
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cardiovasc. Med. , 13 October 2021
Sec. Heart Valve Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.706123
This article is part of the Research Topic Valvular Heart Failure: A new disease entity of the atrioventricular valves View all 4 articles
Concomitant tricuspid regurgitation (TR) is common in patients with mitral regurgitation (MR). While current guidelines recommend repair of both valves at the time of surgery when feasible, high risk patients are often undertreated, leading to significant morbidity and mortality. With advances in transcatheter edge-to-edge repair (TEER) devices and technique, combined TEER for treating significant MR and TR has emerged as a new tool for heart failure management. Recent evidence has shed light on which patients with severe TR should be targeted for transcatheter intervention either in isolation or in combination with a MV TEER procedure and allows for expanded treatment options in patients who otherwise would be limited to medical management. Technological advancements remain ahead of robust clinical data, and thus randomized clinical studies in patients with severe MR and TR will be instrumental in determining the best approach in treating these patients with transcatheter therapies.
Transcatheter edge-to-edge repair (TEER) with the MitraClip (Abbott Vascular, Santa Clara, USA) has been demonstrated to be safe and effective in treating severe mitral regurgitation (MR) of both degenerative and functional etiologies (1, 2). Similarly, TEER using the PASCAL system (Edwards Lifesciences, Irvine, USA) is associated with excellent survival, improved functional status and quality of life in MR patients at 1 year (3). Tricuspid valve (TV) TEER has been shown to be safe and effective using the TriClip device (Abbott Vascular, Santa Clara, USA) (4) and an early feasibility study using PASCAL for TV TEER recently reported encouraging 30-day outcomes (5).
Functional (secondary) tricuspid regurgitation (TR) is the most frequently encountered etiology for TR and refers to regurgitation not related to primary organic tricuspid valve disease (6). Multiple studies have shown increasing TR severity is associated with worse survival regardless of age, left (LV) or right ventricular (RV) dysfunction and pulmonary hypertension (7). Both late residual TR seen after left-sided valve valve surgery (8) and isolated severe TR (6) carry excess mortality and morbidity.
Significant TR may not improve predictably after treatment of the left-sided valve lesion and reduced RV afterload; thus, TR should be managed as part of the index procedure (8–12). MV repair alone is often associated with an initial improvement in TR and RV function. However, the result may be temporary, with frequent recurrence or progression of TR and most of the available data comes from surgical literature (8–12). In contrast, concomitant TV repair effectively and durably eliminates severe TR and improves RV function, supporting a more aggressive approach to important functional TR (12, 13). There is emerging data on the impact of isolated MV TEER and to a lesser extent combined MV and TV TEER on clinical outcomes in patients with both severe MR and TR (Table 1). The aim of this article is to review the existing data on feasibility and benefits of combined transcatheter mitral and tricuspid repair and highlight the important considerations for patient selection and procedural success.
