- 1Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 2National Clinical Research Center for Cardiovascular Diseases, Capital Medical University, Beijing, China
- 3Beijing Advanced Innovation Center for Big Data-Based Precision Medicine for Cardiovascular Diseases, Capital Medical University, Beijing, China
- 4Department of Radiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 5Cardiovascular Research Centre, Royal Brompton Hospital, London, United Kingdom
- 6National Heart and Lung Institute, Imperial College London, London, United Kingdom
Purpose: Left ventricular global function index (LVGFI) assessed using cardiac magnetic resonance (CMR) seems promising in the prediction of clinical outcomes. However, the role of the LVGFI is uncertain in patients with heart failure (HF) with dilated cardiomyopathy (DCM). To describe the association of LVGFI and outcomes in patients with DCM, it was hypothesized that LVGFI is associated with decreased major adverse cardiac events (MACEs) in patients with DCM.
Materials and Methods: This prospective cohort study was conducted from January 2015 to April 2020 in consecutive patients with DCM who underwent CMR. The association between outcomes and LVGFI was assessed using a multivariable model adjusted with confounders. LVGFI was the primary exposure variable. The long-term outcome was a composite endpoint, including death or heart transplantation.
Results: A total of 334 patients (mean age: 55 years) were included in this study. The average of CMR-LVGFI was 16.53%. Over a median follow-up of 565 days, 43 patients reached the composite endpoint. Kaplan–Meier analysis revealed that patients with LVGFI lower than the cutoff values (15.73%) had a higher estimated cumulative incidence of the endpoint compared to those with LVGFI higher than the cutoff values (P = 0.0021). The hazard of MACEs decreased by 38% for each 1 SD increase in LVGFI (hazard ratio 0.62[95%CI 0.43–0.91]) and after adjustment by 46% (HR 0.54 [95%CI 0.32–0.89]). The association was consistent across subgroup analyses.
Conclusion: In this study, an increase in CMR-LVGFI was associated with decreasing the long-term risk of MACEs with DCM after adjustment for traditional confounders.
Introduction
The most common causes of heart failure (HF) include ischemia dilated cardiomyopathy (IDCM) and non-ischemia dilated cardiomyopathy (NIDCM) (1). Both DCMs involve left ventricular (LV) dilation and contractile dysfunction, referring to cardiac remodeling underlying the morphological substrate of the clinical syndrome of HF, and are associated with adverse clinical outcomes in pathological conditions (2). A previous observational study (3) showed eccentric hypertrophy, in which myocardial mass was increased with cavity enlargement in 94% of the patients with DCM. Thus, cardiac remodeling patterns differ in LV volume, mass, and function in patients with HF. The increased LV mass is the same as the increased LV volume and depressed LV systolic function (ventricles exhibit eccentric hypertrophy), which is crucial for prognosis (4). Thus, the comprehensive evaluation of cardiac performance requires a combination of the structure and function measurement.
Numerous studies have demonstrated that the measurement of LV ejection fraction (LVEF) as a marker of global systolic myocardial function is a robust predictor of morbidity and mortality in patients with DCM (5, 6). However, LVEF does not account for the correlation between LV mass and dimension, and hence, it lacks the comprehensive evaluation of cardiac performance (7).
CMR imaging is the gold standard for evaluating cardiac function and morphology and plays a central role in the diagnosis of HF, the assessment of prognosis, and monitoring of therapy (8, 9). Recently, a new marker of LV global function index (LVGFI) from CMR that comprehensively incorporates LV volume and mass, as well as global systolic function, has been introduced to assess cardiac performance (7). In previous studies, LVGFI is a new and promising parameter that can predict adverse outcomes in healthy individuals (10). It is determined by CMR after STEMI, rendering it a robust predictor of MACEs at long-term follow-up of 3.1 years (11). However, the role of the LVGFI is unknown in patients with HF due to DCM. Herein, we hypothesized that a high LVGFI is associated with a decrease in MACEs in patients with DCM.
Materials and Methods
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (12).
Study Population and Design
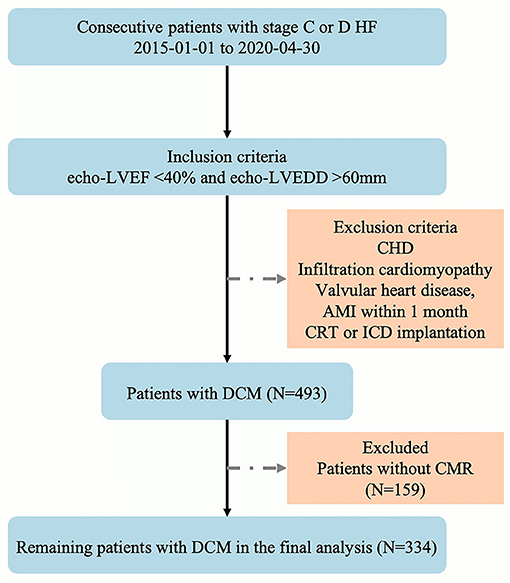
Patients with stage C or D HF were identified from January 1, 2015 to April 30, 2020 and enrolled non-selectively and consecutively in this prospective observational cohort study at the Department of Heart Failure Program at Anzhen Hospital (Capital Medical University, Beijing, China). The current study enrolled 493 patients with DCM according to the World Health Organization/International Society guidelines and the Federation of Cardiology (13). According to the inclusion criteria of the study, patients with LVEF <40%, and LV end-diastolic diameter (LVEDD) >60 mm on echocardiography were enrolled. On the other hand, patients with congenital heart disease, infiltrative cardiomyopathy, valvular heart disease, acute myocardial infarction (MI) within 1 month, or a history of CRT and ICD implantation were excluded from this study. Supplementary Table 1 provides the complete list of epidemiological, clinical, biological variables, electrocardiogram, and imaging variables recorded for each patient.
