
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 20 January 2022
Sec. Coronary Artery Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.736868
Background: Vascular inflammation plays an important role in the pathogenesis and development of acute coronary syndrome (ACS). However, studies on the association between elevated pentraxin-3 level and adverse outcomes in patients with ACS have yielded controversial results. The purpose of this meta-analysis was to assess the value of elevated pentraxin-3 level as an inflammatory marker for predicting adverse outcomes in patients with ACS.
Methods: Two authors systematically searched the articles indexed in PubMed, Embase, CNKI, Wanfang, and VIP databases up to March 31, 2021. Studies reporting the association of elevated pentraxin-3 level at the acute phase with cardiovascular mortality, all-cause mortality, or cardiac events (cardiac death, non-fatal myocardial infarction, revascularization, or heart failure) in patients with ACS were included.
Results: A total of 8,775 ACS patients from 12 studies were identified and analyzed. When compared the lowest pentraxin-3 level, ACS patients with the highest pentraxin-3 level conferred an increased risk of cardiovascular mortality [risk ratio (RR) 2.10; 95% CI 1.44–3.06], all-cause mortality (RR 1.99; 95% CI 1.46–2.71), and cardiac events (RR 1.74; 95% CI 1.32–2.29), even after adjustment for some important confounders. Subgroup analysis indicated that the association of elevated pentraxin-3 level with cardiac events appeared to be stronger in ST-segment elevation myocardial infarction patients (RR 2.72; 95% CI 1.69–4.36) than in all patients with ACS (RR 1.59; 95% CI 1.10–2.29).
Conclusions: Elevated pentraxin-3 level is possibly an independent predictor of adverse outcomes in patients with ACS. Assessment of pentraxin-3 level at the acute phase can provide important information for early risk stratification of ACS.
Acute coronary syndrome (ACS) refers to a group of conditions, namely, ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina. Despite advances in evidence-based therapies, ACS remains a major cause of morbidity and mortality around the world (1). However, risk prediction of morbidity and mortality is still challenging for patients with ACS.
The common cause of ACS is atherosclerotic plaque rupture or erosion with inflammation and subsequent coronary thrombosis (2). Increased local and systemic inflammation plays an important role in the development of ACS (3). Many inflammatory markers have been identified as strong and independent predictors of cardiovascular events in patients with ACS (4). Pentraxin-3 is mainly synthesized by macrophages, neutrophils, endothelial cells, and other cell types in response to acute inflammatory stimuli (5, 6). According to the primary structure of the subunit, the pentraxin family is divided into short pentraxins and long pentraxins (7, 8). Although from the same protein family as C-reactive protein (CRP), pentraxin-3 is predominantly expressed in atherosclerotic plaques (9). Therefore, pentraxin-3 may be a promising biomarker for inflammatory vascular disease. Apart from its diagnostic value (10, 11), several studies (12–19) have examined the role of pentraxin-3 level as a predictor of adverse outcomes in patients with ACS. However, the utility of pentraxin-3 as a prognostic biomarker remained conflicting in this population (20–22).
A previous meta-analysis has assessed the predictive value of elevated pentraxin-3 level in patients with coronary artery disease (CAD) (23). However, the results of this meta-analysis were limited by a small number of studies in the ACS subgroup. To clarify the pentraxin-3 as a prognostic biomarker, we undertook this meta-analysis to evaluate the value of elevated pentraxin-3 level for predicting adverse outcomes in ACS patients.
This meta-analysis was performed in accordance with the checklists of the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement (24). Two reviewers independently searched the articles indexed in PubMed, Embase, CNKI, Wanfang, and VIP databases up to March 31, 2021, using the following keywords in combination: “pentraxin-3” AND “acute coronary syndrome” OR “acute myocardial infarction” OR “unstable angina.” Reference lists of pertinent articles were manually reviewed to identify additional eligible studies.
