
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 10 November 2021
Sec. Lipids in Cardiovascular Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.757738
This article is part of the Research Topic Chronic Inflammation, Oxidative Stress and Lipoprotein Metabolism in Cardio-Pulmonary Continuum View all 7 articles
 Arjun Sinha1,2
Arjun Sinha1,2 Adovich S. Rivera2,3
Adovich S. Rivera2,3 Simran A. Chadha4
Simran A. Chadha4 Sameer Prasada1
Sameer Prasada1 Anna E. Pawlowski5
Anna E. Pawlowski5 Edward Thorp6
Edward Thorp6 Matthew DeBerge6
Matthew DeBerge6 Rosalind Ramsey-Goldman1
Rosalind Ramsey-Goldman1 Yvonne C. Lee1
Yvonne C. Lee1 Chad J. Achenbach1
Chad J. Achenbach1 Donald M. Lloyd-Jones1,2
Donald M. Lloyd-Jones1,2 Matthew J. Feinstein1,2*
Matthew J. Feinstein1,2*Background: Chronic inflammatory diseases (CIDs) are considered risk enhancing factors for coronary heart disease (CHD). However, sparse data exist regarding relative CHD risks across CIDs.
Objective: Determine relative differences in CHD risk across multiple CIDs: psoriasis, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), human immunodeficiency virus (HIV), systemic sclerosis (SSc), and inflammatory bowel disease (IBD).
Methods: The cohort included patients with CIDs and controls without CID in an urban medical system from 2000 to 2019. Patients with CIDs were frequency-matched with non-CID controls on demographics, hypertension, and diabetes. CHD was defined as myocardial infarction (MI), ischemic heart disease, and/or coronary revascularization based on validated administrative codes. Multivariable-adjusted Cox models were used to determine the risk of incident CHD and MI for each CID relative to non-CID controls. In secondary analyses, we compared CHD risk by disease severity within each CID.
Results: Of 17,049 patients included for analysis, 619 had incident CHD (202 MI) over an average of 4.4 years of follow-up. The multivariable-adjusted risk of CHD was significantly higher for SLE [hazard ratio (HR) 1.9, 95% confidence interval (CI) 1.2, 3.2] and SSc (HR 2.1, 95% CI 1.2, 3.9). Patients with SLE also had a significantly higher risk of MI (HR 3.6, 95% CI 1.9, 6.8). When CIDs were categorized by markers of disease severity (C-reactive protein for all CIDs except HIV, for which CD4 T cell count was used), greater disease severity was associated with higher CHD risk across CIDs.
Conclusions: Patients with SLE and SSc have a higher risk of CHD. CHD risk with HIV, RA, psoriasis, and IBD may only be elevated in those with greater disease severity. Clinicians should personalize CHD risk and treatment based on type and severity of CID.
Individuals living with chronic inflammatory diseases (CIDs) have an increased risk of coronary heart disease (CHD) and myocardial infarction (MI) (1, 2). In primary prevention guidelines from the European Society of Cardiology and American Heart Association, certain CIDs such as psoriasis, rheumatoid arthritis (RA), human immunodeficiency virus (HIV), and systemic lupus erythematosus (SLE) are recognized as risk-enhancing factors for atherosclerotic cardiovascular disease (ASCVD) (3, 4). Emerging data also suggest that CHD risk is elevated in other CIDs such as systemic sclerosis (SSc) and inflammatory bowel disease (IBD) (5–7). While grouping CIDs together rightly emphasizes the role of inflammation in CHD, each CID is distinct with respect to its immunologic and inflammatory dysfunction. There is also a wide range of disease severity within each CID. Thus, the degree of risk factor modification for primary prevention should match the risk of CHD based on CID type and severity. However, prior studies investigating CHD risk in inflammatory conditions either investigated only a single CID or aggregated several heterogeneous CIDs into groups (8).
Given pathophysiological and clinical heterogeneity among CIDs, which may lead to distinct patterns of CHD risk, more granular investigation of CHD risks for specific CIDs is needed. Therefore, we compared risks for incident CHD, including MI, across several disaggregated CIDs (RA, HIV, psoriasis, SLE, SSc, and IBD) in a large metropolitan health system. We also investigated associations of inflammatory phenotype severity with CHD risk within CIDs.
The Northwestern Medicine Enterprise Data Warehouse (NMEDW) was used to create an electronic health record-based cohort of persons with CIDs and non-CID controls receiving regular outpatient care in the Northwestern Medicine (NM) system, which serves the large, diverse metropolitan area of Chicago, Illinois (9). As described previously, regular outpatient care was defined as at least 1 CID specialty and/or primary care outpatient visit at least once every 2 years between baseline (the first in-person outpatient encounter) and censoring dates (death, CHD, or most recent in-person outpatient encounter at least 1 year after baseline date if more than 2 years passed between outpatient visits) (9). Controls were defined as persons in care without CIDs and were 1:1 frequency-matched with the CID population on the combination of the following variables: age, sex, race, insurance status, baseline year, hypertension, and diabetes. The overall cohort included 38,097 patients (Figure 1). However, the primary analyses were conducted in nested cohort of patients with cholesterol levels available within 1 year of baseline date, n = 17,049. Patients with baseline CHD and missing demographic data were excluded from the study. The cohort creation and research protocol were approved by the institutional review board at Northwestern University (Chicago, IL, USA). A waiver of informed consent was received.
