
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 01 December 2021
Sec. Cardiovascular Therapeutics
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.766088
This article is part of the Research Topic Insights in Cardiovascular Therapeutics: 2021 View all 13 articles
 Yong Zhu1
Yong Zhu1 Kesen Liu1
Kesen Liu1 Xiangyun Kong2
Xiangyun Kong2 Jing Nan3
Jing Nan3 Ang Gao1
Ang Gao1 Yan Liu1
Yan Liu1 Hongya Han1*†
Hongya Han1*† Hong Li1
Hong Li1 Huagang Zhu1
Huagang Zhu1 Jianwei Zhang1
Jianwei Zhang1 Yingxin Zhao1*†
Yingxin Zhao1*†Introduction: In-stent restenosis (ISR) remains a challenging issue despite the great advance of drug-eluting stents (DES). In addition, the consensus was lacking regarding the optimal strategy for DES-ISR. Therefore, we aimed to evaluate angiographic and clinical outcomes of the two most effective treatments DES vs. drug-eluting balloon (DCB) for patients with DES-ISR.
Methods: This meta-analysis used the data from the randomized controlled trials (RCTs), which were identified by a systematic search in the databases of PubMed, Embase, and Cochrane Library. Target lesion revascularization (TLR) was regarded as the primary endpoint. In addition, the late angiographic outcomes and other clinical outcomes, namely, cardiac death, myocardial infarction (MI), target vessel revascularization, stent thrombosis, and major adverse cardiac events, were also included for analysis.
Results: Five RCTs with about 1,193 patients were included in this meta-analysis for the analysis. For the primary endpoint, the overall pooled outcomes suggested repeat DES implantation was associated with a significant reduction in the term of TLR compared with DCB angioplasty (risk ratio = 1.53, 95% CI 1.15–2.04, p = 0.003). But no significant difference in angiographic outcomes and other clinical endpoints were observed between DES and DCB. In the subgroup analysis, DCB was inferior to new-generation DES (NG-DES)/everolimus-eluting stent (EES) in the term of TLR. In addition, this non-significant trend was also noted in the subgroup of the paclitaxel-eluting stent (PES) vs. DCB. For the angiographic endpoints, EES, not PES, was associated with larger minimum lumen diameter [mean difference (MD) = −0.25, 95% CI −0.38 to −0.11, p = 0.0003], lower percent diameter stenosis (MD = 7.29%, 95% CI 2.86–11.71%, p = 0.001), and less binary restenosis (OR = 2.20, 95% CI 1.18–4.11, p = 0.01). But NG-DES/EES was comparable to DCB in cardiac death, MI, and stent thrombosis.
Conclusions: For the patients with DES-ISR, treatment with DES, especially NG-DES/EES could reduce the risk of TLR significantly compared to DCB at long-term follow-up.
Drug-eluting stents (DES) were widely used to treat ischemic coronary artery disease (CAD) and it has been recommended as the default choice in patients undergoing percutaneous coronary intervention (PCI) (1–3). Although the antirestenotic performance of DES improves significantly compared to bare-metal stents (BMS), 5–10% of patients treated with DES still suffer from restenosis, especially in complex clinical and anatomic settings (4, 5). Several previous studies also demonstrated patients treated for DES-in-stent restenosis (ISR) may have a worse long-term prognosis compared to these patients with BMS-ISR (6, 7). Therefore, DES restenosis after PCI has become an important and challenging issue in routine clinical practice.
With consistent improvement in the technique, various treatment strategies were performed and tested in those patients with DES-ISR, such as balloon angioplasty, cutting balloon, vascular brachytherapy, rotablation, DES implantation, drug-coated balloon (DCB) angioplasty, and excimer laser (8, 9). Among those strategies, repeat DES implantation and DCB angioplasty have been regarded as the most effective therapeutic options and recommended by current guidelines (Class I, Level A) (3). Although several randomized controlled trials (RCTs) have been designed to compare outcomes of DES vs. DCB in patients presenting with DES-ISR (10–14), few of them were powered for clinical endpoints indeed. In addition, the conclusions obtained from the RCTs were controversial and considerable heterogeneity existed in various aspects of those RCTs such as characteristic of participants, type of restenotic stent, and generation of DES used in the repeat stenting arm (10–14). Furthermore, recent strong pieces of evidence suggested the risk of death increased significantly beyond the 1st year in patients treated with paclitaxel-coated devices for peripheral artery disease (15), which raised concerns on DCB in the field of coronary intervention because almost all DCB used in the routine clinical practice were coated with paclitaxel.
Against this background, a comprehensive meta-analysis of RCTs was performed by us to investigate the angiographic and clinical outcomes of repeat DES vs. DCB angioplasty in patients presenting with DES-ISR.
This meta-analysis was performed according to the preferred reporting items for systematic reviews and meta-analyses.
