- 1Department of Geriatrics, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
- 2Department of Cardiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
Objective: To identify feature immune-related genes (IRGs) in patients with hypertrophic cardiomyopathy (HCM) and verify their ability to diagnose HCM.
Methods: The GSE160997 dataset on cardiac tissue from 18 HCM patients and 5 controls was downloaded from the Gene Expression Omnibus database. A false discovery rate <0.05 and |log2 fold change| >1 were the filters applied to identify the differentially expressed genes (DEGs). The differentially expressed IRGs were the intersection results between the DEGs and an IRG dataset from the IMMPORT database. The protein-protein interaction network of differentially expressed IRGs was constructed, and the top 20 hub genes with the most adjacent nodes in the network were selected. The least absolute shrinkage and selection operator regression algorithm and a random forest algorithm were used to identify the feature IRGs as biomarkers that were then verified against GSE36961.
Results: A total of 1079 DEGs were identified in GSE160997. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses indicated that immune-related mechanisms play an important role in the pathogenesis of HCM. A total of 121 differentially expressed IRGs were identified, and 5 feature IRGs were selected, 4 of which were confirmed as potential biomarkers of HCM by external verification with excellent discrimination ability. A diagnosis model of HCM based on the four feature IRGs was developed and visualized as a nomogram with a C-index of 0.925 (95% confidence interval 0.869–0.981).
Conclusion: Our study identified four feature IRGs as biomarkers for the diagnosis of HCM, offering an innovative perspective of the underlying immune-related pathological molecular mechanisms.
Introduction
Hypertrophic cardiomyopathy (HCM) is a hereditary heart disease that is defined as unexplained, isolated, progressive myocardial hypertrophy, usually accompanied by heart failure or arrhythmia. It is considered the primary underlying condition in sudden cardiac death in young adults and athletes. Mutations in the genes encoding proteins of the cardiomyocytic contractile apparatus are considered the main cause of HCM. However, the relationship between sarcomere mutations and clinical outcomes remains unpredictable in patients with HCM, largely because of genetic and phenotypic heterogeneity (1, 2). During the past two decades, the continuous improvement of gene sequencing technology has enabled the diagnosis of HCM among populations with a family history and in asymptomatic patients through genetic testing to avoid sudden death. This has become a class I recommendation for supplementary testing according to the European Society of Cardiology and American Heart Association/American College of Cardiology guidelines (3, 4).
Sarcomere protein gene (such as MYBPC3, MYH7, TNNT2, TNNI3, TPM1, MYL2, MYL3, and ACTC) mutations have become targets in the genetic testing of HCM. Unfortunately, in the genetic testing of different HCM cohorts, up to 50–68% of patients were negative for sarcomere protein gene mutations (5). The signaling pathways and regulatory networks underlying the pathogenesis of HCM remain unclear to date. Thus, a systematic analysis of its transcriptional map might provide useful information about the overall underlying molecular mechanisms and pathological processes of HCM and aid in the diagnosis and treatment of HCM.
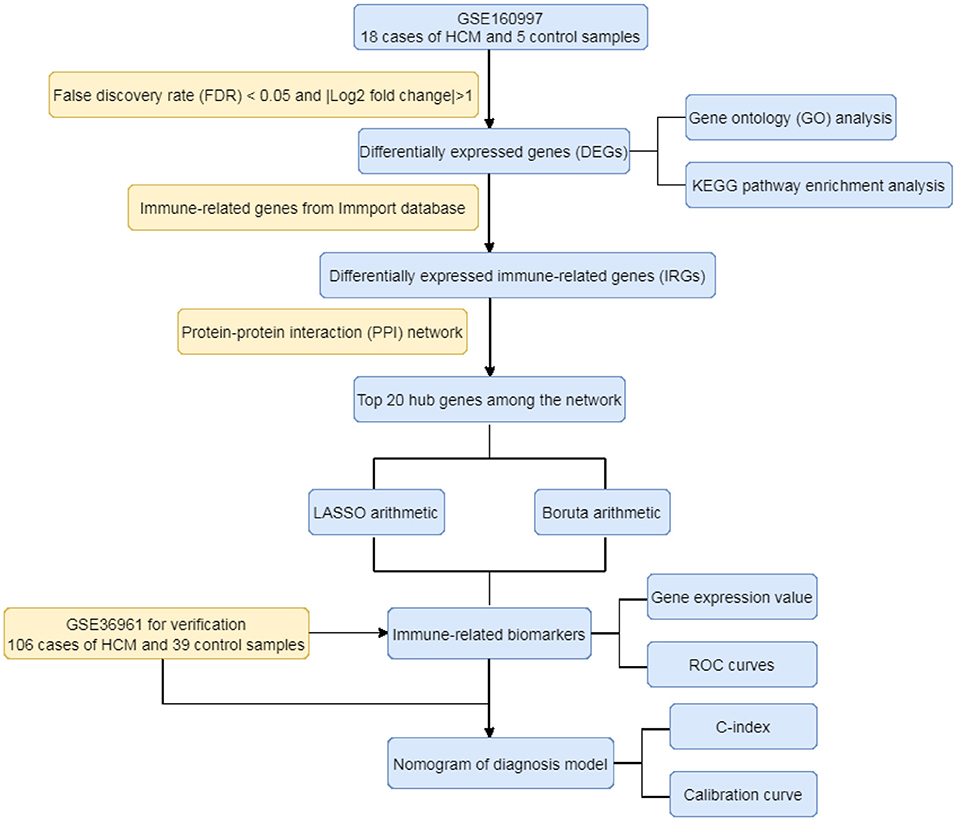
As the flowchart showed in Figure 1, this study aimed to explore the high-throughput sequencing gene expression data of HCM patients and normal samples, construct the differential gene expression profile of HCM, annotate the gene ontology and potential signal pathways, and identify valuable biomarkers that may further the gene test diagnosis of HCM.