The inclusion criteria were as follows: (1) participants with clinical diagnosis of ACS (including STEMI, NSTEMI, and unstable angina); (2) cohort studies and post hoc analysis of clinical trials; (3) acute phase pentraxin-3 level as a predictor; (4) cardiovascular mortality, all-cause mortality, or cardiac events (cardiac death, recurrent myocardial infarction, revascularization, unstable angina pectoris, or heart failure) as an outcome of interests; and (5) reported multivariate-adjusted risk ratio (RR), hazard ratio (HR) or odds ratio (OR) and corresponding 95% CI of adverse outcomes for the highest pentraxin-3 group vs. the lowest pentraxin-3 group. The non-inclusion criteria included: (1) participants were not restricted in patients with ACS (enrolling all types of CAD); (2) risk estimate reported by univariate analysis; (3) analyzed the predictive value of pentraxin-3 level by continuous data; and (4) reviews or conference abstract.
Two reviewers independently scanned the titles or abstracts, retrieved the potentially eligible full-text articles, and evaluated the methodological quality. Any disagreements were resolved by discussion with a third reviewer. The extracted data included: a surname of the first author, time of publication, the origin of patients, study design, type or subtype of patients, sample sizes, age, gender distribution, the definition of cardiac events, cutoff value of the elevated pentraxin-3 level, length of follow-up, maximally adjusted risk summary, adjustment for variables, and items of methodological quality. The study quality was evaluated using a 9-point Newcastle–Ottawa Scale (NOS) (25). Studies with a score of 7 points or over were considered as high-quality.
All data were analyzed using STATA 12.0 (StataCorp, TX, USA). To facilitate meta-analysis, the OR and HR reported in the original studies were directly considered as approximately RR. Heterogeneity between studies was checked using the I2 statistic and Cochran's Q test. A random-effect model was utilized if there was evidence of significant heterogeneity (I2 statistic > 50% and/or P value < 0.1 of Cochran's Q test); otherwise, we used a fixed-effect model for analysis. Subgroup analyses were performed according to study design, ACS subtypes, sample sizes, and length of follow-up. In addition, we conducted a leave-out-one study sensitivity analysis to examine the robustness of the original pooling risk estimate and sources of the heterogeneity. Publication bias was investigated by visual inspection of funnel plot if more than 10 studies were included in the analysis.
Figure 1 summarizes the process of study selection. A total of 786 potentially relevant records were identified by searching the electronic medical databases. After removing duplicated records and reviewing the titles and abstracts, 40 articles were retrieved for full-text evaluation. Of which, 28 articles were further removed after applying the predefined inclusion criteria. Thus, 12 studies (12–17, 21, 22, 26–29) were ultimately included in this meta-analysis.
Table 1 describes the baseline characteristics of the included studies. Two studies (13, 22) adopted the retrospective design and others were prospective studies. A total of 8,775 patients with ACS were identified, with sample sizes ranging from 79 to 5,154. The percentage of male gender ranged from 41.7 to 85.7%. Follow-up duration varied from 30 days to 84 months. According to the NOS criteria, the included studies were grouped as moderate to high quality (6–8 NOS points).
Seven studies (13, 16, 17, 21, 22, 26, 27) reported the association of pentraxin-3 level with cardiac events. A random-effect meta-analysis indicated that elevated pentraxin-3 level was associated with an increased risk of cardiac events (RR 1.74; 95% CI 1.32–2.29; I2 = 48.9%; P = 0.068; Figure 2). Sensitivity analysis did not significantly alter the original predictive significance (data not shown). Subgroup analysis (Table 2) showed that the value of elevated pentraxin-3 in predicting cardiac events was stronger in STEMI patients (RR 2.72) than all patients with ACS (RR 1.59).