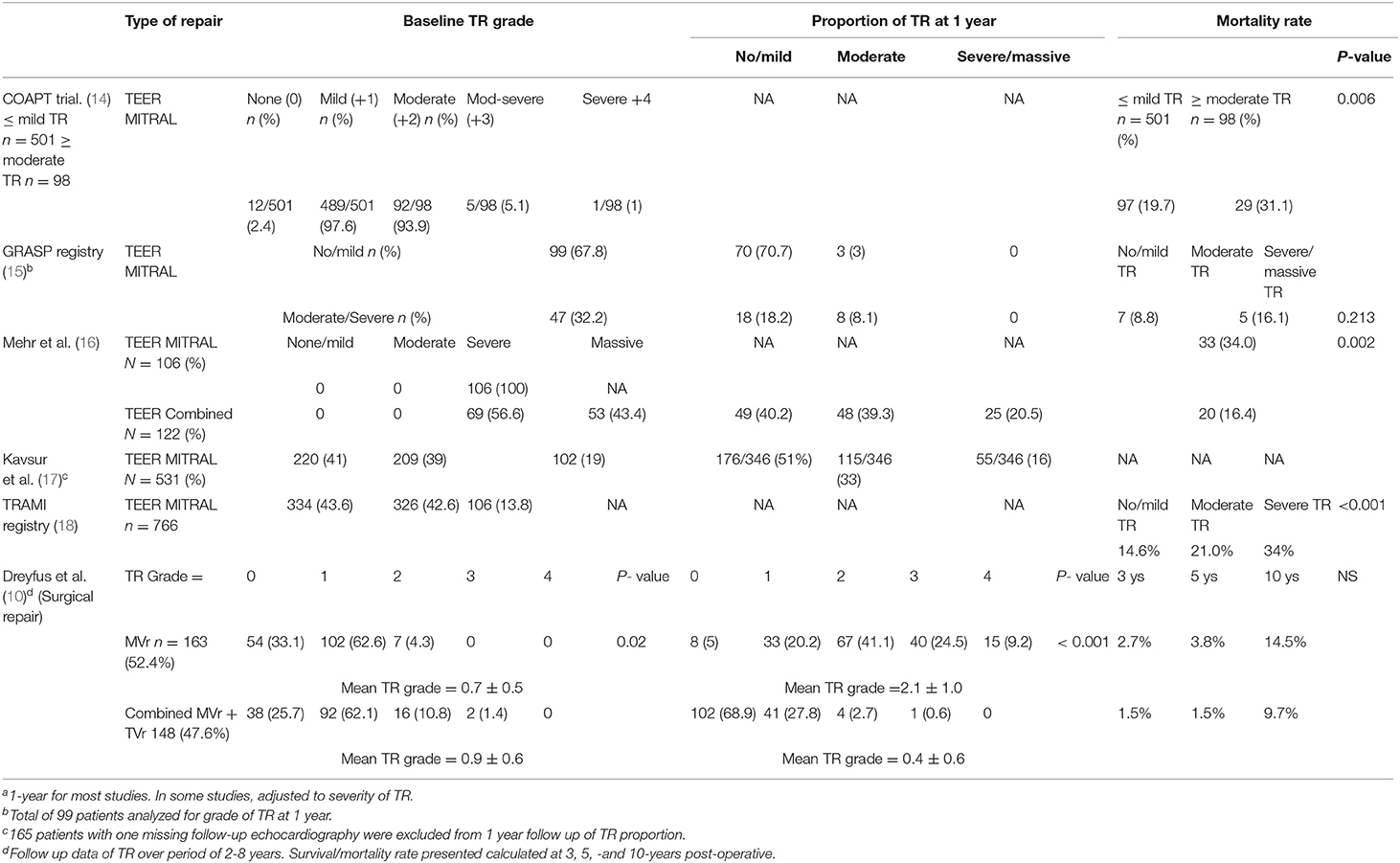
Table 1. Survival and recurrent TR rates of combined mitral and tricuspid repair versus isolated Mitral repair.a
MR is either functional, secondary to annular dilatation and leaflet tethering as seen with ischemic or dilated cardiomyopathies and atrial fibrillation, or primary in the setting of mitral valve apparatus dysfunction in rheumatic heart disease, myxomatous degeneration, infective endocarditis (IE), among other etiologies. Most cases of significant TR are secondary to dilatation of the tricuspid annulus and/or leaflet tethering due to RV remodeling in the setting of pressure or volume overload such as pulmonary hypertension, dilated cardiomyopathies, or atrial fibrillation (19–21). Primary etiologies of TR include rheumatic, IE, congenital (Ebstein's), myxomatous, blunt chest trauma, carcinoid, drugs, and radiation. A growing number of patients develop significant TR from iatrogenic etiologies such as intracardiac device leads and endomyocardial biopsies (22–24).
Echocardiography remains the critical imaging modality in selecting patients for combined MV and TV TEER, which includes complete transthoracic echocardiography (TTE) and transesophageal echocardiographic (TEE) views according to recommendations (25) for a comprehensive cardiac assessment (26). Accurate evaluation of MR and TR severity using quantitative parameters is recommended (27). Quantitative assessment of TR severity has less established cut-off values in comparison with MR severity assessment (27, 28). Hahn et al. has suggested a new 5-grade scale for TR severity grading, expanded to include massive and torrential TR (28). In comparison to imaging the MV, TV imaging is more challenging as the tricuspid leaflets are thinner and present with a variety of morphological variants. Additionally, the TV is an anterior structure in the field far from the TEE probe. While TEE is used for defining the precise mechanism of TR, patient selection and procedural guidance, TTE is essential for assessment of the TV and quantifying RV function under basal conditions (29).
The use of advanced echocardiography features such as multiplanar views and 3-dimensional imaging has significantly improved the accuracy of the diagnosis and invasive management of valvular disease. Using the X-plane mode, two simultaneous orthogonal planes are obtained, allowing visualization of morphological details and precise determination of cardiac valvular lesions (Figures 1A,B). While echocardiographic assessment is sufficient for planning of TEER procedures, cardiac CT is required when planning transcatheter valve replacement procedures of the MV (TMVR) or TV (TTVR). CT imaging is crucial for TMVR as it better assesses the dimensions and geometry of the mitral annulus, determines optimal device landing zones and helps to predict the risk of left ventricular outflow tract obstruction and paravalvular leak. Equally, utilizing CT for planning TTVR procedures allows for accurate assessment of the tricuspid annulus, RA and RV dimensions and inferior vena cava-TV relationship (30).
