optical coherence tomography-guided versus intravascular ultrasound-guided rotational atherectomy in patients with calcified coronary lesions. EuroIntervention. (2020) 16:e313–21. doi: 10.4244/EIJ-D-19-00725
32. Shin DH, Hong SJ, Mintz GS, Kim JS, Kim BK, Ko YG, et al. Effects of intravascular ultrasound- guided versus angiography-guided new-generation drug-eluting stent implantation: meta- analysis with individual patient-level data from 2,345 randomized patients. JACC Cardiovasc Interv. (2016) 9:2232–9. doi: 10.1016/j.jcin.2016.07.021
Keywords: imaging guidance, heavy calcified lesion, rotablator atherectomy, drug-eluting stent implantation, 1-year MACE
Citation: Wongpraparut N, Bakoh P, Anusonadisai K, Wongsawangkit N, Tresukosol D, Chotinaiwattarakul C, Phankingthongkum R, Tungsubutra W, Chunhamaneewat N, Towashiraporn K, Phichaphop A, Panchavinnin P, Pongakasira R and Panchavinnin P (2021) Intravascular Imaging Guidance Reduce 1-Year MACE in Patients Undergoing Rotablator Atherectomy-Assisted Drug-Eluting Stent Implantation. Front. Cardiovasc. Med. 8:768313. doi: 10.3389/fcvm.2021.768313
Received: 01 September 2021; Accepted: 30 September 2021;
Published: 27 October 2021.
Edited by:
Antonios Karanasos, Hippokration General Hospital, GreeceReviewed by:
Petros Dardas, St. Luke's Hospital, GreeceJiang Ming Fam, National Heart Center Singapore, Singapore
Copyright © 2021 Wongpraparut, Bakoh, Anusonadisai, Wongsawangkit, Tresukosol, Chotinaiwattarakul, Phankingthongkum, Tungsubutra, Chunhamaneewat, Towashiraporn, Phichaphop, Panchavinnin, Pongakasira and Panchavinnin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nattawut Wongpraparut, d29uZ3ByYXBhcnV0QHlhaG9vLmNvbQ==
 Nattawut Wongpraparut
Nattawut Wongpraparut Paroj Bakoh1
Paroj Bakoh1 Kawin Anusonadisai
Kawin Anusonadisai Namthip Wongsawangkit
Namthip Wongsawangkit Asa Phichaphop
Asa Phichaphop Rungtiwa Pongakasira
Rungtiwa Pongakasira