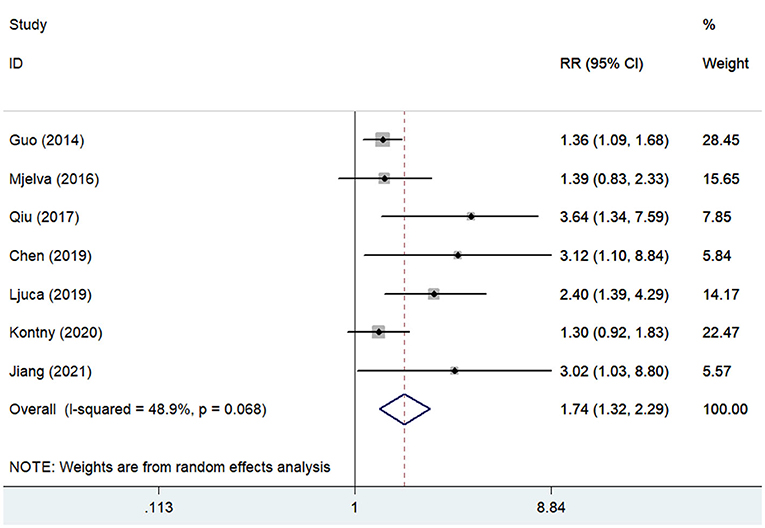
Figure 2. Forest plots showing the pooled multivariate-adjusted RR and 95% CI of cardiac events for the highest pentraxin-3 level vs. the lowest pentraxin-3 level.
Four studies (12, 14, 21, 28) reported the association between pentraxin-3 level and all-cause mortality. A fixed-effect meta-analysis showed that elevated pentraxin-3 level was associated with higher risk of all-cause mortality (RR 1.99; 95% CI 1.46–2.71; I2 = 47.7%; P = 0.125; Figure 3). Sensitivity analysis did not markedly change the originally predictive significance (data not shown).
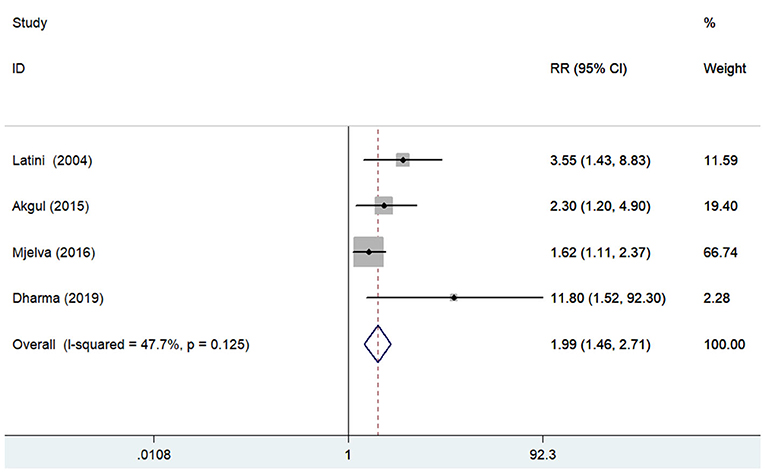
Figure 3. Forest plots showing the pooled multivariate-adjusted RR and 95% CI of all-cause mortality for the highest pentraxin-3 level vs. the lowest pentraxin-3 level.
Four studies (13, 15, 22, 29) reported the association of pentraxin-3 level with cardiovascular mortality. A random-effect meta-analysis showed that elevated pentraxin-3 level was associated with a higher risk of cardiovascular mortality (RR 2.10; 95% CI 1.44–3.06; I2 = 58.4%; P = 0.065; Figure 4). Sensitivity analysis did not significantly change the original predictive significance (data not shown).
