
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 12 November 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.753133
This article is part of the Research Topic Novel Risk Predicting System for Heart Failure View all 16 articles
A correction has been applied to this article in:
Corrigendum: Association of systemic immune-inflammation index with short-term mortality of congestive heart failure: A retrospective cohort study
Purpose: The present study aimed to clarify the potential predictive significance of Systemic immune-inflammation index (SII) in assessing the poor prognosis of critically ill patients with congestive heart failure (CHF).
Methods: Detailed clinical data were extracted from the Multiparameter Intelligent Monitoring in Intensive Care III database after gaining access and building the local platform. The 30- and 90-day and hospital all-cause mortalities of the patient was the primary outcome, and the readmission rate and the occurrence of major cardiovascular adverse events (MACEs) were the secondary outcomes. the Cox proportional hazard model and Logistic regression analysis were selected to reveal the relationship between SII level and the research outcome. Further, the propensity score matching (PSM) analysis was performed to improve the reliability of results by reducing the imbalance across groups.
Results: There were a total of 4,606 subjects who passed the screening process and entered the subsequent analysis. Multivariate regression analysis showed that after adjusting for possible confounders, including age, heart rate, and albumin, etc., the high level of SII was independently associated with 30- and 90-day and hospital mortalities (tertile 3 vs. tertile 1: HR, 95% CIs: 1.23, 1.04-1.45; 1.21, 1.06-1.39; 1.26, 1.05-1.50) and the incidence of MACEs (tertile 3 vs. tertile 1: OR, 95% CI: 1.39, 1.12-1.73) in critically ill patients with CHF, but no significant correlation was found between SII and the readmission rate. Consistently, patients with high SII level still presented a significantly higher short-term mortality than patients with low SII in the PSM subset.
Conclusion: In critically ill patients with CHF, high level of SII could effectively predict high 30- and 90-day and hospital mortalities, as well as the high risk of occurrence of MACEs.
Congestive heart failure (CHF) is defined as a pathological condition in which the cardiac output is insufficient to maintain the perfusion and metabolic needs of various tissues and organs, mainly characterized by the congestion of pulmonary circulation, accompanied by the corresponding clinical manifestations such as shortness of breath and decreased activity tolerance (1). CHF is the severe or terminal stage of most primary cardiovascular diseases and has increasingly become a major public problem threatening human health. In the United States and Europe, more than 1 million heart failure patients are hospitalized each year, and as the population ages, this number is expected to increase by more than 50% in the next 15 years (2). In addition, although some progress has been made in the treatment in recent years, the prognosis of CHF patients is still poor, with 5-year mortality rate as high as 40-50% (3). Therefore, accurate assessment and stratification of prognosis are critical to the clinical management of CHF, and the development of certain prognostic-related biomarkers is also pressing, which can help clinicians to identify high-risk patients early and take more aggressive treatment measures (4).
Although the specific pathogenesis of heart failure remains unclear, abnormal immune activation and chronic inflammation play an important role. The damage, repair and remodeling of myocardium are the important link in the occurrence and development of heart failure, of which immune/inflammatory cells (neutrophils, lymphocytes, etc.) and the inflammatory factors (tumor necrosis factor, interleukin-6, etc.) released by them are involved (5). Inflammatory factors can induce cardiomyocyte hypertrophy, apoptosis and fibrosis, and ultimately lead to adverse cardiac remodeling and progressive left ventricular dysfunction (6). Inflammatory factors have been considered to be biomarkers for poor prognosis of heart failure, and anti-inflammatory therapy is also expected to become a new target for the treatment of heart failure (7).
Systemic immune-inflammation index (SII) is a composite inflammatory indicator that combines three significant immune cells, including neutrophil, lymphocyte, and platelet, and is considered to be an excellent indicator of local immune response and systemic inflammation (8). Neutrophils, platelets and the cytokines they produce are mainly related to non-specific immune responses, while lymphocytes are considered to be mainly related to specific immune pathways. Compared with the absolute count of single immune cells, SII has a better representation in reflecting the inflammation status of the body, with better stability. Until now, SII has been confirmed to be closely correlated with the poor prognosis of a variety of cardiovascular diseases, including coronary artery disease (9), aortic stenosis (10), infective endocarditis (11), etc., showing good application prospects, but no studies have shown that SII is correlated with the prognosis of patients with CHF. In view of this, the present study attempted to elucidate the independent association between SII and the occurrence of adverse events such as short-term mortality in critically ill patients with CHF, so as to provide reference for the prevention and treatment of heart failure.
All data used in the research and analysis of this study are derived from MIMIC-III (Medical Information Mart for Intensive Care III) database, a free and open-access large-scale critical care medicine database developed and run by the Massachusetts Institute of Technology for researchers all over the world. Another advantage of this database, in addition to including the detailed clinical data such as demographic data, vital signs, laboratory tests, and various treatment information, is that it provides accurate death information of all subjects, including time of death in the hospital or within 90 days after discharge, which makes it possible for the clinicians to carry out the prognostic related research. Besides, since this database has been approved by the local Ethics Committee as a whole and the individual identifying information of the research subjects has been deleted, this study no longer needs additional ethical approval.
The subjects of this study are all from the MIMIC- III database (12), and patients who meet all the following requirements are included in the subsequent analysis: (1) Patients diagnosed with CHF based on the ninth revision of the International Classification of Diseases (ICD-9) code (code 428.0); (2) Adult patients 18 years of age and older; (3) First admission to intensive care units (ICU). Patients who met one of the following criteria were excluded from the study: (1) The length of ICU stay was shorter than 24 h; (2) Absence of SII results during hospitalization; (3) Survival time was <0 (the time of death of some organ donors may be earlier than the admission).
The short-term mortality, including the 30- and 90-day and hospital all-cause mortalities of critically ill patients with CHF, were selected as the primary study outcome, while the secondary study outcome events were defined as the patients' readmission rate and the occurrence of major adverse cardiac events (MACEs), which is a composite outcome event, including all-cause death, readmission for acute heart failure, the use of mechanical circulatory support and the implementation of heart transplantation (13).
