- 1Department of Cardiology, Key Laboratory of Cardiac Injury and Repair of Henan Province, First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Administration Department of Henan Medical Association, Zhengzhou, China
Aims: The present study aimed to investigate the prognostic role of derived neutrophil-to-lymphocyte ratio (dNLR) in patients with coronary heart disease (CHD) after PCI.
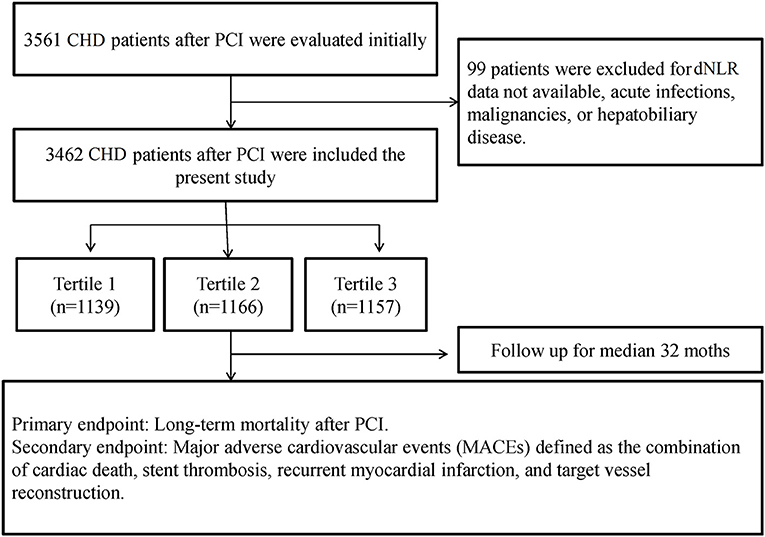
Methods: A total of 3,561 post-PCI patients with CHD were retrospectively enrolled in the CORFCHD-ZZ study from January 2013 to December 2017. The patients (3,462) were divided into three groups according to dNLR tertiles: the first tertile (dNLR < 1.36; n = 1,139), second tertile (1.36 ≥ dNLR < 1.96; n = 1,166), and third tertile(dNLR ≥ 1.96; n = 1,157). The mean follow-up time was 37.59 ± 22.24 months. The primary endpoint was defined as mortality (including all-cause death and cardiac death), and the secondary endpoint was major adverse cardiovascular events (MACEs) and major adverse cardiovascular and cerebrovascular events (MACCEs).
Results: There were 2,644 patients with acute coronary syndrome (ACS) and 838 patients with chronic coronary syndrome (CCS) in the present study. In the total population, the all-cause mortality (ACM) and cardiac mortality (CM) incidence was significantly higher in the third tertile than in the first tertile [hazard risk (HR) = 1.8 (95% CI: 1.2–2.8), p = 0.006 and HR = 2.1 (95% CI: 1.23–3.8), p = 0.009, respectively]. Multivariate Cox regression analyses suggested that compared with the patients in the first tertile than those in the third tertile, the risk of ACM was increased 1.763 times (HR = 1.763, 95% CI: 1.133–2.743, p = 0.012), and the risk of CM was increased 1.763 times (HR = 1.961, 95% CI: 1.083–3.550, p = 0.026) in the higher dNLR group during the long-term follow-up. In both ACS patients and CCS patients, there were significant differences among the three groups in the incidence of ACM in univariate analysis. We also found that the incidence of CM was significantly different among the three groups in CCS patients in both univariate analysis (HR = 3.541, 95% CI: 1.154–10.863, p = 0.027) and multivariate analysis (HR = 3.136, 95% CI: 1.015–9.690, p = 0.047).
Conclusion: The present study suggested that dNLR is an independent and novel predictor of mortality in CHD patients who underwent PCI.
Introduction
Previous studies suggested that systemic inflammation is a predictive marker of clinical outcome of coronary heart disease (CHD) (1–5). Some biomarkers such as C-reactive protein (6, 7) and blood routine test parameters including leukocyte (8, 9) and neutrophil (10–12), which represent systemic inflammation, have been reported to be associated with adverse outcomes of CHD patients. Recently, a novel biomarker, derived neutrophil-to-lymphocyte ratio (dNLR), which was defined as neutrophil count/(leukocyte count—neutrophil count) has been demonstrated to be a predictor of chronic inflammatory diseases and cancers (13–18). Although CHD is a chronic inflammatory disease, a few studies have investigated the relation between dNLR and the long-term outcomes in CHD patients who underwent PCI. In the present study, we aimed to investigate the association of dNLR with the long-term outcomes in Chinese patients with angiographic CHD who underwent PCI.
