- 1Vicerrectorado de Investigación, Universidad San Ignacio de Loyola, Lima, Peru
- 2Programa de Atención Domiciliaria – EsSalud, Lima, Peru
- 3Departamento de Cardiología, Hospital Nacional Edgardo Rebagliati Martins, Lima, Peru
- 4Facultad de Medicina Alberto Hurtado, Universidad Peruana Cayetano Heredia, Lima, Peru
- 5Cardiology Department, Hospital Universitario 12 de Octubre, Madrid, Spain
- 6Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Madrid, Spain
Background: It has been proposed that transcatheter aortic valve replacement (TAVR) may be an option for patients with cancer and severe aortic stenosis. We assessed the association between previous or active cancer and clinical outcomes in TAVR patients.
Methods: We searched four electronic databases from inception to March 05, 2021. The primary outcome was all-cause mortality. Secondary outcomes were cardiovascular mortality, myocardial infarction, stroke, acute kidney injury, pacemaker implantation, major bleeding, and vascular complications. All meta-analyses were performed using a random-effects model. Relative risks (RRs) and adjusted hazard ratios (aHRs) with their 95% confidence interval (95% CI) were pooled.
Results: Thirteen cohort studies involving 255,840 patients were included. The time period for mortality ranged from inpatient to 10 years. Patients with active cancer had a higher risk of all-cause mortality using both crude (RR, 1.46; 95% CI, 1.13–1.88) and adjusted (aHR, 1.79; 95% CI, 1.43–2.25) estimates compared to non-cancer group. In contrast, the risk of cardiovascular mortality (RR, 1.26; 95% CI, 0.58–2.73), myocardial infarction (RR, 0.94; 95% CI, 0.34–2.57), stroke (RR, 0.90; 95% CI, 0.75–1.09), pacemaker implantation (RR, 0.87; 95% CI, 0.50–1.53), acute kidney injury (RR, 0.88; 95% CI, 0.74–1.04), major bleeding (RR, 1.15; 95% CI, 0.80–1.66), and vascular complications (RR, 0.96; 95% CI, 0.79–1.18) was similar between patients with or without cancer.
Conclusion: Our review shows that TAVR patients with active cancer had an increased risk of all-cause mortality. No significant association with secondary outcomes was found.
Introduction
Transcatheter aortic valve replacement (TAVR) has become a safe and effective treatment option for patients with symptomatic severe aortic stenosis (1). It is well-known that a large proportion of patients undergoing TAVR are elderly with multiple comorbidities that may influence their short-term prognosis (1). Among them, it has been estimated that ~20% of TAVR patients have a history of cancer (2). However, cancer patients have often been excluded from pivotal TAVR trials. Given their likely poor survival, the decision as to whether a patient with cancer and severe aortic stenosis is a candidate for TAVR is complex. Moreover, severe aortic stenosis could potentially condemn patients to a higher mortality risk than cancer itself, and access to TAVR may provide a longer life expectancy.
Several studies with mixed results have been reported evaluating the association between cancer and outcomes in TAVR patients (2–5). Moreover, it is unknown whether the outcomes vary if the patients had previous cancer or it was active. Therefore, we performed a systematic review and meta-analysis to assess the association between previous or active cancer and clinical outcomes in patients with severe aortic stenosis treated with TAVR.
Methods
This systematic review was reported according to the 2009 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement (6).
Search Strategy
We searched in four electronic databases (PubMed, Embase, Web of Science, and Scopus) from inception to March 05, 2021. The complete search strategy can be found in Supplementary Table 1. There were no restrictions on publication year or language. The reference lists of included studies and relevant reviews were also screened to identify eligible studies.
Eligibility Criteria
The inclusion criteria were the following: (i) cohort studies evaluating the association between previous or active cancer and clinical outcomes in adult patients (≥18 years old) with severe aortic stenosis treated with transcatheter aortic valve replacement and (ii) studies reporting at least one of the primary or secondary outcomes at any length of follow-up. We only included cohort studies since it is not possible to evaluate cancer as exposure in randomized controlled trials. Cross-sectional studies, case-control studies, case series, case reports, systematic reviews, conference abstracts, and editorials were excluded.
Study Selection
All articles from the search were downloaded and duplicates were removed. Title/abstract and full-texts were independently assessed by two review authors (JTV and GZ). Any disagreement was resolved by a third review author (CDA).
Outcomes
The primary outcome was all-cause mortality. Secondary outcomes were cardiovascular mortality, myocardial infarction, stroke, pacemaker implantation, acute kidney injury, major bleeding, and vascular complications. The study definitions were used for all outcomes.
Data Extraction
Two authors (JTV and GZ) independently extracted the information from each study using a standard data extraction form that was previously piloted. Any disagreement was resolved by a third review author (CDA). The following data were extracted: first author name, year of publication, country, study design, type of population, sample size, age, sex, comorbidities, the timing of cancer, follow-up duration, and primary and secondary outcomes.
Risk of Bias Assessment
Two review authors (CDA and JTV) independently assessed the risk of bias of each cohort study using the Newcastle-Ottawa Scale (NOS) tool (7). Any disagreement was resolved by consensus. The NOS tool rates cohort studies based on three domains: selection, comparability, and outcome. The selection domain consists of four items: representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, and demonstration that the outcome of interest was not present at the start of the study. The comparability domain consists of one item: comparability of cohorts on the basis of the design or analysis. The outcome domain consists of three items: assessment of outcome, was follow-up long enough for outcomes to occur, and adequacy of follow-up of cohorts. Each item is scored with zero, one, or two stars. Overall, each study was judged as follows: low risk of bias (8–9 stars), moderate risk of bias (5–7 stars), and high risk of bias (0–4 stars) (8).
Statistical Analysis
All meta-analyses were performed using a random-effects model. The between-study variance (tau2) was estimated using the Paule-Mandel method (9). Unadjusted relative risks (RRs) and adjusted hazard ratios (aHRs) with their 95% confidence intervals (CIs) were pooled. We have combined the aHRs from each study as reported. Statistical heterogeneity was evaluated using the Chi-square test (p < 0.10 as threshold) and the I2 statistic (10). Heterogeneity was defined as follows: low if I2 <30, moderate if I2 = 30–60%, and high if I2 > 60%. Publication bias was assessed using the visual inspection of funnel plots and Egger's test if 10 or more studies were available (10). Subgroups analyses were performed according to the timing of cancer (previous history of cancer or active cancer). The test for subgroup differences (interaction test) was considered statistically significant if the p-value was <0.10 as previously recommend (10, 11). For the main meta-analyses, a two-tailed p < 0.05 was used for statistical significance. We used the package meta from R 3.6.3 (www.r-project.org) for all meta-analyses.
