- 1Division of Cardiology, Chungbuk National University Hospital, Cheongju, South Korea
- 2Division of Cardiology, Yonsei University Health System, Seoul, South Korea
Background: It is unclear whether atrial fibrillation (AF) catheter ablation (AFCA) improves the left ventricular (LV) diastolic function. We evaluated the 1-year change in the H2FPEF score, which reflects the degree of LV diastolic function, after AFCA among patients with a normal LV systolic function.
Methods and Results: We included 1,471 patients (30.7% female, median age 60 years, paroxysmal-type AF 68.6%) who had available H2FPEF scores at baseline and at 1-year after AFCA to evaluate the 1-year change in the H2FPEF score (ΔH2FPEF score[1−yr]) after AFCA. Baseline high H2FPEF scores (≥6) were independently associated with the female sex, left atrium (LA) diameter, LV mass index, pericardial fat volume, and a low estimated glomerular filtration rate. One year after AFCA, decreased ΔH2FPEF scores[1−yr] were associated with baseline H2FPEF scores of ≥6 [OR, 4.19 (95% CI, 2.88–6.11), p < 0.001], no diabetes [OR, 0.60 (95% CI, 0.37–0.98), p = 0.04], and lower pericardial fat volume [OR, 0.99 (95% CI, 0.99–1.00), p = 0.003]. Increased ΔH2FPEF scores[1−yr] were associated with a baseline H2FPEF score of <6 [OR, 3.54 (95% CI, 2.08–6.04), p < 0.001] and sustained AF after a recurrence within 1 year [SustainAF[1−yr]; OR, 1.89 (95% CI, 1.01–3.54), p = 0.048]. Throughout a 56-month median follow-up, an increased ΔH2FPEF score[1−yr] resulted in a poorer rhythm outcome of AFCA (at 1 year, log-rank p = 0.003; long-term, log-rank p = 0.010).
Conclusions: AFCA appears to improve LV diastolic dysfunction. However, SustainAF[1−yr] may contribute to worsening LV diastolic dysfunction, and it was shown by increased ΔH2FPEF scores[1−yr], which was independently associated with higher risk of AF recurrence rate after AFCA.
Clinical Trial Registration: ClinicalTrials.gov Identifier: NCT02138695.
Introduction
Atrial fibrillation (AF) and underlying heart failure (HF) have been emerging topics of importance in the field of cardiovascular disease over the past 3 decades and frequently overlap (1, 2). Specifically, AF has been shown to follow HF with preserved ejection fraction (HFpEF) more frequently than HF with reduced ejection fraction (HFrEF) due to the differences in the left ventricular (LV) diastolic dysfunction and the left atrial (LA) remodeling process (2, 3). Prior studies have shown improvements in the LV systolic function (4), performance and quality of life (5), and mortality (6) after AF catheter ablation (AFCA) in HFrEF patients, suggesting that a reduction in AF may be sufficient for a clinical benefit. Nevertheless, there are no specific recommendations for the management of AF in HFpEF patients, and data regarding the efficacya of AFCA in patients with a normal LV systolic function and LV diastolic dysfunction are relatively limited. Although there have been a few studies reporting an improvement in the LV diastolic function after AFCA by maintaining sinus rhythm (7, 8), they adopted conventional approaches that used mainly symptoms and the LV ejection fraction (LVEF) for the diagnosis of HFpEF with various diagnostic accuracies (9). Recently, a novel scoring system has been developed, the H2FPEF score (10), which can estimate the probability of the underlying HFpEF through six clinical and echocardiographic characteristics, and can be feasibly applied in clinical practice. The aim of this study was to better understand the factors by which LV diastolic function worsens or improves after AF rhythm control by AFCA. In this study, we used the H2FPEF score at two time points, before and 1 year after the AFCA. We aimed to compare the cardiac structural and functional changes within a year and to evaluate the rhythm outcomes both within a year and over the long-term using the H2FPEF score.
