- 1Department of Nephrology, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
- 2Med-X Center for Materials, Sichuan University, Chengdu, China
Aims: We aimed to assess the association between dietary inflammation index (DII) and abdominal aortic calcification (AAC) in US adults aged ≥40 years.
Methods: Data were obtained from the 2013–2014 National Health and Nutrition Examination Survey (NHANES). Participants who were <40 years old and missing the data of DII and AAC were excluded. DII was calculated based on a 24-h dietary recall interview for each participant. AAC score was quantified by assessing lateral spine images and severe AAC was defined as AAC score >6. Weighted multivariable regression analysis and subgroup analysis were preformed to estimate the independent relationship between DII with AAC score and severe AAC.
Results: A total of 2,897 participants were included with the mean DII of −0.17 ± 2.80 and the mean AAC score of 1.462 ± 3.290. The prevalence of severe AAC was 7.68% overall, and participants in higher DII quartile tended to have higher rates of severe AAC (Quartile 1: 5.03%, Quartile 2: 7.44%, Quartile 3: 8.38%, Quartile 4: 10.46%, p = 0.0016). A positive association between DII and AAC score was observed (β = 0.055, 95% CI: 0.010, 0.101, p = 0.01649), and higher DII was associated with an increased risk of severe AAC (OR = 1.067, 95% CI: 1.004, 1.134, p = 0.03746). Subgroup analysis indicated that this positive association between DII and AAC was similar in population with differences in gender, age, BMI, hypertension status, and diabetes status and could be appropriate for different population settings.
Conclusion: Higher pro-inflammatory diet was associated with higher AAC score and increased risk of severe AAC. Anti-inflammatory dietary management maybe beneficial to reduce the risk of AAC.
Introduction
Vascular calcification (VC) is a pathology characterized by ectopic calcification in the vessel wall of muscular or elastic arteries (1), which can be commonly observed in patients with diabetes, chronic kidney disease (CKD), hypertension, osteoporosis, etc. (2–4). It contributes to a series of cardiovascular diseases (CVDs) including atherosclerosis, hypertension, aortic stenosis, and coronary heart disease and associated with a poor prognosis including higher risk of mortality, adverse cardiovascular events (stroke, acute myocardial infarction, peripheral vascular diseases, etc.), and other comorbidities (5). Meanwhile, severe calcification poses a great challenge to the treatment. There is currently no validated effective treatment for VC, and the underlying mechanisms have not been fully elucidated. Sodium thiosulfate has been reported as a potential treatment method, but more studies are still necessary (6). Djuric et al. found that sodium thiosulfate positively affected calcification progress in iliac arteries and heart valves but failed to hinder the calcification progress of abdominal aorta in a small randomized controlled trial (7). Further studies are still needed to validate the potential clinical application. After the onset of CVDs accompanied with VC, it is difficult to reverse the diseased vessels. Additionally, VC itself can hinder its treatment. For example, coronary artery disease complicated with severe calcification was still a problem in percutaneous coronary intervention treatment (8). Both clinical and epidemiological data showed that VC was closely related to higher cardiovascular disease risk and mortality, thus, the prevention and management of VC is of great significance (9, 10).
The abdominal aorta is a site prone to atherosclerosis and calcification, which could act as a good indicator of VC in patients and predicted both cardiovascular and all-cause mortality (11–13). It has been reported that the prevalence of abdominal aortic calcification (AAC) increased with age, ranging from 60% at age 65–69 years to 96% at 85 years and older. In addition, patients with AAC showed an increased risk of cardiovascular death and all-cause mortality than those without AAC (14). To evaluate the severity of calcified vessels, Kauppila et al. described an AAC grading quantification method (AAC score) by using lateral lumbar radiography in a Framingham study subgroup and found it could predict all-cause mortality and non-fatal cardiovascular events in hemodialysis patients independently (15, 16). Recently, the development of imaging technology helps us to understand VC more comprehensively. In 2009, the Kidney Disease: Improving Global Outcomes (KDIGO) suggested that abdominal lateral X-ray film should be used instead of CT as the basic imaging examination to evaluate whether abdominal aorta calcification exists and suggested that AAC score could reflect aortic calcification in peritoneal dialysis patients (17).
As a potential target for therapeutic measures, the management of diet is the most basic treatment in clinical practice and the dietary influence on health has aroused wide attention. Shivappa et al. developed the dietary inflammatory index (DII), a literature-derived, population-based dietary score summarizing the effect of dietary parameters on inflammation, which categorizes an individual's diet into proinflammatory and anti-inflammatory diet (18). A positive higher DII score means a more proinflammatory effect and a negative lower DII score indicates a more anti-inflammatory effect. Previous studies have documented a direct association between DII and a higher risk for cancer, metabolic syndrome, sarcopenia, etc. (19, 20). Li et al. reported that dietary patterns with a higher proinflammatory potential were associated with higher risk of coronary heart disease and strokes, and reducing the dietary inflammatory potential may potentially provide an effective strategy for CVD prevention (21).
Although the mechanisms involved in VC are complex and related to multiple factors, current studies have mainly found that inflammation can promote VC (22). However, the relationship between dietary inflammatory potential and abdominal aortic calcification has not been reported before. Using data from the National Health and Nutrition Examination Survey (NHANES), the aim of this study was to explore the association of dietary inflammatory potential and AAC, which may shed new light on the management of VC in clinical practice.
Materials and Methods
Study Population
We obtained data from the NHANES, which is a cross-sectional study to evaluate the nutrition and health status of non-institutionalized population in the USA on a repeated 2-year cycle conducted by the National Center for Health Statistics (NCHS) (23). All NHANES data are publicly available at https://www.cdc.gov/nchs/nhanes/.
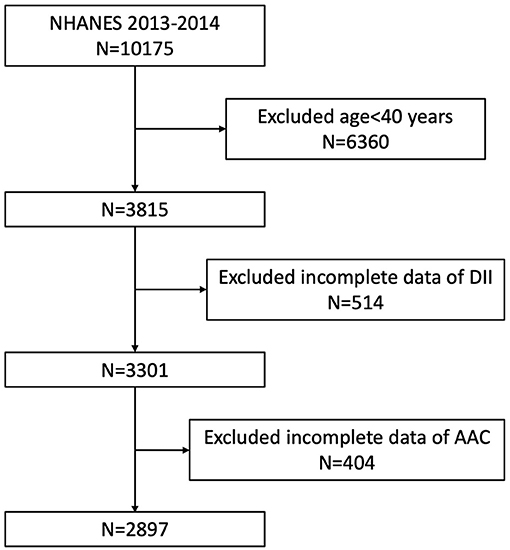
Our study was based on the data from NHANES 2013 to 2014, since only this cycle included both data on abdominal artery calcification and dietary inflammatory index. Because the data about AAC score was not available for participants aged <40 years (they were excluded for the DXA scan in NHANES 2013–2014), we enrolled participants aged ≥40 years with complete data both on AAC score and DII in our analysis. A total of 10,175 individuals were enrolled at first, after exclusion of participants aged <40 years old (n = 6,360), missing the dietary data relating to DII (n = 514), or missing the data of AAC (n = 404), 2,897 subjects aged ≥40 years were included in our final analysis (Figure 1).