A systematic literature search was performed by us in the databases of PubMed, Embase, and Cochrane Library to identify all relevant articles published from inception to June 19, 2021. The three types of search terms (and their similar terms) used in this study were listed as follows: in-stent restenosis OR coronary stent restenosis OR stent restenosis OR restenosis OR ISR; drug-eluting stent OR drug eluting stent OR drug-coated stent OR drug coated stent OR everolimus-eluting stent OR everolimus eluting stent OR everolimus-coated stent OR everolimus coated stent OR zotarolimus-eluting stent OR zotarolimus eluting stent OR zotarolimus-coated stent OR zotarolimus coated stent OR sirolimus-eluting stent OR sirolimus eluting stent OR sirolimus-coated stent OR sirolimus coated stent OR paclitaxel-eluting stent OR paclitaxel eluting stent OR paclitaxel-coated stent OR paclitaxel coated stent OR DES OR EES OR ZES OR SES OR PES; drug-coated balloon OR drug coated balloon OR drug-eluting balloon OR drug eluting balloon OR paclitaxel-coated balloon OR paclitaxel coated balloon OR paclitaxel-eluting balloon OR paclitaxel eluting balloon OR sirolimus-coated balloon OR sirolimus coated balloon OR sirolimus-eluting balloon OR sirolimus eluting balloon OR DCB OR PCB OR SCB. In addition, the reference list of eligible studies and review articles were reviewed by us to identify additional publications as well.
Eligible studies were identified independently by two researchers (YZ and KSL) with the assistance of EndNote software according to the pre-specified PICOS criteria. The PICOS criteria were as follows: (1) patients: patients presenting with DES-ISR; (2) intervention: treatment with DCB; (3) comparison: repeat DES implantation; (4) outcomes: long-term (≥1 year) clinical endpoints and/or follow-up angiographic endpoints; and (5) study design: RCTs.
The primary endpoint was target lesion revascularization (TLR), which was defined as any revascularization procedure involving the target lesion. Other clinical outcomes, namely, cardiac death, myocardial infarction (MI), target vessel revascularization (TVR), stent thrombosis (definite or probable), and major adverse cardiac events (MACEs), which was a composite of death, MI, and TLR, were considered as secondary endpoints. Notably, a composite of cardiac death, target vessel MI, and clinically driven TLR reported in the RCT BIOLUX was regarded as MACEs for final analysis.
For the angiographic endpoints, in-segment measurements (the treated area plus its 5 mm proximal/distal edges), namely, minimum lumen diameter (MLD), percent diameter stenosis (DS%), and late lumen loss (LLL) were adopted for the analysis. In addition, binary restenosis, which was defined as >50% diameter stenosis in the segment, was also determined for the analysis.
The data extraction was performed by one author (YZ) with the standardized form recording the key items and verified by another researcher (XYK). For the included studies, the basic characteristics such as lead authors, publication years, period of recruitment, sample size, type of DCB/DES used, follow-up time, and reported outcomes were all collected. In addition to those, the clinical, lesion, procedural characteristics, and dual antiplatelet therapy (DAPT) protocol of the study population were also recorded by us.
Two independent researchers (YL and AG) were responsible for quality assessment. In addition, the quality of RCTs was assessed using revised Jadad's score, which is reliable and convenient. Notably, the discrepancies encountered in processes of study selection, data extraction, and quality assessment were resolved by discussion with the senior researcher (YXZ).
The heterogeneity between included studies was assessed by the Cochrane Q test (p < 0.1 indicates significance) and I2 statistic. When there was no significant heterogeneity across the studies (p > 0.1 and I2 < 50%), the principal measures risk ratio (RR) and mean difference (MD) with the corresponding 95% CI were calculated with the Mantel-Haenszel/inverse variance fixed-effects model. If significant heterogeneity was detected, the random-effects model was performed. In addition, sensitivity analysis or subgroup analysis was also performed approximately to further confirm the conclusions obtained. But the publication bias was not examined in this study because the eligible studies were limited (<10). All the statistical analyses included in this meta-analysis were performed using Review Manager version 5.3 (The Nordic Cochrane Center, Copenhagen, Denmark). In addition, p < 0.05 was considered as statistical significance.
As demonstrated in Figure 1, the search strategy used in this meta-analysis yielded 1,827 records, of which 1,501 records were left for screening after removing duplicates. After title and abstract screening, a total of 167 full-text articles were assessed for eligibility. In the end, eight articles from five RCTs were included for the final analysis after excluding 159 additional records by reviewing full-text articles (5, 10–14, 16, 17).
As present in Tables 1, 2, five multicenter RCTs (eight articles) with about 1,193 patients enrolled from 2009 to 2016 were finally included in this meta-analysis (5, 10–14, 16, 17). In addition, the included RCTs were published from 2013 to 2018. In those included studies, the angiographic follow-up period was 6–9 months, and the follow-up period for clinical outcomes ranges from 12 to 36 months. In addition to these contents, the primary endpoints/objective, inclusion criteria, and exclusion criteria of the eligible studies were also summarized and presented in Table 2.
According to data obtained from the included studies (Table 3), baseline demographics, lesion, procedure characteristics, and DAPT protocol (except RIBS IV) between the DCB and DES groups were similar. In addition, from Table 3, we should realize that almost no patients with target lesions located in the left main artery were included in those eligible RCTs.