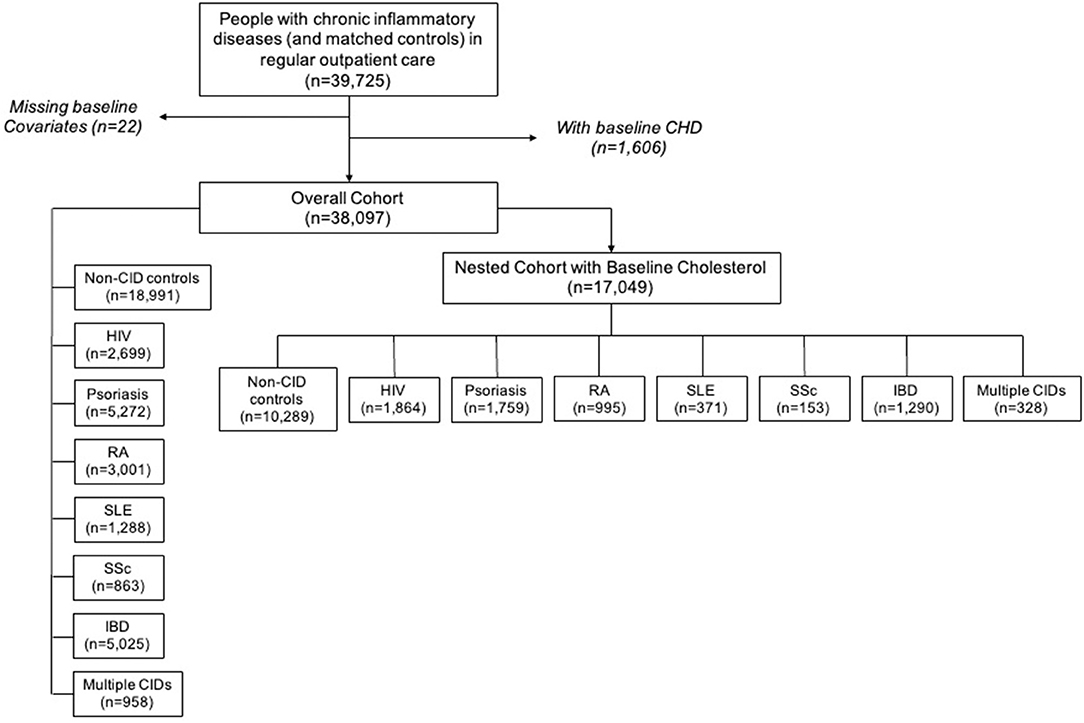
Figure 1. Chronic inflammatory disease cohort creation flow diagram. CHD, coronary heart disease; CID, chronic inflammatory disease; HIV, human immunodeficiency virus; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; IBD, inflammatory bowel disease.
Adults age 18 years and older with CIDs were identified during the observation period from January 1, 2000 to January 1, 2019 using previously validated criteria: two or more International Classification of Diseases-9 (ICD-9) or ICD-10 diagnosis codes within a 2 year period were required for SSc (10), IBD (11, 12), and psoriasis (13, 14); SLE required three diagnosis codes in three separate months as described previously (15, 16); and RA required two diagnosis codes and a prescription for a disease-modifying antirheumatic drug as defined previously (17, 18). HIV was defined by validated methods including positive HIV-1 antibody or other serology, plasma HIV RNA (viral load) level at or above the lower limit of detection, and/or at least three instances in which both HIV viral load and CD4 T lymphocyte cell count/mL3 (CD4) were ordered concurrently (19, 20). ICD codes used as part of the above definitions are included in the Supplementary Methods. Individuals who met criteria for multiple CID diagnoses were evaluated separately.
Baseline was defined as the time of the first clinic visit with a qualifying diagnosis code. Age and data regarding sex, race/ethnicity, and insurance were obtained from the baseline visit. Baseline hypertension was defined using validated administrative codes (ICD-9 401–405 and ICD-10 I10–I15) on any date prior to 1 year after the baseline date (21, 22). Measured blood pressure values were not used to define hypertension given the heterogeneity of visit types and potential for systematic differences in measurement across exposure groups (23, 24). Baseline diabetes was defined using validated ICD-9 or ICD-10 administrative codes (ICD-9 250 and ICD-10 E10–E11, E13) and either hemoglobin A1c > 6.5% or prescription of antidiabetic medications on any date prior to 1 year after the baseline date (25). Current smoking status was obtained at baseline based on self-reported patient history. Baseline total cholesterol was obtained from the closest measurement to baseline date, within 1 year of baseline date. All data analyzed are derived from data obtained during routine clinical care, including cholesterol levels which were derived from standard clinical cholesterol assays (total cholesterol, triglycerides, high-density lipoprotein cholesterol, and calculated low-density lipoprotein cholesterol). Body-mass index (BMI) was calculated (kg/m2) from the closest measurements to baseline date. Deaths were confirmed by a combination of electronic health record chart review and linkage to the national death index and social security death index.
Incident CHD was defined as a new diagnosis of myocardial infarction (MI), angina, coronary revascularization, or other ischemic heart disease using validated ICD definitions, which have demonstrated high levels of agreement with expert chart review (Supplementary Methods) (26–29).
The period up to 90 days after baseline was treated as a blanking period due to the possibility that pre-existing CHD was not noted in the medical record until sometime shortly after baseline; therefore, people with CHD diagnosed within 90 days of the baseline date were excluded. Continuous and categorical variables were reported as mean ± standard deviation and frequency, respectively. Between-group comparisons of continuous and categorical variables were performed using ANOVA and Pearson's chi-squared tests, respectively. Follow-up period was defined as the time between baseline date and censoring date, which was the first of: (1) first encounter for CHD, (2) death, or (3) the most recent face-to-face encounter through September 9, 2019.
CHD was investigated as a composite outcome for the primary analysis. Unadjusted incidence rates for each individual CID and controls were estimated using a quasi-Poisson model to account for overdispersion of the data given varying lengths of observation periods. Cox proportional hazards models were used to analyze associations of each CID with incident CHD with non-CID controls serving as the reference group. The primary model adjusted for baseline demographics and clinical covariates including age, sex, race/ethnicity, insurance status, baseline year, diabetes, hypertension, current smoking, total cholesterol, statin use, and systemic steroid use. Secondary analyses using the same modeling approach were also performed separately with MI as the only outcome (rather than the composite of CHD, which includes MI).