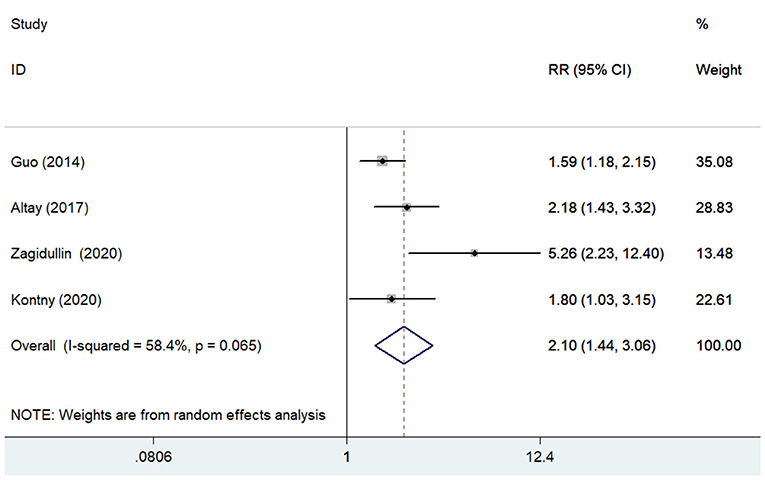
Figure 4. Forest plots showing the pooled multivariate-adjusted RR and 95% CI of cardiovascular mortality for the highest pentraxin-3 level vs. the lowest pentraxin-3 level.
Because of less than recommended an arbitrary number of 10 studies, we did not construct the funnel plots to examine the likelihood of publication bias (30).
The main finding of the current meta-analysis indicates that elevated blood level of pentraxin-3 is significantly associated with a higher risk of cardiac events, cardiovascular mortality, and all-cause mortality in patients with ACS, even after adjustment for confounders. ACS patients with the highest pentraxin-3 level conferred ~74%, 1.99-fold, and 2.1-fold higher risk of cardiac events, all-cause mortality, and cardiovascular mortality, respectively.
In addition to categorical data analysis of pentraxin-3 level, each ten-fold increase in pentraxin-3 level was associated with a 3.86-fold higher risk of cardiac events in patients with NSTEMI and unstable angina (19). Moreover, per 50% increase in acute phase pentraxin-3 level was associated with a 7% higher risk of cardiovascular mortality after adjustment for confounders in patients with ACS (11). These findings further supported the predictive role of elevated pentraxin-3 level in patients with ACS. However, the controversial result remained using continuous variable analysis of pentraxin-3 in patients with non-ST-elevation ACS (20).
Acute coronary syndrome (ACS) includes heterogeneous patient populations. Our subgroup analysis showed that the value of elevated pentraxin-3 in predicting cardiac events appeared to be stronger in STEMI patients compared with all ACS patients. This result may be correlated with a higher median pentraxin-3 level in STEMI than in NSTEMI patients (31). Pentraxin-3 level was significantly correlated with infarct size (32) and thrombus burden (28). When we grouped the studies by the length of follow-up, the value of pentraxin-3 in predicting cardiac events was evident in studies with short-term follow-up than those with relatively long-term follow-up. This finding suggested that the pentraxin-3 level may provide important information for the early risk stratification of ACS patients. Notably, the results of subgroup analysis should be interpreted with caution due to the limited number of studies analyzed.
Pentraxin-3 is an early biomarker of local inflammation in the vasculature (33). Blood pentraxin-3 level positively correlated with cardiac troponin I, creatine kinase-MB, and high-sensitivity CRP (15, 27). Elevated pentraxin-3 level may reflect an acute inflammatory response induced by myocardial injury. Moreover, the pentraxin-3 level was correlated with plaque vulnerability (34). Both the degree of inflammation and plaque vulnerability contribute to the adverse clinical events. Pentraxin-3 and CRP may represent different inflammatory processes of atherosclerosis. The increased blood level of the pentraxin protein family member CRP was also associated with a higher long-term risk of recurrent cardiovascular events and mortality in patients with ACS (35). An interesting issue is whether the predictive value of pentraxin-3 is superior to CRP. Considering pentraxin-3 level peaked earlier than CRP (22, 36), therefore it may be a better prognostic marker than CRP in acute phase ACS (19, 21, 26). Also, the pentraxin-3 level was more closely associated with the angiographic complexity and severity of coronary disease compared with CRP (37).