In this meta-analysis, a total of 816 target lesions treated successfully by either DCB or DES were followed by angiography range from 6 to 9 months. Overall, no significant differences in MLD, DS%, LLL, and binary restenosis were detected between the DES and DCB groups. However, subsequent subgroup analysis demonstrated a significant trend toward an increment in MLD (MD = −0.25 mm, 95 CI −0.38 to −0.11 mm, p = 0.0003; I2 = 0%, p = 0.70, Figure 2A) was observed in the everolimus-eluting stents (EES) group. In addition, patients receiving EES were associated with significantly lower DS% (MD = 7.29, 95% CI 2.86–11.71, p = 0.001; I2 = 0%, p = 0.84, Figure 2B) and the risk of binary restenosis (OR = 2.20, 95% CI 1.18–4.11, p = 0.01; I2 = 0%, p = 0.33, Figure 2D) compared to the patients treated with DCB. However, no significant difference in LLL was noted between DES and DCB (Figure 2C).
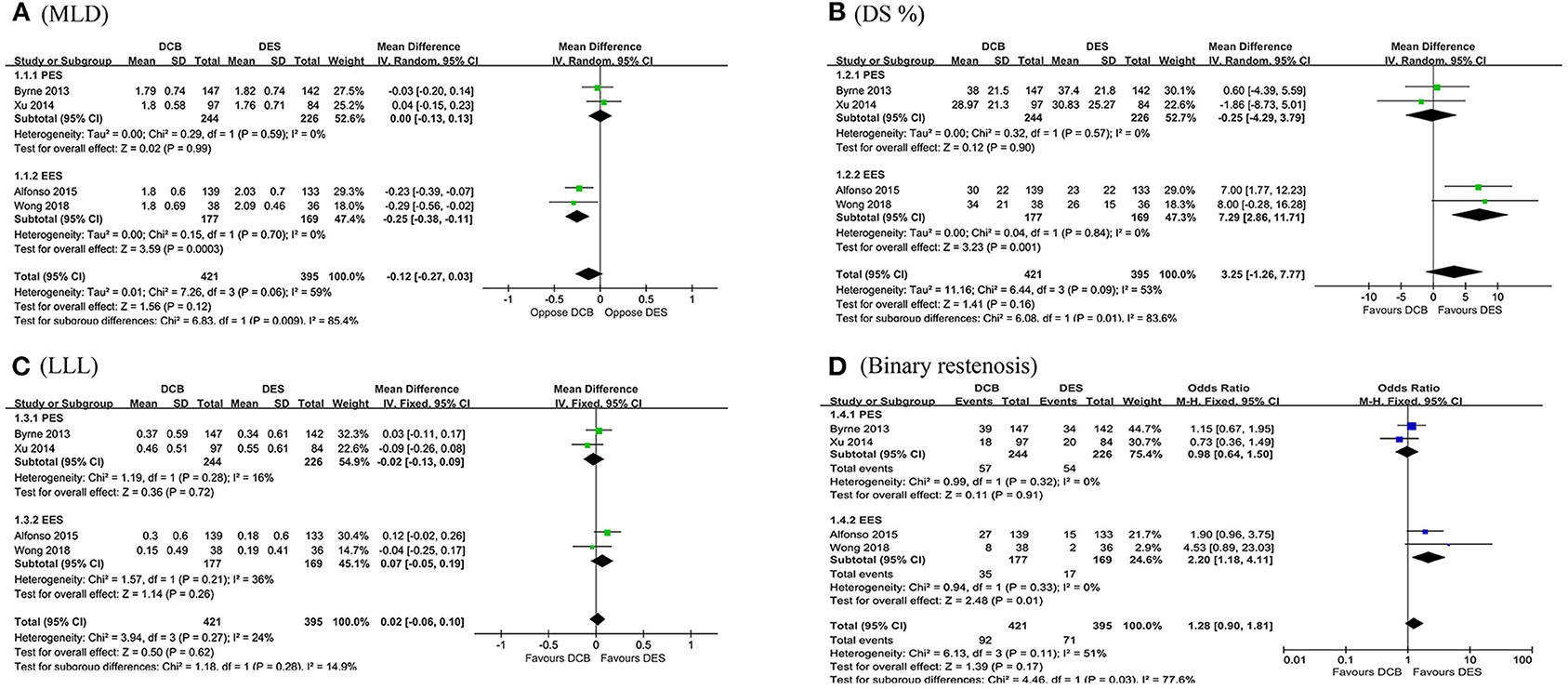
Figure 2. Forest plot of comparing angiographic outcomes in MLD (A), DS% (B), LLL (C), and binary restenosis (D) between repeat DES implantation and DCB angioplasty. MLD, minimum lumen diameter; DS%, percent diameter stenosis; LLL, late lumen loss; DES, drug-eluting stents; DCB, drug-coated balloon; PES, paclitaxel-eluting stents; EES, everolimus-eluting stents.
In this section, the longest available clinical follow-up periods were considered for the analysis and the primary endpoint TLR was reported in the five RCTs including 1,092 patients. After pooling the data of 573 patients receiving DCB vs. 519 patients receiving DES, this meta-analysis revealed repeat DES implantation was associated with reduced TLR compared to DCB angioplasty in patients presenting with DES-ISR (RR = 1.53, 95% CI 1.15–2.04, p = 0.003, Figure 3), with low heterogeneity across the trials (I2 = 0%, p = 0.50). In the subgroup analysis, a strong trend toward a decrease in TLR (RR = 1.36, 95% CI 0.96–1.91, p = 0.08; I2 = 0%, p = 0.56, Figure 4) was even noted in paclitaxel-eluting stent (PES) group, although this difference was not statistically significant compared to DCB.