Materials and Methods
Data Source and Differentially Expressed Gene Analyses
There are five comparatively valuable datasets that contain more than 10 samples of HCM in GEO database, namely GSE160997 (6), GSE143786 (7), GSE148602 (8), GSE130036 (9), and GSE36961. Among these five datasets, GSE160997, GSE130036, and GSE36961 focus on transcriptome information and a previous study of our team had explored GSE130036 (10). The high-throughput sequencing dataset GSE160997 used in this study contained data regarding myocardial tissue of 18 HCM patients and 5 healthy controls. All data were statistically analyzed and visualized using R software (version 4.0.3) and its relevant packages. The original data of all samples were normalized and differentially expressed genes (DEGs) were identified using the following filters: a false discovery rate [FDR (11)] <0.05 and |Log2 fold change| >1 analyzed with the limma package (12). The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses of DEGs were further explored by the “ClusterProfiler” (13) and “org.Hs.eg.db” (14) packages. The results were visualized with the “ggplot2” (15) package.
Immune-Related Gene Analyses
A dataset with 1,793 unique immune-related genes (IRGs) that had been confirmed in previous studies as being involved in the natural immune process was downloaded from the IMMPORT database [(16); https://www.immport.org/shared/]. The differentially expressed IRGs were the intersection results between the DEGs and the 1793 IRGs. The protein-protein interaction (PPI) network of differentially expressed IRGs was constructed using the STRING database [(17); https://string-db.org/, Version:11.0], with the highest confidence level defined as an interaction score of more than 0.9. Subsequently, the top 20 hub genes with the most adjacent nodes in the PPI network were selected.
Feature IRGs Identified and Correlation Analysis
The least absolute shrinkage and selection operator (LASSO) regression arithmetic and Boruta arithmetic (18) were used independently to identify the feature IRGs from the 20 hub genes. The former is a regression analysis algorithm that uses regularization to improve prediction accuracy (19). The latter is a random forest method that can provide a numerical estimate of feature importance (20). In addition, the R “corrplot” package (21) was used to analyze a potential correlation among the selected feature IRGs.
Feature IRGs Verification and Diagnosis Model Constructed
The selected feature IRGs were further verified using the independent dataset GSE36961 with 106 HCM patients and 39 healthy controls. The gene expression values were displayed by boxplots, and receiver-operating characteristic (ROC) curves were used to assess the discrimination ability of the feature IRGs. A five-fold cross-validation was conducted 500 times on the GSE36961 cohort to conform the reliability of the area under the ROC curves (AUC) of the selected biomarkers. Moreover, an online webtool NetworkAnalyst (22) was used to further explore the potential miRNA—feature IRG regulatory network and transcription factor (TF)—feature IRG regulation mechanisms. Finally, a diagnosis model combining the selected biomarkers was developed by logistic regression and visualized as a nomogram (23). The C-statistic and calibration curves were used to evaluate the identification ability and calibration of the model.
Results
DEGs Between HCM and Normal Samples
After the normalization of GSE160997 dataset (Figures 2A,B), a total of 1,079 DEGs were identified in the GSE160997 dataset, and filtering resulted in 618 significantly upregulated and 461 significantly downregulated DEGs (Figure 2C). The top 30 DEGs were displayed in a heatmap (Figure 2D). The GO annotation and KEGG pathway enrichment analyses of DEGs are illustrated in Figures 2E,F. It became obvious that inflammation and immune-related signaling pathways and biological regulatory functions played a dominant role in the enrichment results of the DEGs, which prompted us to further explore the IRGs among the DEGs.
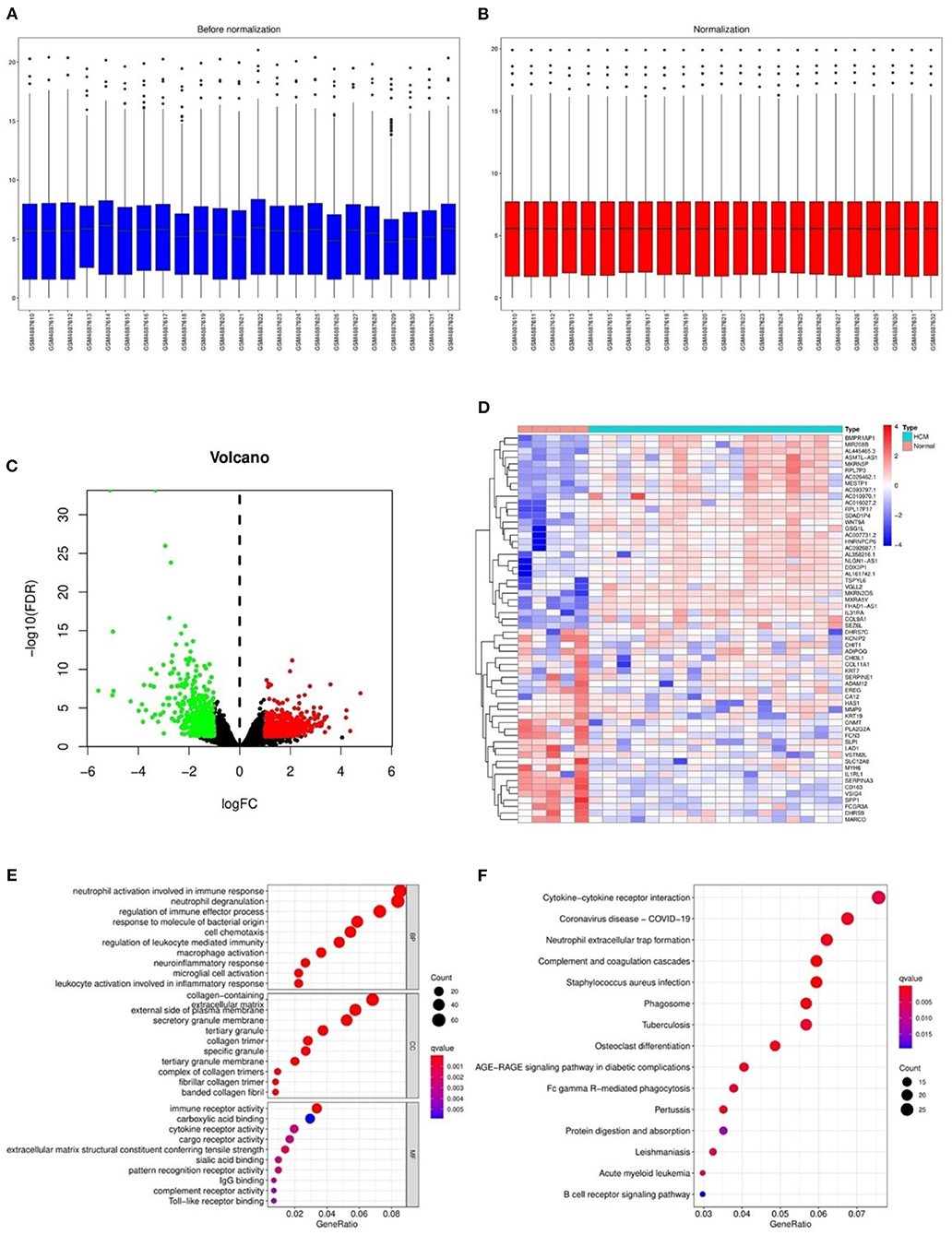
Figure 2. The differentially expressed genes in GSE160997 and the Gene Ontology annotation and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis. (A) The boxplot of GSE160997 before normalization. (B) The boxplot of GSE160997 after normalization. (C) The Volcano plot of 1,079 DEGs, with 618 significantly upregulated (red) and 461 significantly downregulated (green). (D) The heatmap of top 30 DEGs. (E) The gene ontology (GO) annotation of DEGs. (F) The Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of DEGs.