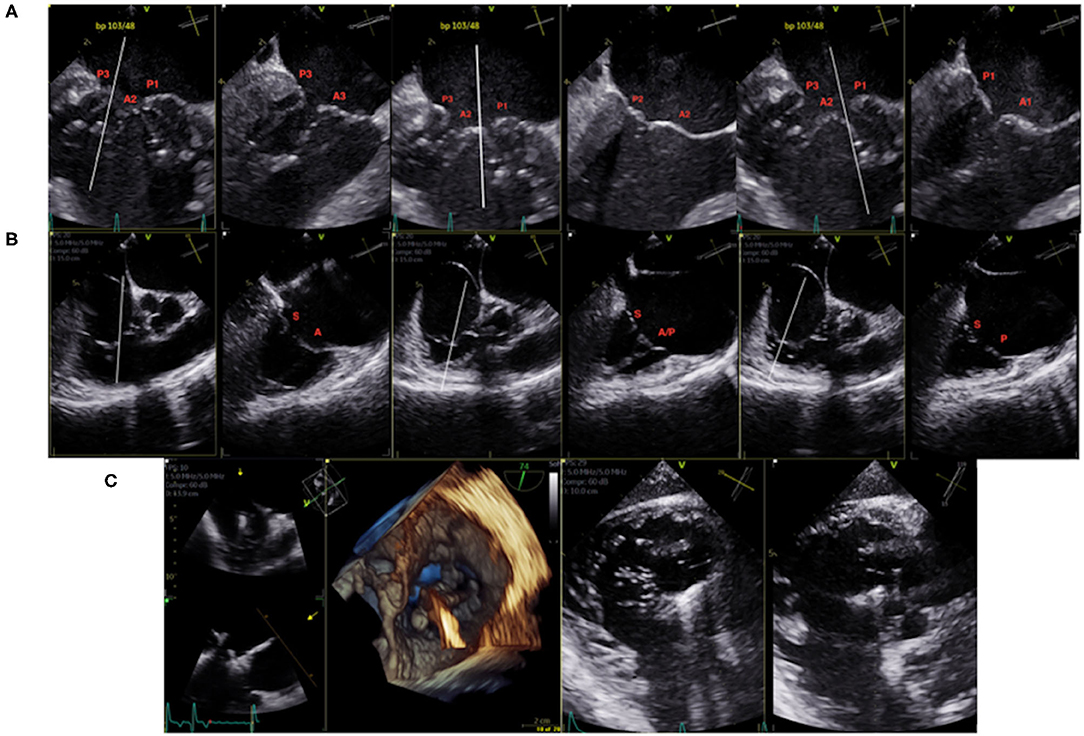
Figure 1. (A) 2D TEE X-plane of the mitral valve pre-procedural mapping (bi-commissural view). (B) 2D TEE X-plane of the tricuspid valve pre-procedural mapping (bi-commissural view). (C) TEE 3D and trans-gastric intraprocedural guidance of clip insertion in the anterior/septal position.
Interventional echocardiography is an emerging and growing field and is essential for the guidance of many transcatheter structural heart procedures. Similar to other transcatheter valve interventions, combined MV and TV TEER procedural success relies on adequate visualization of the cardiac structures and skilled intraprocedural guidance, with a focus on device positioning and successful grasping of the MV and TV leaflets. Imaging quality will depend not only on numerous patient characteristics (e.g., shadowing from prosthetic valves, hypertrophied interatrial septum, massive atria, horizontal orientation of the heart, chest/spine deformities, esophageal anatomy/pathologies), but also on the device used for repair.
For streamlined intraprocedural guidance, the rapid transition between mid-esophageal multiplanar views to transgastric to 3-D imaging of the valve is required. Furthermore, standardizing imaging views of the valves allows for efficient communication between the interventionalist and the interventional echocardiographer, increasing the rate of procedural success while decreasing procedure time. In general, transcatheter TV procedures are more challenging than those on the MV as the TV leaflets are often difficult to simultaneously visualize on 2D echocardiography (31), thus 3D echocardiography and transgastric views are mandatory for effective procedural guidance (Figure 1C). Lower-esophageal views may also be helpful. Many procedural imaging steps for MV TEER also apply to TV TEER, however transgastric views in particular are essential to guide TV TEER (32) as they provide excellent visualization of TV leaflet morphology, coaptation gaps, device landing zones, and location of the predominant TR jet (32, 33). Furthermore, transgastric views provide confirmation of complete leaflet insertion into the center of the clip prior to device deployment. When TEE is inconclusive, on-table TTE imaging may provide confirmation of adequate leaflet insertion. More recently, intracardiac echocardiography has been used for isolated TEER procedures on both the mitral (34) and tricuspid (35, 36) valves. The use of 4D ICE for mitral procedures is useful in patients with contraindications to TEE or in whom the risk of general anesthesia is too high, or specifically in the case of TV interventions, when TEE imaging is suboptimal.
Postprocedural assessment by TEE in the immediate post-deployment phase of the procedure is crucial in assessing the reduction in regurgitation severity and to rule out procedural complications (e.g., single leaflet device attachment (SLDA) or pericardial effusion). Follow up postprocedural TTE targets the assessment of long term success in terms of sustained reduction of regurgitation, ventricular reverse remodeling, reduction in afterload and stability of the device (37).
In the presence of concomitant severe MR and TR, double surgical valve intervention is indicated in patients with acceptable risk (38). Current guidelines recommend combined TV surgery in patients with severe TR (Stages C and D) undergoing left-sided valve surgery and in patients with progressive TR (Stage B) with either (1) tricuspid annular dilatation (TAD, tricuspid annulus end-diastolic diameter >4.0 cm) or (2) prior signs and symptoms of right-sided HF (39). TR has been shown to progress after MV surgery over years in several studies and is associated with poor outcomes (8, 13, 40). Several studies have reported consistent results suggesting that ≥2+ TR should be treated concomitantly with MV surgery (8, 11, 40), with a hazard ratio of up to 2.5 for persistent heart failure (HF) after MV surgery if significant TR was present preoperatively (8).
