
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 01 December 2021
Sec. Cardiovascular Surgery
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.759826
This article is part of the Research Topic Insights in Heart Surgery: 2021 View all 20 articles
 Sheng Zhang1†
Sheng Zhang1† Dan Zheng1†
Dan Zheng1† Xiao-Qiong Chu1†
Xiao-Qiong Chu1† Yong-Po Jiang1
Yong-Po Jiang1 Chun-Guo Wang2
Chun-Guo Wang2 Qiao-Min Zhang1
Qiao-Min Zhang1 Lin-Zhu Qian1
Lin-Zhu Qian1 Wei-Ying Yang1
Wei-Ying Yang1 Wen-Yuan Zhang1
Wen-Yuan Zhang1 Tao-Hsin Tung3*
Tao-Hsin Tung3* Rong-Hai Lin1*
Rong-Hai Lin1*Background: Cardiac surgery is associated with a substantial risk of major adverse events. Although carbon dioxide (CO2)-derived variables such as venous-to-arterial CO2 difference (ΔPCO2), and PCO2 gap to arterial–venous O2 content difference ratio (ΔPCO2/C(a−cv)O2) have been successfully used to predict the prognosis of non-cardiac surgery, their prognostic value after cardiopulmonary bypass (CPB) remains controversial. This hospital-based study explored the relationship between ΔPCO2, ΔPCO2/C(a−cv)O2 and organ dysfunction after CPB.
Methods: We prospectively enrolled 114 intensive care unit patients after elective cardiac surgery with CPB. Patients were divided into the organ dysfunction group (OI) and non-organ dysfunction group (n-OI) depending on whether organ dysfunction occurred or not at 48 h after CPB. ΔPCO2 was defined as the difference between central venous and arterial CO2 partial pressure.
Results: The OI group has 37 (32.5%) patients, 27 of which (23.7%) had one organ dysfunction and 10 (8.8%) had two or more organ dysfunctions. No statistical significance was found (P = 0.84) for ΔPCO2 in the n-OI group at intensive care unit (ICU) admission (9.0, 7.0–11.0 mmHg), and at 4 (9.0, 7.0–11.0 mmHg), 8 (9.0, 7.0–11.0 mmHg), and 12 h post admission (9.0, 7.0–11.0 mmHg). In the OI group, ΔPCO2 also showed the same trend [ICU admission (9.0, 8.0–12.8 mmHg) and 4 (10.0, 7.0–11.0 mmHg), 8 (10.0, 8.5–12.5 mmHg), and 12 h post admission (9.0, 7.3–11.0 mmHg), P = 0.37]. No statistical difference was found for ΔPCO2/C(a−cv)O2 in the n-OI group (P = 0.46) and OI group (P = 0.39). No difference was detected in ΔPCO2, ΔPCO2/C(a−cv)O2 between groups during the first 12 h after admission (P > 0.05). Subgroup analysis of the patients with two or more failing organs compared to the n-OI group showed that the predictive performance of lactate and Base excess (BE) improved, but not of ΔPCO2 and ΔPCO2/C(a−cv)O2. Regression analysis showed that the BE at 8 h after admission (odds ratio = 1.37, 95%CI: 1.08–1.74, P = 0.009) was a risk factor for organ dysfunction 48 h after CBP.
Conclusion : ΔPCO2 and ΔPCO2/C(a−cv)O2 cannot be used as reliable indicators to predict the occurrence of organ dysfunction at 48 h after CBP due to the pathophysiological process that occurs after CBP.