Methods
Study Design and Population
In the present study, we enrolled 3,561 CHD patients who underwent PCI from January 2013 to December 2017 in the First Affiliated Hospital of Zhengzhou University. All the participants were CHD patients who underwent coronary angiograph and at least one stent being implanted. Both ACS (n = 2,644) and CCS (n = 838) were involved in this study. The diagnostic criteria of ACS were described previously. ACS includes unstable angina, ST segment elevation myocardial infarction, and non-ST segment elevation myocardial infarction. All the patients were administered with standard-dose double antiplatelet, statins, and β receptor blockers before PCI if there are no contraindications.
The patients with serious heart failure, hematological diseases, acute infections, and malignant tumor were excluded from the study. The Declaration of Helsinki was complied in the present study, and the protocol was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. The need to obtain informed consent from eligible patients was waived by the ethics committee for the retrospective design of the study.
Ninety-nine patients were excluded due to hematologic parameters (data not available) or acute infections. Finally, there were 3,462 patients who were enrolled to the present study. A flowchart of inclusion and exclusion of patients is shown in Figure 1.
Definition of Derived Neutrophil-To-Lymphocyte Ratio
The basal blood collection was performed before PCI. Routine blood testing performed in accordance with standard methods was as described previously. Briefly, 2 ml of venous blood samples were collected in standardized dipotassium EDTA tubes. The blood routine test was measured using an automated blood counter within 2 h of collection to minimize variations due to sample aging. We calculated the dNLR according to the formula as follows: neutrophil count/(leukocyte count—neutrophil count) as described previously (13–15).
Endpoints
In the present study, we defined a primary endpoint and several secondary endpoints. The primary endpoint was the long-term mortality, including all-cause death and cardiac death. The secondary endpoints include major adverse cardiac events (MACE) and the major adverse cardiac and cerebrovascular events (MACCEs) as described previously (19–21).
Follow-Up
We carried out the follow-up at least 18 months for each patient. The longest follow-up time is 80 months. All the patients were followed either by office visits or by telephone contacts as necessary.
Statistical Analyses