Through the PostgreSQL software (version 9.6, https://www.postgresql.org/), we extracted detailed clinical data of the research subjects from the MIMIC-III database, including demographic data (age, gender, race, etc.), comorbidities (hypertension, diabetes mellitus, atrial fibrillation, etc.), vital signs (heart rate, blood pressure, respiratory rate, etc.), severity of illness scores [the sequential organ failure assessment (SOFA) and the simplified acute physiology score II (SAPSII) scores], laboratory tests (blood routine, electrolytes, etc.) and intervention measures (dialysis, mechanical ventilation, etc.). And SII was calculated as follows: SII = platelet × neutrophil/lymphocyte (14).
After separately extracting the required tables from the MIMIC-III database, the Stata software (version 16, https://www.stata.com/) was used to process and merge these original table and generate a complete table that can be used for subsequent analysis. Use the “winsorize” function to identify and process outliers, and fill in missing values through multiple compensation methods.
The continuous variables were presented in the form of mean ± standard deviation (SD) or median (interquartile range). If continuous variables satisfy both normal distribution and homogeneity of variance, t-test was used for analysis, while the Mann-Whitney U test was conducted if the normal distribution or homogeneity of variance were not satisfied. Categorical variables are expressed in the form of the number of cases (percentage), with the chi-square test (or Fisher's exact method) for analysis.
We constructed a generalized additive model (GAM) to determine the non-linear relationship between SII and 30- and 90-day all-cause mortalities in critically ill patients with CHF. In addition, we also visually show the relationship between SII and patients' survival through the Kaplan-Meier (K-M) curve, and use the Log-rank test for hypothesis testing.
In order to relieve the interference of possible confounding factors on the results, we completed univariate and multivariate regression analysis to further clarify the relationship between SII and outcome variables. In the crude model, no variables were adjusted. In model I, the age, gender, and race variables were adjusted, while model II further adjusted other 12 variables on the basis of these variables of model II, including heart rate, blood urea nitrogen (BUN), albumin, troponin T (cTnT), N-terminal probrain natriuretic peptide (NT-proBNP), urine output of first day, cardiac index, the type of first ICU admission, the use of mechanical ventilation and vasopressor, pneumonia, and liver diseases. The selection of confounding factors follows the following principles (15): (1) A certain factor has an influence of more than 10% on the research variable; (2) Some factors may have a significant impact on the outcome variable based on past experience. Besides, we used the variance inflation factor (VIF) to test the multicollinearity between variables with 5 as the threshold, and the variables with a high degree of collinearity, serum chloride, were deleted to avoid over-fitting of the model. The Cox regression analysis was used to determine the relationship between SII and short-term mortalities in critically ill patients with CHF, while Logistic regression analysis was used to analyze the association between SII and readmission rates or MACEs.
To further enhance the credibility of our analysis, we performed the subgroup analysis and propensity score matching (PSM) analysis. First, By the subgroup analysis, determine whether the correlation between the SII and the high 30-day all-cause mortality in critically ill patients with CHF was consistent across various subgroups stratified mainly by comorbidities, and reflect the stability of SII as a prognostic marker. The optimal cut-off value of SII for the short-term mortality was determined by the X-tile (version 3.6.1, Yale University School of Medicine) software, and then the entire study population was divided into two groups, namely the high SII group and the low SII group. In this study, all variables with uneven distribution between the two groups of patients were included in the PSM model as covariates, and the corresponding propensity score was calculated by Logistic regression. Then the two groups of individuals with similar propensity scores were matched by 1:1 using nearest neighbor matching method with a caliper width of 0.05, and the histogram of the propensity score distribution was draw, which was carried out through the Stata software. Then, K-M curves were depicted and Cox regression analysis was conducted in the matching cohort after PSM, which further validated SII as an independent risk factor for poor prognosis in the critically ill patients with CHF.
The statistical analysis above was conducted by EmpowerStats software (version 2.20, http://www.empowerstats.com/cn/, X&Y solutions, Inc, Boston, MA) and R software (version 3.4.3). p < 0.05 (two-sided) was considered statistically significant. Besides, in the regression analysis, we corrected p-value with false discovery rate (FDR) to avoid the false positives with multiple comparisons and used the false discovery rate (FDR) of 0.05 as cutoff.
The study subjects were screened according to the process mentioned above, and 4,606 subjects were finally included (Figure 1). The demographic data, vital signs, comorbidities, treatment, scores and laboratory examinations and other relevant information between survivors and non-survivors' cohorts were shown in Table 1 in detail. Besides, the clinical characteristics of critical ill patients with CHF across three group divided according to SII levels were presented in Supplementary Table 1. Overall, the median age of the study subjects included was 74.91 years old, mainly male patients, reaching 2,436, accounting for 52.89%. And the majority of the subjects were whites, accounting for 72.71%, blacks only 7.21%, and other races including Asians accounted for 20.08%. The duration of follow-up was 90 days, of which 3,226 survived and 1,380 died due to various reasons before the end of the study. SII (213.97 vs. 163.11, p < 0.001) was significantly higher in the non-survivor cohort. Compared with the survivor group, subjects in the non-survivor cohort were older (median age: 78.94 vs. 72.70 years old, p < 0.001), with more unstable vital signs, higher incidence of comorbidities such as valvular heart disease (VHD), atrial fibrillation, and renal failure, as well as higher SOFA and SAPSII scores.