This meta-analysis has potential implications for clinical practice. Detection of blood pentraxin-3 level has the potential to identify high-risk ACS patients. ACS patients with elevated pentraxin-3 level should be received more closely monitor and active evidence-based therapies. Correspondingly, ACS patients with a high level of pentraxin-3 may potentially benefit from anti-inflammatory therapies. However, future well-designed clinical trials are necessary to support this hypothesis.
There were several potential limitations in our meta-analysis. First, a single measurement of the blood level of pentraxin-3 rather than a dynamic monitor may have resulted in selection bias. Second, different cut-off points of pentraxin-3 elevation used across studies prevent clinicians to identify those in need of closely monitor and active treatment. Future studies should further determine the optimal cut-off value for pentraxin-3 elevation. Third, we failed to analyze the predictive role of pentraxin-3 by continuous data because of the small number of such studies. Fourth, the majority of study outcomes were limited by considerable and at least moderate heterogeneity (~50% or more), which may be partly interpreted by the different cut-off points of pentraxin-3 elevation, the definition of outcomes, subtypes of ACS, or length of follow-up. Finally, we pooled the studies reporting different risk estimates (RR, OR, and HR) and approximated OR and HR to RR. This approach may have led to a slight overestimation of actual predictive value.
This meta-analysis suggests that the blood level of pentraxin-3 as an inflammatory marker possibly independently predicts cardiac events, cardiovascular mortality, and all-cause mortality in patients with ACS. Detection of acute-phase pentraxin-3 level can provide important information for early risk stratification of ACS.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
DG: study conception, design, and revised the article critically for important intellectual content. YF and RH: acquisition of data, analysis, and interpretation of data. YF and CM: statistical analysis. YF drafted the article. All authors read the final approval of the version to be published.
This work is supported by (1) the Jiangsu Innovative Team Leading Talent Fund (CXTDC2016006, QNRC2016446); (2) the Zhenjiang Key Research and Development Fund (SH2021038), and (3) the Suqian Science and Technology Support Project Fund (K201907).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. (2018) 137:e67–e492. doi: 10.1161/CIR.0000000000000558
2. Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. (2017) 136:1155–66. doi: 10.1161/CIRCULATIONAHA.117.029870
3. Sager HB, Nahrendorf M. Inflammation: a trigger for acute coronary syndrome. Q J Nucl Med Mol Imaging. (2016) 60:185–93.
4. Fiechter M, Ghadri JR, Jaguszewski M, Siddique A, Vogt S, Haller RB, et al. Impact of inflammation on adverse cardiovascular events in patients with acute coronary syndromes. J Cardiovasc Med. (2013) 14:807–14. doi: 10.2459/JCM.0b013e3283609350
5. Bonacina F, Baragetti A, Catapano AL, Norata GD. Long pentraxin 3: experimental and clinical relevance in cardiovascular diseases. Mediators Inflamm. (2013) 2013:725102. doi: 10.1155/2013/725102
6. Casula M, Montecucco F, Bonaventura A, Liberale L, Vecchie A, Dallegri F, et al. Update on the role of Pentraxin 3 in atherosclerosis and cardiovascular diseases. Vascul Pharmacol. (2017) 99:1–12. doi: 10.1016/j.vph.2017.10.003
7. Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. (2005) 23:337–66. doi: 10.1146/annurev.immunol.23.021704.115756
8. Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol. (2008) 28:1–13. doi: 10.1007/s10875-007-9126-7