Despite improvements in surgical technique, anesthesia, management, and postoperative care, cardiac surgery is still associated with a substantial risk of major adverse events (1). Early identification of risk factors and interventions reduce the occurrence of complications. Venous-to-arterial carbon dioxide (CO2) difference (ΔPCO2) is a parameter that reflects tissue hypoperfusion in critically ill patients who are insufficiently resuscitated (2). The PCO2 gap to arterial–venous O2 content difference ratio (ΔPCO2/C(a−cv)O2) has also been described as an indicator of the relationship between oxygen delivery (DO2) and oxygen consumption (VO2) (3). Although they have been successfully used to guide fluid resuscitation in patients with sepsis (4, 5) and predict prognosis of non-cardiac surgery patients (6, 7), the predictive value of CO2-derived variables after cardiopulmonary bypass surgery (CPB) is still controversial (8, 9). CO2-derived variables may be unable to inform on tissue ischemia because they do not track VO2 changes in cardiac surgical patients (10). Hyperlactatemia is related to tissue hypoxia but is also affected by factors such as catecholamines and metabolic rate. A previous study found that base excess (BE) is superior to lactate levels for the prediction of ICU mortality after cardiac surgery (11). Organ dysfunction is central to the pathogenesis of death and disability in critically ill patients, and the prognosis becomes worse as the number of failed organs increases. Therefore, it is more meaningful to use organ dysfunction as a clinical outcome variable because of the potential relationship. Although there are many studies on CO2-derived variables and clinical outcome, few studies have explored the prognostic value of CO2-derived variables basing on organ dysfunction. Organ dysfunction is considered to be an effective new outcome indicator in cardiac surgical patients, especially when the number of cases required is relatively small (12, 13).
The main objective of this study was to investigate relationship between CO2-derived variables and organ dysfunction occurring during the early phases after elective cardiac surgery with CPB. The secondary objective was to compare these variables with BE and lactate, which are usually associated with tissue hypoperfusion.
We conducted a prospective observational study in a 39-bed mixed intensive care unit (ICU) of a university-affiliated Hospital. Our clinical trial was registered in the China Clinical Trial Registry (registration number: ChiCTR-ROC-17010727). Ethics approval was obtained from the Ethics Committee of Taizhou Hospital, Zhejiang Province. Written informed consent was obtained from all patients. The clinical trial started in February 2017 and ended in August 2018.
Adults (≥18 years old) admitted to ICU immediately after CBP, were included if an arterial line and a central venous catheter (confirmed by X-ray) had been inserted. After initiation of the trial, we removed one of the eligibility criteria: “Factors affecting the accuracy of cardiac output monitoring: aortic regurgitation, atrial fibrillation, aortic balloon counter pulsation, high dose norepinephrine,” because it was not necessary and would have reduced our sample size. The exclusion criteria were: emergency surgery, pregnancy, chronic renal insufficiency with dialysis, preoperative acute or chronic liver failure, hematologic diseases, misplacement of the central venous catheter, and death within 48 h.
Intraoperative and ICU management was conducted according to local protocols and international guidelines. Ventilator settings were as follows: control mode, tidal volume 6–8 mL/kg, positive end-expiratory pressure ≤ 5 cmH2O, respirations 14–16 times/min, fraction of inspired oxygen 40%. Analgesia was achieved with continuous infusion of fentanyl (0.5–10 mg/kg/h) targeting Critical Care Pain Observation Tool scores of 0–2. Anesthesia was maintained with a continuous infusion of propofol (1–4 mg/kg/h) targeting Richmond Agitation-Sedation Scale scores of−2–0. Vasoactive drugs and fluid infusions were adjusted according to mean arterial pressure of 60–70 mmHg. Infusion of red blood cells was prescribed as needed to maintain hemoglobin (Hb) concentration ≥9.0 g/dL. Crystalloid solution (Lactated Ringer's solution or 0.9% saline) or 20% albumin was added for volume expansion if necessary.
We prospectively collected preoperative, intraoperative, and postoperative variables, including clinical characteristics, duration of surgery, CBP duration, vital signs, fluid balance, vasoactive-inotropic score, routine blood tests, blood gas results, and clinical biochemistry. The specific data collection time points were at ICU admission and 4 h (H4), 8 h (H8), 12 h (H12), 24 h (H24), and 48 h (H48) after ICU admission. White blood cell count, hemoglobin concentration, platelet count, clinical biochemistry, chest X-rays, or CT scans were reviewed to evaluate organ function at ICU admission, H24, and H48. The length of stay in ICU, length of ventilator use, European System for Cardiac Operative Risk Evaluation II, Acute Physiology and Chronic Health Evaluation Score II (APACHE-II), and sequential organ failure score were recorded at ICU admission, H24, and H48. Paired arterial and central venous blood gases, arterial blood lactate, base excess (BE), and Hb levels were measured at ICU admission, H4, H8, and H12 using an automated analyzer (ABL800 Flex, Radiometer Medical Aps, Aakandevej 21, DK-2700 Bronshoj, Denmark).