This study protocol was approved by the Human Subjects Review Committee at Anzhen Hospital (Approval No. 2013007X). The patient's identity remained anonymous, and the requirement for informed consent was waived due to the observational design of this study, as reported elsewhere (14).
CMR Protocol and Variables Definition
CMR acquisition was performed on a 3.0 T scanner (Magnetom Verio; Siemens AG Healthcare, Erlangen, Germany or Discovery™ MR750w, General Electric Healthcare, Waukesha, WI, USA) with retrospective electrocardiogram gating and 32-channel phased-array coil. The steady-state free precession breath-hold cine images with 25 reconstructed phases were acquired. The detailed scanning parameters of cine images have been described previously (15).
One experienced and independent radiologist blinded to clinical data gathered the CMR readings for all standard cardiac parameters and LVGFI. The LVGFI was the primary exposure variable in this study and acquired by drawing endocardial and epicardial borders on the short-axis cine images from basal slice to apical segment (Figure 1). It was defined according to the following formula for each subject:
where the LV stroke volume (LVSV) was calculated by LVEDV-LVESV, and the LV global volume (LVGV) was defined as the sum of the mean LV cavity volume (LVEDV+LVESV)/2 and the myocardium volume. The LV myocardial volume was calculated as the LV myocardial mass divided by the specific myocardial density (1.05 g/mL). Further details regarding CMR data assessment are described previously (15).

Figure 1. Representative example of left ventricular global function index (LVGFI) in a patient with dilated cardiomyopathy. Endocardial (red) and epicardial (green) borders were drawn on the short-axis cine images from basal segment to apical segment and then three-dimensional (3D) model of the left ventricle in end-diastole and end-systole were generated automatically.
Covariates
The selection of covariates is based on our previous studies (1, 9, 15), and those examining the risk factors for HF (16). Laboratory (17) and electrocardiogram (18) examinations provided data on hemoglobin (HgB), white blood cell counts (WBC), ion concentration, renal function, the level of brain natriuretic peptide (BNP), atrial fibrillation (AF) (19), and LBBB.
Follow-Up and Study Endpoints
The attending physician of the patients had to fill out clinical information, and the biochemical and imaging data were obtained using the Empower DataWeb data collection management system (X&Y Solutions, Inc., Boston, MA, USA). The patients were followed up at a 3-month interval with the clinical outpatient visit or by contact with the patient's family members. The status of the events was updated on the Empower DataWeb each time.
The main outcome was the composite of major adverse cardiac events (MACEs), including cardiovascular death and cardiac transplantation. Cardiovascular death was defined as HF, fatal MI, sudden death, and ischemic or hemorrhagic stroke. The causes of death were based on the physician's judgment. The patients were followed up until the beginning of May 2020.
Statistical Analysis
All statistical analyses were performed using EmpowerStats (www.empowerstats.com, X&Y Solutions, Inc. Boston MA) and R software version 3.6.1 (http://www.R-project.org). The two-side alpha level was set at 0.05. Continuous variables are presented as mean ± SD or medians and interquartile ranges (IQRs), while categorical variables are presented as frequencies and percentages. Box-Cox transformation was used to assess the skewed distribution variable. We used multiple imputations based on five replications and a chained equation approach method in the R approach to account for missing data. We also performed sensitivity analyses to compare the distributions of variables with missing data at observed complete case data and the pooling datasets with variables from multiple imputations.
Firstly, generalized additive models (GAMs) were used to identify the LVGFI and outcome correlations after adjusting the most covariates because LVGFI was a continuous variable (Supplementary Figure 1). GAM also assessed and explored the linear correlation between MACEs and confounders (Supplementary Figure 2) to fit the best model. Secondly, univariate Cox models were used to evaluate the association between each significant variable and MACEs in patients with DCM within 5 years of follow-up. Thirdly, multivariate Cox models were used to examine whether CMR-LVGFI had an independent effect on MACEs in patients with DCM within follow-up. When added to this model, the covariates altered the effect estimate of >10%, and covariates of known clinical importance were adjusted while omitting the collinear variables (20). Finally, the subgroup analysis deduced whether potential risk factors had an impact on the results. The interactions among the subgroups were examined using the multivariate-adjusted Cox model.