We performed an exploratory analysis of incident CHD risk by disease severity within each CID. We used baseline (measured within 1 year from baseline date) C-reactive protein (CRP) levels as proxy-measures of disease severity in all CIDs except HIV, for which baseline CD4 T cell level was used as a surrogate for immune dysregulation. CD4 T cell count was used as the marker due to its known association with immune dysfunction, inflammation, and CHD in HIV (30, 31). CRP was used as the marker for the remaining CIDs as it is a clinical biomarker of general inflammation and CHD risk in the general population, as well as a marker commonly measured for a number of CIDs. We determined the risk of incident CHD for each tertile of disease severity within each CID using Cox-proportional hazard models, using the non-CID group as a standard reference group across CIDs.
After using the nested cohort with available baseline cholesterol levels for the primary analyses, we repeated our analyses in the overall cohort by removing baseline total cholesterol as an inclusion criterion to increase the available sample size. Thus, total cholesterol was not included in the model for these secondary analyses. Two-sided p ≤ 0.05 were considered significant. All statistical analyses were performed using R (The R Foundation for Statistical Computing) version 3.5.1.
The nested cohort included 17,049 unique patients: 10,289 non-CID controls, 1,864 with HIV, 1,759 with psoriasis, 995 with RA, 153 with SSc, 371 with SLE, 1,290 with IBD, and 328 with multiple CIDs (Figure 1). One-third of the patients with multiple CIDs had either SLE or SSc. Demographics and clinical characteristics for each group in the nested cohort are presented in Table 1. There were several expected differences in demographics: patients with RA and SSc were older; patients with RA, SSc, and SLE were predominantly women while patients with HIV were predominantly men; and there were more Black adults with HIV and SLE. There were also differences in risk factors: patients with RA, SSc, and SLE had higher baseline rates of hypertension; patients with RA and psoriasis had higher baseline rates of diabetes; and the HIV cohort had the highest prevalence of active smokers. Baseline characteristics of the overall cohort (including persons without baseline cholesterol levels) are presented in Supplementary Table 1.
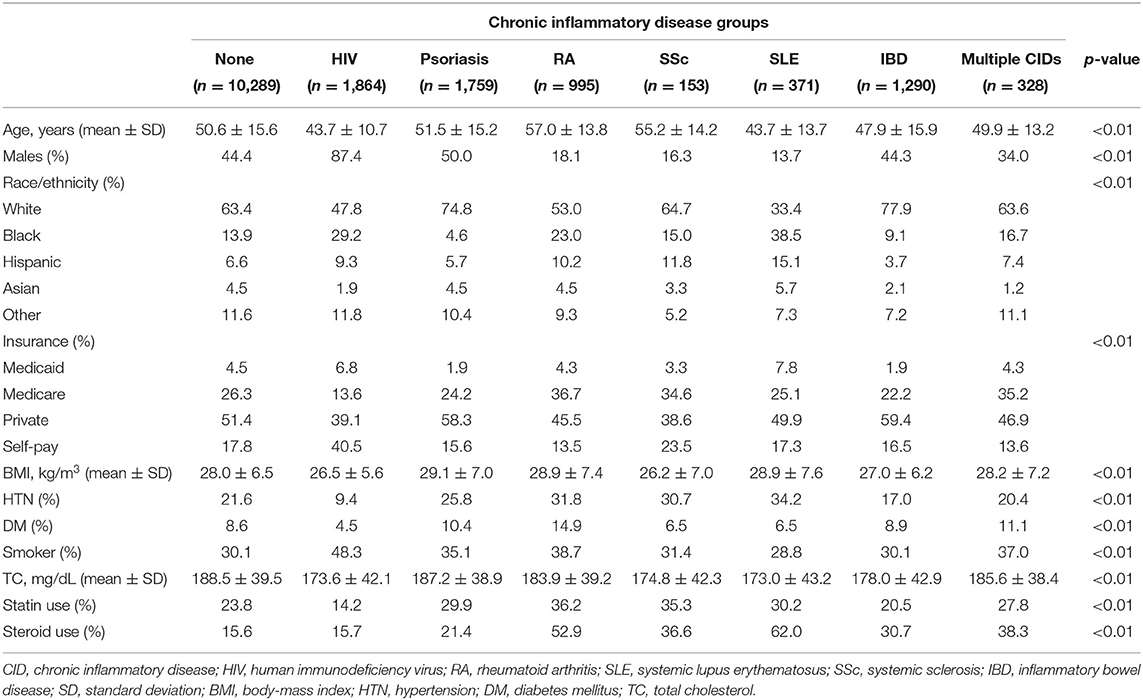
Table 1. Baseline clinical characteristics of controls and chronic inflammatory disease groups in the nested cohort.
There were 619 incident CHD events including 202 incident MI events over an average of 4.1 years of follow-up in the nested cohort. The crude incidence rates for CHD and MI for each CID and those with multiple CIDs in the nested cohort are described in Table 2. In the overall cohort, there were 1,196 incident CHD events including 451 incident MI events over an average of 4.1 years of follow-up. Incidence rates are provided in Supplementary Table 2; patterns across CIDs were largely similar as in the nested cohort.
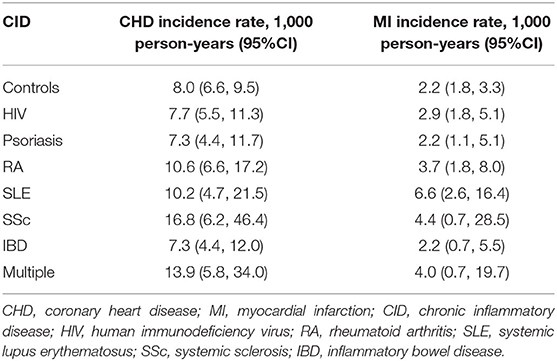
Table 2. Crude coronary heart disease and myocardial infarction incidence rates per 1,000 person-years for controls and chronic inflammatory disease groups in the nested cohort.
