
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 23 August 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.732715
Objective: Both serum uric acid (SUA) levels and lipid components, such as LDL, HDL, and Lp(a), have been reported to associate with CAD. However, the influence of SUA status at different concentrations of lipid indices for the risk of myocardial revascularization (MRT) in ACS patients is currently unknown.
Methods: We retrospectively analyzed a hospital-based sample of 14,234 ACS patients with no previous history of percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery. All patients went for coronary angiography. Binary logistic regression models were performed, and the odds ratios (OR) at 95% confidence interval (CIs) were used to approximate the associated risk of UA and lipid profile for myocardial revascularization, with the lowest quartile/tertile serving as the reference category.
Results: Overall, 8,818 (61.9%) patients undergone MRT out of 14,234 patients. Elevated SUA and HDL were negatively associated with an increased likelihood of MRT during admission (P < 0.001). However, LDL and Lp(a) levels were positively associated with MRT among ACS patients. Furthermore, interaction analyses between SUA and lipid profiles, particularly LDL and Lp(a), compared with those in the lowest quartile of SUA levels, show that patients in higher SUA quartiles grouped by lipid components had a significantly lower chance of undergoing MRT, with the lowest OR (95%CI) for subjects being 0.222 (0.170-0.290), 0.478 (0.374-0.612), and 0.604 (0.468-0.780) in LDL tertiles, being 0.671(0.523-0.862), 0.316(0.242-0.413), and 0.410 (0.310-0.542) in Lp(a) tertiles, respectively. In the three tertiles of HDL levels, the incidence of MRT dropped steadily as SUA levels increased. Also, we further analyzed ACS patients without diabetes. Compared with the first quartile of SUA levels, the risks of MRT were significantly lower in different tertiles of lipids components [LDL, Lp(a), HDL].
Conclusion: An increase in SUA levels may decrease the chance of undergoing MRT in ACS patients, even in those with increased Lp(a) and LDL-c. Elevated serum uric acid may play a protective role during an acute stage of ACS.
Coronary artery disease (CAD) is one of the main causes of death all over the world, especially in Asian countries, where its morbidity and mortality are higher than those in Western countries (1, 2). Though recent advances in drug therapy and revascularization have significantly reduced the mortality associated with coronary artery disease, still more is required from the scientific community. Up-to-date, the involvement of a variety of risk factors in the pathogenesis and development of CAD is recognized. Of the already identified risk factors, the status of uric acid (UA) and lipid indices are gaining momentum.
Serum uric acid (SUA) is an end product in the degradation of the purine nucleotides adenine and guanine, and its levels in plasma have been shown to be increased in CAD (3–6) and associated with hypercholesterolemia (7–10). In previous studies, it was suggested that elevated SUA is associated with demonstrable antioxidant activity (11–13). As a potent scavenger of free radicals, the concentration of SUA rises in the condition of acute and chronic oxidative stress. However, many studies revealed the potentially harmful effects of UA (e.g., local tissue damage), partly mediated by an increase in free radical activity (14, 15). Consequently, UA is more known for its role in the development of CAD (3, 16, 17), and its CAD-related adverse outcomes (5). However, a recent study revealed that UA restores endothelial function in patients with type 1 diabetes and regular smokers (18). Also, another study observed an increase in serum antioxidant capacity in healthy volunteers following a systemic UA administration (19). Importantly, in acute myocardial infarction, acute oxidative stress has been observed, in which the subsequent rise of SUA concentration reaches maximal concentrations within a short interval of time (several minutes or hours) (20). This indicates that there is ongoing controversy as to whether the acute increase in UA itself is sufficient to implicate a potential protective benefit during acute coronary syndrome (ACS) or the effect of such an acute increase of UA can be affected by the lipid status of the patients during ACS.
According to a Mendelian randomization study, lipid concentrations including lipoprotein (a) [Lp(a)], low-density lipoprotein (LDL), and triglyceride-rich lipoprotein/residual lipoprotein cholesterol were speculated to participate in the pathogenesis of coronary heart disease (21, 22). Considering the close relationship between UA and other conventional cardiovascular risk factors (such as hyperlipidemia, obesity, hypertension, and diabetes) and the strong antioxidant capacity of SUA, the relationship between SUA and acute coronary stenosis (ACS) is complicated (16, 23, 24). Besides, to the extent of our knowledge, there is no data that report the interaction of SUA and the different levels of lipid indices in myocardial revascularization (MRT). Therefore, this study aimed to explore the relationship between UA/lipid panel and MRT, and whether SUA predicts the risk of myocardial revascularization at different lipid levels in ACS patients.
This population-based retrospective cohort study was conducted between January 2011 and July 2020 at the cardiovascular department of the First Affiliated Hospital of Dalian Medical University (FAHDM), Dalian City, Liaoning Province of China. The clinical data for this study were retrieved from the Electronic Medical Record Research Database (EMRRD) of FAHDM. The EMRRD is established to create a computerized clinical archive, where clinical notes are regularly updated.
The present study recruited patients with chest pain who satisfy a minimum age of 18 years with no previous history of percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery, and had a complete record of physical examination with complete coronary angiography data. In the first phase of the selection of the participants, we consecutively recruited 18,682 adults who presented with chest pain related to the non-trauma cause. In the second phase, we excluded patients with stable angina (n = 1,069), pulmonary embolism (n = 670), aortic dissection (n = 600), and unidentified sudden death (n = 432), and screened a total of 15,911 patients who were eligible for ACS diagnosis. Further, we excluded patients who refused to perform coronary angiography (n = 169), had a severe heart/renal disease (n = 278), had incomplete data (n = 332) on key clinical covariates, had a history of MI (myocardial infarction)/CABG (coronary artery bypass graft) (n = 670), and were contraindicated for coronary angiography (n = 228). Finally, this study included a total of 14,234 ACS patients. Figure 1 depicts a moderate summary of the study participants' selection. The research was carried out in conformity with the standards of the Helsinki Declaration. The requirement for informed consent was waived as the data was generated from the electronic database of the hospital and the study was approved by the FAHDMU institutional review board.
Electronic health records were used to obtain information on demographic and clinical factors such as age, gender, lifestyle, and medication use. The blood samples were collected before coronary angiography at admission and were biochemically tested for blood glucose level, blood cell counts, and serum concentrations of total cholesterol (TC), triglycerides, high-density lipoprotein cholesterol (HDL-c), lipoprotein(a) [Lp(a)], and low-density lipoprotein cholesterol (LDL-c), SUA, and creatinine at the FAHDMU laboratory using the standard protocols. Hypertension was defined as systolic blood pressure at least 140 mmHg, or DBP at least 90 mmHg, and/or a self-reported history of hypertension with/without antihypertensive medication. Diabetes mellitus was defined as current use of oral antidiabetic medications or insulin, fasting serum glucose > 6.5 mmol/L after fasting for a minimum of 8 h, and/or a self-reported history of diabetes (25). Smokers were considered those participants with a current history of smoking or reported a lifetime consumption of >100 cigarettes (26, 27).
