- 1Department of Medicine, Graduate School, Kyung Hee University, Seoul, South Korea
- 2Division of Nephrology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, South Korea
- 3Division of Nephrology, Department of Internal Medicine, Kyung Hee University, Seoul, South Korea
- 4Division of Nephrology, Department of Internal Medicine, Korea University College of Medicine, Seoul, South Korea
- 5Division of Nephrology, Department of Internal Medicine, Veterans Health Service Medical Center, Seoul, South Korea
Background: Vascular adhesion protein-1 (VAP-1) is an oxidative enzyme of primary amines that facilitates the transmigration of inflammatory cells. Its oxidative and inflammatory effects are prominently increased in pathological conditions, such as metabolic, atherosclerotic, and cardiac diseases. However, the clinical significance of circulating VAP-1 levels in hemodialysis (HD) patients is unclear.
Methods: A total of 434 HD patients were enrolled in a prospective multicenter cohort study between June 2016 and April 2019. Plasma VAP-1 levels were measured at the time of data entry, and the primary endpoint was defined as a composite of cardiovascular (CV) and cardiac events.
Results: Circulating VAP-1 levels were positively correlated with plasma levels of cardiac remodeling markers, including brain natriuretic peptide, galectin-3, and matrix metalloproteinase-2. Multivariable logistic regression analysis revealed that patients with higher circulating VAP-1 levels were more likely to have left ventricular diastolic dysfunction [odds ratio, 1.40; 95% confidence interval [CI], 1.04–1.88]. The cumulative event rate of the composite of CV events was significantly greater in VAP-1 tertile 3 than in VAP-1 tertiles 1 and 2 (P = 0.009). Patients in tertile 3 were also associated with an increased cumulative event rate of cardiac events (P = 0.015), with a 2.06-fold higher risk each for CV (95% CI, 1.10–3.85) and cardiac (95% CI, 1.03–4.12) events after adjusting for multiple variables.
Conclusions: Plasma VAP-1 levels were positively associated with left ventricular diastolic dysfunction and the risk of incident CV and cardiac events in HD patients. Our results indicate that VAP-1 may aid clinicians in identifying HD patients at a high risk of CV events.
Introduction
Patients receiving hemodialysis (HD) have substantial retention of uremic toxins, which lead to a number of adverse metabolic processes (1). Oxidative stress and inflammation are representative pathophysiologic processes of uremic complications and are major contributors of cardiovascular (CV) complications in HD patient (2–5), by promoting myocardial stiffening and left ventricular (LV) hypertrophy and inducing endothelial dysfunction and progression of atherosclerosis (5–7). These conditions severely increase the risk of CV complications, which have become leading causes of death in HD patients (8).
Vascular adhesion protein-1 (VAP-1) is a semicarbazide-sensitive amine oxidase that catalyzes the oxidative deamination of primary amines, which generates free radicals and causes oxidative stress (9, 10). It also facilitates the transmigration of inflammatory cells and worsens injuries in inflamed tissues (9, 11). These deleterious roles are prominently enhanced in pathological conditions. Circulating VAP-1 levels are increased in septic, metabolic, and autoimmune diseases, and higher VAP-1 levels increase the risk of atherosclerotic events and CV mortality (12–14). In patients with impaired renal function, excessive concentrations and abundant substrates of VAP-1 were observed, the latter of which undergo uncontrolled deamination and oxidative stress (14, 15). Furthermore, VAP-1 is suggested to play a pivotal role in HD patients because many dialysis-specific factors upregulate inflammatory processes (15, 16). Therefore, VAP-1 may be critically involved in the occurrence of adverse CV events in HD patients through oxidative stress and inflammation.
However, the clinical significance of VAP-1 has rarely been evaluated in HD patients, and no reports have investigated the prognostic significance of VAP-1. In this study, we investigated the association between circulating VAP-1 levels and risk of incident adverse CV events in HD patients, along with the relationship of echocardiographic parameters and circulating cardiac biomarkers with VAP-1 levels.
Materials and Methods
Study Population
All data in this study were obtained from the K-cohort registry, which is a multicenter, internet-based, prospective cohort of HD patients in Korea designed to investigate the prognostic markers of CV complications and mortality. Patients from six general hospitals were enrolled if they were aged >18 years and received regular 4-h HD prescriptions per session that occurred thrice a week for at least 3 months. The exclusion criteria were as follows: pregnancy, hematological malignancy, presence of a solid tumor, and a life expectancy of <6 months. A total of 637 patients were recruited between June 2016 and April 2019, and 434 patients with whole plasma samples at the time of enrollment were included. The study protocol was approved by the local ethics committee (KHNMC 2016-04-039), and the study was conducted in accordance with the principles of the Declaration of Helsinki. All involved participants signed written informed consent forms before enrollment.
The patients were classified into three groups based on the circulating VAP-1 levels as follows: tertile 1, <343.2 ng/mL; tertile 2, 343.2– <438.2 ng/mL; and tertile 3, ≥438.2 ng/mL. All patients were prospectively followed up for specific clinical events after baseline assessments. Patient follow-up was censored at the time of transfer to peritoneal dialysis, kidney transplantation, loss of follow-up, or patient consent withdrawal.
Data Collection and Outcome Measures
Information on baseline demographic factors, laboratory data, dialysis, and concomitant medications were collected from medical records and interviews. Information on comorbidities were investigated and used to calculate the Charlson comorbidity index score (17). Fasting blood samples for laboratory data and enzymatic measurements were collected before the start of HD in a midweek session.