The Information of Immune-Related Genes
A total of 121 differentially expressed IRGs between the DEGs and an IRG dataset from the IMMPORT database were identified (Figure 3A). These 121 differentially expressed IRGs formed a close PPI network interaction at a confidence score >0.9 (Figure 3B). The top 20 hub genes with the most adjacent nodes in the PPI network are listed in Figure 3C, and the detailed information is listed in Table 1.
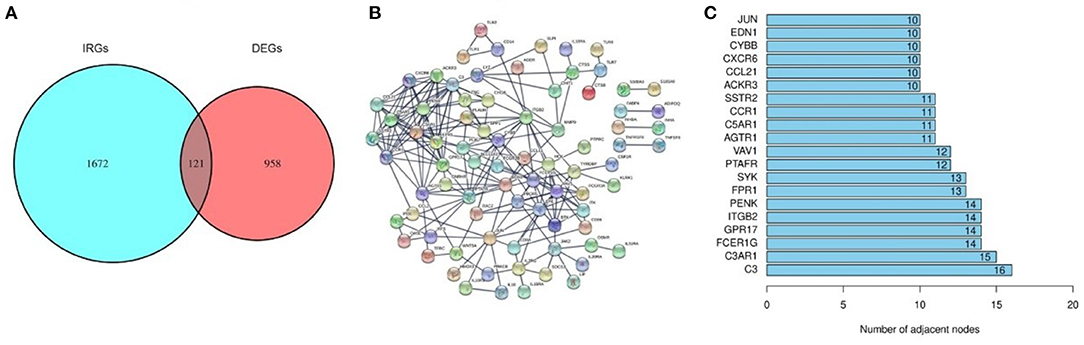
Figure 3. The differentially expressed Immune-related genes and PPI network. (A) The intersection result of DEGs and IRGs. (B) The protein-protein interaction (PPI) network of 121 differentially expressed IRGs. (C) The top 20 genes with most adjacent nodes among the PPI network.
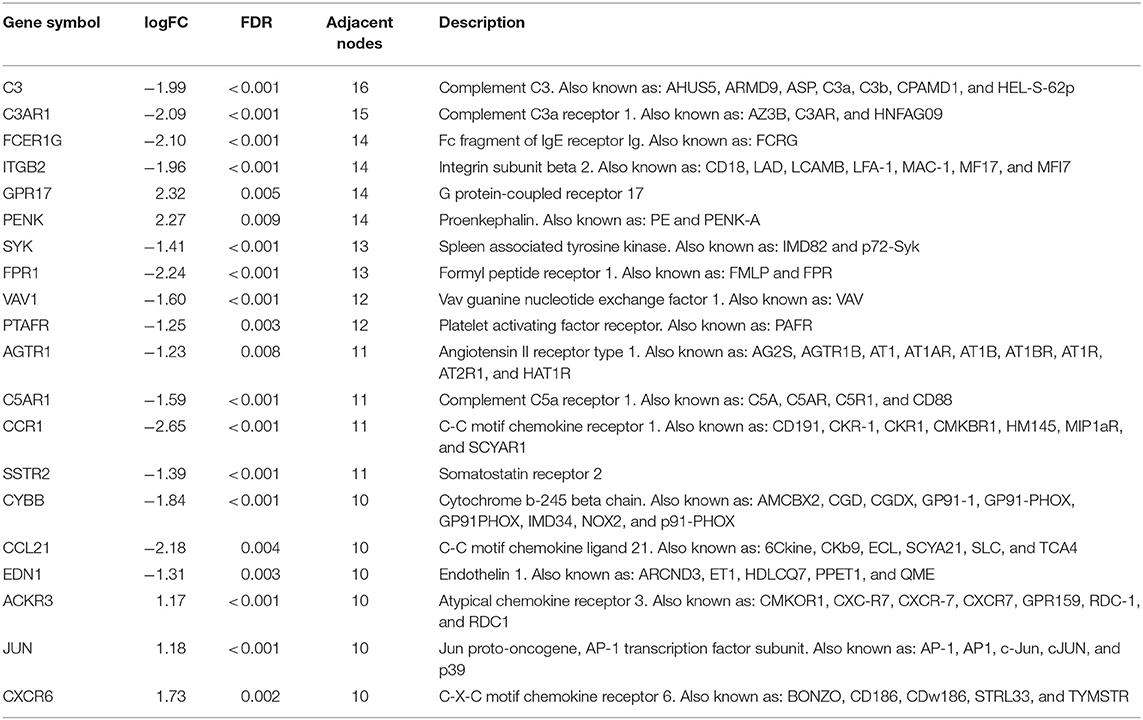
Table 1. The detail information of the top-20 immune-related genes with most adjacent nodes in protein-protein interaction network.
