
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 13 January 2022
Sec. Coronary Artery Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.756379
This article is part of the Research TopicInsights in Coronary Artery Disease: 2021View all 15 articles
 Miaohan Qiu1,2
Miaohan Qiu1,2 Yi Li2
Yi Li2 Kun Na2,3
Kun Na2,3 Zizhao Qi2,4
Zizhao Qi2,4 Sicong Ma2,5
Sicong Ma2,5 He Zhou2,6
He Zhou2,6 Xiaoming Xu2
Xiaoming Xu2 Jing Li2,4
Jing Li2,4 Kai Xu2
Kai Xu2 Xiaozeng Wang2
Xiaozeng Wang2 Yaling Han2*
Yaling Han2*Backgrounds: A plug-and-play standardized algorithm to identify the ischemic risk in patients with coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI) could play a valuable step to help a wide spectrum of clinic workers. This study intended to investigate the ability to use the accumulation of multiple clinical routine risk scores to predict long-term ischemic events in patients with CAD undergoing PCI.
Methods: This was a secondary analysis of the I-LOVE-IT 2 (Evaluate Safety and Effectiveness of the Tivoli drug-eluting stent (DES) and the Firebird DES for Treatment of Coronary Revascularization) trial, which was a prospective, multicenter, and randomized study. The Global Registry for Acute Coronary Events (GRACE), baseline Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX), residual SYNTAX, and age, creatinine, and ejection fraction (ACEF) score were calculated in all patients. Risk stratification was based on the number of these four scores that met the established thresholds for the ischemic risk. The primary end point was ischemic events at 48 months, defined as the composite of cardiac death, nonfatal myocardial infarction, stroke, or definite/probable stent thrombosis (ST).
Results: The 48-month ischemic events had a significant trend for higher event rates (from 6.61 to 16.93%) with an incremental number of risk scores presenting the higher ischemic risk from 0 to ≥3 (p trend < 0.001). In addition, the categories were associated with increased risk for all components of ischemic events, including cardiac death (from 1.36 to 3.15%), myocardial infarction (MI) (from 3.31 to 9.84%), stroke (3.31 to 6.10%), definite/probable ST (from 0.58 to 1.97%), and all-cause mortality (from 2.14 to 6.30%) (all p trend < 0.05). The net reclassification index after combined with four risk scores was 12.5% (5.3–20.0%), 9.4% (2.0–16.8%), 12.1% (4.5–19.7%), and 10.7% (3.3–18.1%), which offered statistically significant improvement in the performance, compared with SYNTAX, residual SYNTAX, ACEF, and GRACE score, respectively.
Conclusion: The novel multiple risk score model was significantly associated with the risk of long-term ischemic events in these patients with an increment of scores. A meaningful improvement to predict adverse outcomes when multiple risk scores were applied to risk stratification.
Personalized medicine is a medical model that separates patients into different groups with tailored medical decisions, practices, and interventions based on their predicted risk of disease. Theoretically, taking a series of risk factors into account to evaluate the individual risk of patients with coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI) before the decision-making process was superior to “one-size-fits-all” approaches (1–3).
Recently a variety of risk scoring systems, as comprehensive predicted tools for risk assessment, have been developed to support physicians in clinical practice for these patients (4–7). However, to our knowledge, there was not a robust, interoperable, and universal risk score that could be extended to different populations, which is mainly caused by prediction algorithms derived from different cohorts and a complex and time-varying clinical process (8–11). Meanwhile, previous studies demonstrate that an additive value of one risk score combined with a biomarker, angiographic characteristic, and with another risk score to risk predicting (12–14).
Thus, we sought to investigate whether using a strategy assisted by the accumulation of multiple clinical routine risk algorithms could improve the ability of discrimination to predict long-term ischemic events in patients with CAD undergoing PCI.
This was a secondary analysis of the I-LOVE-IT 2 (Evaluate Safety and Effectiveness of the Tivoli drug-eluting stent (DES) and the Firebird DES for Treatment of Coronary Revascularization; NCT01681381) trial, which was a prospective, multicenter, randomized, assessor-blinded, and non-inferiority study that compared a biodegradable polymer sirolimus-eluting stent (BP-SES, Tivoli, Essen Tech, Beijing, China) with a durable polymer sirolimus-eluting stent (DP-SES, Firebird 2, MicroPort, Shanghai, China). Study details have been previously described (15, 16). In brief, between October 2012 and June 2013, a total of 2,737 patients presenting with stable CAD or acute coronary syndromes (ACS) were randomly assigned to undergo PCI with either BP-SES or DP-SES at a 2:1 ratio at 32 centers in China. Patients who were randomized to the BP-SES group were additionally re-randomized to a 6- or 12-month DAPT group at a 1:1 ratio. All patients were discharged with a prescription for at least 100 mg aspirin indefinitely and 75 mg clopidogrel for 6 or 12 months after stent implantation. Qualitative and quantitative coronary angiography (including Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) score and residual SYNTAX score) were centrally evaluated by a blinded independent core laboratory (CCRF, Beijing, China) using QAngio XA Version 7.3 Analysis Software (Medis Medical Imaging System, Leiden, The Netherlands). The study complies with the provisions of the Declaration of Helsinki, and the study protocol was approved by the institutional review board at each participating site. All patients provided written informed consent.