Materials and Methods
Study Subjects
The study protocol adhered to the Declaration of Helsinki and was approved by the institutional review board of the Yonsei University Health system. All patients provided written informed consent for inclusion in the Yonsei AF Ablation Cohort Database (ClinicalTrials.gov Identifier: NCT02138695). From January 2009 to September 2019, 1,471 patients with a diagnosis of AF and a normal LVEF were identified as having clinical and echocardiographic information for the calculation of the H2FPEF score before AFCA and 1 year after AFCA. All patients underwent AFCA, and the indications for the AFCA complied with the latest guidelines (11). The exclusion criteria for the study were as follows: (1) a reduced LVEF, defined as <50%; (2) a follow-up duration <12 months; and (3) a repeat ablation within a year (Figure 1).
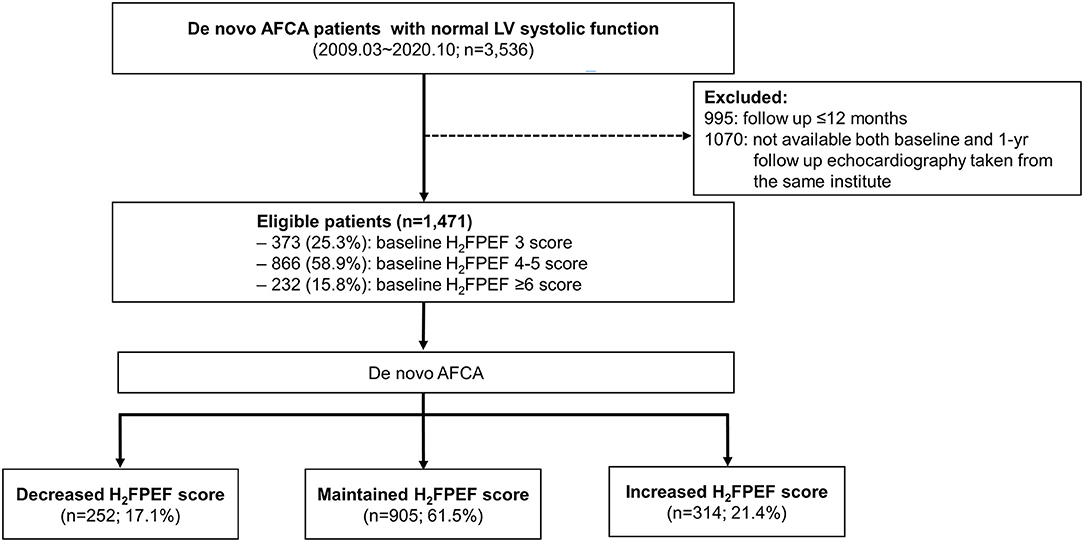
Figure 1. Screening and study flowchart of the patient selection. AF, atrial fibrillation; AFCA, atrial fibrillation catheter ablation; LV, left ventricular.
Calculating the H2FPEF Score at Baseline and 1 Year After the Atrial Fibrillation Catheter Ablation
The H2FPEF score has six domains based on clinical and echocardiographic values: heaviness (body mass index >30 kg/m2, 2 points), hypertension (on two or more antihypertensive medicines, 1 point), atrial fibrillation (paroxysmal or persistent, 3 points), pulmonary hypertension (Doppler echocardiographic estimated pulmonary artery systolic pressure >35 mmHg, 1 point), elderly status (age >60 years, 1 point), and filling pressure (Doppler echocardiographic E/Em > 9, 1 point). The baseline H2FPEF scores were obtained within 3 months prior to the AFCA, and the 1-year H2FPEF scores were obtained 1 year after the AFCA with all clinical and echocardiographic values.