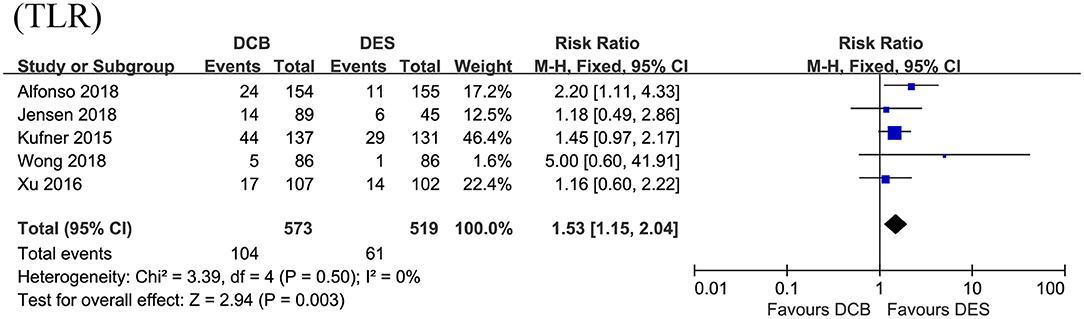
Figure 3. Forest plot of comparing the risk of TLR between repeat DES implantation and DCB angioplasty. TLR, target lesion revascularization; DES, drug-eluting stents; DCB, drug-coated balloon.
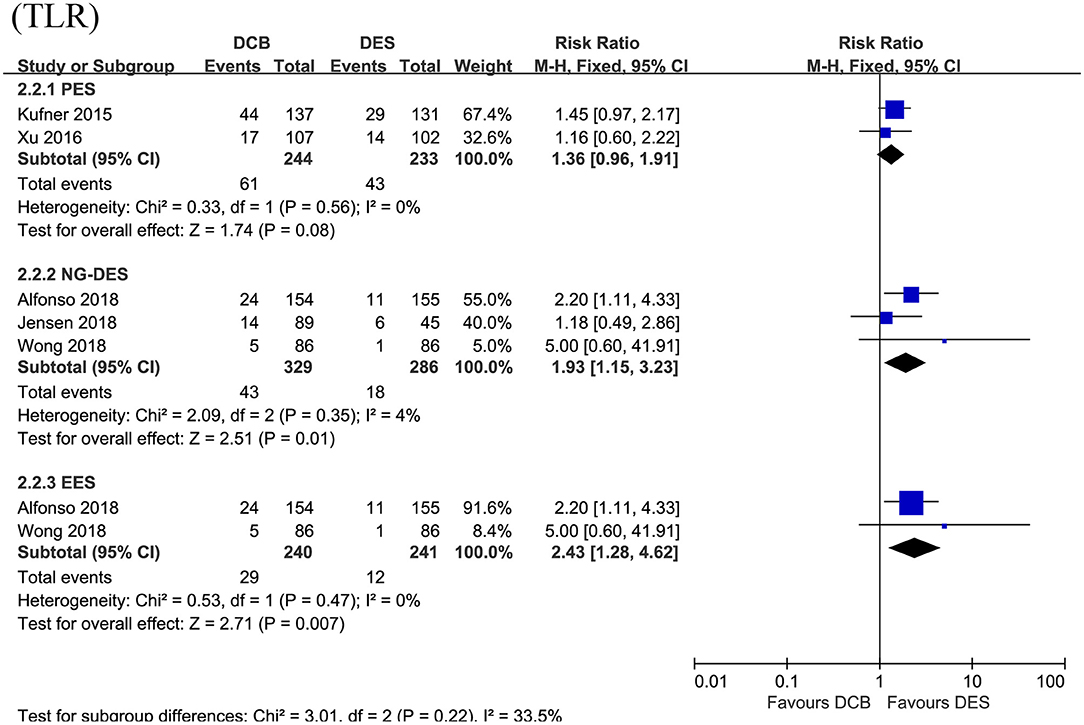
Figure 4. Subgroup analysis for the primary endpoint TLR according to the type of DES used. TLR, target lesion revascularization; DES, drug-eluting stents; PES, paclitaxel-eluting stents; NG-DES, new-generation DES; EES, everolimus-eluting stents.
For the secondary outcomes, as demonstrated in Figure 5, no significant differences in MACEs, cardiac death, MI, and stent thrombosis were noted between the DCB group and the DES group in this meta-analysis. However, repeat DES implantation may be superior to DCB angioplasty in reducing the risk of TVR (RR = 1.50, 95% CI 1.11–2.04, p = 0.009; I2 = 28%, p = 0.24, Figure 5). In the subgroup analysis (Table 4), we also found repeat revascularization with EES, compared with DCB angioplasty, could reduce the risk of MACEs (RR = 1.58, 95% CI 1.03–2.43, p = 0.04). However, in terms of cardiac death, DES especially PES may be inferior to DCB.