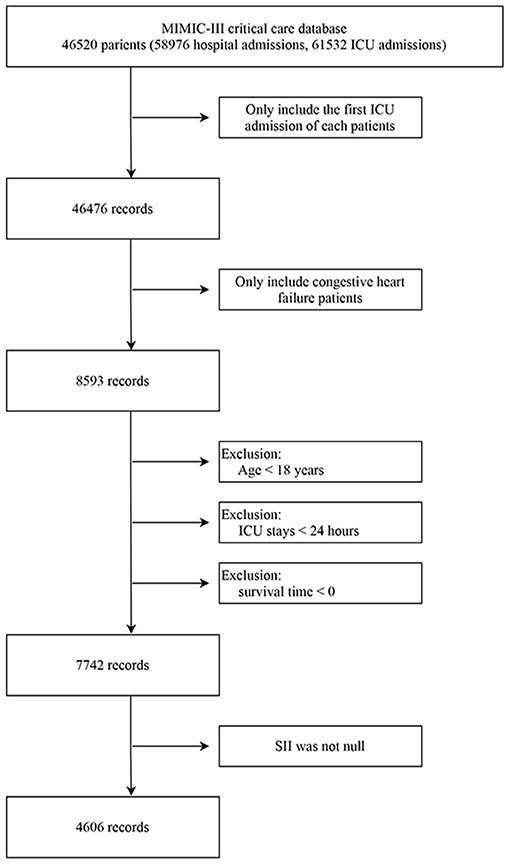
Figure 1. Workflow of exclusion and inclusion as utilized to select the final 4,606 patients. ICU, intensive care unit. SII, systemic immune-inflammation index.
GAM analysis was used to identify the non-linear correlation between SII and the short-term all-cause mortality of patients, which showed that there was a U-shaped curve relationship between SII and 30-day (Figure 2A) and 90-day (Figure 2B) all-cause mortalities in critically ill patients with CHF, indicating that higher or lower SII may be associated with increased mortality. The K-M survival curve (Figure 3) also showed that compared with the low SII group, patients with high SII level had a lower overall survival rate and shorter survival time, with a statistically significant p-value.
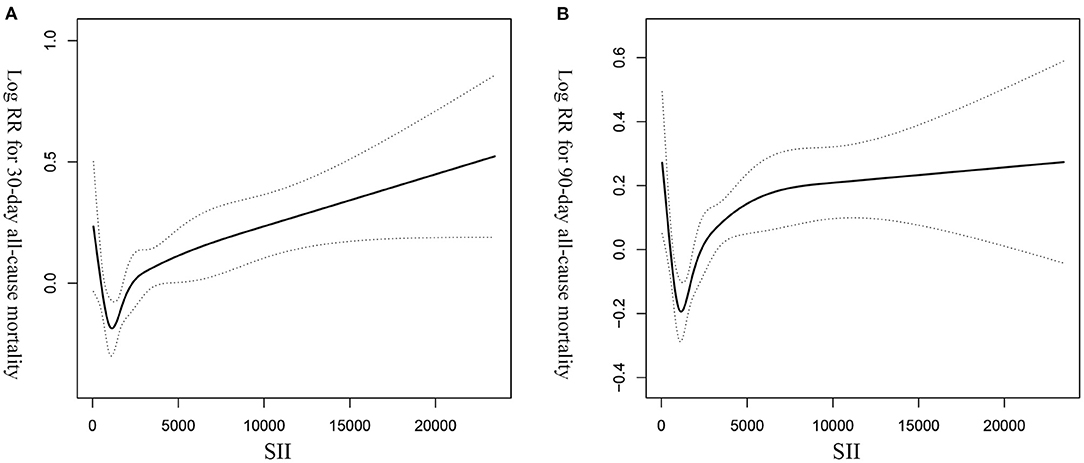
Figure 2. The non-linear curve fitting of the association between SII and 30- (A) and 90- (B) day all-cause mortalities in critically ill patients with CHF after adjusting for age, gender, race, and other potential variables. SII, systemic immune-inflammation index. CHF, congestive heart failure.
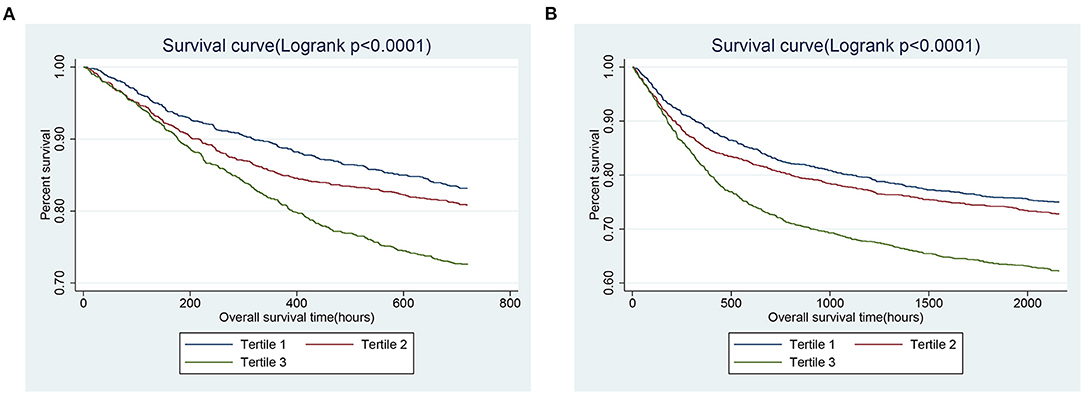
Figure 3. Kaplan-Meier survival curves showing the 30- (A) and 90- (B) day all-cause mortalities stratified by SII tertile in critically ill patients with CHF. SII, systemic immune-inflammation index. CHF, congestive heart failure.
In order to verify the independent relationship between SII and the poor short-term mortality of CHF patients, we performed the univariate and multivariate Cox regression analysis (Table 2), with SII stratified by tertiles. In crude model, the third tertile of SII increased significantly the risk of 30- (HR, 95% CI: 1.73, 1.48-2.03) and 90-day (HR, 95% CI: 1.65, 1.45-1.88) and hospital (HR, 95% CI: 1.61, 1.36-1.91) all-cause mortalities compared with the first tertile. In multivariate model I, after adjusting for age, sex, and race, the third tertile of SII group also suffered from the higher risk of 30- (HR, 95% CI: 1.63, 1.40-1.91) and 90-day (HR, 95% CI: 1.57, 1.38-1.79) and hospital (HR, 95% CI: 1.45, 1.22-1.72) all-cause mortalities. In model II, in addition to adjusting for the variables in model I and other possible confounders such as pneumonia, the use of dialysis, and BUN level, high levels of SII were still strongly associated with poor 30- (HR, 95% CI: 1.23, 1.04-1.45) and 90-day (HR, 95% CI: 1.21, 1.06-1.39) and hospital (HR, 95% CI: 1.26, 1.05-1.50) all-cause mortalities.