In the nested cohort, we observed a significantly higher risk of incident CHD in patients with SLE (HR 2.0, 95% CI 1.2, 3.2), SSc (HR 2.1, 95% CI 1.2, 3.9), and multiple CIDs (HR 2.0, 95% CI 1.2, 3.4) compared with non-CID controls after multivariable adjustment. Patients with HIV, psoriasis, RA, or IBD did not have a significantly higher risk of incident CHD than non-CID controls (Figure 2). When the analysis was repeated in the larger overall cohort after removing baseline cholesterol from the inclusion criteria, the findings were similar (Supplementary Figure 1). Patients with SLE, SSc, and multiple CIDs had a significantly higher risk of incident CHD compared with controls after adjustment.
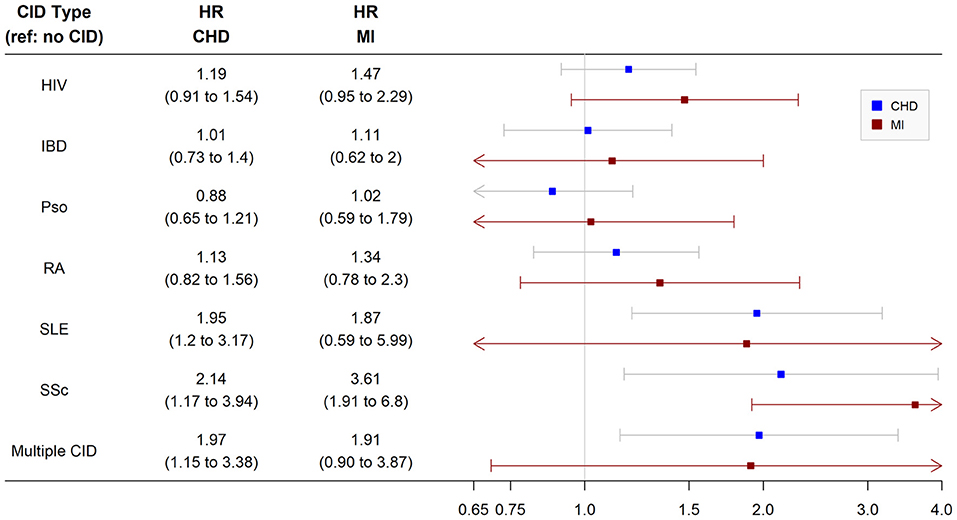
Figure 2. Risk of incident coronary heart disease and myocardial infarction among chronic inflammatory disease groups. Analysis performed in nested cohort. Cox proportional hazard ratios adjusted for age, sex, race/ethnicity, insurance, baseline year, hypertension, diabetes, current smoking, total cholesterol, statin use, and systemic steroid use. CHD, coronary heart disease; MI, myocardial infarction; HR, hazard ratio; CID, chronic inflammatory disease; HIV, human immunodeficiency virus; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; IBD, inflammatory bowel disease.
In secondary analyses of the nested cohort using the MI-only endpoint, only patients with SLE had a significantly higher multivariable-adjusted risk of incident MI (HR 3.6, 95% CI 1.9, 6.8) than non-CID controls. Other groups including SSc and multiple CIDs did not have a significantly higher risk of incident MI (Figure 2). When the analysis was repeated in the larger overall cohort after removing baseline cholesterol from the inclusion criteria, patients with SLE as well as SSc, HIV, and multiple CIDs had a significantly higher risk of incident MI than non-CID controls after adjustment (Supplementary Figure 1). Of note, there were no significant interactions between CID and sex or race/ethnicity with respect to CHD or MI in the nested or overall cohort.
We next investigated associations of levels of inflammation within CID groups with risk of incident CHD and MI. Patients with HIV were divided into tertiles based on baseline CD4 T cell levels and the remaining CIDs were divided into tertiles based on baseline CRP levels. The risk of incident CHD was assessed in both the nested and overall cohorts. The risk of incident MI was assessed only in the overall cohort given insufficient MI events in the nested cohort with available CRP levels. In addition, individuals with multiple CIDs were excluded from this analysis given the use of different biomarkers for HIV (CD4 T cell count) and the other CIDs (CRP).
In the nested cohort, the risk of incident CHD was significantly higher in all three groups of patients with SLE—regardless of baseline CRP level—compared with controls. While the risk of incident CHD was not significantly higher in the other CID subgroups, there was a pattern by which higher inflammatory burden (higher CRP tertile or lower CD4 tertile) was associated with numerically higher CHD risk across CIDs (Figure 3). Findings were similar in the overall cohort (Supplementary Figure 2). In the overall cohort, the risk of incident MI was also significantly higher in all three groups of patients with SLE compared with controls (Figure 4). Additionally, the risk of incident MI was significantly higher in patients with HIV and RA with proxy-markers of high inflammatory burden, with ~2-fold higher risks for MI (vs. non-CID controls) for HIV patients in the lowest CD4 tertile (HR 2.0, 95% CI 1.2, 3.3) and RA patients in the highest CRP tertile (HR 2.1, 95% CI 1.0, 4.4).