Exposure and Outcome Definitions
DII score was designed as exposure variable. It was designed to evaluate the inflammatory potential of diets based on the proinflammatory and anti-inflammatory properties of the dietary components (18). Dietary data in NHANES were obtained by a 24-h dietary recall interview and have been validated by the Nutrition Methodology Working Group (24). We calculated the DII based on the 24-h dietary recall data and used this to assess the dietary inflammatory potential for each individual. A positive higher DII suggested a proinflammatory effect and negative lower DII suggested an anti-inflammatory effect of diet (18). Twenty-eight food parameters were available in NHANES 2013–2014 and were used to calculate DII, namely, alcohol, β-carotene, cholesterol, carbohydrates, energy, fats, fibers, folic acid, iron, magnesium, zinc, selenium, vitamin A, vitamin B-6, vitamin B-12, vitamin C, vitamin D, vitamin E, mono-unsaturated fatty acid, protein, niacin, riboflavin, n-3 fatty acid, n-6 fatty acid, poly-unsaturated fatty acid, saturated fat, caffeine, and thiamin. Inflammatory effect scores for each dietary parameter were calculated, and the sum of the scores of each food parameter for each individual was the DII score. It has been proven that using food parameters <30 to calculate DII would not reduce its predictive ability (25, 26). DII was analyzed as a continuous variable, and participants were divided to quartiles based on the DII score for further analysis.
AAC score and severe AAC were designed as outcome variables. AAC score was used to evaluate the severity of calcified vessels, and a higher AAC score indicated a more severe vascular calcification condition. It was quantified according to the Kauppila score system by assessing lateral lumbar spine images (vertebrae L1 to L4) obtained using dual-energy X-ray absorptiometry (DXA, Densitometer Discovery A, Hologic, Marlborough, MA, USA) (16). In the Kauppila scoring method for AAC, both the anterior and posterior aortic walls were divided into four segments corresponding to the region in front of the lumbar vertebrae L1 to L4. In these four segments, aortic calcification is visually either a diffuse white spot of the aorta extending to the anterior and/or posterior aortic walls or a white linear calcification of the anterior and/or posterior aortic walls. Different calcification severity of each segment corresponded to different scores according to the calcific deposit proportions, and the sum of scores for all segments was the final AAC score, resulting in a range from “0 to 6” for each segment and “0 to 24” for the total score (16). According to previous studies, we defined severe AAC as a total AAC score >6, which was considered significant aortic calcification and has been widely used as a cutoff point in previous studies (16, 27, 28). In NHANES 2013–2014, participants who were aged <40 years old, pregnant, self-reported history of radiographic contrast material (barium) use in the past 7 days, weighted over 450 lbs., and a Harrington Rod in the spine for scoliosis were excluded for DXA scans, and their AAC score data were not available.
Covariates
Continuous variables in our study included age (year), body mass index (BMI, kg/m2), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), and serum creatinine (mg/dl). Categorical variables included gender, race, education level, ratio of family income to poverty (RIP), hypertension, diabetes, congestive heart failure (CHF), and smoke. All detailed measurement process of these variables was publicly available at www.cdc.gov/nchs/nhanes/.
Statistical Analysis
Statistical analyses were conducted according to CDC guidelines (29). All analysis was performed using appropriate NHANES sampling weights and accounted for complex multistage cluster survey design. Continuous variables were presented as mean with standard deviation, and categorical variables were presented as frequency or percentage. Weighted Student's t-test (for continuous variables) or weighted chi-square test (for categorical variables) was employed to assess the differences in groups divided by DII (quartiles). Weighted multiple regression analysis was preformed to estimate the independent relationship between DII scores and AAC (including AAC score and severe AAC) in three different models. In model 1, no covariates were adjusted. Model 2 was adjusted for gender, age, and race. Model 3 was adjusted for gender, age, race, education level, PIR, SBP, DBP, BMI, serum creatinine, hypertension, diabetes, congestive heart failure, and smoke. Subgroup analysis was performed by stratified multivariate regression analysis. In addition, weighted generalized additive models and smooth curve fittings were used to address the non-linearity of DII and AAC. p < 0.05 was considered statistically significant. All analysis was preformed using Empower software (www. empowerstats.com; X&Y solutions, Inc., Boston MA) and R version 3.4.3 (http://www.R-project.org, The R Foundation).