The SPSS 22.0 for windows statistical software (SPSS Inc., Chicago, IL, USA) was used to analyze the data. The continuous data were presented as mean ± standard deviation (mean ± SD). The t-test was utilized to analyze the differences between normally distributed numeric variables, while the Mann–Whitney U-test was used to analyze the difference between non-normally distributed variables. The categorical data were presented as frequencies and percentages. Chi-square (χ2) test was employed for the comparison of categorical variables. The patients were divided into three groups according to the dNLR tertiles: the first tertile (dNLR < 1.36; n = 1,139), second tertile (1.36 ≥ dNLR < 1.96; n = 1,166), and third tertile dNLR ≥ 1.96; n = 1,157). We utilized the Kaplan–Meier analysis to analyze the cumulative incidence of long-term outcomes among three groups. The log-rank test was used to compare the difference between each group. To adjust the confounders, we used the multivariable Cox regression analysis to evaluate the predictive value of dNLR and to calculate the hazard ratios (HRs) and its 95% confidence intervals (CIs). Two-side p < 0.05 was considered significant.
Result
Baseline Characteristics of Three Groups
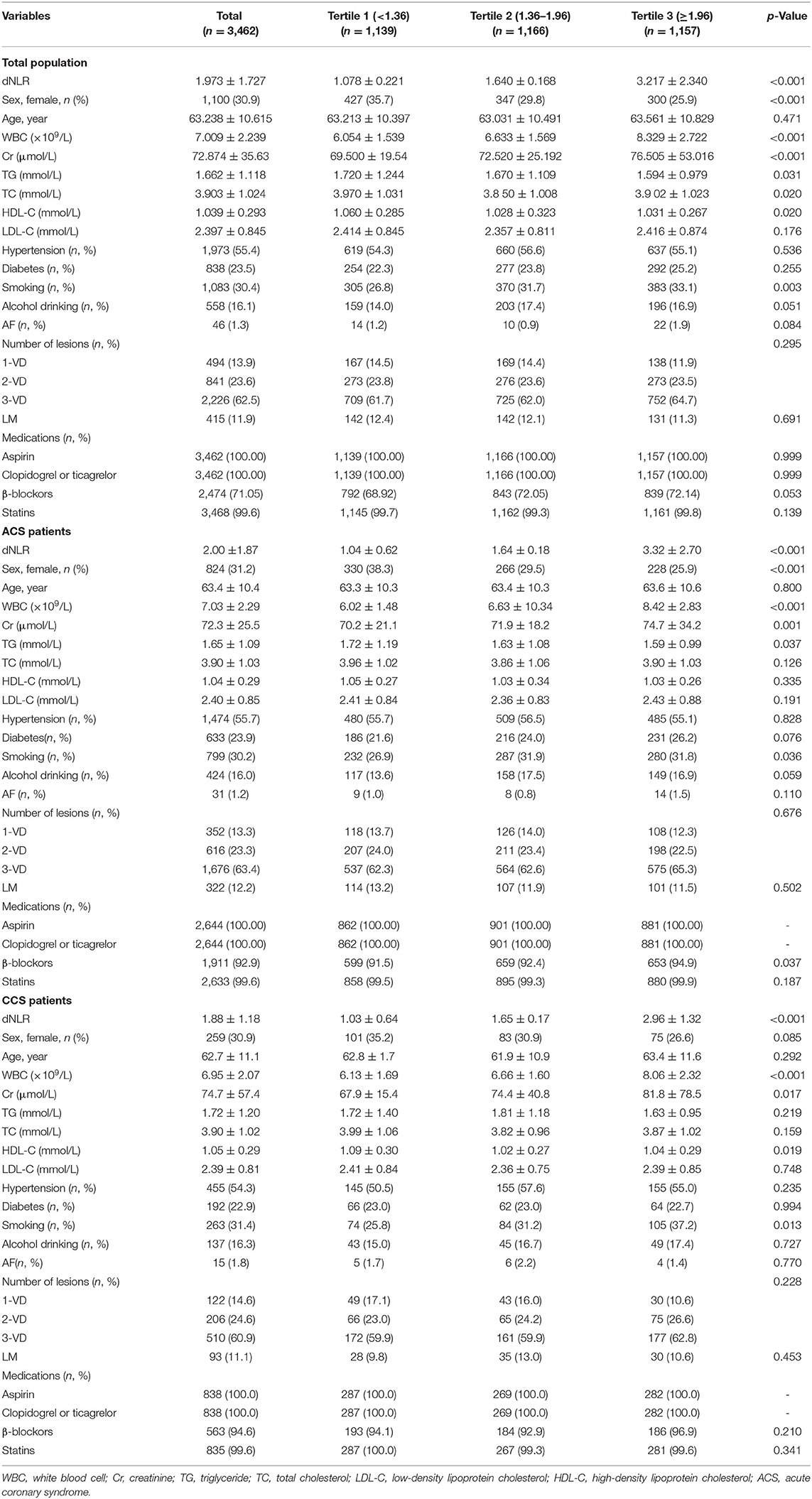
The study included 3,462 patients who were divided into three groups according to the dNLR tertiles: the first tertile (dNLR < 1.36; n = 1,139), second tertile (1.36 ≥ dNLR < 1.96; n = 1,166), and third tertile (dNLR ≥ 1.96; n = 1,157). The baseline data are shown in Table 1. In the total patients, there were significant differences among these three tertiles in dNLR, sex, white blood cell (WBC), creatinine (Cr), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and smoking (all p < 0.05). We did not find a significant difference among these three groups in age, hypertension, diabetes mellitus, and alcohol drinking. We also showed the baseline data characteristics of ACS and CCS patients in Table 1.
Comparison of Clinical Outcomes Among Groups
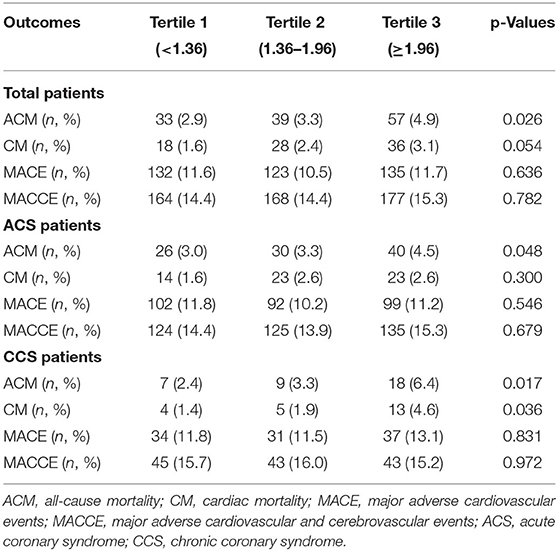
Table 2 shows the incidence of ACM in the three groups. In the total population, there were 33 (2.9%) ACMs in the first tertile dNLR group and 57 (4.9%) ACMs in the third tertile group. The difference was significant (p = 0.026). We did not find a significant difference between the three groups in the incidence of CM, MACEs, and MACCEs. In ACS patients, the incidence of ACM was higher in the third tertile group than that in the first tertile group (4.5 vs. 3.0%, p = 0.048). In the CCS patients, both ACM (6.4 vs. 2.4%, p = 0.017) and CM (4.6 vs. 1.4%, p = 0.036) had significant frequency in the third tertile group than that in the first tertile group.