Time-dependent receiver operating characteristic curves (ROCs) were plotted to set the optimal cutoff of CMR-LVGFI for the primary composite endpoint based on the Youden index. The optimal cutoff values of LVGFI for the analyzed events were based on the highest sum of sensitivity and specificity. The event-free curves were based on Kaplan–Meier analysis stratified by LVGFI optimal cutoffs and compared using the log-rank test.
Results
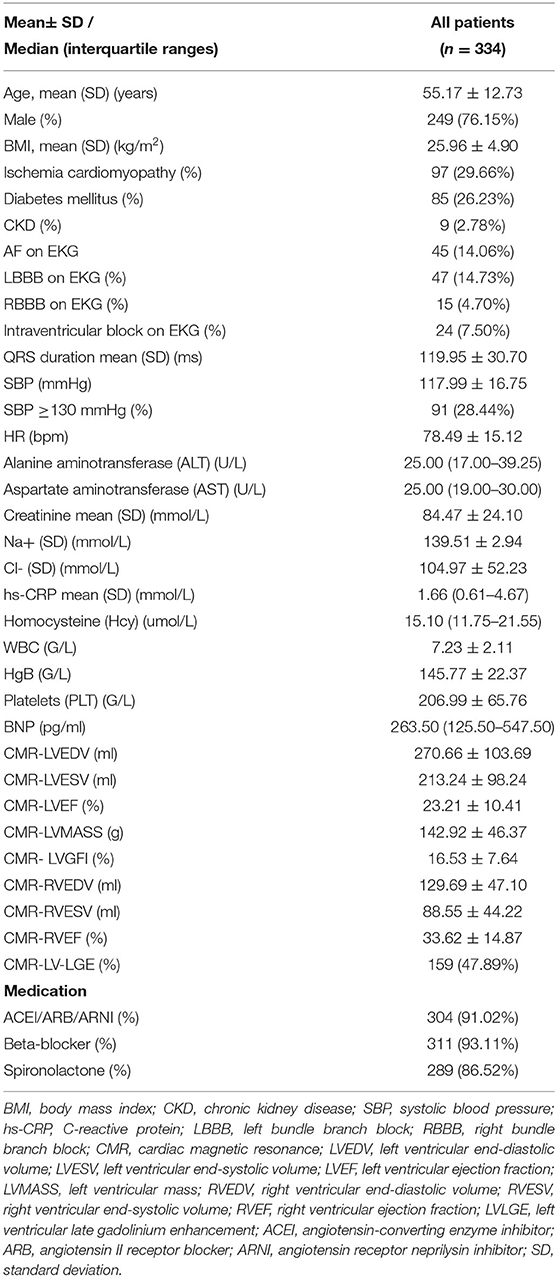
Between January 1, 2015 and April 30, 2020, a total of 493 patients with stage C or D HF who met the above inclusion criteria were enrolled in this study. However, 159 patients did not complete the CMR examination, and the final analysis included 334 patients (Figure 2) with DCM with stage C or D HF. The mean age of the patients was 55 ± 13 years, and 76.15% were males. In approximately one-third of patients, HF is caused by ischemic cardiomyopathy. Among the patients in the cohort study, 14% showed AF or LBBB on EKG, the average QRS interval was 119.95 ± 30.70 ms, and the mean LVEF and RVEF on CMR was 23.21 ± 10.41% and 33.62 ± 14.87%, respectively. The median BNP level was 263.50 pg/mL, as determined in a central laboratory. Furthermore, the concomitant guideline-based medical therapy was well used in the cohort study: 91.57% of the cohort was prescribed either ACEI or ARB or ARNI, 95.83% was prescribed a β-blocker, and 89.58% was prescribed an aldosterone antagonist (Table 1).
CMR-LVGFI Levels and the Risk of MACEs in Patients With DCM
A total of 43 patients developed MACEs in the median follow-up period of 565 days, consisting of 41 patient deaths and two heart transplantation patients. In the present study, we established a linear inverse correlation between LVGFI and Log RR for MACEs after adjusting for sex, age, BMI, ischemia cardiomyopathy, LBBB on EKG, QRS duration, RBBB on EKG, intraventricular block on EKG, AF on EKG; Na+, Cl–, BNP, Hb, creatinine, WBC, LV-LGE, RVEDV, and RVEF, respectively (Supplementary Figure 1).