Feature IRGs Identified and Correlation Analysis
The LASSO arithmetic selected 6 feature IRGs (Figures 4A,B) and the Boruta arithmetic 14 feature IRGs (Figure 4C). Five feature IRGs (ACKR3, AGTR1, CCR1, CYBB, and JUN) were identified by both methods (Figure 4D). The correlation analysis result displaying the Pearson correlation values indicated that there was a strong positive correlation between CYBB and CCR1 and a moderate positive correlation between ACKR3 and JUN (Figure 4E).
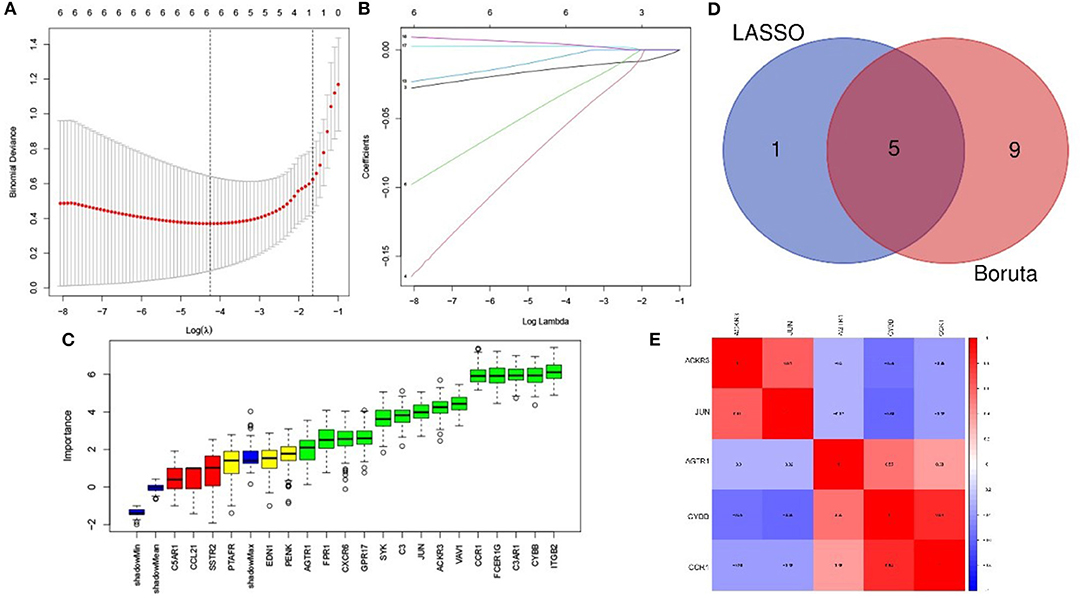
Figure 4. Feature immune-related genes identified and correlation analysis. (A) The optimal lambda selection by LASSO. (B) The coefficient profiles of the six hub genes. (C) Feature IRGs selected by Boruta arithmetic. Genes in green are regarded as important feature IRGs while neutral in yellow and are rejected in red according to the score of feature importance. (D) The intersection result of LASSO and Boruta arithmetic. (E) The correlation analysis of the feature IRGs.
Feature IRG Verification and Diagnosis Model Constructed
The gene expression values of the five selected IRGs in GSE36961 are illustrated in Figure 5, and the original data are presented in the Supplementary File. It is worth noting that JUN was highly expressed in the normal controls in GSE36961, which was contrary to the previous result. The gene expressions of the remaining four IRGs (ACKR3, AGTR1, CCR1, and CYBB) were consistent with the previous analysis and were statistically significant, suggesting them as potential diagnostic biomarkers of HCM. The AUC of the four biomarkers were all remarkable, indicating their ability to contribute to the diagnosis of HCM (Figure 6). The results of 500 times five-fold cross-validation show that the average AUC scores of CYBB, CCR1, AGTR1 and ACKR3 were 0.883, 0.856, 0.842, and 0.865 respectively.
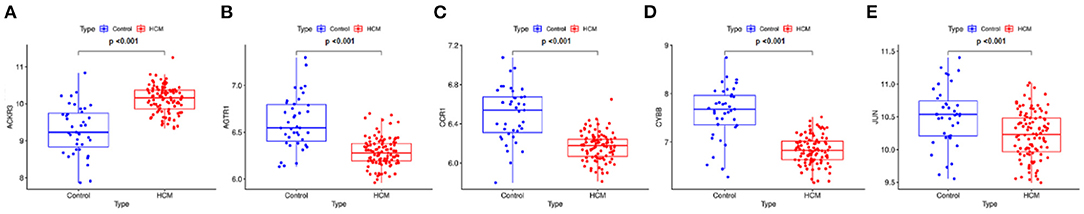
Figure 5. Gene expression of the five-feature immune-related genes. The gene expression value of the five feature IRGs. (A) Boxplot of ACKR3 in GSE36961. (B) Boxplot of AGTR1 in GSE36961. (C) Boxplot of CCR1 in GSE36961. (D) Boxplot of CYBB in GSE36961. (E) Boxplot of JUN in GSE36961.