Results
Study Selection
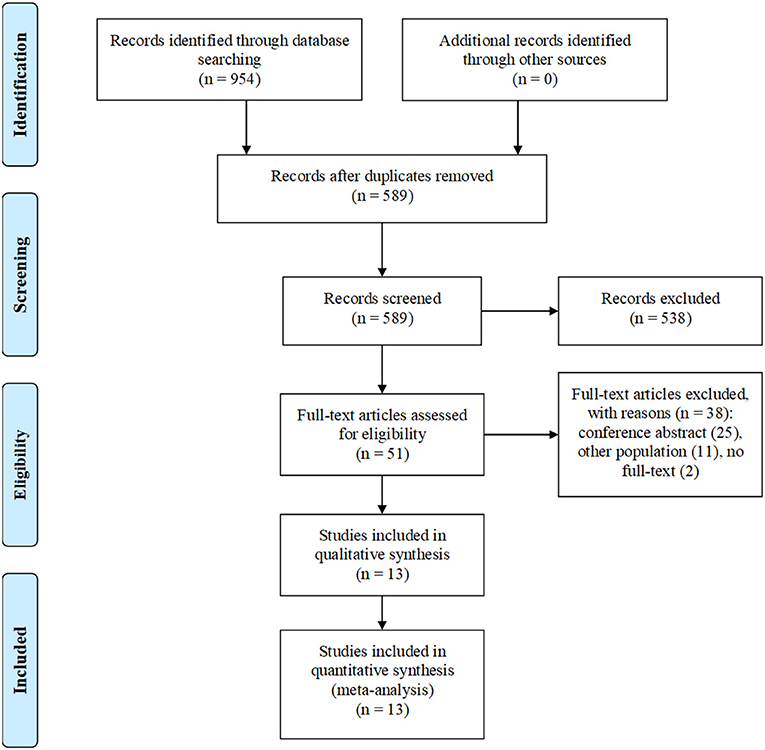
Our electronic search retrieved 954 articles. After the removal of 365 duplicates, 589 articles underwent title/abstract screening, of which 51 articles were included in the full-text screening. Finally, a total of 13 studies were selected (Figure 1) (2–5, 12–20).
Study Characteristics
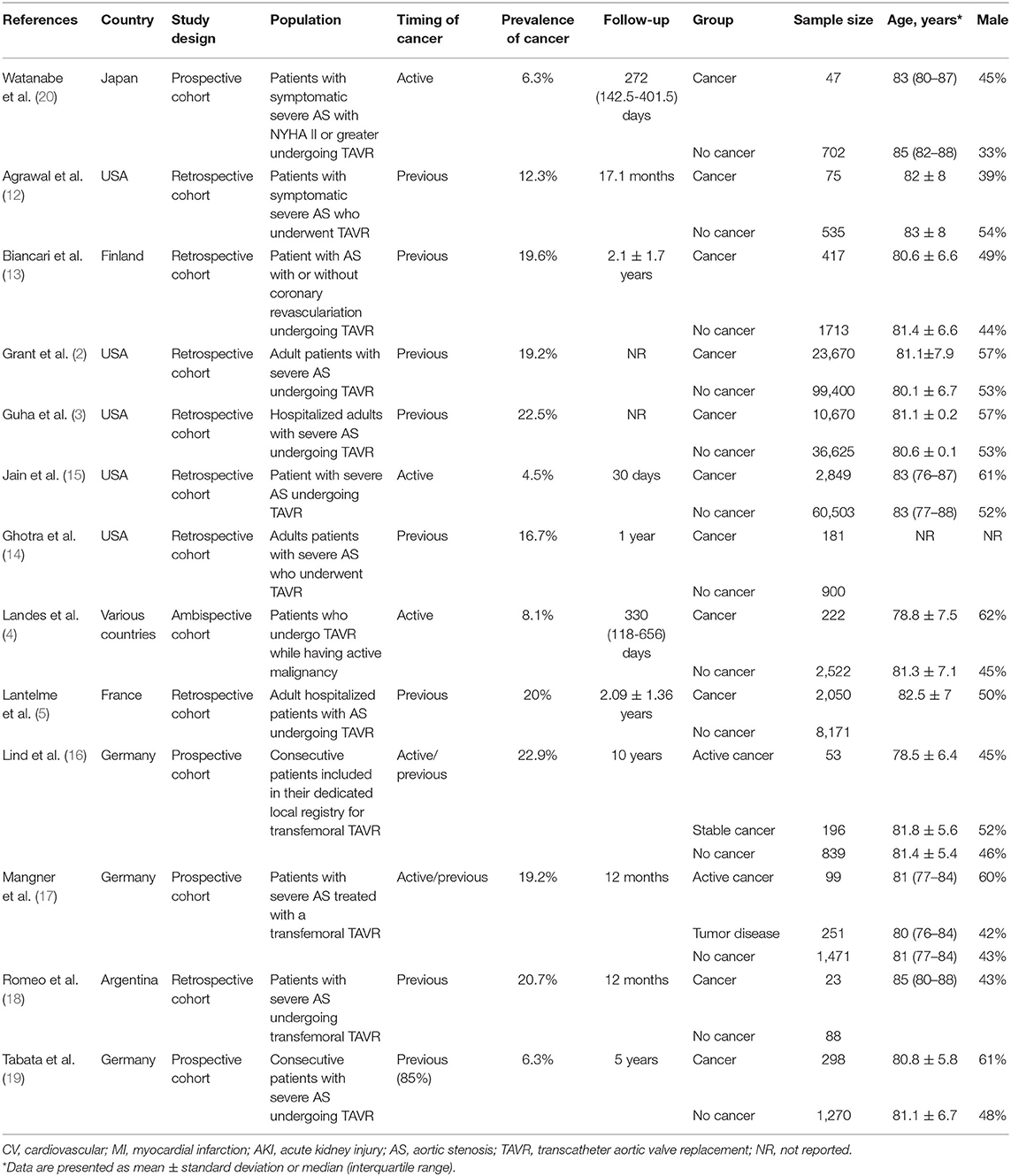
The main characteristics of the 13 cohort studies (n = 255.840 patients) are shown in Table 1. Eight studies were retrospective, four were prospective, and one was ambispective. Most studies were conducted in the United States of America (n = 4) and Germany (n = 3). The mean age ranged from 78.5 to 83 years and 50% were men. The most common comorbidities were hypertension (85%), dyslipidemia (60%), coronary artery disease (44%), and diabetes (32%). Active cancer was assessed in five studies and previous cancer in 10 studies. The prevalence of cancer in TAVR patients ranged from 4.5 to 22.9% across studies. The follow-up ranged from 1 month to 10 years. The median Society of Thoracic Surgeons (STS) score ranged from 3 to 8.1 across eight studies. The type of transcatheter aortic valve device was reported in six studies. The use of a self-expandable valve varied from 18 to 76% and a balloon-expandable valve from 24 to 100%. The access route of TAVR ranged from 82 to 100% across six studies. Information on the type of cancer was available in 10 studies (Supplementary Table 2). The most frequent cancer types were hematologic (25%), breast (22%), prostate (21%), and lung (18%). Only four studies reported data on cancer staging and treatment (Supplementary Table 2). The proportion of patients with metastases ranged from 6 to 31% across studies. Antineoplastic treatment was reported in 29–83% of cases. The adjusted effect estimates and adjusted variables of each study are described in Supplementary Table 3. The adjusted variables were not uniform across studies. The variables most commonly adjusted were age, sex, New York Heart Association scale, STS score, and coronary artery disease. None of the effect estimates were adjusted for time.