The primary endpoint was a composite of incident CV events and mortality, which included cardiac events such as coronary artery disease requiring coronary artery bypass surgery or percutaneous intervention, myocardial infarction, heart failure, ventricular arrhythmia, cardiac arrest, and sudden death, as well as cerebral infarction, cerebral hemorrhage, and peripheral vascular occlusive diseases requiring revascularization or surgical intervention. All-cause mortality events were recorded. The secondary endpoints were the correlation of VAP-1 levels with LV diastolic dysfunction, which was defined as peak early diastolic flow velocity (E)/peak early diastolic tissue velocity (E') of >15 on echocardiography, and levels of circulating cardiac markers.
Echocardiographic Measurements
Among the enrolled patients, 214 (49.3%) underwent echocardiography [61 (42.4%) in tertile 1, 75 (51.7%) in tertile 2, and 78 (53.8%) in tertile 3]. Cardiologists and trained sonographers examined two-dimensional and M-mode echocardiographs based on the recommendations of the American Society of Echocardiography (18). LV end-diastolic diameter (LVDd), LV end-systolic diameter, LV posterior wall thickness, and interventricular septal thickness were measured in the M-mode echocardiogram. LV mass was estimated using the Devereux formula, with the body surface area as the index. LV end-diastolic and LV end-systolic volumes, LV ejection fraction, and left atrial dimensions were determined in apical two- and four-chamber views. E and peak late diastolic flow velocity (A) was determined from the mitral valve inflow velocity curve using pulsed-wave Doppler ultrasonography. E′ was measured from the septal aspect of the mitral annulus using tissue Doppler. The E/A and E/E′ ratios were calculated.
Measurements of Circulating Cardiac Markers and VAP-1 Levels
Baseline plasma samples for the measurement of N-terminal pro-B-type natriuretic peptide (NT-proBNP), brain natriuretic peptide (BNP), matrix metalloproteinase-2 (MMP-2), and VAP-1 were collected using ethylenediaminetetraacetic acid-treated tubes. After centrifugation for 15 min at 1,000 × g at room temperature, the samples were stored at 80°C until use. Enzyme-linked immunosorbent assay was performed using Magnetic Luminex® Screening Assay multiplex kits (R&D Systems, Inc., Minneapolis, MN, USA).
Statistical Analysis
Data are expressed as means ± standard deviations (SDs) or medians (interquartile ranges). Differences among the three groups were identified using analysis of variance or Kruskal-Wallis test. Tukey post-hoc test and Mann-Whitney U-test with Bonferroni correction were used to identify intergroup differences. Chi-square test or Fisher's exact test was used to compare the categorical variables. Log-transformed high-sensitivity C-reactive protein (hsCRP) values were used because of the skewed data distribution. Correlation between the VAP-1 levels and continuous variables was evaluated using Spearman's analyses. Binary logistic regression analysis was used to assess the association between the VAP-1 levels and LV diastolic dysfunction. A Cox proportional hazards model was constructed to identify independent variables related to CV and cardiac events and all-cause mortality. Parameters significantly associated with weight in the univariable analysis and clinically