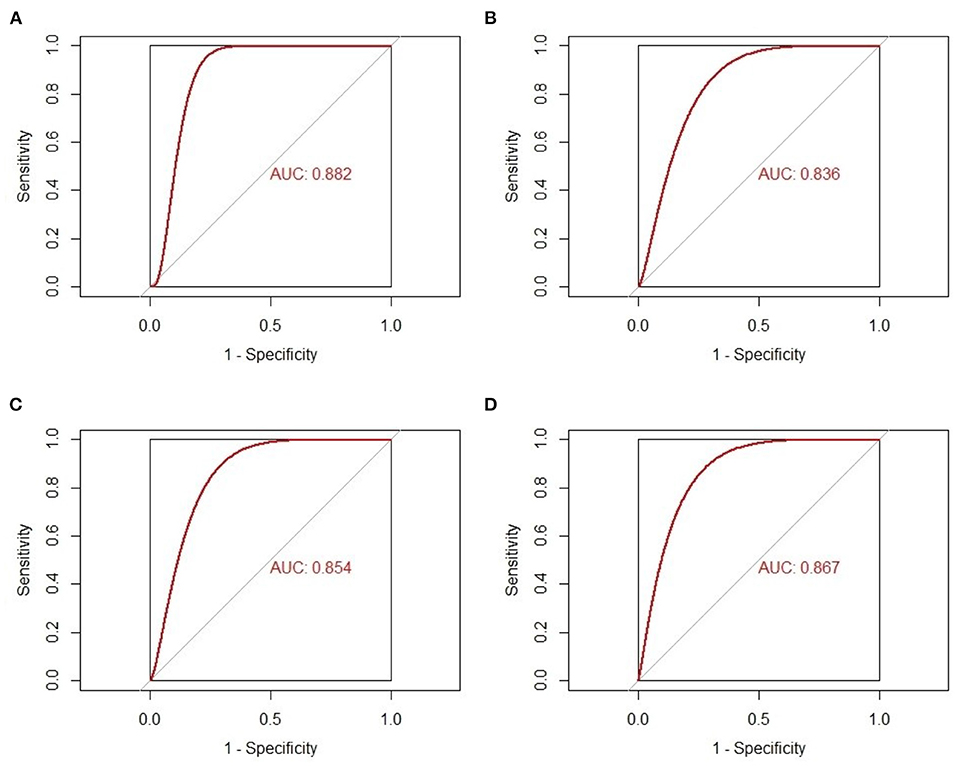
Figure 6. The receiver-operating characteristic curves of the four-feature immune-related genes as biomarkers of hypertrophic cardiomyopathy. (A) AUC of CYBB in GSE36961. (B) AUC of AGTR1 in GSE36961. (C) AUC of CCR1 in GSE36961. (D) AUC of ACKR3 in GSE36961.
The miRNA—feature IRG regulatory network and transcription factor (TF)—feature IRG regulatory network were listed as Figure 7. The four IRGs were transformed into binary variables according to the median value of gene expression, based on which a diagnosis model was constructed by logistic regression that was visualized as a nomogram (Figure 8A). The C-index of the diagnosis model was 0.925 (95% confidence interval 0.869–0.981) with appropriate calibration (Figure 8B).
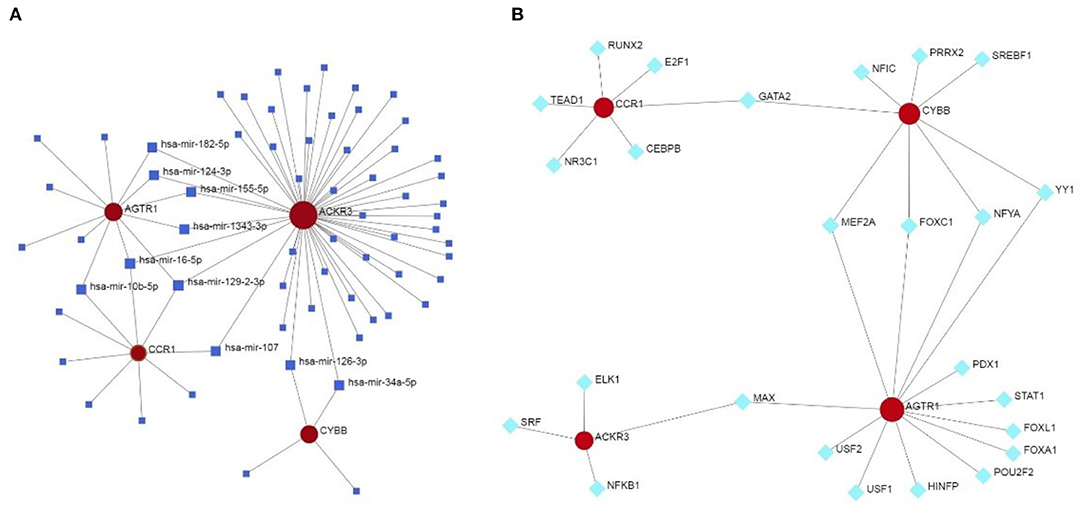
Figure 7. The miRNA—feature IRG regulatory network and transcription factor (TF)—feature IRG regulatory network. (A) The miRNA—feature IRG regulatory network. (B) The transcription factor (TF)—feature IRG regulatory network.
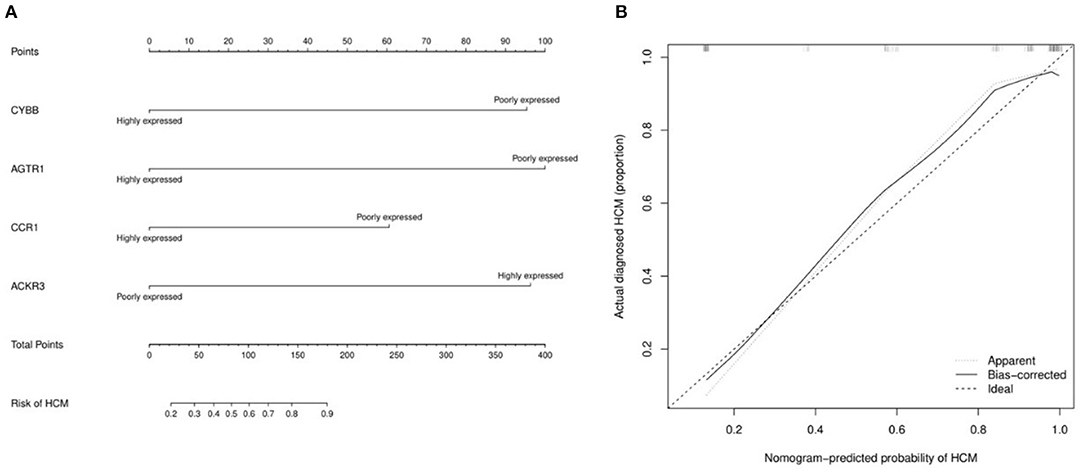
Figure 8. The nomogram and calibration curve of the diagnosis model for hypertrophic cardiomyopathy based on four feature immune-related genes. (A) Nomogram for the diagnosis model of HCM. The observed value of each feature gene was assigned a certain number of points by drawing a vertical line toward the top points scale. The sum of the points for each gene corresponded to the individual risk of HCM. (B) Calibration curve for the diagnosis model of HCM.
