
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Cardiovasc. Med. , 12 October 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.745549
This article is part of the Research Topic CardioNeurology: Basic, translational and clinical research View all 20 articles
 Wenbin Lu1
Wenbin Lu1 Yu Wang1
Yu Wang1 Lijuan Chen1
Lijuan Chen1 Yongjun Li1
Yongjun Li1 Rui Zhang1
Rui Zhang1 Zhongpu Chen1
Zhongpu Chen1 Jinchuan Yan2
Jinchuan Yan2 Mingming Yang1
Mingming Yang1 Bing Han3
Bing Han3 Zhirong Wang4
Zhirong Wang4 Shenghu He5
Shenghu He5 Lianglong Chen6
Lianglong Chen6 Xiang Wu7
Xiang Wu7 Hesong Zeng8
Hesong Zeng8 Likun Ma9
Likun Ma9 Guoping Shi10
Guoping Shi10 Jianrong Yin11
Jianrong Yin11 Jiyan Chen12
Jiyan Chen12 GenShan Ma1*
GenShan Ma1*Background: Warfarin, along with aspirin and clopidogrel, has long been recommended for patients with atrial fibrillation (AF) who are undergoing percutaneous coronary intervention with a drug-eluting stent (PCI-DES). However, this triple therapy has been known to increase the risk of bleeding complications. Meanwhile, there is no evidence from prospective trials on the use of ticagrelor in a dual therapy. We here aimed to compare the antiplatelet drug ticagrelor as a dual antithrombotic agent to aspirin and clopidogrel in bleeding events.
Methods: In this multicenter, active-controlled, open-label, randomized trial, patients with AF taking warfarin who had undergone PCI-DES were randomly assigned to the ticagrelor therapy group (Dual group) or the clopidogrel plus aspirin therapy group (Triple group). The primary and secondary endpoints were overall bleeding events and major bleeding events, respectively, according to the Thrombolysis in Myocardial Infarction (TIMI) criteria at 6 months. Cardiovascular events [re-PCI, surgical bypass, myocardial infarction (MI), heart failure, rehospitalization due to angina pectoris, stent thrombosis and death due to cardiovascular causes] at 6 months were also recorded.
Results: A total of 296 patients from 12 medical centers in China were randomized after PCI-DES to either the Dual therapy group (n = 148) or the Triple group (n = 146) for 6 months. The overall incidence of bleeding events at 6 months was 36.49% in the Dual therapy group and 35.62% in the Triple group [hazard ratio, 0.930; 95% confidence interval (CI), 0.635 to 1.361; P = 0.7088]. The incidence of the secondary endpoint over 6 months was 4.73% in the Dual therapy group and 1.37% in the Triple group (hazard ratio, 0.273; 95% CI, 0.057 to 1.315; P = 0.1056). Cardiovascular event occurrence was also comparable in both groups at 6 months (18.24 vs. 16.44%; hazard ratio, 0.845; 95% CI, 0.488 to 1.465; P = 0.5484).
Conclusions: The incidence of total bleeding events in AF patients treated with ticagrelor was comparable to that in patients treated with clopidogrel plus aspirin at 6 month; Meanwhile, the incidence of cardiovascular events were also comparable between the groups.
Clinical Trial Registration: MANJUSRI, ClinicalTrials.gov# NCT02206815, 2014, August 1st
Chronic treatment with oral anticoagulants (e.g., warfarin) is essential for most atrial fibrillation (AF) patients with CHA2DS2VASc scores ≥ 2. Treatment of patients with AF who have undergone percutaneous coronary intervention with a drug-eluting stent (PCI-DES) further requires follow-up antiplatelet therapy to minimize thrombotic events. Triple therapy, such as warfarin plus dual antiplatelet agents (DAPT), aspirin and clopidogrel, has been used for many years in patients with AF who have undergone PCI-DES (1).
The European Society of Cardiology recommended a short period of therapy with VKA, aspirin, and clopidogrel for such patients (2). The 2019 AHA/ACC/HRS guidelines for the management of patients with AF suggested that “Triple therapy should be administered to these patients with bare-metal stents for 1–3 months and much longer in patients with a drug-eluting stent (3–6 months) followed by one anticoagulant plus clopidogrel 75 mg/day or aspirin 100 mg/day” (3).
RCT studies as well as retrospective analyses all suggested that the combination of oral anticoagulation with a P2Y12 inhibitor and aspirin in patients with AF undergoing PCI-DES is associated with a high bleeding risk (4, 5). Triple antithrombotic therapy, particularly if consisting of a OAC, aspirin and a P2Y12 inhibitor, is associated with a increasing of bleeding, including major and intracranial hemorrhages (6). However, pooled data from meta-analysis (7) have shown a possible increase of ischemic events in the dual therapy with clopidogrel, for example Galli et al. confirmed that dual therapy with clopidogrel with a significant increase of stent thrombosis risk in the overall population and a significant 43% increase of MI in the ACS/PCI subgroup (8) sparkling the interest for the use of alternative antithrombotic agents. In addition to this, clopidogrel, but not ticagrelor, is characterized by an interindividual variability in the pharmacodynamic profile, leading to insufficient platelet inhibition and increased ischemic events in up to 40% of treated patients and thus Most recent investigations support the clinical benefit of a genetic guided selection of antiplatelet therapy in patients undergo PCI (i.e., switching to prasugrel or ticagrelor) (9, 10).