While surgical data suggests that repairing the TV after addressing the predominant MV disease does not pose a significant additional surgical risk, some patients with mild preoperative TR might benefit from isolated MV surgery alone. This is evidenced by a study in 1,900 patients with degenerative MV disease with a structurally normal TV, of which 67 underwent a combined repair procedure. In those with mild preoperative TR, <20% of patients developed 2+ or greater TR at 3 years (13). In contrast, Kwak et al. showed that even with mild or moderate degrees of secondary TR, which is commonly not corrected at the time of left-sided valve surgery, may progress over time in ~25% of patients and result in reduced long-term functional outcome and survival (9).
Historically, surgical TV repair durability has been inconsistent, with recurrence rates of significant TR in up to 40% of cases depending on the technique performed (41). Ring annuloplasty has been shown to be superior to other repair techniques (DeVega suture annuloplasty, combined ring annuloplasty plus edge-to-edge suture or suture bicuspidization procedure), with >85% of patients being free from ≥2+ TR at 10 years (38). Importantly, reoperation for isolated TR after left-sided valve surgery is associated with a high perioperative mortality rates between 10 and 25% (42–44).
Several predictors for persistence or progression of TR have been reported and may assist with patient selection for a combined procedure. These include TAD (at end-diastole >40 mm diameter or 21 mm/m2 diameter on preoperative TTE; >70 mm diameter on direct intraoperative measurement of the inter-commissural distance), degree of RV dysfunction or remodeling, leaflet tethering height, pulmonary artery hypertension, AF, and intra-annular RV pacemaker or implantable cardioverter-defibrillator leads (10–12, 45–49). As it has been shown that addressing both significant MR and TR concomitantly leads to improvement in RV function (13), mirroring such results from the surgical literature with a combined TEER procedure is likely to improve patient outcomes.
Understanding the relative benefit of TEER for TR based on etiology is important in deciding when to intervene on the tricuspid valve with TEER. Recently, a study of 159 patients undergoing TTVr evaluated the impact of TR etiology on outcomes (50). Those with TR in the setting of severe MR made up almost 50% of the cohort, with the remaining patients TR etiology attributed to atrial fibrillation, pulmonary hypertension or chronic dialysis. TR secondary to MR or atrial fibrillation showed a lower primary endpoint of death, HF hospitalization or reintervention after intervention when compared to those with TR secondary to dialysis or pulmonary hypertension. Patients with dialysis-related TR had the greatest mortality with TTVr (33% at 1 year), while those with pulmonary hypertension had the highest rate of the primary endpoint of death, HF hospitalization, or reintervention. These results were consistent irrespective of whether patients underwent an isolated or combined TEER procedure (50). Thus, considering the underlying etiology of TR is important, and this study suggests that those with severe MR derive a significant benefit from TEER, and such patients should be considered for a combined TEER procedure.
Although evidence is still limited, recently published registry data suggests that a combined TEER procedure is safe, effective and likely improves clinical outcomes (Table 1). In a small study of 27 high risk patients with severe MR and TR, undergoing a combined TEER procedure was associated with a lower rate of HF hospitalization, higher cardiac output, and reduction in N-terminal pro-B-type natriuretic peptide levels when compared to a matched control group undergoing MV TEER alone (51). In a subsequent larger study of 122 patients with severe MR and TR undergoing a combined TEER procedure from the TriValve Registry (52) demonstrating that not only did isolated tricuspid valve TEER was associated low procedural mortality and significant improvement in clinical outcomes, but that a combined TEER procedure was associated with a lower (16.4%) 1-year all-cause mortality when compared to matched patients from the TRAMI Registry (34.0%) (18) treated with isolated mitral TEER. On multivariate analysis, combined TEER was associated with a nearly 50% lower mortality rate (HR 0.52) after correcting for confounding variables (16). These promising data provide the basis for randomized trials to further evaluate the impact of combined TEER on clinical outcomes.
Recently, a large retrospective study of patients with baseline TR ranging from none/mild (41%), moderate (39%), and severe (19%) who underwent MitraClip for severe MR revealed several important findings pertaining to patient selection for a combined TEER procedure. First, TR improvement was associated with a lower rate of HF hospitalization at 2 years with a hazard ratio of 0.6. Second, patients with TAD (≥34 mm) at follow up had a higher rate of HF hospitalization. Third, TR was more likely to improve after MitraClip when the TAD decreased on follow up echocardiography. Fourth, that patients with atrial fibrillation were less likely to experience a decrease in TAD (and thus TR) and finally that MR > grade II at discharge was associated with lack of improvement in TR (17). Thus, when considering patients with severe MR and TR for a combined TEER procedure, those with TAD and atrial fibrillation should be favored for this approach. Interestingly, this study showed no association with baseline pulmonary hypertension and TR improvement, in contrast to two other smaller studies, one of which included patients with smaller TAD (53) and the other with larger TAD (54).
Becoming facile with TEER for treating MR is key to performing TEER for severe TR. Nevertheless, there remains a learning curve that needs to be overcome in order to optimize procedural success and improve patient outcomes. It has been shown that the learning curve when treating MR with MitraClip is steepest from 25 to 50 cases (55). In a retrospective review of 22 patients treated with combined TEER, procedure duration in the first tertile was significantly longer by 80 min (223 ± 13 vs. 143 ± 23 min) when compared to the third tertile. In addition, there was less residual TR comparing the beginning to the end of the study period (56). Although previous experience with MV TEER facilitates more rapid adoption of TV TEER by operators, imagers must also face a learning curve of tricuspid procedural guidance, which has its own unique challenges.
