- Department of Cardiovascular Surgery, Beijing Aortic Disease Center, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
Background: This study aimed to evaluate the early and long-term outcomes of a single center using a frozen elephant trunk (FET) procedure for chronic type B or non-A non-B aortic dissection.
Methods: From February 2009 to December 2019, 79 patients diagnosed with chronic type B or non-A non-B aortic dissection who underwent the FET procedure were included in the present study. We analyzed operation mortality and early and long-term outcomes, including complications, survival and interventions.
Results: The operation mortality rate was 5.1% (4/79). Spinal cord injury occurred in 3.8% (3/79), stroke in 2.5% (2/79), and acute renal failure in 5.1% (4/79). The median follow-up time was 53 months. The overall survival rates were 96.2, 92.3, 88.0, 79.8, and 76.2% at 1/2, 1, 3, 5 and 7 years, respectively. Moreover, 79.3% of patients did not require distal aortic reintervention at 7 years. The overall survival in the subacute group was superior to that in the chronic group (P = 0.047).
Conclusion: The FET technique is a safe and feasible approach for treating chronic type B and non-A non-B aortic dissection in patients who have contraindications for primary endovascular aortic repair. The technique combines the advantages of both open surgical repair and endovascular intervention, providing comparable early and long-term follow-up outcomes and freedom from reintervention.
Introduction
According to current expert consensus, patients with uncomplicated type B aortic dissection (AD) are suggested for medical treatment and periodic clinical and imaging surveillance. Surgical repair and interventional treatment are considered options for complicated type B or non-A non-B AD (1–3). Recently, thoracic endovascular aortic repair (TEVAR) has been recommended as the first-line treatment for complicated type B or non-A non-B AD because of its lower morbidity and mortality (4). However, TEVAR may not be feasible for patients who have an unfavorable aortic anatomy, a lack of a sufficient proximal landing zone, a high risk of retrograde type A aortic dissection (RTAAD) and/or concomitant with a proximal aortic lesion.
The frozen elephant trunk (FET) procedure may be an alternative treatment for these kinds of patients, according to the recent recommendations published by the European Society for Vascular Surgery and the European Association for Cardio-Thoracic Surgery (5, 6). The FET procedure combines the advantages of open surgery and endovascular treatment, by which total arch replacement (TAR) and descending aortic dissection repair (7, 8) can be performed simultaneously. The FET procedure can eliminate the risk of type Ia endoleak and RTAAD, benefit the thrombosis of false lumen, enable positive aortic remodeling and improve prognosis (9). However, there have been few reports on the outcomes of chronic type B or non-A non-B AD using the FET procedure, and the long-term outcomes, in particular, have not been reported (10).
Therefore, we retrospectively reviewed the experience in our center on the treatment of complicated type B or non-A non-B aortic dissection using the FET procedure according to the most recent published recommendations (5, 6). This study was conducted in accordance with the rules and checklist of the STROBE statement.
Patients and Methods
This study was performed in accordance with the Declaration of Helsinki (2013). The Ethics Committees of Beijing Anzhen Hospital, Capital Medical University, approved this retrospective study (2020100X).
Patients
From February 2009 to December 2019, 79 consecutive patients diagnosed with chronic type B or non-A non-B aortic dissection underwent open surgical repair using TAR combined with FET under hypothermic cardiopulmonary bypass (CPB) and antegrade selective cerebral perfusion (ASCP) via median sternotomy in Beijing Anzhen Hospital. Computed tomographic angiography (CTA) and echocardiography were performed to evaluate and confirm the diagnosis preoperatively.
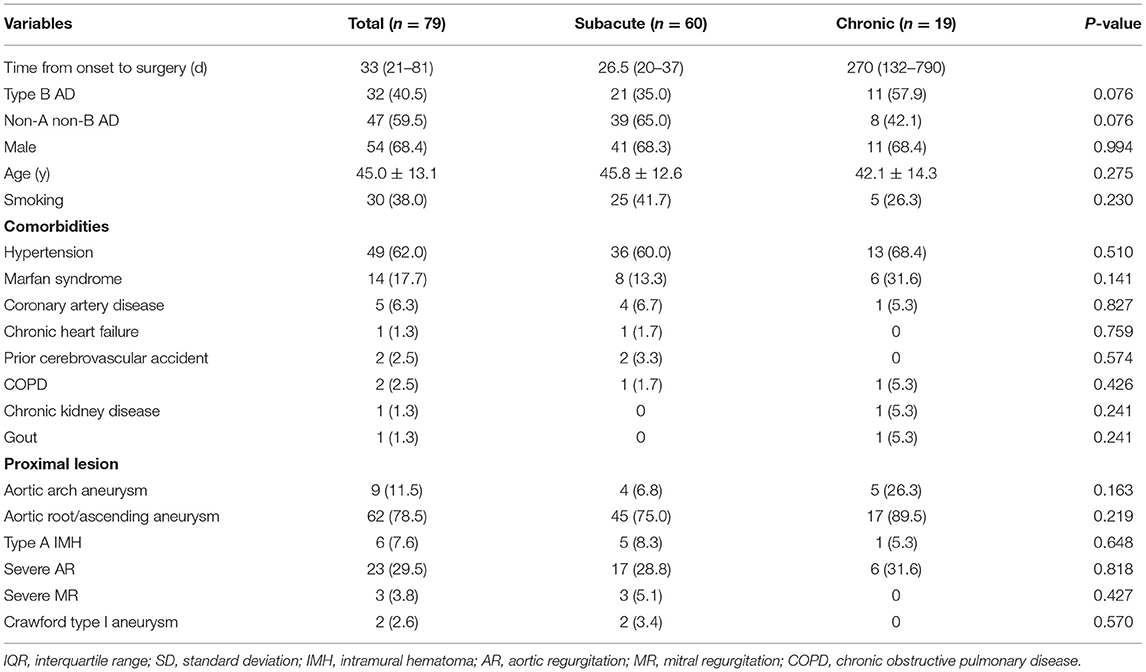
According to the definition of the STROAGE guidelines, there were 60 subacute dissections (75.9%, 14–90 days from the appearance of symptoms) and 19 chronic dissections (24.1%, >90 days) (11). The mean age was 45.0 ± 13.1 years, and 54 patients were male (68.4%). Hypertension was the most frequent risk factor in this cohort, which was observed in 49 patients (62.0%). Marfan syndrome was noted in 14 (17.7%), along with coronary artery disease in 5 (6.3%), chronic heart failure in 1 (1.3%), respiratory disease and prior cerebrovascular accident in 2 each (2.5%) and chronic kidney disease in 1 (1.3%). Sixty-two patients (78.5%) had ascending/root aortic aneurysms, aortic arch aneurysms in 9 cases (11.5%), severe aortic valve regurgitation in 23 cases (29.5%) and severe mitral valve regurgitation in 3 cases (3.8%). Four patients had undergone a previous aortic procedure, including the Bentall procedure in 3 (3.8%), mitral valvuloplasty in 1 (1.3%), ascending aortic replacement in 1 (1.3%) and abdominal aortic replacement in 1 (1.3%). More detailed parameters are presented in Table 1.