Recently, randomized clinical trials and recent evidence have supported the ESC recommendation for dual antithrombotic therapy in patients with AF who have undergone PCI-DES (6, 11). As is well-known, the recent guidelines (2018–2020 ECS Guidelines) supported the current trend of antithrombotic therapy using a combination of one antiplatelet drug and one anticoagulant (12–14), for example,2020 ESC Guidelines presented that In AF patients with ACS undergoing an uncomplicated PCI, early cessation (<1 week) of aspirin and continuation of dual therapy with an OAC and a P2Y12 inhibitor for up to 12 months is recommended if the risk of stent thrombosis is low or if concerns about bleeding risk prevail over concerns about risk of stent thrombosis, irrespective of the type of stent used. However,1 week triple therapy with clopidogrel can be associated to increased ischemic events.
The TWILIGHT trial proved that ticagrelor monotherapy was associated with a lower incidence of clinically relevant bleeding than ticagrelor plus aspirin, without higher risks of death, myocardial infarction, or stroke, again suggesting that ticagrelor is a more potent antiplatelet drug (15, 16).
The recent AUGUSTUS study was designed for AF patients who had ACS or had undergone PCI and were taking a P2Y12 inhibitor to receive apixaban or warfarin and aspirin or a matching placebo for 6 months (17, 18). However, more than 90% of the individuals in this trial were treated with clopidogrel instead of more potent agents, such as ticagrelor. Among published clinical studies such as PIONEER trial, ENTRUST trial and Re-Dual trial, the Re-Dual study included the most patients received ticagrelor were only 12% (19–21). The use of ticagrelor can potentially overcome the increased rate of ischemic events as the rate of non-responders to this drug is very low.
On the other hand, triple therapy with ticagrelor in these previous trials was somewhat unfounded and in particular lacked the support of ESC guidelines. Thus, the rationale for our study is that ticagrelor were used only in a minority of the 4 RCTs on the topic (PIONEER, RE-DUAL, AUGUSTUS and ENTRUST-AF), which implied that there are not many data from prospective studies on a dual therapy including this potent P2Y12 inhibitors.
Therefore, we performed the present study to compare the combination of ticagrelor and warfarin with traditional triple therapy, with the aim to evaluate the safety and efficacy of ticagrelor as one of dual antithrombins.
The trial rationale and design were published previously (22). In brief, the MANJUSRI trial was a prospective, multicenter, open-label, randomized clinical trial that compared the potent antiplatelet drug ticagrelor with the traditional antithrombotic drugs clopidogrel plus aspirin in patients with AF taking warfarin and receiving PCI-DES (all of the patients in the trial received drug-eluting stents). This clinical trial was registered with ClinicalTrials.gov (NCT02206815). The study was performed in accordance with the provisions of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. The institutional ethics committee associated with the participating centers approved the trial protocol. All of the patients provided written informed consent.
Eligible patients were recruited from 12 participating centers in China from September 2014 to February 2019. The inclusion criteria were men or nonpregnant women ≥ 18 and ≤ 75 years of age with a severe coronary lesion (≥ 75% angiographically or fractional flow reserve, FFR <0.8) with an indication for coronary stent implantation and patients with a CHA2DS2-VASc score ≥ 2. The exclusion criteria are listed in Supplementary Table 1. CHA2DS2-VASc scores, which reflect the risk of stroke, and HAS-BLED scores, which reflect the risk of bleeding, were assessed in all of the patients.
Patients who met all of the inclusion criteria and none of the exclusion criteria were randomly assigned at a 1:1 ratio to receive ticagrelor and warfarin (Dual therapy group) or clopidogrel + aspirin + warfarin (Triple group) just after PCI-DES. The randomization sequence and allocation were accomplished using sealed envelopes containing a computer-generated sequence (generated at ZhongDa Hospital Affiliated with Southeast University). The randomized enrollment progress and patient distribution are shown in Supplementary Figure 1. All of the patients in the trial took warfarin, and the doses were adjusted to reach a target international normalized ratio (INR) within the range of 2.0 to 2.5. Before PCI-DES, a loading dose of 180 mg of ticagrelor or 300 mg of clopidogrel and 300 mg of aspirin were administered. After PCI-DES, patients on Triple group received clopidogrel 75 mg/day + aspirin 100 mg/day, whereas patients in the Dual therapy group received ticagrelor 90 mg/bid. The use of proton pump inhibitors to reduce the risk of bleeding was recommended but not mandatory. Follow-ups were performed during routinely scheduled outpatient visits at 1, 3, and 6 months and via telephone calls at 15 days and 7 months after randomization. After 6 months, the patients were transitioned from their 2-trial interventions to receive antiplatelet and anticoagulant therapy at the discretion of the attending physician according to the local standard of care.