Vasoactive-inotropic score (14) and CO2-derived and O2-derived variables were calculated as follows:
Vasoactive-inotropic score = dopamine (μg/kg/min) + dobutamine (μg/kg/min) + 10 × milrinone (μg/kg/min)
+ 100 × epinephrine (μg/kg/min) + 100 × norepinephrine (μg/kg/min) + 10,000 × vasopressin (μg/kg/min)
The primary outcome variable was organ dysfunction at H48. Briefly, H48 organ dysfunction included: acute respiratory distress syndrome (PaO2/fraction of inspired oxygen <300 mmHg or PaO2 <60 mmHg requiring non-invasive ventilation or invasive mechanical ventilation support), acute kidney injury [Kidney Disease Improving Global Outcomes (KDIGO) level ≥1], acute tissue hypoperfusion (presence of tachycardia and hypotension associated with a central venous oxygen saturation <65%, cardiac index ≤ 2.2 L/min/m2), cardiac arrest (15) (cessation of cardiac mechanical activity, as confirmed by the absence of circulation signs), arrhythmia (15) (atrial fibrillation for ≥1 min was recorded, analyzed, and defined as “postoperative atrial fibrillation;” ventricular tachycardia and ventricular fibrillation were recorded by continuous ECG monitoring during the intensive care stay), acute neurologic dysfunction (stroke, seizure, persistent delirium, and Glasgow coma score below 12). According to the presence or absence of organ dysfunction at 48 h after CPB, the patients were divided into organ dysfunction group (OI) and non-organ dysfunction group (n-OI). The OI group was further divided into two subgroups: OI-1 including patients with one organ dysfunction, and OI-2 including patients with two or more organ dysfunctions.
GPower software version 3.0.10 was applied to estimate required sample size for this study. This study used repeated-measure, between factors for the analysis. The study effect size was set at 0.25, power was set at 90%, alpha value was set at 0.05, and four -time measurements. Based on these, a minimum total sample of 108 subjects is required.
The statistical analysis was carried out using IBM SPSS 20 software. The Kolmogorov–Smirnoff test evaluated the normality of continuous variables and found that many continuous variables were non-normally distributed. Consequently, all continuous variables were expressed as median. The Mann–Whitney U test or Kruskal–Wallis test was used for group comparison, and repeated-measures ANOVA was used for intragroup comparison. Chi-square test (or Fisher's exact test, when appropriate) was used to compare categorical variables. GraphPad prism version 8.0.2 statistical software was used for boxplot charts.
According to the occurrence of organ dysfunction at H48, the patients were divided into n-OI and OI groups, with the OI group being further subdivided into OI-1 and OI-2 subgroups according to the number of organs with evidence of dysfunction at H48. Comparisons took place within the OI group, within the n-OI group, between the OI and n-OI groups, and between the n-OI and OI-2 subgroups. The ROC curve analysis was carried out to assess the predictive performance of the variables independently associated with H48 organ dysfunction. The area under the curve (AUC) was calculated based on their 95% confidence intervals (CIs).
In a subsequent analysis, variables were introduced into the logistic regression model if significantly associated with H48 organ dysfunction at the univariate analysis, when P-value was < 0.05. A Hosmer–Lemeshow test was used to assess the goodness of fit of the model.
A total of 139 patients who fulfilled the criteria were screened and 114 patients were included (Figure 1). A total of 77 (67.5%) patients formed the n-OI group, and 37 (32.5%) patients comprised the OI group, of which 23.7% (27/114) had one organ dysfunction, 8.8% (10/114) had two or more organ dysfunctions. Compared to the OI group, the n-OI group had shorter length of stay in hospital [10.0 (8.5–14.0) vs. 13.0 (10.0–18.0) days, P = 0.01], shorter length of ICU stay [42.0 (24.0–48.0) vs. 47.0 (27.0–66.5) h, P = 0.02], and lower H24 and H48 sequential organ failure scores [3 (2–5) vs. 5 (4–7), P = 0.01] and [4 (3–5) vs. 5 (3–6), P = 0.02, respectively]. No statistical difference in the duration of mechanical ventilation was detected between the two groups of patients [19.0 (16.4–21.0) vs. 20.2 (18.5–22.8) h, P = 0.07] (Table 1).
The length of ventilator usage in all patients was 19.5 (range: 17.0–21.0) h. In order to reduce the influence of spontaneous breathing on CO2-derived variables after weaning, ICU admission, H4, H8, and H12 time points were selected for analysis.