Echocardiographic Measurement and Three-Dimensional Computed Tomography
Transthoracic echocardiography was conducted in all patients using commercially available devices (Vivid 7 or Vivid E9 from GE Healthcare, Chicago, IL, USA, or iE 33 from Philips, Amsterdam, the Netherlands) as recommended by the American Society of Echocardiography (12). Standard images were obtained in the parasternal and apical views through two-dimensional (2D), Doppler, and M-mode imaging, including the LA anteroposterior diameter and the LV end-systolic and end-diastolic dimensions (LVESD and LVEDD). The early Doppler mitral inflow (E) was recorded using pulsed waves from the apical window, with a 1- to 3-mm pulsed Doppler sample volume placed between the tips and mitral leaflets during diastole. The early diastolic mitral annular velocity (Em) was recorded as the peak early diastolic tissue velocity using color Doppler tissue imaging of the septal mitral annulus. The ratio of the early diastolic mitral inflow velocity to the early diastolic mitral annular velocity (E/Em) was calculated. Tricuspid regurgitation (TR) and estimated right atrial (RA) pressure were evaluated using the recommended methods, and the right ventricular systolic pressure (RVSP) was calculated as 4 × (TR jet)2 + estimated RA pressure (13). The initial and 1 year after AFCA, the echocardiographies used to estimate the H2FPEF scores were those performed during an elective visit on stable medication.
Three-dimensional spiral computed tomography (CT) (64-channel, Light Speed Volume CT from GE Healthcare, Chicago, IL, USA, or Brilliance 63 from Philips, Amsterdam, the Netherlands) was performed in all patients, and the scans were analyzed using an imaging-processing workstation (Aquarius; TeraRecon, Inc., Foster City, CA, USA). The LA volume and pericardial fat volume measurements have been described in previous studies (14, 15).
Electrophysiologic Characterization and Radiofrequency Catheter Ablation
Intracardiac electrograms were obtained using the Prucka CardioLab™ Electrophysiology system (GE Healthcare, Chicago, IL, USA). A 3D electro-anatomical map (Ensite NavX; Abbott Laboratories, Chicago, IL, USA; CARTO3; Johnson & Johnson Inc., USA) was generated using a circumferential pulmonary vein-mapping catheter through a long sheath (Schwartz left 1; Abbott Laboratories, Chicago, IL, USA) and by merging the 3D geometry generated by the electroanatomic mapping system with the corresponding 3D spiral CT images. Left atrium electrogram voltage maps were generated during high right atrial pacing at 500 ms to prevent rate-dependent activation changes and by measuring mean peak-to-peak voltage as previously described (16). All patients underwent a de novo procedure with a circumferential pulmonary vein isolation (CPVI). The endpoint of the CPVI was the electric isolation of the PV potentials and bidirectional block of the PVs. We tested whether there was an immediate recurrence of AF within 10 min after cardioversion with an isoproterenol infusion (5–20 μg/min depending on the ß-blocker used with a target sinus heart rate of 120 bpm) to find extra-PV foci triggers, then confirmed successful CPVI 30 min after the initial isolation. Extra-PV foci triggers under an isoproterenol infusion were ablated as much as possible if they were consistent and reproducible. Then, we ended the de novo procedure. The detailed procedural techniques and strategies for the AFCA have been presented in our previous studies (17, 18).
Post-ablation Management and Rhythm Follow-Up
The patients were discharged without any antiarrhythmic drugs (AADs) with the exception of those who had symptomatic frequent atrial premature beats, non-sustained atrial tachycardia (AT), early recurrence of AF on telemetry during the admission period, or recurrent extra-PV foci triggers after the AFCA procedure (13.7%). The clinical and cardiac rhythm information was obtained regularly from an outpatient clinic at 1, 3, 6, and 12 months, and every 6 months thereafter (or whenever symptoms developed). All patients underwent electrocardiogram recordings at every visit, and 24-h Holter monitoring was performed at 3 and 6 months, and every 6 months thereafter, according to the latest guidelines (11). AF recurrence was defined as any episode of AF or AT of at least 30 s in duration. Any ECG documentation of an AF recurrence within the 3-month blanking period was diagnosed as an early recurrence, and an AF recurrence of more than 3 months after the AFCA was diagnosed as a clinical recurrence. We evaluated the time point of the clinical recurrence as follows: within 1 year as a short-term and beyond 1 year as a long-term recurrence. We also estimated the quality of the AF control after the AFCA. We defined patients with sustained AF/AT as those who remained in a sustaining AF/AT rhythm (>30 s) on the final follow-up after the AFCA despite AADs or electrical cardioversion.