The results of univariate analyses of MACEs are summarized in Table 2. The univariate analyses showed that ischemia cardiomyopathy, QRS duration, AF on EKG, creatinine, BNP, Na+, Cl-, CMR-LVEDV, CMR-LVESV, CMR-LVMASS, and CMR-LVGFI was associated with a significant increase in the incidence of MACEs. Next, we performed a multivariate Cox regression analysis to further explore CMR-LVGFI as a prognostic marker. In the multivariable analysis shown in Table 3, CMR-LVGFI level is negatively associated with the risk of MACEs in Model I (hazard ratio adjusted (HRadj) 0.51 per 1 SD increment, 95% confidence interval (CI): 0.33–0.77; P = 0.0013) after adjusting for age, sex, and BMI. This was also true in Model II (HRadj 0.54 per 1 SD increment, 95% CI: 0.35–0.82; P = 0.0044) after adjusting for sex, age, BMI, and ischemia cardiomyopathy, LBBB on EKG, QRS duration, RBBB on EKG, intraventricular block on EKG, and AF on EKG; Model III (HRadj 0.63 per 1 SD increment, 95% CI: 0.42–0.96; P = 0.0302) after adjusting for sex, age, BMI, Na+, Cl–, BNP, Hb, creatinine, white blood cell; Model IV (HRadj 0.93 per 1 SD increment, 95% CI: 0.87–0.98; P = 0.0126), after adjusting for sex, age, BMI, LV-LGE, RVEDV, RVEF; Model V (HRadj 0.54 per 1 SD increment, 95% CI: 0.32–0.89; P = 0.01654) was obtained after adjusting for ischemia cardiomyopathy, LBBB on EKG, QRS duration, RBBB on EKG, intraventricular block on EKG, AF on EKG, Na+, Cl–, BNP, Hb, creatinine, white blood cell, LV-LGE, RVEDV, and RVEF.
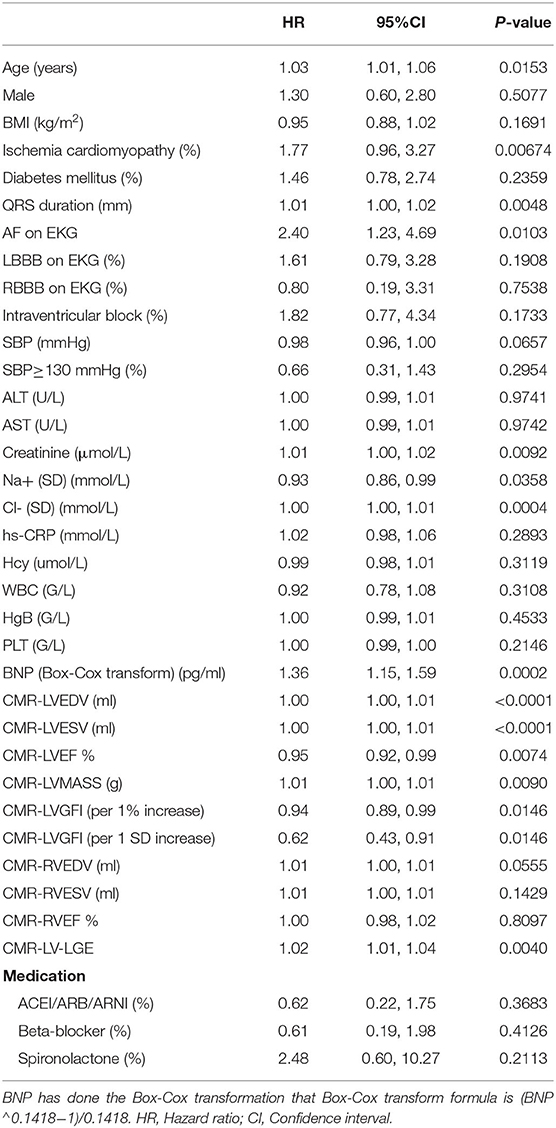
Table 2. Univariate Cox analysis for MACEs in patients with HF within the median follow-up period of 565 days.
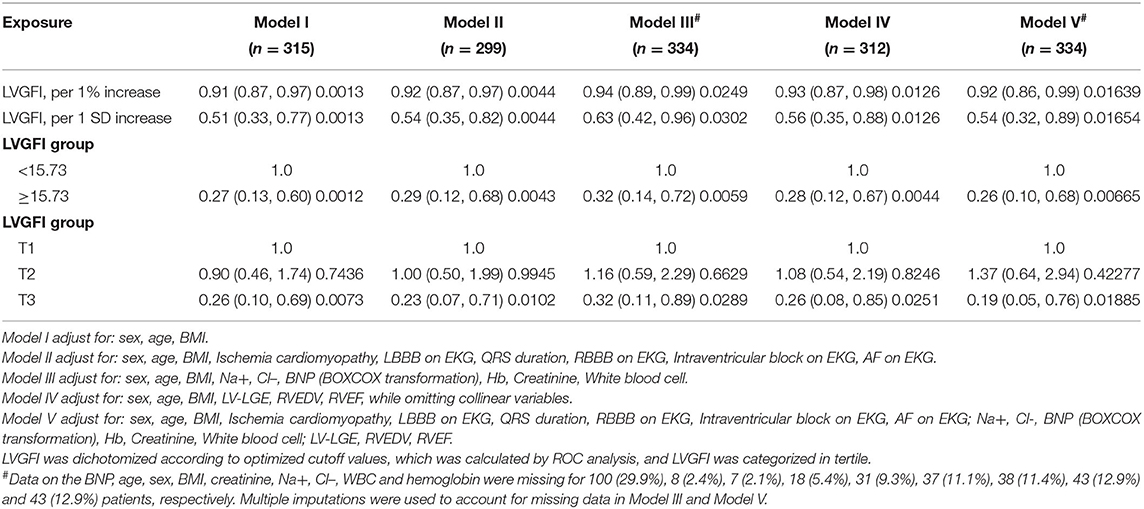
Table 3. Multivariate Cox analysis with CMR- LVGFI for MACEs in patients with HF within the median follow-up period of 565 days.