Surgical Technique
The detailed surgical technique of TAR combined with FET was described previously (12, 13). Island technique arch reconstruction was performed when there was no involvement of the brachiocephalic trunk and the left common carotid artery. Detailed procedures were described previously (14, 15).
The length of the stented graft (MicroPort Medical Co Ltd, Shanghai, China) was 10 cm, the diameter was 24–30 mm, and the graft had 3 and 1-cm stent-free vascular grafts for sutures in the proximal and distal edges, respectively. The size of the stented graft was determined according to the diameter of the proximal descending aorta of healthy individuals matched for age, sex, and height. The diameter of the stented graft was larger than the true lumen but slightly smaller than the entire chosen aorta.
Briefly, after a median sternotomy, the brachiocephalic vessels and the transverse arch were dissected and exposed. The left sternocleidomastoid muscle and other cervical muscle groups were partially transected if necessary. The left subclavian artery (LSCA) and the left common carotid artery (LCCA) were carefully separated and exposed, and care was taken to avoid injury to the thoracic duct; then, the thoracic duct was ligated if necessary.
Right axillary artery cannulation was used for CPB and ASCP. Cooling was initiated when CPB was established. Cold hyperkalemic cardioplegic solution was used for cardiac arrest. Then, valvular repair or replacement and proximal aortic operations were performed during the cooling phase. When the nasopharyngeal temperature was under 25°C, circulatory arrest was performed. Unilateral ASCP was initiated with a flow rate of 5–10 mL·kg−1·min−1 by the right axillary artery. Unilateral ASCP was considered to be adequate for cerebral circulation in the left hemisphere when the left radial artery pressure was 20 mmHg and there was recurrent bleeding through the LCCA; otherwise, bilateral ASCP was performed.
The anterior wall of the aortic arch was incised up to the origin of the LCCA, and the incision was performed ~0.5 cm distal to the origin of the IA and the LCCA. No dissection of the innominate artery (IA) or the LCCA was confirmed intraoperatively. The stented graft was inserted into the true lumen of the descending aorta and deployed. The stent-free vascular graft was pulled and trimmed to a semioval shape to match the aortic arch wall containing the IA and the LCCA. A running suture was performed within the stent-free vascular graft between the LSCA and the LCCA using 4-0 Prolene, and the inner suture was performed counterclockwise while the outer suture was performed clockwise. Thus, the residual aortic wall and the stent-free vascular graft formed a circular opening. Finally, the distal end of the ascending aortic graft was anastomosed to the circular opening using open distal anastomosis. After anastomosis, the CPB gradually resumed to normal flow, and rewarming was started. The left subclavian artery was transected 0.5–1.0 cm distal to the origin, and the proximal segment was sutured using 5-0 Prolene. The LSCA was anastomosed to the LCCA in an end-to-side fashion (Figure 1).
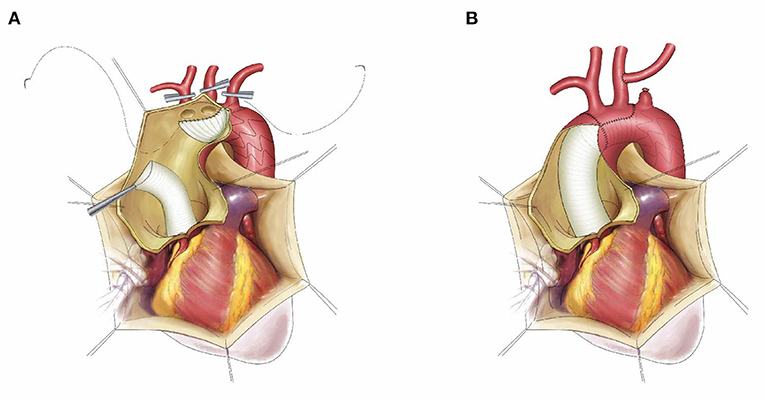
Figure 1. The schematic diagram of frozen elephant trunk with “island technique” and left subclavian aorta to left common carotid artery transposition. The stented graft was inserted into the true lumen of descending aorta, the stent-free vascular graft was trimmed to a semi-oval shape containing the innominate artery and left common carotid artery, then the inner suture was performed counterclockwise while the outer suture was performed clockwise (A); The final result after suture, left subclavian aorta to left common carotid artery transposition (B).