For these analyses, four risk scores, which were supported by an extensive, rigorous external validation process, and/or endorsed by current guidelines, were used to predict the ischemic risk after PCI, as follows. (1) Discharge Global Registry for Acute Coronary Events (GRACE) score (17) was calculated and described based on age, history of congestive heart failure, history of myocardial infarction (MI), resting heart rate, systolic blood pressure, ST-segment depression, initial serum creatinine, elevated cardiac enzymes, and PCI in-hospital. Patients were considered intermediate to high ischemic risk for scores ≥88 (18). (2) Baseline SYNTAX score is a comprehensive angiographic scoring system that is derived entirely from the coronary anatomy and lesion characteristics, which was designed to quantify lesion complexity before the procedure (19). The baseline SYNTAX Score may aid in assessing the ischemic events, including cardiac death, MI, and target vessel revascularization (20). The baseline SYNTAX score value of 13 is considered an optimal cutoff point depending on the prognosis of patients (20). (3) Age, creatinine, and ejection fraction (ACEF) score developed by Ranucci et al. (21) was a simple tool for predicting in-hospital mortality in patients undergoing elective cardiac surgery. Meanwhile, a previous study showed that the ACEF score had a good discriminative in patients undergoing PCI (22). The ACEF score ≥1.0225 might be useful and applicable for risk stratification in these populations with respect to the long-term clinical prognosis (22). (4) Residual SYNTAX score (rSS) was first proposed by Généreux et al. (23), which was calculated based on the remaining obstructive coronary disease after treatment with PCI. The rSS could be used to quantify the burden and complexity of residual CAD after the procedure. The rSS of >0 was associated with long-term ischemic outcomes, including all-cause mortality and MI (24, 25). Risk score calculations are shown in Supplementary Appendix S1.
The method of risk stratification in the current study was calculated using the number of these four scores (called ACE-SYNTAX score) that met the thresholds for the intermediate- or high-risk, ranging from 0 to ≥3, logically a total of four categories, in all patients.
All clinical and laboratory variables included in the present analysis were prospectively collected. The multiple risk score model was developed to predict ischemic events at 48 months, defined as the composite of cardiac death, nonfatal MI, stroke, or definite/probable stent thrombosis (ST). The definitions of those endpoints were described in the previous report (15, 16). All patients were followed-up with by telephone or hospital visits at 1, 6, 9, 12, and 18 months, and annually for up to 5 years. All clinical events were adjudicated by a blinded independent clinical events committee.
Patient characteristics were stratified according to the risk stratification of risk scores. Continuous variables are presented as mean ± SD; categorical variables are displayed as counts and percentages. Comparisons were performed with a chi-square test for categorical variables and one-way ANOVA for continuous variables. Testing for trends in event rates across risk scores was done with the Cochran–Armitage test. Time-to-event data with estimated event rates measured with the Kaplan–Meier method were compared using the log-rank test. An individual risk score was evaluated for the discrimination for 4-year ischemic events. The discrimination of individual risk score was measured by the receiver operator characteristic curve (ROC) with the area under the curve (AUC), which ranges from 0.50 (no discrimination) to 1.0 (perfect discrimination). The net reclassification improvement (NRI) analysis was performed to assess the improved ability of combined risk scores for risk stratification over the single score (26). To deal with the missing components of the risk scores that occurred at random, multiple imputations were performed using chained equations. Missing values were predicted on the basis of all other clinical variables. The Cox regression estimates from each imputed dataset were averaged together to produce an overall hazard ratio (HR) computed using the Rubin rule. Unless otherwise specified, a 2-sided p-value of < 0.05 was considered to indicate statistical significance. Statistical analysis was performed using SAS software version 9.3 (SAS Institute, Cary, NC, USA).
A total of 2,207 patients with 3,027 lesions were selected and calculated ACE-SYNTAX score and were analyzed in the study. ACEF score was not fully evaluable in 342 patients due to the missing data of ejection fraction (240 cases) or creatinine clearance (102 cases). Meanwhile, the GRACE score could not be calculated in 188 cases with a lack of cardiac enzymes. The outcomes of these 530 patients excluded from the analysis are shown in Supplementary Table S1.
The baseline SYNTAX score ranged from 0 to 55, with a mean ± SD of 11.9 ± 8.3, and a median of 10.0 (6.0–16.0). The residual SYNTAX score ranged from 0 to 53, with a mean ± SD of 3.4 ± 5.2, and a median of 0.0 (0.0–5.0). The ACEF score ranged from 0.4 to 7.6, with a mean ± SD of 1.3 ± 0.8, and a median of 1.0 (0.9–1.2). The GRACE score ranged from 16 to 153, with a mean ± SD of 76.0 ± 21.5, and a median of 77.0 (60.0–91.0). By using the previously validated cutoffs described in the methods, 831 patients (37.65%) based on baseline SYNTAX score, 1,053 patients (47.71%) based on residual SYNTAX score, 995 patients (45.08%) based on ACEF score, and 650 patients (29.45%) based on GRACE score met the thresholds for the intermediate or high-risk category. A Venn diagram was shown to demonstrate the coexistence of conditions of these risk scores (Supplementary Figure S1).