Between January 2011 and July 2020, coronary angiography was performed for all patients at admission using digital subtraction angiography. The physician used the Seldinger method to puncture the radial artery or femoral artery and applied the Judkins method to perform left and right coronary angiography. The presence of arterial stenosis was examined from four major coronary artery branches (left main, left anterior descending, left circumflex, and right coronary artery). The degree of stenosis was graded based on the presence of a plaque and stenosis of the lumen (28). The myocardial revascularization in ACS patients was obtained based on the ESC/EACTS Guidelines on myocardial revascularization (29–31). A severe coronary artery disease was defined as at least 1 coronary stenosis of more than 75%. According to the hospital protocol, two experienced interventional cardiologists who were blinded to the clinical data should review the coronary angiography results. If there was any disagreement between the two interventional cardiologists, images were reviewed and adjudicated by a senior interventional cardiologist to reach a consensus before the data recorded in the EMRRD.
Data were analyzed using SPSS version 23.0 (SPSS Inc. Chicago, IL, USA). The study population was stratified into quartiles based on gender-specific SUA levels and tertiles based on LDL, Lp(a), and HDL tertiles. The respective cut-off values of SUA (Q1, Q2, Q3, and Q4), LDL (T1, T2, and T3), Lp(a) (T1, T2, and T3), and HDL (T1, T2, and T3) in males and females are given in the footnote of each table. Test variables were summarized using mean ± SD for continuous variables, and percentiles for categorical variables. Student t-tests were performed to compare the normally distributed data. When variables were non-normal distributions, the Mann-Whitney U-test was applied and expressed as median (Interquartile range). The Chi-square test (χ2) was used to analyze categorical data. Binary logistic regression models were performed, and the odds ratios (OR) at 95% confidence intervals (CIs) were utilized to approximate the associated risk of UA and lipid profile for myocardial revascularization, with the lowest quartile/tertile serving as the reference category. Pearson correlation coefficient were used to evaluate the linear relationship between uric acid and lipid profiles among ACS patients. A two-sided P < 0.05 was considered statistically significant.
This study included 14,234 patients. Baseline demographic and clinical characteristics according to quartiles of serum UA are listed in Table 1. The mean values of SUA, Lp(a), TC, LDL were significantly higher in Q4 than in Q1–Q3. However, the mean values of creatinine and HDL levels were significantly lower in Q1 than in Q2–Q4. Patients with elevated SUA were younger (P < 0.001), with higher proportion of smokers (P = 0.017), alcohol drinkers (P = 0.022), but with lower prevalence of hypertension (P < 0.001), diabetes mellitus (P < 0.001). Patients in the first quartile were more often in therapy with myocardial revascularization at admission (P < 0.001). Patients in Q1of SUA with significant CAD at coronary angiography had a more frequent involvement of LCX (P < 0.001), LAD (P < 0.001), and RCA (P < 0.001). Patients in Q2 of SUA had a more frequent involvement of LCA (P < 0.001).
Overall, myocardial revascularization was implemented in 8,818 (61.9%) patients out of 14,234 patients. The prevalence of myocardial revascularization was significantly decreased from 70.5% in the first quartile to 57% in the fourth quartile of SUA, respectively (Figure 2A). Based on HDL tertiles, the prevalence of myocardial revascularization was also significantly decreased from 65.6 to 56.3% with an increase in HDL tertiles (Figure 2B). Myocardial revascularization, on the other hand, increased considerably from 56.7% in the low tertile to 64.4 and 64.8% in the middle and upper tertiles of LDL, respectively (Figure 2C). Likely, the prevalence of myocardial revascularization increased considerably from 61% in the low tertile of Lp(a) to 61.5 and 63.3 in the middle and upper tertiles, respectively (Figure 2D).
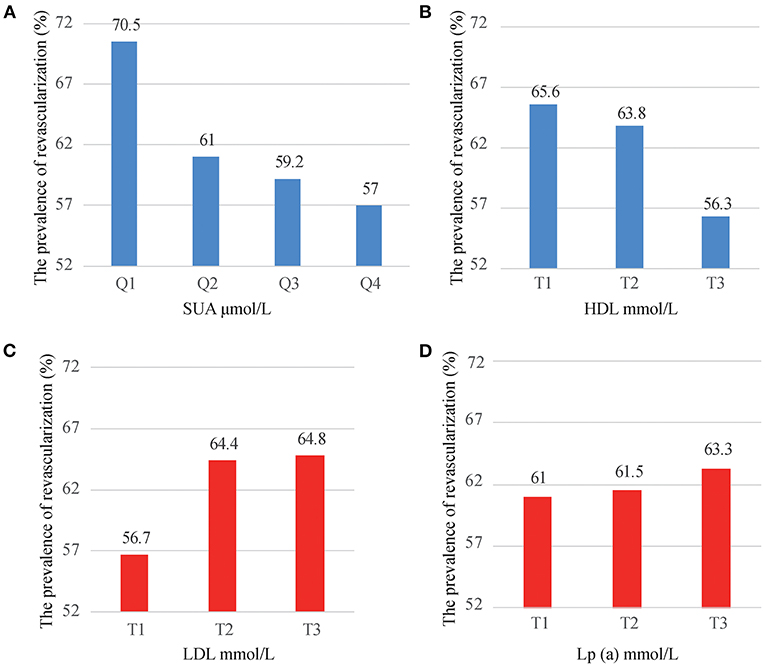
Figure 2. The prevalence of myocardial revascularization grouped by SUA quartiles (A), HDL tertiles (B), LDL tertiles (C), and Lp(a) tertiles (D).
Table 2 presents the ORs of myocardial revascularization among ACS patients grouped by the quartiles of SUA levels. We observed that, compared with the first quartile of SUA, the risk association of myocardial revascularization was decreased with the increase of SUA levels. This decrease in risk of myocardial revascularization persisted even after adjusting for age, gender, smoking, HDL, LDL, TG, Glucose, Creatinine, statin use, hypertension. Compared with the first quartile, the adjusted OR (95%CI) for the patients in Q2, Q3, and Q4 were 0.614 (0.528-0.714), 0.551 (0.474-0.639) and 0.477 (0.411-0.554), respectively (P for trend <0.001).