Study Endpoints and Follow-Up
The primary study endpoints were operation death and late death. Overall in-hospital mortality was defined as death within 30 days after surgery and hospital death. Late mortality was defined as all-cause death beyond 30 days after surgery during follow-up. Secondary endpoints included distal aortic reoperations and complications. Distal aortic reoperation referred to any reinterventions, including open surgeries or endovascular interventions on the distal aorta. Complications included stroke, endoleak, limb ischemia and spinal cord injury.
All discharged patients were followed up regularly through clinic visits, phone calls, emails or letters. All survivors were recommended to undergo periodic CTA scans of the entire aorta (Figure 2).
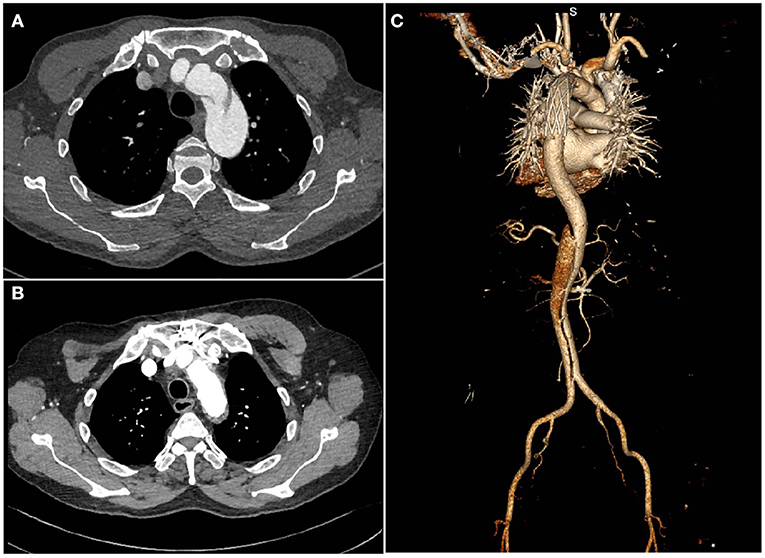
Figure 2. Computed tomography images of a patient with chronic type B dissection with aortic arch involvement (A) preoperatively (B,C), postoperatively.
Statistical Analysis
All analyses in the study were performed using SPSS software version 25.0 (IBM Corporation, Chicago, USA) and GraphPad Prism for Windows 8.0 (La Jolla, CA, USA.). Continuous variables are summarized as the mean ± standard deviation (SD) or median with interquartile range (IQR) according to their normality and were compared using Student's t-test or the Mann-Whitney U test. Categorical variables are expressed as numbers (percentage) and were compared using the Pearson χ2 test or Fisher's exact test. Survival analysis and freedom from distal aortic reoperation were estimated using the Kaplan-Meier method. Competing risk analysis was performed based on the methods described by Blackstone et al. (16). A 2-sided significance level with a P < 0.05 was considered statistically significant.
Results
Surgical Data
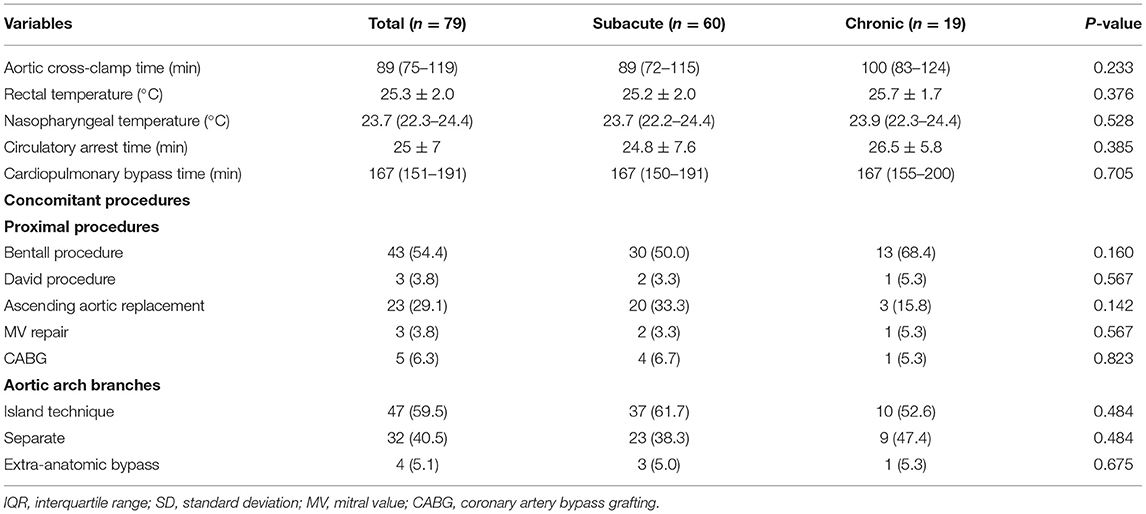
All patients underwent elective surgery and completed it successfully. Most patients (59.5%) underwent “island technique” aortic arch branch anastomosis (Table 2). Approximately half of the patients (54.4%) required the Bentall procedure, 23 patients (29.1%) underwent ascending aortic replacement, and 3 patients (3.8%) underwent the David procedure. Other concomitant procedures included mitral valve repair in 3 patients (3.8%) and coronary artery bypass grafting in 5 patients (6.3%). Extra anatomic bypass occurred in 4 cases (5.1%), including ascending aorta-femoral aorta bypass in 2 patients (2.8%), axillary artery-axillary artery bypass in 1 patient and ascending aorta-axillary artery bypass in 1 patient (1.4%).