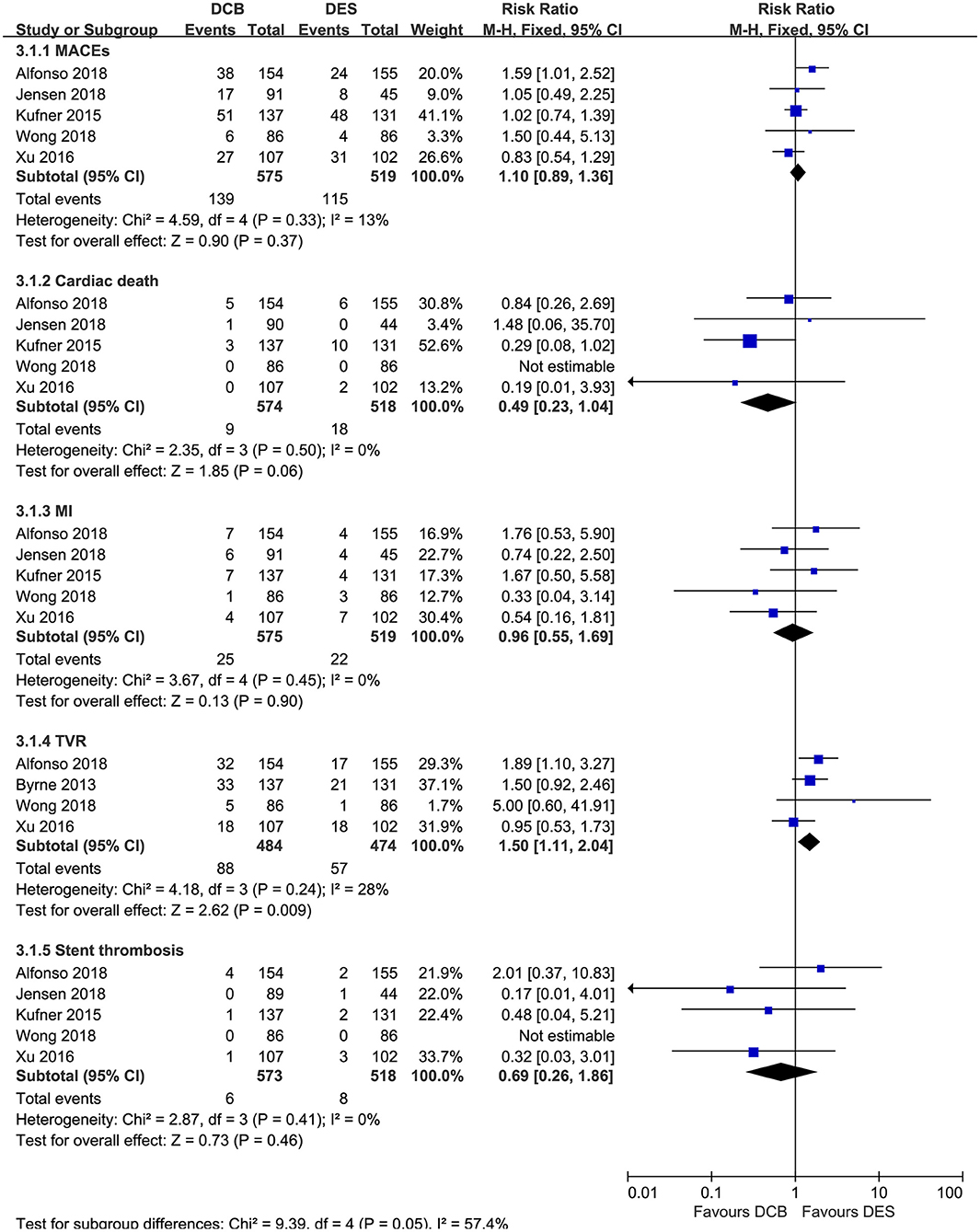
Figure 5. Forest plot comparing secondary outcomes between repeat DES implantation and DCB angioplasty. DES, drug-eluting stents; DCB, drug-coated balloon; MACEs, major adverse cardiac events; MI, myocardial infarction; TVR, target vessel revascularization.
In this meta-analysis, the publication bias was not assessed by funnel plot because of the limited number of included studies. The sensitivity analysis, which was performed by sequentially omitting one trial at a time, also confirmed that DES outperformed DEB in terms of the primary endpoints TLR. However, after excluding the study from Alfonso et al. the difference in TVR loses significance (RR = 1.34, 95% CI 0.93–1.94, p = 0.12).
This meta-analysis comprehensively compared the angiographic and clinical endpoints of the two different strategies (DCB vs. DES) for patients presenting with DES-ISR in routine clinical practice. In addition, the major findings were listed as follows: (1) EES, not PES, was superior to DCB in angiographic endpoints, with larger MLD, lower DS%, and less binary restenosis. (2) For the clinical endpoints, the overall pooled outcomes demonstrated revascularization with DES significantly reduced the risk of TLR and TVR compared to DCB angioplasty. (3) In addition to TLR and TVR, EES was also associated with lower MACEs than DCB in the subgroup analysis.
Despite the great advance in primary and secondary prevention, CAD remains the leading cause of mortality worldwide (18, 19). PCI based on DES is the most commonly used strategy for myocardial revascularization in patients with CAD at present (1, 3). Although the efficacy of DES in the prevention of restenosis improves significantly compared to BMS, DES-ISR still develops in 5–10% of patients after DES deployment (5, 20). In addition, the pathophysiology underlying DES-ISR may be more complex. In addition to neointimal hyperplasia and stent under-expansion, which were considered as the dominant risk factors for BMS-ISR, neo-atherosclerosis seems to be another factor contributing to DES-ISR (8, 9). Furthermore, DES-ISR continues to be a therapeutic challenge and trials designed to determine the optimal treatment strategy for patients with DES-ISR were limited.