Risk of Bias Assessment
According to the NOS tool, eight studies were scored as low risk of bias and five studies as the moderate risk of bias (Supplementary Table 4).
All-Cause Mortality
In 13 studies (n = 255.796), the risk of all-cause mortality was similar between patients with and without cancer (RR, 1.13; 95% CI, 0.95–1.35; I2 = 94%) (Figure 2). The funnel plot did not show asymmetry and the Egger's test was not significant (p = 0.68) (Supplementary Figure 1).
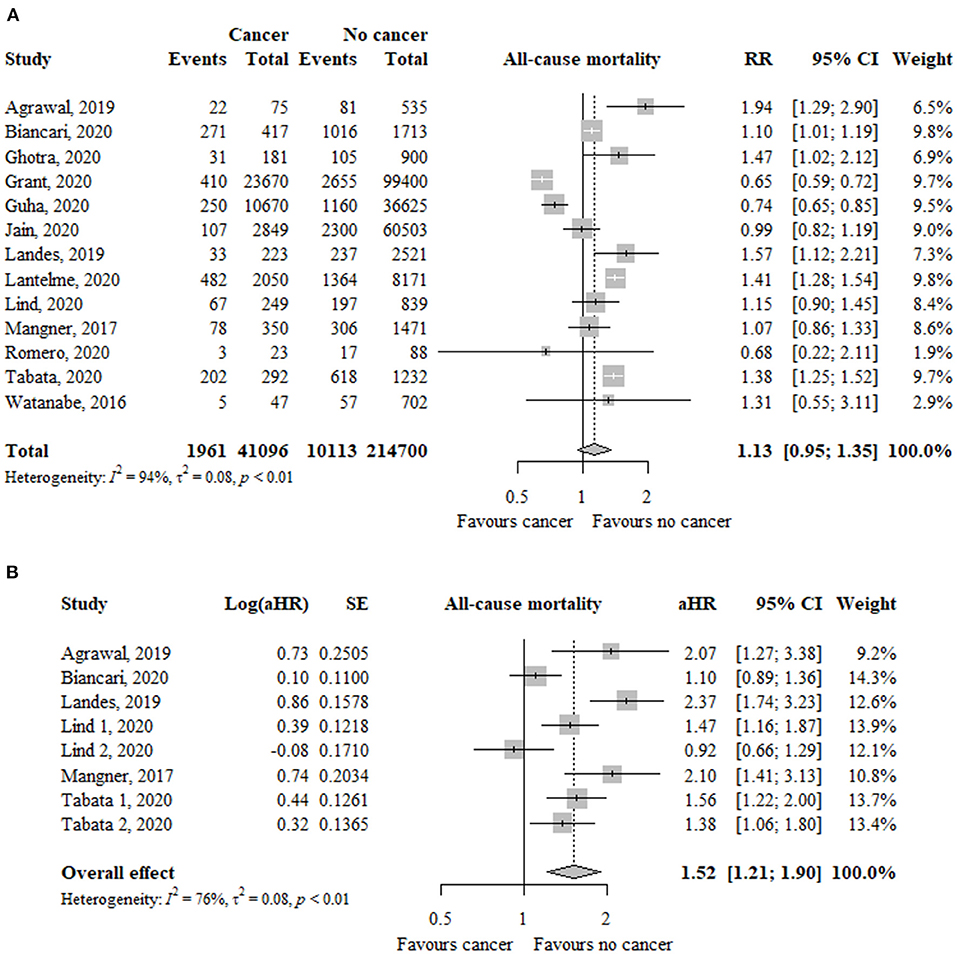
Figure 2. Association between cancer and all-cause mortality in TAVR patients using (A) risk ratios and (B) adjusted hazard ratio as effect measures. RR, relative risk; aHR, adjusted hazard ratio; CI, confidence interval; TAVR, transcatheter aortic valve replacement.
In eight studies (n = 9.917), using adjusted estimates, the risk of all-cause mortality was significantly higher in the cancer group compared to the non-cancer group (aHR, 1.52; 95% CI, 1.21–1.90; I2 = 76%) (Figure 2).