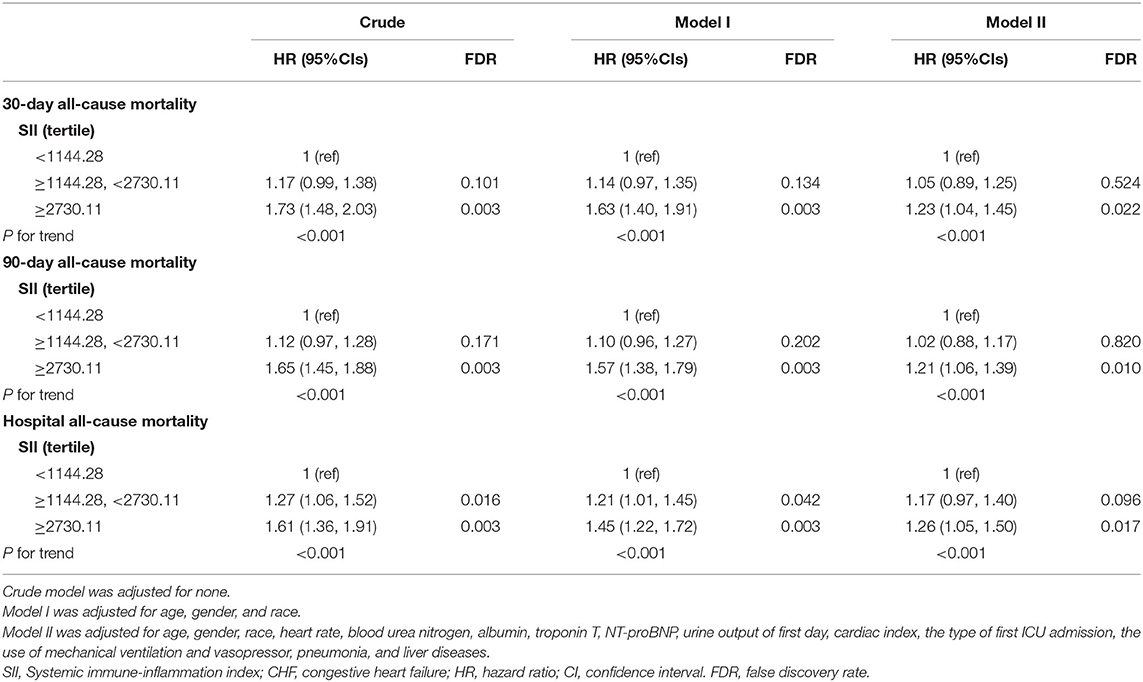
Table 2. The univariate and multivariate Cox regression analysis exploring the association of SII tertile with short-term mortality of critically ill patients with CHF.
For MACEs, a similar trend was observed. In model II (Table 3), patients with an SII ≥ 2730.11 were still at higher incidence of MACEs (OR, 95% CI: 1.39, 1.12-1.73). However, in the multivariate regression analysis, there was no obvious independent correlation between SII and readmission rate in the critically ill patients with CHF (tertile 3 vs. tertile 1: OR, 95% CI: 0.97, 0.81-1.15).
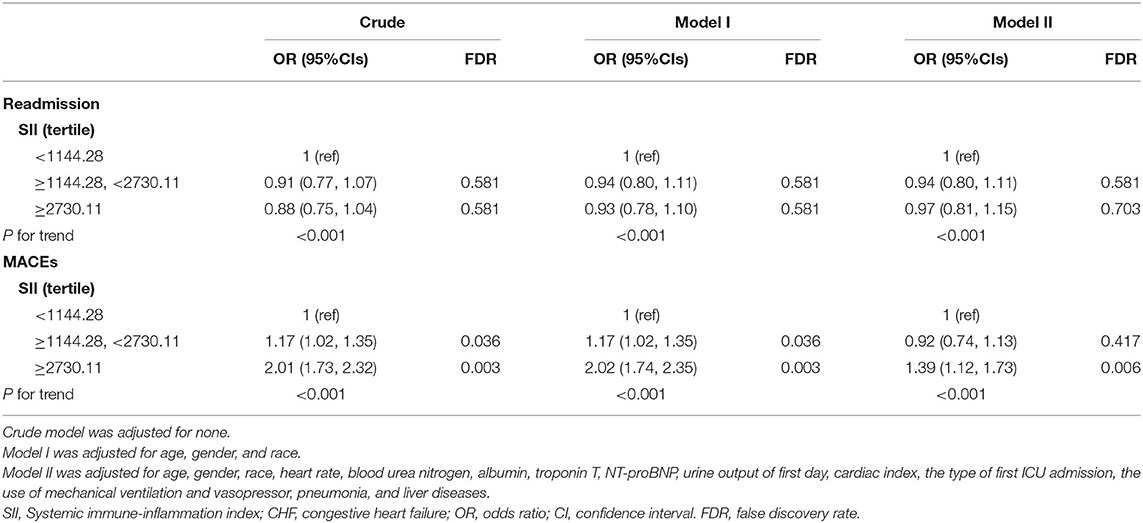
Table 3. The univariate and multivariate Logistic regression analysis exploring the association of SII tertile with readmission and MACEs of critically ill patients with CHF.
The subgroup analysis was conducted to reveal the correlation between SII and 30-day mortality across comorbidities and different parameters, and the results were shown in Table 4. First, the results of the study showed that in all subgroups, the increase in SII level was closely related to the increase in the 30-day all-cause mortality of critically ill patients with CHF. Besides, most of the stratification factors have not been found to have a significant impact on the relationship between the SII and 30-day all-cause mortality (interaction p-value > 0.05), except for hyperlipemia (p = 0.006) and acute myocardial infarction (AMI, p = 0.016).
