
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 25 November 2021
Sec. Cardiovascular Surgery
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.721264
Background: Previous clinical studies and meta-analysis evaluating the influence of dexmedetomidine on postoperative atrial fibrillation showed inconsistent results. We performed an updated meta-analysis to evaluate the influence of dexmedetomidine on incidence of postoperative atrial fibrillation after cardiac surgery.
Methods: Randomized controlled trials that evaluated the potential influence of dexmedetomidine on the incidence of atrial fibrillation after cardiac surgery were obtained by search of PubMed, Embase, and Cochrane's Library databases from inception to April 12, 2021. A random-effects model incorporating the potential publication bias was used to pool the results. Influences of patient or study characteristics on the efficacy of dexmedetomidine on atrial fibrillation after cardiac surgery were evaluated by meta-regression and subgroup analyses.
Results: Fifteen studies with 2,733 patients were included. Pooled results showed that dexmedetomidine significantly reduced the incidence of atrial fibrillation compared to control (OR: 0.72, 95% CI: 0.55–0.94, p = 0.02) with mild heterogeneity (I2 = 26%). Subgroup analysis showed that dexmedetomidine significantly reduced the incidence of atrial fibrillation in studies from Asian countries (OR: 0.41, 95% CI: 0.26–0.66, p < 0.001), but not in those from non-Asian countries (OR: 0.89, 95% CI: 0.71–1.10, p = 0.27; p for subgroup difference = 0.004). Meta-regression analysis showed that the mean age and proportion of male patients may modify the influence of dexmedetomidine on POAF (coefficient = 0.028 and 0.021, respectively, both p < 0.05). Subgroup analysis further showed that Dex was associated with reduced risk of atrial fibrillation after cardiac surgery in studies with younger patients (mean age ≤ 61 years, OR = 0.44, 95% CI: 0.28–0.69, p = 0.004) and smaller proportion of males (≤ 74%, OR = 0.55, 95% CI: 0.36–0.83, p = 0.005), but not in studies with older patients or larger proportion of males (p for subgroup difference = 0.02 and 0.04).
Conclusions: Current evidence supports that perioperative administration of dexmedetomidine may reduce the risk of incidental atrial fibrillation after cardiac surgery, particularly in Asians.
Post-operative atrial fibrillation (POAF) is a common complication, which affects up to 35% of patients after cardiac surgery (1, 2). Clinically, POAF has been related with a higher incidence of neurocognitive dysfunction, prolonged hospital stay, and increased risk of all-cause mortality in these patients (3). Current risk factors for POAF after cardiac surgery include aging, diabetes mellitus, and impaired cardiac function etc. (4). However, no definite strategy has been confirmed as an effective preventative method for POAF (5, 6). Dexmedetomidine (Dex) is well-applied perioperative medication for patients that received cardiac surgery (7). Pharmacologically, Dex is a highly selective α2-adrenoreceptor agonist which exerts various clinical efficacies during perioperative periods, such as sedation, analgesia, anti-anxiety, and diuresis (8, 9). Besides, use of Dex is suggested to reduce POAF after cardiac surgeries in some previous randomized controlled trials (RCTs) (10–12), although other RCTs generally did not support a preventative efficacy of Dex for POAF (13–24). A few meta-analyses have been performed to evaluate the influence of Dex on POAF after cardiac surgery. Early meta-analyses including RCTs before 2017 consistently showed that Dex was not associated with reduced incidence of POAF after cardiac surgeries (25–29), while a recent meta-analysis including RCTs up to 2018 showed that Dex was effective in preventing POAF after cardiac surgeries (30). However, results of this meta-analysis are confounded by including two RCTs with overlapped patients (10, 31), which both showed a significant preventative efficacy of Dex on POAF. Besides, three RCTs have been published since the last meta-analysis (12, 23, 24), including a large-scale RCT which suggested a lack of preventative efficacy of Dex on POAF (24). Accordingly, we performed an updated meta-analysis to systematically evaluate the possible influence of Dex on POAF after cardiac surgery. Multiple predefined subgroup analyses were also performed to explore the potential influences of patient or study characteristics on the outcome.
We followed the instructions of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (32) and the Cochrane Handbook guidelines (33) during the designing, performing, and reporting of the meta-analysis.