The primary endpoint of the study was the occurrence of overall bleeding events (minimal, minor, and major) during the first 6 months of follow-up. The secondary endpoint was the occurrence of major bleeding events during the 6-month follow-up. Bleeding events in the trial were assessed according to the Thrombolysis in Myocardial Infarction (TIMI) bleeding criteria (23–26): (1) Major: any intracranial bleeding; clinically overt signs of hemorrhage associated with a drop in hemoglobin of ≥5-g/dL or a ≥15% absolute decrease in hematocrit; and fatal bleeding; (2) Minor: clinically overt signs (including imaging), resulting in a hemoglobin drop of 3- to <5-g/dL or a ≥10% decrease in hematocrit; no observed blood loss: ≥4 g/dL decrease in the hemoglobin concentration or ≥12% decrease in hematocrit; requiring medical attention: any overt sign of hemorrhage that met one of the following criteria and did not meet the criteria for a major or minor bleeding event, as defined above; requiring intervention: medical treatment to stop or treat bleeding, including changing the dose of the study drug; leading to or prolonging hospitalization; and prompting evaluation (unscheduled visit to a healthcare professional and diagnostic testing); (3) Minimal: any overt bleeding event not meeting the criteria above; any clinically overt sign of hemorrhage (including imaging) associated with a <3-g/dL decrease in hemoglobin concentration or <9% decrease in hematocrit.
Cardiovascular events reported were re-PCI (percutaneous coronary intervention) or surgical bypass, MI (myocardial infarction), heart failure and rehospitalization due to angina pectoris, stent thrombosis and death due to cardiovascular causes, all of which were in accordance with the Academic Research Consortium criteria. Standardized questions were used to assess cardiovascular events, and the use of medications. A committee of clinical events that was unaware of treatment allocations adjudicated and verified all events that required medical attention from the medical records of the referring doctors and hospitals.
Data management and statistical analyses were performed using SAS software, version 9.4 (SAS Institute, USA).
Based on previous research mainly retrospective analysis at the time, we anticipated a bleeding complication rate of 18% in the dual ticagrelor therapy group and 30% in the clopidogrel plus aspirin group at 6 months. The proportion of patients dropping out of the treatment and the control groups was assumed to be 5%. The sample size was estimated at 296 subjects.
The primary and secondary endpoints were the first occurrences of events. The primary analysis compared the time from randomization to the first occurrence of any event in the composite endpoint using the log-rank test.
The null and alternative hypotheses were the following.
H0: The distribution of the first occurrence of bleeding in the 2 groups is the same.
H1: The distribution of the first occurrence of bleeding in the 2 groups is not the same.
The hypotheses were tested at an overall significance level of 5% (2 sided). Patients who failed to record any event at the primary composite efficacy endpoint were censored at the close of the study (i.e., date of the end-of-treatment visit) or at the time of the last available information, if not earlier. Kaplan-Meier and Cox proportional hazard regression estimates of the cumulative risk for the first occurrence of any event were calculated in the 2 groups. The hazard ratio and 95% confidence intervals (CIs) are reported.
The sponsors of the study played no role in the entire process, including the study design, data collection, and data analysis. The corresponding author is fully responsible for the trial and manuscript publishing.
From September 2014 to February 2019, a total of 296 patients from 12 sites in China were enrolled. Totals of 148 and 146 AF patients were assigned to the ticagrelor therapy group (Dual group) and clopidogrel plus aspirin (Triple group) therapy group, respectively (Figure 1). After a follow-up period of 6 months for both groups, the final collection of follow-up data occurred on February 20, 2019. A total of 3 patients died during the study period. The direct cause of death of a patient in the ticagrelor therapy group was heart failure; One patient died of trauma following a fall in Triple therapy group, and the other also in the Triple group died of acute exacerbation of renal insufficiency.
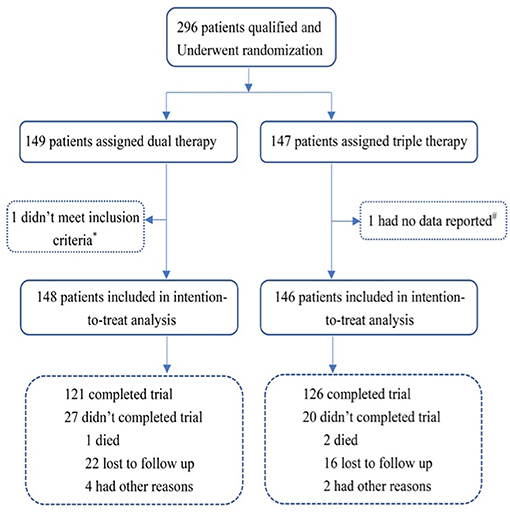
Figure 1. Randomization and follow-up. *One patient in the ticagrelor therapy group did not meet the inclusion criteria immediately after randomization; #one patient in the Triple therapy group had no data reported. Dual therapy: ticagrelor therapy group; Triple therapy: clopidogrel plus aspirin group.
The mean age of the patients in the trial was 69.39 years old. In all, 65.54% of patients in the Dual therapy group and 62.33% in the Triple therapy group were male. Clinical comorbidities, including hypertension, diabetes, and history of stroke/TIA, and the use of medication were comparable between the 2 groups (Table 1). The average CHA2DS2-VASc score was 3.41 in the ticagrelor therapy group and 3.22 in the Triple therapy group. The mean HAS-BLED scores were 2.01 in the ticagrelor therapy group and 1.97 in the triple therapy group. The mean INR at randomization was 2.08 in the ticagrelor therapy group and 2.13 in the Triple therapy group, respectively. Baseline procedural characteristics were similar between the two groups, including PCI-related vessels and periprocedural treatments, are shown in Table 2.