Although repeat stenting with DES and DCB angioplasty has been recommended by current guidelines, the consensus was lacking regarding the best treatment for DES-ISR. Bajraktari et al. performed a meta-analysis of seven studies (three of which were RCTs) and reported DEB was comparable to DES in terms of clinical outcomes (MACEs, cardiac death, MI, stent thrombosis, TLR, and TVR) for the treatment of DES-ISR (21). However, Giacoppo et al. reported, in the meta-analysis of individual patient data from all available RCTs (DAEDALUS), DCB angioplasty is significantly associated with a higher risk of TLR compared to repeat stenting with DES for the treatment of DES-ISR at 3-year follow-up (22). In addition, the primary safety endpoint (a composite of all-cause mortality, MI, and target lesion thrombosis) was similar between the two groups (22). In consistent with the study DAEDALUS, this study also confirmed repeat DES implantation was superior to DCB angioplasty in terms of TLR. Moreover, this trend still persisted when we compared PES with DCB, but it was marginally significant. To extend previous studies, this meta-analysis further confirmed EES, not PES, outperforms DCB in terms of MLD, DS%, and binary restenosis.
Our conclusions were reliable and stable because we only included RCTs and the sensitivity analysis and subgroup analysis were performed. But we should keep in mind our conclusions may not generalize to the patients with target lesions located in the left main artery or aorto-ostial coronary. Because few such patients were included in this study. Therefore, multicenter, prospective, and RCTs are needed to figure out this knowledge gap.
DES, especially new-generation (NG-DES)/EES, was superior to DCB in reducing the risk of TLR in this meta-analysis. But treatment with DCB for DES-ISR could avoid multilayers of metal stents in the coronary artery, which poses difficulty for further treatment of recurrent restenosis and is associated with a poor prognosis (23–25). In addition, DCB may be more suitable for patients who are intolerable of long-term DAPT or at a high risk of bleeding (26, 27). Therefore, further investigating and refining DCB technology were warranted. First, future RCTs should focus on the strategy of DCB angioplasty combined with neointimal modification by scoring/cutting balloon, rotablation, and excimer laser. In addition, Kufner et al. have confirmed in patients with DES-ISR, neointimal modification with scoring balloon before DCB was superior over DCB angioplasty alone (28). Second, to extend the previous study from Ali et al. (29), RCTs with large sample sizes and extended clinical follow-up time are required to verify the efficacy and safety of sirolimus-coated balloon (SCB) in patients with DES-ISR. Third, compared to DCB, bioresorbable vascular scaffolds (BVS) could prevent early recoil without adding another layer of metal. Alfonso et al. have confirmed BVS was similar to DCB but inferior to EES in late angiographic and clinical results for the treatment of any ISR in RIBS trial VI (30). In addition, Moscarella et al. also reported BVS was associated with a numerically higher rate of device-oriented cardiovascular events (DOCE) compared with EES while a similar rate compared to DCB for the treatment for any ISR in the BIORESOLVE-ISR Study (31). But the value of BVS in the scenario of DES-ISR remains unsettled and future trials are needed to investigate it.
Several limitations should be acknowledged in this meta-analysis. First, the type of restenotic DES, generation of DES used, time from implantation to admit for DES-ISR, and antithrombotic strategy after intervention for DES-ISR were not uniformly across the included RCTs, which may cause heterogeneity. Second, this meta-analysis only included five RCTs. Therefore, some subgroups were not powered to detect differences and publication bias was not evaluated. Third, this meta-analysis was based on the study level rather than the patient level. In addition, the ratio of follow-up angiography at the scheduled time was suboptimal and the exact reasons were not reported. Finally, the follow-up time for the main analysis was limited up to 3 years in this meta-analysis. Future studies with an extended follow-up duration could provide an additional value to assess the efficacy and safety of DES vs. DCB for DES-ISR.
Angioplasty with DCB was moderately less effective than repeat DES implantation in reducing the TLR for patients with coronary DES-ISR at long-term follow-up. In addition, this trend was more obvious when compared with NG-DES/EES. In addition, repeat stenting with EES could also provide better angiographic outcomes than DCB angioplasty. To confirm our findings, RCTs with a large sample size and extended follow-up duration are required in the future.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Data analysis, interpretation, and manuscript writing were performed by YZhu. Literature search, study selection, data extraction, and quality assessment were performed by YZhu, KL, XK, YL, AG, and YZha. YZha, JZ, and HH were responsible for the conception and design of the study. JN and HL revised the manuscript carefully. All authors read and approved the final manuscript.
