
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 23 September 2021
Sec. Cardiac Rhythmology
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.741377
 Ching-Yao Chou1,2
Ching-Yao Chou1,2 Fa-Po Chung1,3
Fa-Po Chung1,3 Hung-Yu Chang3,4
Hung-Yu Chang3,4 Yenn-Jiang Lin1,3
Yenn-Jiang Lin1,3 Li-Wei Lo1,3
Li-Wei Lo1,3 Yu-Feng Hu1,3
Yu-Feng Hu1,3 Tze-Fan Chao1,3
Tze-Fan Chao1,3 Jo-Nan Liao1,3
Jo-Nan Liao1,3 Ta-Chuan Tuan1,3
Ta-Chuan Tuan1,3 Chin-Yu Lin1,3
Chin-Yu Lin1,3 Ting-Yung Chang1,3
Ting-Yung Chang1,3 Chih-Min Liu1,3
Chih-Min Liu1,3 Cheng-I Wu1,3
Cheng-I Wu1,3 Sung-Hao Huang1,3,5
Sung-Hao Huang1,3,5 Chun-Chao Chen1
Chun-Chao Chen1 Wen-Han Cheng1,3
Wen-Han Cheng1,3 Shin-Huei Liu1,3
Shin-Huei Liu1,3 Isaiah Carlos Lugtu1,6
Isaiah Carlos Lugtu1,6 Ankit Jain1,7
Ankit Jain1,7 An-Ning Feng3,4
An-Ning Feng3,4 Shih-Lin Chang1,3*
Shih-Lin Chang1,3* Shih-Ann Chen1,3,8
Shih-Ann Chen1,3,8Background: Surgical scars cause an intra-atrial conduction delay and anatomical obstacles that facilitate the perpetuation of atrial flutter (AFL). This study aimed to investigate the outcome and predictor of recurrent atrial tachyarrhythmia after catheter ablation in patients with prior cardiac surgery for valvular heart disease (VHD) who presented with AFL.
Methods: Seventy-two patients with prior cardiac surgery for VHD who underwent AFL ablation were included. The patients were categorized into a typical AFL group (n = 45) and an atypical AFL group (n = 27). The endpoint was the recurrence of atrial tachyarrhythmia during follow-up. A multivariate analysis was performed to determine the predictor of recurrence.
Results: No significant difference was found in the recurrence rate of atrial tachyarrhythmia between the two groups. Patients with concomitant atrial fibrillation (AF) had a higher recurrence of typical AFL compared with those without AF (13 vs. 0%, P = 0.012). In subgroup analysis, typical AFL patients with concomitant AF had a higher incidence of recurrent atrial tachyarrhythmia than those without it (53 vs. 14%, P = 0.006). Regarding patients without AF, the typical AFL group had a lower recurrence rate of atrial tachyarrhythmia than the atypical AFL group (14 vs. 40%, P = 0.043). Multivariate analysis showed that chronic kidney disease (CKD) and left atrial diameter (LAD) were independent predictors of recurrence.
Conclusions: In our study cohort, concomitant AF was associated with recurrence of atrial tachyarrhythmia. CKD and LAD independently predicted recurrence after AFL ablation in patients who have undergone cardiac surgery for VHD.
In patients with prior cardiac surgery, atrial flutter (AFL) may develop with the critical isthmus located at the region bounded by surgical scar and the anatomical structure of the right or left atrium (1–5). Radiofrequency (RF) catheter ablation could be considered as the first-line therapy for atypical AFL when compared with antiarrhythmic drugs in patients with cardiac surgery history (6). It had been demonstrated that atypical AFL after surgery for congenital heart disease (CHD) could be successfully ablated in 50–90% of circuits with traditional entrainment or a three-dimensional (3D) mapping system (1–4, 7, 8). As for acquired heart disease, Aktas et al. had compared the ablation outcome of AFL between patients with and without prior cardiac surgery. The patients with prior cardiac surgery were likely to have a lower freedom from AFL and atrial fibrillation (AF) after AFL ablation than those without. The type of cardiac surgery was not emphasized in this study (9). Nabar et al. demonstrated that the success rate of ablation of atrial arrhythmia after open heart surgery (included CHD and valvular surgery) is ≥90%. The mean following period was relatively short term (12 months) (10). Enriquez et al. reported that macro-reentry was the predominant mechanism of atrial tachycardia (AT) in patients with mitral valve (MV) surgery. Repeated procedure could achieve a favorable outcome (11).
Our present study aimed to analyze the long-term outcomes after AFL catheter ablation in patients with prior cardiac surgery for valvular heart disease (VHD), including mitral, tricuspid, and aortic valve surgery and concomitant coronary artery bypass surgery (CABG). The type of flutter, impact of AF, and electrophysiologic mechanism were analyzed. Moreover, the predictors of recurrent atrial tachyarrhythmia for this patient group were determined.
This is a retrospective study that enrolled patients with a history of surgical intervention for VHD who underwent AFL catheter ablation. Two high-volume institutions in Taiwan were included in this study, namely, Taipei Veterans General Hospital and Cheng Hsin General Hospital. Eighty-two patients with a history of surgical intervention for VHD were included, all of whom received AFL catheter ablation between July 2008 and April 2019. Surgical intervention for VHD was defined as mitral valve, tricuspid valve, and aortic valve replacement or repair. Patients who received a concomitant CABG were also included. Clinical AF was defined as the diagnosis of AF before AFL catheter ablation. Concomitant AF ablation was not performed due to absence of clinical symptoms. The exclusion criteria included patients with AF who received pulmonary vein isolation (PVI) (n = 5), patients with focal AT who received catheter ablation (n = 3), and patients who received an incomplete procedure due to complications or unstable hemodynamics (n = 2). Finally, 72 patients were enrolled in the study. The patients were categorized into two groups according to the type of AFL catheter ablation they received: the typical AFL group (patients with typical AFL only; n = 45) and the atypical AFL group (patients who had an atypical AFL for catheter ablation, with or without concomitant typical AFL; n = 27). Baseline and electrophysiological characteristics were collected and analyzed.