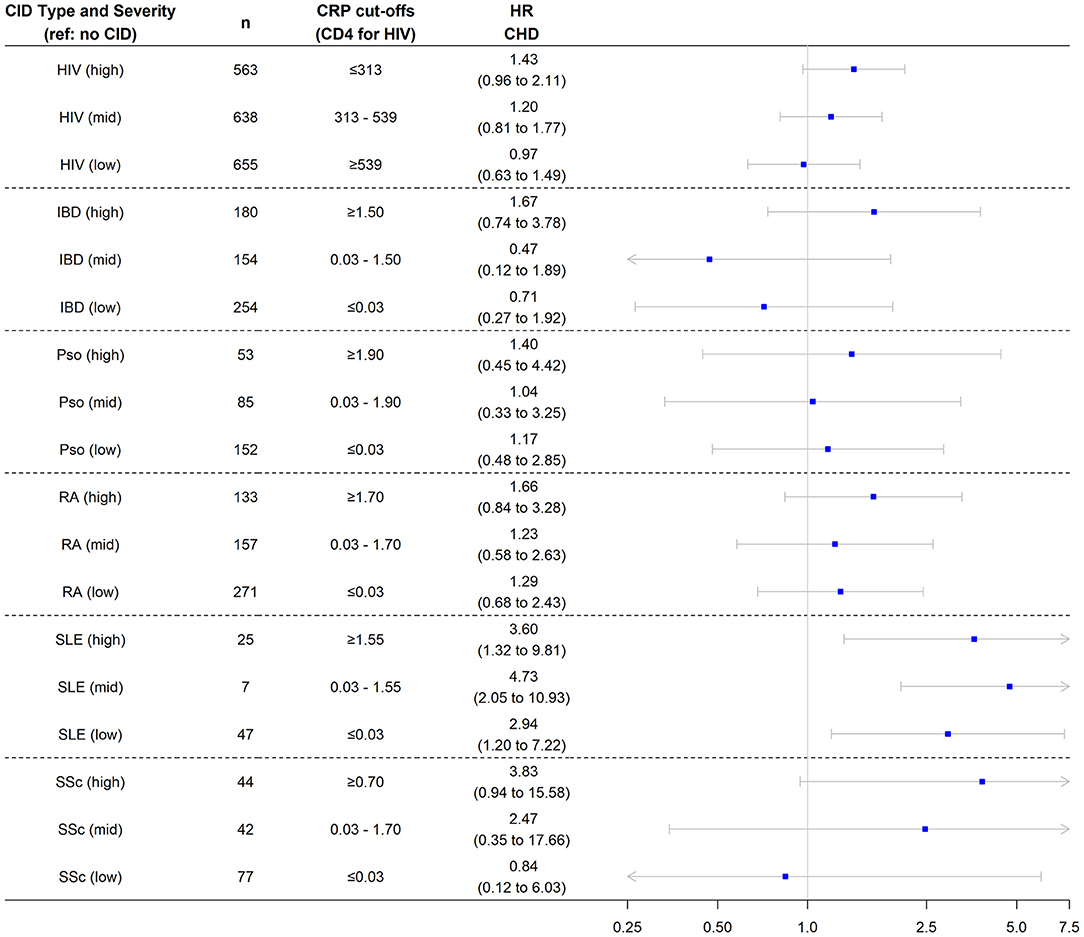
Figure 3. Risk of incident coronary heart disease among chronic inflammatory disease groups stratified by disease severity. Analysis performed in nested cohort. Cox proportional hazard ratios adjusted for age, sex, race/ethnicity, insurance, baseline year, hypertension, diabetes, current smoking, total cholesterol, statin use, and systemic steroid use. CHD, coronary heart disease; HR, hazard ratio; CID, chronic inflammatory disease; HIV, human immunodeficiency virus; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; IBD, inflammatory bowel disease.
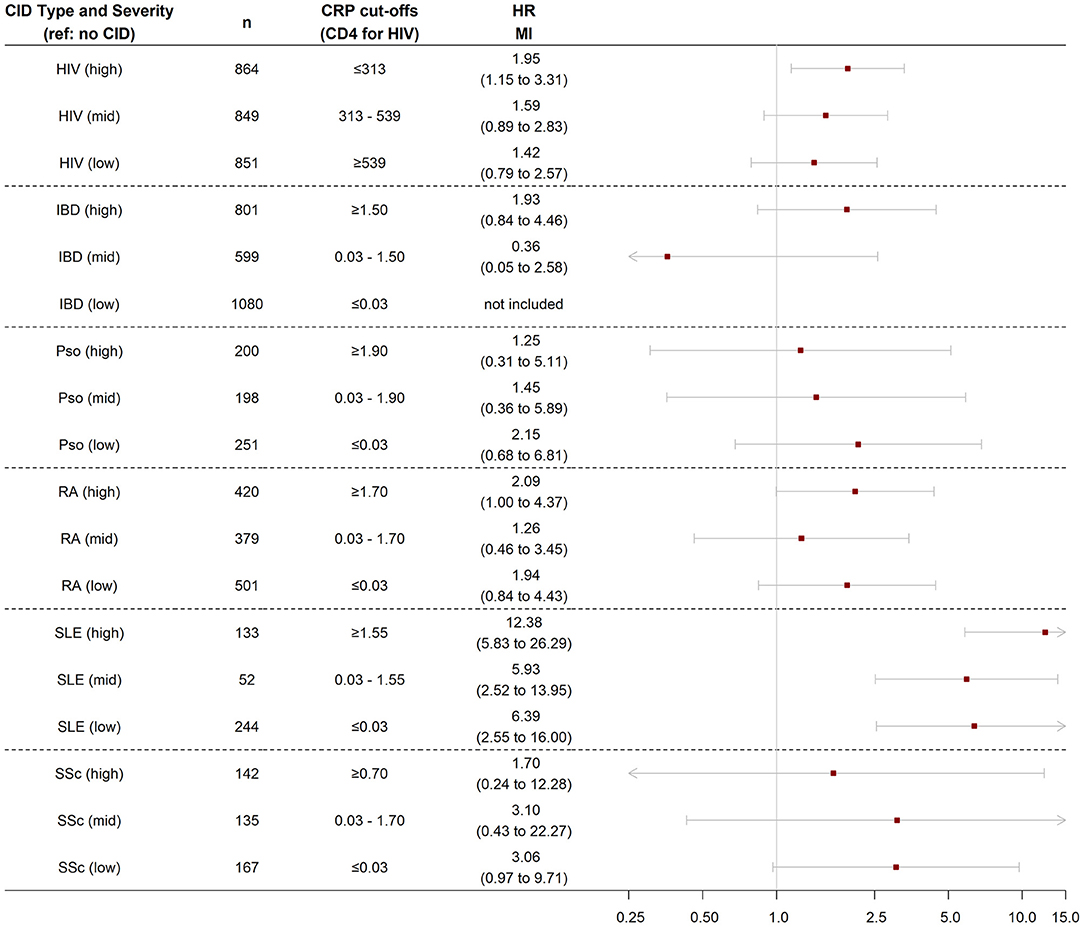
Figure 4. Risk of incident myocardial infarction among chronic inflammatory disease groups stratified by disease severity. Analysis performed in nested cohort. Cox proportional hazard ratios adjusted for age, sex, race/ethnicity, insurance, baseline year, hypertension, diabetes, current smoking, statin use, and systemic steroid use. MI, myocardial infarction; HR, hazard ratio; CID, chronic inflammatory disease; HIV, human immunodeficiency virus; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; IBD, inflammatory bowel disease.