This work was supported by the grant from the National Key Research and Development Program of China (2017YFC0908800), the Beijing Municipal Administration of Hospitals Ascent Plan (DFL20150601) and Mission Plan (SML20180601), and the Beijing Municipal Health Commission Project of Science and Technology Innovation Center (PXM2019_026272_000006) (PXM2019_026272_000005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DES, drug-eluting stents; CAD, coronary artery disease; PCI, percutaneous coronary intervention; BMS, bare-metal stents; DCB, drug-coated balloon; RCTs, randomized controlled trials; TLR, target lesion revascularization; MI, myocardial infarction; TVR, target vessel revascularization; MACEs, major adverse cardiac events; MLD, minimum lumen diameter; DS%, percent diameter stenosis; LLL, late lumen loss; RR, risk ratio; MD, mean difference; CI, confidence interval; EES, everolimus-eluting stents; PES, paclitaxel-eluting stents; NG-DES, new-generation DES; SCB, sirolimus-coated balloon; BVS, bioresorbable vascular scaffolds.
1. Stefanini GG, Holmes DR, Jr. Drug-eluting coronary-artery stents. N Engl J Med. (2013) 368:254–65. doi: 10.1056/NEJMra1210816
2. Serrador Frutos AM, Jiménez-Quevedo P, Pérez De Prado A, PanÁlvarez-Ossorio M. Spanish cardiac catheterization and coronary intervention registry. 26th Official report of the spanish society of cardiology working group on cardiac catheterization and interventional cardiology (1990-2016). Rev Esp Cardiol (Engl Ed). (2017) 70:1110–20. doi: 10.1016/j.rec.2017.10.017
3. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy855
4. Basavarajaiah S, Naganuma T, Latib A, Sticchi A, Ciconte G, Panoulas V, et al. Treatment of drug-eluting stent restenosis: comparison between drug-eluting balloon versus second-generation drug-eluting stents from a retrospective observational study. Catheter Cardio Interv. (2016) 88:522–8. doi: 10.1002/ccd.26368
5. Alfonso F, Pérez-Vizcayno MJ, Cuesta J, García Del Blanco B, García-Touchard A, López-Mínguez JR, et al. 3-Year clinical follow-up of the RIBS IV clinical trial. JACC Cardiovasc Interv. (2018) 11:981–91. doi: 10.1016/j.jcin.2018.02.037
6. Steinberg DH, Gaglia MA, Pinto Slottow TL, Roy P, Bonello L, De Labriolle A, et al. Outcome differences with the use of drug-eluting stents for the treatment of in-stent restenosis of bare-metal stents versus drug-eluting stents. Am J Cardiol. (2009) 103:491–5. doi: 10.1016/j.amjcard.2008.09.107
7. Alfonso F, Pérez-Vizcayno MJ, García Del Blanco B, García-Touchard A, López-Mínguez J, Masotti M, et al. Everolimus-eluting stents in patients with bare-metal and drug-eluting in-stent restenosis. Circulation Cardiovasc Interv. (2016) 9:e003479. doi: 10.1161/CIRCINTERVENTIONS.115.003479
8. Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol. (2014) 63:2659–73. doi: 10.1016/j.jacc.2014.02.545
9. Shlofmitz E, Iantorno M, Waksman R. Restenosis of drug-eluting stents. Circulation Cardiovasc Interv. (2019) 12:e007023. doi: 10.1161/CIRCINTERVENTIONS.118.007023
10. Byrne RA, Neumann F, Mehilli J, Pinieck S, Wolff B, Tiroch K, et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet. (2013) 381:461–7. doi: 10.1016/S0140-6736(12)61964-3
11. Xu B, Gao RL, Wang J'An, Yuejin Y, Chen SL, Liu B, et al. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis: results from the PEPCAD China ISR trial. JACC Cardiovasc Interv. (2014) 7:204–11. doi: 10.1016/j.jcin.2013.08.011
12. Alfonso F, Perez-Vizcayno MJ, Cardenas A, Garcia DBB, Garcia-Touchard A, Lopez-Minguez JR, et al. A Prospective randomized trial of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis of drug-eluting stents: the RIBS IV randomized clinical trial. J Am Coll Cardiol. (2015) 66:23–33. doi: 10.1016/j.jacc.2015.04.063
13. Wong YTA, Kang DY, Lee JB, Rha SW, Hong YJ, Shin ES, et al. Comparison of drug-eluting stents and drug-coated balloon for the treatment of drug-eluting coronary stent restenosis: a randomized RESTORE trial. Am Heart J. (2018) 197:35–42. doi: 10.1016/j.ahj.2017.11.008
14. Jensen CJ, Richardt G, Tölg R, Erglis A, Skurk C, Jung W, et al. Angiographic and clinical performance of a paclitaxel-coated balloon compared to a second-generation sirolimus-eluting stent in patients with in-stent restenosis: the BIOLUX randomised controlled trial. EuroIntervention. (2018) 14:1096–103. doi: 10.4244/EIJ-D-17-01079
15. Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. (2018) 7:e011245. doi: 10.1161/JAHA.118.011245
16. Kufner S, Cassese S, Valeskini M, Neumann FJ, Schulz-Schupke S, Hoppmann P, et al. Long-term efficacy and safety of paclitaxel-eluting balloon for the treatment of drug-eluting stent restenosis: 3-year results of a randomized controlled trial. JACC Cardiovasc Interv. (2015) 8:877–84. doi: 10.1016/j.jcin.2015.01.031