This retrospective cohort study obtained ethical approval from the institutional review board of the Taipei Veterans General Hospital and was conducted in full compliance with national ethical and regulatory guidelines (11).
On surface 12-lead electrocardiography, a typical or atypical AFL could be identified by the flutter wave (12). Detailed electrophysiologic study and mapping has been described in our previous studies (13–15). Three-dimensional electroanatomical mapping systems were used in 26 (96.3%) of 27 patients in the atypical AFL group and 4 (8.9%) of 45 patients in the typical AFL group. The EnSite mapping system (St. Jude Medical, Minneapolis, MN, USA), Carto mapping system (Biosense-Webster Inc., Diamond Bar, CA, USA), Rhythmia (Boston Scientific Corporation, Middlesex County, MA, USA), and ablation catheters were used at the discretion of the treating electrophysiologist. The AFL circuit was confirmed using electroanatomical mapping and entrainment maneuvers.
Typical flutter was defined as common cavotricuspid isthmus (CTI)-dependent flutter including both counterclockwise (common) and clockwise (reverse common) variants, with the circuit originally described as a broad active wavefront rotating around the tricuspid annulus (16). With the traditional catheter mapping, concealed entrainment from the tricuspid isthmus was performed to confirm or exclude CTI-dependent flutter. A return cycle length after transient entrainment is equal to baseline cycle length (<20 ms difference) when pacing the flutter isthmus, right atrial roof, and anterior and septal right atrial walls (17). With the 3D mapping system, activation mapping should reveal the propagation around the tricuspid annulus. Ablation was performed by creating a linear lesion from the tricuspid annulus to the inferior vena cava. RF current was delivered through an 8-mm non-irrigated catheter using a temperature control mode with a maximal temperature of 60°C or 4-mm open-irrigated catheter using a power control mode with a maximal power of 30–40 W. Successful lesion formation was defined as a 90% reduction in electrogram amplitude. The endpoint of ablation was the bidirectional block across the line of ablation which was confirmed by differential pacing.
Atypical AFL was defined as the existence of non-CTI-dependent, macro-reentry circuit, which is common in the setting of prior atrial surgical scar. With the traditional catheter mapping, entrainment maneuver was performed at multiple sites and postpacing interval (PPI) not exceeding the cycle length by more than 20 ms was considered to be part of the circuit (18). With the 3D mapping system, activation mapping was used to reveal the flutter circuit. For right AFL, RF energy was delivered at the critical isthmus connecting to the anatomical obstacles (tricuspid annulus, inferior vena cava, or superior vena cava) until a 90% reduction in local electrogram amplitude was achieved and bidirectional block was observed. For left AFL, a transseptal puncture was performed with fluoroscopy guidance, and the activated clotting time was kept at ≥300 s with intravenous unfractionated heparin. RF energy was delivered to the critical isthmus connecting to the anatomical obstacles. In patients with scar-related AFL, the ablation strategy was to ablate the clinical AFL and any other potential flutter channel that could be identified. The endpoint of ablation was determined on the basis of AFL termination or interruption during ablation and negative inducibility of clinical AFL by programmed extra stimuli from the CS catheter with intravenous isoproterenol (1–5 μg/min) infused to achieve at least a 20% heart rate increment (19). The subsequent AFLs occurred owing to the interruption of the initial AFL circuit during ablation or induced by programmed stimulation after elimination of initial AFL.
After discharge following the index ablation procedure, the patients were followed up at 2 weeks and then regularly every 1–3 months at our cardiology outpatient clinic. Antiarrhythmic medicines were prescribed for 4–8 weeks after the procedure to prevent early recurrence of AF/AFL within 3 months. The blanking period was defined as within 3 months after ablation (20). Follow-up with 24-h Holter monitoring or 1-week cardiac event monitoring was performed 3 months after the ablation procedure and at any time if the patients experienced symptoms suggestive of tachyarrhythmia. Long-term efficacy was assessed based on resting surface 12-lead electrocardiograms (ECG), 24-h Holter monitoring records, and/or 1-week cardiac event monitoring records. Any atrial tachyarrhythmia such as AF, AFL, and AT lasting ≥30 s was defined as a recurrence. The follow-up period was up to September 30, 2019.
Continuous variables are expressed as mean ± standard deviation (SD). Categorical variables were compared using the chi-square test. Hazard ratio was calculated using univariate and multivariate logistic regression analyses with a stepwise analysis and confirmation. In the multivariate regression analysis, factors with P < 0.1 in the univariate analysis were selected for adjustment. The variables entered in the multivariate logistic regression analysis included chronic kidney disease (CKD) and left atrial diameter (LAD). A two-sided P < 0.05 was considered significant for all statistical determinations. All analyses were performed using IBM Statistical Product and Service Solutions (SPSS) Version 20.
The baseline characteristics are shown in Table 1. The flowsheet of the inclusion, exclusion, classification of subgroups, recurrence number, and patterns is provided in Figure 1. There were 45 patients in the typical AFL group and 27 patients in the atypical AFL group. No significant differences in baseline characteristics were found between these two groups, except for the higher incidence of hyperlipidemia in the atypical AFL group.