Among 2,207 patients, the risk score of 514 (23.3%) patients who failed to reach any of the four scores cutoff value was defined as zero. The number of other groups, 1 to ≥3 risk-score, were 553 (25.1%), 632 (28.6%), and 508(23.0%), respectively. The overall distribution of incremental risk-score categories was displayed in Supplementary Figure S2. The baseline demographics and calculation of risk scores are reported in Table 1. The antiplatelet therapy during the follow-up period is shown in Supplementary Table S2. The lesion characteristics and procedural results are shown in Table 2 stratified across cumulative risk-score categories.
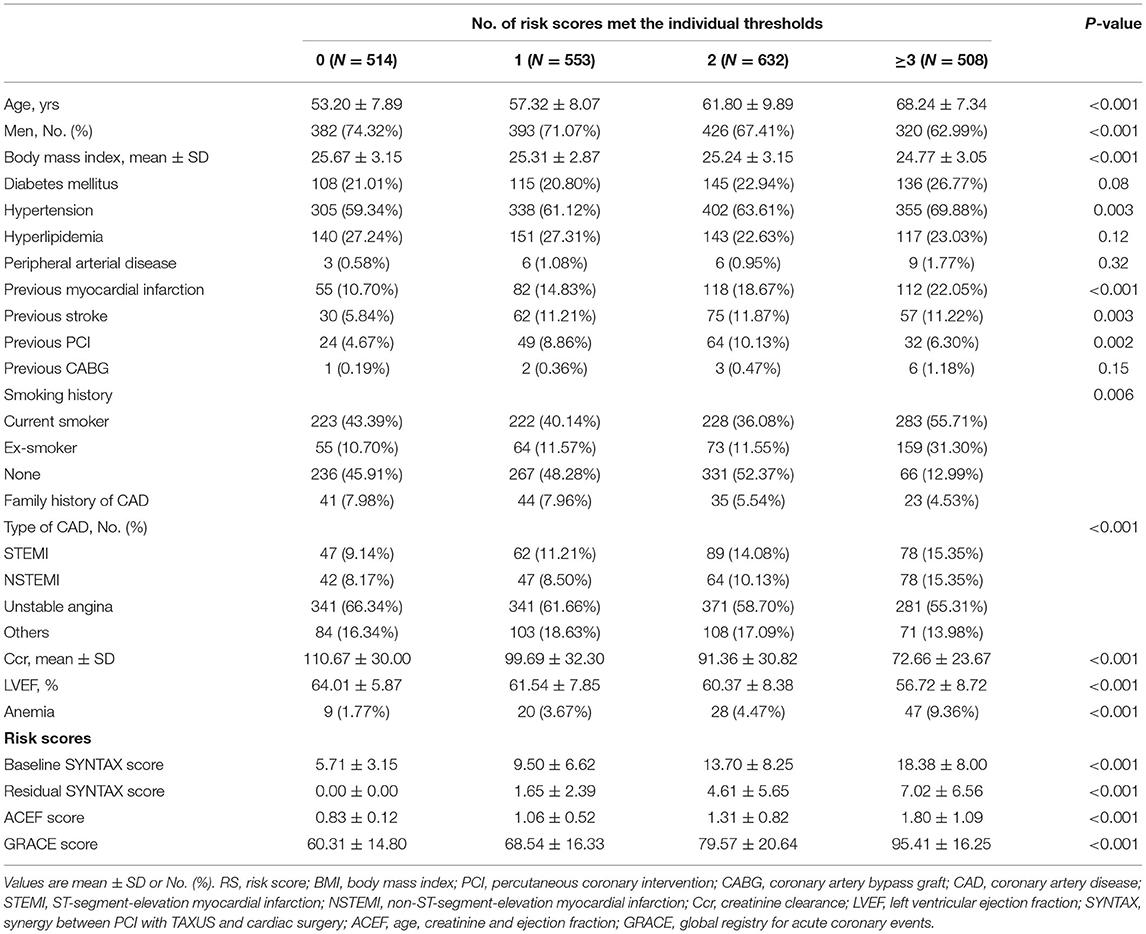
Table 1. Baseline demographics and score calculation stratified across cumulative risk-score categories.