Results
Baseline Characteristics of Participants
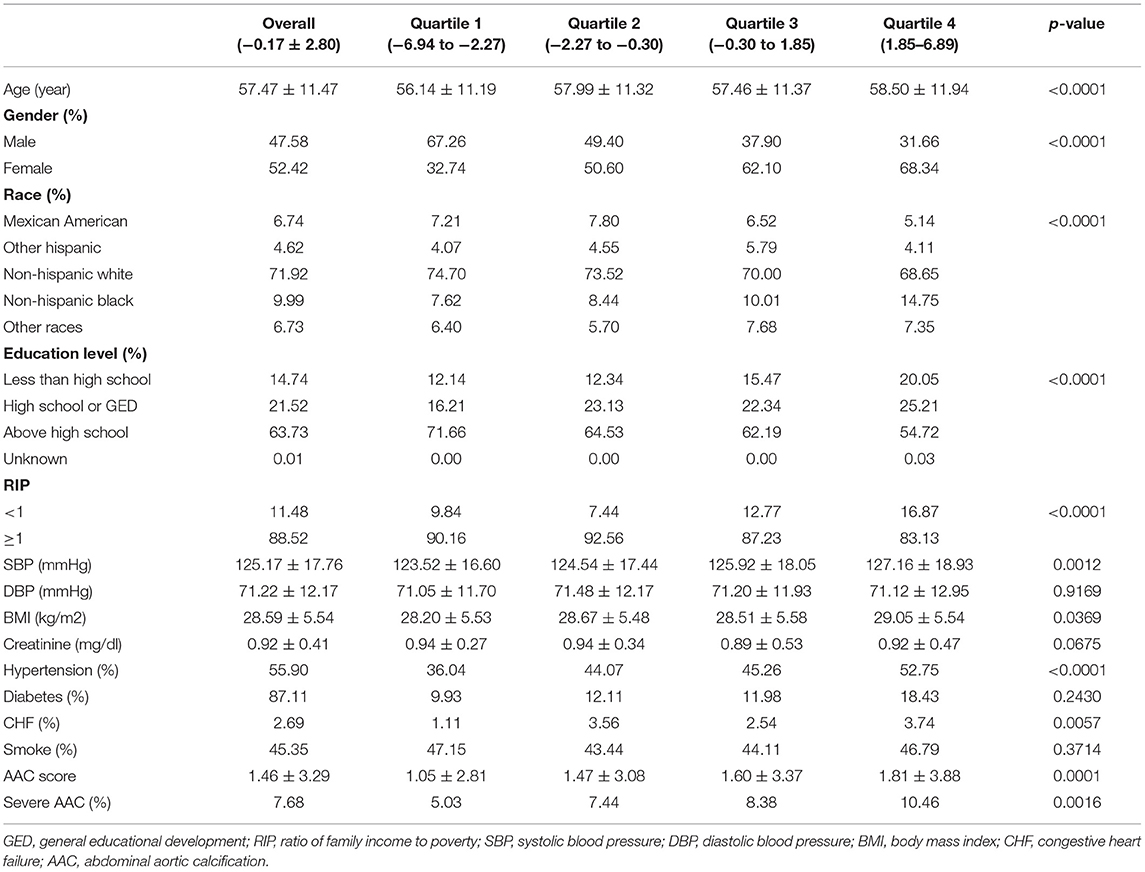
The weighted demographic characteristics and other covariates of included participants are shown in Table 1. A total of 2,897 participants were included in our study, of whom 47.58% were males and 52.42% were females with an average age of 57.47 ± 11.47 years old. The mean of DII was −0.17 ± 2.80, and the ranges of DII for quartiles 1–4 were −6.94 to −2.27, −2.27 to −0.30, −0.30 to 1.85, and 1.85 to 6.89, respectively. Among different quartiles of DII, significant differences were observed in gender, race, education level, PIR, SBP, BMI, whether having hypertension, congestive heart failure, AAC score, and the prevalence of severe AAC, while the difference between quartiles in DBP, serum creatinine, diabetes, and smoke status did not meet the statistical significance. The average AAC score was 1.462 ± 3.290 for the whole participants, and the mean AAC score was 1.811 ± 3.884 for the highest quartile and 1.049 ± 2.806 for the lowest quartile. The prevalence of severe AAC was 7.68% overall, and participants in higher DII quartile tended to have higher rates of severe AAC (Quartile 1: 5.03%, Quartile 2: 7.44%, Quartile 3: 8.38%, Quartile 4: 10.46%, p = 0.0016).
Higher DII Was Associated With Higher AAC Score and Higher Risk of Severe AAC
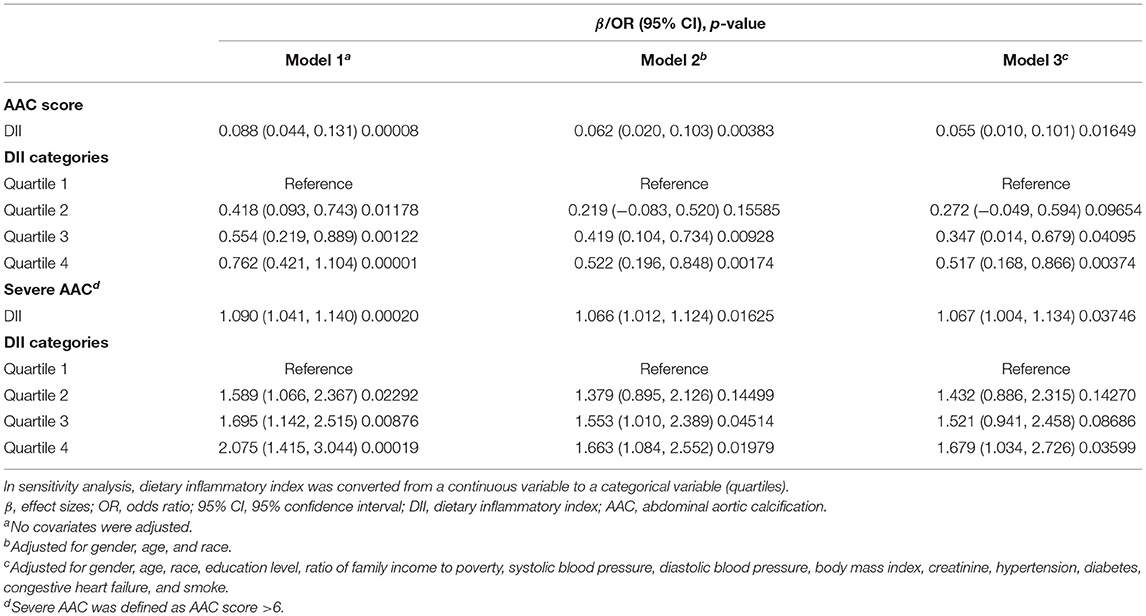
We employed weighted multiple regression analysis to assess the association between DII and AAC score in three different models. In model 1, no covariates were adjusted. Model 2 was adjusted for gender, age, and race. Model 3 was adjusted for gender, age, race, education level, PIR, SBP, DBP, BMI, serum creatinine, hypertension, diabetes, congestive heart failure, and smoke. A positive association between DII and AAC was observed both in model 1 (β = 0.088, 95% CI: 0.044, 0.131, p = 0.00008) and model 2 (β = 0.062, 95% CI: 0.020, 0.103, p = 0.00383). In fully adjusted model (model 3), the positive association was still stable (β = 0.055, 95% CI: 0.010, 0.101, p = 0.01649), indicating that each unit of increased DII score was associated with 0.055 increased unit of AAC score, respectively. We also converted DII from a continuous variable to a categorical variable (quartiles) to conduct the sensitivity analysis. Compared with the lowest DII scores quartile (Quartile 1), the AAC score increased with the higher DII quartiles group. The mean AAC score of the highest DII quartile (Quartile 4) was 0.517 unit higher compared with the lowest quartile (β = 0.517, 95% CI: 0.168, 0.866; p = 0.00374), respectively (Table 2).