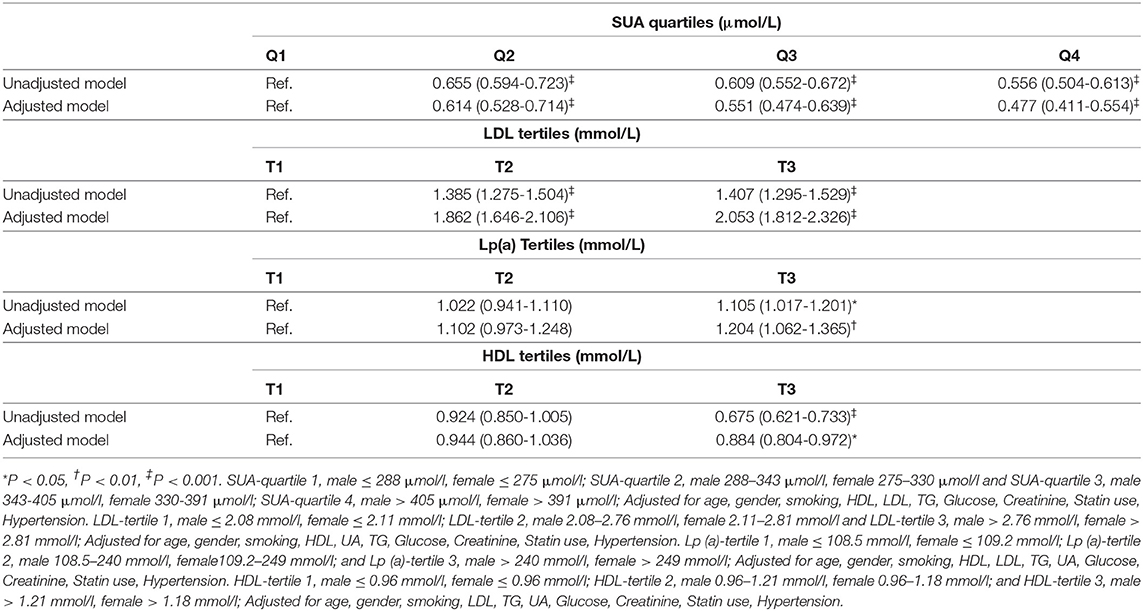
Table 2. The risk estimate for the myocardial revascularization based on the SUA quartiles, HDL tertiles, LDL tertiles, and Lp (a) tertiles.
Compared with the first tertile of LDL, the risk association of myocardial revascularization was increased with the increase of LDL levels. The risk association remained after adjustment for potential confounders including as age, gender, smoking, HDL, SUA, TG, Glucose, Creatinine, statin use, and hypertension. The adjusted OR (95% CI) of myocardial revascularization in 3rd tertile compared to the 1st tertile of LDL was 2.053 (1.812, 2.326; P < 0.001). When grouped by Lp(a) tertiles, the third tertile of Lp(a) levels was associated with a 1.204-fold increased risk of myocardial revascularization compared to those in the 1st tertile of Lp(a) after adjusting for a variety of confounding variables [the adjusted OR (95% CI) = 1.204 (1.062, 1.365; P = 0.004)] including, age, gender, smoking, HDL, LDL, TG, SUA, Glucose, Creatinine, statin use, hypertension. After adjusting for several confounding factors, including, age, gender, smoking, LDL, TG, SUA, Glucose, Creatinine, statin use, and hypertension, the third tertile of HDL was linked with a 0.884-fold lower risk of myocardial revascularization compared to those in the first tertile of HDL [the adjusted OR (95% CI) = 0.884 (0.804, 0.972; P = 0.011)] (Table 2). However, as shown in Supplementary Table 1, the linear relationships between uric acid and lipid profiles among ACS patients were not significant.
To calculate the interaction impact between SUA and LDL, we estimated the OR and 95% CI for myocardial revascularization in those patients grouped by LDL tertiles along with their matched SUA quartiles. The regression analysis indicated that patients in the first tertile of LDL had a gradually lower risk of myocardial revascularization as their SUA levels increased. Also, ACS patients at the higher quartiles of SUA had a lower risk for myocardial revascularization across second and third tertiles of LDL. The lowest OR (95% CI) for the subjects in tertiles 1, 2, and 3 were 0.222 (0.170-0.290), 0.478 (0.374-0.612), and 0.604 (0.468-0.780), respectively. The ORs linked with an elevated SUA among participants classified by tertiles of LDL were shown in Figures 3A–C. Similarly, the patients at lower tertiles of LDL grouped by SUA shows the lowest risk of been treated with myocardial revascularization (Figure 3D).
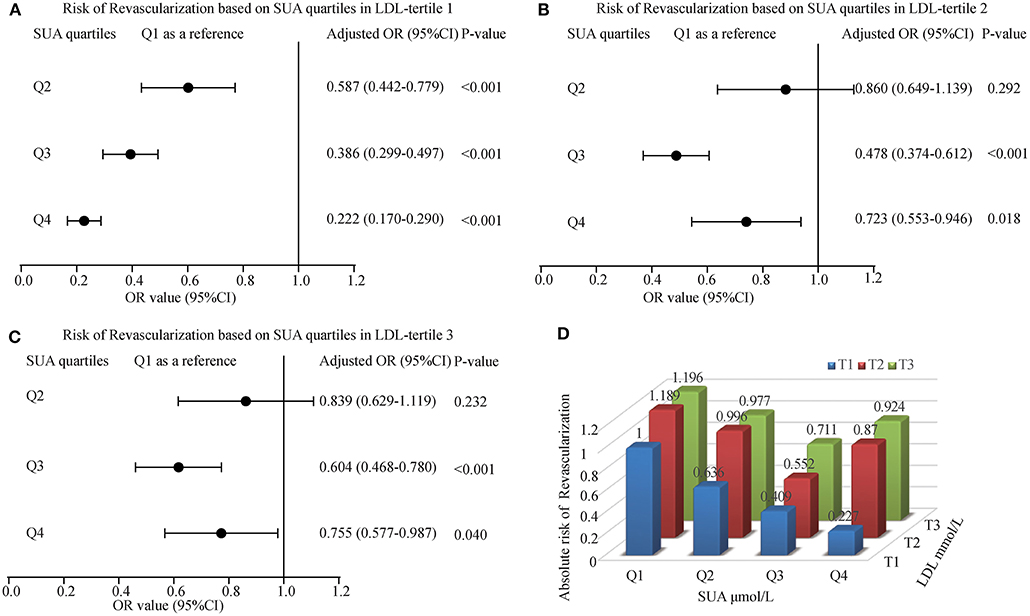
Figure 3. The risk of myocardial revascularization (MRT) based on baseline SUA quartiles in ACS patients. (A) Adjusted odd ratios for MRT in LDL-tertile 1; (B) Adjusted odd ratios for MRT in LDL-tertile 2; (C) Adjusted odd ratios for MRT in LDL-tertile 3; (D) Adjusted odd ratios for MRT in each group by quartiles of the SUA and tertiles of the LDL. Adjusted for age, gender, smoking, HDL, TG, Glucose, Creatinine, Statin use, and Hypertension.