fundamental parameters were included in the multivariable models. Formal tests for the interaction between VAP-1 levels and predefined subgroups were conducted in addition to the main effects of the fully adjusted models. We modeled the association between VAP-1 levels and the hazard ratio to predict CV events. We used three knots and restricted cubic spline transformations to continuous measures. We calculated the sample size using standard formulas based on the number of patients to obtain the adequate statistical power for the primary endpoint and show a different composite event-free survival rate with an α-level of 0.05, β error of 0.20, and hazard ratio of 1.5. The minimum required sample size in each group was 98 patients. Statistical significance was set at P < 0.05. Statistical analyses were performed using the SPSS software (version 22.0; SPSS, IBM Corp., Armonk, NY, USA) and R software (version 3.6.2).
Results
Baseline Demographic Characteristics and Laboratory Data
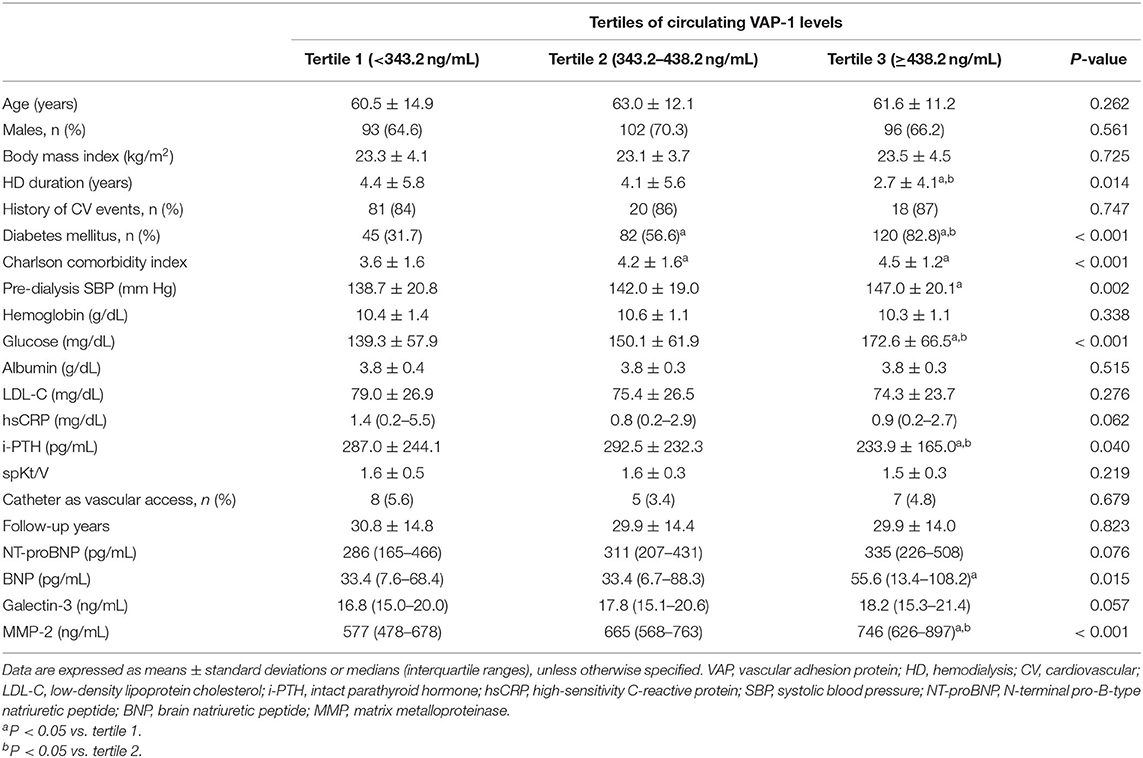
The mean VAP-1 level was 386.0 (range, 318.1–484.6) ng/mL, with mean VAP-1 levels in tertiles 1, 2, and 3 of 281.8 ng/mL (range, 242.6–318.5 ng/mL), 385.5 ng/mL (range, 365.6– 411.0 ng/mL), and 523.6 ng/mL (range, 484.6–601.4 ng/mL), respectively. The baseline clinical characteristics, demographics, and laboratory results are shown in Table 1. Patients in tertile 3 had a shorter HD history, higher prevalence of diabetes mellitus, higher Charlson comorbidity index, higher pre-dialysis systolic blood pressure (SBP), and lower intact parathyroid hormone (i-PTH) than those in tertile 1. Regarding the circulating cardiac markers, patients in tertile 3 showed the highest BNP and MMP-2 levels. Plasma MMP-2 levels were moderately correlated with plasma VAP-1 levels, whereas BNP and galectin-3 showed weak positive correlations (Supplementary Table S1). In contrast, hsCRP levels demonstrated a weak negative correlation with circulating VAP-1 levels.
Relationship Between Plasma VAP-1 Levels and LV Diastolic Dysfunction in Hemodialysis Patients
Baseline echocardiographic measurements are presented in Supplementary Table S2. LVDd was significantly different among the tertiles, with the highest E wave and E/A and E/E' ratios observed in patients in VAP-1 tertile 3. We constructed univariable and multivariable binary logistic regression models to determine the association between VAP-1 and LV diastolic dysfunction (Table 2). In the univariable analysis, circulating VAP-1 level increment per SD [dds ratio [OR], 1.51; 95% confidence interval [CI], 1.15–2.00; P = 0.004] and Charlson comorbidity index (OR, 1.32; 95% CI, 1.08–1.62; P = 0.006) were significantly associated with an increased risk of LV diastolic dysfunction. Age, male sex, pre-dialysis SBP, and NT-proBNP increments per SD showed a borderline significant association with LV diastolic dysfunction. In the multivariable binary logistic regression model, the Charlson comorbidity index (OR, 1.24; 95% CI, 1.01–1.53; P = 0.045) and serum VAP-1 level (OR, 1.40; 95% CI, 1.04–1.88; P = 0.028) remained statistically significant as independent determinants of LV diastolic dysfunction in HD patients.
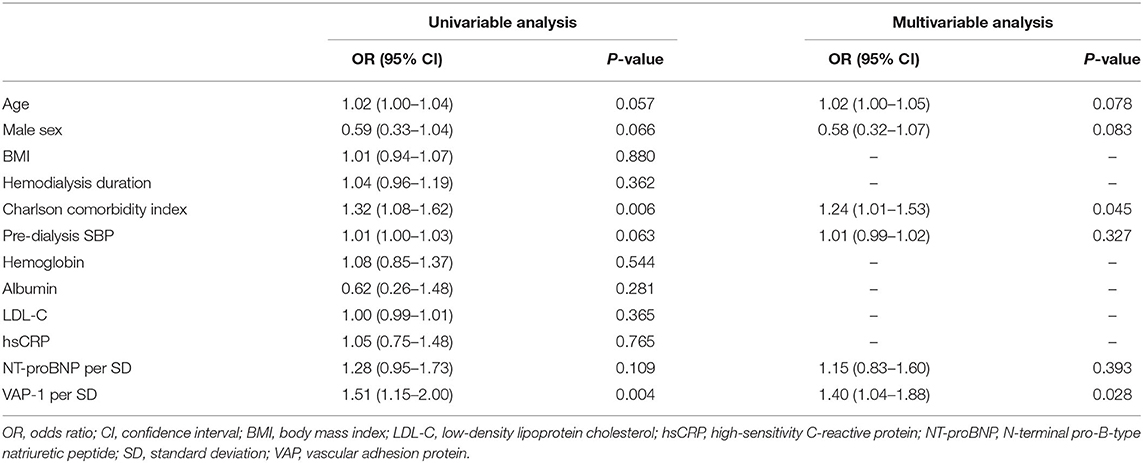
Table 2. Relationship between vascular adhesion protein-1 levels and left ventricle diastolic dysfunction.