When planning TV TEER procedures, the choice of device can present a challenge given that these devices were developed for the mitral valve, whereas tricuspid valve leaflets are thinner and more fragile and severe TR tends to involve larger leaflet malcoaptation gaps. Some evidence from MV TEER has shown that the XTR clip leads to more SLDA and leaflet tearing (57), however the length of the XTR device is theoretically ideal when treating severe TR as most patients have malcoaptation gaps. The use of MitraClip XTR vs. NTR devices has been shown to achieve a higher procedural success rate (TR ≤ 2+ 80% vs. 70%), was able to treat TR with larger coaptation defects including those with torrential TR and lead to a greater reduction in TR. Importantly, the SLDA rates were similar (5%) (58) to those in the TRILUMINATE trial (4).
The MitraClip™ G4 system offers 2 new clip sizes (NTW and XTW) which measure 6 mm in the center, 50% wider than the 4-mm width of the NT/XT clip. The G4's larger grasping capability in addition to the option for independent leaflet grasping would be advantageous for treating TR, as recently reported (59, 60). Furthermore, the dedicated TriClip G4 system was recently given CE Mark and Health Canada approval for use in treating severe TR. Head-to-head studies of the TriClip and PASCAL systems for combined or isolated TV TEER awaits further investigation.
There are several advantages in considering a combined TEER procedure. First, a combined approach closely mirrors what is recommended in the guidelines for surgical intervention for TR in the setting of severe MR (39). Second, it avoids a second invasive procedure using general anesthesia in elderly and potentially frail patients. Third, it appears to reduce HF hospitalization and mortality, however a randomized clinical trial is clearly needed in this realm to better answer these and other important clinical questions. Finally, a combined procedure may reduce costs aside from avoiding a second procedure if the operator uses the same delivery system in treating both valves. Conversely, staged TV TEER procedures for only those whose TR persists after 1 month avoids unnecessary procedures. While some TEER procedures for combined TR and MR can be accomplished with the MitraClip guide (58), an advantage is offered by using the dedicated TriClip guide, as is being used in the TRILUMINATE Trial. Use of the MitraClip guide in performing TV TEER and conversely, the TriClip guide in performing MV TEER is off label. One perceived advantage of the PASCAL system is that it circumvents the need for different TEER guide catheters for treating both the MV and TV, however data for combined TEER with this device is sparse.
Although currently the most common interventional technique, TEER has anatomical limitations for the treatment of every patient with MR and TR. In future, a tailored approach using an expanded toolbox will allow more nuanced device selection based on valve anatomy and disease stage. Combined valve repair (TEER, annuloplasty, chordal implants) and replacement, or combined valve replacement may become a reality with advances in device development and procedural technique. Furthermore, future studies are needed to identify patients who will benefit the most from this treatment, the optimal anatomic features and standardized procedural success criteria associated with positive clinical outcomes.
As residual significant TR after MV TEER is an independent predictor for increased mortality, developing transcatheter therapies and having a more robust understanding of which patients will benefit from a combined TEER procedure is important. Small studies have demonstrated that a combined approach is safe, effective, and has shown promising short-term results, however randomized clinical trials are needed in this realm, especially regarding durability and persistent improvement in outcomes. Recent data has shed additional light on the deleterious effects of residual TR after MV TEER, including the implications of tricuspid annular dimensions and concomitant atrial fibrillation which will help guide patient selection for a combined procedure. There are several advantages to considering a combined procedure, both from clinical and cost perspectives. While there is a learning curve associated with both MV and TV TEER, the recent CE mark of the TriClip G4 and PASCAL systems will facilitate TV TEER in clinical practice. Ultimately, patients with concomitant severe MR and TR who are not ideal surgical candidates should be considered for a combined TEER procedure, using the available data to determine their suitability for this attractive therapeutic approach.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
NF has received speaker honoraria from Abbott Vascular and is a consultant for Edwards Lifesciences.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. (2011) 364:1395–406. doi: 10.1056/NEJMoa1009355
2. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. (2018) 379:2307. doi: 10.1056/NEJMoa1806640
3. Webb JG, Hensey M, Szerlip M, Schäfer U, Cohen GN, Kar S, et al. 1-year outcomes for transcatheter repair in patients with mitral regurgitation from the CLASP study. J Am Coll Cardiol Intv. (2020) 13:2344–57. doi: 10.1016/j.jcin.2020.06.019
4. Nickenig G, Weber M, Lurz P, von Bardeleben RS, Sitges M, Sorajja P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. (2019) 394:2002–11. doi: 10.1016/S0140-6736(19)32600-5
5. Kodali S, Hahn RT, Eleid MF, Kipperman R, Smith R, Lim DS, et al. Feasibility study of the transcatheter valve repair system for severe tricuspid regurgitation. J Am Coll Cardiol. (2021) 4:345–56.