Discussion
HCM is a common inherited cardiovascular disease that occurs in one of 500 people in the general population and leads to heart failure, fatal arrhythmia, and stroke. Mutations in genes encoding proteins of the cardiomyocytic contractile apparatus are considered the main cause of HCM. Genetic tests focusing on sarcomere protein genes have been recommended in current guidelines and experience widespread adoption. However, with the accumulation of experimental data (24–26), approximately two-thirds of HCM patients were shown not to have sarcomere protein mutations. Therefore, it is necessary to explore alternative molecular mechanisms and identify more valuable biomarkers of HCM by utilizing contemporary methods to analyze biological complexity with an emphasis on network medicine (27).
According to bioinformatics analysis, the GO annotation and KEGG enrichment analysis of the DEGs in GSE160997 were dominated by immune and inflammatory response mechanisms, such as neutrophil and macrophage activation, neutrophil degranulation, regulation of leukocyte-mediated immunity, and immune effector processes. Currently, inflammation and immunity attract substantial interest with regard to their role in the pathophysiology of cardiovascular diseases. Some previous studies (28–31) suggested that immune and inflammatory response mechanisms may play an important role in myocardial hypertrophy and remodeling. In contrast, most research has focused on heart failure and myocardial remodeling after acute myocardial infarction, with extremely limited evidence on HCM.
The preliminary results of bioinformatics analysis enabled further exploration of IRGs as biomarkers of HCM. A dataset with 1,793 unique IRGs was downloaded from the IMMPORT database and used to identify the IRGs among the DEGs in GSE160997. A total of 121 IGRs were selected from the 1,079 DEGs, and 5 feature IRGs were identified as candidate biomarkers by both the LASSO and Boruta arithmetic. Finally, four feature IRGs were confirmed as biomarkers with an excellent AUC score using the GSE36961 dataset of HCM patients and 39 healthy controls. Our novel diagnosis model of HCM constructed based on these four feature IRGs showed high discrimination ability (C-index = 0.925) and favorable calibration. This pioneering research offers a new perspective on the identification of potential biomarkers that might aid in the diagnosis of HCM.
Angiotensin II receptor type 1 (AGTR1) is a significant effector of the renin-angiotensin-aldosterone system, which is associated with cardiovascular diseases, especially cardiac remodeling. The system consists of two regulatory pathways: a pathological pro-inflammatory pathway and a protective anti-inflammatory pathway. AGTR1, involved in the former, can promote vasoconstriction, cell proliferation, inflammation, oxidative stress, hypertrophy, and fibrosis, and angiotensin II–type 1 receptor blockers are widely used to prevent cardiac remodeling after acute myocardial infarction and heart failure (32, 33).
Atypical chemokine receptor 3 (ACKR3) (34), previously known as C-X-C chemokine receptor type 7 (CXCR7), has emerged as a potential hub gene in cardiac development in animal experiments (35–37). Nevertheless, animal studies have focused on the effects of ACKR3 knockout on cardiac development. In this study, the expression of ACKR3 in HCM patients was significantly higher than that in the normal controls in both GSE160997 and GSE36961. At present, no study has explored the effect of a high expression of ACKR3 on cardiac development or structural remodeling, which may be a promising area of research going forward.
CCR1 gene encodes a protein that acts as a chemokine receptor. This protein and its ligands macrophage inflammatory protein 1 (MIP-1, CCL3) and regulated on activation normal T expressed and secreted protein (RANTES, CCL5) can be expressed in monocytes/macrophages/T lymphocytes, whose signaling is the key to recruit effector immune cells to inflammatory sites. Liehn et al. proved that in model mice with myocardial infarction, the deletion of CCR1 can reduce tissue inflammatory injury and protect the myocardium (38). Data from animal and human studies show that CCR1 and its ligands are central mediators of a variety of inflammatory diseases, making the receptor an attractive candidate for therapeutic intervention. At present, CCR1 receptor antagonists are mainly used in clinical trials for the treatment of rheumatoid arthritis (39). Batis et al. selectively blocks the CCR1 receptor through met RANTES therapy, confirming that a treatment strategy based on regulating CCR1/CCR5 mediated cell migration and/or effector function may help limit cardiac tissue damage during chronic Chagas disease (40).
The protein encoded by cytochrome b-245 beta chain (CYBB) gene, also named NOX-2, is a component of cytochrome b-245, which plays an important role in the development of phagocytic microbicidal oxidase system. A consensus has been reached that the deficiency of CYBB leads to decreased NADPH oxidase activity in phagocytes, and neutrophils phagocytize bacteria but do not effectively kill bacteria, and that this might be the potential pathogenesis of chronic granulomatous disease (41). In addition, the gene has also been reported to be associated with ventricular hypertrophy and arrhythmia caused by hypoxia and high-fat diet (42–44).
The strengths of this study are (a) the innovative perspective that immune and inflammation may underlie the molecular mechanism of HCM and (b) the identification of IRGs as diagnostic biomarkers using bioinformatics analysis of transcriptome datasets of HCM patients and healthy controls. Nonetheless, some limitations of this study need to be recognized. First, the study was a retrospective data analysis and lacked detailed clinical or prognostic information, such as the thickness of the ventricular septum and the occurrence of major adverse cardiovascular events in patients with HCM. This limited the further exploration of the relevant genes determining these clinical features or outcomes. Second, although the four feature biomarkers were verified by an independent dataset of sufficient size and achieved excellent discrimination, a larger dataset is essential for verification in the future. Third, the different transcriptome datasets were detected by different sequencing platforms. The baselines of the different transcriptome datasets were inconsistent, indicating that they may only determine whether the expression of characteristic genes is abnormal without the ability to identify a generally applicable reference range.
Conclusion
The systematic analysis of the transcriptional profiles of HCM and exploration of the potential underlying molecular mechanisms led to the identification of four feature IRGs as biomarkers of HCM. Based on these, a diagnosis model of HCM was developed that brings a novel aspect to the current understanding of the pathological processes and required genetic testing in HCM.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
XZ and GL were involved in the conception and design of the study. XZ was responsible for visualization and article writing. RH provided scientific supervision. All authors reviewed and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the Gene Expression Omnibus (GEO) database for the precious data used for free in scientific research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.752559/full#supplementary-material
Abbreviations
HCM, hypertrophic cardiomyopathy; GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; DEGs, differentially expressed genes; IRG, immune-related gene; PPI, protein-protein interaction; LASSO, least absolute shrinkage and selection operator; ROC curve, receiver operator characteristics curve.