Prognostic Utility of the VAP-1 Level in Hemodialysis Patients
During a mean follow-up of 30.3 months, 61 deaths (14.1%) and 77 adverse CV events (17.7%) occurred. Regarding CV events, coronary artery disease occurred in 36 patients, heart failure in 7, ventricular arrhythmia in 1, cardiac arrest in 9, sudden death in 9, CV events in 9, and peripheral vascular occlusive diseases in 6. VAP-1 tertile 3 had the highest cumulative CV event rate (P = 0.009; Figure 1A) and a greater cumulative rate of cardiac events (P = 0.015; Figure 1B). The cumulative event rate of patient mortality did not differ among VAP-1 tertiles (P = 0.747).
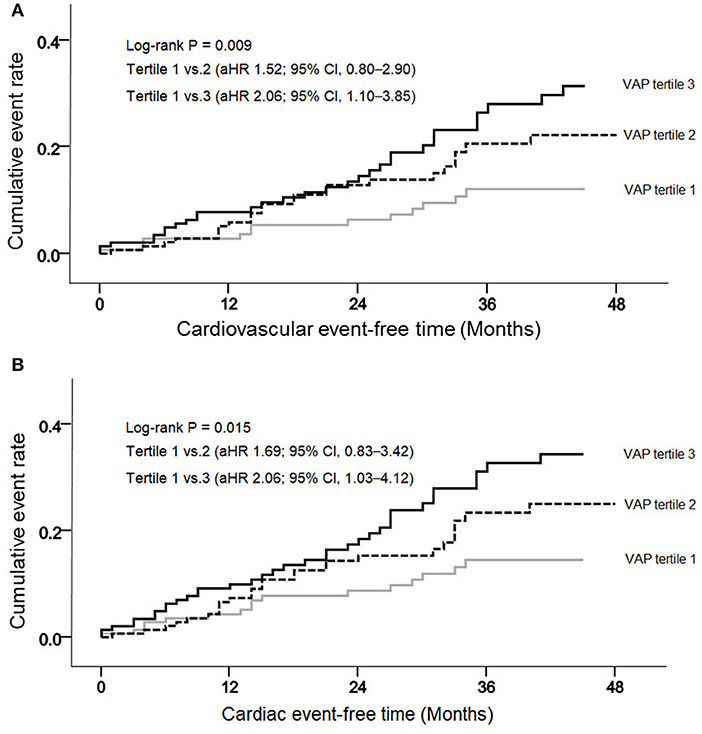
Figure 1. Cumulative cardiovascular (A) and cardiac (B) event rates, according to the vascular adhesion protein-1 (VAP-1) levels.
Univariable Cox regression analysis revealed that VAP-1 tertile 3 was significantly associated with an increased risk of the composite of CV events [hazard ratio [HR], 2.51; 95% CI, 1.39–4.54; P = 0.002] (Table 3). In the multivariable Cox regression analysis, VAP-1 tertile 3 was significantly associated with a 2.06-fold higher risk of CV events (95% CI, 1.10–3.85; P = 0.025) and VAP-1 increment per SD was significantly associated with a 1.31-fold higher risk of CV events (95% CI, 1.05–1.64; P = 0.019). The risk of cardiac events and patient mortality was further investigated. Patients in VAP-1 tertile 3 had a 2.06-fold higher risk of cardiac disease after adjustment for multiple variables (95% CI, 1.03–4.12; P = 0.041). VAP-1 increment per SD was also significantly associated with the risk of cardiac events (HR, 1.29; 95% CI, 1.01–1.64; P = 0.038). However, among patients in VAP-1 tertile 3, VAP-1 levels were not significantly associated with the risk of mortality. To evaluate potential linear associations, we evaluated the association between VAP-1 and the risk of composite of CV events and cardiac events during follow-up. The restricted cubic spline model after multiple adjustments showed gradually increasing HRs for both CV and cardiac events with increasing VAP-1 levels (Figure 2).
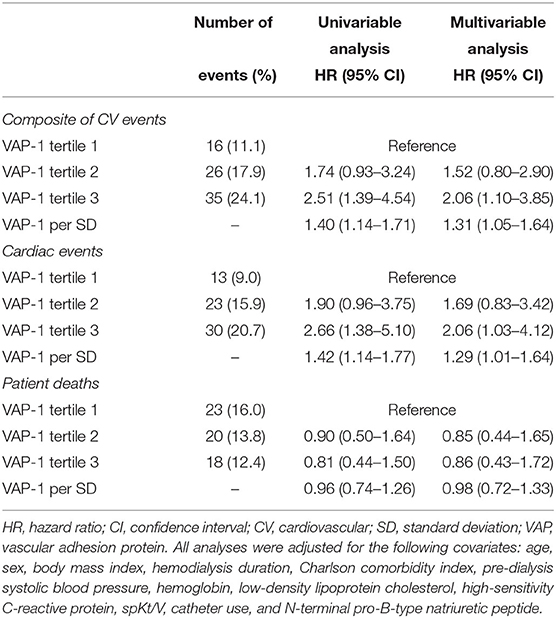
Table 3. Hazard ratios of plasma vascular adhesion protein-1 levels for cardiovascular events, cardiac events, and mortality.