9. Savchenko A, Imamura M, Ohashi R, Jiang S, Kawasaki T, Hasegawa G, et al. Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J Pathol. (2008) 215:48–55. doi: 10.1002/path.2314
10. Kume N, Mitsuoka H, Hayashida K, Tanaka M. Pentraxin 3 as a biomarker for acute coronary syndrome: comparison with biomarkers for cardiac damage. J Cardiol. (2011) 58:38–45. doi: 10.1016/j.jjcc.2011.03.006
11. Demir MT, Baydin A, Amanvermez R, Erenler AK, Guzel M, Yucel O. Comparison of pentraxin-3 and ischemia-modified albumin with troponin in early diagnosis of acute coronary syndrome. Bratisl Lek Listy. (2018) 119:509–12. doi: 10.4149/BLL_2018_093
12. Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. (2004) 110:2349–54. doi: 10.1161/01.CIR.0000145167.30987.2E
13. Guo R, Li Y, Wen J, Li W, Xu Y. Elevated plasma level of pentraxin-3 predicts in-hospital and 30-day clinical outcomes in patients with non-ST-segment elevation myocardial infarction who have undergone percutaneous coronary intervention. Cardiology. (2014) 129:178–88. doi: 10.1159/000364996
14. Akgul O, Baycan OF, Bulut U, Somuncu MU, Pusuroglu H, Ozyilmaz S, et al. Long-term prognostic value of elevated pentraxin 3 in patients undergoing primary angioplasty for ST-elevation myocardial infarction. Coron Artery Dis. (2015) 26:592–7. doi: 10.1097/MCA.0000000000000280
15. Altay S, Cakmak HA, Kemaloglu Oz T, Ozpamuk Karadeniz F, Turer A, Erer HB, et al. Long-term prognostic significance of pentraxin-3 in patients with acute myocardial infarction: 5-year prospective cohort study. Anatol J Cardiol. (2017) 17:202–9. doi: 10.14744/AnatolJCardiol.2016.7307
16. Qiu CH Li ZC, Lu Z, Xue LX. The predictive value of plasma long pentraxin3 on recent adverse cardiovascular event in ST? segment elevation myocardial infarction population. J Trop Med. (2017) 17:405–8.
17. Chen MF. Clinical Significance of Changes in the Levels of Pentraxin3 and Chemokine 16 in Acute Coronary Syndrome. Master's thesis of Inner Mongolia Medical University (2019).
18. Tomandlova M, Jarkovsky J, Tomandl J, Kubkova L, Kala P, Littnerova S, et al. Prognostic value of pentraxin-3 level in patients with STEMI and its relationship with heart failure and markers of oxidative stress. Dis Markers. (2015) 2015:159051. doi: 10.1155/2015/159051
19. Matsui S, Ishii J, Kitagawa F, Kuno A, Hattori K, Ishikawa M, et al. Pentraxin 3 in unstable angina and non-ST-segment elevation myocardial infarction. Atherosclerosis. (2010) 210:220–5. doi: 10.1016/j.atherosclerosis.2009.10.033
20. Eggers KM, Armstrong PW, Califf RM, Johnston N, Simoons ML, Venge P, et al. Clinical and prognostic implications of circulating pentraxin 3 levels in non ST-elevation acute coronary syndrome. Clin Biochem. (2013) 46:1655–9. doi: 10.1016/j.clinbiochem.2013.08.014
21. Mjelva OR, Ponitz V, Brugger-Andersen T, Grundt H, Staines H, Nilsen DW. Long-term prognostic utility of pentraxin 3 and D-dimer as compared to high-sensitivity C-reactive protein and B-type natriuretic peptide in suspected acute coronary syndrome. Eur J Prev Cardiol. (2016) 23:1130–40. doi: 10.1177/2047487315619733
22. Kontny F, Andersen T, Ueland T, Akerblom A, Lakic TG, Michelsen AE, et al. Pentraxin-3 vs C-reactive protein and other prognostic biomarkers in acute coronary syndrome: a substudy of the platelet inhibition and patients outcomes (PLATO) trial. Eur Heart J Acute Cardiovasc Care. (2020) 9:313–22. doi: 10.1177/2048872619846334
23. Chu Y, Teng J, Feng P, Liu H, Wang F, Li X. Pentraxin-3 in coronary artery disease: a meta-analysis. Cytokine. (2019) 119:197–201. doi: 10.1016/j.cyto.2019.03.017
24. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
25. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed May 28, 2021).