The ΔPCO2 of the n-OI group at the four time points were 9.0 (7.0–11.0), 9.0 (7.0–11.0), 9.0 (7.0–11.0), and 9.0 (7.0–11.0) mmHg (P = 0.84), and those of the OI group were 9.0 (8.0–12.8), 10.0 (7.0–11.0), 10.0 (8.5–12.5), and 9.0 (7.3–11.0) mmHg (P = 0.37). The ΔPCO2/C(a−cv)O2 of the n-OI group at the four time points were 2.0 (1.6–2.7), 2.1 (1.7–2.8), 2.0 (1.5–2.3), and 2.0 (1.7–2.4) mmHg/mL (P = 0.46), and those of the OI group were 2.1 (1.7–2.9), 2.2 (1.8–2.5), 2.1 (1.8–2.6), and 1.8 (1.6–2.5) mmHg/mL (P = 0.39) (Table 2), respectively. There were no statistical differences in the CO2-derived variables at any of the four time points (P > 0.05) between the n-OI and OI groups (Figure 2). Statistically significant differences were observed intragroup for pH, PO2, BE, Hb concentration, fraction of inspired oxygen, lactate level, fluid balance, and body temperature at all four time points (Table 2).
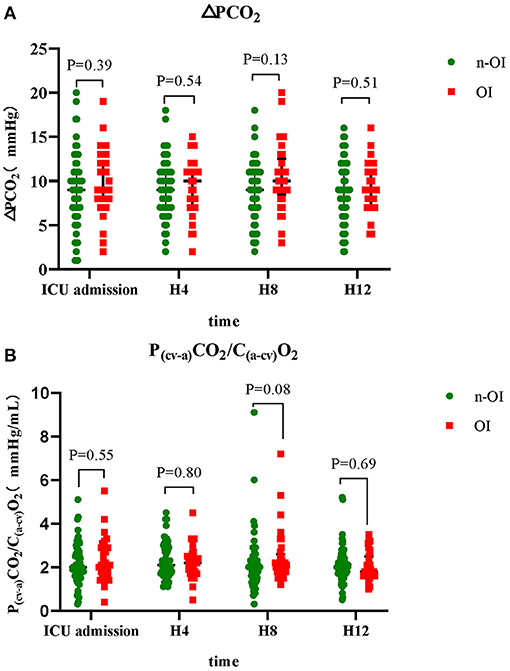
Figure 2. Dynamic changes of carbon dioxide-derived variables of n-OI and OI groups at the four time points. (A) central venous-to-arterial carbon dioxide difference (ΔPCO2) (B) ΔPCO2 to arterial-central venous oxygen content difference rate (ΔPCO2/C(a−cv)O2). GraphPad prism version 8.0.2 statistical software was used to construct box charts (median with interquartile range). The Mann–Whitney U test was used for intergroup comparison.
Regarding specific time point variables, ΔPCO2, ΔPCO2/C(a−cv)O2, lactate and BE had no predictive value (Table 3). Comparison of the subgroup OI-2 to the n-OI group demonstrated that lactate and BE had limited predictive value (Table 4). After adjusting for age (median, ≤ 58, >58 years old) and gender, H8 BE (odds ratio = 1.37, 95% CI: 1.08–1.74, P = 0.009) was a risk factor for H48 organ dysfunction after CPB.
We found that the ΔPCO2/C(a−cv)O2 and ΔPCO2 did not differ significantly between the two groups at different time points during the first 12 h after ICU admission. Elevated ΔPCO2 and ΔPCO2/C(a−cv)O2 were common phenomena after CPB. The ΔPCO2/C(a−cv)O2 and ΔPCO2 cannot be used to predict H48 organ dysfunction. The predictive performance of lactate and BE was significantly enhanced as the number of dysfunctioning organs increased, although it was limited.