6. Topilsky Y, Nkomo VT, Vatury O, Michelena HI, Letourneau T, Suri RM, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging. (2014) 7:1185–94. doi: 10.1016/j.jcmg.2014.07.018
7. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. (2004) 43:405–9. doi: 10.1016/j.jacc.2003.09.036
8. Ruel M, Rubens FD, Masters RG, Pipe AL, Bedard P, Mesana TG. Late incidence and predictors of persistent or recurrent heart failure in patients with mitral prosthetic valves. J Thorac Cardiovasc Surg. (2004) 128:278–83. doi: 10.1016/j.jtcvs.2003.11.048
9. Kwak JJ, Kim YJ, Kim MK, Kim HK, Park JS, Kim KH, et al. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J. (2008) 155:732–7. doi: 10.1016/j.ahj.2007.11.010
10. Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. (2005) 79:127–32. doi: 10.1016/j.athoracsur.2004.06.057
11. Navia JL, Brozzi NA, Klein AL, Ling LF, Kittayarak C, Nowicki ER, et al. Moderate tricuspid regurgitation with left-sided degenerative heart valve disease: to repair or not to repair? Ann Thorac Surg. (2012) 93:59–67; discussion 68–59. doi: 10.1016/j.athoracsur.2011.08.037
12. Benedetto U, Melina G, Angeloni E, Refice S, Roscitano A, Comito C, et al. Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J Thorac Cardiovasc Surg. (2012) 143:632–8. doi: 10.1016/j.jtcvs.2011.12.006
13. Desai RR, Vargas Abello LM, Klein AL, Marwick TH, Krasuski RA, Ye Y, et al. Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure. J Thorac Cardiovasc Surg. (2013) 146:1126–32.e1110. doi: 10.1016/j.jtcvs.2012.08.061
14. Hahn RT, Asch F, Weissman NJ, Grayburn P, Kar S, Lim S, et al. Impact of tricuspid regurgitation on clinical outcomes: the COAPT trial. J Am Coll Cardiol. (2020) 76:1305–14. doi: 10.1016/j.jacc.2020.07.035
15. Ohno Y, Attizzani GF, Capodanno D, Cannata S, Dipasqua F, Immé S, et al. Association of tricuspid regurgitation with clinical and echocardiographic outcomes after percutaneous mitral valve repair with the MitraClip System: 30-day and 12-month follow-up from the GRASP Registry. Eur Heart J Cardiovasc Imaging. (2014) 15:1246–55. doi: 10.1093/ehjci/jeu114
16. Mehr M, Karam N, Taramasso M, Ouarrak T, Schneider S, Lurz P, et al. Combined tricuspid and mitral versus isolated mitral valve repair for severe MR and TR: an analysis from the TriValve and TRAMI registries. JACC Cardiovasc Interv. (2020) 13:543–50. doi: 10.1016/j.jcin.2019.10.023
17. Kavsur R, Iliadis C, Spieker M, Brachtendorf BM, Tiyerili V, Metze C, et al. Fate of tricuspid regurgitation in patients undergoing transcatheter edge-to-edge mitral valve repair. EuroIntervention. (2021) 41: ehaa946–1926. doi: 10.1093/ehjci/ehaa946.1926
18. Kalbacher D, Schafer U, von Bardeleben RS, Zuern CS, Bekeredjian R, Ouarrak T, et al. Impact of tricuspid valve regurgitation in surgical high-risk patients undergoing MitraClip implantation: results from the TRAMI registry. EuroIntervention. (2017) 12:e1809–16. doi: 10.4244/EIJ-D-16-00850
19. Park SJ, Gentry JL 3rd, Varma N, Wazni O, Tarakji KG, Mehta A, et al. Transvenous extraction of pacemaker and defibrillator leads and the risk of tricuspid valve regurgitation. JACC Clin Electrophysiol. (2018) 4:1421–8. doi: 10.1016/j.jacep.2018.07.011
20. Mediratta A, Addetia K, Yamat M, Moss JD, Nayak HM, Burke MC, et al. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc Imaging. (2014) 7:337–47. doi: 10.1016/j.jcmg.2013.11.007
21. Addetia K, Harb SC, Hahn RT, Kapadia S, Lang RM. Cardiac implantable electronic device lead-induced tricuspid regurgitation. JACC Cardiovasc Imaging. (2019) 12:622–36. doi: 10.1016/j.jcmg.2018.09.028
22. Lin G, Nishimura RA, Connolly HM, Dearani JA, Sundt TM. 3rd, Hayes DL. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. (2005) 45:1672–5. doi: 10.1016/j.jacc.2005.02.037
23. Chang JD, Manning WJ, Ebrille E, Zimetbaum PJ. Tricuspid valve dysfunction following pacemaker or cardioverter-defibrillator implantation. J Am Coll Cardiol. (2017) 69:2331–41. doi: 10.1016/j.jacc.2017.02.055