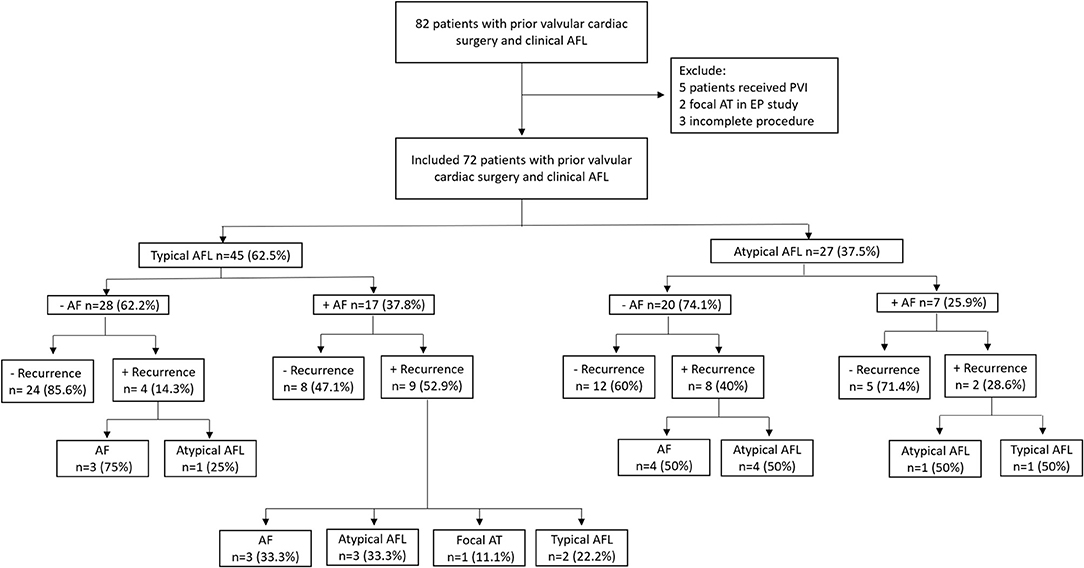
Figure 1. Flowsheet of the inclusion, classification of subgroups, recurrence number, and patterns in our study cohort. AFL, atrial flutter; AF, atrial fibrillation; AT, atrial tachycardia; PVI, pulmonary vein isolation; EP, electrophysiology.
The circuit number and the location of the isthmus of AFL in our study cohort are shown in Table 2. The mean number of reentrant circuits was 1.39 ± 0.72 in the total study population, while it was 2.04 ± 0.85 in the atypical AFL group. CTI-dependent AFL occurred in 17 patients in the atypical AFL group (17/27, 63.0%) and in 62 patients out of our total study cohort (62/72, 86.1%).
An example of circuits in typical and atypical AFL demonstrated by the 3D mapping system is shown in Figure 2. In comparison of the typical and atypical AFL group, the mean follow-up period was 24.3 ± 26.0 and 18.0 ± 13.3 months, respectively (P = 0.18). No significant differences in the prevalence of clinical AF, recurrence rate of atrial tachyarrhythmia, and type of recurrent atrial tachyarrhythmias (including AFL, atypical AFL, AF, and focal AT) were found between the two groups (Table 3). The Kaplan–Meier analysis revealed no significant difference in the recurrence-free survival of atrial tachyarrhythmia between the typical and atypical AFL groups (Figure 3A).
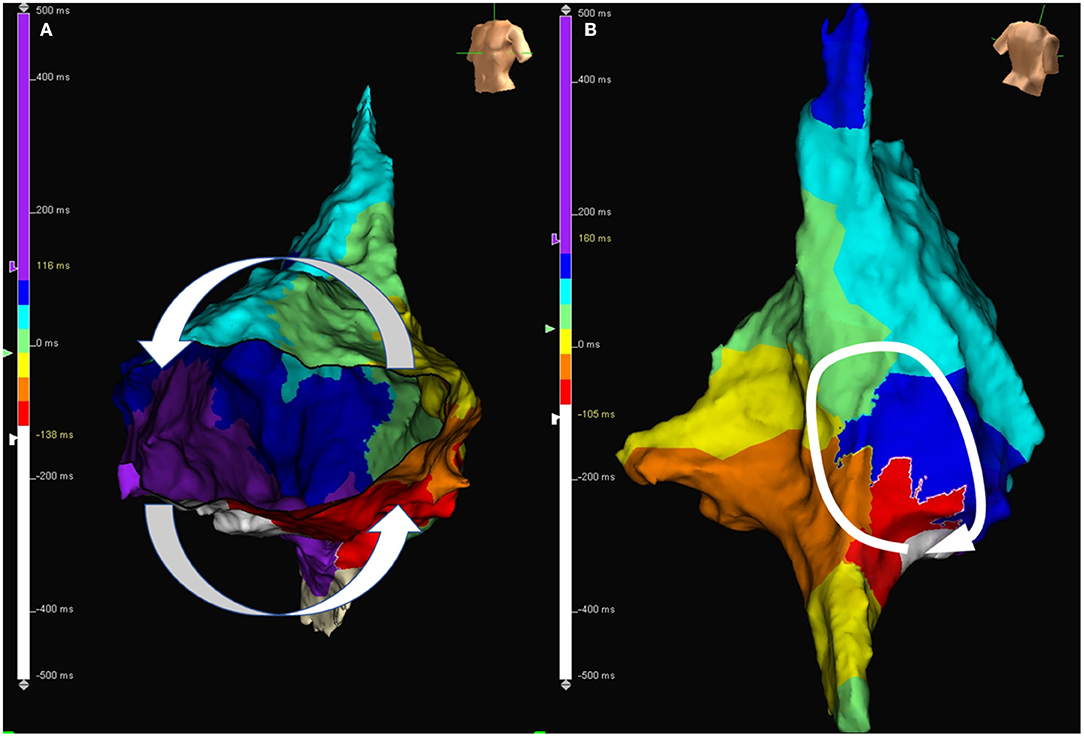
Figure 2. (A) Activation map during AFL in a patient with typical AFL. The activation wave front travels through cavotricuspid isthmus and goes around the tricuspid annulus. (B) Activation map during atypical atrial flutter in a patient with atypical AFL. The activation wave front goes around the surgical scar located at the right posterior free wall. AFL, atrial flutter.