To the best of our knowledge, this study is the first to analyze the association of both ΔPCO2 and ΔPCO2/Ca−cvO2 on 48 h-organ dysfunction after adult cardiac surgery. In our study, the ΔPCO2 value, CPB time, aortic clamping time, and sequential organ failure score at ICU admission were similar to Guinot et al. study (16). They also found that ΔPCO2 is not predictive of postoperative complications or mortality. Their research did not provide information on the tidal volume and pCO2, which are thought to affect the accuracy of ΔPCO2 (17). Our study has very specific ventilator settings, which make up for this deficiency. Additionally, we found that rewarming, Hb dilution, and pH change were universal after CPB, which might have affected the accuracy of our estimations (18, 19). According to the literature, pCO2 may be elevated due to the accumulation of CO2 in tissues caused by reperfusion after CPB (20). Another study with negative results was reported by Morel et al. (21), who believe that ΔPCO2 is difficult to interpret due to the sudden variation of many parameters interfering with tissue perfusion. Such studies with negative results may have a common feature that outcomes may occur before measurement such as vasoplegia, heart failure, and acute renal failure. Furthermore, the complication itself may lead to an increased ΔPCO2 and, according to the time of measurement, the arrow of causation could be reversed (22). In our study, the median sequential organ failure score was 5, 4, and 3 at ICU admission, and 24 and 48 h post admission, respectively. Therefore, some of the organ dysfunction was suspected to have occurred during surgery. However, we believe that outcomes to be reliable as organ dysfunction generally results in poor outcomes (prolonged ICU stay, prolonged hospital stay).
A review of the literature also found studies with positive results. Mukai et al. (23) found that the ΔPCO2 at the end of cardiac surgery was a moderate predictor of postoperative complications (area under the curve [AUC]: 0.731; 95% confidence interval [CI] 0.588–0.874), at a 6.8 mmHg cut off with a sensitivity of 72.0% and specificity of 70.6%. Complications are defined as MMOM (major organ morbidity and mortality) (24), as follows: death, stroke requiring drug treatment, renal failure requiring dialysis, prolonged mechanical ventilation (more than 48 h postoperatively), re-operation, and deep sternal infection. It is worth noting that measurements were obtained at the end of surgery and the definition of complications were easy to identify. Hence, we cannot exclude that perioperative ΔPCO2 could be predictive of outcome (21). A previous study reported that ΔPCO2/C(a−cv)O2 is not associated with respiratory quotient (25). In our study, ΔPCO2/C(a−cv)O2 was not associated with the existence of tissue hypoxia effectively because of its poor relationship with lactate (R2=0.067). ΔPCO2/C(a−cv)O2 was grouped according to whether it was ≥1.4 mmHg/mL, and no statistical significance were detected in the length of the ventilator, LOS in ICU, or the 24 h and 48 h sequential organ failure score (data not provided). That is probably largely because of the influence of CPB on the activation of inflammation in vivo, ischemia-reperfusion injury, myocardial suppression, and other factors, thus, a microcirculation disorder exists that can last for 72 h (26). From our research, we have observed many factors that affect CO2-derived indicators after cardiac surgery in the real world, including CBP itself (27), translocation, endothelial dysfunction (28, 29), and microcirculation alterations (30) may directly or indirectly cause complications, making the interpretation of the results more complicated.
Hyperlactatemia is common after cardiopulmonary bypass, and it can be influenced by many factors. Patients undergoing CPB are exposed to the “elution effect,” i.e., the release of lactic acid from the lungs (31) and the liver due to organ malfunction associated with CPB (32). A recent study has demonstrated that hyperlactatemia may be also in relation to tissue metabolic uncoupling (33). Therefore, lactic acid does not always reflect tissue hypoxia. The predictive value of lactate after CPB surgery is limited. This study demonstrated that postoperative lactic acidemia is common, with progressively decreasing levels of lactic acid observed in both groups. After adjustment for confounding factors, compared to ΔPCO2 and ΔPCO2/C(a-cv)O2, lactic acid has a moderate ability to predict two or more organ dysfunction. In addition, BE is the amount of base in mmol required to titrate 1 L of whole arterial blood to a pH of 7.40, with the sample fully saturated with oxygen at 37°C and a PaCO2 of 40 mmHg. We also found that BE was a more robust predictor of H48 organ dysfunction than lactic acid, and H8 BE was a risk factor for dysfunction of two or more organs (odds ratio = 1.37), whereas lactic acid was not. A high negative value of BE may be associated with renal dysfunction, metabolic acidosis, and shock, but the factors associated with the reduced systemic BE in patients after cardiac surgery are yet to be clarified (34). We speculated that lactic acidosis might be one of the main reasons leading to the high negative value of BE because a good correlation was established between BE and lactic acid (R2 = 0.599, P < 0.05), which could also explain the low susceptibility of BE to interference from other factors.