We explored the effect between SUA and Lp(a) interaction in myocardial revascularization. With an increase in SUA levels, the risk of myocardial revascularization was decreased gradually among patients present in T1 of Lp(a) category (OR = 0.773, 0.678, 0.671 for Q2, Q3, and Q4 of SUA) and T2 of Lp(a) category (OR = 0.545, 0.495, 0.316 for Q2, Q3, and Q4 of SUA). The forest plots presented the ORs associated with an elevated SUA in participants grouped by Lp(a) tertiles are illustrated in Figures 4A–C. However, at the T3 Lp(a) level, the third quartile of SUA has the lowest risk [OR (95%CI) = 0.410 (0.310-0.542)]. Likewise, patients in the lower tertiles of Lp(a) grouped by SUA also had a decreased likelihood of undergoing MRT, with patients in T1 having the lowest risk (OR = 0.769, 0.693, and 0.679 for Q2, Q3, and Q4, respectively) (Figure 4D).
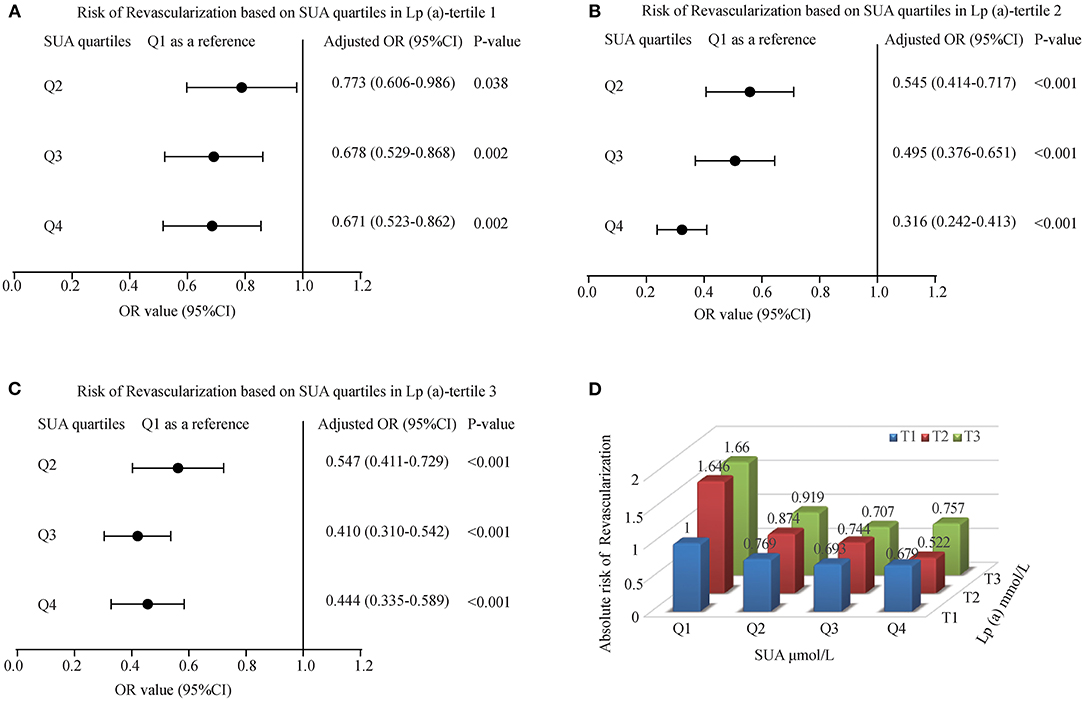
Figure 4. The risk of myocardial revascularization (MRT) based on baseline SUA quartiles in ACS patients. (A) Adjusted odd ratios for MRT in Lp(a)-tertile 1; (B) Adjusted odd ratios for MRT in Lp(a)-tertile 2; (C) Adjusted odd ratios for MRT in Lp(a)-tertile 3; (D) Adjusted odd ratios for MRT in each group by quartiles of the SUA and tertiles of the Lp(a). Adjusted for age, gender, smoking, HDL, LDL, TG, Glucose, Creatinine, Statin use, and Hypertension.
Figure 5 showed the interaction effect between elevated SUA and HDL. With an increase in SUA levels, the risk of myocardial revascularization was decreased gradually when ACS patients were grouped based on HDL tertiles. The ORs (95% CI) for MRT in the tertiles of HDL for those patients in the fourth quartile of SUA were 0.506 (0.417-0.614), 0.563 (0.467-0.679), and 0.681 (0.560-0.827), respectively. Figure 5D likewise, illustrated that the highest quartile of SUA levels had the lowest risk of undergoing MRT, with patients in Q4 having the lowest risk of MRT (OR = 0.538, 0.522, and 0.503 for T1, T2, and T3, respectively).
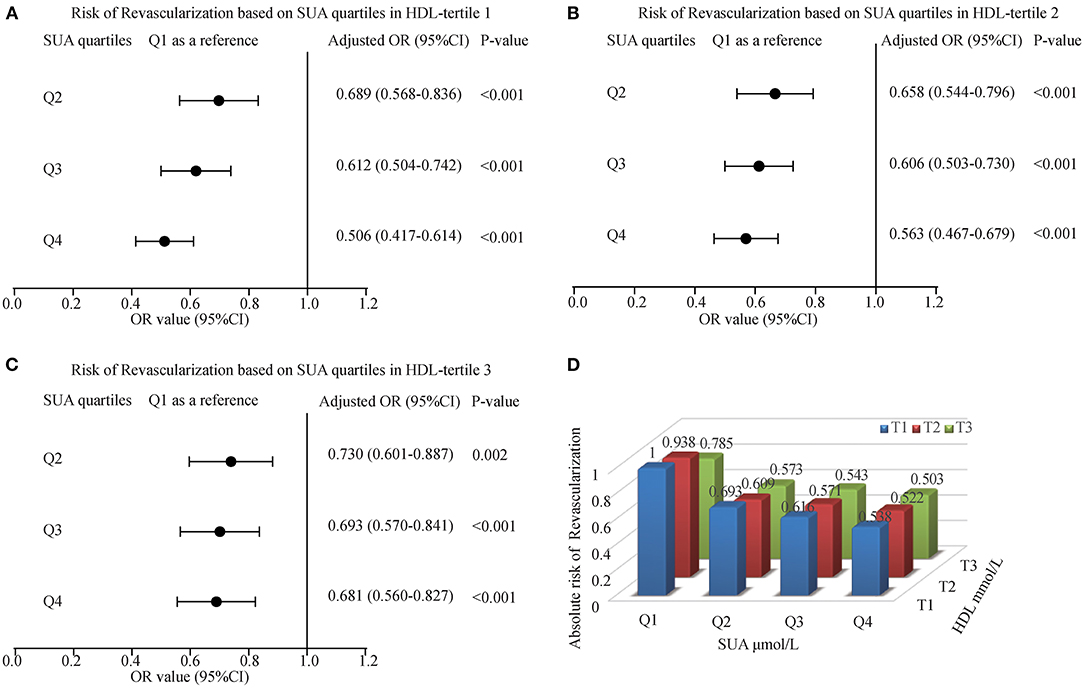
Figure 5. The risk of myocardial revascularization (MRT) based on baseline SUA quartiles in ACS patients. (A) Adjusted odd ratios for MRT in HDL-tertile 1; (B) Adjusted odd ratios for MRT in HDL-tertile 2; (C) Adjusted odd ratios for MRT in HDL-tertile 3; (D) Adjusted odd ratios for MRT in each group by quartiles of the SUA and tertiles of the HDL. Adjusted for age, gender, smoking, LDL, TG, Glucose, Creatinine, Statin use, and Hypertension.