24. Paniagua D, Aldrich HR, Lieberman EH, Lamas GA, Agatston AS. Increased prevalence of significant tricuspid regurgitation in patients with transvenous pacemakers leads. Am J Cardiol. (1998) 82:1130–2, A1139. doi: 10.1016/S0002-9149(98)00567-0
25. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
26. Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. (2013) 26:921–64. doi: 10.1016/j.echo.2013.07.009
27. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. (2017) 30:303–71. doi: 10.1016/j.echo.2017.01.007
28. Zoghbi WA, Asch FM, Bruce C, Gillam LD, Grayburn PA, Hahn RT, et al. Guidelines for the evaluation of valvular pair or replacement: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. (2019) 32:431–475. doi: 10.1093/ehjci/jex139
29. Soliman OI, McGhie JS, Anwar AM, Strachinaru M, Geleijnse ML, ten Cate FJ. Tricuspid valve disease: imaging using transthoracic echocardiography. In: Practical Manual of Tricuspid Valve Diseases Cham: Springer International Publishing (2018). p. 79–115. doi: 10.1007/978-3-319-58229-0_5
30. Naoum C, Blanke P, Cavalcante JL, Leipsic J. Cardiac computed tomography and magnetic resonance imaging in the evaluation of mitral and tricuspid valve disease: implications for transcatheter interventions. Circ Cardiovasc Imaging. (2017) 10:e005331. doi: 10.1161/CIRCIMAGING.116.005331
31. Anwar AM, Geleijnse ML, Soliman OI, McGhie JS, Frowijn R, Nemes A, et al. Assessment of normal tricuspid valve anatomy in adults by realtime three-dimensional echocardiography. Int J Cardiovasc Imaging. (2007) 23:717–24. doi: 10.1007/s10554-007-9210-3
32. Soliman OI, ten Cate JF, Hahn RT. Imaging of the tricuspid valve: transoesophageal echocardiograph. In: Soliman OI, editor, Practical Manual of Tricuspid Valve Diseases. New York, NY (2018). p. 117–26. doi: 10.1007/978-3-319-58229-0_6
33. Hahn RT. State-of-the-Art Review of Echocardiographic Imaging in the evaluation and treatment of functional tricuspid regurgitation. Circ Cardiovasc Imaging. (2016) 9:e005332. doi: 10.1161/CIRCIMAGING.116.005332
34. Sanchez CE, Yakubov SJ, Singh G, Rogers JH, Kander NH, Tang GHL. 4-dimensional intracardiac echocardiography in transcatheter mitral valve repair with the mitraclip system. JACC Cardiovasc Imaging. (2021) S1936-878X(20)31030-5. doi: 10.1016/j.jcmg.2020.08.042. [Epub ahead of print].
35. Fam NP, Samargandy S, Gandhi S, Eckstein J. Intracardiac echocardiography for guidance of transcatheter tricuspid edge-to-edge repair. EuroIntervention. (2018) 14:e1004–5. doi: 10.4244/EIJ-D-18-00672
36. Tang GHL, Yakubov SJ, Sanchez Soto CE. 4-Dimensional intracardiac echocardiography in transcatheter tricuspid valve repair with the MitraClip system. JACC Cardiovasc Imaging. (2020) 13:1591–600. doi: 10.1016/j.jcmg.2019.10.024
37. Soliman OI. Chang CC, Modine T. Echocardiographic imaging in transcatheter mitral and tricuspid valve therapies. Cardiac Interventions Today. (2019) 13:47–55.
38. Navia JL, Nowicki ER, Blackstone EH, Brozzi NA, Nento DE, Atik FA, et al. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg. (2010) 139:1473–82.e1475. doi: 10.1016/j.jtcvs.2010.02.046
39. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint committee on clinical practice guidelines. Circulation. (2020) 143:e35–71. doi: 10.1161/CIR.0000000000000932
40. Matsunaga A, Duran CM. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation. (2005) 112:I453–7. doi: 10.1161/CIRCULATIONAHA.104.524421
41. Faggion Vinholo T, Mori M, Mahmood SUB, Mullan CW, Weininger G, Yousef S, et al. Combined valve operations in the aortic and mitral positions with or without added tricuspid valve repair. Semin Thorac Cardiovasc Surg. (2020) 32:665–72. doi: 10.1053/j.semtcvs.2020.02.010
42. Staab ME, Nishimura RA, Dearani JA. Isolated tricuspid valve surgery for severe tricuspid regurgitation following prior left heart valve surgery: analysis of outcome in 34 patients. J Heart Valve Dis. (1999) 8:567–74.