After a 6-month follow-up, 54 patients (36.49%) in the ticagrelor therapy group and 52 patients (35.62%) in the Triple group experienced bleeding events. The time-to-first bleeding event analysis revealed comparable bleeding rates between the 2 groups [hazard ratio (HR), 0.930; 95% CI, 0.635 to 1.361; P = 0.7088] (Figure 2A). Similarly, 7 (4.73%) patients in the ticagrelor therapy group and 2 (1.37%) patients in the Triple group experienced major bleeding events (HR, 0.273; 95% CI, 0.057 to 1.315; P = 0.1056) according to the TIMI bleeding classifications (Figure 2B).
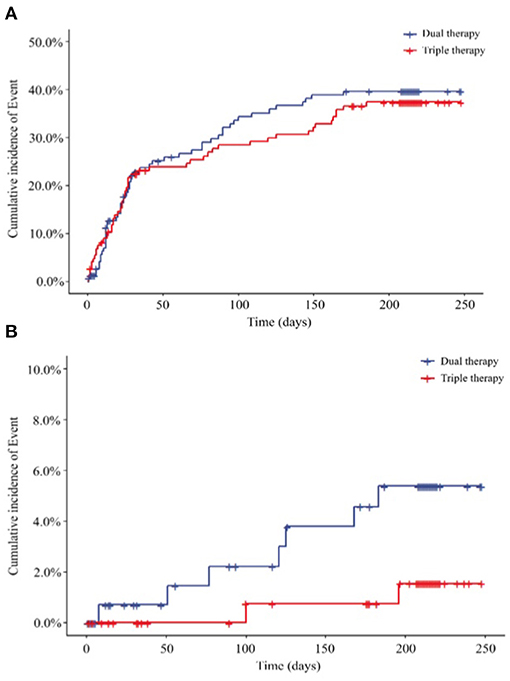
Figure 2. Endpoints, (A) Primary outcome for the incidence of total bleeding (HR 0.930; 95% CI, 0.635 to 1.361; P = 0.7088); (B) secondary outcome for the incidence of major bleeding (HR, 0.273; 95% CI, 0.057 to 1.315; P = 0.1056) HR, hazard ratio. Dual therapy: ticagrelor therapy group; triple therapy: clopidogrel plus aspirin group.
The majority of bleeding events in the trial were minor bleeding events mainly concentrated in the nose (27.78 vs. 21.15%) and mouth (29.63 vs. 32.69%) in the ticagrelor therapy and triple therapy groups (Table 3). We observed comparable distributions and proportions of bleeding events between the 2 groups. The occurrence of multiple bleeding events especially twice bleeding seems more frequently in the Triple therapy group (36.54%) and it was 20.37% in the ticagrelor therapy group, but showed no significant difference between the groups.
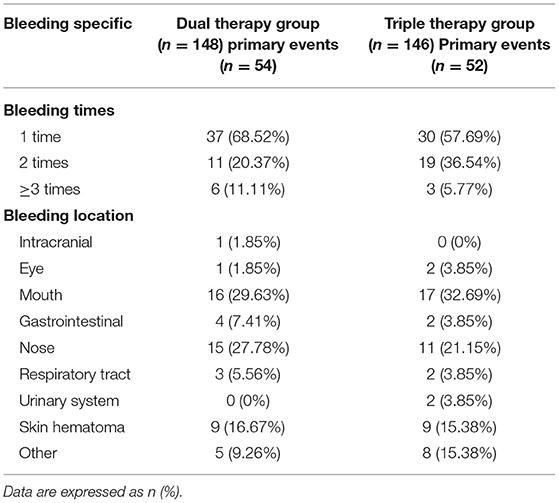
Table 3. Bleeding Times and Specific Location of Bleeding in 2 Groups of Patients; “Others” were cases in which the bleeding site could not be identified, but were identified as bleeding by the research team due to Hb decrease.
According to the data analysis and patients lost to follow-up or other reasons, we obtained 247 patients for the per protocol set (PPS). In the set, 48 of the 121 patients (39.67%) in the ticagrelor therapy group experienced total bleeding events compared with 46 of the 126 (36.51%) patients in the Triple group, representing comparable bleeding rates between the 2 groups (HR, 0.889; 95% CI, 0.593 to 1.332; P = 0.5684) (Figure 3A; Supplementary Table 2). Considering the secondary endpoints in the PPS, 6 (4.96%) patients in the ticagrelor therapy group experienced major bleeding events, whereas 2 (1.59%) patients in the Triple therapy group experienced major bleeding events. Time-to-event analysis showed no significant difference (HR, 0.313; 95% CI, 0.063 to 1.552; P = 0.1550) (Figure 3A; Supplementary Table 2) between the 2 groups.