To better confirm the effect of SUA level on myocardial revascularization, we further analyzed ACS patients without diabetes. Compared with the first quartile of SUA levels, the risks of myocardial revascularization were significantly lower in different tertiles of lipids components (LDL, Lp(a), HDL). Figure 6 showed the interaction effect of elevated SUA and lipids, including LDL, Lp(a), HDL. Figures 6A,D,G showed that the lowest ORs (95%CI) for myocardial revascularization in SUA quartiles categorized by LDL tertiles were 0.701 (0.572, 0.859) for Q4 of T1, 0.493 (0.399, 0.609) for Q3 of T2, 0.548 (0.441-0.681) for Q3 of T3. Similarly, in Lp(a) tertiles, the lowest ORs (95%CI) for myocardial revascularization associated with SUA were 0.626 (0.509, 0.769) for Q3 of T1, 0.517 (0.417, 0.640) for Q4 of T2, and 0.594 (0.481-0.733) for Q3 of T3 (Figures 6B,E,H). When the patients were categorized based on HDL tertiles, those participants present at the highest quartile of SUA levels had the lowest risk of myocardial revascularization (Figures 6C,F,I). After excluding patients with diabetes mellitus, our results showed that the increase of SUA was still associated with the decreased risk of myocardial revascularization at different concentrations of lipid indices, suggesting that DM status did not influence the outcome of the data.
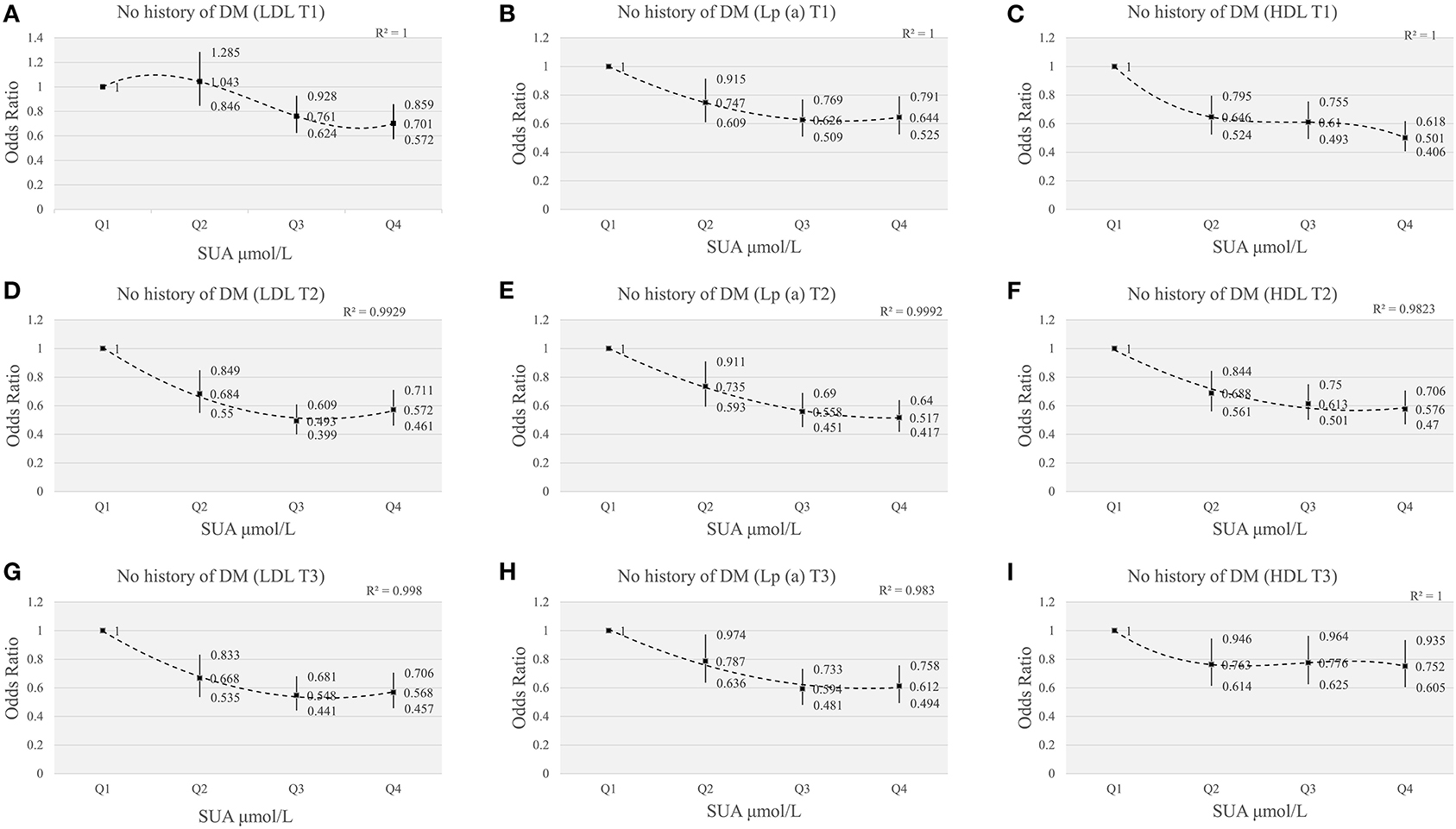
Figure 6. The risk of myocardial revascularization based on SUA quartiles grouped by LDL/Lp(a)/HDL tertiles in ACS patients without DM. (A,D,G) adjusted for age, gender, smoking, HDL, TG, Glucose, Creatinine, Statin use, Hypertension; (B,E,H) adjusted for age, gender, smoking, HDL, LDL, TG, Glucose, Creatinine, Statin use, Hypertension; (C,F,I) adjusted for age, gender, smoking, LDL, TG, Glucose, Creatinine, Statin use, Hypertension.
In this retrospective study, elevated SUA and HDL were found to be inversely linked with an increased chance of MRT during admission of 14,234 ACS patients from the hospital registry. Whereas, LDL and Lp(a) levels were positively associated with MRT among ACS patients. Also, the interaction analyses between SUA and lipid profiles, particularly [LDL, and Lp(a)] show that patients in higher SUA quartiles grouped by the lipid components had a significantly decreased chance of undergoing MRT. Even after the adjustment for possible confounders, this risk association of SUA and lipid components with MRT continues.