This study has some limitations. First, this was a single-center study, and its results may not be generalizable. Second, the sample size was relatively small, and no deaths occurred among the study patients. Third, because the group classification was based solely on the occurrence of organ dysfunction, a misclassification bias may have occurred. However, our subgroup analysis was based on the number of failing organs and resulted in similar conclusions. Fourth, Pcv−aCO2/C(a−cv)O2 cannot replace Pmv−aCO2/C(a−cv)O2 (mv: mixed venous blood) and might have underestimated the CO2 exchange from splanchnic circulation. Finally, it was not easy to detect when the outcome of interest, i.e., organ dysfunction, first occurred relative to the measurement. Continuous measurements or change over time may have more prognostic value than a single time point. However, the dynamic changes of ΔPCO2 at t0 and T6 time points were not found to be associated with postoperative complications or mortality in children after CPB (16).
In conclusion, ΔPCO2 and ΔPCO2/C(a−cv)O2 cannot be used as reliable indicators to predict the occurrence of organ dysfunction at 48 h after CBP, which is due to the complex pathophysiological processes after CBP.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethics Committee of Taizhou Hospital, Zhejiang Province. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SZ: data curation, investigation, resources, writing—original draft, validation, and funding acquisition. DZ: data curation, investigation, and methodology. X-QC: data curation, investigation, methodology, and software. Y-PJ, L-ZQ, W-YY, and W-YZ: data curation, investigation, and resources. C-GW: data curation and formal analysis. Q-MZ: data curation and resources. T-HT: design of the study and performed data synthesis of the revised stage. R-HL: conceptualization, data curation, formal analysis, funding acquisition, software, validation, writing—review, and editing. All authors have read and approved the manuscript.
This study was supported by the Medical and Health Science and Technology Project, Zhejiang Province (2017KY163). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors kindly thank Dr. Zhang Meixian for her contribution to establishing the database.
ΔPCO2/C(a−cv)O2, Arterial-venous O2 content difference ratio; CO2, carbon dioxide; CPB, cardiopulmonary bypass; Hb, hemoglobin; ICU, intensive care unit; n-OI, non-organ dysfunction group; OI, organ dysfunction group; ΔPCO2, venous-to-arterial CO2 difference.
1. D'Agostino RS, Jacobs JP, Badhwar V, Fernandez FG, Paone G, Wormuth DW, et al. The society of thoracic surgeons adult cardiac surgery database: 2018 update on outcomes and quality. Ann Thorac Surg. (2018) 105:15–23. doi: 10.1016/j.athoracsur.2017.10.035
2. Mallat J, Lemyze M, Tronchon L, Vallet B, Thevenin D. Use of venous-to-arterial carbon dioxide tension difference to guide resuscitation therapy in septic shock. World J Crit Care Med. (2016) 5:47–56. doi: 10.5492/wjccm.v5.i1.47
3. Mekontso-Dessap A, Castelain V, Anguel N, Bahloul M, Schauvliege F, Richard C, et al. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med. (2002) 28:272–7. doi: 10.1007/s00134-002-1215-8
4. Ospina-Tascón GA, Umaña M, Bermúdez W, Bautista-Rincón DF, Hernandez G, Bruhn A, et al. Combination of arterial lactate levels and venous-arterial CO2 to arterial-venous O2 content difference ratio as markers of resuscitation in patients with septic shock. Intensive Care Med. (2015) 41:796–805. doi: 10.1007/s00134-015-3720-6
5. Ospina-Tascón GA, Bautista-Rincón DF, Umaña M, Tafur JD, Gutiérrez A, García AF, et al. Persistently high venous-to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock. Crit Care. (2013) 17:R294. doi: 10.1186/cc13160