Determine the optimal cut-off value of SII for 90-day all-cause mortality of critically ill patients with CHF through X-tile software, which is 5533.4. Subjects were divided into two groups according to the cut-off value, and their clinical characteristics were summarized in Table 5, most of which were unevenly distributed across the two groups. In order to effectively balance these confounding factors and improve the credibility of our results, we conducted a PSM analysis with 1:1 matching. After PSM, 602 pairs of research objects were generated and the difference of almost all variables were balanced between the two groups, with a good matching performance (Figure 4). At the same time, after PSM, compared with the low SII level group, subjects in the high SII level group still underwent higher 30- (24.9 vs. 33.2%, p = 0.002) and 90-day (35.0 vs. 42.0%, p = 0.013) and hospital (22.4 vs. 27.7%, p = 0.033) all-cause mortalities, with shorter survival time (Table 5; Figure 5). Besides, after adjusting for these covariable in the model II, the increased SII was also associated with increased risk of short-term all-cause mortality in critically ill patients with CHF after PSM and the results were shown in Figure 6 in the form of a forest map.
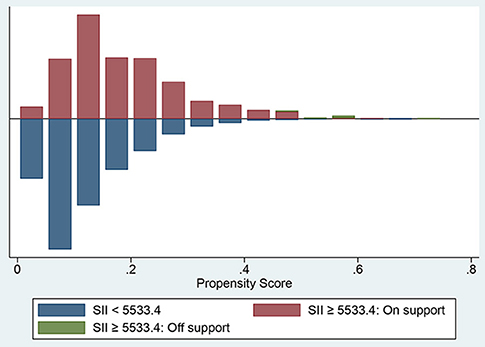
Figure 4. The histogram presenting the range of propensity scores and the corresponding number of matches in high (red) and low (blue) SII groups. SII, systemic immune-inflammation index.
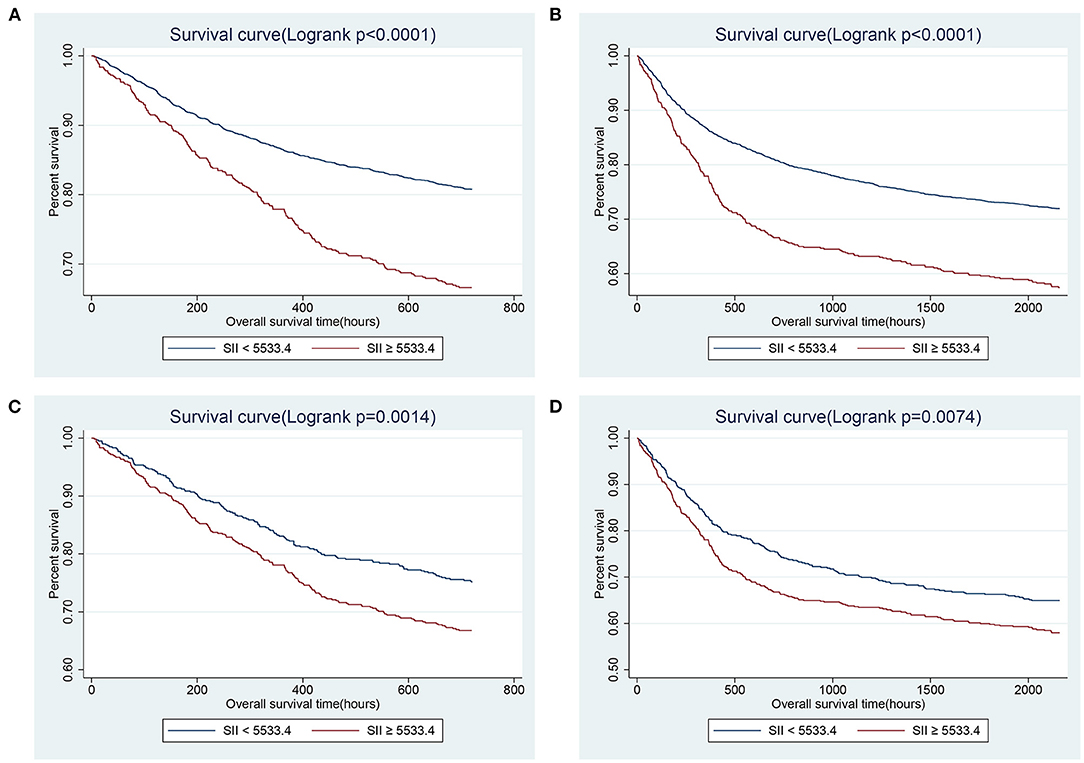
Figure 5. Kaplan-Meier survival curves showing the 30- (A) and 90- (B) day all-cause mortalities stratified by cut-off value of SII in critically ill patients with CHF before PSM. (C,D) presented the correlation between SII and 30- and 90-day mortalities after PSM, separately. SII, systemic immune-inflammation index. CHF, congestive heart failure. PSM, propensity score matching.