The time-dependent ROC curves that we used for bootstrap resampling (times = 500) were constructed to determine the optimal cutoff of CMR-LVGFI for the primary composite with a 15.73% endpoint (Supplementary Figure 3). The dichotomized CMR-LVGFI value by 15.73 was used in Kaplan–Meier survival analysis (Figure 3), which showed a significant difference among patients stratified by the LVGFI value by 15.73%. A decreased LVGFI (LVGFI <15.73%) was associated with a reduced MACE-free survival (log-rank P = 0.0021).
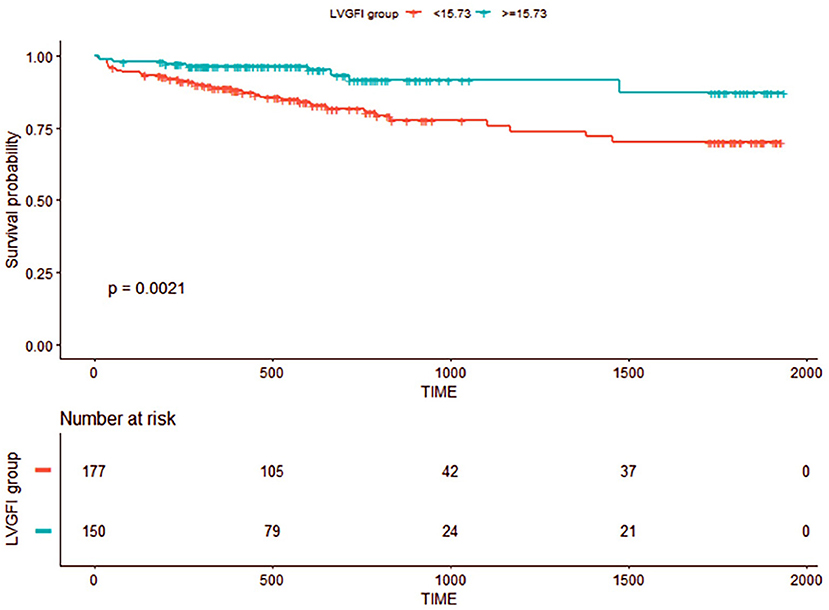
Figure 3. Kaplan–Meier event-free survival curve. Numbers that do not add up to 334 are attributed to the missing data for LVGFI.
Subgroup Analysis Between CMR-LVGFI Levels and MACEs in Patients With Stage C or D HF With DCM
To evaluate the potential influence of other factors, the subgroup analysis was conducted after stratifying the patients by age, BMI, and SBP, sex, diabetes mellitus, LBBB on EKG, ischemia cardiomyopathy, AF on EKG, and CMR-LV-LGE (Table 4). Notably, all subgroups demonstrated a similar overall correlation between CMR-LVGFI levels and the risk of MACEs in patients with stage C or D HF with DCM.
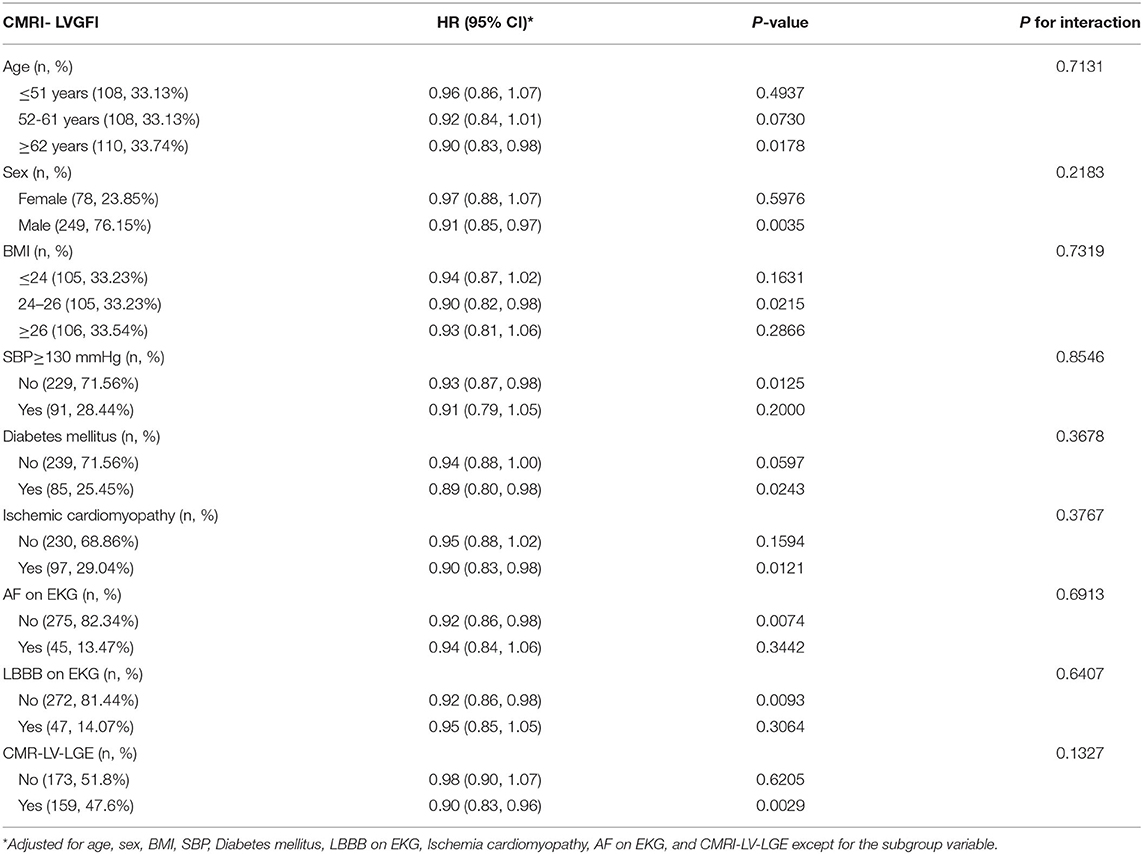
Table 4. Effects of CMR- LVGFI on MACEs in patients with HF in each subgroup by multivariable Cox model within the median follow-up period of 565 days.