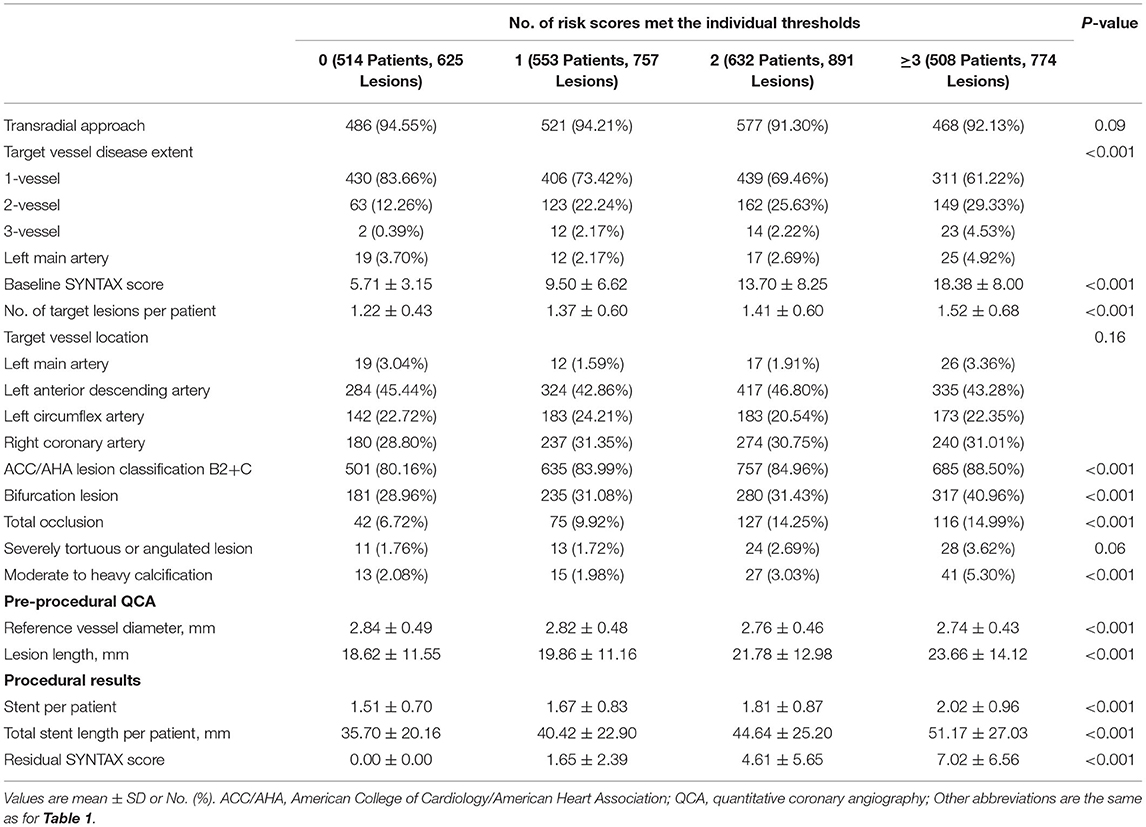
Table 2. Lesion characteristics and procedural results stratified across cumulative risk-score categories.
The 48-month ischemic events had a significant trend for higher event rates (from 6.61 to 16.93%) with incremental risk-score categories from 0 to ≥3 (ptrend < 0.001). The categories were also associated with increased risk for all components of ischemic events, including cardiac death (from 1.36 to 3.15%, ptrend = 0.025), all MI (from 3.31 to 9.84%, ptrend < 0.001), stroke (3.31 to 6.10%, ptrend = 0.013), definite/probable ST (from 0.58 to 1.97%, ptrend = 0.035), TVMI (from 2.92 to 8.66%, ptrend < 0.001), and all-cause mortality (from 2.14 to 6.30%, ptrend < 0.001) at 48 months with a significant trend according to risk-score categories (Table 3).
Using the multiple imputations for the missing values (ejection fraction, creatinine clearance, and cardiac enzymes), a total of 20 imputed datasets were generated. The trend of 48-month ischemic events was robust in each imputed dataset (all ptrend < 0.001, Supplementary Table S3).
There were consistent findings measured with the Kaplan–Meier method. The incidence of ischemic events, all-cause mortality at 4 years experienced a significant increase with the cumulative number of risk scores (both p < 0.001 by log-rank test). The landmark analysis showed that the patients with the higher cumulative risk-score were associated with a higher risk of ischemic events in the intervals of 0–30 days as well as 30 days to 4 years (from 2.1 to 7.68%, log-rank p < 0.001, and from 4.5 to 9.3%, log-rank p = 0.003, respectively) (Figure 1 and Supplementary Table S4). A sensitive analysis was performed and showed that the incidence of 48-month ischemic events had a consistent tendency with incremental risk-score categories with incremental risk-score categories (Supplementary Table S5).
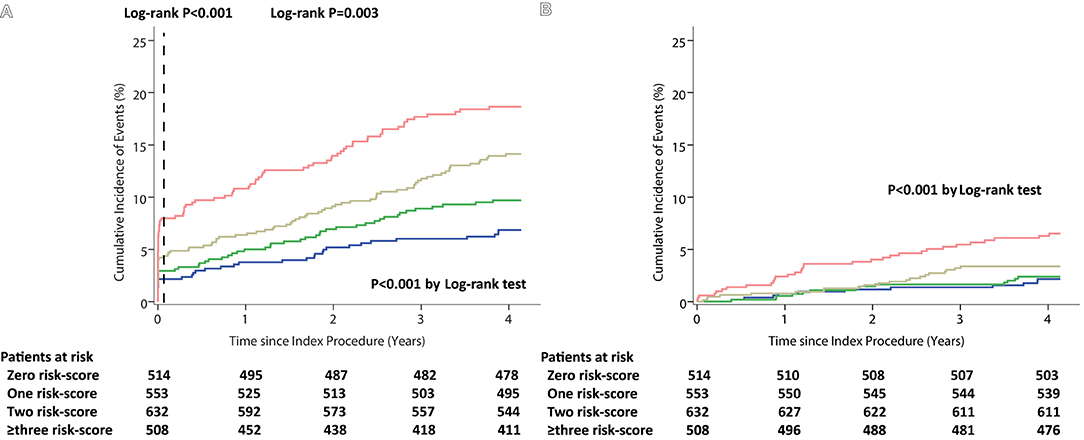
Figure 1. Kaplan–Meier curves during follow-up for 48-month Outcomes among the Cumulative Risk-score Categories. (A) Ischemic events (with 30-day landmark analysis). (B) All-cause Mortality.