PubMed, Embase, and the Cochrane's Library (Cochrane Center Register of Controlled Trials) databases were searched for relevant studies with a combined strategy of: (1) “dexmedetomidine”; (2) “heart” OR “cardiac surgery” OR “coronary artery bypass graft” OR CABG OR “atrial fibrillation” OR arrhythmia OR “cardiac”; and (3) “random” OR “randomized” OR “randomised” OR “randomly.” Only clinical studies were considered. The references of related reviews and original articles were also searched as complementation. The latest database search was performed on April 12, 2021.
Inclusion criteria were: (1) peer-reviewed articles in English; (2) designed as parallel-group RCTs; (3) included adult patients scheduled for open heart surgery who were randomly allocated to a perioperative administration of Dex or a non-Dex control group; and (4) reported the incidence of POAF during the index hospitalization. For studies with overlapped patients, the one with the largest sample size was included (33). Reviews, studies including children or neonates, preclinical studies, observational studies, and repeated reports were excluded.
Study search, data extraction, and quality evaluation were conducted by two independent authors. If disagreement occurred, it was resolved by consensus between the two authors. We extracted data regarding study information (first author, publication year, and study country), study design (blind or open-label), patient information [number of participants, mean age, gender, body weight or body mass index (BMI), and surgery type], details of Dex administration (regimen, timing and duration), and components of controls. Quality evaluation was achieved using the Cochrane's Risk of Bias Tool (33) according to the following aspects: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessors; (5) incomplete outcome data; (6) selective outcome reporting; and (7) other potential bias.
Incidence of POAF in each arm was evaluated via odds ratio (OR) and its 95% confidence intervals (CIs). We used the Cochrane's Q test to detect the heterogeneity, and significant heterogeneity was suggested if P < 0.10 (34). The I2 statistic was also calculated, and an I2 > 50% reflected significant heterogeneity. Pooled analyses were calculated using a random-effect model because this method could incorporate the influence of potential heterogeneity and retrieves a more generalized result (33). Sensitivity analysis by excluding one study at a time was used to determine the robustness of the finding. Subgroup analyses were performed to determine the potential influences of study characteristics on the results of meta-analysis, including country of the study, type of surgery, mean age of the patients, proportions of the males, with or without loading dose of Dex, timing of Dex administration, regimen of controls, or score of study quality. Meta-regression analyses were also performed to evaluate the potential influences of mean age of the patients and proportions of the males on the effect of Dex on POAF. Medians of continuous variables were used as cut-off values for defining of subgroups. Publication bias was evaluated by visual inspection of funnel plots, and the Egger's regression asymmetry test (35). P-values < 0.05 were considered statistically significant. The RevMan (Version 5.1; Cochrane, Oxford, UK) and Stata software (Version 12.0; Stata, College Station, TX) were applied for statistical analyses.
In summary, 1,478 articles were obtained through the database search. After exclusion of duplicate studies, 1,331 articles were screened. Among them, 1,281 articles were subsequently excluded based on titles and abstracts primarily because these studies were irrelevant. Among the 50 potentially relevant articles, 35 were further excluded via full-text review based on reasons listed in Figure 1. Finally, 15 RCTs (10–24) were included into the meta-analysis.
Table 1 shows the characteristics of the included studies. Overall, 15 RCTs (10–24) with 2,733 patients who received cardiac surgery – 1,388 patients in the Dex group and 1,345 patients in the control group - were included. These studies were published between 1997 and 2020. All of the studies included patients that underwent open-heart cardiac surgery. Among them, ten RCTs included patients who underwent elective coronary artery bypass grafting (CABG) (11–21), while the other five included patients who underwent mixed type of open-heart surgeries, including CABG, valvular surgeries, and aortic surgeries (10, 16, 22–24). The sample sizes of the included RCTs varied between 64 and 794. The mean ages of the patients varied between 52 and 75 years, with proportions of male ranging from 32 to 90%. Mean body weight of the patients was reported in five studies (11, 13–15, 22), while BMI was reported in another five studies (10, 16, 17, 21, 24). Dex was administered only during the procedure of surgery in two studies (13, 23), only during the stay in Intensive Care Unit (ICU) in nine studies (10, 12, 15–17, 19–22), and during the surgery and ICU in four studies (11, 14, 18, 24). A loading dose and subsequent continuously intravenous administration of Dex was applied five studies (13–15, 19, 22), and in the remaining ten studies, no leading dose of Dex was applied (10–12, 16–18, 20, 21, 23, 24). As for the controls, in six studies, Dex was compared to placebo (11, 13, 17, 18, 20, 24), while in the others, comparisons were between Dex and active controls including propofol (10, 12, 14, 15, 21–23), morphine (16), and remifentanil (19).