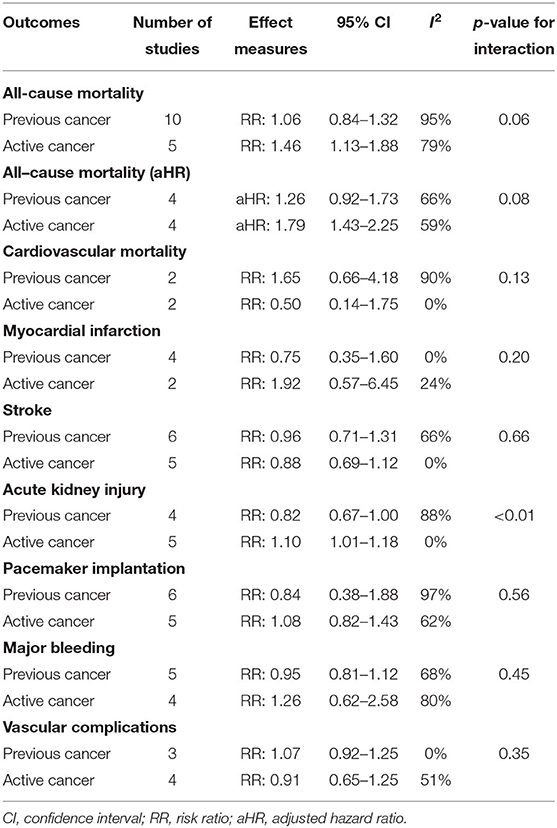
Only patients with active cancer, but no previous cancer, had a significantly increased risk of all-cause mortality using unadjusted (RR, 1.46; 95% CI, 1.13–1.88; I2 = 79%) and adjusted (aHR, 1.79; 95% CI, 1.43–2.25; I2 = 59%) effect estimates (Table 2). The test for subgroup differences suggests that there is a statistically significant subgroup effect using unadjusted (p = 0.06) and adjusted (p = 0.08) effect estimates.
Cardiovascular Mortality
In four studies (n = 6.233), the risk of cardiovascular mortality was not significantly different between patients with and without cancer (RR, 1.26; 95% CI, 0.58–2.73; I2 = 76%) (Figure 3).
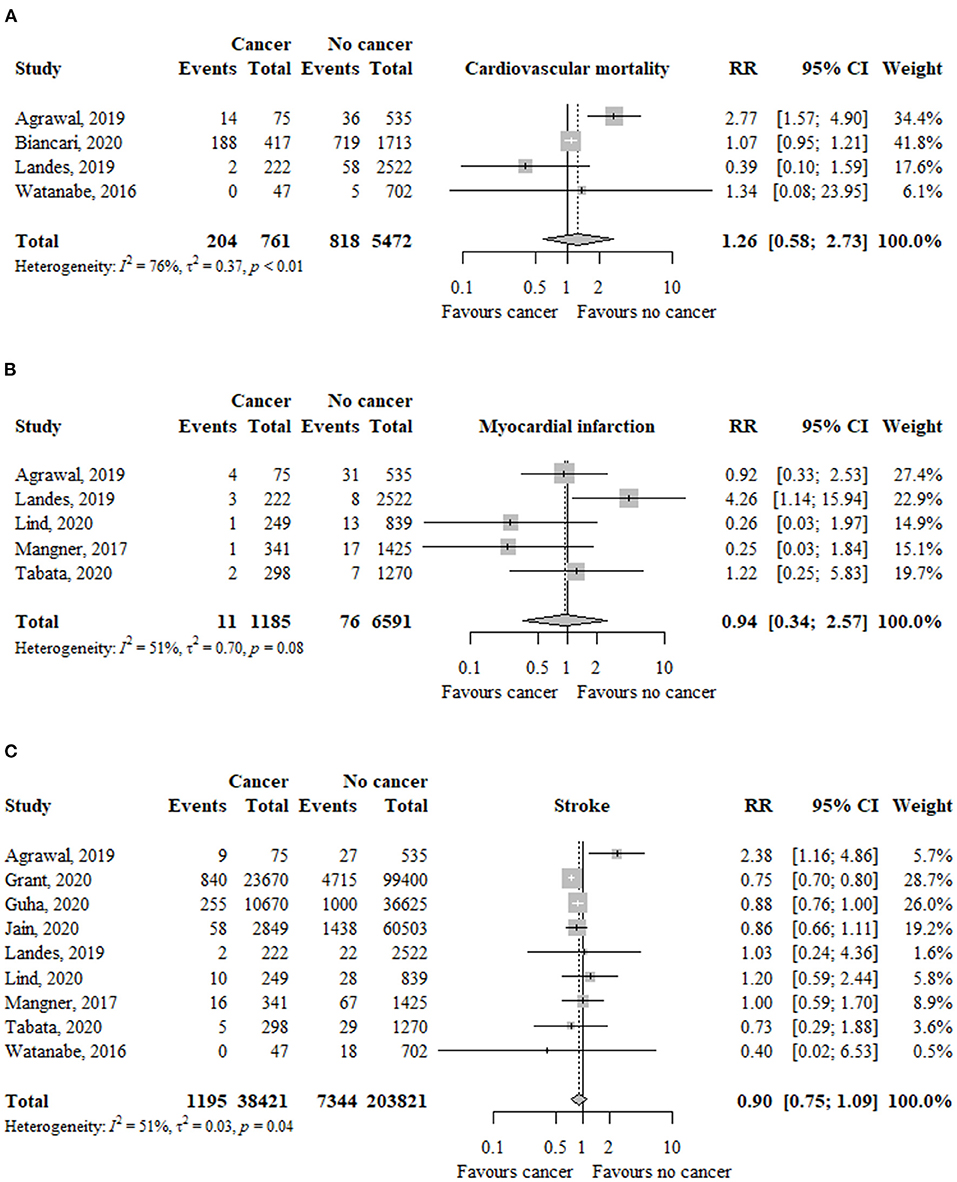
Figure 3. Association between cancer and (A) cardiovascular mortality, (B) myocardial infarction, and (C) stroke in TAVR patients. RR, relative risk; aHR, adjusted hazard ratio; CI, confidence interval; TAVR, transcatheter aortic valve replacement.
The risk of cardiovascular mortality was similar among patients with previous (RR, 1.65; 95% CI, 0.66–4.18; I2 = 90%) or active (RR, 0.50; 95% CI, 0.14–1.75; I2 = 0%) cancer compared to patients without cancer (Table 2). The test for subgroup differences was not significant (p = 13).