Statistical Analysis
The baseline characteristics of the patients were compared using descriptive statistics and presented as median (interquartile interval) values for continuous variables and as numbers (percentages) for categorical variables. To compare the baseline characteristics according to the baseline H2FPEF and the 1-year change in the H2FPEF score (ΔH2FPEF score[1−yr]) categories, the Mantel–Haenszel chi-squared test was used for categorical variables, and the Kruskal–Wallis H test was used for continuous variables. To identify the factors associated with the baseline H2FPEF and ΔH2FPEF score[1−yr], univariate and multivariable logistic regression analyses were performed. Multivariable Cox proportional hazard analyses were performed to evaluate the association of the baseline H2FPEF and ΔH2FPEF score[1−yr] with a clinical recurrence in both the short- and long-term periods. A multivariable regression analysis included those variables with significant p-values of < 0.1 in the univariate analysis. The Cox proportional hazards assumption was tested based on Schoenfeld residuals. Two-sided p-values < 0.05 were considered statistically significant. The statistical analyses were conducted using SAS version 9.4 (SAS Institute) and R version 4.0.0 (R Foundation for Statistical Computing) software.
Results
Characteristics of the Patients With High H2FPEF Scores
Table 1 summarizes patient characteristics depending on the H2FPEF score. In the 1,471 patients, the median (IQR) age was 60 (53, 68) years, 30.7% were female, and 68.6% had paroxysmal-type AF. The baseline H2FPEF scores were 3 points in 373 patients (25.3%), 4–5 points in 866 (58.9%), and ≥6 points in 232 (15.8%) patients. Patients with higher H2FPEF scores were older and had a higher body mass index, hypertension, estimated RVSP, and higher E/Em, as those variables that composed this score. In the higher H2FPEF score-group, the CHA2DS2-VASc score (p < 0.001), prevalence of diabetes (p < 0.001), a prior