The median CPB and aortic cross-clamp times were 167 (151–191) min and 89 (75–119) min, respectively (Table 2). The mean rectal temperature and circulatory arrest time were 25.3 ± 2.0°C and 25 ± 7 mins, respectively, and the median nasopharyngeal temperature was 23.6 (22.1–24.3)°C.
Morbidity and Mortality
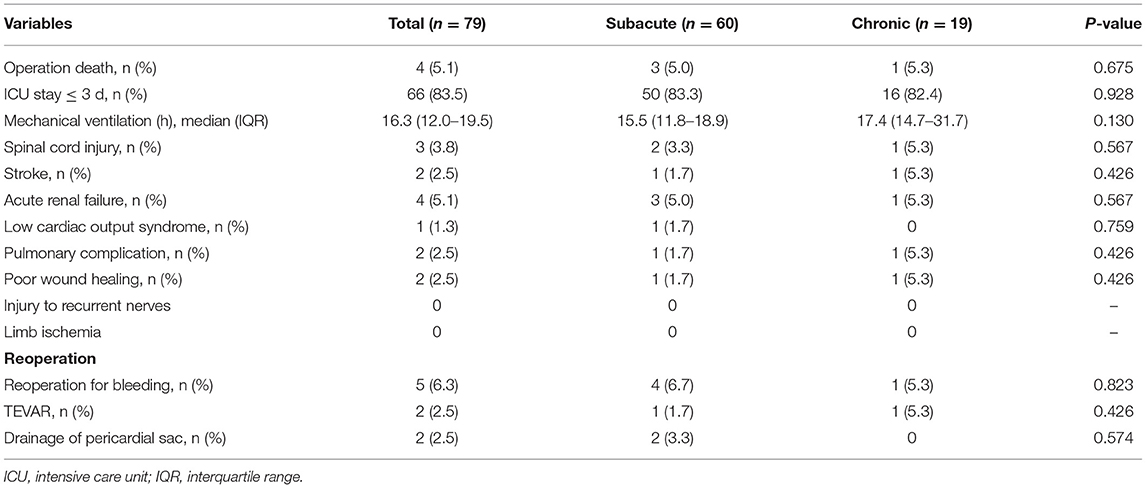
The early postoperative results are presented in Table 3. Four patients (5.1%) died within 30 days. Two patients died of stroke; 1 patient suffered from heart failure and renal failure postoperatively, and it was difficult to maintain their blood pressure. The patient eventually died; 1 patient suffered from respiratory failure, renal failure and intestinal bleeding, his family members requested transfer to a local hospital, and the patient eventually died.
Reoperation for bleeding occurred in 5 patients (6.3%). Two patients (2.5%) required TEVAR because of intimal tears distal to the FET and poor reopening in the distal part of the FET. Two patients (2.5%) required resternotomy because of acute cardiac tamponade. Four patients (5.1%) experienced renal failure and needed continuous renal replacement therapy (CRRT). Spinal cord injury occurred in 3 patients (3.8%), including two cases of paraparesis and one of paraplegia. Poor wound healing occurred in 2 patients (2.5%). There was no recurrent laryngeal nerve injury case. The median time of mechanical ventilation was 16.3 (12.0–19.5) h. Most patients (83.5%) stayed in the intensive care unit within 72 h. The detailed parameters are presented in Table 3.
Follow-Up
Among the 75 patients, 66 patients (88.0%) completed the follow-up. The median follow-up time was 53 months [range 3–144 months; 95% confidence interval (CI) 37.88–67.12]. A total of 10 patients died during the follow-up period, with causes that included aortic-related events (n = 5), cerebral hemorrhage (n = 2) and sudden death (n = 3) for unknown reasons. The overall survival rates were 96.2% (95% CI, 88.7–98.8%), 92.3% (95% CI, 83.7–96.5%), 88.0% (95% CI, 78.1–93.6%), 79.8% (95% CI, 65.8–88.6%) and 76.2% (95% CI, 60.5–86.3%) at 1/2, 1, 3, 5, and 7 years, respectively (Figure 3A). The overall survival rate of patients in the subacute group was higher than that in the chronic group (P = 0.0467) (Figure 3B). The freedom from distal operation rates were 97.3% (95% CI, 89.8–99.3%), 97.3% (95% CI, 89.8–99.3%), 87.8% (95% CI, 75.9–94.1%), 84.3% (95% CI, 66.9–92.2%) and 79.3% (95% CI, 61.3–89.7%) at 1/2, 1, 3, 5, and 7 years, respectively (Figure 4).