As for severe AAC, we also observed that increased DII was associated with a higher risk of severe AAC (model 1, OR = 1.090, 95% CI: 1.041, 1.140, p = 0.00020; model 2, OR = 1.066, 95% CI: 1.012, 1.124, p = 0.01625; model 3, OR = 1.067, 95% CI: 1.004, 1.134, p = 0.03746). In model 3, which was adjusted for all covariates, our results indicated that each unit of increased DII score was associated with 6.7% increased risk of severe AAC. In sensitivity analysis, the adjusted OR (reference to Quartile 1) was 1.679 (95% CI: 1.034, 2.726, p = 0.03599) for Quartile 4, suggesting a stable positive relationship between increased DII and increased risk of severe AAC with statistical significance (Table 2).
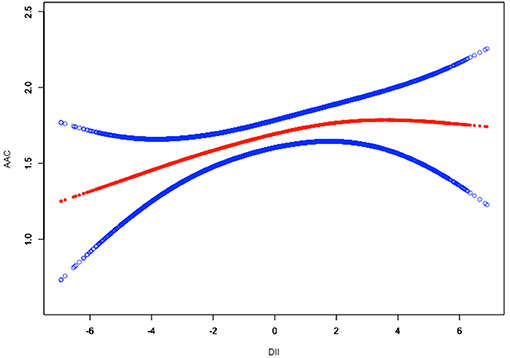
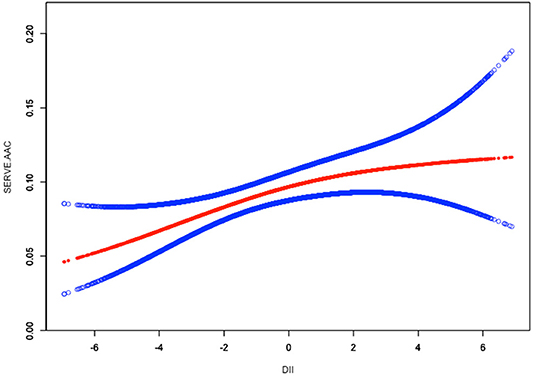
To assess the non-linear relationship between DII and ACC, weighted generalized additive models and smooth curve fittings were employed for further study. The results indicated that there was no non-linear relationship between DII and AAC score (Figure 2). A similar result was observed in the association between DII and severe AAC (Figure 3).
DII Positively Associated With Higher AAC Score in Different Subgroups
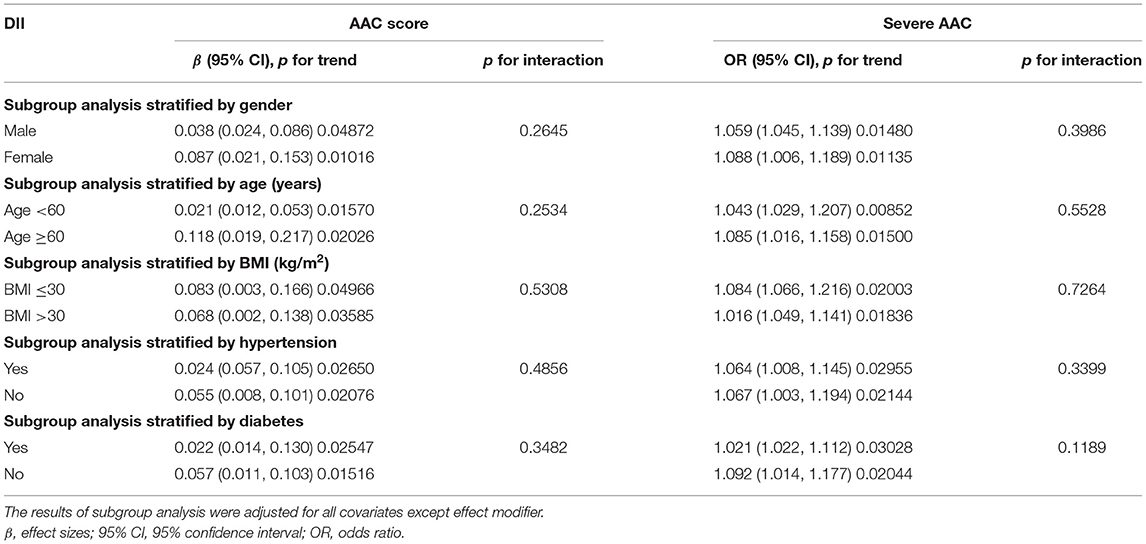
Subgroup analysis was conducted to further explore the association between DII and AAC score in different population setting including gender, age, BMI, hypertension, and diabetes (Table 3). We also performed an interaction test to evaluate if there was any significant dependence of the effect modifier on this association. p for interaction >0.05 means no significant dependence was observed. In our analysis, no correlation with the p for interaction meeting the statistical significance was detected (all p for interaction >0.05), indicating that the magnitude of the association was the same for different population settings.
Regarding the correlation between DII and AAC score, in subgroups stratified by gender, age, BMI, hypertension, and diabetes, the positive associations between DII and AAC score in different subgroups were all significant (p for trend < 0.05) and p for interaction >0.05, suggesting that the correlation between DII scores and AAC score was similar in population with differences in gender, age, BMI, hypertension status, and diabetes status.
DII Positively Associated With Increased Risk of Severe AAC in Different Subgroups
As for the association between DII and severe ACC, the results of subgroup stratified by gender demonstrated that DII was positively associated with higher risk of severe AAC both in female subgroup (OR = 1.088, p for trend = 0.01135) and male subgroup (OR = 1.059, p for trend = 0.01480) with p for interaction of 0.3986, indicating that there was no significant dependence of gender on this positive association between DII and severe AAC.
Similarly, we did not find any significant dependence on age, BMI, hypertension, diabetes status (all p for interaction >0.05). These results indicated that the positive association between DII and the risk of severe AAC was similar in population with differences in gender, age, BMI, hypertension status, and diabetes status and could be appropriate for various population settings as well (Table 3).
Discussion
In this cross-sectional study including 2,897 adults, a significant positive association between DII with AAC score and severe ACC was observed, indicating that a proinflammatory diet may contribute to higher AAC score and increased risk of severe AAC. The associations between the exposure variable and outcomes were still stable in subgroups stratified by gender, age, BMI, hypertension status, and diabetes status, suggesting that this positive association may be appropriate for different population settings.