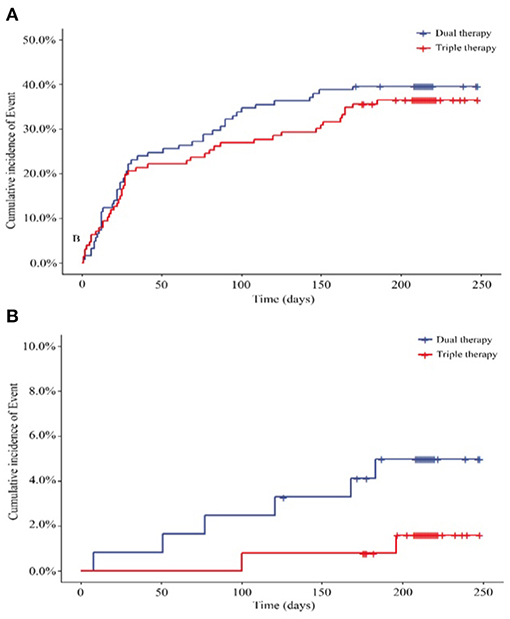
Figure 3. (A) Primary outcome for the incidence of total bleeding in PPS (N = 247) (HR, 0.889; 95% CI, 0.593 to 1.332; P = 0.5684); (B) Secondary outcome for the incidence of major bleeding (HR, 0.313; 95% CI, 0.063 to 1.552; P = 0.1550). HR, hazard ratio. Dual therapy: ticagrelor therapy group; triple therapy: clopidogrel plus aspirin group.
Cardiovascular events, including re-PCI, surgical bypass, MI, heart failure, rehospitalization due to angina pectoris, stent thrombosis and death due to cardiovascular causes, at the end of the 6-month follow-up are shown in Figure 4; Table 4. We reported cardiovascular events in 27 (18.24%) patients in the ticagrelor therapy group, whereas 24 (16.44%) patients in the Triple group experienced these events (HR, 0.845; 95% CI, 0.488 to 1.465; P = 0.5484).
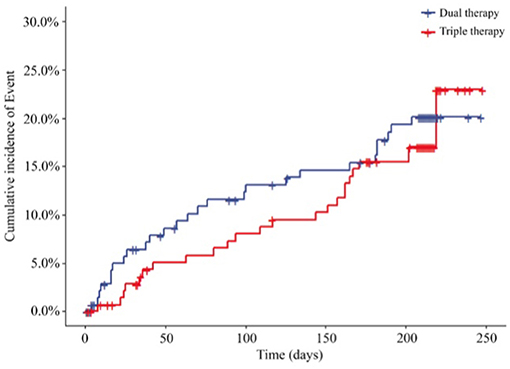
Figure 4. Incidence of cardiovascular event outcomes of the 2 groups (HR, 0.845; 95% CI, 0.488 to 1.465; P = 0.5484). HR, hazard ratio. Dual therapy: ticagrelor therapy group; triple therapy: clopidogrel plus aspirin group.
Furthermore, at the end of the 6-month follow-up in the PPS, 22 (18.18%) patients in the ticagrelor therapy group experienced cardiovascular events compared to 20 events (15.87%) in the Triple group. Time-to-event analysis showed no significant difference (HR, 0.844; 95% CI, 0.461 to 1.547; P = 0.5836) (Figure 5; Supplementary Table 3) between the 2 groups.
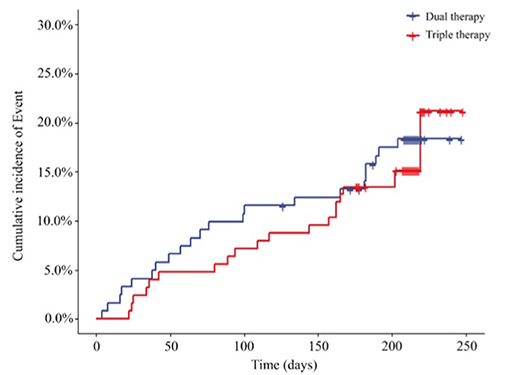
Figure 5. Incidence of cardiovascular event outcomes of the two groups (PPS, N = 247) (HR, 0.844; 95% CI, 0.461 to 1.547; P = 0.5836). HR, hazard ratio. Dual therapy: ticagrelor therapy group; triple therapy: clopidogrel plus aspirin group.
There are many issues surrounding antithrombotic treatment of patients with AF undergoing PCI that remain unresolved. Whether ticagrelor as a part of dual therapy would exhibit favorable safety and efficacy is unknown. Some meta-analysis suggested that the use of ticagrelor as part of dual or triple antithrombotic therapy is associated with significantly higher rates of clinically relevant hemorrhagic complications compared with clopidogrel (27).
What's different is that our trial primarily confirmed the safety of ticagrelor as a dual therapy regimen didn't increasing bleeding rates, especially total bleeding rates. We further found that most of the total TIMI bleeding events were minimal and minor events. This result indicates that ticagrelor, even with its potent antithrombotic effect, did not increase the risk of fatal bleeding after 6 months as dual therapy when combined with warfarin and administered to AF patients after they underwent PCI-DES.