We determined the risks of incident CHD and MI in several distinct CIDs, enabling comparison of CHD risk between disaggregated CID subtypes. We found that patients with SLE, SSc, or multiple CIDs had ~2-fold higher risks of incident CHD than patients without CIDs. Patients with SLE also had an ~4-fold higher risk of incident MI compared with non-CID controls. Generally, there was a pattern across CIDs of higher inflammation severity being associated with higher CHD risk. Patients with HIV and RA with high baseline levels of inflammation/immune dysregulation had ~2-fold higher risks for incident MI than non-CID controls.
Our results have meaningful implications for CHD risk stratification in patients with these CIDs. Currently, primary prevention guidelines appropriately note that the pooled cohort equation for ASCVD risk estimation may underestimate risk in inflammatory conditions, explicitly mentioning HIV, RA, SLE, and psoriasis (3). However, the relative differences in risk observed in our cohort emphasize the need to individualize CHD risk assessment based on type and severity of CID. The strength of association of SLE with CHD and MI was particularly noteworthy: SLE patients had a 2-fold increased risk of CHD and 4-fold increased risk of MI relative to non-CID controls—even after multivariable adjustment—and SLE patients with higher CRP levels had a 12-fold higher MI risk compared with non-CID controls. Compared with SLE, the increased risk of CHD with SSc was similar while the increased risk of MI was smaller in magnitude. Although patients with HIV, RA, IBD, and psoriasis did not have significantly higher risk for CHD compared with non-CID controls in the overall groups, the risk of MI was 2-fold higher in patients with HIV and RA with markers of advanced immune dysregulation (lower CD4 count for HIV) and inflammation (CRP for RA).
From a clinical and epidemiological standpoint, our findings are consistent with studies evaluating isolated CIDs and CHD risk. Population-based studies have demonstrated ~2 to 3-fold increased risks of MI and ASCVD in patients with SLE and SSc (5, 6, 32, 33). Multiple studies in patients with RA have demonstrated a 1.5 to 3-fold higher risk of MI (34, 35). In patients with HIV, the risk of MI correlates with CD4 T cell count. Prior analysis from the Veterans Aging Cohort Study showed a near 2-fold increased risk of MI in patients with CD4 T cell count <200 cells/mm3, similar to what we observed in patients with CD4 T cell count in the lowest tertile (<312 cells/mm3) (36). While prior studies have demonstrated an increased risk of MI and ASCVD with psoriasis, the increased risk is primarily present in those with severe psoriasis (37–39). We likely did not observe a significantly elevated risk in our patients with psoriasis because of inclusion of individuals with mild disease. We also did not observe a significant difference in CHD risk in patients with IBD after multivariable adjustment. But for both psoriasis and IBD, we observed trends toward higher CHD risk in those with higher CRP levels, although this numerically higher risk did not meet statistical significance. These findings suggest that systemic inflammation, more commonly seen with SLE, SSc, RA, and HIV, has a stronger effect on CHD and MI risk than localized inflammation, more commonly seen with IBD and psoriasis.
Biologically, comparison across CIDs also allows for potential insights into inflammatory mechanisms that may contribute to ASCVD. Our findings suggest that patients with SLE, especially those with severe disease, may have greater plaque instability. An inflammatory pathway central to SLE pathophysiology is the production of neutrophil extracellular traps (NETs). Dysregulation in NET homeostasis contributes to persistent inflammation in many CIDs, especially SLE (40–42). NETs can contribute to plaque instability through direct endothelial damage and activation of NOD-like receptor protein 3 (NLRP3) inflammasome (43). The NLRP3 inflammasome is the primary regulator of interleukin (IL)-1β, which in turn stimulates IL-6 production (44). Targeting this pathway with therapies such as Canakinumab and colchicine has shown a reduction in cardiovascular events in high risk general population (45–47). These therapies, along with further understanding of NET dysregulation, may help refine therapeutic (mechanistic) and population (CID subtype) targets to improve cardiovascular outcomes in patients with CIDs, especially SLE.
There are important limitations to our study. This was an electronic health record-based study in a single large medical system, thus making selection bias, selection of controls, and loss to follow-up potential concerns. We sought to address this by refining the inclusion criteria to require regular outpatient follow-up, as well as frequency-matching by key demographic and clinical risk factors. We further adjusted for these matching variables (given heterogeneity between CIDs) along with current smoking, total cholesterol level, statin use (although data were limited regarding statin dose and timing), and systemic steroid use. However, we were not able to include other immunosuppressive therapies used to treat these conditions, which may impact CHD risk. Despite these cohort-related limitations, including limited size, we are not aware of a large enough prospective cohort consisting of persons of similar scale and diversity of CIDs that would enable a similar comparative analysis. Other limitations include CHD and MI event ascertainment, which was based on administrative codes; although they have been demonstrated to have high levels of agreement with expert chart review as they are more likely to be discrete episodes that can be more readily captured (29). Lastly, we used CRP levels as a proxy-marker of disease severity given its widespread available and because standardized disease severity scales based on symptoms and physical examination were not widely available across CIDs in this study.