Discussion
This large prospective cohort study investigated the association between CMR-derived LVGFI and outcomes in patients with stage C or D HF with DCM in China. The current findings demonstrated that the long-term risk of the composite endpoint in patients with DCM over a median follow-up period of 565 days was significantly negatively associated with CMR-LVGFI integrating LV structure with global function. For every 1 SD increase in CMR-LVGFI, a HR of the composite endpoint after adjusting the clinical, laboratory, electrocardiogram, and CMR routine variables decreased by 46%, which was evident in all the subgroups after adjustments.
The changes in LV structure and geometry, termed cardiac remodeling, involve chamber dilation and/or hypertrophy. The patterns of LV remodeling were classified as concentric hypertrophy and remodeling, physiological hypertrophy, and eccentric hypertrophy and remodeling. Fifty years ago, Linzbach (21) proposed the concept of LV structural remodeling and developed a classification system for LV remodeling patterns. In 1965, Grant et al. (3) defined eccentric hypertrophy as an increased myocardial mass with LV chamber enlargement. Based on the M-mode echocardiography data from 4,975 participants in the Framingham Heart Study, Savage et al. (22) described two subtypes of LV eccentric hypertrophy, referred to as “eccentric dilated” and “eccentric non-dilated.” Typically, patients with depressed systolic function exhibit LV enlargement, accompanied by eccentric hypertrophy. A previous observational study showed that the most common pattern of LV structural remodeling is eccentric hypertrophy, in which myocardial mass is increased with chamber dilatation; this phenomenon was detected in 94% of the patients with DCM (23). Thus, those with a dilated ventricle might have eccentric hypertrophy and low EF. Additionally, LV remodeling is accompanied by decreased cardiac function and is also associated with adverse outcomes. Furthermore, this remodeling pattern has been emphasized by the HF guidelines (24) and has become the target of HF treatment (25, 26). However, the relative importance of structural vs. functional abnormalities in a failing heart is currently under intensive research focus. The vast majority of studies emphasized LVEF as a maker of global systolic myocardial function, deeming it a robust predictor of morbidity and mortality in patients with DCM (5). However, LVEF only focuses on the cardiac functional status and does not account for the LV geometry and hypertrophy. Importantly, a comprehensive definition of cardiac performance requires more than a single measure of structure or function. LVGFI is a combination of structural and functional parameters and provides a rational and comprehensive description of cardiac performance.
LVGFI was initially introduced by Mewton et al. (10). The assessment of LVGFI incorporates elements of LV cavity size and mass, reflecting cardiac remodeling. Currently, it is a promising LV function index for screening, surveillance in individuals with CVD (cardiovascular disease), and risk-stratification in the general population (7, 10). Previous studies have shown that LVGFI by ECHO (7) and CMR (10) was independently associated with the subsequent development of HF, cardiovascular events, and a combined endpoint of all adverse events in community-dwelling individuals. Our results are consistent with these findings and could be extrapolated to patients with DCM. To the best of our knowledge, this is the first comprehensive study evaluating the correlation between CMR-LVGFI and future cardiovascular events in patients with DCM. Moreover, the study included patients with ischemic or non-ischemic cardiomyopathy. These associations persisted even after accounting for confounding and stratification factors, indicating that LVGFI is a reliable LV functional index because it reflects the comprehensive cardiac performance combining LV global systolic performance information with anatomical LV parameters. However, no significant differences were detected between the areas under the curve (AUC) obtained with LVGFI and LVEF on the MACEs (LVGFI ROC area: 0.653, LVEF ROC area: 0.659, P = 0.6) in our study (Supplementary Figure 4). Interestingly, three differences were noted between our and previous studies. Firstly, our study population comprised of patients with DCM, while that in the previous study was a normal community population. Secondly, the measurement method of LVGFI in our study was novel with reliable CMR imaging techniques, while that in the previous study was based on echocardiography. Finally, the primary outcome in our study was cardiovascular death and cardiac transplantation, while the primary endpoint in the previous study was incident symptomatic HF. Furthermore, from a large multicenter CMR study with patients of acute MI treated with percutaneous coronary intervention, LVGFI did not provide incremental prognostic information about LVEF (27).