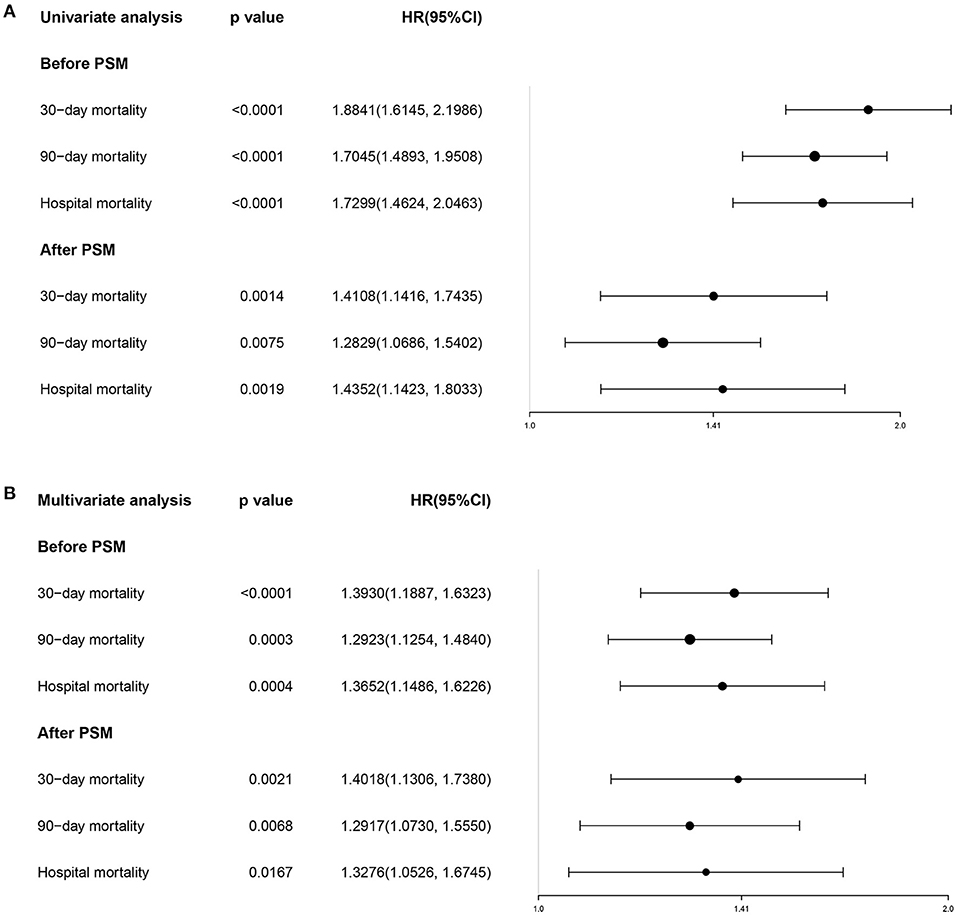
Figure 6. The forest plot showing the univariate (A) and multivariate (B) Cox regression for effects of SII on short-term mortality in the critically ill patients with CHF before and after PSM. SII, systemic immune-inflammation index. CHF, congestive heart failure. PSM, propensity score matching.
CHF is widespread all over the world, with gradually increasing incidence with age, and has become one of the diseases with the highest rates of hospitalizations and deaths in the world, bringing about enormous medical expenditure and social burden, especially in developing countries (16). In addition to exploring promising treatments, the development of novel prognostic markers for risk stratification of patients is also of great value for improving the prognosis of patients. As we all know, as a commonly used indicator of heart failure, NT-proBNP can make a good performance in prognostic evaluation. However, the clinical applications of NT-proBNP for risk assessment has some drawbacks and problems. First, compared to the real value, the detection of NT-proBNP tend to be underestimated because of the short half-life. Moreover, the test of NT-proBNP may be affected by various factors such as age, gender, and kits (17). Therefore, the search for more possible biomarkers has gradually attracted the attention of researchers and clinicians.
In the present study, we extracted the clinical data of 4,606 critically ill patients who diagnosed with CHF between 2001 and 2012 in MIMIC-III database, and analyzed the relationship between SII and short-term prognosis by univariate and multivariate regression analyses for the first time. And the results have verified that SII could be an independent biomarker for poor short-term prognosis of CHF. There was a non-linear relationship between SII and 30- and 90-day mortalities. And the higher SII level, the higher risk of 30- and 90-day and hospital mortalities, as well as the higher incidence of MACEs. The correlation remained significant after adjusting for possible confounders, stratifying according to comorbidities, and PSM matching, respectively.
Inflammation, a widely accepted hallmark of heart failure, has been proven to play an indispensable role in the pathogenesis of heart failure (18). The increase and activation of inflammation-related cytokines not only present a reflection of the activation of inflammation in vivo, but have also been identified as being associated with poor prognosis in heart failure. Tumor necrosis factor α (TNF-α) was the first cytokine found to be significantly elevated in the serum of patients with heart failure, which could directly induce myocardial apoptosis and necrosis, bringing about ventricular adverse remodeling (19). The elevated levels of TNF-α often indicated impaired cardiac systolic function and poor long-term survival (20). In addition, interleukin 6 (IL-6), another classic cytokine mainly derived from monocytes, could be significantly increased in patients with left ventricular systolic dysfunction without clinical symptoms, and may be a sensitive indicator for the early diagnosis of heart failure (21). IL-6 was also positively correlated with the severity of heart failure, the concentration of which was negatively correlated with left ventricular ejection fraction and overall survival (22). Although these inflammatory molecules had good performance in the prognostic evaluation of patients, the expensive detection cost limited their clinical application. As a novel biomarker, SII can systematically and comprehensively reflect the status of inflammation in vivo. Blood routine is a routine examination for almost all hospitalized patients. It is simple, fast, and inexpensive. Compared with NT-proBNP, cytokines and other prognostic indicators, SII can effectively screen high-risk patients, guide the formulation of individualized treatment plans and improve the prognosis of patients without additional cost, with a broad application prospect, especially in underdeveloped areas.