In this study, our data suggested that elevated SUA were associated with a decreased likelihood of MRT among ACS patients. In the past, high levels of SUA was speculated to increase mortality and major adverse cardiovascular events in patients with acute myocardial infarction (32). On the contrary, a recent study by Zhang et al. reported that SUA (300 μmol/L) can alleviate the damage of acute hypoxia (33). This indicates the theory behind SUA is paradoxical in view of its association with acute and chronic CAD. More and more new clinical studies have found that uric acid has a neuroprotective effect. Each milligram per deciliter increase in blood uric acid increases the probability of excellent clinical outcomes by 12% in patients with acute ischemic stroke (34). Brouns et al. found that lower UA during the first week after stroke was associated with a more severe stroke, poor stroke evolution, and poor long-term stroke prognosis (35). A clinical study on the Chinese population demonstrated that a proper increase of serum uric acid is conducive to the prognosis of young patients with cerebral infarction (36). Uric acid is also beneficial to the prognosis of cerebral infarction, according to recent investigations by Wu et al. (37) and Liu et al. (38). The available clinical trial data supporting UA as a form of neuroprotective drug for ischemic stroke was summarized by Li et al. (39). Therefore, it is important to explore the relationship between the increase of SUA levels in patients with acute ACS and myocardial revascularization.
The role of oxidative stress in the development of atherosclerosis cannot be overstated (40). Interestingly, UA contributes up to 60% of free-radical scavenging in human serum (41), and its potential as a medicinal antioxidant has already been noted (42). Our study found that ACS patients with high SUA values, regardless of their different categories of lipid profile, had a lower likelihood of undergoing MRT. According to the previous studies, acute oxidative stress due to tissue ischemia and cell redox state alteration occurs in ischemic stroke, acute lower limb ischemia, and myocardial infarction (43–46). The subsequent elevation of SUA concentration due to ischemic tissue metabolism of adenosine (47–49), loss of nitric oxide-induced inhibition of xanthine oxidase (50), and impaired oxidative metabolism (51) are all similar findings in these states. In this regard, SUA increase could be a preventive response to counteract the negative consequences of free radical activity and oxidative stress.
High levels of LDL cholesterol and Lp(a) have a strong correlation with CAD, especially in acute myocardial infarction (52–54). Also, Lp(a) is considered not only a risk factor for atherosclerotic diseases but also speculated to closely associate with the pathophysiology of thrombotic diseases (55). Furthermore, an epidemiological study found that Lp(a) > 30 mg/dl was linked to an increased risk of coronary heart disease (52). On the contrary, CAD is negatively associated with low HDL cholesterol. Importantly, lipid-lowering therapy has proven to significantly reduce the morbidity and mortality of CVDs, which confirm the deleterious effect of hypercholesterolemia. In the present study, our findings show that high LDL and Lp(a) levels were positively associated with MRT among ACS patients, whereas HDL-c was negatively associated with MRT. But, the risk of MRT decreased when participants were categorized based on their SUA levels, even among the subjects present at the highest tertiles of LDL-c and Lp(a).
We investigated the relationship between SUA level and lipid indices in ACS patients, taking into account the direct effect of SUA, which is known to be a product of xanthine-oxidoreductase activity, and the role of elevated LDL-c and Lp(a) in modifying the pathophysiology of ACS. Most patients in the top quartiles of SUA levels had a decreased risk of MRT, according to the interaction assessment in those patients grouped by the different lipid indices tertiles and their corresponding quartiles of SUA. These results show that a subsequent rise of SUA concentration in ACS may indicate a protective response. Because it has been demonstrated that the physiologic effect of UA can be strengthened by short-term administration to prevent oxidative and free radical-mediated tissue damage, and that early administration of mixed antioxidants improved cardiovascular hemodynamics significantly (56). Also, a shred of evidence has documented that the administration of SUA significantly increases the ex vivo antioxidant capacities, complying with the normal and low oxidant stress, of healthy volunteers with lower SUA baseline concentrations (4). Another study reported that long exposure to moderately high SUA concentration appears important, as the acute elevation in tumor lysis syndrome does not necessarily cause gout even at concentrations beyond 1,200 uM (57). Collectively, these pieces of evidence indicate that the acute increase in SUA as a physiologic response can play an important role in maintaining hemostatic equilibrium, presumably via the ROS pathway. Therefore, the observed lower risk of MRT among ACS patients present at the top quartiles of SUA (despite the high levels of lipid profile/indices) confirms that the acute elevation of SUA may play a protective role in ACS patients in a short time.
Hyperuricemia and dyslipidemia often coexist in CVD patients (8–10). Paradoxically, dyslipidemia is more often due to other conditions, like hypertension or diabetes than SUA itself, in the presence of chronic hyperuricemia. Chronic oxidative stress is present in patients with hypercholesterolemia, diabetes, and hypertension, and it is speculated to play a key role in the development and progression of atherosclerosis (58). Compared to the above-mentioned risk factors, many researchers reported a positive association between hyperuricemia and incident DM (59–61). Therefore, to avoid selection bias, and to better confirm the effect of SUA level on myocardial revascularization, we further analyzed ACS patients without diabetes. We still observed that ACS patients free of DM with high levels of SUA had a lower likelihood of MRT.
The current study has certain advantages and disadvantages. To the best of our knowledge, no study has looked into the interaction between SUA and lipid indices in MRT with ACS using admission data. As a result, it's unclear if the association between high SUA levels and lipid indices [LDL-c, Lp(a), and HDL-c] amplifies or squeezes the risk of MRT in ACS patients. This study, however, should be interpreted with some limitations. The single-centered retrospective design has limited the cause-and-effect relationship between SUA/lipid profiles and the risk of MRT in ACS. Also, our data cannot provide data on the long-term effects of SUA on ACS prognosis. Similarly, due to the nature of our study, we do not have detailed information on the previous history of SUA, which otherwise could help to further understand the difference of SUA levels prior to ACS and during ACS occurrences.