Myocardial Infarction
In five studies (n = 7.776), the risk of myocardial infarction was not significantly different between patients with and without cancer (RR, 0.94; 95% CI, 0.34–2.57; I2 = 51%) (Figure 3).
The risk of myocardial infarction was not significantly different between patients with previous (RR, 0.75; 95% CI, 0.35–1.60; I2 = 0%) or active (RR, 1.92; 95% CI, 0.57–6.45; I2 = 24%) cancer compared to patients without cancer (Table 2). The test for subgroup differences was not significant (p = 20).
Stroke
In nine studies (n = 242.242), the risk of myocardial infarction was similar between patients with and without cancer (RR, 0.90; 95% CI, 0.75–1.09; I2 = 51%) (Figure 3).
The risk of stroke was similar between patients with previous (RR, 0.96; 95% CI, 0.71–1.31; I2 = 66%) or active (RR, 0.88; 95% CI, 0.69–1.12; I2 = 0%) cancer compared to patients without cancer (Table 2). The test for subgroup differences was not significant (p = 66).
Acute Kidney Injury
In seven studies (n = 240.073), the risk of acute kidney injury was not significantly different between patients with and without cancer (RR, 0.88; 95% CI, 0.74–1.04; I2 = 94%) (Figure 4).
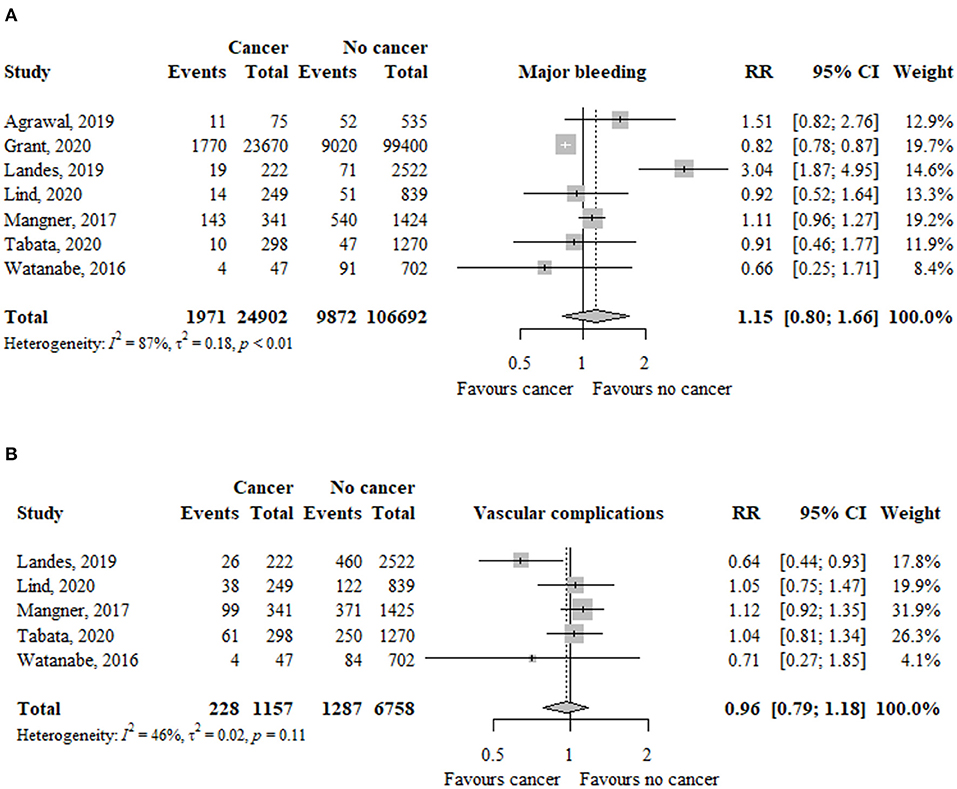
Figure 4. Association between cancer and (A) acute kidney injury and (B) pacemaker implantation in TAVR patients. RR, relative risk; aHR, adjusted hazard ratio; CI, confidence interval; TAVR, transcatheter aortic valve replacement.
The risk of acute kidney injury was significantly higher in patients with active cancer (RR, 1.10; 95% CI, 1.01–1.18; I2 = 0%), but no previous cancer (RR, 0.82; 95% CI, 0.67–1.00; I2 = 88%), compared to patients without cancer (Table 2). The test for subgroup differences was significant (p < 0.01).
Pacemaker Implantation
In nine studies (n = 244.987), the risk of pacemaker implantation was similar between patients with and without cancer (RR, 0.87; 95% CI, 0.50–1.53; I2 = 96%) (Figure 4).
The risk of pacemaker implantation was similar between patients with previous (RR, 0.84; 95% CI, 0.38–1.88; I2 = 97%) or active (RR, 1.08; 95% CI, 0.82–1.43; I2 = 62%) cancer compared to patients without cancer (Table 2). The test for subgroup differences was not significant (p = 56).
Major Bleeding
In seven studies (n = 131.594), the risk of major bleeding was not significantly different between patients with and without cancer (RR, 1.15; 95% CI, 0.80–1.66; I2 = 87%) (Figure 5).