The mechanisms of the relationship between SII and adverse prognosis of CHF remains unclear, and possible explanations are as follows. Recently, it has been revealed that circulating immune cells and their subtypes had a vital indicative effect on the prognosis of cardiovascular diseases (23). SII was a prognostic indicator that integrates three circulating immune cells, including neutrophils, lymphocytes, and platelets, the increase of which indicates either a relative increase in platelets and neutrophils counts or a relative decrease in lymphocytes. Neutrophils are the main component of white blood cells, accounting for 60-70% of the total, and play an important role in the non-specific immune system. In the inflammatory response, neutrophils react rapidly, with strong chemotaxis and phagocytosis, and participate in the progression of various cardiovascular diseases, including heart failure (24). In addition, neutrophils could contribute to oxidative stress and endothelial dysfunction by releasing a large amount of myeloperoxidase, NADPH oxidase, and so on (25). According to a community-based study (26), absolute neutrophils can effectively predict the increased incidence of acute decompensated heart failure after AMI, and it was also an independent risk factor for death (27). Lymphocytes mainly consist of two subtype including T and B cells, which were often associated with the specific immunity. Several previous studies have reported that lymphocyte counts in patients with heart failure were lower than in the normal population and lymphopenia served as an independent predictor of poor survival of patients with chronic and advanced heart failure (28, 29). Lymphopenia was considered to be an important feature of systemic inflammation, which may be caused by the following mechanisms. First, the circulating lymphocytes were attracted into the cardiac tissue, leading to its redistribution. Then, lymphopenia was related to the activation of renin-angiotensin-aldosterone hormone and the adrenergic nervous system, which could exert pro-apoptotic effects on lymphocytes (28). Platelets were differentiated from the mononuclear-phagocyte system and were the key mediator linking the two pathological processes of inflammation and thrombosis (30). On the one hand, platelets were the main effector molecules for hemostasis after cardiovascular injury. On the other hand, the activation of platelets plays a major role in pathogenic thrombosis and participates in the pathogenesis of many groups of cardiovascular diseases, including coronary heart disease (31). Moreover, Platelets interacted with leukocytes and their subtypes (neutrophil, lymphocytes, etc.) and endothelial cells and activated them, inducing monocyte adhesion and transport, releasing inflammatory factors such as TNF-α and IL-1, which together promoted local inflammation and fibrosis in heart failure (31). The study by Kandis et al. (32) demonstrated that mean platelet volume (MPV), indicating the activation of platelets, increased significantly in decompensated heart failure patients. In addition, MPV at admission was independently associated with the hospital and 6-month mortalities.
Besides, the subgroup analysis was also conducted, and the results demonstrated that the association was stable and consistent between the high level of SII and the poor 30-day mortality across CHF patients with different comorbidities or severity scores. We also noted that, among the critically ill patients with CHF, patients complicated with AMI and hyperlipidemia had a higher risk of 30-day mortality, and this risk was higher for higher SII, which implied that SII may be more valuable for the prognostic evaluation of CHF patients with AMI or hyperlipidemia. Similarly, the previous study has also shown that in patients with coronary heart disease, the high level of SII indicated a high risk of cardiac death in the future, as well as the high incidence of non-fatal stroke and heart failure (9). Although there was no research on the correlation between SII and hyperlipidemia, the interconnection of hyperlipidemia and inflammation involved in the pathogenesis of cardiovascular disease has been recognized (33).
As a retrospective cohort study, the existence of confounding factors cannot be ignored, and it was difficult to intuitively and accurately judge the correlation between SII level and the prognosis of CHF patients. Another highlight of this study was the use of PSM analysis to reduce the imbalance of confounding factors across different groups. After PSM, the most baseline characteristics were well-balanced between these two groups, except for the use of dialysis, the type of first ICU admission, and the peripheral vascular diseases. More importantly, the conclusion that high levels of SII were independently related to high short-term mortality remained stand before and after PSM, which improved the reliability of SII as a prognostic marker of CHF.
But at the same time, there were certain limitations in this study. First, the present study was conducted at a single center and did not validate the prognostic value of SII in a validation cohort. Second, the clinical data were collected retrospectively from databases and it was difficult to ensure that variables were evenly distributed across groups, although multiple regression models were conducted to adjust for confounders and PSM analysis was used to minimize inter-group differences in baseline characteristics. Third, some significant variables may be omissive due to the lack of data. Last, this study was unable to determine the underlying mechanism of the association between high SII and poor prognosis of CHF patients, and further experiments are necessary.
In summary, the high level of SII is closely related to the poor short-term prognosis in critically ill patients with CHF, including 30- and 90-day and hospital all-cause mortalities, as well as the occurrence of MACEs, and is expected to be a simple and effective prognostic evaluation indicator.
Publicly available datasets were analyzed in this study. This data can be found at: https://mimic.mit.edu.
The studies involving human participants were reviewed and approved by the Institutional Review Boards of Massachusetts Institute of Technology (Cambridge, MA, USA) and Beth Israel Deaconess Medical Center (Boston, MA, USA). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
ZY and LZ conceived and designed the study. YT and XZ collected and analyzed the clinical data. YT drafted the manuscript. YF and QC participated in the implementation of statistical methods in this study and put forward constructive suggestions. ZL and HL reviewed the study and participated in the interpretation of the results. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
This study was supported by the National Natural Science Foundation of China (81873416 and 82100071), the Key Research and Development Program of Hunan Province (2020SK2065), and Natural Science Foundation Project of Hunan Province (2020JJ4634).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.753133/full#supplementary-material
CHF, Congestive heart failure; SII, Systemic immune-inflammation index; MIMIC-III, Medical Information Mart for Intensive Care III; ICD-9, The ninth revision of the International Classification of Diseases; ICU, Intensive care unit; MACEs, Major adverse cardiac events; SOFA, The sequential organ failure assessment; SAPSII, The simplified acute physiology score II; SD, Standard deviation; GAM, Generalized additive model; K-M curve, Kaplan-Meier curve; BUN, Blood urea nitrogen; cTnT, Troponin T; NT-proBNP, N-terminal probrain natriuretic peptide; VIF, Variance inflation factor; PSM, Propensity score matching; VHD, Valvular heart disease; AMI, Acute myocardial infarction; TNF-α, Tumor necrosis factor α; IL-6, Interleukin 6; MPV, Mean platelet volume; FDR, False discovery rate.