An increase in SUA levels may decrease the need for myocardial revascularization, even in those with increased Lp(a) and LDL-c. Elevated serum uric acid may play a protective role in ACS patients in the short term. Further study is required to confirm the observed association.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Dalian Medical University institutional review board. Written informed consent was not required for this study, in accordance with the local legislation and institutional requirements.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This research was funded by the National Natural Science Foundation of China (grant numbers 81900439 and 81970286), the Science Foundation of Doctors of Liaoning Province (grant number 2020-BS-197), the Chang Jiang Scholars Program (grant number T2017124), the Dalian Talents Innovation Supporting Project (grant number 2018RD09), and the Liao Ning Revitalization Talents Program (grant number XLYC2002096).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.732715/full#supplementary-material
1. Hata J, Kiyohara Y. Epidemiology of stroke and coronary artery disease in Asia. Circ J. (2013) 77:1923–32. doi: 10.1253/circj.CJ-13-0786
2. Vaisi-Raygani A, Ghaneialvar H, Rahimi Z, Nomani H, Saidi M, Bahrehmand F, et al. The angiotensin converting enzyme D allele is an independent risk factor for early onset coronary artery disease. Clin Biochem. (2010) 43:1189–94. doi: 10.1016/j.clinbiochem.2010.07.010
3. Duran M, Kalay N, Akpek M, Orscelik O, Elcik D, Ocak A, et al. High levels of serum uric acid predict severity of coronary artery disease in patients with acute coronary syndrome. Angiology. (2012) 63:448–52. doi: 10.1177/0003319711426868
4. Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. (2000) 283:2404–10. doi: 10.1001/jama.283.18.2404
5. Li YH, Lin GM, Lin CL, Wang JH, Chen YJ, Han CL. Relation of serum uric acid and body mass index to mortality in high-risk patients with established coronary artery disease: a report from the ET-CHD registry, 1997-2006. J Cardiol. (2013) 62:354–60. doi: 10.1016/j.jjcc.2013.06.002
6. Okura T, Higaki J, Kurata M, Irita J, Miyoshi K, Yamazaki T, et al. Elevated serum uric acid is an independent predictor for cardiovascular events in patients with severe coronary artery stenosis: subanalysis of the Japanese Coronary Artery Disease (JCAD) Study. Circ J. (2009) 73:885–91. doi: 10.1253/circj.CJ-08-0828
7. Berkowitz D. Blood lipid and uric acid interrelationships. JAMA. (1964) 190:856–8. doi: 10.1001/jama.1964.03070220062023
8. Kuwabara M, Borghi C, Cicero AFG, Hisatome I, Niwa K, Ohno M, et al. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: a five-year cohort study in Japan. Int J Cardiol. (2018) 261:183–8. doi: 10.1016/j.ijcard.2018.03.045
9. Peng TC, Wang CC, Kao TW, Chan JY, Yang YH, Chang YW, et al. Relationship between hyperuricemia and lipid profiles in US adults. Biomed Res Int. (2015) 2015:127596. doi: 10.1155/2015/127596
10. Teng F, Zhu R, Zou C, Xue Y, Yang M, Song H, et al. Interaction between serum uric acid and triglycerides in relation to blood pressure. J Hum Hypertens. (2011) 25:686–91. doi: 10.1038/jhh.2010.112
11. Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. (1986) 235:747–54. doi: 10.1042/bj2350747
12. Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA, Uric acid and oxidative stress. Curr Pharm Des. (2005) 11:4145–51. doi: 10.2174/138161205774913255
13. Hink HU, Santanam N, Dikalov S, McCann L, Nguyen AD, Parthasarathy S, et al. Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol. (2002) 22:1402–8. doi: 10.1161/01.ATV.0000027524.86752.02
14. Diaz MN, Frei B, Vita JA, Keaney JF Jr. Antioxidants and atherosclerotic heart disease. N Engl J Med. (1997) 337:408–16. doi: 10.1056/NEJM199708073370607
15. Halliwell B. The antioxidant paradox. Lancet. (2000) 355:1179–80. doi: 10.1016/S0140-6736(00)02075-4
16. Moriarity JT, Folsom AR, Iribarren C, Nieto FJ, Rosamond WD. Serum uric acid and risk of coronary heart disease: atherosclerosis risk in communities (ARIC) study. Ann Epidemiol. (2000) 10:136–43. doi: 10.1016/S1047-2797(99)00037-X
17. Ndrepepa G, Braun S, King L, Hadamitzky M, Haase HU, Birkmeier KA, et al. Association of uric acid with mortality in patients with stable coronary artery disease. Metabolism. (2012) 61:1780–6. doi: 10.1016/j.metabol.2012.05.014
18. Waring WS, McKnight JA, Webb DJ, Maxwell SR. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes. (2006) 55:3127–32. doi: 10.2337/db06-0283
19. Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol. (2001) 38:365–71. doi: 10.1097/00005344-200109000-00005
20. Werns SW, Lucchesi BR. Myocardial ischemia and reperfusion: the role of oxygen radicals in tissue injury. Cardiovasc Drugs Ther. (1989) 2:761–9. doi: 10.1007/BF00133206
21. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. (2009) 301:2331–9. doi: 10.1001/jama.2009.801
22. Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. (2010) 31:2844–53. doi: 10.1093/eurheartj/ehq386
23. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. (1999) 131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003
24. Wheeler JG, Juzwishin KD, Eiriksdottir G, Gudnason V, Danesh J. Serum uric acid and coronary heart disease in 9,458 incident cases and 155,084 controls: prospective study and meta-analysis. PLoS Med. (2005) 2:e76. doi: 10.1371/journal.pmed.0020076
25. Pearson TA, Palaniappan LP, Artinian NT, Carnethon MR, Criqui MH, Daniels SR, et al. Prevention, American heart association guide for improving cardiovascular health at the community level, 2013 update: a scientific statement for public health practitioners, healthcare providers, and health policy makers. Circulation. (2013) 127:1730–53. doi: 10.1161/CIR.0b013e31828f8a94
26. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of atherosclerosis: objectives and design. Am J Epidemiol. (2002) 156:871–81. doi: 10.1093/aje/kwf113
27. Ford ES, Giles WH, Mokdad AH. The distribution of 10-Year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol. (2004). 43:1791–6. doi: 10.1016/j.jacc.2003.11.061
28. Liu F, Hui S, Hidru TH, Jiang Y, Zhang Y, Lu Y, et al. The prevalence, distribution, and extent of subclinical atherosclerosis and its relation with serum uric acid in hypertension population. Front Cardiovasc Med. (2021) 8:638992. doi: 10.3389/fcvm.2021.638992
29. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.15829/1560-4071-2019-8-151-226
30. Taggart DP, Boyle R, de Belder MA, Fox KA. The 2010 ESC/EACTS guidelines on myocardial revascularisation. Heart. (2011) 97:445–6. doi: 10.1136/hrt.2010.216135
31. Authors/Task, Force m., Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V . ESC/EACTS Guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. (2014) 35:2541–619. doi: 10.1093/eurheartj/ehu278