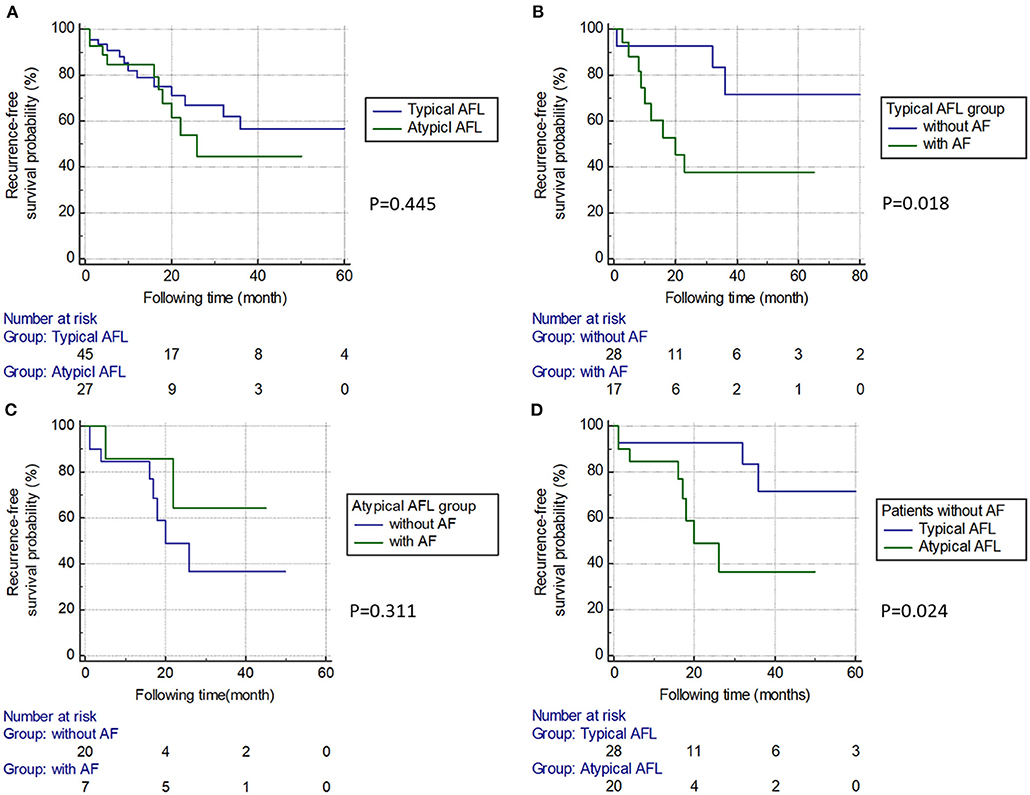
Figure 3. Kaplan–Meier survival curve between subgroups. (A) The typical and atypical AFL groups. There was no significant difference in the recurrence-free survival rate between the two groups. (B) Subgroups of patients with or without AF in the typical AFL group. Patients with AF had a higher recurrence rate than those without. (C) Subgroups of patients with or without AF in the atypical AFL group. There was no significant difference in the recurrence-free survival rate between the two groups. (D) In patients without AF, the atypical AFL group had a higher recurrence rate than the typical AFL group. AFL, atrial flutter; AF, atrial fibrillation.
As shown in Table 4, among 72 patients, 23 had concomitant AF (31.9%) before enrollment. Patients with concomitant AF had a trend toward a higher recurrence rate of atrial tachyarrhythmia compared to those without concomitant AF (P = 0.074). The recurrence rate of typical AFL was higher in patients with concomitant AF than those without concomitant AF (P = 0.012).
In the typical AFL group, 17 out of 45 (37.8%) cases had a concomitant AF, and these patients had a significantly higher recurrence rate of atrial tachyarrhythmia than those without concomitant AF (P = 0.006; Table 4 and Figure 3B). In the atypical AFL group, we found no significant difference in the recurrence rate of atrial tachyarrhythmia between the patients with and without concomitant AF (P = 0.331; Table 4 and Figure 3C).
In patients without concomitant AF (n = 48), the atypical AFL group had a higher recurrence rate of atrial tachyarrhythmia than the typical AFL group (P = 0.043; Table 4 and Figure 3D).
Among the 72 patients, 40 patients received single-valve surgery and 32 received multiple-valve surgery. Ten patients received concomitant on-pump CABG. The total number of valves treated by surgery in our study population was 110. The surgical detail is shown in Table 5.
In the comparison of the single-valve and multiple-valve groups, the occurrence rate of atypical AFL was the same (15/40 vs. 12/32, P = 1). In the single-valve surgery group, there was a trend toward an increased occurrence of atypical AFL in patients who underwent mitral valve (MV) surgery, when compared to aortic valve (AV) surgery (44.4 and 16.7%, P = 0.07, respectively). Only one patient received lone tricuspid valve (TV) surgery and developed atypical AFL.
LA flutter occurred in 10 patients in our study cohort (10/72, 13.9%), whereas the remaining 62 patients had RA flutter only (62/72, 86.1%). LA flutter was highly associated with prior MV surgery (9/10, 90%).
Table 6 shows the results of the univariate and multivariate Cox regression analyses of each risk factor. In the univariate analysis, CKD and LAD were associated with the recurrence of atrial tachyarrhythmia (P = 0.017 and 0.029, respectively). In the multivariate analysis, CKD and LAD were both identified as independent predictors of recurrent atrial tachyarrhythmia (P = 0.02, hazard ratio 2.73 and P = 0.04, hazard ratio 1.06, respectively).
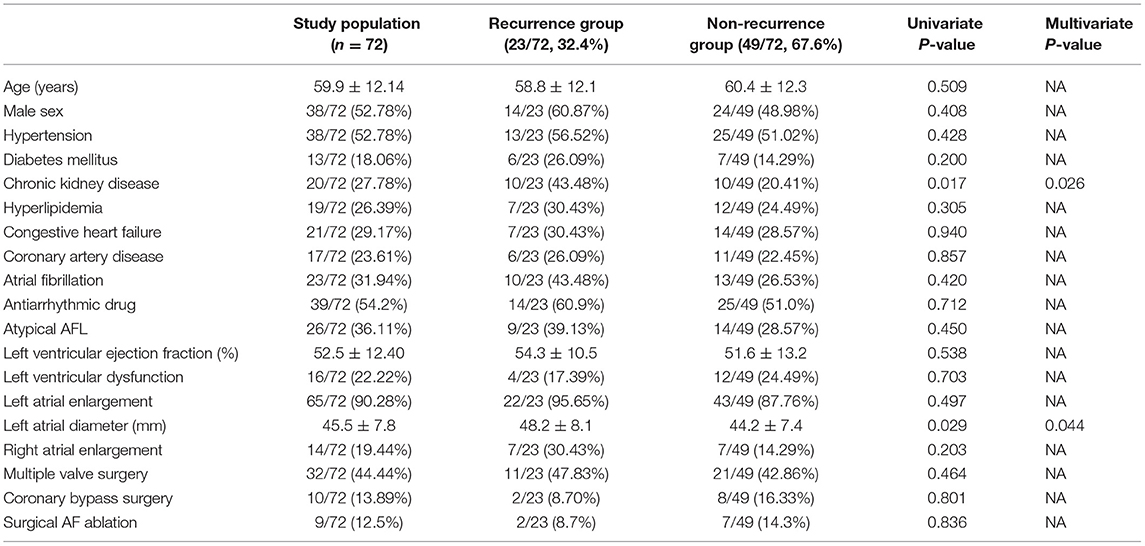
Table 6. Univariate and multivariate Cox regression analyses for each risk factor of recurrent atrial tachyarrhythmia.