Our findings meaningfully add to prior literature and allow for relative comparison between and within each CID. CHD risk was elevated in patients with SLE and SSc, regardless of level of inflammation. Patients with severe SLE had a markedly elevated risk of MI. In contrast, the risk of MI was elevated in patients with HIV and RA with high levels of inflammation/immune dysregulation. Our study provides the initial basis to include some of these distinctions in future ASCVD primary prevention guidelines and emphasizes the need to understand inflammatory mechanisms specific to each CID that contribute to CHD and MI.
Dataset obtained from Northwestern Medicine Enterprise Data Warehouse. De-identified dataset is available upon request to authorized individuals. Requests to access these datasets should be directed to bWF0dGhld2pmZWluc3RlaW5Abm9ydGh3ZXN0ZXJuLmVkdQ==.
The studies involving human participants were reviewed and approved by Institutional review board at Northwestern University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
AS designed the research study and wrote the manuscript. AR performed the analyses. AP generated the dataset. SC, SP, ET, MD, RR-G, YL, CA, and DL-J contributed to the discussion and edited the manuscript. MF designed the research study and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the American Heart Association Fellow-to-Faculty Award (16FTF31200010; PI: Feinstein). National Institutes of Health R01HL156792 (PI: Feinstein) and UL1TR001422 (PI: Lloyd-Jones) and AS was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number T32HL069771. YL and RR-G are funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases under award number P30 AR072579.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the Northwestern Medicine Enterprise Data Warehouse for logistical support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.757738/full#supplementary-material
1. Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol. (2011) 27:174–82. doi: 10.1016/j.cjca.2010.12.040
2. Kaplan MJ. Management of cardiovascular disease risk in chronic inflammatory disorders. Nat Rev Rheumatol. (2009) 5:208–17. doi: 10.1038/nrrheum.2009.29
3. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. (2019). 74:e177–e232. doi: 10.1016/j.jacc.2019.03.010
4. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Euro Heart J. (2016). 37:2315–81. doi: 10.1093/eurheartj/ehw106
5. Butt SA, Jeppesen JL, Torp-Pedersen C, Sam F, Gislason GH, Jacobsen S, et al. Cardiovascular manifestations of systemic sclerosis: a danish nationwide cohort study. J Am Heart Assoc. (2019) 8:e013405. doi: 10.1161/JAHA.119.013405
6. Man A, Zhu Y, Zhang Y, Dubreuil M, Rho YH, Peloquin C, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis. (2013) 72:1188–93. doi: 10.1136/annrheumdis-2012-202007
7. Sun HH, Tian F. Inflammatory bowel disease and cardiovascular disease incidence and mortality: a meta-analysis. Euro J Prevent Cardiol. (2018) 25:1623–31. doi: 10.1177/2047487318792952
8. Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. (2014) 130:837–44. doi: 10.1161/CIRCULATIONAHA.114.009990
9. Prasada S, Rivera A, Nishtala A, Pawlowski AE, Sinha A, Bundy JD, et al. Differential associations of chronic inflammatory diseases with incident heart failure. JACC Heart Fail. (2020) 8:489–98. doi: 10.1016/j.jchf.2019.11.013
10. Valenzuela A, Yaqub A, Fiorentino D, Krishnan E, Chung L. Validation of the ICD-9-CM code for systemic sclerosis using updated ACR/EULAR classification criteria. Scand J Rheumatol. (2015) 44:253–5. doi: 10.3109/03009742.2015.1008038
11. Ma C, Moran GW, Benchimol EI, Targownik LE, Heitman SJ, Hubbard JN, et al. Surgical rates for crohn's disease are decreasing: a population-based time trend analysis and validation study. Am J Gastroenterol. (2017) 112:1840–8. doi: 10.1038/ajg.2017.394
12. Thirumurthi S, Chowdhury R, Richardson P, Abraham NS. Validation of ICD-9-CM diagnostic codes for inflammatory bowel disease among veterans. Dig Dis Sci. (2010) 55:2592–8. doi: 10.1007/s10620-009-1074-z
13. Lofvendahl S, Theander E, Svensson A, Carlsson KS, Englund M, Petersson IF. Validity of diagnostic codes and prevalence of physician-diagnosed psoriasis and psoriatic arthritis in southern Sweden–a population-based register study. PLoS ONE. (2014) 9:e98024. doi: 10.1371/journal.pone.0098024
14. Asgari MM, Wu JJ, Gelfand JM, Salman C, Curtis JR, Harrold LR, et al. Validity of diagnostic codes and prevalence of psoriasis and psoriatic arthritis in a managed care population, 1996-2009. Pharmacoepidemiol Drug Saf. (2013) 22:842–9. doi: 10.1002/pds.3447
15. Walunas TL, Jackson KL, Chung AH, Mancera-Cuevas KA, Erickson DL, Ramsey-Goldman R, et al. Disease outcomes and care fragmentation among patients with systemic lupus erythematosus. Arthritis Care Res. (2017) 69:1369–76. doi: 10.1002/acr.23161
16. Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus. (2010) 19:741–3. doi: 10.1177/0961203309356289
17. Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. (2011) 13:R32. doi: 10.1186/ar3260
18. Singh JA, Holmgren AR, Noorbaloochi S. Accuracy of veterans administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum. (2004) 51:952–7. doi: 10.1002/art.20827
19. Felsen UR, Bellin EY, Cunningham CO, Zingman BS. Development of an electronic medical record-based algorithm to identify patients with unknown HIV status. AIDS Care. (2014) 26:1318–25. doi: 10.1080/09540121.2014.911813