43. Kwon DA, Park JS, Chang HJ, Kim YJ, Sohn DW, Kim KB, et al. Prediction of outcome in patients undergoing surgery for severe tricuspid regurgitation following mitral valve surgery and role of tricuspid annular systolic velocity. Am J Cardiol. (2006) 98:659–61. doi: 10.1016/j.amjcard.2006.03.047
44. Zack CJ, Fender EA, Chandrashekar P, Reddy YNV, Bennett CE, Stulak JM, et al. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol. (2017) 70:2953–60. doi: 10.1016/j.jacc.2017.10.039
45. Van de Veire NR, Braun J, Delgado V, Versteegh MI, Dion RA, Klautz RJ, et al. Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J Thorac Cardiovasc Surg. (2011) 141:1431–9. doi: 10.1016/j.jtcvs.2010.05.050
46. Calafiore AM, Gallina S, Iaco AL, Contini M, Bivona A, Gagliardi M, et al. Mitral valve surgery for functional mitral regurgitation: should moderate-or-more tricuspid regurgitation be treated? a propensity score analysis. Ann Thorac Surg. (2009) 87:698–703. doi: 10.1016/j.athoracsur.2008.11.028
47. Chan V, Burwash IG, Lam BK, Auyeung T, Tran A, Mesana TG, et al. Clinical and echocardiographic impact of functional tricuspid regurgitation repair at the time of mitral valve replacement. Ann Thorac Surg. (2009) 88:1209–15. doi: 10.1016/j.athoracsur.2009.06.034
48. Kim JB, Yoo DG, Kim GS, Song H, Jung SH, Choo SJ, et al. Mild-to-moderate functional tricuspid regurgitation in patients undergoing valve replacement for rheumatic mitral disease: the influence of tricuspid valve repair on clinical and echocardiographic outcomes. Heart. (2012) 98:24–30. doi: 10.1136/heartjnl-2011-300403
49. Yilmaz O, Suri RM, Dearani JA, Sundt TM 3rd, Daly RC, Burkhart HM, et al. Functional tricuspid regurgitation at the time of mitral valve repair for degenerative leaflet prolapse: the case for a selective approach. J Thorac Cardiovasc Surg. (2011) 142:608–13. doi: 10.1016/j.jtcvs.2010.10.042
50. Schlotter F, Orban M, Rommel KP, Besler C, von Roeder M, Braun D, et al. Aetiology-based clinical scenarios predict outcomes of transcatheter edge-to-edge tricuspid valve repair of functional tricuspid regurgitation. Eur J Heart Fail. (2019) 21:1117–25. doi: 10.1002/ejhf.1547
51. Besler C, Blazek S, Rommel KP, Noack T, von Roeder M, Luecke C, et al. Combined mitral and tricuspid versus isolated mitral valve transcatheter edge-to-edge repair in patients with symptomatic valve regurgitation at high surgical risk. JACC Cardiovasc Interv. (2018) 11:1142–51. doi: 10.1016/j.jcin.2018.04.010
52. Taramasso M, Alessandrini H, Latib A, Asami M, Attinger-Toller A, Biasco L, et al. Outcomes after current transcatheter tricuspid valve intervention: mid-term results from the international trivalve registry. JACC Cardiovasc Interv. (2019) 12:155–65. doi: 10.1016/j.jcin.2018.10.022
53. Frangieh AH, Gruner C, Mikulicic F, Attinger-Toller A, Tanner FC, Taramasso M, et al. Impact of percutaneous mitral valve repair using the MitraClip system on tricuspid regurgitation. EuroIntervention. (2016) 11:e1680–6. doi: 10.4244/EIJV11I14A320
54. Toyama K, Ayabe K, Kar S, Kubo S, Minamishima T, Rader F, et al. Postprocedural changes of tricuspid regurgitation after MitraClip therapy for mitral regurgitation. Am J Cardiol. (2017) 120:857–61. doi: 10.1016/j.amjcard.2017.05.044
55. Eleid MF, Reeder GS, Malouf JF, Lennon RJ, Pislaru SV, Nkomo VT, et al. The learning curve for transcatheter mitral valve repair with MitraClip. J Interv Cardiol. (2016) 29:539–45. doi: 10.1111/joic.12326
56. Mahowald MK, Pislaru SV, Reeder GS, Padang R, Michelena HI, Mankad SV, et al. Institutional learning experience for combined edge-to-edge tricuspid and mitral valve repair. Catheter Cardiovasc Interv. (2020) 96:1323–30. doi: 10.1002/ccd.28856
57. Doldi PM, Brinkmann I, Orban M, Stolz L, Orban M, Stocker T, et al. Percutaneous edge-to-edge repair of severe mitral regurgitation using the MitraClip XTR versus NTR system. Clin Cardiol. (2021) 44:708–14. doi: 10.1002/clc.23599
58. Ali FM OG, Edwards J, Connelly KA, Fam NP. Comparison of transcatheter tricuspid valve repair using the MitraClip NTR and XTR systems. Int J Cardiol. (2021) 327:156–62. doi: 10.1016/j.ijcard.2020.11.073
59. Fam NP, Ali FM, Hassanin M, Ong G. Transcatheter tricuspid valve repair with the modified TriClip/MitraClip G4 system. Eurointervention. (2021) 17:e441–2. doi: 10.4244/EIJ-D-20-01295
Keywords: mitral regugitation, tricuspid regurgitation, heart failure, transcatheter, combined approach
Citation: Burke L, Hassanin M, Ong G and Fam N (2021) A Practical Approach to Combined Transcatheter Mitral and Tricuspid Valve Intervention. Front. Cardiovasc. Med. 8:706123. doi: 10.3389/fcvm.2021.706123
Received: 06 May 2021; Accepted: 12 September 2021;
Published: 13 October 2021.
Edited by:
Yan Topilsky, Tel Aviv Sourasky Medical Center, IsraelReviewed by:
Gabriele Tamagnini, Villa Torri Hospital, ItalyCopyright © 2021 Burke, Hassanin, Ong and Fam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neil Fam, bmVpbC5mYW1AdW5pdHloZWFsdGgudG8=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.