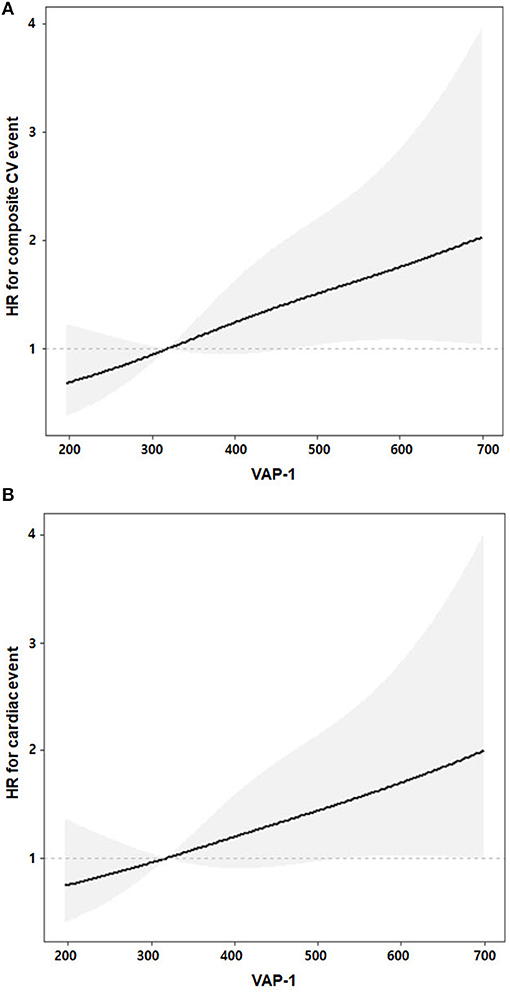
Figure 2. Linear associations of VAP-1 and adjusted risks of the composite of cardiovascular (A) and cardiac (B) events. Gray shaded areas represent 95% confidence intervals. The adjusted multiple variables were age, sex, body mass index, hemodialysis duration, Charlson comorbidity index, pre-dialysis systolic blood pressure, hemoglobin, low-density lipoprotein cholesterol, high-sensitivity C-reactive protein, spKt/V, catheter use, and N-terminal pro-B-type natriuretic peptide.
The relationship between VAP-1 levels and the composite of incident CV events was further investigated in subgroups stratified by the presence of diabetes mellitus and NT-proBNP levels, with cut-offs defined as median values in each parameter (>386.5 ng/mL for VAP-1 and >313.3 pg/mL for NT-proBNP, respectively; Table 4). Multivariable analysis showed that higher VAP-1 levels were associated with an increased risk of CV events both in patients with (HR, 2.39; 95% CI, 1.14–5.03; P = 0.022) and without (HR, 2.57; 95% CI, 1.05–6.30; P = 0.039) diabetes mellitus. There was a significant interaction between higher VAP-1 levels and diabetes mellitus in association with CV events (HR, 0.51; 95% CI, 0.29–0.90; P for interaction = 0.02). Compared to those with low NT-proBNP levels, patients with high VAP-1 and NT-proBNP levels showed an increased risk of CV events (HR, 1.99; 95% CI, 1.01–3.89; P = 0.046), whereas those with isolated high NT-proBNP levels did not show this association. There was no significant interaction between VAP-1 and NT-proBNP levels (HR, 1.57; 95% CI, 0.59–4.18; P for interaction = 0.36).
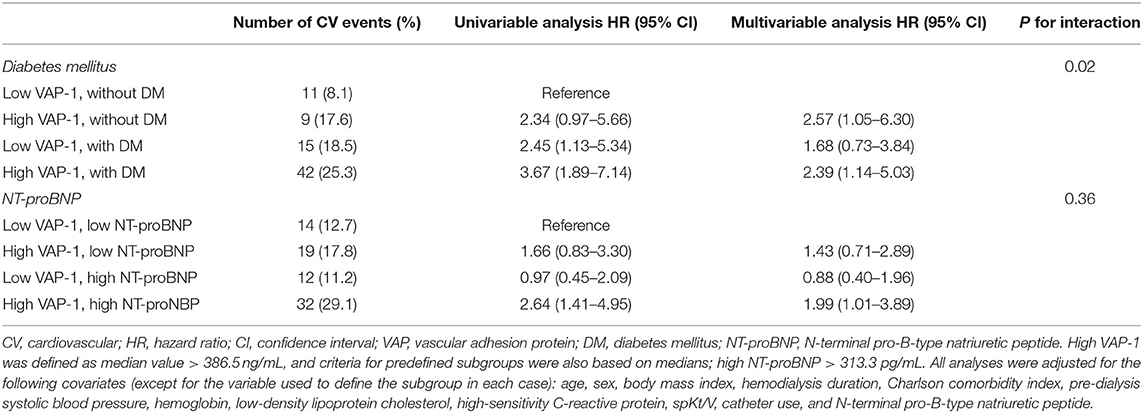
Table 4. Hazard ratios of plasma vascular adhesion protein-1 levels for the composite of cardiovascular events according to predefined subgroups.
Discussion
In this prospective cohort study, we investigated the associations of plasma VAP-1 levels with cardiac dysfunction and CV outcomes in HD patients. Plasma VAP-1 levels were positively associated with circulating cardiac biomarker levels and LV diastolic dysfunction. Patients in VAP-1 tertile 3 had the greatest risk of a higher composite of CV events, and this association remained significant after adjusting for established CV risk factors. Taken together, our findings suggest that plasma VAP-1 may be a novel biomarker of incident CV events in HD patients.
High blood pressure and hyperglycemia were representative metabolic disorders that increased plasma VAP-1 levels in individuals without renal impairment (13, 19–23). In the present study, serum glucose level and pre-dialysis SBP were the highest among patients in VAP-1 tertile 3, suggesting that the relationship among blood pressure, serum glucose, and VAP-1 levels is consistent in HD patients. Because VAP-1 promoted the inflammatory process, the positive correlation between plasma VAP-1 levels and hsCRP was expected. However, we showed that the correlation between VAP-1 and hsCRP was negative and the correlation power was highly weak, implying that higher plasma VAP-1 levels may not merely be a secondary reflection of systemic inflammation in HD patients.