It was shown that the 4-year rate of ischemic events was significantly higher in the patients with cumulative risk-score 2 to ≥3, leaving cumulative risk-score 0 as the reference [(HR: 2.05, 95% CI, 1.38–3.06), and (HR: 2.72, 95% CI, 1.83–4.05), respectively], whereas, this did not differ in patients with cumulative risk-scores 0 and 1 (HR: 1.41, 95% CI, 0.92–2.18) (Figure 2).
The ischemic events at 48 months stratified by one and different combinations of risk score (s) are illustrated in Figure 3 and Supplementary Figure S3. Using cumulative risk-score categories could discriminate the risk of ischemia better than any single risk score, especially in patients with lower and higher ischemic risk. The combination with two risk scores of baseline SYNTAX and GRACE score has good discrimination to predict the 48-month ischemic events in all kinds of two risk scores. The combination with three risk scores of baseline SYNTAX, residual SYNTAX, and GRACE score and baseline SYNTAX, ACEF, and GRACE score has a better ability to assess the ischemic risk.
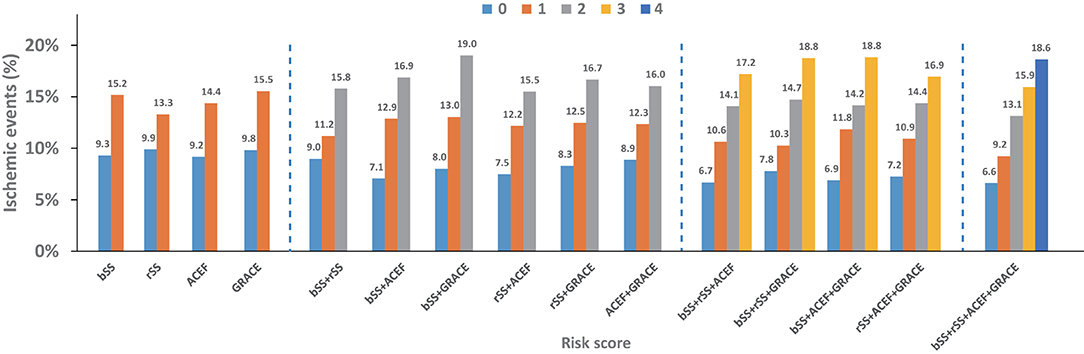
Figure 3. Four-year ischemic events stratified by individual and different combination of risk score (s).
The ROC curves for 48-month ischemic events of the individual and an incremental number of risk scores, as continuous variables, are shown in Supplementary Figure S4. The discrimination of individual risk score was moderate, with AUC from 0.55 to 0.58. The AUC of an incremental number of risk scores was 0.61 (0.57–0.64). The best cutoffs of GRACE, baseline SYNTAX, ACEF, and residual SYNTAX score to predict 48-month ischemic events risk were 87, 12.5, 1.11, and 1 point(s), respectively. The optimal threshold of the ACE-SYNTAX model was two points for ischemic events at 48 months. Comparing with the baseline and residual SYNTAX score, the AUC of incremental number of risk scores at 48-month ischemic events had a significant improvement [0.57 (0.53–0.61), p = 0.038 and 0.55 (0.51–0.59), p = 0.001, respectively]. There was no significant improvement in AUC of ROC when compared ACEF and GRACE score with cumulative risk score [0.58 (0.54–0.62), p = 0.16 and 0.57 (0.54–0.61), p = 0.08, respectively]. Reclassification of patients into risk categories according to the occurrence of 48-month ischemic events is summarized in Supplementary Table S6. The NRI after combined with four risk scores was 12.5% (5.3–20.0%), 9.4% (2.0–16.8%), 12.1% (4.5–19.7%), and 10.7% (3.3–18.1%), which offers statistically significant improvement in the performance, compared with SYNTAX, residual SYNTAX, ACEF, and GRACE score, respectively.
The current study, which included data from a prospective, multicenter, and randomized trial, is the first study to investigate the feasibility and effectiveness of the management strategy (ACE-SYNTAX score) that combined with multiple risk scores could modify the discrimination to predict the long-term prognosis of patients with CAD undergoing PCI. The main findings of this analysis were as follows: (1) as the clinical routine risk scores, the baseline SYNTAX, residual SYNTAX, ACEF, and GRACE score demonstrated a certain value with respect to predicted long-term ischemic risk in CAD patients with stents implantation, with moderate discrimination; (2) the risk of ischemic events, including cardiac death, MI, stroke, definite/probable ST, and all-cause mortality have a significant increasing trend with incremental risk-score categories in these patients; and (3) using combinatory of predicting algorithms properly could play a valuable step to help clinicians identify the risk of these patients with implementation of sufficient treatments both in the post-procedure and long-term period, especially in these with lower or higher risk.