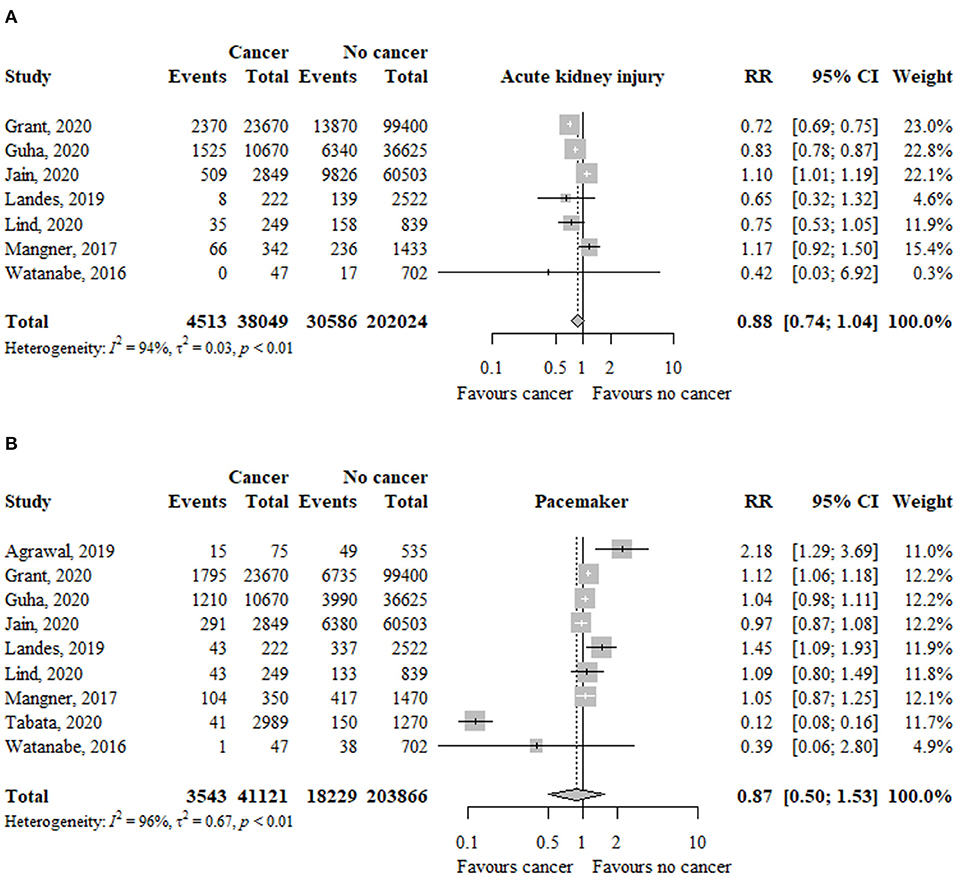
Figure 5. Association between cancer and (A) major bleeding and (B) vascular complications in TAVR patients. RR, relative risk; aHR, adjusted hazard ratio; CI, confidence interval; TAVR, transcatheter aortic valve replacement.
The risk of major bleeding was not significantly different between patients with previous (RR, 0.95; 95% CI, 0.81–1.12; I2 = 97%) or active (RR, 1.26; 95% CI, 0.62–2.58; I2 = 80%) cancer compared to patients without cancer (Table 2). The test for subgroup differences was not significant (p = 45).
Vascular Complications
In five studies (n = 7.915), the risk of vascular complications was similar between patients with and without cancer (RR, 0.96; 95% CI, 0.79–1.18; I2 = 46%) (Figure 5).
The risk of vascular complications was similar between patients with previous (RR, 1.07; 95% CI, 0.92–1.25; I2 = 0%) or active (RR, 0.91; 95% CI, 0.65–1.25; I2 = 51%) cancer compared to patients without cancer (Table 2). The test for subgroup differences was not significant (p = 35).
Discussion
Main Findings
In the present meta-analysis, we provide a comprehensive overview of the association between previous or active cancer and mortality and TAVR complications in 255.840 patients with severe aortic stenosis who underwent TAVR from 13 cohort studies. The main study findings can be summarized as follows: (1) only TAVR patients with active cancer had an increased risk of all-cause mortality using unadjusted and adjusted effect estimates; (2) the association between cancer (either previous or active) and cardiovascular mortality in patients who underwent TAVR was not significant; (3) complications after TAVR, such as acute myocardial infarction, need for pacemaker implantation, major bleeding and vascular complications, occurred similarly in cancer patients regardless of cancer activity, as in those without cancer.
Association Between Cancer and Mortality After TAVR
Cancer is an increasingly frequent comorbidity in patients with cardiovascular diseases, with shared risk factors, such as obesity, a processed diet, smoking, or physical inactivity (21). This is also the case of aortic stenosis, the most common valve disease (22). In addition, cancer treatments, especially chest radiation therapy (23, 24), pose a specific risk for the development of aortic valve disease. Therefore, it is to be expected that in the coming years we will often have to deal with patients with severe aortic stenosis and cancer and make important decisions regarding the treatment of both entities. Latency to the presentation of valvular heart disease from cancer therapies is often over 20 years (23), so many patients with cancer have an inactive or stabilized tumor disease.
The contributions of this meta-analysis are relevant since, in patients with severe aortic stenosis who underwent TAVR implantation, only those who had active cancer had higher mortality from all causes. Patients with cancer present a higher risk of mortality and more life-threatening health conditions than the general population. The higher mortality has been attributed to both cardiovascular and non-cardiovascular conditions (25), but recently particular attention has been paid to cardiovascular disease-related deaths (26, 27), especially in certain subpopulations of cancer patients (26–28). As it has been described, mortality in cancer patients is strongly conditioned by the type of tumor (29). For those cancers of high malignancy (i.e., lung or pancreas), mortality is more likely due to cancer itself, but in others such as prostate, intestinal, or breast cancer, they present a high risk of mortality not attributable to cancer (29). In cancer patients over 60 years old, cardiovascular diseases are the main cause of death (29, 30). This elderly population is more prone to develop severe aortic stenosis, which can be more lethal than many malignancies if left untreated (31). Gastrointestinal (mainly colorectal cancer), breast, and prostate cancer were the most common malignancies in patients with severe aortic stenosis treated with TAVR (4, 13, 14, 17), and these tumors appear at older ages and tend to have a more indolent progression. Indeed, a study showed that breast cancer and prostate cancer were not associated with an increased risk of all-cause mortality (13). Regarding non-cardiac causes of death in patients with active cancer, the more commonly described are cerebrovascular disease, infections, liver failure, kidney disease (29), and cancer-related mortality, which represents ~50% of cancer patient's deaths (especially in those with progressive malignancies in stage III to IV) (4, 17). TAVR can be a reasonable option in patients with previous cancer, considering the stage of initial cancer and the duration of remission. In patients with active cancer there a comprehensive multidisciplinary assessment aimed to select candidates for TAVR should be conducted, given their higher risk of mortality in the mid- and long-term.
After relieving the aortic stenosis with the TAVR procedure, cardiovascular mortality was equaled in the three groups of patients (non-cancer, previous or active cancer), regardless of the history of cancer. The importance of managing aortic valve disease in cancer patients is that TAVR would allow a treatment directed to cancer with surgery, chemotherapy, targeted cancer therapies, or biological anticancer drugs that would improve the malignancy prognosis (4). In any case, it seems clear that an adequate selection of candidates for TAVR allows good cardiovascular results even in patients with active cancer, who may achieve a reasonable life expectancy after having solved a serious treatable disease, such as aortic stenosis, with a procedure with manageable complications.