Table 2 shows the details of study quality evaluation. Nine of the included studies were double-blind (11–13, 16, 17, 20, 22–24), while the rest were single-blind (18) or open-label (10, 14, 15, 19, 21). Methods of random sequence generation were reported in seven studies (10, 12, 15, 16, 22–24), and information of allocation concealment was reported in five studies (11, 14, 21, 22, 24). The quality of the included studies varied, with a quality score from 3 to 7.
Mild heterogeneity was detected among the included RCTs (p for Cochrane's Q test = 0.16, I2 = 26%). Pooled results with a random-effect model showed that Dex significantly reduced the incidence of POAF after cardiac surgery compared to control (OR: 0.72, 95% CI: 0.55–0.94, p = 0.02; Figure 2A). Sensitivity analysis by excluding one study at a time did not significantly affect the results (OR: 0.72–0.80, p all < 0.05). Subgroup analysis according to the origin of the study showed that Dex significantly reduced the incidence of atrial fibrillation in studies from Asian countries (OR: 0.41, 95% CI: 0.26–0.66, p < 0.001), but not in those from non-Asian countries (OR: 0.89, 95% CI: 0.71–1.10, p = 0.27; p for subgroup difference = 0.004; Figure 2B), which fully resolved the heterogeneity (I2 = 0% for both subgroups). Results of meta-regression analysis showed that the mean age and proportion of male patients may modify the influence of dexmedetomidine on POAF (coefficient = 0.028 and 0.021, respectively, both p < 0.05; Table 3). Further subgroup analyses showed that that Dex was associated with reduced POAF in studies with younger patients (mean age ≤ 61 years, OR: 0.44, 95% CI: 0.28–0.69, p = 0.004) and smaller proportion of males (≤74%, OR: 0.55, 95% CI: 0.36–0.83, p = 0.005; Table 4), but not in studies with older patients or larger proportion of males (p for subgroup difference = 0.02 and 0.04, respectively). The results were not significantly affected by difference in study characteristics such as type of surgery, with or without loading dose of Dex, timing of Dex administration, regimen of controls, or score of study quality (Table 4; p for subgroup analyses all > 0.05).
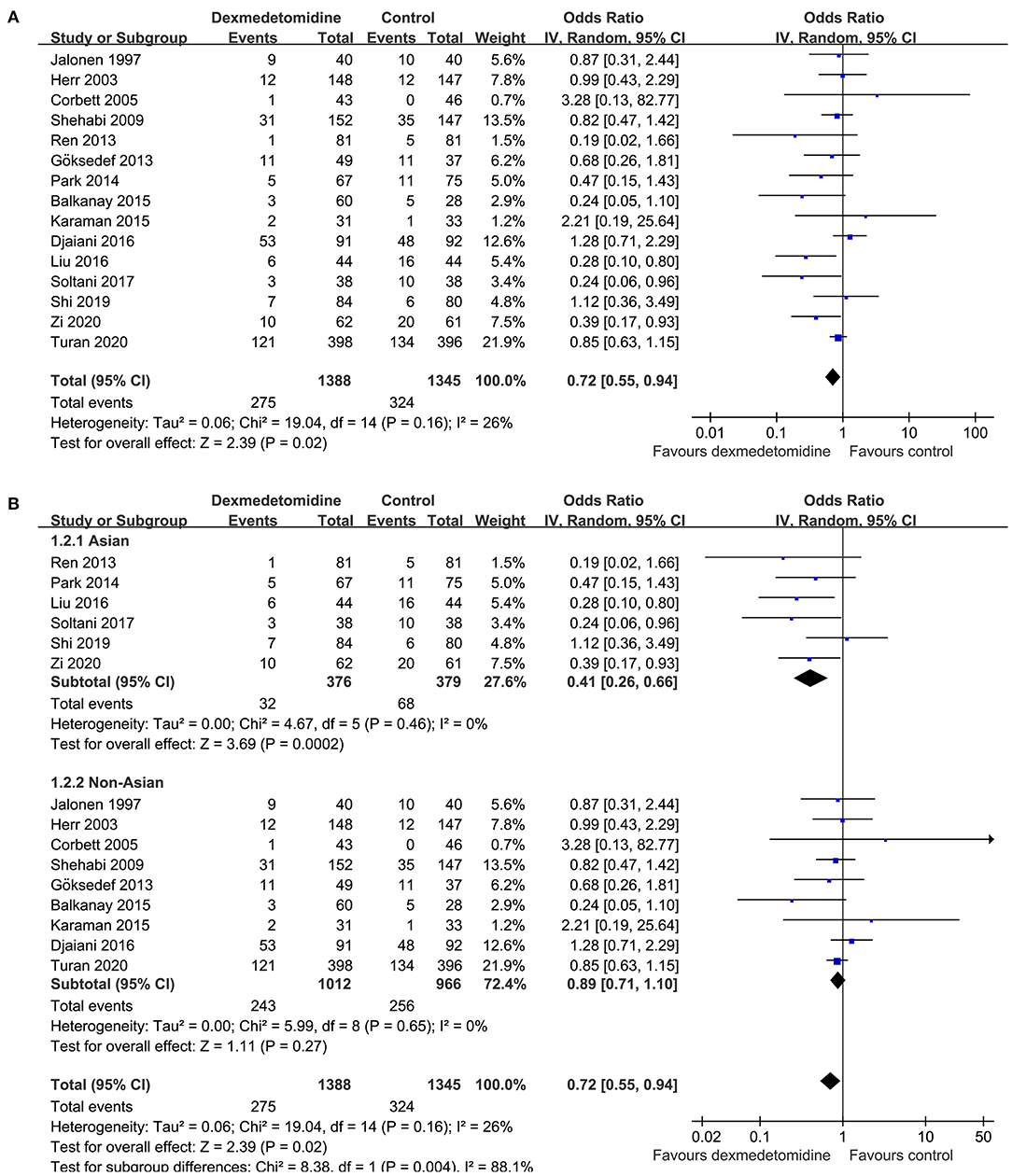
Figure 2. Forest plots for the meta-analysis comparing the influences of dexmedetomidine and controls on the incidence of POAF after cardiac surgery; (A) overall meta-analysis; and (B) subgroup analysis according to the origin of the studies.