As we all know, the prognosis of patients with CAD is determined by baseline risk factors and the use of guideline-indicated therapies. The appropriately-stratified for these patients after stents implantation, which is a significant management challenge, has the potential to achieve the optimal individualized treatment and improve long-term outcomes (27–29). Thus, it is no doubt that using risk prediction algorithms to stratify the patients according to their estimated risk of future ischemic events could assist clinicians in selecting the optimal intensity and/or duration of secondary prevention therapy in decision-making. However, the gaps between guidelines and clinical practices were that the evidence-recommended tools to predict risk might be not universally applicable and robust. The main reason might be the timeliness of the risk scores, though it might be difficult to solve. The most frequently-used risk scores in our routine clinical practice, such as GRACE and SYNTAX scores, are developed from several years back in time. As the newest risk assessment tools, such as dual antiplatelet therapy (DAPT) and Predicting complications in patients undergoing stent implantation and subsequent Dual antiplatelet therapy (PRECISE-DAPT) scores, the data used to build them from randomized controlled trials or observational studies are still more than 5 years (4, 5). However, clinical practice and technology have advanced at a breathless pace. Over recent decades, medicine has drastically evolved with wider clinical use of more advanced diagnostic and therapeutic techniques, they might misestimate the risk of the disease (30). Meanwhile, the complex interaction between residual risk after PCI and the therapeutic benefit of secondary prevention management increases the complexity of risk stratification in these patients. Although no clinical risk tool is perfect, making use of the scores appropriately could provide convincing evidence in helping clinicians make individualized decisions for their patients. Utility of the accumulation of multiple risk scores could overcome the situation, having a significant improvement to discriminate risk of patients with a better classification.
Of note, evidence from a prior study suggests that medical management based on risk stratification was significantly associated with improved long-term prognosis, nevertheless, the benefits decreased with increasing estimated risk (31). The utilization of risk scores tends to be more successful in improving the reclassification of risk, which may enable monitoring and mobilizing clinical practice managers. Admittedly, there was no significant improvement in AUC of ROC at 48-month ischemic events when compared ACEF and GRACE scores with cumulative risk scores. However, it is intelligible that there is just integrating several validated risk scores without any other factors implantation. Still, the accumulative scores were proved a better net reclassification of risk compared with each single score. As we all know, adjustment of the weight of variables and bringing new factors into the risk score could increase the performance when the algorithm is insufficiently accurate in different races/ethnicity. Both above mentioned methods needed a series of cohorts to re-develop and re-validate the score. Our study demonstrates a plug-and-play strategy to risk assessment, which is especially suitable for these without established tools to carry out.
There is no escaping the fact that physicians routinely overestimated the risk of cardiac events and overvalued the benefits of invasive and secondary prevention management with a strong reliance on their intuition (32–34). Undoubtedly, predicting the adverse risk based on objectively quantified clinical algorithms could provide superior risk discrimination (32, 34). The number of prediction tools, as well as the presence of overlapping risk scores in the same clinical scenarios, is the blowout of a sharp increase, which makes it difficult to select using a universal and interoperable scoring system for the cardiologists. Indeed, in the clinical routine practice, using multiple complex algorithms could be challenging and cumbersome to compute.
However, with the improvement of the digital hospital and laboratory information system and the advent of machine learning based on deep neural networks, an approach may be a viable solution to generate detailed data in high-volume capacity (35, 36). It is convenient to capture all factors relating to the scores in the electronic medical records (EMR), calculate them automatically, and then quickly preset the predicting risk of patients in the system in auto (37, 38). It should be noted that dichotomizing continuous risk scores into a regression model might not be the optimal choice, which could induce a potential risk of inaccuracy. However, predictive accuracy for ischemic events was similar to continuation and dichotomization measurements in our research. Considering the clinical applicability without excessive consumption of accuracy, it might be acceptable to transfer continuous variables into binary variables.
The current study is limited by its post-hoc nature. As a retrospective analysis, the results of our study are hypothesis-generating. Thus, it is essential to confirm our findings in several specifically designed trials. Second, even with digital hospital and laboratory information systems, it is still complicated for clinicians to carry out too many risk-assessing tools. Therefore, in order to increase availability, what needs to be done further is investigating the proper combination of risk scores in different races/ethnicity. Third, the patients with missing data of risk scores were excluded in our study, which could have biased the estimates. Nevertheless, multiple imputations were performed to address it. The results were consistent between before and after imputation.
The guideline-indicated ischemic risk scores displayed reasonable predictive performance in CAD patients with DES implantation. The novel multiple risk score model was significantly associated with the risk of long-term ischemic events in these patients with an increment of scores. A meaningful improvement to predict adverse outcomes when multiple risk scores were applied to risk stratification. Further studies are needed to confirm these findings.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of General Hospital of Northern Theater Command. The patients/participants provided their written informed consent to participate in this study.
MQ designed a statistical plan and verified the underlying data, and was in charge of manuscript writing and statistical analysis. YL designed a statistical plan and verified the underlying data. YL, KN, ZQ, SM, HZ, XX, JL, KX, and XW contributed to recruiting subjects during the whole period of study. YH contributed to the leadership of the whole process of study conduction and acted as the key role of initiating, designing, conducting, and concluding the study. All authors contributed to the article and approved the submitted version.