To our knowledge, this is the first study assessing the association between the dietary inflammatory potential and AAC. Previous studies have revealed the influence of various dietary factors on calcification. Experimental studies have shown the roles of high phosphorus and calcium diets as promoters of calcification, and magnesium and vitamin K intakes as inhibitory factors (30–34). Obesity and metabolic syndrome could also promote VC, and vitamin E can protect against calcification in uremic rats (35–37). In addition, Lu et al. reported that high serum selenium concentration was positively associated with a mean AAC score and an increased risk of severe AAC among non-institutionalized US adults independently (38).
As one of the most appropriate approaches to understand the relationship between diet and the risk of various diseases, dietary pattern analysis has been regarded as a more robust and valid measure of dietary intake in the longer term (39). It has been demonstrated that a proinflammatory diet can increase systemic inflammation (40). For example, the “Western” diet containing high intake in red meat, high-fat dairy products, refined grains, and simple carbohydrates, has been reported to be associated with higher levels of CRP and interleukin (IL)-6 (41, 42). In contrast, the Dietary Approaches to Stop Hypertension (DASH) diet emphasized intake of fruits, vegetables, low fat dairy foods, and reduced saturated and total fat, which can suppress the inflammation process (43). Likewise, the Mediterranean diet pattern, which is high in fruit, vegetables, and healthy fats, has been reported to be associated with reduced concentrations of CRP (44). Recently, DII has been gradually explored by more scholars as a variable risk factor on health and disease in a number of specialized areas, including cardiovascular disease, metabolic syndrome (19, 45), cancer (46–48), obstetrics (49), digestive system disease (50, 51), etc. Relevant to our study, inflammation was regarded to play a crucial role in VC.
Traditionally, VC was classified into two forms, depending on the place where the mineral deposit is located. Intimal calcification was closely related to lipid deposits, and the clinically relevant infiltration of inflammatory cells, with obstructive arterial disease (34), and medial calcification was associated with aging, diabetes mellitus, hypertension, osteoporosis, and CKD (22). It is recognized as a cell-mediated, active process involving a series of related genes and proteins, with an imbalance of promoters and inhibitors. With the advances in the field of VC pathogenesis, our current understanding of the mechanism of VC included chronic inflammation, autophagy defects, endoplasmic reticulum stress, mitochondrial dysfunction, and its dynamics (52). The exact mechanism of the positive association between DII and AAC in our study still remains unclear. A possible explanation to support our results might be the effect of diet on proinflammatory markers such as IL-1, IL-6, and tumor necrosis factor-α (TNF-α) (18, 53). Proinflammatory diet could increase the level of inflammation markers, and the increased inflammation level promotes VC (22). Previous studies have indicated that IL-1β (54), IL-6 (55), and TNF-α (56) could stimulate vascular smooth muscle cell (VSMC) calcification through various pathways. The increased IL-1β release could modulate proinflammatory signaling cascades in VSMCs to promote calcification through NF-kappa B pathway, WNT/β-catenin pathway, etc. (57, 58). IL-6 could also get involved in the VSMC calcification by inducing oxidative stress and activating RANKL, STAT3, BMP-2-WNT/β-catenin pathway, etc. (59–62). TNF-α mediated calcification via the upregulation of BMP-2 signaling and triggered the osteo-/chondrogenic transformation of VSMCs with an interplay with MSX-2 up-expression, WNT/β-catenin pathway, and oxidative stress (63–65). Proinflammatory macrophages also have been reported to further play a key role in VC progression (66). It could be inferred that proinflammatory diet may increase the inflammation level, and the continuously activated inflammatory state could lead to the activation of inflammation-related signaling pathways, macrophage infiltration, and T-lymphocyte activation, which in turn activated the differentiation of VSMCs into osteoblastic phenotype and calcium salt deposition, resulting in the progress of vascular calcification.
One of the strengths of our study is that it was based on a nationwide, population-based sampling survey data and appropriate weighting of survey participants was adopted, making the findings widely usable in the US population. In addition, considering the relationship of ACC with some other risk factors, several covariables related to AAC including gender, age, BMI, serum creatinine, hypertension, diabetes, congestive heart failure, smoke, etc. were included in the adjusted model for weighted multiple regression analysis (67–69). Moreover, our large sample size allowed us to perform subgroup analyses. Our results indicated that the positive association between DII and AAC was similar in population with differences in gender, age, BMI, hypertension status, and diabetes status. Given the fact that aging was originally one of the strongest drivers of VC in the elderly, the prevalence of AAC was expected to be increased among older population (70). For the subgroup analysis stratified by age, we found that DII was positively associated with higher AAC score (β = 0.021, p for trend = 0.01570) and higher risk of severe AAC (OR = 1.043, p for trend = 0.00852) in the <60 year-old subgroup. In the ≥60-year-old group, we observed a similar result (for AAC score, β = 0.118, p for trend = 0.02026; for severe AAC, OR = 1.085, p for trend = 0.01500). Interaction test indicated that the magnitude of the association was the same for population with different ages (for AAC score, p for interaction = 0.2534; for severe AAC, p for interaction = 0.5528). Indeed, for each unit of increased DII, a 4.3% and 8.5% increased risk of severe AAC was observed for participants aged <60 and ≥60 years, which was consistent with the fact that older population showed a higher AAC prevalence and had higher risks of AAC.
The limitations of this study cannot be ignored. Firstly, since NHANES was a cross-sectional survey in US population, we could not obtain a causal relationship between dietary inflammatory potential with AAC, and the ability of our findings to be generalized to a common population or other ethnic groups may be limited. Therefore, prospective studies with a larger sample size are required to clarify the causality. Secondly, we cannot completely rule out residual confounders due to unmeasured or unknown variables. For example, even in the non-hospitalized population, there is a possibility that medication may affect VC, such as vitamin K antagonists (71) and statins (58, 72). In addition, the data on AAC scores for participants aged <40 years old was not available in NHANES 2013–2014. Thus, we could not analyze this association for wider age groups. The detailed molecular mechanisms supporting our findings are still uncertain and need more laboratory-based real experiments. Finally, the DII was calculated from a detailed and validated dietary questionnaire, but the diet was subject to change over time, thus we cannot account for dietary changes. Since the DII was calculated based on the 24-h dietary recall data, some misclassification of the exposure and recall bias were inevitable. Thus, further researches are still needed to gain insight into the long-term association between dietary inflammatory potential and AAC.