2019 AHA/ACC/HRS guidelines suggested that in patients with AF at increased risk of stroke (based on CHA2 DS2 -VASc risk score of 2 or greater) who have undergone PCI with stenting for ACS, double therapy with a P2Y12 inhibitor (clopidogrel or ticagrelor) and dose-adjusted vitamin K antagonist is reasonable to reduce the risk of bleeding as compared with triple therapy (3). Our results provide further evidence support and supplement for the clinical application of dual therapy with different P2Y12 inhibitor. On the other hand our results are consistent with and support the trends of using dual drug regimen in patients with atrial fibrillation and PCI-DES, as recommended by recent guidelines (12, 28, 29). In the “North American Perspective 2016–2021 Update” regarding antithrombotic treatment for these patients, triple therapy was only recommended during the peri-PCI period and dual therapy as soon as possible after hospital discharge (30–32). In other words, our study suggests a possibility that a slightly stronger dual antithrombotic with ticagrelor therapy might be used immediately after PCI in AF patients, instead of 1 week to 1 month of triple antithrombotic therapy.
The MANJUSRI trial showed that, among AF patients who had undergone PCI-DES, the anticoagulation regimen warfarin plus ticagrelor resulted in comparable total bleeding events compared with Triple therapy. The difference in risk between the ticagrelor therapy group and the Triple therapy group was 6.8% (0.87 percentage points) over ~6 months of treatment. Compared to previously published trials, bleeding rates (33, 34) in our trial appeared to be amplified. This might be attributed to several factors, firstly, patients in triple group received a relatively longer time of Antithrombotic therapy, then nearly one-third of the patients in our study used glycoprotein receptor antagonists during the perioperative period and Glycoprotein IIbIIIa receptor antagonists seemed to be used more frequently in the trial of ticagrelor group.
The rates of major TIMI bleeding were higher in the ticagrelor therapy group than in the Triple group, but the difference was not statistically significant. The difference in risk between the groups was 27.5% (3.36 percentage points), however in our trial, we must consider the particularity of certain cases themselves. In the ticagrelor therapy group, only 1 of 7 major bleeding patients suffered from intracranial bleeding, and the others all experienced gastrointestinal bleeding. However, it is important to note that 2 of the patients who experienced major bleeding were at high risk at the time of enrollment. One patient had a HASBLED score ≥3, and the other patient had an INR of 4.43. Another 3 patients' specific situations were similar to those of the 2 patients in the Triple group. In conclusion, these findings suggest a relatively balanced risk of bleeding in the prevention of thromboembolism, at least to some extent, providing clinicians with one more regimen option when considering a AF patient's risk of bleeding and the risk of thromboembolic events after PCI-DES.
The strategies for dual therapy with ticagrelor that we tested incorporated two changes relative to the previous trials of antithrombotic therapy. The first change is the incorporation of ticagrelor as part of the dual therapy regimen for all patients with AF just after PCI-DES. The proportion of patients taking ticagrelor in our trial (every patient in the dual therapy group took oral ticagrelor, so the usage rate was 100%) was greater to that in the few other trials that have investigated ticagrelor as part of the regimens in patients with atrial fibrillation and PCI-DES (3–5.5% in the PIONEER trial; 7–8% in ENTRUST trial) (15, 16). The second change refers to the use of warfarin as the basic anticoagulant for the two groups of patients, instead of NOVC. This change was mainly based on economic reasons given that most Chinese patients prefer warfarin due to its relatively lower price, and at the time of enrollment, more than 90% of the patients were taking warfarin once daily as antithrombotic therapy for AF (35, 36); Secondly, access to NOACs in China was possible only after 2016.
There several limitations in the trial though we are the first trial that systematically evaluated the safety of ticagrelor as part of a dual antithrombotic regimen. First, the primary analyses showed that the safety of ticagrelor was similar to that of Triple therapy, and the result just reached a point of non-inferiority, rather than superiority, as anticipated. This outcome might be related to the potent antiplatelet effect of ticagrelor as discussed (37), and longer duration of antithrombotic administration as well as more additional antithrombotic administration during perioperative period. Secondly, We did not compare the risk of bleeding between the two groups at 1 week and 1 month, because current guidelines only recommend the shorter-term triple antithrombotic therapy (6, 28, 29, 38). Thirdly, the rates of primary endpoints of bleeding in this study were higher than those in the previous trials on this topic; in addition to over verified minor bleeding as discussed above, we suspected that to some extent this outcome might be related to increased variability in INR during the follow-up process and Asians being prone to bleeding when treated with warfarin (39, 40). In the end, the number of patients in our trial was relatively small for various reasons, including research funding, thus restricting the power of the study to detect worthwhile differences between the two groups. In addition, the progress of the trial was relatively slow due to physicians' and patients' concerns about various issues, such as safety in subcenters during the first year. However, though the trial did take us almost 5 years to complete, the patients were not highly selected, all of the data in the trial met the random requirements and the inclusion and exclusion criteria, and the two groups of patients were relatively homogenous.
Patients with AF undergoing PCI-DES treated with ticagrelor and warfarin in this trial experienced an incidence of total bleeding events that was comparable to those receiving a traditional triple antithrombotic regimen consisting of clopidogrel, aspirin, and warfarin. In this study we found that a dual antithrombotic therapy with ticagrelor is associated with similar incidence of bleeding and ischemic events as compared to a triple therapy with clopidogrel among AF patients undergoing PCI, however, we did not demonstrated dual therapy using ticagrelor reduced bleeding events compared to triple therapy. Further studies are warranted to shed light on the potential benefit of implementing a dual antithrombotic therapy with potent P2Y12 inhibitor in this setting.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by ICE for Clinical research of Zhongda Hospital Affiliated to Southeast University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The study was supported by AstraZeneca Pharmaceutical Co., Ltd. (No. ISSBRIL0256).