We found no significant difference in the recurrence rate of atrial tachyarrhythmia between the typical and atypical AFL groups in our cohort. Patients with concomitant AF had a higher incidence of recurrent typical AFL than those without it. In the typical AFL group, patients with concomitant AF had a higher recurrence rate of atrial tachyarrhythmia than those without it. Among patients without concomitant AF, the atypical AFL group had a higher recurrence rate than the typical AFL group. LA flutter was less common than RA flutter and was highly associated with prior MV surgery. CKD and LAD were identified as predictors of recurrence of atrial tachyarrhythmia in patients who received AFL ablation after undergoing surgery for VHD.
Previous studies showed that typical AFL was the most common atrial arrhythmia and demonstrated that patients who had undergone cardiac surgery had higher recurrence rates of AFL (12 vs. 1%) and AF (28 vs. 16%) after AFL catheter ablation than those who had not undergone cardiac surgery (9, 10). Similarly, our present study showed that majority of the total study population (86%) had a CTI-dependent AFL. In the atypical AFL group, CTI-dependent AFL also occurred in 63.0% of the patients. The overall recurrence rate of atrial tachyarrhythmia in our study population was 31.9%. Surgical incisions, either left atriotomy or a trans-septal approach, provided a substrate for atrial arrhythmias, which may have contributed to the perpetuation of AFL (1–5, 23). A higher recurrence rate of atrial tachyarrhythmia after AFL ablation could be explained by the arrhythmogenic substrate in patients after cardiac surgery for VHD (24). As a result, these patients should be closely followed up even after successful elimination of the AFL during the ablation procedure.
Several studies have reported that successful AFL ablation decreased the AF recurrence in 50–75% of patients with a history of AF (25–33). One meta-analysis also demonstrated that the recurrence of AF was 52.7% in patients which a history of AF after a mean of 16 months following period (34). In our present study, the recurrence rate of AF was 12.5% after AFL ablation in patients with concomitant AF; however, the recurrence rate of AFL was 29.2% (12.5% typical AFL, 16.7% atypical AFL). In agreement with previous studies, AFL catheter ablation might be associated with decreased AF recurrence in our study cohort. The higher recurrence rate of AFL might be due to difference in arrhythmic substrate between patients with or without prior cardiac surgery. The underlying mechanism of the effects of AFL ablation on the reduction of AF recurrence rate remains unknown. We hypothesized that the presence of AFL could be a trigger to initiate other types of atrial tachyarrhythmias, and AFL could transform into AF over time. In contrast, in the typical AFL group, patients with concomitant AF had a higher recurrence rate of atrial tachyarrhythmia than those without it (52.9 vs. 14.3%). The presence of clinical AF may represent the diseased substrate and led to perpetuation of atrial tachyarrhythmia (35, 36).
Among patients without AF, a higher recurrence rate was found in the atypical AFL group than the typical AFL group in our study. This finding might be explained by atrial remodeling which is associated with a higher recurrence rate of atrial tachyarrhythmia. Patients with atypical AFL or multiple reentrant circuits might have a more complex and abnormal substrate than those with typical AFL only (14, 21).
Cardiac surgery was associated with a high occurrence of atrial arrhythmias. AF and AFL have been reported in up to 15–40% of patients in the postoperative period following CABG, and a higher occurrence was found in valve surgery (37–50%) (37). RA incision and atriotomy were very common in valve surgery, and surgical scar contributed to more incidence of atypical AFL when compared with RA appendage cannulation which was performed in CABG. Since the location of the AFL circuit was highly associated with different surgical incision, RA flutter was much more common than LA flutter (38, 39). The occurrence of LA flutter was highly associated with LA surgical approach for MV surgery (23, 38–40). In our study cohort, LA flutter occurred in only 13% (10/72) of patients, and 90% (9/10) of these patients had MV surgery. This result was compatible with previous studies. Our study also demonstrated that the number of valves receiving surgery, concomitant CABG, and surgical AF ablation did not affect the occurrence of atypical AFL or recurrence after AFL ablation. Notably, in our cohort of single-valve surgery, there was a trend toward a higher occurrence of atypical AFL in MV surgery than AV surgery (P = 0.07).
CKD and LAD were predictors of recurrent atrial tachyarrhythmia in our present study. The correlation between CKD and AF has been demonstrated. The prevalence of AF in patients with CKD was found to be between 18 and 21.2%, and concomitant AF and CKD had a negative effect on prognosis (41–44). In patients with CKD without a preexisting AF, AF was detected in 7.7% of the patients after 5 years of follow-up (44). Several risk factors for developing atrial arrhythmias in patients with CKD have been reported: increased inflammation, lower serum calcium level, increased platelet count, and higher ferritin level (42, 45, 46). These factors could enhance the arrhythmogenic substrate contributing to the recurrence of atrial tachyarrhythmia after AFL ablation.
LA enlargement is involved in the mechanism of atrial arrhythmia. Several score systems including LA diameter have been used to evaluate the progression of AF (47). LA size was also an independent predictor of atrial tachyarrhythmia recurrence after AF ablation. One meta-analysis study showed three important predictors of recurrence after AF ablation: valvular AF, LAD longer than 50 mm, and recurrence within 30 days after ablation (48). LA volume seemed to be a more accurate predictor of recurrence than the type of AF (paroxysmal or persistent) (49–51). In agreement with previous evidence, our study suggested that LAD played an important role to predict the recurrence of atrial tachyarrhythmia in patients with cardiac surgery for VHD.