Conclusion
In this cross-sectional study with 2,897 adults included, a significant positive association between DII and AAC was observed, indicating that higher consumption of proinflammatory diet might contribute to a higher AAC score and increased risk of severe AAC. Our finding suggests that a proper anti-inflammatory dietary management maybe beneficial to lower the risk of vascular calcification in adults. However, further research and clinical settings are still needed to validate for potential application.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
Ethics Statement
The studies involving human participants were reviewed and approved by The NCHS Ethic Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZQ: data analysis, software, and writing (original draft). KC: formal analysis and writing (original draft). RL: methodology and writing (original draft). LJ and QY: formal analysis and software. BS: conceptualization, funding acquisition, and writing (reviewing and editing). All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82000702), the Science and Technology Achievement Transformation Fund of West China Hospital of Sichuan University (Grant No. CGZH19006), the 1.3.5 project for disciplines of excellence from West China Hospital of Sichuan University (Grant No. ZYJC21010), and Med+ Biomaterial Institute of West China Hospital/West China School of Medicine of Sichuan University (Grant No. ZYME20001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Chichen Zhang for his assistance in data processing.
References
1. Ngai D, Lino M, Bendeck MP. Cell-matrix interactions and matricrine signaling in the pathogenesis of vascular calcification. Front Cardiovasc Med. (2018) 5. doi: 10.3389/fcvm.2018.00174
2. Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitushighlights. Arterioscler Thromb Vasc Biol. (2016). 37:191–204 doi: 10.1161/ATVBAHA.116.306256
3. Paloian NJ, Giachelli CM. A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol. (2014) 307:F891. doi: 10.1152/ajprenal.00163.2014
4. Zhu J, Guo F, Zhang J, Mu C. Relationship of calcification in the carotid or coronary arteries and osteoporosis in the elderly. Minerva Med. (2018) 110:12–17 doi: 10.23736/S0026-4806.18.05632-X
5. Qin Z, Liao R, Xiong Y, Jiang L, Li J, Wang L, et al. A narrative review of exosomes in vascular calcification. Ann Transl Med. (2021) 9:579. doi: 10.21037/atm-20-7355
6. O'Neill WC. Sodium thiosulfate: mythical treatment for a mysterious disease? Clin J Am Soc Nephrol. (2013) 8:1068–9. doi: 10.2215/CJN.04990513
7. Djuric P, Dimkovic N, Schlieper G, Djuric Z, Pantelic M, Mitrovic M, et al. Sodium thiosulphate and progression of vascular calcification in end-stage renal disease patients: a double-blind, randomized, placebo-controlled study. Nephrol Dial Transplant. (2020) 35:162–9. doi: 10.1093/ndt/gfz204
8. Olivari Z, Rubartelli P, Piscione F, Ettori F, Fontanelli A, Salemme L, et al. Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions: data from a multicenter, prospective, observational study (TOAST-GISE). J Am Coll Cardiol. (2003) 41:1672–8. doi: 10.1016/S0735-1097(03)00312-7
9. Criqui MH, Denenberg JO, Ix JH, Cc Lelland RLM, Wassel CL, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. (2015) 311:271–8. doi: 10.1001/jama.2013.282535
10. Martin SS, Blaha MJ, Blankstein R, Agatston A, Rivera JJ, Virani SS, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. (2014) 129:77. doi: 10.1161/CIRCULATIONAHA.113.003625
11. Martino F, Loreto PD, Giacomini D, Kaushik M, Ronco C. Abdominal aortic calcification is an independent predictor of cardiovascular events in peritoneal dialysis patients. Ther Apher Dial. (2013) 17:448–453. doi: 10.1111/j.1744-9987.2012.01084.x
12. Eun YH, Geun PB, Seok HH, Sungjin C, Whee PC, Yang CW, et al. The prognostic value of abdominal aortic calcification in peritoneal dialysis patients. Int J Med Sci. (2013) 10:617–23. doi: 10.7150/ijms.5773
13. Mäkelä SM, Asola M, Hadimeri H, Heaf JG, Heiro M, Kauppila L, et al. Abdominal aortic calcifications predict survival in peritoneal dialysis patients. Perit Dial Int. (2018) 38:366–73. doi: 10.3747/pdi.2017.00043
14. Rodondi N, Taylor BC, Bauer DC, Lui LY, Vogt MT, Fink HA, et al. Association between aortic calcification and total and cardiovascular mortality in older women. J Int Med. (2007) 261:238–44. doi: 10.1111/j.1365-2796.2007.01769.x
15. Wilson PWF, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. (2001) 103:1529–34. doi: 10.1161/01.CIR.103.11.1529
16. Kauppila LI, Polak JF, Cupples LA, Hannan MT, Wilson P. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. (1997) 132:245–50. doi: 10.1016/S0021-9150(97)00106-8
17. KDIGOC-MW Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. (2009) 113:S1. doi: 10.1038/ki.2009.188
18. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert J. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
19. Miguel RC, Maira BR, Miguel MG. The role of dietary inflammatory index in cardiovascular disease, metabolic syndrome and mortality. Int J Mol Sci. (2016) 17: doi: 10.3390/ijms17081265
20. Geng JW, Deng LH, Qiu S, Bian HY, Cai BY, Jin K, et al. Dietary inflammatory potential and risk of sarcopenia: data from national health and nutrition examination surveys. Aging US. (2021) 13:1913–28. doi: 10.18632/aging.202141
21. Li J, Lee DH, Hu J, Tabung FK, Li Y, Bhupathiraju SN, et al. Dietary inflammatory potential and risk of cardiovascular disease among menand women in the U.S. J Am Coll Cardiol. (2020) 76:2181–93. doi: 10.1016/j.jacc.2020.09.535
22. Sage AP, Tintut Y, De Mer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. (2010) 7:528. doi: 10.1038/nrcardio.2010.115
23. Skubisz C, Kern A. National Health and Nutrition Examination Survey[M]: Plan and, Aps Meeting. Springer, New York (2013).
24. SUSNCf Health. Plan and operation of the third national health and nutrition examination survey, 1988-94. Vital Health Stat. (1994).