The funnel plots were symmetrical, suggesting low-risk of publication bias (Figure 3). Egger's regression tests showed similar results (p = 0.301).
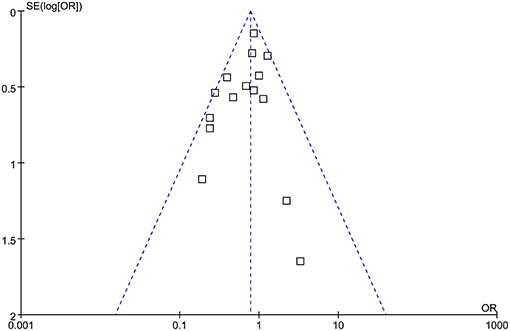
Figure 3. Funnel plots for the meta-analysis comparing the influences of dexmedetomidine and controls on the incidence of POAF after cardiac surgery.
In this updated meta-analysis of RCTs, we found that perioperative use of Dex is associated with 28% reduction of POAF after cardiac surgeries. The results seem to be stable since sensitivity analysis by excluding one study a time showed consistent results. Besides, subgroup analysis showed that Dex was associated with reduced POAF in Asian studies, but not in non-Asian studies. Moreover, both meta-regression and subgroup analyses showed that Dex was associated with reduced POAF in studies with younger patients and smaller proportion of males, but not in studies with older patients or larger proportion of males. These findings indicated that perioperative administration of Dex may reduce the risk of POAF after cardiac surgery, particularly in Asians. Moreover, age and sex of the patients may affect the potential preventative efficacy of Dex on POAF.
Compared with previous meta-analyses of the same fields, our study has multiple strengths. Firstly, 15 up-to-date RCTs were included, which represents the most recent understanding of the influence of Dex on POAF after cardiac surgeries. In addition, strict inclusion criteria were applied, which avoided the confounding effects of including studies with overlapped patients. Finally, multiple sensitivity and subgroup analyses were performed to shown the stability of the results. Results of meta-analysis are consistent with the previous observed potential antiarrhythmic efficacy of Dex. An early study showed that the potential anti-arrhythmic efficacy of Dex could be related to its inhibition on sympatholytic properties and activation of the vagus nerve (36). A subsequent retrospective case series supported that Dex may have novel antiarrhythmic properties for the acute termination of reentrant supraventricular tachycardia (37). A recent experimental study in rat model of ischemic cardiomyopathy (ICM) showed that Dex conferred anti-arrhythmic effects in the context of ICM via upregulation of connexin 43 and suppression of inflammation and fibrosis (38). Future studies are needed to determine the exact pharmacological mechanisms that underlying the possible anti-arrhythmic efficacy of Dex.