Nevertheless, the present study has several limitations. This is a single-center study with a limited number of patients that might restrict the generalizability of these results. Based on prospective data collection and with adequate control of potential confounding factors, it is necessary to test the hypothesis in a large real-world cohort. Since the attending physician determined the cause of death, a subjective bias cannot be ruled out, which is similar to the current cohort study, unless systematic autopsy becomes the standard. Finally, the data of the BNP covariate was missing in 29.9% of the participants. However, statistical techniques employed multiple imputations to address the issue of missing data, and sensitivity analyses indicated that our assumptions regarding the patterns of missing data were reasonable (Supplementary Table 2).
Conclusion
The LVGFI, integrating LV structure with global function, is independently associated with the long-term risk of MACEs in patients with stage C or D HF with DCM during follow-up. This suggested that LVGFI is a reliable LV functional and prognostic index because it reflects the cardiac performance at different degrees of structural LV remodeling.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Subjects Review Committee at Anzhen Hospital (Approval No. 2013007X). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
LX and JD are the guarantors of integrity of the entire study. TL, JD, and LX are responsible for the study concepts and design. TL, ZZ, KB, YG, HW, RW, WL, SC, YL, and YS performed the experimental studies and prepared the first draft of the manuscript, which was critically revised by GY, DF, JD, and LX. TL, ZZ, KB, YG, and HW analyzed and interpreted the data. TL and ZZ performed statistical analysis and all the results were checked by JD and LX. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (U1908211), the National Key Research and Development Program of China (2016YFC1300300 and 2016YFC1301002), and the Capital's Funds for Health Improvement and Research Foundation of China (2020-1-1052). This study was also supported in part by the British Heart Foundation (TG/18/5/34111, PG/16/78/32402), the European Research Council Innovative Medicines Initiative (DRAGON, H2020-JTI-IMI2 101005122), the AI for Health Imaging Award (CHAIMELEON, H2020-SC1-FA-DTS-2019-1 952172), and the UK Research and Innovation Future Leaders Fellowship (MR/V023799/1).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Xinlin Chen and Changzhong Chen (Department of Epidemiology and Biostatistics, X&Y solutions Inc. in Boston) for their helpful comments about the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.751907/full#supplementary-material
References
1. Liu T, Ma X, Liu W, Ling S, Zhao L, Xu L, et al. Late gadolinium enhancement amount as an independent risk factor for the incidence of adverse cardiovascular events in patients with stage C or D heart failure. Front Physiol. (2016) 7:484. doi: 10.3389/fphys.2016.00484
2. Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. (2011) 58:1733–40. doi: 10.1016/j.jacc.2011.07.022
3. Grant C, Greene DG, Bunnell IL. Left ventricular enlargement and hypertrophy. A clinical and angiocardiographic study. Am J Med. (1965) 39:895–904.
4. Feild BJ, Baxley WA, Russell RO Jr, Hood WP Jr, Holt JH, Dowling JT, et al. Left ventricular function and hypertrophy in cardiomyopathy with depressed ejection fraction. Circulation. (1973) 47:1022–31. doi: 10.1161/01.CIR.47.5.1022
5. Solomon SD, Claggett B, Desai AS, Packer M, Zile M, Swedberg K, et al. Influence of ejection fraction on outcomes and efficacy of Sacubitril/Valsartan (LCZ696) in heart failure with reduced ejection fraction: the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure (PARADIGM-HF) trial. Circ Heart Fail. (2016) 9:e002744. doi: 10.1161/CIRCHEARTFAILURE.115.002744
6. Hollenberg SM, Warner Stevenson L, Ahmad T, Amin VJ, Bozkurt B, Butler J, et al. 2019 ACC college of cardiology solution set oversight committee expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: a report of the American. J Am Coll Cardiol. (2019) 74:1966–2011. doi: 10.1016/j.jacc.2019.08.001
7. Nwabuo CC, Moreira HT, Vasconcellos HD, Mewton N, Opdahl A, Ogunyankin KO, et al. Left ventricular global function index predicts incident heart failure and cardiovascular disease in young adults: the coronary artery risk development in young adults (CARDIA) study. Eur Heart J Cardiovasc Imaging. (2019) 20:533–40. doi: 10.1093/ehjci/jey123
8. Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol. (2009) 54:1407–24. doi: 10.1016/j.jacc.2009.04.094