This study received funding from AstraZeneca Pharmaceutical Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge medical writing assistance provided by Ms. Sunita Rana and Dr. Kaushik Subramanian, Indegene Pvt Ltd., Bengaluru, India.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.745549/full#supplementary-material
1. Mishra A, Singh M, Acker WW, Kamboj S, Sporn D, Stapleton D, et al. Antithrombotic therapy in patients with atrial fibrillation and coronary artery disease undergoing percutaneous coronary intervention. J Cardiovasc Pharmacol. (2019) 74:82–90. doi: 10.1097/FJC.0000000000000697
2. European Heart Rhythm Association, European Association for Cardio- Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY, Schotten U, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European society of cardiology (ESC). Eur Heart J. (2010) 31:2369–429. doi: 10.1093/eurheartj/ehq278
3. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. (2019) 140:e125–51. doi: 10.1161/CIR.0000000000000665
4. Haller PM, Sulzgruber P, Kaufmann C, Geelhoed B, Tamargo J, Wassmann S, et al. Bleeding and ischaemic outcomes in patients treated with dual or triple antithrombotic therapy: systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. (2019) 5:226–36. doi: 10.1093/ehjcvp/pvz021
5. Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, et al. Safety and efficacy outcomes of double vs. Triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. (2019) 40:3757–67. doi: 10.1093/eurheartj/ehz732
6. Khan SU, Osman M, Khan MU, Khan MS, Zhao D, Mamas MA, et al. Dual versus triple therapy for atrial fibrillation after percutaneous coronary intervention: a systematic review and meta-analysis. Ann Intern Med. (2020) 172:474–83. doi: 10.7326/M19-3763
7. Galli M, Andreotti F, D'Amario D, Vergallo R, Montone RA, Porto I, et al. Dual therapy with direct oral anticoagulants significantly increases the risk of stent thrombosis compared to triple therapy. Eur Heart J Cardiovasc Pharmacother. (2020) 6:128–9. doi: 10.1093/ehjcvp/pvz030
8. Galli M, Andreotti F, Porto I, Crea F. Intracranial haemorrhages vs. Stent thromboses with direct oral anticoagulant plus single antiplatelet agent or triple antithrombotic therapy: a meta-analysis of randomized trials in atrial fibrillation and percutaneous coronary intervention/acute coronary syndrome patients. Europace. (2020) 22:538–46. doi: 10.1093/europace/euz345
9. Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D'Amario D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. (2021) 397:1470–83. doi: 10.1016/S0140-6736(21)00533-X
10. Galli M, Franchi F, Rollini F, Cavallari LH, Capodanno D, Crea F, et al. Genetic testing in patients undergoing percutaneous coronary intervention: rationale, evidence and practical recommendations. Expert Rev Clin Pharmacol. (2021) 14:963–78. doi: 10.1080/17512433.2021.1927709
11. Eyileten C, Postula M, Jakubik D, Toma A, Mirowska-Guzel D, Patti G, et al. Non-Vitamin K Oral anticoagulants (NOAC) versus vitamin k antagonists (VKA) for atrial fibrillation with elective or urgent percutaneous coronary intervention: a meta-analysis with a particular focus on combination type. J Clin Med. (2020) 9:1120. doi: 10.3390/jcm9041120
12. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
13. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. (2018) 39:1330–93. doi: 10.1093/eurheartj/ehy136
14. Lip GYH, Collet JP, Haude M, Byrne R, Chung EH, Fauchier L, et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace. (2019) 21:192–3. doi: 10.1093/europace/euy174
15. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without Aspirin in high-risk patients after PCI. N Engl J Med. (2019) 381:2032–42. doi: 10.1056/NEJMoa1908419
16. Baber U, Zafar MU, Dangas G, Escolar G, Angiolillo DJ, Sharma SK, et al. Ticagrelor with or without aspirin after PCI: the TWILIGHT platelet substudy. J Am Coll Cardiol. (2020) 75:578–86. doi: 10.1016/j.jacc.2019.11.056
17. Lopes RD, Vora AN, Liaw D, Granger CB, Darius H, Goodman SG, et al. An open-Label, 2 ×2 factorial, randomized controlled trial to evaluate the safety of apixaban vs. Vitamin K antagonist and aspirin vs. placebo in patients with atrial fibrillation and acute coronary syndrome and/or percutaneous coronary intervention: Rationale and design of the AUGUSTUS trial. Am Heart J. (2018) 200:17–23. doi: 10.1016/j.ahj.2018.03.001
18. Jones DW, Minhas S, Fierro JJ, Ardeshna D, Rawal A, Cave B, et al. From WOEST to AUGUSTUS: a review of safety and efficacy of triple versus dual antithrombotic regimens in patients with atrial fibrillation requiring percutaneous coronary intervention for acute coronary syndrome. Ann Transl Med. (2019) 7:405. doi: 10.21037/atm.2019.08.21
19. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. (2016) 375:2423–34. doi: 10.1056/NEJMoa1611594
20. Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. (2019) 394:1335–43. doi: 10.1016/S0140-6736(19)31872-0
21. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, et al. RE-DUAL PCI steering committee and investigators. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. (2017) 377:1513–24. doi: 10.1056/NEJMoa1708454
22. Lu W, Chen L, Wang Y, Yao Y, Fu C, Zuo P, et al. Rationale and design of MANJUSRI trial: a randomized, open-label, active-controlled multicenter study to evaluate the safety of combined therapy with ticagrelor and warfarin in AF subjects after PCI-DES. Contemp Clin Trials. (2015) 40:166–71. doi: 10.1016/j.cct.2014.12.002
23. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding academic research consortium. Circulation. (2011) 123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449
24. Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. (2005) 96:1200–6. doi: 10.1016/j.amjcard.2005.06.056
25. Bovill EG, Terrin ML, Stump DC, Berke AD, Frederick M, Collen D, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI), Phase II trial. Ann Intern Med. (1991) 115:256–65. doi: 10.7326/0003-4819-115-4-256
26. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2007) 357:2001–15. doi: 10.1056/NEJMoa0706482
27. Andreou I, Briasoulis A, Pappas C, Ikonomidis I, Alexopoulos D. Ticagrelor versus clopidogrel as part of dual or triple antithrombotic therapy: a systematic review and meta-analysis. Cardiovasc Drugs Ther. (2018) 32:287–94. doi: 10.1007/s10557-018-6795-9
28. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European society of cardiology (ESC) and of the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
29. Núñez-Gil IJ, Riha H, Ramakrishna H. Review of the 2017 European society of cardiology's guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation and focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with the European association for cardio-thoracic surgery. J Cardiothorac Vasc Anesth. (2019) 33:2334–43. doi: 10.1053/j.jvca.2018.09.032
30. Angiolillo DJ, Bhatt DL, Cannon CP, Eikelboom JW, Gibson CM, Goodman SG, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a north American perspective: 2021 update. Circulation. (2021) 143:583–96. doi: 10.1161/CIRCULATIONAHA.120.050438
31. Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention: a north american perspective-2016 update. Circ Cardiovasc Interv. (2016) 9:e004395. doi: 10.1161/CIRCINTERVENTIONS.116.004395
32. Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a north American perspective-2018 update. Circulation. (2018) 138:527–36. doi: 10.1161/CIRCULATIONAHA.118.034722
33. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al. AUGUSTUS investigators. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. (2019) 380:1509–24. doi: 10.1056/NEJMoa1817083
34. Moustafa A, Khan MS, Marei A, Alsamman MA, Baig M, Saad M. Safety and efficacy of dual versus triple antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention: a meta-analysis. Avicenna J Med. (2020) 10:232–40. doi: 10.4103/ajm.ajm_40_20
35. Wang Z, Tang Z, Zhu W, Ge L, Ge J. Efficacy and safety of traditional Chinese medicine on thromboembolic events in patients with atrial fibrillation: a systematic review and meta-analysis. Complement Ther Med. (2017) 32:1–10. doi: 10.1016/j.ctim.2017.03.006
36. Li J, Wang L, Hu J, Xu G. Warfarin use and the risks of stroke and bleeding in hemodialysis patients with atrial fibrillation: a systematic review and a meta-analysis. Nutr Metab Cardiovasc Dis. (2015) 25:706–13. doi: 10.1016/j.numecd.2015.03.013
37. Navarese EP, Khan SU, Kołodziejczak M, Kubica J, Buccheri S, Cannon CP, et al. Comparative efficacy and safety of oral P2Y12 inhibitors in acute coronary syndrome: network meta-analysis of 52 816 patients from 12 randomized trials. Circulation. (2020) 142:150–60. doi: 10.1161/CIRCULATIONAHA.120.046786
38. Kuno T, Ueyama H, Takagi H, Ando T, Numasawa Y, Briasoulis A, et al. Meta-analysis of antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am J Cardiol. (2020) 125:521–7. doi: 10.1016/j.amjcard.2019.11.022
39. Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. (2014) 111:789–97. doi: 10.1160/TH13-11-0948
Keywords: antithrombic therapy, atrial fibrillation, drug eluting stent, percutaneous coronary intervention, coronary artery disease
Citation: Lu W, Wang Y, Chen L, Li Y, Zhang R, Chen Z, Yan J, Yang M, Han B, Wang Z, He S, Chen L, Wu X, Zeng H, Ma L, Shi G, Yin J, Chen J and Ma G (2021) Antithrombotic Therapy With Ticagrelor in Atrial Fibrillation Subjects After Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 8:745549. doi: 10.3389/fcvm.2021.745549
Received: 22 July 2021; Accepted: 20 September 2021;
Published: 12 October 2021.
Edited by:
Andre Rodrigues Duraes, Federal University of Bahia, BrazilReviewed by:
Yuichi Saito, Chiba University Hospital, JapanCopyright © 2021 Lu, Wang, Chen, Li, Zhang, Chen, Yan, Yang, Han, Wang, He, Chen, Wu, Zeng, Ma, Shi, Yin, Chen and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: GenShan Ma, bWFnZW5zaGFuQHNldS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.