Results of subgroup analyses showed that Dex reduced POAF after cardiac surgery in Asian studies, but not in non-Asian studies. Previous studies showed that Caucasians are associated with higher risk of POAF after cardiac surgery than patients of other ethnicity, including the Asians (39, 40). In our meta-analysis, the incidence of POAF in patients of control group from non-Asian countries was also higher than that from Asian countries (24.1 vs. 17.9%). These findings may suggest that Dex is more effective in reducing POAF after cardiac surgery in low-risk populations. Subgroup analyses also showed that Dex was associated with reduced POAF in studies with younger patients and smaller proportion of males, but not in studies with older patients or larger proportion of males, suggesting the potential preventative role of Dex on POAF may be more effective in young patient and in the females. The possible reasons for the findings are also to be determined. It has been shown that advanced age is a risk factor for POAF after cardiac surgery (41). Besides, men have been suggested to have higher incidence of POAF than women after CABG (42). Taken together, results of subgroup analysis may suggest that Dex was more effective for the prevention of POAF in low-risk patients. Since high-risk patients such as aged men are likely to have multiple comorbidities for POAF, and a single intervention of Dex may not be effective. These findings should be validated in large-scale RCTs.
Currently, Dexmedetomidine was recommended to be applied with a loading dose (0.4–1 μg/kg) and continuous infusion (0.2–0.8 μg/kg/h). Results of subgroup analysis showed no difference in reducing POAF after cardiac surgery between studies with and without a loading dose of Dex. Considering the possible adverse effect related with loading dose of Dex (such as hemodynamic instability and possible induction of severe arrhythmia) (43), continuous Dex infusion may be adequate for these patients. In addition, influence of Dex infusion timing on POAF is also clinically important. Although the subgroup difference was not significant, results of subgroup analysis indicated that Dex infusion in ICU after the surgery may be adequate for prevention of POAF (Table 4, 9 studies, OR = 0.64). Future large-scale RCTs are needed to determine whether post-operative use of Dex confers similar efficacy in preventing of POAF as compared to pre-operative use of Dex.
Our study has some limitations. First, the meta-analysis was based on data from study level rather than individual patient data. Accordingly, influences of a few key characteristics on the possible preventative role of Dex for POAF could not be analyzed in our meta-analysis, such as left atrial volume (44), renal function (45), and concurrent cardiovascular medications including perioperative use of beta-blockers, amiodarone, and electrolyte replacement protocol for K and Mg etc. (46), which have all been suggested to affect the risk of POAF after cardiac surgery. Moreover, the sample sizes of the included RCTs varied significantly, most of which were of small scale. Therefore, large-scale RCTs are still needed to validate the findings of the meta-analysis. In addition, weight is one of critical risks leading POAF after cardiac surgery (47). However, only five of the included studies reported body weight, while another five reported BMI. The limited available datasets and differences in body weight and BMI reported in the studies prevented further analyses in subgroup analyses. Besides, quality of some included studies is not good, which may affect the reliability of the findings. However, subgroup analysis did not support that difference in quality score may significantly affect the results. Finally, the optimal regimens of Dex administration for prevention POAF remains to be determined, such as dose, initiating time, and duration. Future studies are warranted in this regard.
In conclusion, results of this updated meta-analysis suggested that perioperative administration of Dex may reduce the risk of POAF after cardiac surgery, particularly in Asians. More studies are warranted to determine whether the age and sex of the patients may affect the potential preventative efficacy of Dex on POAF.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
SP, GC, and PL contributed to the conception and design of the study. SP and HY performed literature search, study identification, quality evaluation, and data extraction. SP, JW, and HY performed the statistical analysis. SP, HY, GC, and PL wrote the first draft of the manuscript. All authors contributed to manuscript revision, and read and approved the submitted version.