25. Shivappa N Steck SE Hurley TG Hussey JR Ma Y Ockene IS. A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. (2014) 17:1825–33. doi: 10.1017/S1368980013002565
26. Park Y, Choi MK, Lee SS, Shivappa N, Han K, Steck SE, et al. Dietary inflammatory potential and risk of mortality in metabolically healthy and unhealthy phenotypes among overweight and obese adults. Clin Nutr. (2018). 38:682–688. doi: 10.1016/j.clnu.2018.04.002
27. Gorriz JL, Molina P, Cerveron MJ, Vila R, Boyer J, Nieto J, et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol. (2015) 10:654–66. doi: 10.2215/CJN.07450714
28. Chen W, Eisenberg R, Mowrey WB, Wylie-Rosett J, Abramowitz MK, Bushinsky DA, et al. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol Dial Transpl. (2020) 35:1171–8. doi: 10.1093/ndt/gfz134
29. Johnson CL, Paulose-Ram R, Ogden CL, Carroll M, Curtin LR. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat. (2013) 161:1–24.
30. Alisson M, Luz G, Dirce M, Fernanda DA, Maria M, Paulo L, et al. Association between dietary intake and coronary artery calcification in non-dialysis chronic kidney disease: the PROGREDIR study. Nutrients. (2018) 10:372. doi: 10.3390/nu10030372
31. Custódio MR, Koike MK, Neves KR, dos Reis LM, Graciolli FG, Neves CL, et al. Parathyroid hormone and phosphorus overload in uremia: impact on cardiovascular system. Nephrol Dial Transpl. (2011) 27:1437–45. doi: 10.1093/ndt/gfr447
32. Ling LW, Michael L, Chu EY, Foster BL, Bartley BA, Somerman MJ, et al. High phosphate feeding promotes mineral and bone abnormalities in mice with chronic kidney disease. Nephrol Dial Transpl. (2013) 28. 1:62–69. doi: 10.1093/ndt/gfs333
33. Zaragatski E, Grommes J, Schurgers LJ, Langer S, Kennes L, Tamm M, et al. Vitamin K antagonism aggravates chronic kidney disease-induced neointimal hyperplasia and calcification in arterialized veins: role of vitamin K treatment? Kidney Int. (2016). 89:601–611. doi: 10.1038/ki.2015.298
34. De Schutter TM, Behets GJ, Geryl H, Peter ME, Steppan S, Gundlach K, et al. Effect of a magnesium-based phosphate binder on medial calcification in a rat model of uremia. Kidney Int. (2013) 83:1109–17. doi: 10.1038/ki.2013.34
35. Rios R, Raya AI, Pineda C, Rodriguez M, Lopez I, Aguilera-Tejero E. Vitamin E protects against extraskeletal calcification in uremic rats fed high fat diets. BMC Nephrol. (2017) 18:374. doi: 10.1186/s12882-017-0790-4
36. Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. (2004) 15:2208–18. doi: 10.1097/01.ASN.0000133041.27682.A2
37. Peralta-Ramirez A, Montes D, Raya AI, Pineda C, Lopez I, Guerrero F, et al. Vitamin E protection of obesity-enhanced vascular calcification in uremic rats. Am J Physiol Renal Physiol. (2014) 306:422–9. doi: 10.1152/ajprenal.00355.2013
38. Lu YY, Chen WL. Clinical relevance of serum selenium levels and abdominal aortic calcification. Biol Trace Elem Res. (2020). 199:2803–10 doi: 10.1007/s12011-020-02405-3
39. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. (2002) 13:3. doi: 10.1097/00041433-200202000-00002
40. Na W, Kim M, Sohn C. Dietary inflammatory index and its relationship with high-sensitivity C-reactive protein in Korean: data from the health examinee cohort. J Clin Biochem Nutr. (2018) 62:83–88. doi: 10.3164/jcbn.17-22
41. Bordoni A, Danesi F, Dardevet D, Dupont D, Vergères G. Dairy products and inflammation: a review of the clinical evidence. Crit Rev Food Sci Nutr. (2015) 57:2497–525. doi: 10.1080/10408398.2014.967385
42. Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among iranian women. J Nutr. (2007) 137:992–8. doi: 10.1093/jn/137.4.992
43. Soltani S, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr. (2018). 37:542–550. doi: 10.1016/j.clnu.2017.02.018
44. Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The mediterranean diet, its components, cardiovascular disease. Am J Med. (2015) 128:229–38. doi: 10.1016/j.amjmed.2014.10.014
45. Peng M, Wang L, Xia Y, Tao L, Xu G. High dietary inflammatory index is associated with increased plaque vulnerability of carotid in patients with ischemic stroke. Stroke. 51:2983–9. doi: 10.1161/STROKEAHA.120.029035
46. Shivappa N, Zucchetto A, Montella M, Serraino D, Hébert J. Inflammatory potential of diet and risk of colorectal cancer: a case-control study from Italy. Bri J Nutr. (2015) 114:1–7. doi: 10.1017/S0007114515001828
47. Young C, Jeonghee L, Jae O, Aesun S, Jeongseon K. Dietary inflammatory index and risk of colorectal cancer: a case-control study in Korea. Nutrients. (2016) 8:469. doi: 10.3390/nu8080469
48. Zahedi H, Djalalinia S, Asayesh H, Mansourian M, Qorbani M. A higher dietary inflammatory index score is associated with a higher risk of incidence and mortality of cancer: a comprehensive systematic review and meta-analysis. Int J Prev Med. (2020) 11:15. doi: 10.4103/ijpvm.IJPVM_332_18
49. Chen LW, Aubert AM, Shivappa N, Bernard JY, Mensink-Bout SM, Geraghty AA, et al. Associations of maternal dietary inflammatory potential and quality with offspring birth outcomes: an individual participant data pooled analysis of 7 European cohorts in the ALPHABET consortium. PLos Med. (2021) 18:22. doi: 10.1371/journal.pmed.1003491
50. Shivappa N, Hébert JR, Rashvand S, Rashidkhani B, Hekmatdoost A. Inflammatory potential of diet and risk of ulcerative colitis in a case-control study from Iran. Nutr Cancer. (2016) 68:404–09. doi: 10.1080/01635581.2016.1152385
51. Salari-Moghaddam A, Keshteli AH, Esmaillzadeh A, Adibi P. Adherence to the pro-inflammatory diet in relation to prevalence of irritable bowel syndrome. Nutr J. (2019) 18. doi: 10.1186/s12937-019-0487-6
52. Lee SJ, Lee IK, Jeon JH. Vascular calcification-new insights into its mechanism. Int J Mol Sci. (2020) 21:32. doi: 10.3390/ijms21082685