17. Xu B, Qian J, Ge J, Wang J, Chen F, Chen J, et al. Two-year results and subgroup analyses of the PEPCAD China in-stent restenosis trial: a prospective, multicenter, randomized trial for the treatment of drug-eluting stent in-stent restenosis. Catheter Cardio Interv. (2016) 87:624–9. doi: 10.1002/ccd.26401
18. Da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira ÂC, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. (2019) 18:89. doi: 10.1186/s12933-019-0893-2
19. Sun Z, Cao Y, Li H. Multislice computed tomography angiography in the diagnosis of coronary artery disease. J Geriatr Cardiol. (2011) 8:104–13. doi: 10.3724/SP.J.1263.2011.00104
20. Bønaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygård O, et al. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. (2016) 375:1242–52. doi: 10.1056/NEJMoa1607991
21. Bajraktari G, Jashari H, Ibrahimi P, Alfonso F, Jashari F, Ndrepepa G, et al. Comparison of drug-eluting balloon versus drug-eluting stent treatment of drug-eluting stent in-stent restenosis: a meta-analysis of available evidence. Int J Cardiol. (2016) 218:126–35. doi: 10.1016/j.ijcard.2016.05.040
22. Giacoppo D, Alfonso F, Xu B, Claessen BEPM, Adriaenssens T, Jensen C, et al. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis: a comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study). Eur Heart J. (2020) 41:3715–28. doi: 10.1093/eurheartj/ehz861
23. Singh AD, Singal AK, Mian A, Kapadia SR, Hedrick DP., Kanaa'N A, et al. Recurrent drug-eluting stent in-stent restenosis: a state-of-the-art review of pathophysiology, diagnosis, and management. Cardiovasc Revasc Med. (2020) 21:1157–63. doi: 10.1016/j.carrev.2020.01.005
24. Wang G, Zhao Q, Chen Q, Zhang X, Tian L, Zhang X. Comparison of drug-eluting balloon with repeat drug-eluting stent for recurrent drug-eluting stent in-stent restenosis. Coron Artery Dis. (2019) 30:473–80. doi: 10.1097/MCA.0000000000000784
25. Kawamoto HM, Ruparelia NMD, Latib AM, Miyazaki TM, Sato KM, Mangieri AM, et al. Drug-coated balloons versus second-generation drug-eluting stents for the management of recurrent multimetal-layered in-stent restenosis. JACC Cardiovasc interv. (2015) 8:1586–94. doi: 10.1016/j.jcin.2015.04.032
26. Roncalli J, Godin M, Boughalem K, Shayne J, Piot C, Huret B, et al. Paclitaxel drug-coated balloon after bare-metal stent implantation, an alternative treatment to drug-eluting stent in high bleeding risk patients (the panelux trial). J Invasive Cardiol. (2019) 31:94–100.
27. Scheller B. Antithrombozytäre therapie und PCI. Herz. (2014) 39:819–21. doi: 10.1007/s00059-014-4158-2
28. Kufner S, Joner M, Schneider S, Tolg R, Zrenner B, Repp J, et al. Neointimal modification with scoring balloon and efficacy of drug-coated balloon therapy in patients with restenosis in drug-eluting coronary stents: a randomized controlled trial. JACC Cardiovasc Interv. (2017) 10:1332–40. doi: 10.1016/j.jcin.2017.04.024
29. Ali RM, Abdul Kader MASK, Wan Ahmad WA, Ong TK, Liew HB, Omar A, et al. Treatment of coronary drug-eluting stent restenosis by a sirolimus- or paclitaxel-coated balloon. JACC Cardiovasc Interv. (2019) 12:558–66. doi: 10.1016/j.jcin.2018.11.040
30. Alfonso F, Cuesta J, Pérez-Vizcayno MJ, García Del Blanco B, Rumoroso JR, Bosa F, et al. Bioresorbable vascular scaffolds for patients with in-stent restenosis: the RIBS VI study. JACC Cardiovasc interv. (2017) 10:1841–51. doi: 10.1016/j.jcin.2017.06.064
31. Moscarella E, Tanaka A, Ielasi A, Cortese B, Coscarelli S, De Angelis MC, et al. Bioresorbable vascular scaffold versus everolimus-eluting stents or drug eluting balloon for the treatment of coronary in-stent restenosis: 1-Year follow-up of a propensity score matching comparison (the BIORESOLVE-ISR Study). Catheter Cardio Inter. (2018) 92:668–77. doi: 10.1002/ccd.27473
Keywords: in-stent restenosis, drug-eluting stent, drug-eluting balloon, randomized controlled trial, target lesion revascularization
Citation: Zhu Y, Liu K, Kong X, Nan J, Gao A, Liu Y, Han H, Li H, Zhu H, Zhang J and Zhao Y (2021) Comparison of Drug-Coated Balloon Angioplasty vs. Drug-Eluting Stent Implantation for Drug-Eluting Stent Restenosis in the Routine Clinical Practice: A Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 8:766088. doi: 10.3389/fcvm.2021.766088
Received: 28 August 2021; Accepted: 31 October 2021;
Published: 01 December 2021.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Emily Spangler, University of Alabama at Birmingham, United StatesCopyright © 2021 Zhu, Liu, Kong, Nan, Gao, Liu, Han, Li, Zhu, Zhang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongya Han, aGh5MTIzMTAwQDE2My5jb20=; Yingxin Zhao, enlpbmd4aW5taUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.