Association Between Cancer and TAVR Complications
This large-scale analysis allowed us to examine the association between cancer history and main TAVR complications. The risk of post-procedural complications was very similar in patients with active/previous cancer and non-cancer patients. However, it should be noted that information was scarce for some outcomes, limiting the robustness of the effect estimates. Only acute kidney injury occurred more frequently in patients with active cancer. Acute kidney injury is a common complication after TAVR, which may occur in half of the cases (although incidence varies widely) (32). Acute kidney injury after TAVR is multifactorial in origin: administration of iodinated contrast agents, bleeding and anemia, microembolisms, hypotension, or nephrotoxic drugs, among others. In addition, predisposing factors such as chronic kidney disease or previous heart failure play a role (32, 33). Although cancer patients tend to be younger and have fewer comorbidities (2), there are several cancer-related mechanisms underlying the higher risk of acute kidney injury, including a number of conventional chemotherapeutic agents, tumor infiltration, immune response, or volume depletion, among others (34).
The type of cancer may also play a role in the risk of post-TAVR acute kidney injury (3). The importance of this finding is that acute kidney injury after TAVR is associated with higher mortality, especially in those patients who develop stage III acute kidney injury (33), so preventive measures aimed at avoiding or minimizing kidney damage should be established early in cancer patients, especially those with active disease avoiding dehydration and withdrawing possible nephrotoxic drugs in the peri-intervention period.
In relation to other post-TAVR complications (stroke, pacemaker implantation, acute myocardial infarction, or bleeding), the risk was low and similar in the three groups of patients (without cancer, with active or previous cancer) (2–4, 12, 16, 17, 19, 20).
Limitations
Our review has some limitations. First, since all included studies were observational and most were retrospective, there is a risk of confounding bias. Although we pooled unadjusted and adjusted effect estimates, there is a risk of residual confounding. Second, our findings are not extensible to patients treated with surgical aortic valve replacement as age and surgical risks are different from TAVR patients. Third, the heterogeneity was high among studies. Possible reasons include different sample sizes, heterogeneous definitions of bleeding and acute kidney injury, different types of cancer, and various lengths of follow-up. Fourth, information on the type, stage, and treatment of cancer was poorly reported across studies. Thus, it was not possible to assess the impact of these known prognostic factors on all-cause mortality. Fifth, since only a few studies were available for meta-analyses of some outcomes (e.g., cardiovascular mortality, myocardial infarction, and vascular complications) and their subgroups, pooled effect estimates for these outcomes should be interpreted with caution. Finally, it should be taken into account that possibly in patients with active cancer referred for TAVR implantation there may be a bias related to the prognosis of the malignancy itself since valve replacement would not have been considered in those with a very reduced life expectancy, or those in whom cancer treatment is not feasible. Therefore, life expectancy may have been overestimated in some patients with active cancer.
Conclusion
Our meta-analysis shows that cancer patients present similar cardiovascular outcomes and post-procedural complications after TAVR. In patients with previous stable cancer, the overall prognosis is very similar to those without a history of cancer. Patients with active cancer presented higher all-cause mortality, which may be related to cancer itself, but TAVR should not systematically be denied to these groups of patients. A comprehensive evaluation involving a multidisciplinary team of cardiologists and oncologists aimed to select candidates for TAVR given their higher risk of mortality in the mid- and long-term is desirable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.763557/full#supplementary-material
References
1. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2021) 77:e25–197. doi: 10.1016/j.jacc.2020.11.035
2. Grant JK, Vincent L, Ebner B, Maning J, Singh H, Olorunfemi O, et al. In-hospital outcomes in patients with a history of malignancy undergoing transcatheter aortic valve implantation. Am J Cardiol. (2021) 142:109–15. doi: 10.1016/j.amjcard.2020.11.029
3. Guha A, Dey AK, Arora S, Cavender MA, Vavalle JP, Sabik JF III, et al. Contemporary trends and outcomes of percutaneous and surgical aortic valve replacement in patients with cancer. J Am Heart Assoc. (2020) 9:e014248. doi: 10.1161/JAHA.119.014248
4. Landes U, Iakobishvili Z, Vronsky D, Zusman O, Barsheshet A, Jaffe R, et al. Transcatheter aortic valve replacement in oncology patients with severe aortic stenosis. JACC Cardiovasc Interv. (2019) 12:78–86. doi: 10.1016/j.jcin.2018.10.026
5. Lantelme P, Lacour T, Bisson A, Herbert J, Ivanes F, Bourguignon T, et al. Futility risk model for predicting outcome after transcatheter aortic valve implantation. Am J Cardiol. (2020) 130:100–07. doi: 10.1016/j.amjcard.2020.05.043
6. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
7. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed April 15, 2021).
8. Dai XC, Yang XX, Ma L, Tang GM, Pan YY, Hu HL. Relationship between fluoroquinolones and the risk of aortic diseases: a meta-analysis of observational studies. BMC Cardiovasc Disord. (2020) 20:49. doi: 10.1186/s12872-020-01354-y
9. Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. (2016) 7:55–79. doi: 10.1002/jrsm.1164
10. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Wiley-Blackwell (2021).
11. Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Global Health. (2019) 7:192–98. doi: 10.1016/j.cegh.2018.05.005
12. Agrawal N, Kattel S, Waheed S, Kapoor A, Singh V, Sharma A, et al. Clinical outcomes after transcatheter aortic valve replacement in cancer survivors treated with ionizing radiation. Cardiooncology. (2019) 5:8. doi: 10.1186/s40959-019-0044-7
13. Biancari F, Dahlbacka S, Juvonen T, Virtanen MPO, Maaranen P, Jaakkola J, et al. Favorable outcome of cancer patients undergoing transcatheter aortic valve replacement. Int J Cardiol. (2020) 315:86–9. doi: 10.1016/j.ijcard.2020.03.038
14. Ghotra AS, Monlezun DJ, Boone D, Jacob R, Poosti K, Loghin C, et al. Outcomes of patients undergoing transcatheter aortic valve implantation with incidentally discovered masses on computed tomography. Am J Cardiol. (2020) 132:114–18. doi: 10.1016/j.amjcard.2020.07.003
15. Jain V, Saad AM, Gad MM, Bansal A, Abdelfattah O, Farwati M, et al. Outcomes of cancer patients undergoing transcatheter aortic valve replacement. JACC. (2020) 2:506–08. doi: 10.1016/j.jaccao.2020.05.023
16. Lind A, Totzeck M, Mahabadi AA, Jánosi RA, El Gabry M, Ruhparwar A, et al. Impact of cancer in patients undergoing transcatheter aortic valve replacement: a single-center study. JACC. (2020) 2:735–43. doi: 10.1016/j.jaccao.2020.11.008
17. Mangner N, Woitek FJ, Haussig S, Holzhey D, Stachel G, Schlotter F, et al. Impact of active cancer disease on the outcome of patients undergoing transcatheter aortic valve replacement. J Interv Cardiol. (2018) 31:188–96. doi: 10.1111/joic.12458
18. Romeo FJ, Seropian IM, Chiabrando JG, Raleigh JV, Smietniansky M, Cal M, et al. Additive prognostic value of carbohydrate antigen-125 over frailty in patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. (2021) 97:E263–e73. doi: 10.1002/ccd.29067
19. Tabata N, Al-Kassou B, Sugiura A, Kandt J, Shamekhi J, Stundl A, et al. Prognostic impact of cancer history in patients undergoing transcatheter aortic valve implantation. Clin Res Cardiol. (2020) 109:1243–50. doi: 10.1007/s00392-020-01615-y
20. Watanabe Y, Kozuma K, Hioki H, Kawashima H, Nara Y, Kataoka A, et al. Comparison of results of transcatheter aortic valve implantation in patients with versus without active cancer. Am J Cardiol. (2016) 118:572–7. doi: 10.1016/j.amjcard.2016.05.052
21. Johnson CB, Davis MK, Law A, Sulpher J. Shared risk factors for cardiovascular disease and cancer: implications for preventive health and clinical care in oncology patients. Can J Cardiol. (2016) 32:900–7. doi: 10.1016/j.cjca.2016.04.008
22. Kornowski R, Landes U. The double jeopardy of aortic stenosis in cancer patients. Eur Heart J Qual Care Clin Outcomes. (2018) 4:150–51. doi: 10.1093/ehjqcco/qcy016
23. Stewart MH, Jahangir E, Polin NM. Valvular heart disease in cancer patients: etiology, diagnosis, and management. Curr Treat Options Cardiovasc Med. (2017) 19:53. doi: 10.1007/s11936-017-0550-6
24. Zafar MR, Mustafa SF, Miller TW, Alkhawlani T, Sharma UC. Outcomes after transcatheter aortic valve replacement in cancer survivors with prior chest radiation therapy: a systematic review and meta-analysis. Cardiooncology. (2020) 6:8. doi: 10.1186/s40959-020-00062-y
25. Suh E, Stratton KL, Leisenring WM, Nathan PC, Ford JS, Freyer DR, et al. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: a retrospective cohort analysis from the childhood cancer survivor study. Lancet Oncol. (2020) 21:421–35. doi: 10.1016/S1470-2045(19)30800-9
26. Oh CM, Lee D, Kong HJ, Lee S, Won YJ, Jung KW, et al. Causes of death among cancer patients in the era of cancer survivorship in Korea: Attention to the suicide and cardiovascular mortality. Cancer Med. (2020) 9:1741–52. doi: 10.1002/cam4.2813
27. Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. (2019) 394:1041–54. doi: 10.1016/S0140-6736(19)31674-5
28. Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. (2019) 40:3889–97. doi: 10.1093/eurheartj/ehz766
29. Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol. (2017) 28:400–07. doi: 10.1093/annonc/mdw604
30. Stoltzfus KC, Zhang Y, Sturgeon K, Sinoway LI, Trifiletti DM, Chinchilli VM, et al. Fatal heart disease among cancer patients. Nat Commun. (2020) 11:2011. doi: 10.1038/s41467-020-15639-5
31. Okura Y, Ishigaki S, Sakakibara S, Yumoto C, Hashitate M, Sekine C, et al. Prognosis of cancer patients with aortic stenosis under optimal cancer therapies and conservative cardiac treatments. Int Heart J. (2018) 59:750–58. doi: 10.1536/ihj.17-320
32. Scherner M, Wahlers T. Acute kidney injury after transcatheter aortic valve implantation. J Thorac Dis. (2015) 7:1527–35. doi: 10.3978/j.issn.2072-1439.2015.06.14
33. Julien HM, Stebbins A, Vemulapalli S, Nathan AS, Eneanya ND, Groeneveld P, et al. Incidence, predictors, and outcomes of acute kidney injury in patients undergoing transcatheter aortic valve replacement: insights from the society of thoracic surgeons/american college of cardiology national cardiovascular data registry-transcatheter valve therapy registry. Circ Cardiovasc Interv. (2021) 14:e010032. doi: 10.1161/CIRCINTERVENTIONS.120.010032
Keywords: aortic stenosis, TAVR, cancer, systematic review, mortality
Citation: Diaz-Arocutipa C, Torres-Valencia J, Zavaleta-Camacho G and Vicent L (2021) Association Between Previous or Active Cancer and Clinical Outcomes in TAVR Patients: A Systematic Review and Meta-Analysis of 255,840 Patients. Front. Cardiovasc. Med. 8:763557. doi: 10.3389/fcvm.2021.763557
Received: 24 August 2021; Accepted: 12 October 2021;
Published: 02 November 2021.
Edited by:
Cezar Angi Iliescu, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Dominique Monlezun, University of Texas MD Anderson Cancer Center, United StatesPietro Ameri, University of Genoa, Italy
Copyright © 2021 Diaz-Arocutipa, Torres-Valencia, Zavaleta-Camacho and Vicent. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos Diaz-Arocutipa, Y2RpYXphckB1c2lsLmVkdS5wZQ==
 Carlos Diaz-Arocutipa
Carlos Diaz-Arocutipa Javier Torres-Valencia
Javier Torres-Valencia Gabriela Zavaleta-Camacho
Gabriela Zavaleta-Camacho Lourdes Vicent
Lourdes Vicent