First, this was a retrospective study, and therefore, there were limitations inherent in retrospective data analysis and interpretation. Second, our study included 32% of the patients with preprocedural AF who received index AFL ablation without concomitant AF ablation. The reason why concomitant AF ablation was not done for those patients was because the clinical and predominant arrhythmia was AFL which caused the major symptom in our study population. Based on the decision of physicians, only AFL ablation was performed for those patients. Moreover, antiarrhythmic drugs were used in significant proportions of the included patients, and it is unclear whether we can exclude potentially associated AF. Third, follow-up with 24-h Holter monitoring or 1-week cardiac event monitoring was performed 3 months after the ablation procedure and whenever the patients experienced symptoms suggestive of tachyarrhythmia. The recurrence rate in our study may be underestimated because of asymptomatic arrhythmia during long-term follow-up. Finally, some patients received cardiac surgery in other hospitals not within the two centers in this study or had surgery a long time ago; hence, surgical records were not available for review for details. Although the AFL mechanism and isthmus location could be provided by the 3D mapping system, there was limitation to determine the relationship between the surgical technique and the flutter circuits in each case. Therefore, the classification of our study groups into typical and atypical AFL might be oversimplified for the complex nature of these patients.
In our study cohort, we found no significant difference in the recurrence rate of atrial tachyarrhythmia between patients with typical and atypical AFL who had postsurgery VHD. In the typical AFL group, patients with AF had a higher recurrence rate of atrial tachyarrhythmia than those without AF. Among patients without concomitant AF, the atypical AFL group had a higher recurrence rate than the typical AFL group. LA flutter was less common than RA flutter and was highly associated with prior MV surgery. CKD and LAD were identified as independent predictors for recurrence of atrial tachyarrhythmia in patients who received AFL ablation with prior surgery for VHD. Close follow-up and monitoring of recurrent atrial tachyarrhythmia in this cohort are warranted.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This retrospective cohort study obtained ethical approval from the institutional review board of the Taipei Veterans General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
C-YC: data collection and writing. H-YC, C-YL, T-YC, C-ML, C-IW, S-HH, C-CC, W-HC, S-HL, IL, and AJ: help for data collection. F-PC, Y-JL, L-WL, Y-FH, T-FC, J-NL, T-CT, A-NF, and S-AC: supervision and manuscript correction. S-LC: writing review and edition. All authors contributed to the article and approved the submitted version.
This work was supported by Taipei Veterans General Hospital grants (V108C-055, V108C-032, C17-095, C19-027, V107C-041, V106C-056, VGHUST107-G1-7-1, MOST 107-2314-B-010-061-MY2, MOST 106-2314-B-010-046-MY3, MOST 106-2314-B-010-035-MY3, MOST 105-2314-B-075-036, MOST104-2314-B-075-0, and MOST104-2314-B-075-024-MY3) and the SZU-YUAN Research Foundation of Internal Medicine.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kalman JM, VanHare GF, Olgin JE, Saxon LA, Stark SI, Lesh MD. Ablation of 'incisional' reentrant atrial tachycardia complicating surgery for congenital heart disease. Use of entrainment to define a critical isthmus of conduction. Circulation. (1996) 93:502–12. doi: 10.1161/01.CIR.93.3.502
2. Akar JG, Kok LC, Haines DE, DiMarco JP, Mounsey JP. Coexistence of type I atrial flutter and intra-atrial re-entrant tachycardia in patients with surgically corrected congenital heart disease. J Am College Cardiol. (2001) 38:377–84. doi: 10.1016/S0735-1097(01)01392-4
3. Chan DP, Van Hare GF, Mackall JA, Carlson MD, Waldo AL. Importance of atrial flutter isthmus in postoperative intra-atrial reentrant tachycardia. Circulation. (2000) 102:1283–9. doi: 10.1161/01.CIR.102.11.1283
4. Lesh MD, Kalman JM, Saxon LA, Dorostkar PC. Electrophysiology of “incisional” reentrant atrial tachycardia complicating surgery for congenital heart disease. Pacing Clin Electrophysiol. (1997) 20:2107–11. doi: 10.1111/j.1540-8159.1997.tb03638.x
5. Nakagawa H, Shah N, Matsudaira K, Overholt E, Chandrasekaran K, Beckman KJ, et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: isolated channels between scars allow “focal” ablation. Circulation. (2001) 103:699–709. doi: 10.1161/01.CIR.103.5.699
6. Natale A, Newby KH, Pisano E, Leonelli F, Fanelli R, Potenza D, et al. Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J Am College Cardiol. (2000) 35:1898–904. doi: 10.1016/S0735-1097(00)00635-5
7. Delacretaz E, Ganz LI, Soejima K, Friedman PL, Walsh EP, Triedman JK, et al. Multi atrial maco-re-entry circuits in adults with repaired congenital heart disease: entrainment mapping combined with three-dimensional electroanatomic mapping. J Am College Cardiol. (2001) 37:1665–76. doi: 10.1016/S0735-1097(01)01192-5
8. Triedman JK, Alexander ME, Berul CI, Bevilacqua LM, Walsh EP. Electroanatomic mapping of entrained and exit zones in patients with repaired congenital heart disease and intra-atrial reentrant tachycardia. Circulation. (2001) 103:2060–5. doi: 10.1161/01.CIR.103.16.2060