The I-LOVE-IT 2 trial was sponsored by Essen Technology (Beijing, China), and our study was also supported by the National Key Research and Development Program of China (2016YFC1301300 and 2016YFC1301303).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JT declared a shared affiliation, with two of the authors ZQ and JL to the handling editor at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors appreciate the dedicated efforts of clinical research collaborators in the I-LOVE-IT 2 trial organization.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.756379/full#supplementary-material
1. Costa F, Tijssen JG, Ariotti S, Giatti S, Moscarella E, Guastaroba P, et al. Incremental value of the CRUSADE, ACUITY, and HAS-BLED risk scores for the prediction of hemorrhagic events after coronary stent implantation in patients undergoing long or short duration of dual antiplatelet therapy. J Am Heart Assoc. (2015) 4. doi: 10.1161/JAHA.115.002524
2. Bueno H, Fernandez-Aviles F. Use of risk scores in acute coronary syndromes. Heart. (2012) 98:162–8. doi: 10.1136/heartjnl-2011-300129
3. Tahir UA, Yeh RW. Individualizing dual antiplatelet therapy duration after percutaneous coronary intervention: from randomized control trials to personalized medicine. Expert Rev Cardiovasc Ther. (2017) 15:681–93. doi: 10.1080/14779072.2017.1362980
4. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. (2016) 315:1735–49. doi: 10.1001/jama.2016.3775
5. Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. (2017) 389:1025–34. doi: 10.1016/S0140-6736(17)30397-5
6. Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol. (2016) 67:2224–34. doi: 10.1016/j.jacc.2016.02.064
7. Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. (2004) 291:2727–33. doi: 10.1001/jama.291.22.2727
8. Harada Y, Michel J, Lohaus R, Mayer K, Emmer R, Lahmann AL, et al. Validation of the DAPT score in patients randomized to 6 or 12 months clopidogrel after predominantly second-generation drug-eluting stents. Thromb Haemost. (2017) 117:1989–99. doi: 10.1160/TH17-02-0101
9. Song L, Guan C, Yan H, Qiao S, Wu Y, Yuan J, et al. Validation of contemporary risk scores in predicting coronary thrombotic events and major bleeding in patients with acute coronary syndrome after drug-eluting stent implantations. Catheter Cardiovasc Interv. (2018) 91:573–81. doi: 10.1002/ccd.27468
10. Ueda P, Jernberg T, James S, Alfredsson J, Erlinge D, Omerovic E, et al. External validation of the DAPT Score in a nationwide population. J Am Coll Cardiol. (2018) 72:1069–78. doi: 10.1016/j.jacc.2018.06.023
11. Zhao XY, Li JX, Tang XF, Xu JJ, Song Y, Jiang L, et al. Validation of predictive value of patterns of nonadherence to antiplatelet regimen in stented patients thrombotic risk score in chinese population undergoing percutaneous coronary intervention: a prospective observational study. Chin Med J. (2018) 131:2699–704. doi: 10.4103/0366-6999.245263
12. Zhao X, Jiang L, Xu L, Tian J, Xu Y, Zhao Y, et al. Predictive value of in-hospital white blood cell count in Chinese patients with triple-vessel coronary disease. Eur J Prev Cardiol. (2019) 26:872–82. doi: 10.1177/2047487319826398
13. Reindl M, Reinstadler SJ, Tiller C, Kofler M, Theurl M, Klier N, et al. ACEF score adapted to ST-elevation myocardial infarction patients: the ACEF-STEMI score. Int J Cardiol. (2018) 264:18–24. doi: 10.1016/j.ijcard.2018.04.017
14. Morici N, Tavecchia GA, Antolini L, Caporale MR, Cantoni S, Bertuccio P, et al. Use of PRECISE-DAPT score and admission platelet count to predict mortality risk in patients with acute coronary syndrome. Angiology. (2019) 70:867–77. doi: 10.1177/0003319719848547
15. Han Y, Xu B, Jing Q, Lu S, Yang L, Xu K, et al. A randomized comparison of novel biodegradable polymer- and durable polymer–coated cobalt-chromium sirolimus-eluting stents. JACC Cardiovasc Interv. (2014) 7:1352–60. doi: 10.1016/j.jcin.2014.09.001
16. Han Y, Xu B, Xu K, Guan C, Jing Q, Zheng Q, et al. Six versus 12 months of dual antiplatelet therapy after implantation of biodegradable polymer sirolimus-eluting stent: randomized substudy of the I-LOVE-IT 2 trial. Circ Cardiovasc Interv. (2016) 9:e003145. doi: 10.1161/CIRCINTERVENTIONS.115.003145
17. Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. (2006) 333:1091. doi: 10.1136/bmj.38985.646481.55
18. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2011) 32:2999–3054. doi: 10.1093/eurheartj/ehr236
19. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. (2005) 1:219–27. Available online at: https://eurointervention.pcronline.com/article/the-syntax-score-an-angiographic-tool-grading-the-complexity-of-coronary-artery-disease
20. Palmerini T, Genereux P, Caixeta A, Cristea E, Lansky A, Mehran R, et al. Prognostic value of the SYNTAX score in patients with acute coronary syndromes undergoing percutaneous coronary intervention: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage StrategY) trial. J Am Coll Cardiol. (2011) 57:2389–97. doi: 10.1016/j.jacc.2011.02.032