20. Feinstein MJ, Steverson AB, Ning H, Pawlowski AE, Schneider D, Ahmad FS, et al. Adjudicated heart failure in HIV-infected and uninfected men and women. J Am Heart Assoc. (2018) 7:e009985. doi: 10.1161/JAHA.118.009985
21. Pace R, Peters T, Rahme E, Dasgupta K. Validity of health administrative database definitions for hypertension: a systematic review. Can J Cardiol. (2017) 33:1052–9. doi: 10.1016/j.cjca.2017.05.025
22. Teixeira PL, Wei WQ, Cronin RM, Mo H, VanHouten JP, Carroll RJ, et al. Evaluating electronic health record data sources and algorithmic approaches to identify hypertensive individuals. J Am Med Inform Assoc. (2017) 24:162–71. doi: 10.1093/jamia/ocw071
23. Ali S, Rouse A. Practice audits: reliability of sphygmomanometers and blood pressure recording bias. J Hum Hypertens. (2002) 16:359–61. doi: 10.1038/sj.jhh.1001384
24. Rouse A, Marshall T. The extent and implications of sphygmomanometer calibration error in primary care. J Hum Hypertens. (2001) 15:587–91. doi: 10.1038/sj.jhh.1001241
25. Wilke RA, Berg RL, Peissig P, Kitchner T, Sijercic B, McCarty CA, et al. Use of an electronic medical record for the identification of research subjects with diabetes mellitus. Clin Med Res. (2007) 5:1–7. doi: 10.3121/cmr.2007.726
26. Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. (2008) 43:1424–41. doi: 10.1111/j.1475-6773.2007.00822.x
27. Patel AB, Quan H, Welsh RC, Deckert-Sookram J, Tymchak W, Sookram S, et al. Validity and utility of ICD-10 administrative health data for identifying ST- and non-ST-elevation myocardial infarction based on physician chart review. CMAJ Open. (2015) 3:E413–8. doi: 10.9778/cmajo.20150060
28. McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS ONE. (2014) 9:e92286. doi: 10.1371/journal.pone.0092286
29. Fishman E, Barron J, Dinh J, Jones WS, Marshall A, Merkh R, et al. Validation of a claims-based algorithm identifying eligible study subjects in the ADAPTABLE pragmatic clinical trial. Contemp Clin Trials Commun. (2018) 12:154–60. doi: 10.1016/j.conctc.2018.11.001
30. Negredo E, Massanella M, Puig J, Pérez-Álvarez N, Gallego-Escuredo JM, Villarroya J, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: clinical implications. Clin Infect Dis. (2010) 50:1300–8. doi: 10.1086/651689
31. Ho JE, Scherzer R, Hecht FM, Maka K, Selby V, Martin JN, et al. The association of CD4+ T-cell counts and cardiovascular risk in treated HIV disease. AIDS. (2012) 26:1115–20. doi: 10.1097/QAD.0b013e328352ce54
32. Avina-Zubieta JA, To F, Vostretsova K, De Vera M, Sayre EC, Esdaile JM. Risk of myocardial infarction and stroke in newly diagnosed systemic lupus erythematosus: a general population-based study. Arthritis Care Res. (2017) 69:849–56. doi: 10.1002/acr.23018
33. Hermansen ML, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology. (2017) 56:709–15. doi: 10.1093/rheumatology/kew475
34. Lindhardsen J Ahlehoff O Gislason GH Madsen OR Olesen JB Torp-Pedersen C . The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis. (2011) 70:929–34. doi: 10.1136/ard.2010.143396
35. del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. (2001) 44:2737–45. doi: 10.1002/1529-0131(200112)44:12 <2737::AID-ART460>3.0.CO;2-#
36. Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. (2013) 173:614–22. doi: 10.1001/jamainternmed.2013.3728
37. Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. (2009) 145:700–3. doi: 10.1001/archdermatol.2009.94
38. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. (2006) 296:1735–41. doi: 10.1001/jama.296.14.1735
39. Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the general practice research database. Eur Heart J. (2010) 31:1000–6. doi: 10.1093/eurheartj/ehp567
40. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. (2018) 18:134–47. doi: 10.1038/nri.2017.105
41. Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. (2010) 107:9813–8. doi: 10.1073/pnas.0909927107
42. Dieker J, Tel J, Pieterse E, Thielen A, Rother N, Bakker M, et al. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis. Arthritis Rheumatol. (2016) 68:462–72. doi: 10.1002/art.39417
43. Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. (2013) 190:1217–26. doi: 10.4049/jimmunol.1202388
44. Ridker PM. From CANTOS to CIRT to COLCOT to clinic: will all atherosclerosis patients soon be treated with combination lipid-lowering and inflammation-inhibiting agents? Circulation. (2020) 141:787–9. doi: 10.1161/CIRCULATIONAHA.119.045256
45. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
46. Tardif JC Kouz S Waters DD Bertrand OF Diaz R Maggioni AP . Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. (2019) 381:2497–505. doi: 10.1056/NEJMoa1912388
Keywords: coronary heart disease, lupus (SLE), systemic sclerosis, inflammation, rheumatoid arthritis, HIV–human immunodeficiency virus, psoriasis
Citation: Sinha A, Rivera AS, Chadha SA, Prasada S, Pawlowski AE, Thorp E, DeBerge M, Ramsey-Goldman R, Lee YC, Achenbach CJ, Lloyd-Jones DM and Feinstein MJ (2021) Comparative Risk of Incident Coronary Heart Disease Across Chronic Inflammatory Diseases. Front. Cardiovasc. Med. 8:757738. doi: 10.3389/fcvm.2021.757738
Received: 12 August 2021; Accepted: 22 October 2021;
Published: 10 November 2021.
Edited by:
Alexander V. Sorokin, National Heart, Lung, and Blood Institute (NHLBI), United StatesCopyright © 2021 Sinha, Rivera, Chadha, Prasada, Pawlowski, Thorp, DeBerge, Ramsey-Goldman, Lee, Achenbach, Lloyd-Jones and Feinstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew J. Feinstein, bWF0dGhld2pmZWluc3RlaW5Abm9ydGh3ZXN0ZXJuLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.