9. Aktas MK, Khan MN, Di Biase L, Elayi C, Martin D, Saliba W, et al. Higher rate of recurrent atrial flutter and atrial fibrillation following atrial flutter ablation after cardiac surgery. J Cardiovasc Electrophysiol. (2010) 21:760–5. doi: 10.1111/j.1540-8167.2009.01709.x
10. Nabar A, Timmermans C, Medeiros A, Polymeropoulos K, Crijns HJ, Rodriguez LM. Radiofrequency ablation of atrial arrhythmias after previous open-heart surgery. Europace. (2005) 7:40–9. doi: 10.1016/j.eupc.2005.07.001
11. Enriquez A, Santangeli P, Zado ES, Liang J, Castro S, Garcia FC, et al. Postoperative atrial tachycardias after mitral valve surgery: Mechanisms and outcomes of catheter ablation. Heart Rhythm. (2017) 14:520–6. doi: 10.1016/j.hrthm.2016.12.002
12. Chang S-L, Chen S-A. Right and left atrial macroreentrant tachycardias. Cardiac Electrophysiol Clinics. (2014) 6:469–81. doi: 10.1016/j.ccep.2014.05.004
13. Chen SA, Chiang CE, Wu TJ, Tai CT, Lee SH, Cheng CC, et al. Radiofrequency catheter ablation of common atrial flutter: comparison of electrophysiologically guided focal ablation technique and linear ablation technique. J Am College Cardiol. (1996) 27:860–8. doi: 10.1016/0735-1097(95)00565-X
14. Chang SL, Tai CT, Lin YJ, Wongcharoen W, Lo LW, Lee KT, et al. The role of left atrial muscular bundles in catheter ablation of atrial fibrillation. J Am College Cardiol. (2007) 50:964–73. doi: 10.1016/j.jacc.2007.05.026
15. Chang SL, Tsao HM, Lin YJ, Lo LW, Hu YF, Tuan TC, et al. Differentiating macroreentrant from focal atrial tachycardias occurred after circumferential pulmonary vein isolation. J Cardiovasc Electrophysiol. (2011) 22:748–55. doi: 10.1111/j.1540-8167.2010.02002.x
16. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. (2017) 14:e275–444. doi: 10.1016/j.hrthm.2017.05.012
17. Saoudi N, Cosio F, Waldo A, Chen SA, Iesaka Y, Lesh M, et al. A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases; a Statement from a Joint Expert Group from The Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. (2001) 22:1162–82. doi: 10.1053/euhj.2001.2658
18. Chang SL, Lin YJ, Tai CT, Lo LW, Tuan TC, Udyavar AR, et al. Induced atrial tachycardia after circumferential pulmonary vein isolation of paroxysmal atrial fibrillation: electrophysiological characteristics and impact of catheter ablation on the follow-up results. J Cardiovasc Electrophysiol. (2009) 20:388–94. doi: 10.1111/j.1540-8167.2008.01358.x
19. Higa S, Tai CT, Lin YJ, Liu TY, Lee PC, Huang JL, et al. Focal atrial tachycardia: new insight from noncontact mapping and catheter ablation. Circulation. (2004) 109:84–91. doi: 10.1161/01.CIR.0000109481.73788.2E
20. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Heart Rhythm. (2017). 14:e445–94. doi: 10.1093/europace/eux275
21. Lin YJ, Higa S, Tai CT, Chang SL, Lee KT, Lo LW, et al. Role of the right atrial substrate in different types of atrial arrhythmias. Heart Rhythm. (2009) 6:592–8. doi: 10.1016/j.hrthm.2009.02.019
22. Tai CT, Chen SA. Electrophysiological mechanisms of atrial flutter. JCMA. (2009) 72:60–7. doi: 10.1016/S1726-4901(09)70024-3
23. Tambeur L, Meyns B, Flameng W, Daenen W. Rhythm disturbances after mitral valve surgery: comparison between left atrial and extended trans-septal approach. Cardiovasc Surg. (1996) 4:820–4. doi: 10.1016/S0967-2109(96)00054-3
24. Markowitz SM, Brodman RF, Stein KM, Mittal S, Slotwiner DJ, Iwai S, et al. Lesional tachycardias related to mitral valve surgery. J Am College Cardiol. (2002) 39:1973–83. doi: 10.1016/S0735-1097(02)01905-8
25. Saoudi N, Atallah G, Kirkorian G, Touboul P. Catheter ablation of the atrial myocardium in human type I atrial flutter. Circulation. (1990) 81:762–71. doi: 10.1161/01.CIR.81.3.762
26. Feld GK, Fleck RP, Chen PS, Boyce K, Bahnson TD, Stein JB, et al. Radiofrequency catheter ablation for the treatment of human type 1 atrial flutter. Identification of a critical zone in the reentrant circuit by endocardial mapping techniques. Circulation. (1992) 86:1233–40. doi: 10.1161/01.CIR.86.4.1233
27. Cosio FG, Lopez-Gil M, Goicolea A, Arribas F, Barroso JL. Radiofrequency ablation of the inferior vena cava-tricuspid valve isthmus in common atrial flutter. Am J Cardiol. (1993) 71:705–9. doi: 10.1016/0002-9149(93)91014-9
28. Hsieh MH, Tai CT, Chiang CE, Tsai CF, Yu WC, Chen YJ, et al. Recurrent atrial flutter and atrial fibrillation after catheter ablation of the cavotricuspid isthmus: a very long-term follow-up of 333 patients. J Intervent Cardiac Electrophysiol. (2002) 7:225–31. doi: 10.1023/a:1021392105994
29. Philippon F, Plumb VJ, Epstein AE, Kay GN. The risk of atrial fibrillation following radiofrequency catheter ablation of atrial flutter. Circulation. (1995) 92:430–5. doi: 10.1161/01.CIR.92.3.430
30. Paydak H, Kall JG, Burke MC, Rubenstein D, Kopp DE, Verdino RJ, et al. Atrial fibrillation after radiofrequency ablation of type I atrial flutter: time to onset, determinants, and clinical course. Circulation. (1998) 98:315–22. doi: 10.1161/01.CIR.98.4.315
31. Katritsis D, Iliodromitis E, Fragakis N, Adamopoulos S, Kremastinos D. ablation therapy of type i atrial flutter may eradicate paroxysmal atrial fibrillation. Am J Cardiol. (1996) 78:345–7. doi: 10.1016/S0002-9149(96)00291-3