LV diastolic dysfunction is commonly identified in HD patients and is associated with high CV mortality (24–28). In this study, the E/E' ratio was used as a criterion for LV diastolic dysfunction because an E/E' ratio of >15 has been reported as a strong predictor of CV events in HD patients (29, 30). We found that a higher VAP-1 level was independently associated with an increased risk of LV diastolic dysfunction. In addition, higher VAP-1 levels were correlated with an increased risk of cardiac events after adjusting for multiple confounders, indicating that VAP-1 levels may reflect structural changes in cardiac pathology and that VAP-1 could be a potential biomarker of incident cardiac events in HD patients. Furthermore, we found a significant correlation between plasma VAP-1 and MMP-2, one of the main mediators of pathologic extracellular matrix remodeling and fibrosis in several cardiac diseases (31–33). Previous studies have reported that MMP-2 is involved in LV diastolic dysfunction because circulating MMP-2 levels were correlated with the E/E' ratio (32, 33).
Patients in VAP-1 tertile 3 had a higher cumulative incidence of the composite of CV events than those in VAP-1 tertile 1. In addition, Cox regression analysis showed that high plasma VAP-1 levels were independently associated with a significantly increased risk of CV events, even after adjustments for possible confounders, including SBP and the Charlson comorbidity index. These findings suggest that VAP-1 may be a useful indicator for screening HD patients at a high risk of CV events. Positive correlations of plasma VAP-1 with traditional risk factors, including glucose levels, pre-dialysis SBP, and LV diastolic dysfunction, further support its usefulness in predicting CV outcomes. Notably, plasma VAP-1 levels did not predict all-cause mortality, despite their significant association with CV events. A possible explanation for this discrepancy might be that more than two-third (65.6%) of mortalities in this study were not attributed to CV events.
Subgroup analysis showed that higher plasma VAP-1 levels were associated with an increased risk of CV events in both patients with and without diabetes mellitus. However, the HR of higher VAP-1 levels was lower in HD patients with diabetes mellitus than in HD patients without diabetes mellitus (P for interaction = 0.02), indicating that the predictive power of VAP-1 may differ based on the presence of diabetes mellitus. Considering that more HD patients are developing diabetes mellitus (34, 35), a lower predictive power of plasma VAP-1 levels in this subpopulation could be a disadvantage as a prognostic biomarker of CV events. Although the underlying mechanism of this phenomenon could not be assessed in this study, we speculate that hyperglycemia-induced upregulation of unfavorable molecules, such as plasma proinflammatory cytokines and advanced glycation end products, reduce the relative contributions of plasma VAP-1 to the incidence of CV events (36, 37).
The clinical utility of NT-proBNP as a cardiac biomarker in HD patients had been inconclusive, partly because of intra-patient variations and different cut-off values used across studies (38–40). An observational study also reported that elevated NT-proBNP levels are likely caused by intravascular volume expansion rather than cardiac dysfunction in stable HD patients, further complicating the interpretations of its clinical value in HD patients with hypervolemia (41). Our subgroup analysis showed that increased VAP-1 and NT-proBNP levels were associated with a significantly higher risk of composite CV events, whereas isolated NT-proBNP levels were not (Table 4). These findings indicate that plasma VAP-1 levels can help differentiate those at a high risk of adverse CV outcomes among HD patients exhibiting increased baseline NT-proBNP levels.
Our study had some limitations. Analyses for individual CV events could not be performed because of the limited number of events and short follow-up period. Echocardiographic data were obtained in a small proportion of enrolled patients (49.3%), although the analysis of available data revealed an evident association between high plasma VAP-1 levels and LV diastolic dysfunction. Moreover, multivariable analysis might not have controlled all relevant confounding factors, and thus these were not thoroughly assessed in this study.
In conclusion, our study demonstrated that plasma VAP-1 levels were associated with circulating markers of cardiac remodeling, as well as a greater risk of LV diastolic dysfunction. In addition, higher plasma VAP-1 levels were correlated with an increased risk of future CV events in HD patients. Our results indicate that VAP-1 might help overcome the limitations of traditional risk factors in the setting of end-stage renal disease and help clinicians identify HD patients at a high risk of CV events.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by KyungHee University Hospital IRB. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DKK, YHL, and HSH constructed the research questions and designed the analysis. JSK, YGK, S-YL, SYA, D-YL, KHJ, S-HL, and J-YM conducted the data collection. DKK, YHL, J-YM, and HSH drafted the manuscript. All authors reviewed the results, commented on the manuscript, read, and approved the final manuscript.