32. Xu Q, Zhang M, Abeysekera IR, Wang X. High serum uric acid levels may increase mortality and major adverse cardiovascular events in patients with acute myocardial infarction. Saudi Med J. (2017) 38:577–85. doi: 10.15537/smj.2017.6.17190
33. Zhang B, Yang N, Lin SP, Zhang F. Suitable concentrations of uric acid can reduce cell death in models of OGD and cerebral ischemia-reperfusion injury. Cell Mol Neurobiol. (2017) 37:931–9. doi: 10.1007/s10571-016-0430-8
34. Chamorro A, Obach V, Cervera A, Revilla M, Deulofeu R, Aponte JH. Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke. (2002) 33:1048–52. doi: 10.1161/hs0402.105927
35. Brouns R, Wauters A, Van De Vijver G, De Surgeloose D, Sheorajpanday R, De Deyn PP. Decrease in uric acid in acute ischemic stroke correlates with stroke severity, evolution and outcome. Clin Chem Lab Med. (2010) 48:383–90. doi: 10.1515/CCLM.2010.065
36. Zhang B, Gao C, Yang N, Zhang W, Song X, Yin J, et al. Is elevated SUA associated with a worse outcome in young Chinese patients with acute cerebral ischemic stroke? BMC Neurol. (2010) 10:82. doi: 10.1186/1471-2377-10-82
37. Wu H, Jia Q, Liu G, Liu L, Pu Y, Zhao X, et al. Decreased uric acid levels correlate with poor outcomes in acute ischemic stroke patients, but not in cerebral hemorrhage patients. J Stroke Cerebrovasc Dis. (2014) 23:469–75. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.007
38. Liu X, Liu M, Chen M, Ge QM, Pan SM. Serum uric acid is neuroprotective in Chinese patients with acute ischemic stroke treated with intravenous recombinant tissue plasminogen activator. J Stroke Cerebrovasc Dis. (2015) 24:1080–6. doi: 10.1016/j.jstrokecerebrovasdis.2015.01.011
39. Li R, Huang C, Chen J, Guo Y, Tan S. The role of uric acid as a potential neuroprotectant in acute ischemic stroke: a review of literature. Neurol Sci. (2015) 36:1097–103. doi: 10.1007/s10072-015-2151-z
40. Maxwell SR, Lip GY. Free radicals and antioxidants in cardiovascular disease. Br J Clin Pharmacol. (1997) 44:307–17. doi: 10.1046/j.1365-2125.1997.t01-1-00594.x
41. Maxwell SR, Thomason H, Sandler D, Leguen C, Baxter MA, Thorpe GH, et al. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur J Clin Invest. (1997) 27:484–90. doi: 10.1046/j.1365-2362.1997.1390687.x
42. Waring WS, Maxwell S. Diagnosis of molybdenum cofactor deficiency. Lancet. (1999) 353:675–6. doi: 10.1016/S0140-6736(05)75474-X
43. De Scheerder IK, van de Kraay AM, Lamers JM, Koster JF, de Jong JW, Serruys PW. Myocardial malondialdehyde and uric acid release after short-lasting coronary occlusions during coronary angioplasty: potential mechanisms for free radical generation. Am J Cardiol. (1991) 68:392–5. doi: 10.1016/0002-9149(91)90838-C
44. Hayashi T, Sakurai M, Itoyama Y, Abe K. Oxidative damage and breakage of DNA in rat brain after transient MCA occlusion. Brain Res. (1999) 832:159–63. doi: 10.1016/S0006-8993(99)01409-2
45. Hill MF, Singal PK. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation. (1997) 96:2414–20. doi: 10.1161/01.CIR.96.7.2414
46. Mathru M, Dries DJ, Barnes L, Tonino P, Sukhani R, Rooney MW. Tourniquet-induced exsanguination in patients requiring lower limb surgery. An ischemia-reperfusion model of oxidant and antioxidant metabolism. Anesthesiology. (1996) 84:14–22. doi: 10.1097/00000542-199601000-00003
47. Costa F, Sulur P, Angel M, Cavalcante J, Haile V, Christman B, et al. Intravascular source of adenosine during forearm ischemia in humans: implications for reactive hyperemia. Hypertension. (1999) 33:1453–7. doi: 10.1161/01.HYP.33.6.1453
48. Raatikainen MJ, Peuhkurinen KJ, Hassinen IE. Contribution of endothelium and cardiomyocytes to hypoxia-induced adenosine release. J Mol Cell Cardiol. (1994) 26:1069–80. doi: 10.1006/jmcc.1994.1127
49. Waring WS, Webb DJ, Maxwell SR. Uric acid as a risk factor for cardiovascular disease. QJM. (2000) 93:707–13. doi: 10.1093/qjmed/93.11.707
50. Zulueta JJ, Sawhney R, Yu FS, Cote CC, Hassoun PM. Intracellular generation of reactive oxygen species in endothelial cells exposed to anoxia-reoxygenation. Am J Physiol. (1997) 272:L897–902. doi: 10.1152/ajplung.1997.272.5.L897
51. Leyva F, Anker S, Swan JW, Godsland IF, Wingrove CS, Chua TP, et al. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur Heart J. (1997) 18:858–65. doi: 10.1093/oxfordjournals.eurheartj.a015352
52. Berg K, Dahlen G, Frick MH. Lp(a) lipoprotein and pre-beta1-lipoprotein in patients with coronary heart disease. Clin Genet. (1974) 6:230–5. doi: 10.1111/j.1399-0004.1974.tb00657.x
53. Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr., DePalma SM . 2017 Focused Update of the (2016). ACC Expert Consensus Decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. (2017) 70:1785–822. doi: 10.1016/j.jacc.2017.07.745
54. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. (2014) 129(25 Suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a
55. McLean JW, Tomlinson JE, Kuang WJ, Eaton DL, Chen EY, Fless GM, et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. (1987) 330:132–7. doi: 10.1038/330132a0
56. Galley HF, Howdle PD, Walker BE, Webster NR. The effects of intravenous antioxidants in patients with septic shock. Free Radic Biol Med. (1997) 23:768–74. doi: 10.1016/S0891-5849(97)00059-2
57. Leach M, Parsons RM, Reilly JT, Winfield DA. Efficacy of urate oxidase (uricozyme) in tumour lysis induced urate nephropathy. Clin Lab Haematol. (1998) 20:169–72. doi: 10.1046/j.1365-2257.1998.00124.x
58. Lonn EM, Yusuf S. Evidence based cardiology: emerging approaches in preventing cardiovascular disease. BMJ. (1999) 318:1337–41. doi: 10.1136/bmj.318.7194.1337
59. Bhole V, Choi JW, Kim SW, de Vera M, Choi H. Serum uric acid levels and the risk of type 2 diabetes: a prospective study. Am J Med. (2010) 123:957–61. doi: 10.1016/j.amjmed.2010.03.027
60. Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. (2008) 31:361–2. doi: 10.2337/dc07-1276
Keywords: serum uric acid, myocardial revascularization, lipid indices, ACS, risk factors
Citation: Lin Y, Hidru TH, Fan R, Gao J, Li H, Yang X and Xia Y (2021) The Relationship Between Serum Uric Acid at Different Concentrations of Lipid Indices and the Risk of Myocardial Revascularization in Patients With Acute Coronary Syndrome: A Retrospective Analysis. Front. Cardiovasc. Med. 8:732715. doi: 10.3389/fcvm.2021.732715
Received: 29 June 2021; Accepted: 02 August 2021;
Published: 23 August 2021.
Edited by:
Yan Zhang, Peking University, ChinaReviewed by:
Xin Tu, Huazhong University of Science and Technology, ChinaCopyright © 2021 Lin, Hidru, Fan, Gao, Li, Yang and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunlong Xia, eXVubG9uZ194aWFAMTI2LmNvbQ==; Xiaolei Yang, eWFuZ3hsMTAxMkB5ZWFoLm5ldA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.