References
1. Wolf CM. Hypertrophic cardiomyopathy: genetics and clinical perspectives. Cardiovasc Diagnosis Ther. (2019) 9:S388–415. doi: 10.21037/cdt.2019.02.01
2. Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. (2013) 381:242–55 doi: 10.1016/S0140-6736(12)60397-3
3. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2020) 76:3022–55
4. UK COM. 2014 esc guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J. (2014) 35:2733–79. doi: 10.1093/eurheartj/ehu284
5. Ko C, Arscott P, Concannon M, Saberi S, Day SM, Yashar BM, et al. Genetic testing impacts the utility of prospective familial screening in hypertrophic cardiomyopathy through identification of a nonfamilial subgroup. Genet Med. (2018) 20:69–75. doi: 10.1038/gim.2017.79
6. Maron BA, Wang R-S, Shevtsov S, Drakos SG, Arons E, Wever-Pinzon O, et al. Individualized interactomes for network-based precision medicine in hypertrophic cardiomyopathy with implications for other clinical pathophenotypes. Nat Commun. (2021) 12:873. doi: 10.1038/s41467-021-21146-y
7. Guo Q, Wang J, Sun R, Gu W, He Z, Chen Q, et al. Identification of circulating hub long noncoding RNAs associated with hypertrophic cardiomyopathy using weighted correlation network analysis. Mol Med Rep. (2020) 22:4637–44. doi: 10.3892/mmr.2020.11566
8. Guo Q, Wang J, Sun R, He Z, Chen Q, Liu W, et al. Comprehensive construction of a circular RNA-associated competing endogenous RNA network identified novel circular RNAs in hypertrophic cardiomyopathy by integrated analysis. Front Genet. (2020) 11:764. doi: 10.3389/fgene.2020.00764
9. Liu X, Ma Y, Yin K, Li W, Chen W, Zhang Y, et al. Long non-coding and coding RNA profiling using strand-specific RNA-seq in human hypertrophic cardiomyopathy. Sci Data. (2019) 6:90. doi: 10.1038/s41597-019-0094-6
10. Zheng X, Yang Y, Fu CH, Huang R. Identification and verification of promising diagnostic biomarkers in patients with hypertrophic cardiomyopathy associate with immune cell infiltration characteristics. Life Sci. (2021) 285:119956. doi: 10.1016/j.lfs.2021.119956
11. Korthauer K, Kimes PK, Duvallet C, Reyes A, Subramanian A, Teng M, et al. A practical guide to methods controlling false discoveries in computational biology. Genome Biol. (2019) 20:118. doi: 10.1186/s13059-019-1716-1
12. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. (2015) 43:e47. doi: 10.1093/nar/gkv007
13. Yu G, Wang L-G, Han Y, He Q-Y. Clusterprofiler: an r package for comparing biological themes among gene clusters. Omics. (2012) 16:284–7. doi: 10.1089/omi.2011.0118
14. Carlson M, Falcon S, Pages H, Li N. org.Hs.eg.db: Genome Wide Annotation for Human. R package version (2019). p. 3.
15. Ginestet C. Ggplot2: elegant graphics for data analysis. J R Statist Soc Ser A. (2011) 174:245. doi: 10.1111/j.1467-985X.2010.00676_9.x
16. Bhattacharya S, Andorf S, Gomes L, Dunn P, Schaefer H, Pontius J, et al. ImmPort: disseminating data to the public for the future of immunology. Immunol Res. (2014) 58:234–9. doi: 10.1007/s12026-014-8516-1
17. Mering CV, Huynen M, Jaeggi D, Schmidt S, Bork P, Snel B. String: a database of predicted functional associations between proteins. Nucleic Acids Res. (2003) 31:258–61. doi: 10.1093/nar/gkg034
18. Kursa MB, Rudnicki WR. Feature selection with the Boruta package. J Stat Softw. (2010) 36:1–13. doi: 10.18637/jss.v036.i11
19. Zhao E, Xie H, Zhang Y. Predicting diagnostic gene biomarkers associated with immune infiltration in patients with acute myocardial infarction. Front Cardiovasc Med. (2020) 7:586871. doi: 10.3389/fcvm.2020.586871
20. Zheng X, Huang R, Liu G, Jia Z, Chen K, He Y. Development and verification of a predictive nomogram to evaluate the risk of complicating ventricular tachyarrhythmia after acute myocardial infarction during hospitalization: a retrospective analysis. Am J Emerg Med. (2020) 46:462–8. doi: 10.1016/j.ajem.2020.10.052
21. Wei T, Simko V, Levy M, Xie Y, Jin Y, Zemla J. Corrplot: Visualization of a Correlation Matrix. R package version 0.73. (2013). p. 230.