9. Liu T, Song D, Dong J, Zhu P, Liu J, Liu W, et al. Current understanding of the pathophysiology of myocardial fibrosis and its quantitative assessment in heart failure. Front Physiol. (2017) 8:238. doi: 10.3389/fphys.2017.00238
10. Mewton N, Opdahl A, Choi EY, Almeida AL, Kawel N, Wu CO, et al. Left ventricular global function index by magnetic resonance imaging–a novel marker for assessment of cardiac performance for the prediction of cardiovascular events: the multi-ethnic study of atherosclerosis. Hypertension. (2013) 61:770–8. doi: 10.1161/HYPERTENSIONAHA.111.198028
11. Eitel I, Pöss J, Jobs A, Eitel C, de Waha S, Barkhausen J, et al. Left ventricular global function index assessed by cardiovascular magnetic resonance for the prediction of cardiovascular events in ST-elevation myocardial infarction. J Cardiovasc Magn Reson. (2015) 17:62. doi: 10.1186/s12968-015-0161-x
12. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. (2014) 12:1500–24. doi: 10.1016/j.ijsu.2014.07.014
13. Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, et al. Report of the 1995 world health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation. (1996) 93:841–2. doi: 10.1161/01.CIR.93.5.841
14. Filion KB, Azoulay L, Platt RW, Dahl M, Dormuth CR, Clemens KK, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. (2016) 374:1145–54. doi: 10.1056/NEJMoa1506115
15. Liu T, Gao Y, Wang H, Zhou Z, Wang R, Chang SS, et al. Association between right ventricular strain and outcomes in patients with dilated cardiomyopathy. Heart. (2020) 107:1233−9. doi: 10.1136/heartjnl-2020-317949
16. Kenchaiah S, Vasan RS. Heart failure in women–insights from the Framingham Heart Study. Cardiovasc Drugs Ther. (2015) 29:377–90. doi: 10.1007/s10557-015-6599-0
17. Chaudhry SP, Stewart GC. Advanced heart failure: prevalence, natural history, and prognosis. Heart Fail Clin. (2016) 12:323–33. doi: 10.1016/j.hfc.2016.03.001
18. Leong KT, Wong LY, Aung KC, Macdonald M, Cao Y, Lee S, et al. Risk stratification model for 30 day heart failure readmission in a multiethnic South East Asian Community. Am J Cardiol. (2017) 119:1428–32. doi: 10.1016/j.amjcard.2017.01.026
19. Karnik AA, Gopal DM, Ko D, Benjamin EJ, Helm RH. Epidemiology of atrial fibrillation and heart failure: a growing and important problem. Cardiol Clin. (2019) 37:119–29. doi: 10.1016/j.ccl.2019.01.001
20. Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. (2014) 348:g14. doi: 10.1136/bmj.g14
21. Linzbach AJ. Heart failure from the point of view of quantitative anatomy. Am J Cardiol. (1960) 5:370–82. doi: 10.1016/0002-9149(60)90084-9
22. Savage DD, Garrison RJ, Kannel WB, Levy D, Anderson SJ, Stokes J 3rd, et al. The spectrum of left ventricular hypertrophy in a general population sample: the Framingham study. Circulation. (1987) 75:I26–33.
23. Gaasch WH, Delorey DE, St John Sutton MG, Zile MR. Patterns of structural and functional remodeling of the left ventricle in chronic heart failure. Am J Cardiol. (2008) 102:459–62. doi: 10.1016/j.amjcard.2008.03.081
24. van der Meer P, Gaggin HK, Dec GW. ACC/AHA versus ESC guidelines on heart failure: JACC guideline comparison. J Am Coll Cardiol. (2019) 73:2756–68. doi: 10.1016/j.jacc.2019.03.478
25. Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. (2019) 322:1077–84. doi: 10.1001/jama.2019.12843
26. Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, et al. Association of change in N-Terminal Pro-B-Type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. (2019) 322:1085–95. doi: 10.1001/jama.2019.12821
Keywords: dilated cardiomyopathy, cardiac magnetic resonance imaging, left ventricular global function index, association analysis, clinical outcomes
Citation: Liu T, Zhou Z, Bo K, Gao Y, Wang H, Wang R, Liu W, Chang S, Liu Y, Sun Y, Firmin D, Yang G, Dong J and Xu L (2021) Association Between Left Ventricular Global Function Index and Outcomes in Patients With Dilated Cardiomyopathy. Front. Cardiovasc. Med. 8:751907. doi: 10.3389/fcvm.2021.751907
Received: 14 August 2021; Accepted: 26 October 2021;
Published: 16 November 2021.
Edited by:
Lilei Yu, Wuhan University, ChinaReviewed by:
Jinwei Tian, The Second Affiliated Hospital of Harbin Medical University, ChinaXiaohu Li, First Affiliated Hospital of Anhui Medical University, China
Copyright © 2021 Liu, Zhou, Bo, Gao, Wang, Wang, Liu, Chang, Liu, Sun, Firmin, Yang, Dong and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Xu, bGVpeHUyMDAxQGhvdG1haWwuY29t; Jianzeng Dong, anpfZG9uZ0AxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Tong Liu
Tong Liu Zhen Zhou4†
Zhen Zhou4† Wei Liu
Wei Liu Guang Yang
Guang Yang Jianzeng Dong
Jianzeng Dong Lei Xu
Lei Xu