This study was supported by the Pudong New Area Commission of Science Technology of Shanghai (PKJ2018-Y17).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Greenberg JW, Lancaster TS, Schuessler RB, Melby SJ. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardiothorac Surg. (2017) 52:665–72. doi: 10.1093/ejcts/ezx039
2. Aguilar M, Dobrev D, Nattel S. Postoperative atrial fibrillation: features, mechanisms, and clinical management. Card Electrophysiol Clin. (2021) 13:123–32. doi: 10.1016/j.ccep.2020.11.010
3. Woldendorp K, Farag J, Khadra S, Black D, Robinson B, Bannon P. Postoperative atrial fibrillation after cardiac surgery: a meta-analysis. Ann Thorac Surg. (2020) 111:544–54 doi: 10.1016/j.athoracsur.2020.10.055
4. Rezaei Y, Peighambari MM, Naghshbandi S, Samiei N, Ghavidel AA, Dehghani MR, et al. Postoperative atrial fibrillation following cardiac surgery: from pathogenesis to potential therapies. Am J Cardiovasc Drugs. (2020) 20:19–49. doi: 10.1007/s40256-019-00365-1
5. Gudbjartsson T, Helgadottir S, Sigurdsson MI, Taha A, Jeppsson A, Christensen TD, et al. New-onset postoperative atrial fibrillation after heart surgery. Acta Anaesthesiol Scand. (2020) 64:145–55. doi: 10.1111/aas.13507
6. Ronsoni RM, Souza AZM, Leiria TLL, Lima GG. Update on management of postoperative atrial fibrillation after cardiac surgery. Braz J Cardiovasc Surg. (2020) 35:206–10. doi: 10.21470/1678-9741-2019-0164
7. Abowali HA, Paganini M, Enten G, Elbadawi A, Camporesi EM. Critical review and meta-analysis of postoperative sedation after adult cardiac surgery: dexmedetomidine versus propofol. J Cardiothorac Vasc Anesth. (2021) 35:1134–42. doi: 10.1053/j.jvca.2020.10.022
8. Lee S. Dexmedetomidine: present and future directions. Korean J Anesthesiol. (2019) 72:323–30. doi: 10.4097/kja.19259
9. Tasbihgou SR, Barends CRM, Absalom AR. The role of dexmedetomidine in neurosurgery. Best Pract Res Clin Anaesthesiol. (2021) 35:221–9. doi: 10.1016/j.bpa.2020.10.002
10. Liu X, Zhang K, Wang W, Xie G, Fang X. Dexmedetomidine sedation reduces atrial fibrillation after cardiac surgery compared to propofol: a randomized controlled trial. Crit Care. (2016) 20:298. doi: 10.1186/s13054-016-1480-5
11. Soltani G, Jahanbakhsh S, Tashnizi MA, Fathi M, Amini S, Zirak N, et al. Effects of dexmedetomidine on heart arrhythmia prevention in off-pump coronary artery bypass surgery: a randomized clinical trial. Electron Physician. (2017) 9:5578–87. doi: 10.19082/5578
12. Zi J, Fan Y, Dong C, Zhao Y, Li D, Tan Q. Anxiety administrated by dexmedetomidine to prevent new-onset of postoperative atrial fibrillation in patients undergoing off-pump coronary artery bypass graft. Int Heart J. (2020) 61:263–72. doi: 10.1536/ihj.19-132
13. Jalonen J, Hynynen M, Kuitunen A, Heikkila H, Perttila J, Salmenpera M, et al. Dexmedetomidine as an anesthetic adjunct in coronary artery bypass grafting. Anesthesiology. (1997) 86:331–45. doi: 10.1097/00000542-199702000-00009
14. Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. (2003) 17:576–84. doi: 10.1016/S1053-0770(03)00200-3
15. Corbett SM, Rebuck JA, Greene CM, Callas PW, Neale BW, Healey MA, et al. Dexmedetomidine does not improve patient satisfaction when compared with propofol during mechanical ventilation. Crit Care Med. (2005) 33:940–5. doi: 10.1097/01.CCM.0000162565.18193.E5
16. Shehabi Y, Grant P, Wolfenden H, Hammond N, Bass F, Campbell M, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study). Anesthesiology. (2009) 111:1075–84. doi: 10.1097/ALN.0b013e3181b6a783
17. Göksedef D, Balkanay O, meroglu S, Talas Z, Arapi B, Junusbekov Y, et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Turk J Thoracic Cardiovasc Surg. (2013) 21, 594–602. doi: 10.5606/tgkdc.dergisi.2013.7645