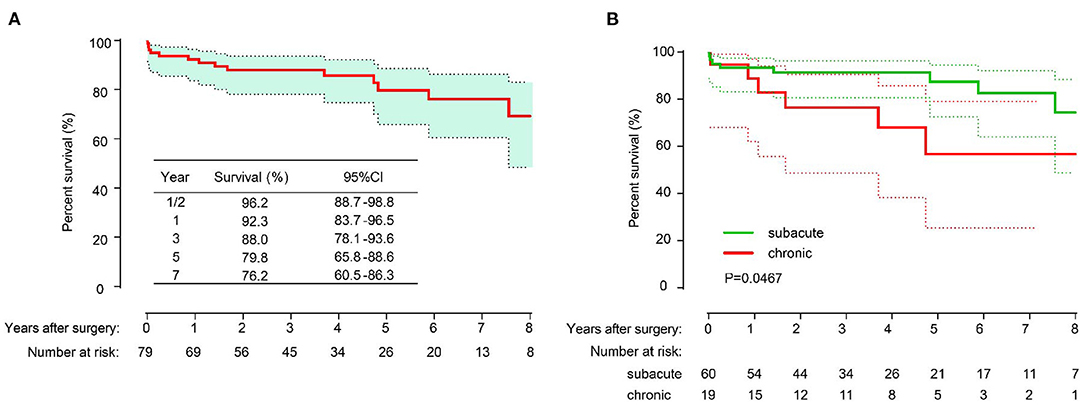
Figure 3. The long-term overall survival of all patients (A); Freedom from distal aortic reoperation of all patients (B).
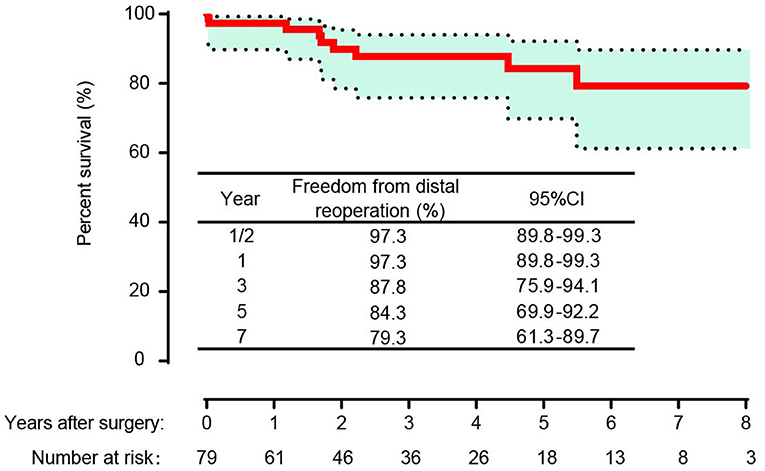
Figure 4. Survival in patients with subacute and chronic type B aortic dissection and compared with the log-rank test.
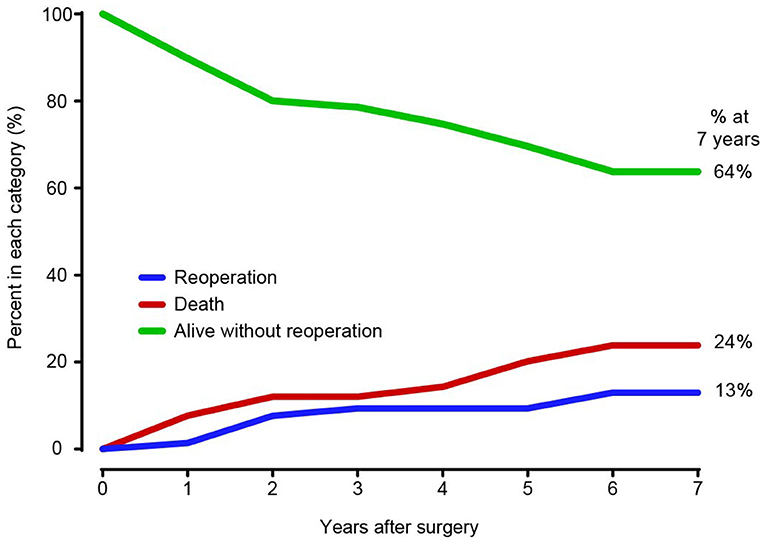
In the competing risk analysis, the incidence rates of distal aortic reintervention at 7 years were 13 and 24% for late mortality and 64% for survival without distal aortic reoperation (Figure 5). A total of 11 patients underwent distal aortic intervention during the follow-up period. Thoracoabdominal aortic aneurysm repair was performed in 8 patients. TEVAR was performed in two patients for residual dissection and anastomotic leak. One patient underwent EVAR due to an abdominal aortic aneurysm.
Discussion
The FET procedure is an attractive method for patients with complicated chronic type B or non-A non-B AD who are unfit for TEVAR. The early and long-term outcomes after the FET procedure are favorable.
In current recommendations, TEVAR is considered to be the optimal treatment for complicated type B AD. However, careful evaluation should be performed to determine whether there are enough proximal landing zones and favorable aortic anatomy. Open surgery may be the optimal option for patients with complicated chronic type B AD concomitant with aortic arch aneurysms or proximal aortic lesions. In patients diagnosed with non-A non-B AD with LCCA involvement only, LSCA to LCCA transposition or bypass should be performed, followed by TEVAR (17), and the outcomes are also satisfactory. However, in cases with aortic arch entry or LSCA to LCCA transposition that does not provide a sufficient proximal landing zone, double transposition or total arch rerouting should be considered, but it would also increase the risk of RTAAD (18, 19). According to previous reports, the high-risk factors for RTAAD include ascending aorta >38 mm, bicuspid aortic valve, arch abnormalities and extensive ascending aortic length (5). For such patients, total arch replacement combined with FET implantation was performed in our center. In this study cohort, most patients (78.5%) had concomitant root/ascending aortic aneurysms, and some had aortic arch aneurysms or valvular lesions. Therefore, our center adopted open surgery to perform the FET procedure, and proximal aortic or valvular lesions were treated.