26. Ljuca F, Hadziefendic B, Jahic E, Tihic N, Lukic S. Pentraxin 3 might be better prognostic serum marker than IL-6, IL-10, and high-sensitivity C-reactive protein for major adverse cardiovascular events in patients with ST-elevation myocardial infarction after bare-metal stent implantation. Saudi Med J. (2019) 40:1202–8. doi: 10.15537/smj.2019.12.24737
27. Jiang N, Zhou S, Wang G, Jiang N, Wang H, Zhao F. Diagnostic value and prognostic significance of CTRP9 combined with pentraxin-3 in acute coronary syndrome. Exp Ther Med. (2021) 21:254. doi: 10.3892/etm.2021.9685
28. Dharma S, Sari NY, Santoso A, Sukmawan R, Rao SV. Association of plasma pentraxin 3 concentration with angiographic and clinical outcomes in patients with acute ST-segment elevation myocardial infarction treated by primary angioplasty. Catheter Cardiovasc Interv. (2020) 96:1233–9. doi: 10.1002/ccd.28626
29. Zagidullin N, Motloch LJ, Gareeva D, Hamitova A, Lakman I, Krioni I, et al. Combining novel biomarkers for risk stratification of two-year cardiovascular mortality in patients with ST-elevation myocardial infarction. J Clin Med. (2020) 9:jcm9020550. doi: 10.3390/jcm9020550
30. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. (2006) 333:597–600. doi: 10.1136/bmj.333.7568.597
31. George M, Shanmugam E, Srivatsan V, Vasanth K, Ramraj B, Rajaram M, et al. Value of pentraxin-3 and galectin-3 in acute coronary syndrome: a short-term prospective cohort study. Ther Adv Cardiovasc Dis. (2015) 9:275–84. doi: 10.1177/1753944715578405
32. Butt N, Bache-Mathiesen LK, Ushakova A, Nordrehaug JE, Jensen SE, Munk PS, et al. Pentraxin 3 in primary percutaneous coronary intervention for ST elevation myocardial infarction is associated with early irreversible myocardial damage: kinetic profile, relationship to interleukin 6 and infarct size. Eur Heart J Acute Cardiovasc Care. (2020) 9:302–12. doi: 10.1177/2048872620923641
33. Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. (2002) 22:e10–4. doi: 10.1161/01.ATV.0000015595.95497.2F
34. Tazaki R, Tanigawa J, Fujisaka T, Shibata K, Takeda Y, Ishihara T, et al. Plasma pentraxin3 level is associated with plaque vulnerability assessed by optical coherence tomography in patients with coronary artery disease. Int Heart J. (2016) 57:18–24. doi: 10.1536/ihj.15-248
35. He LP, Tang XY, Ling WH, Chen WQ, Chen YM. Early C-reactive protein in the prediction of long-term outcomes after acute coronary syndromes: a meta-analysis of longitudinal studies. Heart. (2010) 96:339–46. doi: 10.1136/hrt.2009.174912
36. Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. (2000) 102:636–41. doi: 10.1161/01.CIR.102.6.636
Keywords: pentraxin-3, acute coronary syndrome, mortality, cardiac events, meta-analysis
Citation: Fan Y, He R, Man C and Gong D (2022) Utility of Elevated Pentraxin-3 Level as Inflammatory Marker for Predicting Adverse Outcomes in Patients With Acute Coronary Syndrome: A Meta-Analysis. Front. Cardiovasc. Med. 8:736868. doi: 10.3389/fcvm.2021.736868
Received: 06 July 2021; Accepted: 20 December 2021;
Published: 20 January 2022.
Edited by:
Melvin George, SRM Medical College Hospital and Research Centre, IndiaReviewed by:
Naufal Zagidullin, Bashkir State Medical University, RussiaCopyright © 2022 Fan, He, Man and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dandan Gong, Z29uZ2RhbmRhbnpoakAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.