32. Movsowitz C, Callons DJ, Schwartzman D, Gottlieb C, Marchlinski FE. The results of atrial flutter ablation in patients with and without a history of atrial fibrillation. Am J Cardiol. (1996) 78:93–6. doi: 10.1016/S0002-9149(96)00233-0
33. Kumagai K, Tojo H, Noguchi H, Yasuda T, Tamari H, Matsumoto N, et al. Effects of cavotricuspid isthmus catheter ablation on paroxysmal atrial fibrillation. Japan Heart J. (2001) 42:79–89. doi: 10.1536/jhj.42.79
34. Perez FJ, Schubert CM, Parvez B, Pathak V, Ellenbogen KA, Wood MA. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis. Circulation Arrhythmia Electrophysiol. (2009) 2:393–401. doi: 10.1161/CIRCEP.109.871665
35. Huang JL, Tai CT, Lin YJ, Ueng KC, Huang BH, Lee KT, et al. Right atrial substrate properties associated with age in patients with typical atrial flutter. Heart Rhythm. (2008) 5:1144–51. doi: 10.1016/j.hrthm.2008.05.009
36. Tai CT, Chen SA, Chiang CE, Lee SH, Wen ZC, Huang JL, et al. Long-term outcome of radiofrequency catheter ablation for typical atrial flutter: risk prediction of recurrent arrhythmias. J Cardiovasc Electrophysiol. (1998) 9:115–21. doi: 10.1111/j.1540-8167.1998.tb00892.x
37. Fatemi M, Leledy M, Le Gal G, Bezon E, Mondine P, Blanc JJ. Atrial flutter after non-congenital cardiac surgery: incidence, predictors and outcome. Int J Cardiol. (2011) 153:196–201. doi: 10.1016/j.ijcard.2010.08.021
38. Pap R, Kohari M, Makai A, Bencsik G, Traykov VB, Gallardo R, et al. Surgical technique and the mechanism of atrial tachycardia late after open heart surgery. J Intervent Cardiac Electrophysiol. (2012) 35:127–35. doi: 10.1007/s10840-012-9705-2
39. Kohari M, Pap R. Atrial tachycardias occurring late after open heart surgery. Curr Cardiol Rev. (2015) 11:134–40. doi: 10.2174/1573403X10666141013122021
40. Lukac P, Hjortdal VE, Pedersen AK, Mortensen PT, Jensen HK, Hansen PS. Atrial incision affects the incidence of atrial tachycardia after mitral valve surgery. Ann Thoracic Surg. (2006) 81:509–13. doi: 10.1016/j.athoracsur.2005.07.083
41. Ferreira C, Providência R, Ferreira MJ, Gonçalves LM. Atrial fibrillation and non-cardiovascular diseases: a systematic review. Arquivos Brasil Cardiol. (2015) 105:519–26. doi: 10.5935/abc.20150142
42. Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol. (2010) 5:173–81. doi: 10.2215/CJN.03170509
43. Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, et al. Chronic kidney disease and prevalent atrial fibrillation: The Chronic Renal Insufficiency Cohort (CRIC). Am Heart J. (2010) 159:1102–7. doi: 10.1016/j.ahj.2010.03.027
44. Bansal N, Fan D, Hsu Cy, Ordonez JD, Go AS. Incident atrial fibrillation and risk of death in adults with chronic kidney disease. J. Am. Heart Assoc. (2014) 3:1303. doi: 10.1161/JAHA.114.001303
45. Guo Y, Lip GYH, Apostolakis S. Inflammation in atrial fibrillation. J Am College Cardiol. (2012) 60:2263–70. doi: 10.1016/j.jacc.2012.04.063
46. Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. (2003) 107:87–92. doi: 10.1161/01.CIR.0000042700.48769.59
47. Deng H, Bai Y, Shantsila A, Fauchier L, Potpara TS, Lip GYH. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: a systematic review. Clin Res Cardiol. (2017) 106:813–23. doi: 10.1007/s00392-017-1123-0
48. D'Ascenzo F, Corleto A, Biondi-Zoccai G, Anselmino M, Ferraris F, di Biase L, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: a meta-analysis. Int J Cardiol. (2013) 167:1984–9. doi: 10.1016/j.ijcard.2012.05.008
49. Costa FM, Ferreira AM, Oliveira S, Santos PG, Durazzo A, Carmo P, et al. Left atrial volume is more important than the type of atrial fibrillation in predicting the long-term success of catheter ablation. Int J Cardiol. (2015) 184:56–61. doi: 10.1016/j.ijcard.2015.01.060
50. Njoku A, Kannabhiran M, Arora R, Reddy P, Gopinathannair R, Lakkireddy D, et al. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. Europace. (2018) 20:33–42. doi: 10.1093/europace/eux013
51. Nedios S, Kosiuk J, Koutalas E, Kornej J, Sommer P, Arya A, et al. Comparison of left atrial dimensions in CT and echocardiography as predictors of long-term success after catheter ablation of atrial fibrillation. J Intervent Cardiac Electrophysiol. (2015) 43:237–44. doi: 10.1007/s10840-015-0010-8
Keywords: atrial flutter, valvular heart disease, ablation, atrial fibrillation, heart surgery
Citation: Chou C-Y, Chung F-P, Chang H-Y, Lin Y-J, Lo L-W, Hu Y-F, Chao T-F, Liao J-N, Tuan T-C, Lin C-Y, Chang T-Y, Liu C-M, Wu C-I, Huang S-H, Chen C-C, Cheng W-H, Liu S-H, Lugtu IC, Jain A, Feng A-N, Chang S-L and Chen S-A (2021) Prediction of Recurrent Atrial Tachyarrhythmia After Receiving Atrial Flutter Ablation in Patients With Prior Cardiac Surgery for Valvular Heart Disease. Front. Cardiovasc. Med. 8:741377. doi: 10.3389/fcvm.2021.741377
Received: 14 July 2021; Accepted: 25 August 2021;
Published: 23 September 2021.
Edited by:
Hung-Fat Tse, The University of Hong Kong, Hong Kong, SAR ChinaReviewed by:
Hui-Nam Pak, Yonsei University Health System, South KoreaCopyright © 2021 Chou, Chung, Chang, Lin, Lo, Hu, Chao, Liao, Tuan, Lin, Chang, Liu, Wu, Huang, Chen, Cheng, Liu, Lugtu, Jain, Feng, Chang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shih-Lin Chang, c2xjaGFuZzRAdmdodHBlLmdvdi50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.