6. Robin E, Futier E, Pires O, Fleyfel M, Tavernier B, Lebuffe G, et al. Central venous-to-arterial carbon dioxide difference as a prognostic tool in high-risk surgical patients. Crit Care. (2015) 19:227. doi: 10.1186/s13054-015-0917-6
7. Futier E, Robin E, Jabaudon M, Guerin R, Petit A, Bazin JE, et al. Central venous O2 saturation and venous-to-arterial CO2 difference as complementary tools for goal-directed therapy during high-risk surgery. Crit Care. (2010) 14:R193. doi: 10.1186/cc9310
8. Ospina-Tascón GA, Calderón Tapia LE. Venous-arterial CO2 to arterial-venous O2 differences: a physiological meaning debate. J Crit Care. (2018) 48:443–4. doi: 10.1016/j.jcrc.2018.09.030
9. Huette P, Ellouze O, Abou-Arab O, Guinot PG. Venous-to-arterial pCO2 difference in high-risk surgical patients. J Thorac Dis. (2019) 11:S1551–7. doi: 10.21037/jtd.2019.01.109
10. Abou-Arab O, Braik R, Huette P, Bouhemad B, Lorne E, Guinot PG. The ratios of central venous to arterial carbon dioxide content and tension to arteriovenous oxygen content are not associated with overall anaerobic metabolism in postoperative cardiac surgery patients. PLoS ONE. (2018) 13:e0205950. doi: 10.1371/journal.pone.0205950
11. O'Connor E, Fraser JF. The interpretation of perioperative lactate abnormalities in patients undergoing cardiac surgery. Anaesth Intensive Care. (2012) 40:598–603. doi: 10.1177/0310057X1204000404
12. Stoppe C, McDonald B, Benstoem C, Elke G, Meybohm P, Whitlock R, et al. Evaluation of persistent organ dysfunction plus death as a novel composite outcome in cardiac surgical patients. J Cardiothorac Vasc Anesth. (2016) 30:30–8. doi: 10.1053/j.jvca.2015.07.035
13. Heyland DK, Muscedere J, Drover J, Jiang X, Day AG, Canadian Critical Care Trials Group. Persistent organ dysfunction plus death: a novel, composite outcome measure for critical care trials. Crit Care. (2011) 15:R98. doi: 10.1186/cc10110
14. Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. (2010) 11:234–8. doi: 10.1097/PCC.0b013e3181b806fc
15. Jammer I, Wickboldt N, Sander M, Smith A, Schultz MJ, Pelosi P, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European perioperative clinical outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. (2015) 32:88–105. doi: 10.1097/EJA.0000000000000118
16. Guinot PG, Badoux L, Bernard E, Abou-Arab O, Lorne E, Dupont H. Central venous-to-arterial carbon dioxide partial pressure difference in patients undergoing cardiac surgery is not related to postoperative outcomes. J Cardiothorac Vasc Anesth. (2017) 31:1190–6. doi: 10.1053/j.jvca.2017.02.015
17. Mallat J, Mohammad U, Lemyze M, Meddour M, Jonard M, Pepy F, et al. Acute hyperventilation increases the central venous-to-arterial PCO2 difference in stable septic shock patients. Ann Intensive Care. (2017) 7:31. doi: 10.1186/s13613-017-0258-5
18. Dubin A, Ferrara G, Kanoore Edul VS, Martins E, Canales HS, Canullán C, et al. Venoarterial PCO2-to-arteriovenous oxygen content difference ratio is a poor surrogate for anaerobic metabolism in hemodilution: an experimental study. Ann Intensive Care. (2017) 7:65. doi: 10.1186/s13613-017-0288-z
19. Ospina-Tascón GA, Hernández G, Cecconi M. Understanding the venous-arterial CO2 to arterial-venous O2 content difference ratio. Intensive Care Med. (2016) 42:1801–4. doi: 10.1007/s00134-016-4233-7
20. Jakob SM, Groeneveld AB, Teboul JL. Venous-arterial CO2 to arterial-venous O2 difference ratio as a resuscitation target in shock states? Intensive Care Med. (2015) 41:936–8. doi: 10.1007/s00134-015-3778-1
21. Morel J, Grand N, Axiotis G, Bouchet JB, Faure M, Auboyer C, et al. High veno-arterial carbon dioxide gradient is not predictive of worst outcome after an elective cardiac surgery: a retrospective cohort study. J Clin Monit Comput. (2016) 30:783–9. doi: 10.1007/s10877-016-9855-3