As shown in the Figures 2, 3, the Kaplan–Meier survival analysis showed that patients in the third tertile have an increased risk of ACM and CM, compared with patients in the first tertile. This trend was also found in both ACS patients (Figures 2B,C) and CCS patients (Figures 3B,C).

Figure 2. Cumulative Kaplan–Meier estimates of the time to the first adjudicated occurrence of all-cause death. (A) Total patients. (B) Acute coronary syndrome (ACS) patients. (C) Chronic coronary syndrome (CCS) patients.
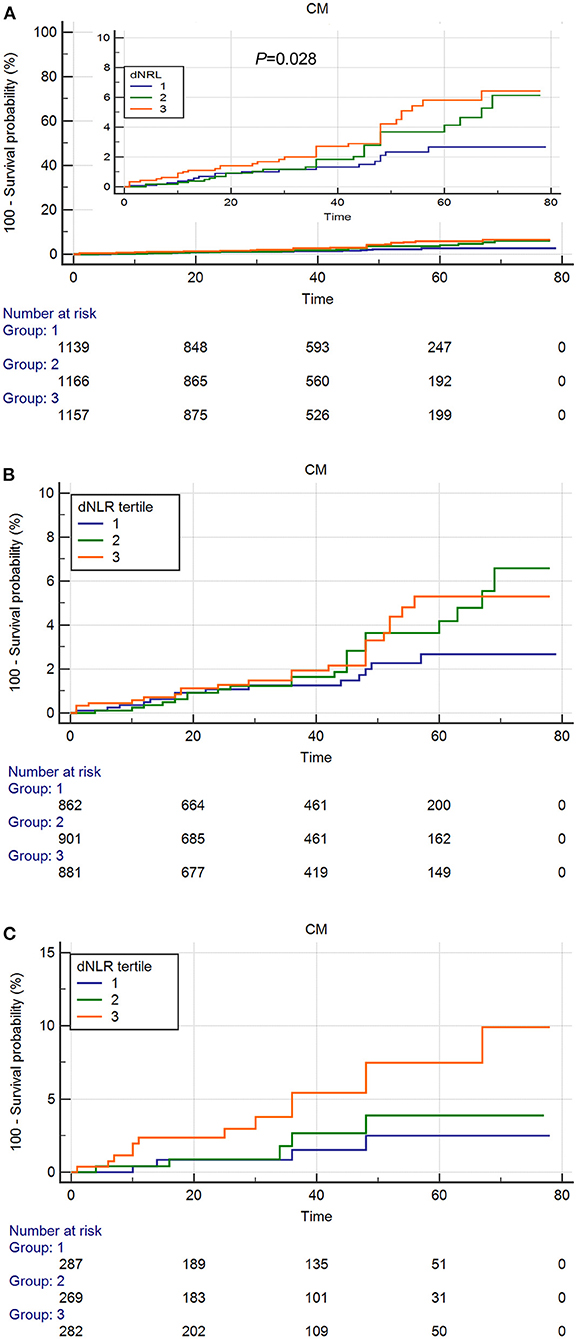
Figure 3. Cumulative Kaplan–Meier estimates of the time to the first adjudicated occurrence of cardiac death. (A) Total Patients. (B) ACS patients. (C) CCS patients.