18. Ren J, Zhang H, Huang L, Liu Y, Liu F, Dong Z. Protective effect of dexmedetomidine in coronary artery bypass grafting surgery. Exp Ther Med. (2013) 6:497–502. doi: 10.3892/etm.2013.1183
19. Park JB, Bang SH, Chee HK, Kim JS, Lee SA, Shin JK. Efficacy and safety of dexmedetomidine for postoperative delirium in adult cardiac surgery on cardiopulmonary bypass. Korean J Thorac Cardiovasc Surg. (2014) 47:249–54. doi: 10.5090/kjtcs.2014.47.3.249
20. Balkanay OO, Goksedef D, Omeroglu SN, Ipek G. The dose-related effects of dexmedetomidine on renal functions and serum neutrophil gelatinase-associated lipocalin values after coronary artery bypass grafting: a randomized, triple-blind, placebo-controlled study. Interact Cardiovasc Thorac Surg. (2015) 20:209–14. doi: 10.1093/icvts/ivu367
21. Karaman Y, Abud B, Tekgul ZT, Cakmak M, Yildiz M, Gonullu M. Effects of dexmedetomidine and propofol on sedation in patients after coronary artery bypass graft surgery in a fast-track recovery room setting. J Anesth. (2015) 29:522–8. doi: 10.1007/s00540-015-1975-2
22. Djaiani G, Silverton N, Fedorko L, Carroll J, Styra R, Rao V, et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. (2016) 124:362–8. doi: 10.1097/ALN.0000000000000951
23. Shi C, Jin J, Qiao L, Li T, Ma J, Ma Z. Effect of perioperative administration of dexmedetomidine on delirium after cardiac surgery in elderly patients: a double-blinded, multi-center, randomized study. Clin Interv Aging. (2019) 14:571–5. doi: 10.2147/CIA.S194476
24. Turan A, Duncan A, Leung S, Karimi N, Fang J, Mao G, et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet. (2020) 396:177–85. doi: 10.1016/S0140-6736(20)30631-0
25. Geng J, Qian J, Cheng H, Ji F, Liu H. The influence of perioperative dexmedetomidine on patients undergoing cardiac surgery: a meta-analysis. PLoS ONE. (2016) 11:e0152829. doi: 10.1371/journal.pone.0152829
26. Liu X, Xie G, Zhang K, Song S, Song F, Jin Y, et al. Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. J Crit Care. (2017) 38:190–6. doi: 10.1016/j.jcrc.2016.10.026
27. Ling X, Zhou H, Ni Y, Wu C, Zhang C, Zhu Z. Does dexmedetomidine have an antiarrhythmic effect on cardiac patients? A meta-analysis of randomized controlled trials. PLoS ONE. (2018) 13:e0193303. doi: 10.1371/journal.pone.0193303
28. Wang G, Niu J, Li Z, Lv H, Cai H. The efficacy and safety of dexmedetomidine in cardiac surgery patients: a systematic review and meta-analysis. PLoS ONE. (2018) 13:e0202620. doi: 10.1371/journal.pone.0202620
29. Zhu Z, Zhou H, Ni Y, Wu C, Zhang C, Ling X. Can dexmedetomidine reduce atrial fibrillation after cardiac surgery? A systematic review and meta-analysis. Drug Des Devel Ther. (2018) 12:521–31. doi: 10.2147/DDDT.S153834
30. Liu Y, Zhang L, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine reduces atrial fibrillation after adult cardiac surgery: a meta-analysis of randomized controlled trials. Am J Cardiovasc Drugs. (2020) 20:271–81. doi: 10.1007/s40256-019-00380-2
31. Liu X, Zhang K, Wang W, Xie G, Cheng B, Wang Y, et al. Dexmedetomidine versus propofol sedation improves sublingual microcirculation after cardiac surgery: a randomized controlled trial. J Cardiothorac Vasc Anesth. (2016) 30:1509–15. doi: 10.1053/j.jvca.2016.05.038
32. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
33. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration (2011). Available online at: www.cochranehandbook.org (accessed May 26, 2021)
34. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
35. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