Funding
This research was supported by grants from the Patient-Centered Clinical Research Coordinating Center, which was funded by the Ministry of Health & Welfare, Republic of Korea (H19C0481 and HC19C0041).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All authors acknowledge the support from Patient-Centered Clinical Research Coordinating Center funded by the Ministry of Health and Welfare, Republic of Korea (H19C0481 and HC19C0041).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.701079/full#supplementary-material
References
1. Rysz J, Franczyk B, Lawinski J, Gluba-Brzozka A. Oxidative stress in ESRD patients on dialysis and the risk of cardiovascular diseases. Antioxidants. (2020) 9:1079. doi: 10.3390/antiox9111079
2. Russa D, Pellegrino D, Montesanto A, Gigliotti P, Perri A, Russa A, et al. Oxidative balance and inflammation in hemodialysis patients: biomarkers of cardiovascular risk? Oxid Med Cell Longev. (2019) 2019:8567275. doi: 10.1155/2019/8567275
3. Hwang HS, Kim JS, Kim YG, Lee SY, Ahn SY, Lee HJ, et al. Circulating PCSK9 level and risk of cardiovascular events and death in hemodialysis patients. J Clin Med. (2020) 9:244. doi: 10.3390/jcm9010244
4. Verma S, Singh P, Khurana S, Ganguly NK, Kukreti R, Saso L, et al. Implications of oxidative stress in chronic kidney disease: a review on current concepts and therapies. Kidney Res Clin Pract. (2021) 40:183–93. doi: 10.23876/j.krcp.20.163
5. Sasaki K, Shoji T, Kabata D, Shintani A, Okute Y, Tsuchikura S, et al. Oxidative stress and inflammation as predictors of mortality and cardiovascular events in hemodialysis patients: the DREAM cohort. J Atheroscler Thromb. (2021) 28:249–60. doi: 10.5551/jat.56069
6. Ren H, Zhou X, Luan Z, Luo X, Han S, Cai Q, et al. The relationship between carotid atherosclerosis, inflammatory cytokines, and oxidative stress in middle-aged and elderly hemodialysis patients. Int J Nephrol. (2013) 2013:835465. doi: 10.1155/2013/835465
7. Hwang HS, Kim JS, Kim YG, Lee YH, Lee DY, Ahn SY, et al. Circulating neprilysin level predicts the risk of cardiovascular events in hemodialysis patients. Front Cardiovasc Med. (2021) 8:684297. doi: 10.3389/fcvm.2021.684297
8. Cozzolino M, Mangano M, Stucchi A, Ciceri P, Conte F, Galassi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. (2018) 33:iii28–34. doi: 10.1093/ndt/gfy174
9. Noonan T, Lukas S, Peet GW, Pelletier J, Panzenbeck M, Hanidu A, et al. The oxidase activity of vascular adhesion protein-1 (VAP-1) is essential for function. Am J Clin Exp Immunol. (2013) 2:172–85.
10. Salmi M, Jalkanen S. VAP-1: an adhesin and an enzyme. Trends Immunol. (2001) 22:211–6. doi: 10.1016/S1471-4906(01)01870-1
11. Salmi M, Jalkanen S. Vascular adhesion protein-1: a cell surface amine oxidase in translation. Antioxid Redox Signal. (2019) 30:314–32. doi: 10.1089/ars.2017.7418
12. Aalto K, Havulinna AS, Jalkanen S, Salomaa V, Salmi M. Soluble vascular adhesion protein-1 predicts incident major adverse cardiovascular events and improves reclassification in a finnish prospective cohort study. Circ Cardiovasc Genet. (2014) 7:529–35. doi: 10.1161/CIRCGENETICS.113.000543
13. Boomsma F, de Kam PJ, Tjeerdsma G, van den Meiracker AH, van Veldhuisen DJ. Plasma semicarbazide-sensitive amine oxidase (SSAO) is an independent prognostic marker for mortality in chronic heart failure. Eur Heart J. (2000) 21:1859–63. doi: 10.1053/euhj.2000.2176
14. Pannecoeck R, Serruys D, Benmeridja L, Delanghe JR, van Geel N, Speeckaert R, et al. Vascular adhesion protein-1: role in human pathology and application as a biomarker. Crit Rev Clin Lab Sci. (2015) 52:284–300. doi: 10.3109/10408363.2015.1050714
15. Wong MY, Saad S, Pollock C, Wong MG. Semicarbazide-sensitive amine oxidase and kidney disease. Am J Physiol Renal Physiol. (2013) 305:F1637–44. doi: 10.1152/ajprenal.00416.2013
16. Nemcsik J, Szoko E, Soltesz Z, Fodor E, Toth L, Egresits J, et al. Alteration of serum semicarbazide-sensitive amine oxidase activity in chronic renal failure. J Neural Transm. (2007) 114:841–3. doi: 10.1007/s00702-007-0698-4
17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
18. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
19. Aalto K, Maksimow M, Juonala M, Viikari J, Jula A, Kahonen M, et al. Soluble vascular adhesion protein-1 correlates with cardiovascular risk factors and early atherosclerotic manifestations. Arterioscler Thromb Vasc Biol. (2012) 32:523–32. doi: 10.1161/ATVBAHA.111.238030
20. Boomsma F, van den Meiracker AH, Winkel S, Aanstoot HJ, Batstra MR, Man in 't Veld AJ, et al. Circulating semicarbazide-sensitive amine oxidase is raised both in type I (insulin-dependent), in type II (non-insulin-dependent) diabetes mellitus and even in childhood type I diabetes at first clinical diagnosis. Diabetologia. (1999) 42:233–7. doi: 10.1007/s001250051143
21. Boomsma F, van Veldhuisen DJ, de Kam PJ, Man in't Veld AJ, Mosterd A, Lie KI, et al. Plasma semicarbazide-sensitive amine oxidase is elevated in patients with congestive heart failure. Cardiovasc Res. (1997) 33:387–91. doi: 10.1016/S0008-6363(96)00209-X