We recommend that the FET procedure be performed if the patients have type B or non-A non-B AD concomitant with the following conditions (20): (i) aortic root or ascending aorta aneurysm; (ii) left common carotid artery dissection involvement or aortic arch aneurysm; (iii) proximal aortic lesion with coronary artery disease or aortic valve disease; (iv) contraindication for TEVAR; and (v) a high risk of RTAAD. The FET procedure for treating type B or non-A non-B AD unfit for TEVAR has some advantages (21). The greatest characteristic was that it combined the advantages of open surgical repair and interventional techniques. First, aortic arch replacement, descending aortic dissection repair and proximal aortic lesion repair can be performed through a median sternotomy in one stage. Second, FET implantation can enlarge the true lumen, promote aortic remodeling and potentially avoid secondary thoracic and abdominal aortic replacement. Third, the FET has extra suture margins at both ends, and anastomosis can be performed for patients who need thoracic and abdominal aortic replacement. Fourth, FET provides a landing zone for secondary TEVAR for those who develop distal stent graft-induced new entry.
The overall in-hospital mortality was 5.1%, which was acceptable for complicated chronic type B or non-A non-B AD, suggesting that the FET procedure is a safe and feasible technique. The overall in-hospital mortality was comparable or superior to those in previous reports (21–24). Takagi et al. (22) reported that the overall in-hospital mortality of chronic type B AD was 3%. Nozdrzykowski et al. (23) reported a single-center experience with 15 patients diagnosed with chronic type B AD who underwent open surgery, and the overall in-hospital mortality was 13.4%. Recently, Weiss et al. (24) reported a retrospective multicenter experience with 57 patients, and the overall in-hospital mortality was 14%. Interestingly, the overall survival rate in the subacute group was superior to that in the chronic group (P = 0.047), which contrasted with a previous report (24). Weiss et al. (24) showed that the overall survival rates between acute and chronic AD were not significantly different (P = 0.65). Recently, our center also reported experience with chronic type A aortic dissection, and the survival rate showed a distinction between the subacute and chronic groups, although there was no significant difference (P = 0.107) (25). The possible explanation may be that chronic B-type dissection itself is often accompanied by dilatation of the descending aorta, which predisposes patients to aortic events. However, this result still needs to be verified objectively, and large-scale studies should be conducted.
Paraplegia remains the most severe complication in aortic surgery because it has serious impacts on the quality of life of patients. In this study, the incidence of spinal cord injury, including paraplegia and paraparesis, was 3.8%, which was similar to the results reported by Weiss et al. (4.0%) (24). Moreover, Preventza et al. (26) found that the incidence of spinal cord injury was 4.7% in a meta-analysis of more than 3,000 patients. Minimizing the circulatory arrest time and FET length above T8 are the key factors in reducing the incidence of spinal cord injury. Furthermore, for high-risk patients, cerebrospinal fluid drainage and neuromonitoring should be performed (27). The incidence of stroke was 2.5%, but all patients with stroke died within 30 days. The results of the meta-analysis indicated that the incidence of stroke was 4.9–6.5% in patients who underwent the FET procedure (28, 29). The presence of bovine aortic arch, preoperative cardiopulmonary resuscitation, aortic valve insufficiency (moderate and severe) and dissection of the common carotid artery were risk factors for postoperative stroke (30, 31). In our study cohort, the incidence of stroke was low compared to those in previous reports (28, 29), which might be due to the small amount of common carotid artery dissection involvement and careful brain protection. Most patients underwent “island technique” arch reconstruction, which preserved autologous vessels and might also have a protective effect on postoperative stroke. However, stroke remains the most devastating complication and warrants further brain protection, including moderate or deep hypothermia, ASCP, cerebral oxygen saturation monitoring and slowing of the rewarming speed (32). Postoperative acute kidney failure is the most common complication following dissection surgery. Minimizing the circulatory arrest time, CPB time and operation time are the key factors to prevent acute kidney failure. For patients with acute renal failure after surgery, early CRRT is recommended, which can partially reverse renal function. The renal resistive index may be helpful for decision-making and improving the prognosis of patients with acute kidney failure (33).
In this series, the overall survival rates and rates of freedom from distal aortic reoperation were 92.3, 79.8, 76.2, and 97.3, 84.3, 79.3% at 1, 5, and 7 years, respectively. There was no cerebral infarction or paraplegia reported. The early and long-term results of this technique for patients with chronic type B or non-A non-B AD were acceptable, which indicates that the FET procedure is a safe and effective approach for such patients. The competing analysis showed that the need for distal aortic reintervention was 13.0% at 7 years, which was comparable with the result reported by Charchyan et al. (20). The authors showed that the cumulative incidence rates of aortic reintervention were 5.6% at 0.5 years and 11.1% at 4 years. Moreover, Weiss et al. (24) showed that 16% of patients with complicated type B aortic dissection required secondary aortic reinterventions at 3 years. In our cohort, it is worth noting that most of the patients who needed reintervention required thoracoabdominal aortic replacement in the follow-up. Moreover, most of the patients (7/8) who required thoracoabdominal aortic replacement had Marfan syndrome, which reminded us of the importance of clinical surveillance and imaging, especially in cases of Marfan syndrome.
Limitations
There are several limitations to the current study. The present study is a retrospective cohort study, which has inherent selection bias. Moreover, it is a single-center study with a small sample size. Studies with larger groups of patients and multiple center trials should be conducted.
Conclusion
The FET procedure is a safe and effective approach for treating complicated chronic type B or non-A non-B AD in patients in whom TEVAR is infeasible. This technique combines the advantages of open surgical techniques and endovascular intervention, allowing simultaneous proximal aortic and aortic arch repair and stabilization of the descending aorta. The early and long-term outcomes were acceptable for such patients. The overall survival rate in the subacute group showed superior outcomes compared with the chronic group, but the results should be objectively understood.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committees of Beijing Anzhen Hospital, Capital Medical University (2020100X). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CL contributed to the conception, data collection, analysis, and drafted the manuscript. RQ, YZ, and SC contributed to the analysis. HL, RG, and YG contributed to the data collection. LS contributed to the conception and writing editing. JZ contributed to the conception, design, acquisition of work, and critically revised the manuscript. All authors approved the submitted version.