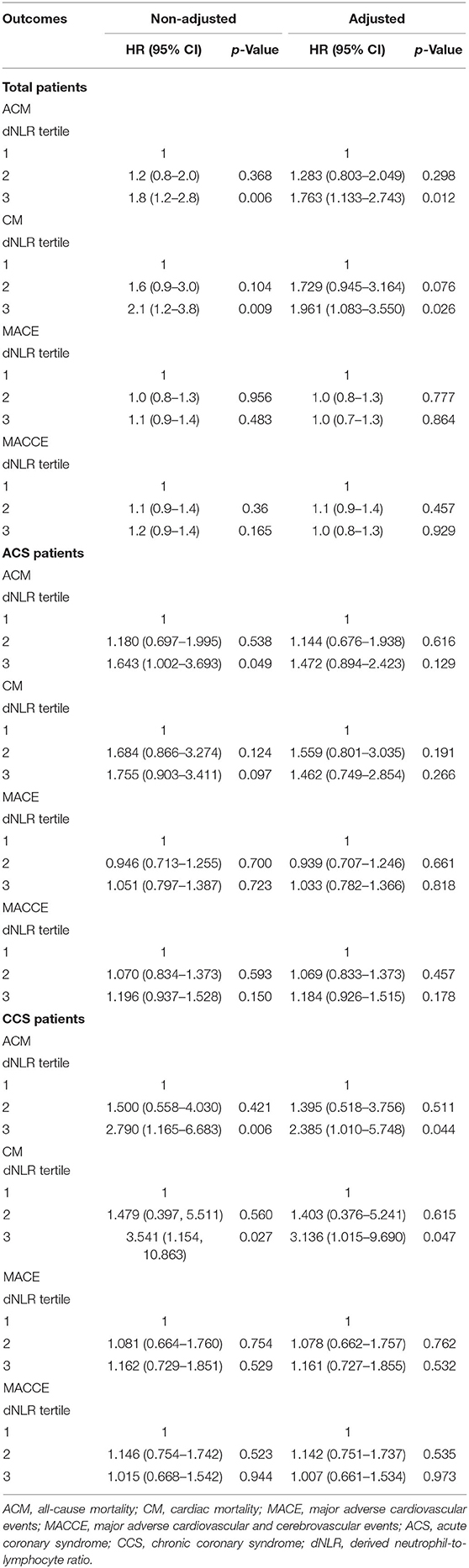
As shown in Table 3, in the total patients, the ACM and CM incidence was significantly lower in the first tertile than that in the third tertile [HR = 1.8 (95% CI: 1.2–2.8), p = 0.006 and HR = 2.1 (95% CI: 1.23–3.8), p = 0.009, respectively]. Multivariate Cox regression analyses suggested that compared with the patients in the first tertile, the risk of ACM was increased 1.763 times (HR = 1.763, 95% CI: 1.133–2.743, p = 0.012), and the risk of CM was increased 1.961 times (HR = 1.961, 95% CI: 1.083–3.550, p = 0.026) in the third tertile dNLR group during the long-term follow-up. All individual dNLR values for survival or not survival are shown in Supplementary Table 1. In both ACS patients and CCS patients, there were significant differences among the three groups in the incidence of ACM in the univariate Cox regression analysis. We also found that the incidence of CM was significantly different among the three groups in the CCS patients in both univariate Cox regression analysis (HR = 3.541, 95% CI: 1.154–10.863, p = 0.027) and multivariate Cox regression analysis (HR = 3.136, 95% CI: 1.015–9.690, p = 0.047).
Discussion
This study suggested that increased dNLR was independently associated with the prognosis of CHD patients who underwent PCI. This is the first study to investigate the relation between dNLR and prognosis in CHD patients.
The circulating cytokines and chemokines were released from elevated neutrophil and during inflammatory responses during which the counts of lymphocyte were declined. The proinflammatory markers including oxidized LDL, proinflammatory cytokines, adhesion molecules, serum amyloid A (SAA), and C-reactive protein (CRP) play an important role in the progress of atherosclerosis (3–5). Therefore, a large number of studies have focused on the relation between inflammatory markers and CHD (2–4). In our study, we used the dNLR, which was calculated as neutrophil count/(leukocyte count—neutrophil count) to predict the mortality of CHD patients who underwent PCI, and found that increased dNLR was associated with high mortality. Neutrophilia reflects the inflammation and the lymphocyte reflects the stress response in human body. Therefore, the dNLR represents a balance between these two paths (22, 23). In our study, we enrolled 3,462 CHD patients who underwent PCI and divided them into three groups according to the dNLR tertiles: the first tertile (dNLR < 1.36; n = 1,139), the second tertile (1.36 ≥ dNLR < 1.96; n = 1,166), and the third tertile (dNLR ≥ 1.96; n = 1,157). We found that the patients in the third tertile have higher mortality. After multivariable Cox regression analysis to adjust the confounders, the dNLR remains an independent predictor of mortality of CHD patients who underwent PCI. In addition, we also found that kidney function is the worst in the third tertile group, which may be an important impact factor of outcomes. A previous study (24) showed that NLR was significantly increased in patients with renal failure, which was in line with our results. Importantly, when we adjusted the Cr variable, the dNLR remains a significant predictive power of mortality.