22. Zhou G, Soufan O, Ewald J, Hancock RE, Basu N, Xia J. Networkanalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. (2019) 47:W234–41. doi: 10.1093/nar/gkz240
23. Deng M, Yin Y, Zhang Q, Zhou X, Hou G. Identification of inflammation-related biomarker Lp-PLA2for patients with COPD by comprehensive analysis. Front Immunol. (2021) 12:670971. doi: 10.3389/fimmu.2021.670971
24. Neubauer S, Kolm P, Ho CY, Kwong RY, Desai MY, Dolman SF, et al. Distinct subgroups in hypertrophic cardiomyopathy in the NHLBI HCM registry. J Am Coll Cardiol. (2019) 74:2333–45. doi: 10.1016/j.jacc.2019.08.1057
25. Bos JM, Will ML, Gersh BJ, Kruisselbrink TM, Ommen SR, Ackerman MJ. Characterization of a phenotype-based genetic test prediction score for unrelated patients with hypertrophic cardiomyopathy. Mayo Clinic Proc. (2014) 89:727–37. doi: 10.1016/j.mayocp.2014.01.025
26. Gruner C, Ivanov J, Care M, Williams L, Moravsky G, Yang H, et al. Toronto hypertrophic cardiomyopathy genotype score for prediction of a positive genotype in hypertrophic cardiomyopathy. Circ Cardiovasc Genet. (2013) 6:19–26. doi: 10.1161/CIRCGENETICS.112.963363
27. Maron BJ, Maron MS, Maron BA, Loscalzo J. Moving beyond the sarcomere to explain heterogeneity in hypertrophic cardiomyopathy: JACC review topic of the week. J Am Coll Cardiol. (2019) 73:1978–86. doi: 10.1016/j.jacc.2019.01.061
28. Shimada YJ, Raita Y, Liang LW, Maurer MS, Hasegawa K, Fifer MA, et al. Comprehensive proteomics profiling reveals circulating biomarkers of hypertrophic cardiomyopathy. Circ Heart Fail. (2021) 14:e007849. doi: 10.1161/CIRCHEARTFAILURE.120.007849
29. Chen R, Ge T, Jiang W, Huo J, Chang Q, Geng J, et al. Identification of biomarkers correlated with hypertrophic cardiomyopathy with co-expression analysis. J Cell Physiol. (2019) 234:21999–2008. doi: 10.1002/jcp.28762
30. Becker RC, Owens AP, Sadayappan S. Tissue-level inflammation and ventricular remodeling in hypertrophic cardiomyopathy. J Thromb Thrombolysis. (2020) 49:177–83. doi: 10.1007/s11239-019-02026-1
31. Kuusisto J, Kärjä V, Sipola P, Kholová I, Peuhkurinen K, Jääskeläinen P, et al. Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart. (2012) 98:1007–13. doi: 10.1136/heartjnl-2011-300960
32. Saravi B, Li Z, Lang CN, Schmid B, Lang FK, Grad S, et al. The tissue renin-angiotensin system and its role in the pathogenesis of major human diseases: quo vadis? Cells. (2021) 10:650. doi: 10.3390/cells10030650
33. De Mello WC. Local renin angiotensin aldosterone systems and cardiovascular diseases. Med Clinics. (2017) 101:117–27. doi: 10.1016/j.mcna.2016.08.017
34. Koenen J, Bachelerie F, Balabanian K, Schlecht-Louf G, Gallego C. Atypical chemokine receptor 3 (ackr3): a comprehensive overview of its expression and potential roles in the immune system. Mol Pharmacol. (2019) 96:809–18. doi: 10.1124/mol.118.115329
35. Yu S, Crawford D, Tsuchihashi T, Behrens TW, Srivastava D. The chemokine receptor cxcr7 functions to regulate cardiac valve remodeling. Dev Dyn. (2011) 240:384–93. doi: 10.1002/dvdy.22549
36. Gerrits H, van Ingen Schenau DS, Bakker NE, van Disseldorp AJ, Strik A, Hermens LS, et al. Early postnatal lethality and cardiovascular defects in cxcr7-deficient mice. Genesis. (2008) 46:235–45. doi: 10.1002/dvg.20387
37. Klein KR, Karpinich NO, Espenschied ST, Willcockson HH, Dunworth WP, Hoopes SL, et al. Decoy receptor cxcr7 modulates adrenomedullin-mediated cardiac and lymphatic vascular development. Dev Cell. (2014) 30:528–40. doi: 10.1016/j.devcel.2014.07.012
38. Liehn E, Merx M, Postea O, Becher S, Djalali-Talab Y, Shagdarsuren E, et al. Ccr1 deficiency reduces inflammatory remodelling and preserves left ventricular function after myocardial infarction. J Cell Mol Med. (2008) 12:496–506. doi: 10.1111/j.1582-4934.2007.00194.x
39. White GE, Iqbal AJ, Greaves DR. Cc chemokine receptors and chronic inflammation—therapeutic opportunities and pharmacological challenges. Pharmacol Rev. (2013) 65:47–89. doi: 10.1124/pr.111.005074
40. Batista AM, Alvarado-Arnez LE, Alves SM, Melo G, Pereira IR, Ruivo LADS, et al. Genetic polymorphism at CCL5 is associated with protection in Chagas' heart disease: antagonistic participation of CCR1+ and CCR5+ cells in chronic chagasic cardiomyopathy. Front Immunol. (2018) 9:615. doi: 10.3389/fimmu.2018.00615
41. Anjani G, Vignesh P, Joshi V, Shandilya JK, Bhattarai D, Sharma J, et al. Recent advances in chronic granulomatous disease. Genes Dis. (2020) 7:84–92. doi: 10.1016/j.gendis.2019.07.010
42. Pena E, Siques P, Brito J, Arribas SM, Böger R, Hannemann J, et al. Nox2 upregulation and p38α MAPK activation in right ventricular hypertrophy of rats exposed to long-term chronic intermittent hypobaric hypoxia. Int J Mol Sci. (2020) 21:8576. doi: 10.3390/ijms21228576
43. Bhatti SN, Li J-M. Nox2 dependent redox-regulation of AKT and ERK1/2 to promote left ventricular hypertrophy in dietary obesity of mice. Biochem Biophys Res Commun. (2020) 528:506–13. doi: 10.1016/j.bbrc.2020.05.162
Keywords: biomarkers, hypertrophic cardiomyopathy, immune-related gene, diagnosis model, random forest—ensemble classifier, LASSO
Citation: Zheng X, Liu G and Huang R (2021) Identification and Verification of Feature Immune-Related Genes in Patients With Hypertrophic Cardiomyopathy Based on Bioinformatics Analyses. Front. Cardiovasc. Med. 8:752559. doi: 10.3389/fcvm.2021.752559
Received: 03 August 2021; Accepted: 28 September 2021;
Published: 26 October 2021.
Edited by:
Alexandre Francois Roy Stewart, University of Ottawa, CanadaReviewed by:
Basavaraj Mallikarjunayya Vastrad, Soniya Education Trust's College of Pharmacy, IndiaThiago Britto-Borges, Heidelberg University Hospital, Germany
Copyright © 2021 Zheng, Liu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruina Huang, wolidedd@gdmu.edu.cn
 Xifeng Zheng
Xifeng Zheng Guangyan Liu1
Guangyan Liu1