22. Koc-Zorawska E, Malyszko J, Zbroch E, Malyszko J, Mysliwiec M. Vascular adhesion protein-1 and renalase in regard to diabetes in hemodialysis patients. Arch Med Sci. (2012) 8:1048–52. doi: 10.5114/aoms.2012.32413
23. Maciorkowska D, Zbroch E, Malyszko J. Circulating renalase, catecholamines, and vascular adhesion protein 1 in hypertensive patients. J Am Soc Hypertens. (2015) 9:855–64. doi: 10.1016/j.jash.2015.08.002
24. Antlanger M, Aschauer S, Kopecky C, Hecking M, Kovarik JJ, Werzowa J, et al. Heart failure with preserved and reduced ejection fraction in hemodialysis patients: prevalence, disease prediction and prognosis. Kidney Blood Press Res. (2017) 42:165–76. doi: 10.1159/000473868
25. Unger ED, Dubin RF, Deo R, Daruwalla V, Friedman JL, Medina C, et al. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. (2016) 18:103–12. doi: 10.1002/ejhf.445
26. Han JH, Han JS, Kim EJ, Doh FM, Koo HM, Kim CH, et al. Diastolic dysfunction is an independent predictor of cardiovascular events in incident dialysis patients with preserved systolic function. PLoS ONE. (2015) 10:e0118694. doi: 10.1371/journal.pone.0118694
27. Smith DH, Thorp ML, Gurwitz JH, McManus DD, Goldberg RJ, Allen LA, et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes. (2013) 6:333–42. doi: 10.1161/CIRCOUTCOMES.113.000221
28. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. (2006) 113:671–8. doi: 10.1161/CIRCULATIONAHA.105.580506
29. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. (2016) 29:277–314. doi: 10.1016/j.echo.2016.01.011
30. Han SS, Cho GY, Park YS, Baek SH, Ahn SY, Kim S, et al. Predictive value of echocardiographic parameters for clinical events in patients starting hemodialysis. J Korean Med Sci. (2015) 30:44–53. doi: 10.3346/jkms.2015.30.1.44
31. Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. (2006) 113:2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865
32. Zile MR, Jhund PS, Baicu CF, Claggett BL, Pieske B, Voors AA, et al. Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction: data from the prospective comparison of ARNI with ARB on management of heart failure with preserved ejection fraction study. Circ Heart Fail. (2016) 9:e002551 doi: 10.1161/CIRCHEARTFAILURE.115.002551
33. Kobusiak-Prokopowicz M, Krzysztofik J, Kaaz K, Jolda-Mydlowska B, Mysiak A. MMP-2 and TIMP-2 in patients with heart failure and chronic kidney disease. Open Med. (2018) 13:237–46. doi: 10.1515/med-2018-0037
34. Oh KH, Kang M, Kang E, Ryu H, Han SH, Yoo TH, et al. The KNOW-CKD study: what we have learned about chronic kidney diseases. Kidney Res Clin Pract. (2020) 39:121–35. doi: 10.23876/j.krcp.20.042
35. Jeon HJ, Bae HJ, Ham YR, Choi DE, Na KR, Ahn MS, et al. Outcomes of end-stage renal disease patients on the waiting list for deceased donor kidney transplantation: a single-center study. Kidney Res Clin Pract. (2019) 38:116–23. doi: 10.23876/j.krcp.18.0068
36. Schottker B, Herder C, Rothenbacher D, Roden M, Kolb H, Muller H, et al. Proinflammatory cytokines, adiponectin, and increased risk of primary cardiovascular events in diabetic patients with or without renal dysfunction: results from the ESTHER study. Diabetes Care. (2013) 36:1703–11. doi: 10.2337/dc12-1416
37. Hegab Z, Gibbons S, Neyses L, Mamas MA. Role of advanced glycation end products in cardiovascular disease. World J Cardiol. (2012) 4:90–102. doi: 10.4330/wjc.v4.i4.90
38. Fahim MA, Hayen A, Horvath AR, Dimeski G, Coburn A, Johnson DW, et al. N-terminal pro-B-type natriuretic peptide variability in stable dialysis patients. Clin J Am Soc Nephrol. (2015) 10:620–9. doi: 10.2215/CJN.09060914
39. Madsen LH, Ladefoged S, Corell P, Schou M, Hildebrandt PR, Atar D. N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease in hemodialysis. Kidney Int. (2007) 71:548–54. doi: 10.1038/sj.ki.5002087
40. Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, et al. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis. (2005) 46:610–20. doi: 10.1053/j.ajkd.2005.06.017
Keywords: vascular adhesion protein-1, hemodialysis, cardiovascular disease, endothelial dysfunction, diastolic dysfunction
Citation: Kim DK, Lee YH, Kim JS, Kim YG, Lee S-Y, Ahn SY, Lee D-Y, Jeong KH, Lee S-H, Hwang HS and Moon J-Y (2021) Circulating Vascular Adhesion Protein-1 Level Predicts the Risk of Cardiovascular Events and Mortality in Hemodialysis Patients. Front. Cardiovasc. Med. 8:701079. doi: 10.3389/fcvm.2021.701079
Received: 27 April 2021; Accepted: 13 August 2021;
Published: 07 September 2021.
Edited by:
Alessio Molfino, Sapienza University of Rome, ItalyCopyright © 2021 Kim, Lee, Kim, Kim, Lee, Ahn, Lee, Jeong, Lee, Hwang and Moon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyeon Seok Hwang, aHdhbmdoc25lQGdtYWlsLmNvbQ==; Ju-Young Moon, anltb29uQGtodS5hYy5rcg==
†These authors have contributed equally to this work and share first authorship
 Dae Kyu Kim
Dae Kyu Kim Yu Ho Lee
Yu Ho Lee Jin Sug Kim
Jin Sug Kim Yang Gyun Kim
Yang Gyun Kim So-Young Lee2
So-Young Lee2 Dong-Young Lee
Dong-Young Lee Sang-Ho Lee
Sang-Ho Lee Ju-Young Moon
Ju-Young Moon