Recently, Agarwal et al. (25) reviewed the prognostic value of NLR across all stages of CHD. In the review of Agarwal, three studies (26–28) reported that higher NLR was associated with increased risk of all-cause death or cardiac death. In addition, two studies (29, 30) from Boag et al. and Spray et al. with up to 5,000 patients showed clearly the impact of lymphopenia on mortality in STEMI patients. Theoretically, for STEMI patients, with the rise in NLR levels, serum troponin will also be increased correspondingly. Severe myocardial injury and ischemia will stimulate the hypothalamus to promote the release of adrenocortic hormones and to increase cortisol level. Cortisol can help to regulate the peripheral leukocyte count. Once the cortisol level increases in vivo, the number of neutral granulocytes will be improved, and the number of lymphocytes will be reduced accordingly, thereby increasing the level of NLR. In addition, lymphopenia is related to age. Also, approximately 70% of lymphocytes in the peripheral blood are produced in the thymus. Therefore, thymus functional degradation may affect the production of lymphocytes. In our study, we utilized multivariable Cox regression analysis to adjust age and other confounders, and dNLR remains a significant predictive value of outcomes.
Several mechanistic pathways may connect NLR to adverse prognosis in CHD patients. First, previous studies (25, 31)suggested that increased neutrophils can result in the production of vascular endothelial growth factor (VEGF), IL-1, IL-6, and IL-17, which all were reported to be associated with adverse outcomes of CHD. Second, lymphocytes have been suggested to play a role in the modulation of the inflammatory response throughout the atherosclerotic process (32). Lymphopenia may be aggravated by redistribution of T cells from the circulation to lymphoid tissues (33); it may induce compensatory proliferation of antigen-experienced T cells, which could increase the risk of cardiovascular disease (34). Third, lymphocytes have a critical role in regulating the inflammatory response to tissue damage, through production of both pro- and anti-inflammatory cytokines.
There were several strengths in our study. First, the long-term follow-up is the main strength of our study. Second, the larger sample size is another strength of our study. Finally, we performed the statistical analysis that used multivariable analysis, which improved the credibility of the study. In addition, there were also some limitations, which are as follows: First, we only collected the baseline data of dNLR; therefore, the influence of dynamic changes of dNLR on the mortality was unclear. Second, there were several patients who were excluded from this study for data not available. Third, our study is a single-center retrospective cohort design; therefore, a multicenter cohort design is needed in the future. Fourth, all the patients were not continuously included in this study, which will be of the selection bias. Finally, we did not collect the revascularization characteristics in this study. Therefore, we cannot analyze the impact of revascularization on outcomes in the present study.
Conclusion
In conclusion, our results suggest that baseline dNLR is a novel independent predictor of mortality in CHD patients who underwent PCI. This founding will help the interventional physician to make clinical decisions on daily practice.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The ethics committee of the First Affiliated Hospital of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
G-QL and W-JZ made substantial contributions to study conception and design and to the drafting and critical revision of the manuscript for important intellectual content. J-HS, X-DZ, WW, Q-QG, J-CZ, and KW made substantial contributions to the study conception and design and critical revision of the manuscript for important intellectual content. Z-YL, F-HS, and LF made substantial contributions to study conception and design. Y-YZ and J-YZ made substantial contributions to study conception and design, drafting and critical revision of the manuscript for important intellectual content, including study supervision. All authors read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (U2004203, 81900263, and 81760043).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.705862/full#supplementary-material
Abbreviations
dNLR, derived neutrophil-to-lymphocyte ratio; PCI, percutaneous coronary intervention; CHD, coronary heart disease; MACE, major adverse cardiac events; MACCEs, major adverse cardiac and cerebrovascular events; HR, hazard ratios; CI, confidence interval.
References
1. Wirtz PH, von Känel R. Psychological stress, inflammation, and coronary heart disease. Curr Cardiol Rep. (2017) 19:111. doi: 10.1007/s11886-017-0919-x
2. Pothineni NVK, Subramany S, Kuriakose K, Shirazi LF, Romeo F, Shah PK, et al. Infections, atherosclerosis, and coronary heart disease. Eur Heart J. (2017) 38:3195–201. doi: 10.1093/eurheartj/ehx362
3. Li H, Sun K, Zhao R, Hu J, Hao Z, Wang F, et al. Inflammatory biomarkers of coronary heart disease. Front Biosci. (2018) 10:508. doi: 10.2741/s508
4. Stewart RAH, Held C, Hadziosmanovic N, Armstrong PW, Cannon CP, Granger CB, et al. Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol. (2017) 70:1689–700. doi: 10.1016/j.jacc.2017.08.017
5. Malhotra A, Redberg RF, Meier P. Saturated fat does not clog the arteries: coronary heart disease is a chronic inflammatory condition, the risk of which can be effectively reduced from healthy lifestyle interventions. Br J Sports Med. (2017) 51:1111–2. doi: 10.1136/bjsports-2016-097285
6. Wensley F, Gao P, Burgess S, Kaptoge S, Di Angelantonio E, Shah T, et al. Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ. (2011) 342:d548. doi: 10.1136/bmj.d548
7. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. (2004) 350:1387–97. doi: 10.1056/NEJMoa032804
8. Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: in-depth review and update. Tex Heart Inst J. (2013) 40:17–29.
9. Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. (2004) 44:1945–56. doi: 10.1016/j.jacc.2004.07.056
10. Alfakry H, Malle E, Koyani CN, Pussinen PJ, Sorsa T. Neutrophil proteolytic activation cascades: a possible mechanistic link between chronic periodontitis and coronary heart disease. Innate Immun. (2016) 22:85–99. doi: 10.1177/1753425915617521
11. Hoyer FF, Nahrendorf M. Neutrophil contributions to ischaemic heart disease. Eur Heart J. (2017) 38:465–72. doi: 10.1093/eurheartj/ehx017
12. Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. (2011) 17:1410–22. doi: 10.1038/nm.2538
13. Song S, Li C, Li S, Gao H, Lan X, Xue Y. Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. Onco Targets Ther. (2017) 10:3145–54. doi: 10.2147/OTT.S138039
14. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GG, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. (2016) 27:732–8. doi: 10.1093/annonc/mdw016
15. Cox S, Hurt C, Grenader T, Mukherjee S, Bridgewater J, Crosby T. The prognostic value of derived neutrophil to lymphocyte ratio in oesophageal cancer treated with definitive chemoradiotherapy. Radiother Oncol. (2017) 125:154–9. doi: 10.1016/j.radonc.2017.08.023
16. Wood G, Grenader T, Nash S, Adams R, Kaplan R, Fisher D, et al. Derived neutrophil to lymphocyte ratio as a prognostic factor in patients with advanced colorectal cancer according to RAS and BRAF status: a post-hoc analysis of the MRC COIN study. Anticancer Drugs. (2017) 28:546–50. doi: 10.1097/CAD.0000000000000488
17. van Kessel KE, de Haan LM., Fransen van de Putte EE, van Rhijn BW, de Wit R, van der Heijden MS, et al. Elevated derived neutrophil-to-lymphocyte ratio corresponds with poor outcome in patients undergoing pre-operative chemotherapy in muscle-invasive bladder cancer 6. Bladder Cancer. (2016) 2:351–60. doi: 10.3233/BLC-160055
18. Ren K, Yin Y, He F, Shao Y, Wang S. Prognostic role of derived neutrophil-to-lymphocyte ratio in surgical triple-negative breast cancer. Cancer Manag Res. (2018) 10:4891–8. doi: 10.2147/CMAR.S180695
19. Zheng YY, Wu TT, Chen Y, Hou XG, Yang Y, Ma X, et al. Gamma-glutamyl transferase-to-platelet ratio as a novel predictor of long-term adverse outcomes in patients after undergoing percutaneous coronary intervention: a retrospective cohort study. Thromb Haemost. (2019) 119:1021–30. doi: 10.1055/s-0039-1681103
20. Zheng YY, Wu TT, Chen Y, Hou XG, Yang Y, Zhang JY, et al. Platelet-to-hemoglobin ratio as a novel predictor of long-term adverse outcomes in patients after percutaneous coronary intervention: a retrospective cohort study. Eur J Prev Cardiol. (2019) 27:2216–9. doi: 10.1177/2047487319870346
21. Zheng YY, Wu TT, Yang Y, Hou XG, Gao Y, Chen Y, et al. Personalized antiplatelet therapy guided by a novel detection of platelet aggregation function in stable coronary artery disease patients undergoing PCI: a randomized controlled clinical trial. Eur Heart J Cardiovasc Pharmacother. (2019) 6:211–21. doi: 10.1093/ehjcvp/pvz059
22. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. (2002) 105:1135–43. doi: 10.1161/hc0902.104353
23. Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. (2016) 14:573–7. doi: 10.1586/14779072.2016.1154788
24. Sargin M, Taşdemir Mete M, Bayer Erdogan S, Kuplay H, Baştopçu M, Bayraktar F, et al. Neutrophil-to-lymphocyte ratio for early renal failure under extracorporeal membrane oxygenation support for postcardiotomy shock. Turk Gogus Kalp Damar Cerrahisi Derg. (2019) 27:314–9. doi: 10.5606/tgkdc.dergisi.2019.17891
25. Agarwal R, Aurora RG, Siswanto BB, Muliawan HS. The prognostic value of neutrophil-to-lymphocyte ratio across all stages of coronary artery disease. Coron Artery Dis. (2021). doi: 10.1097/MCA.0000000000001040. [Epub ahead of print].