21. Ranucci M, Castelvecchio S, Menicanti L, Frigiola A, Pelissero G. Risk of assessing mortality risk in elective cardiac operations: age, creatinine, ejection fraction, and the law of parsimony. Circulation. (2009) 119:3053–61. doi: 10.1161/CIRCULATIONAHA.108.842393
22. Wykrzykowska JJ, Garg S, Onuma Y, de Vries T, Goedhart D, Morel MA, et al. Value of age, creatinine, and ejection fraction (ACEF score) in assessing risk in patients undergoing percutaneous coronary interventions in the “All-Comers” LEADERS trial. Circ Cardiovasc Interv. (2011) 4:47–56. doi: 10.1161/CIRCINTERVENTIONS.110.958389
23. Genereux P, Palmerini T, Caixeta A, Rosner G, Green P, Dressler O, et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol. (2012) 59:2165–74. doi: 10.1016/j.jacc.2012.03.010
24. Qiu M, Li Y, Li J, Xu K, Jing Q, Dong S, et al. Impact of six versus 12 months of dual antiplatelet therapy in patients with drug-eluting stent implantation after risk stratification with the residual SYNTAX score: Results from a secondary analysis of the I-LOVE-IT 2 trial. Catheter Cardiovasc Interv. (2017) 89:565–73. doi: 10.1002/ccd.26948
25. Farooq V, Serruys PW, Bourantas CV, Zhang Y, Muramatsu T, Feldman T, et al. Quantification of incomplete revascularization and its association with five-year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX score. Circulation. (2013) 128:141–51. doi: 10.1161/CIRCULATIONAHA.113.001803
26. Pencina MJ, D'Agostino RB Sr., D'Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. (2008) 27:157–72. doi: 10.1002/sim.2929
27. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
28. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
29. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. (2016) 68:1082–115. doi: 10.1016/j.jacc.2016.03.513
30. Bawamia B, Mehran R, Qiu W, Kunadian V. Risk scores in acute coronary syndrome and percutaneous coronary intervention: a review. Am Heart J. (2013) 165:441–50. doi: 10.1016/j.ahj.2012.12.020
31. Hall M, Bebb OJ, Dondo TB, Yan AT, Goodman SG, Bueno H, et al. Guideline-indicated treatments and diagnostics, GRACE risk score, and survival for non-ST elevation myocardial infarction. Eur Heart J. (2018) 39:3798–806. doi: 10.1093/eurheartj/ehy517
32. Chew DP, Junbo G, Parsonage W, Kerkar P, Sulimov VA, Horsfall M, et al. Perceived risk of ischemic and bleeding events in acute coronary syndromes. Circ Cardiovasc Qual Outcomes. (2013) 6:299–308. doi: 10.1161/CIRCOUTCOMES.111.000072
33. Yan AT, Yan RT, Tan M, Fung A, Cohen EA, Fitchett DH, et al. Management patterns in relation to risk stratification among patients with non-ST elevation acute coronary syndromes. Arch Intern Med. (2007) 167:1009–16. doi: 10.1001/archinte.167.10.1009
34. Bing R, Goodman SG, Yan AT, Fox K, Gale CP, Hyun K, et al. Use of clinical risk stratification in non-ST elevation acute coronary syndromes: an analysis from the CONCORDANCE registry. Eur Heart J Qual Care Clin Outcomes. (2018) 4:309–17. doi: 10.1093/ehjqcco/qcy002
35. Liang H, Tsui BY Ni H, Valentim CCS, Baxter SL, Liu G, et al. Evaluation and accurate diagnoses of pediatric diseases using artificial intelligence. Nat Med. (2019) 25:433–8. doi: 10.1038/s41591-018-0335-9
36. Leyh-Bannurah S-R, Tian Z, Karakiewicz PI, Wolffgang U, Sauter G, Fisch M, et al. Deep learning for natural language processing in urology: state-of-the-art automated extraction of detailed pathologic prostate cancer data from narratively written electronic health records. JCO Clin Cancer Inform. (2018) (2):1–9. doi: 10.1200/CCI.18.00080
37. Ness SL, Manyakov NV, Bangerter A, Lewin D, Jagannatha S, Boice M, et al. JAKE(R) multimodal data capture system: insights from an observational study of autism spectrum disorder. Front Neurosci. (2017) 11:517. doi: 10.3389/fnins.2017.00517
Keywords: coronary artery disease, percutaneous coronary intervention, risk score, ischemic events, drug-eluting stent
Citation: Qiu M, Li Y, Na K, Qi Z, Ma S, Zhou H, Xu X, Li J, Xu K, Wang X and Han Y (2022) A Novel Multiple Risk Score Model for Prediction of Long-Term Ischemic Risk in Patients With Coronary Artery Disease Undergoing Percutaneous Coronary Intervention: Insights From the I-LOVE-IT 2 Trial. Front. Cardiovasc. Med. 8:756379. doi: 10.3389/fcvm.2021.756379
Received: 10 August 2021; Accepted: 06 December 2021;
Published: 13 January 2022.
Edited by:
Masanori Aikawa, Brigham and Women's Hospital and Harvard Medical School, United StatesReviewed by:
Jinwei Tian, The Second Affiliated Hospital of Harbin Medical University, ChinaCopyright © 2022 Qiu, Li, Na, Qi, Ma, Zhou, Xu, Li, Xu, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaling Han, aGFueWFsaW5nQDE2My5uZXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.