Funding
This work was supported by the Beijing Major Science and Technology Projects from the Beijing Municipal Science and Technology Commission (No. Z191100006619093) and the Natural Science Foundation of China (No. 81970393).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. the task force for the diagnosis and treatment of aortic diseases of the European society of cardiology (ESC). Eur Heart J. (2014) 35:2873–926. doi: 10.1093/eurheartj/ehu281
2. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American college of cardiology foundation/American heart association task force on practice guidelines, American association for thoracic surgery, American college of radiology, American stroke association, Society of cardiovascular anesthesiologists, society for cardiovascular angiography and interventions, society of interventional radiology, society of thoracic surgeons, and society for vascular medicine. J Am Coll Cardiol. (2010) 55:e27–129. doi: 10.1016/j.jacc.2010.02.015
3. Shrestha M, Bachet J, Bavaria J, Carrel TP, De Paulis R, Di Bartolomeo R, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg. (2015) 47:759–69. doi: 10.1093/ejcts/ezv085
4. Khoynezhad A, Donayre CE, Omari BO, Kopchok GE, Walot I, White RA. Midterm results of endovascular treatment of complicated acute type B aortic dissection. J Thorac Cardiovasc Surg. (2009) 138:625–31. doi: 10.1016/j.jtcvs.2009.04.044
5. Czerny M, Schmidli J, Adler S, van den Berg JC, Bertoglio L, Carrel T, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg. (2019) 55:133–62. doi: 10.1093/ejcts/ezy313
6. Czerny M, Schmidli J, Adler S, van den Berg JC, Bertoglio L, Carrel T, et al. Editor's choice - current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European association for cardio-thoracic surgery (EACTS) & the European society for vascular surgery (ESVS). Eur J Vasc Endovasc Surg. (2019) 57:165–98. doi: 10.1016/j.ejvs.2018.09.016
7. Karck M, Chavan A, Hagl C, Friedrich H, Galanski M, Haverich A. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg. (2003) 125:1550–3. doi: 10.1016/s0022-5223(03)00045-x
8. Di Bartolomeo R, Murana G, Di Marco L, Alfonsi J, Gliozzi G. Is the frozen elephant trunk frozen?. Gen Thorac Cardiovasc Surg. (2019) 67:111–7. doi: 10.1007/s11748-018-0911-4
9. Kreibich M, Berger T, Morlock J, Kondov S, Scheumann J, Kari FA, et al. The frozen elephant trunk technique for the treatment of acute complicated Type B aortic dissection. Eur J Cardiothorac Surg. (2018) 53:525–30. doi: 10.1093/ejcts/ezx281
10. Kreibich M, Siepe M, Berger T, Kondov S, Morlock J, Pingpoh C, et al. The frozen elephant trunk technique for the treatment of type B and type non-A non-B aortic dissection. Eur J Vasc Endovasc Surg. (2021) 61:107–13. doi: 10.1016/j.ejvs.2020.08.040
11. Rylski B, Pacini D, Beyersdorf F, Quintana E, Schachner T, Tsagakis K, et al. Standards of reporting in open and endovascular aortic surgery (STORAGE guidelines). Eur J Cardiothorac Surg. (2019) 56:10–20. doi: 10.1093/ejcts/ezz145
12. Sun LZ, Qi RD, Chang Q, Zhu JM, Liu YM, Yu CT, et al. Is total arch replacement combined with stented elephant trunk implantation justified for patients with chronic Stanford type A aortic dissection?. J Thorac Cardiovasc Surg. (2009) 138:892–6. doi: 10.1016/j.jtcvs.2009.02.041
13. Sun L, Qi R, Zhu J, Liu Y, Zheng J. Total arch replacement combined with stented elephant trunk implantation: a new “standard” therapy for type a dissection involving repair of the aortic arch?. Circulation. (2011) 123:971–8. doi: 10.1161/CIRCULATIONAHA.110.015081
14. Zhong YL, Qi RD, Ma WG, Ge YP, Qiao ZY, Li CN, et al. Frozen elephant trunk with modified en bloc arch reconstruction and left subclavian transposition for chronic type A dissection. J Thorac Dis. (2018) 10:5376–83. doi: 10.21037/jtd.2018.08.140
15. Zhu JM, Qi RD, Chen L, Liu W, Li CN, Fan ZM, et al. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: Preservation of autologous brachiocephalic vessels. J Thorac Cardiovasc Surg. (2015) 150:101–5. doi: 10.1016/j.jtcvs.2015.03.002
16. Blackstone EH, Naftel DC, Turner ME. The decomposition of time-varying hazard into phases, each incorporating a separate stream of concomitant information. J Am Stat Assoc. (1986) 81:615–24.