26. Wada H, Dohi T, Miyauchi K, Shitara J, Endo H, Doi S, et al. Pre-procedural neutrophil-to-lymphocyte ratio and long-term cardiac outcomes after percutaneous coronary intervention for stable coronary artery disease. Atherosclerosis. (2017) 265:35–40. doi: 10.1016/j.atherosclerosis.2017.08.007
27. Bressi E, Mangiacapra F, Ricottini E, Cavallari I, Colaiori I, Di Gioia G, et al. Impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio on 5-year clinical outcomes of patients with stable coronary artery disease undergoing elective percutaneous coronary intervention. J Cardiovasc Transl Res. (2018) 11:517–23. doi: 10.1007/s12265-018-9829-6
28. Wada H, Dohi T, Miyauchi K, Nishio R, Takeuchi M, Takahashi N, et al. Neutrophil to lymphocyte ratio and long-term cardiovascular outcomes in coronary artery disease patients with low high-sensitivity C-reactive protein level. Int Heart J. (2020) 61:447–53. doi: 10.1536/ihj.19-543
29. Boag SE, Das R, Shmeleva EV, Bagnall A, Egred M, Howard N, et al. T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. J Clin Invest. (2015) 125:3063–76. doi: 10.1172/JCI80055
30. Spray L, Park C, Cormack S, Mohammed A, Panahi P, Boag S, et al. The fractalkine receptor CX3CR1 links lymphocyte kinetics in CMV-seropositive patients and acute myocardial infarction with adverse left ventricular remodeling. Front Immunol. (2021) 12:605857. doi: 10.3389/fimmu.2021.605857
31. Dragu R, Huri S, Zukermann R, Suleiman M, Mutlak D, Agmon Y, et al. Predictive value of white blood cell subtypes for long-term outcome following myocardial infarction. Atherosclerosis. (2008) 196:405–12. doi: 10.1016/j.atherosclerosis.2006.11.022
32. AltinbaS Ö, Demiryürek S, Işik M, Tanyeli Ö, Dereli Y, Görmüş N. Predictive value of neutrophil-to-lymphocyte, aspartate-to-alanine aminotransferase, lymphocyte-to-monocyte and platelet-to-lymphocyte ratios in severity and side of carotid artery stenosis: are those significant? Heart Surg Forum. (2021) 24:E072–8. doi: 10.1532/hsf.3431
33. Wang L, Hong KC, Lin FC, Yang KD. Mycoplasma pneumoniae-associated Stevens-Johnson syndrome exhibits lymphopenia and redistribution of CD4+ T cells. J Formos Med Assoc. (2003) 102:55–8.
Keywords: derived neutrophil-to-lymphocyte ratio, percutaneous coronary intervention, mortality, neutrophil-to-lymphocyte ratio, coronary heart disease
Citation: Liu G-Q, Zhang W-J, Shangguan J-H, Zhu X-D, Wang W, Guo Q-Q, Zhang J-C, Wang K, Liu Z-Y, Song F-H, Fan L, Zheng Y-Y and Zhang J-Y (2021) Association of Derived Neutrophil-To-Lymphocyte Ratio With Prognosis of Coronary Heart Disease After PCI. Front. Cardiovasc. Med. 8:705862. doi: 10.3389/fcvm.2021.705862
Received: 06 May 2021; Accepted: 16 August 2021;
Published: 17 September 2021.
Edited by:
Marat V. Ezhov, Ministry of Health of the Russian Federation, RussiaReviewed by:
Kurt Huber, Wiener Krankenanstaltenverbund, AustriaIoakim Spyridopoulos, Newcastle University, United Kingdom
Copyright © 2021 Liu, Zhang, Shangguan, Zhu, Wang, Guo, Zhang, Wang, Liu, Song, Fan, Zheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Ying Zhang, anl6aGFuZ0B6enUuZWR1LmNu; Ying-Ying Zheng, emhlbmd5aW5nNTI3QDE2My5jb20=
 Gang-Qiong Liu1
Gang-Qiong Liu1 Qian-Qian Guo
Qian-Qian Guo Jian-Chao Zhang
Jian-Chao Zhang Zhi-Yu Liu
Zhi-Yu Liu Ying-Ying Zheng
Ying-Ying Zheng Jin-Ying Zhang
Jin-Ying Zhang