53. Voelkl J, Lang F, Eckardt KU, Amann K, Kuro-o M, Pasch A, et al. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol Life Sci. (2019) 76:2077–91. doi: 10.1007/s00018-019-03054-z
54. Wen CY, Yang XL, Yan ZF, Zhao M, Yue X, Cheng XZ, et al. Nalp3 inflammasome is activated and required for vascular smooth muscle cell calcification. Int J Cardiol. (2013) 168:2242–7. doi: 10.1016/j.ijcard.2013.01.211
55. Kurozumi A, Nakano K, Yamagata K, Okada Y, Nakayamada S, Tanaka Y. IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2. Bone. (2019) 124:53–61. doi: 10.1016/j.bone.2019.04.006
56. Sanchez-Duffhues G, de Vinuesa AG, van de Pol V, Geerts ME, de Vries MR, Janson SGT, et al. Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J Pathol. (2019) 247:333–46. doi: 10.1002/path.5193
57. Ma B, Hottiger MO. Crosstalk between wnt/beta-Catenin and NF-kappa B signaling pathway during inflammation. Front Immunol. (2016) 7. doi: 10.3389/fimmu.2016.00378
58. Saito Y, Nakamura K, Miura D, Yunoki K, Miyoshi T, Yoshida M, et al. Suppression of Wnt signaling and osteogenic changes in vascular smooth muscle cells by eicosapentaenoic. Acid Nutrients. (2017) 9. doi: 10.3390/nu9080858
59. Callegari A, Coons ML, Ricks JL, Rosenfeld ME, Scatena M. Increased calcification in osteoprotegerin-deficient smooth muscle cells: dependence on receptor activator of NF-kappa B ligand and interleukin 6. J Vasc Res. (2014) 51:118–31. doi: 10.1159/000358920
60. Sun MS, Chang Q, Xin MM, Wang Q, Li H, Qian JQ. Endogenous bone morphogenetic protein 2 plays a role in vascular smooth muscle cell calcification induced by interleukin 6 in vitro. Int J Immunopathol Pharmacol. (2017) 30:227–37. doi: 10.1177/0394632016689571
61. Henaut L, Massy ZA. New insights into the key role of interleukin 6 in vascular calcification of chronic kidney disease. Nephrol Dial Transpl. (2018) 33:543–8. doi: 10.1093/ndt/gfx379
62. Lin L, He Y, Xi BL, Zheng HC, Chen Q, Li J, et al. miR-135a suppresses calcification in senescent VSMCs by regulating KLF4/STAT3 pathway. Curr Vasc Pharmacol. (2016) 14:211–8. doi: 10.2174/1570161113666150722151817
63. Rong S, Zhao XZ, Jin XC, Zhang Z, Chen L, Zhu YX, et al. Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein-2 via a mechanism involving the wnt/beta-catenin pathway. Cell Physiol Biochem. (2014) 34:2049–60. doi: 10.1159/000366400
64. Shimizu T, Tanaka T, Iso T, Matsui H, Ooyama Y, Kawai-Kowase K, et al. Notch signaling pathway enhances bone morphogenetic protein 2 (BMP2) responsiveness of msx2 gene to induce osteogenic differentiation and mineralization of vascular smooth muscle cells. J Biol Chem. (2011) 286:19138–48. doi: 10.1074/jbc.M110.175786
65. Liberman M, Johnson RC, Handy DE, Loscalzo J, Leopold JA. Bone morphogenetic protein-2 activates NADPH oxidase to increase endoplasmic reticulum stress and human coronary artery smooth muscle cell calcification. Biochem Biophys Res Commun. (2011) 413:436–41. doi: 10.1016/j.bbrc.2011.08.114
66. Graffouillere L, Deschasaux M, Mariotti F, Neufcourt L, Shivappa N, Hebert JR, et al. Prospective association between the dietary inflammatory index and mortality: modulation by antioxidant supplementation in the SU.VI.MAX randomized controlled trial. Am J Clin Nutr. (2016) 103:878–85. doi: 10.3945/ajcn.115.126243
67. Leow K, Szulc P, Schousboe J, Kiel D, Lewis J. Prognostic value of abdominal aortic calcification: a systematic review and meta-analysis of observational studies. Heart Lung Circ. (2019) 28. doi: 10.1016/j.hlc.2019.06.555
68. Jankovic A, Damjanovic T, Djuric Z, Marinkovic J, Schlieper G, Tosic-Dragovic J, et al. Impact of vascular calcifications on arteriovenous fistula survival in hemodialysis patients: a five-year follow-up. Nephron. (2015) 129:247–52. doi: 10.1159/000380823
69. Forbang NI, Mcclelland RL, Remigio-Baker RA, Allison MA, San Df Ort V, Michos ED, et al. Associations of cardiovascular disease risk factors with abdominal aortic calcium volume and density: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. (2016) 255:54–8. doi: 10.1016/j.atherosclerosis.2016.10.036
70. Zhang Y, Zhao L, Wang N, Li J, He F, Li X, et al. Unexpected role of matrix gla protein in osteoclasts: inhibiting osteoclast differentiation and bone resorption. Mol Cell Biol. (2019). 39. doi: 10.1128/MCB.00012-19
71. Weijs B, Blaauw Y, Rennenberg RJ, Schurgers LJ, Timmermans CC, Pison L, et al. Patients using vitamin K antagonists show increased levels of coronary calcification: an observational study in low-risk atrial fibrillation patients. Eur Heart J. (2011) 32:2555–62. doi: 10.1093/eurheartj/ehr226
72. Bourron O, Phan F, Diallo MH, Hajage D, Aubert CE, Carlier A, et al. Circulating receptor activator of nuclear factor kB ligand and triglycerides are associated with progression of lower limb arterial calcification in type 2 diabetes: a prospective, observational cohort study. Cardiovasc Diabetol. (2020) 19:9. doi: 10.1186/s12933-020-01122-4
Keywords: dietary inflammatory index, abdominal aortic calcification, NHANES, cross-sectional study, likelihood
Citation: Qin Z, Chang K, Liao R, Jiang L, Yang Q and Su B (2021) Greater Dietary Inflammatory Potential Is Associated With Higher Likelihood of Abdominal Aortic Calcification. Front. Cardiovasc. Med. 8:720834. doi: 10.3389/fcvm.2021.720834
Received: 05 June 2021; Accepted: 16 July 2021;
Published: 13 August 2021.
Edited by:
Sanda Maria Cretoiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Qian Yang, First Affiliated Hospital of Chinese PLA General Hospital, ChinaMing-Jie Wang, Fudan University, China
Copyright © 2021 Qin, Chang, Liao, Jiang, Yang and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baihai Su, aW1zYmgmI3gwMDA0MDsxNjMuY29t
 Zheng Qin1,2
Zheng Qin1,2 Baihai Su
Baihai Su