22. Huette P, Beyls C, Mallat J, Martineau L, Besserve P, Haye G, et al. Central venous-to-arterial CO2 difference is a poor tool to predict adverse outcomes after cardiac surgery: a retrospective study. Can J Anaesth. (2021) 68:467–76. doi: 10.1007/s12630-020-01881-4
23. Mukai A, Suehiro K, Kimura A, Funai Y, Matsuura T, Tanaka K, et al. Comparison of the venous-arterial CO2 to arterial-venous O2 content difference ratio with the venous-arterial CO2 gradient for the predictability of adverse outcomes after cardiac surgery. J Clin Monit Comput. (2020) 34:41–53. doi: 10.1007/s10877-019-00286-z
24. Shroyer AL, Coombs LP, Peterson ED, Eiken MC, DeLong ER, Chen A, et al. The society of thoracic surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg. (2003) 75:1856–64. doi: 10.1016/S0003-4975(03)00179-6
25. Bar S, Grenez C, Nguyen M, de Broca B, Bernard E, Abou-Arab O, et al. Predicting postoperative complications with the respiratory exchange ratio after high-risk noncardiac surgery: a prospective cohort study. Eur J Anaesthesiol. (2020) 37:1050–7. doi: 10.1097/EJA.0000000000001111
26. Dekker NAM, Veerhoek D, Koning NJ, van Leeuwen ALI, Elbers PWG, van den Brom CE, et al. Postoperative microcirculatory perfusion and endothelial glycocalyx shedding following cardiac surgery with cardiopulmonary bypass. Anaesthesia. (2019) 74:609–18. doi: 10.1111/anae.14577
27. McGuinness J, Bouchier-Hayes D, Redmond JM. Understanding the inflammatory response to cardiac surgery. Surgeon. (2008) 6:162–71. doi: 10.1016/S1479-666X(08)80113-8
28. Magoon R, Makhija N. Endothelial glycocalyx and cardiac surgery: newer insights. J Cardiothorac Vasc Anesth. (2020) 34:310–1. doi: 10.1053/j.jvca.2019.07.003
29. Brettner F, Chappell D, Schwartz L, Lukasz A, Kümpers P, Becker BF, et al. Vascular endothelial dysfunction during cardiac surgery: on-pump versus off-pump coronary surgery. Eur Surg Res. (2017) 58:354–68. doi: 10.1159/000480431
30. Kara A, Akin S, Ince C. The response of the microcirculation to cardiac surgery. Curr Opin Anaesthesiol. (2016) 29:85–93. doi: 10.1097/ACO.0000000000000280
31. Gasparovic H, Plestina S, Sutlic Z, Husedzinovic I, Coric V, Ivancan V, et al. Pulmonary lactate release following cardiopulmonary bypass. Eur J Cardiothorac Surg. (2007) 32:882–7. doi: 10.1016/j.ejcts.2007.09.001
32. Mustafa I, Roth H, Hanafiah A, Hakim T, Anwar M, Siregar E, et al. Effect of cardiopulmonary bypass on lactate metabolism. Intensive Care Med. (2003) 29:1279–85. doi: 10.1007/s00134-003-1860-6
33. Bar S, Nguyen M, de Broca B, Bernard E, Dupont H, Guinot PG. Risk factors and determinants of intraoperative hyperlactatemia in major non-cardiac surgery. J Clin Anesth. (2021) 74:110359. doi: 10.1016/j.jclinane.2021.110359
Keywords: venous-to-arterial carbon dioxide difference, base excess, lactate, cardiopulmonary bypass, organ dysfunction
Citation: Zhang S, Zheng D, Chu X-Q, Jiang Y-P, Wang C-G, Zhang Q-M, Qian L-Z, Yang W-Y, Zhang W-Y, Tung T-H and Lin R-H (2021) ΔPCO2 and ΔPCO2/C(a–cv)O2 Are Not Predictive of Organ Dysfunction After Cardiopulmonary Bypass Front. Cardiovasc. Med. 8:759826. doi: 10.3389/fcvm.2021.759826
Received: 17 August 2021; Accepted: 09 November 2021;
Published: 01 December 2021.
Edited by:
Hendrik Tevaearai Stahel, Bern University Hospital, SwitzerlandReviewed by:
Pierre-Gregoire Guinot, Centre Hospitalier Regional Universitaire de Dijon, FranceCopyright © 2021 Zhang, Zheng, Chu, Jiang, Wang, Zhang, Qian, Yang, Zhang, Tung and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong-Hai Lin, dHlsaW5yaEAxNjMuY29t; Tao-Hsin Tung, Y2gyODc2QHllYWgubmV0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.