1. Sun D, Zhang F, Ma T, Zhang Y, Liang Z. Atorvastatin alleviates left ventricular remodeling in isoproterenol-induced chronic heart failure in rats by regulating the RhoA/Rho kinase signaling pathway. Pharmacol Rep. (2020) 72:903–11. doi: 10.1007/s43440-020-00085-3
2. Povsic TJ. Emerging therapies for congestive heart failure. Clin Pharmacol Ther. (2018) 103:77–87. doi: 10.1002/cpt.913
3. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. (2011) 306:1669–78. doi: 10.1001/jama.2011.1474
4. Xu M, Zhang L, Song S, Pan L, Muhammad Arslan I, Chen Y, et al. Hydrogen sulfide: Recent progress and perspectives for the treatment of dermatological diseases. J Adv Res. (2021) 27:11–7. doi: 10.1016/j.jare.2020.02.003
5. Li H, Chen C, Wang DW. Inflammatory cytokines, immune cells, and organ interactions in heart failure. Front Physiol. (2021) 12:695047. doi: 10.3389/fphys.2021.695047
6. Bradham WS, Bozkurt B, Gunasinghe H, Mann D, Spinale FG. Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res. (2002) 53:822–30. doi: 10.1016/S0008-6363(01)00503-X
7. Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. (2015) 116:1254–68. doi: 10.1161/CIRCRESAHA.116.302317
8. Wang BL, Tian L, Gao XH, Ma XL, Wu J, Zhang CY, et al. Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clin Chem Lab Med. (2016) 54:1963–9. doi: 10.1515/cclm-2015-1191
9. Yang YL, Wu CH, Hsu PF, Chen SC, Huang SS, Chan WL, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. (2020) 50:e13230. doi: 10.1111/eci.13230
10. Tosu AR, Kalyoncuoglu M, Biter HI, Cakal S, Selcuk M, Cinar T, et al. Prognostic value of systemic immune-inflammation index for major adverse cardiac events and mortality in severe aortic stenosis patients after TAVI. Medicina. (2021) 57:588. doi: 10.3390/medicina57060588
11. Agus HZ, Kahraman S, Arslan C, Yildirim C, Erturk M, Kalkan AK, et al. Systemic immune-inflammation index predicts mortality in infective endocarditis. J Saudi Heart Assoc. (2020) 32:58–64. doi: 10.37616/2212-5043.1010
12. Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
13. Li W, Huang A, Zhu H, Liu X, Huang X, Huang Y, et al. Gut microbiota-derived trimethylamine N-oxide is associated with poor prognosis in patients with heart failure. Med J Aust. (2020) 213:374–9. doi: 10.5694/mja2.50781
14. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
15. Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. (2014) 348:g14. doi: 10.1136/bmj.g14
16. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. (2017) 3:7–11. doi: 10.15420/cfr.2016:25:2
17. Cho JH, Cho HJ, Lee HY, Ki YJ, Jeon ES, Hwang KK, et al. Neutrophil-lymphocyte ratio in patients with acute heart failure predicts in-hospital and long-term mortality. J Clin Med. (2020) 9:557. doi: 10.3390/jcm9020557
18. Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. (2016) 119:159–76. doi: 10.1161/CIRCRESAHA.116.308030
19. Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. (2004) 90:464–70. doi: 10.1136/hrt.2002.007005
20. Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. (2000) 102:3060–7. doi: 10.1161/01.CIR.102.25.3060
21. Plenz G, Song ZF, Tjan TD, Koenig C, Baba HA, Erren M, et al. Activation of the cardiac interleukin-6 system in advanced heart failure. Eur J Heart Fail. (2001) 3:415–21. doi: 10.1016/S1388-9842(01)00137-4
22. Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. (2019) 21:965–73. doi: 10.1002/ejhf.1482
23. Strassheim D, Dempsey EC, Gerasimovskaya E, Stenmark K, Karoor V. Role of inflammatory cell subtypes in heart failure. J Immunol Res. (2019) 2019:2164017. doi: 10.1155/2019/2164017
24. Bonaventura A, Montecucco F, Dallegri F, Carbone F, Luscher TF, Camici GG, et al. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc Res. (2019) 115:1266–85. doi: 10.1093/cvr/cvz084
25. Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, heart failure. Science. (2013) 339:161–6. doi: 10.1126/science.1230719
26. Arruda-Olson AM, Reeder GS, Bell MR, Weston SA, Roger VL. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes. (2009) 2:656–62. doi: 10.1161/CIRCOUTCOMES.108.831024
27. Uthamalingam S, Patvardhan EA, Subramanian S, Ahmed W, Martin W, Daley M, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. (2011) 107:433–8. doi: 10.1016/j.amjcard.2010.09.039
28. Charach G, Grosskopf I, Roth A, Afek A, Wexler D, Sheps D, et al. Usefulness of total lymphocyte count as predictor of outcome in patients with chronic heart failure. Am J Cardiol. (2011) 107:1353–6. doi: 10.1016/j.amjcard.2010.12.049
29. Vaduganathan M, Ambrosy AP, Greene SJ, Mentz RJ, Subacius HP, Maggioni AP, et al. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail. (2012) 5:750–8. doi: 10.1161/CIRCHEARTFAILURE.112.970525
30. Ye GL, Chen Q, Chen X, Liu YY, Yin TT, Meng QH, et al. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: a cohort study. Sci Rep. (2019) 9:10639. doi: 10.1038/s41598-019-47143-2
31. Glezeva N, Gilmer JF, Watson CJ, Ledwidge M. A central role for monocyte-platelet interactions in heart failure. J Cardiovasc Pharmacol Ther. (2016) 21:245–61. doi: 10.1177/1074248415609436
32. Kandis H, Ozhan H, Ordu S, Erden I, Caglar O, Basar C, et al. The prognostic value of mean platelet volume in decompensated heart failure. Emerg Med J. (2011) 28:575–8. doi: 10.1136/emj.2009.088401
Keywords: congestive heart failure, systemic immune-inflammation index, mortality, biomarker, MIMIC-III database
Citation: Tang Y, Zeng X, Feng Y, Chen Q, Liu Z, Luo H, Zha L and Yu Z (2021) Association of Systemic Immune-Inflammation Index With Short-Term Mortality of Congestive Heart Failure: A Retrospective Cohort Study. Front. Cardiovasc. Med. 8:753133. doi: 10.3389/fcvm.2021.753133
Received: 04 August 2021; Accepted: 26 October 2021;
Published: 12 November 2021.
Edited by:
Chen Liu, The First Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Jianqing She, The First Affiliated Hospital of Xi'an Jiaotong University, ChinaCopyright © 2021 Tang, Zeng, Feng, Chen, Liu, Luo, Zha and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihuang Zha, emhhbGlodWFuZ0Bjc3UuZWR1LmNu; Zaixin Yu, eXV6YWl4aW5AMTI2LmNvbQ==
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.