36. Kamibayashi T, Hayashi Y, Mammoto T, Yamatodani A, Sumikawa K, Yoshiya I. Role of the vagus nerve in the antidysrhythmic effect of dexmedetomidine on halothane/epinephrine dysrhythmias in dogs. Anesthesiology. (1995) 83:992–9. doi: 10.1097/00000542-199511000-00013
37. Chrysostomou C, Morell VO, Wearden P, Sanchez-De-Toledo J, Jooste EH, Beerman L. Dexmedetomidine: therapeutic use for the termination of reentrant supraventricular tachycardia. Congenit Heart Dis. (2013) 8:48–56. doi: 10.1111/j.1747-0803.2012.00669.x
38. Wu SJ, Lin ZH, Lin YZ, Rao ZH, Lin JF, Wu LP, et al. Dexmedetomidine exerted anti-arrhythmic effects in rat with ischemic cardiomyopathy via upregulation of connexin 43 and reduction of fibrosis and inflammation. Front Physiol. (2020) 11:33. doi: 10.3389/fphys.2020.00033
39. Nazeri A, Razavi M, Elayda MA, Lee VV, Massumi A, Wilson JM. Race/ethnicity and the incidence of new-onset atrial fibrillation after isolated coronary artery bypass surgery. Heart Rhythm. (2010) 7:1458–63. doi: 10.1016/j.hrthm.2010.06.037
40. Rader F, Van Wagoner DR, Ellinor PT, Gillinov AM, Chung MK, Costantini O, et al. Influence of race on atrial fibrillation after cardiac surgery. Circ Arrhythm Electrophysiol. (2011) 4:644–52. doi: 10.1161/CIRCEP.111.962670
41. Pivatto Junior F, Teixeira Filho GF, Sant'anna JR, Py PM, Prates PR, Nesralla IA, et al. Advanced age and incidence of atrial fibrillation in the postoperative period of aortic valve replacement. Rev Bras Cir Cardiovasc. (2014) 29:45–50. doi: 10.5935/1678-9741.20140010
42. Filardo G, Ailawadi G, Pollock BD, Da Graca B, Sass DM, Phan TK, et al. Sex differences in the epidemiology of new-onset in-hospital post-coronary artery bypass graft surgery atrial fibrillation: a large multicenter study. Circ Cardiovasc Qual Outcomes. (2016) 9:723–30. doi: 10.1161/CIRCOUTCOMES.116.003023
43. Su F, Hammer GB. Dexmedetomidine: pediatric pharmacology, clinical uses and safety. Expert Opin Drug Saf. (2011) 10:55–66. doi: 10.1517/14740338.2010.512609
44. Kislitsina ON, Cox JL, Shah SJ, Malaisrie SC, Kruse J, Liu M, et al. Preoperative left atrial strain abnormalities are associated with the development of postoperative atrial fibrillation following isolated coronary artery bypass surgery. J Thorac Cardiovasc Surg. (2020). doi: 10.1016/j.jtcvs.2020.09.130. [Epub ahead of print].
45. Limite LR, Magnoni M, Berteotti M, Peretto G, Durante A, Cristell N, et al. The predictive role of renal function and systemic inflammation on the onset of de novo atrial fibrillation after cardiac surgery. Eur J Prev Cardiol. (2016) 23:206–13. doi: 10.1177/2047487314564896
46. Zhen-Han L, Rui S, Dan C, Xiao-Li Z, Qing-Chen W, Bo F. Perioperative statin administration with decreased risk of postoperative atrial fibrillation, but not acute kidney injury or myocardial infarction: a meta-analysis. Sci Rep. (2017) 7:10091. doi: 10.1038/s41598-017-10600-x
Keywords: dexmedetomidine, post-operative atrial fibrillation (POAF), perioperative, cardiac surgery, meta-analysis
Citation: Peng S, Wang J, Yu H, Cao G and Liu P (2021) Influence of Dexmedetomidine on Post-operative Atrial Fibrillation After Cardiac Surgery: A Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 8:721264. doi: 10.3389/fcvm.2021.721264
Received: 06 June 2021; Accepted: 26 October 2021;
Published: 25 November 2021.
Edited by:
Sandro Gelsomino, Maastricht University, NetherlandsReviewed by:
Zhichun Gu, Shanghai JiaoTong University, ChinaCopyright © 2021 Peng, Wang, Yu, Cao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ge Cao, Y2FvZ2VfaHgwMTFAMjFjbi5jb20=; Peirong Liu, cGVpcm9uZ2xpdV9zaDdAMjFjbi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.