17. Chen SW, Zhong YL, Qiao ZY, Li CN, Ge YP, Qi RD, et al. One-stage hybrid procedure for distal aortic arch disease: mid-term experience at a single center. J Thorac Dis. (2020) 12:7117–26. doi: 10.21037/jtd-20-2338
18. Chen Y, Zhang S, Liu L, Lu Q, Zhang T, Jing Z. Retrograde type A aortic dissection after thoracic endovascular aortic repair: a systematic review and meta-analysis. J Am Heart Assoc. (2017) 6:4649. doi: 10.1161/JAHA.116.004649
19. Yammine H, Briggs CS, Stanley GA, Ballast JK, Anderson WE, Nussbaum T, et al. Retrograde type A dissection after thoracic endovascular aortic repair for type B aortic dissection. J Vasc Surg. (2019) 69:24–33. doi: 10.1016/j.jvs.2018.04.047
20. Charchyan E, Breshenkov D, Belov Y. Follow-up outcomes after the frozen elephant trunk technique in chronic type B dissection. Eur J Cardiothorac Surg. (2020) 57:904–11. doi: 10.1093/ejcts/ezz348
21. Sun L, Zhao X, Chang Q, Zhu J, Liu Y, Yu C, et al. Repair of chronic type B dissection with aortic arch involvement using a stented elephant trunk procedure. Ann Thorac Surg. (2010) 90:95–100. doi: 10.1016/j.athoracsur.2010.03.048
22. Takagi Y, Ando M, Higuchi Y, Akita K, Tochii M, Ishida M, et al. Recent outcomes of surgery for chronic type B aortic dissection. Ann Vasc Dis. (2010) 3:215–21. doi: 10.3400/avd.oa01030
23. Nozdrzykowski M, Etz CD, Luehr M, Garbade J, Misfeld M. Optimal treatment for patients with chronic Stanford type B aortic dissection: endovascularly, surgically or both?. Eur J Cardiothorac Surg. (2013) 44: e165–74. doi: 10.1093/ejcts/ezt291
24. Weiss G, Tsagakis K, Jakob H, Di Bartolomeo R, Pacini D, Barberio G, et al. The frozen elephant trunk technique for the treatment of complicated type B aortic dissection with involvement of the aortic arch: multicentre early experience. Eur J Cardiothorac Surg. (2015) 47:106–14. doi: 10.1093/ejcts/ezu067
25. Chen Y, Ma WG, Li JR, Zheng J, Liu YM, Zhu JM, et al. Is the frozen elephant trunk technique justified for chronic type A aortic dissection in Marfan syndrome?. Ann Cardiothorac Surg. (2020) 9:197–208. doi: 10.21037/acs.2020.03.10
26. Preventza O, Liao JL, Olive JK, Simpson K, Critsinelis AC, Price MD, et al. Neurologic complications after the frozen elephant trunk procedure: A meta-analysis of more than 3000 patients. J Thorac Cardiovasc Surg. (2020) 160:20–33 e4. doi: 10.1016/j.jtcvs.2019.10.031
27. Ma WG, Zhang W, Wang LF, Zheng J, Ziganshin BA, Charilaou P, et al. Type A aortic dissection with arch entry tear: Surgical experience in 104 patients over a 12-year period. J Thorac Cardiovasc Surg. (2016) 151:1581–92. doi: 10.1016/j.jtcvs.2015.11.056
28. Tian DH, Wan B, Di Eusanio M, Black D, Yan TD. A systematic review and meta-analysis on the safety and efficacy of the frozen elephant trunk technique in aortic arch surgery. Ann Cardiothorac Surg. (2013) 2:581–91. doi: 10.3978/j.issn.2225-319X.2013.09.07
29. Hanif H, Dubois L, Ouzounian M, Peterson MD, El-Hamamsy I, Dagenais F, et al. Aortic arch reconstructive surgery with conventional techniques vs frozen elephant trunk: a systematic review and meta-analysis. Can J Cardiol. (2018) 34:262–73. doi: 10.1016/j.cjca.2017.12.020
30. Dumfarth J, Kofler M, Stastny L, Plaikner M, Krapf C, Semsroth S, et al. Stroke after emergent surgery for acute type A aortic dissection: predictors, outcome and neurological recovery. Eur J Cardiothorac Surg. (2018) 53:1013–20. doi: 10.1093/ejcts/ezx465
31. Zhao H, Ma W, Wen D, Duan W, Zheng M. Computed tomography angiography findings predict the risk factors for preoperative acute ischaemic stroke in patients with acute type A aortic dissection. Eur J Cardiothorac Surg. (2020) 57:912–9. doi: 10.1093/ejcts/ezz351
32. Shirasaka T, Okada K, Kano H, Matsumori M, Inoue T, Okita Y. New indicator of postoperative delayed awakening after total aortic arch replacement. Eur J Cardiothorac Surg. (2015) 47:101–5. doi: 10.1093/ejcts/ezu141
Keywords: non-A non-B aortic dissection, chronic type B aortic dissection, frozen elephant trunk technique, total arch replacement, long term outcomes
Citation: Luo C, Qi R, Zhong Y, Chen S, Liu H, Guo R, Ge Y, Sun L and Zhu J (2021) Early and Long-Term Follow-Up for Chronic Type B and Type Non-A Non-B Aortic Dissection Using the Frozen Elephant Trunk Technique. Front. Cardiovasc. Med. 8:714638. doi: 10.3389/fcvm.2021.714638
Received: 25 May 2021; Accepted: 05 August 2021;
Published: 14 September 2021.
Edited by:
Giovanni Mariscalco, University Hospitals of Leicester NHS Trust, United KingdomReviewed by:
Davut Cekmecelioglu, Cleveland Clinic, United StatesShaojung Li, Taipei Muncipal Wan Fang Hospital, Taiwan
Copyright © 2021 Luo, Qi, Zhong, Chen, Liu, Guo, Ge, Sun and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junming Zhu, YW56aGVuemptQDE2My5jb20=
 Congcong Luo
Congcong Luo Ruidong Qi
Ruidong Qi Junming Zhu
Junming Zhu