stroke (p = 0.004), vascular disease (p < 0.001), or hypertrophic cardiomyopathy (p = 0.007) were higher. The CT-measured LA volume (p < 0.001), pericardial fat volume (p < 0.001), and LA peak pressure (p = 0.004) were higher, and the endocardial bipolar LA voltage (p < 0.001) and eGFR (p < 0.001) were significantly lower in the higher H2FPEF score-group. In the multivariate logistic regression analysis (Supplementary Table 1), high baseline H2FPEF scores (≥6) were independently associated with the female sex [OR, 2.31 (1.35–3.93), p = 0.002], higher left atrial (LA) diameter [OR, 1.09 (1.04–1.13), p < 0.001], LV mass index [OR 1.02 (1.01–1.03), p = 0.002], pericardial fat volume [OR, 1.01 (1.00–1.01), p = 0.015], and lower eGFR [OR, 0.98 (0.97–0.99), p < 0.001].
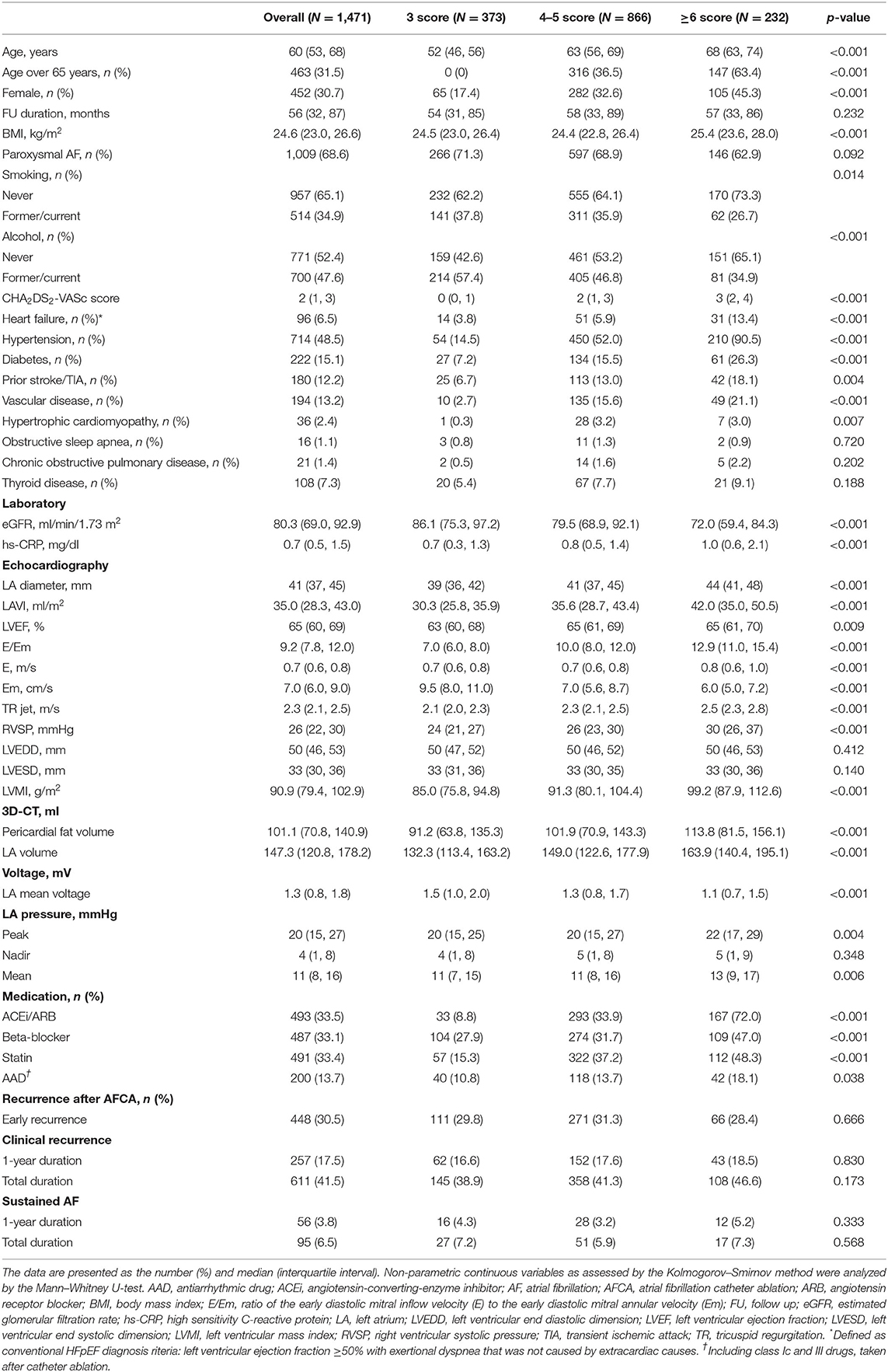
Table 1. Baseline characteristics according to the baseline H2FPEF score stratification in atrial fibrillation (AF) patients with a normal left ventricular ejection fraction (LVEF).
ΔH2FPEF Score[1-yr]
One year after the AFCA, the H2FPEF scores decreased in 17.1% of the patients (252), were maintained in 61.5% (905), and increased in 21.4% (314) (Table 2). A reduction in the ΔH2FPEF score[1−yr] was more commonly observed in patients with high baseline H2FPEF scores (Figure 2A) and was independently associated with baseline H2FPEF scores of ≥6 [OR, 4.19 (2.88–6.11), p < 0.001], the absence of diabetes [OR, 0.60 (0.37–0.98), p = 0.04], a higher LVEF [OR, 1.03 (1.01–1.06), p = 0.011], and a lower pericardial fat volume [OR, 0.99 (0.99–1.00), p = 0.003; Table 3]. Increased ΔH2FPEF scores[1−yr] were less commonly observed in patients with high H2FPEF scores (Figure 2B) and were associated with a baseline H2FPEF score of <6 [OR, 3.54 (2.08–6.04), p < 0.001], sustained AF after a recurrence within a year [SustainAF[1−yr]; OR, 1.89 (1.01–3.54), p = 0.048], the LA volume [OR, 1.00 (1.00–1.01), p = 0.029], and the pericardial fat volume [OR, 1.00 (1.00–1.01), p = 0.032; Table 4].
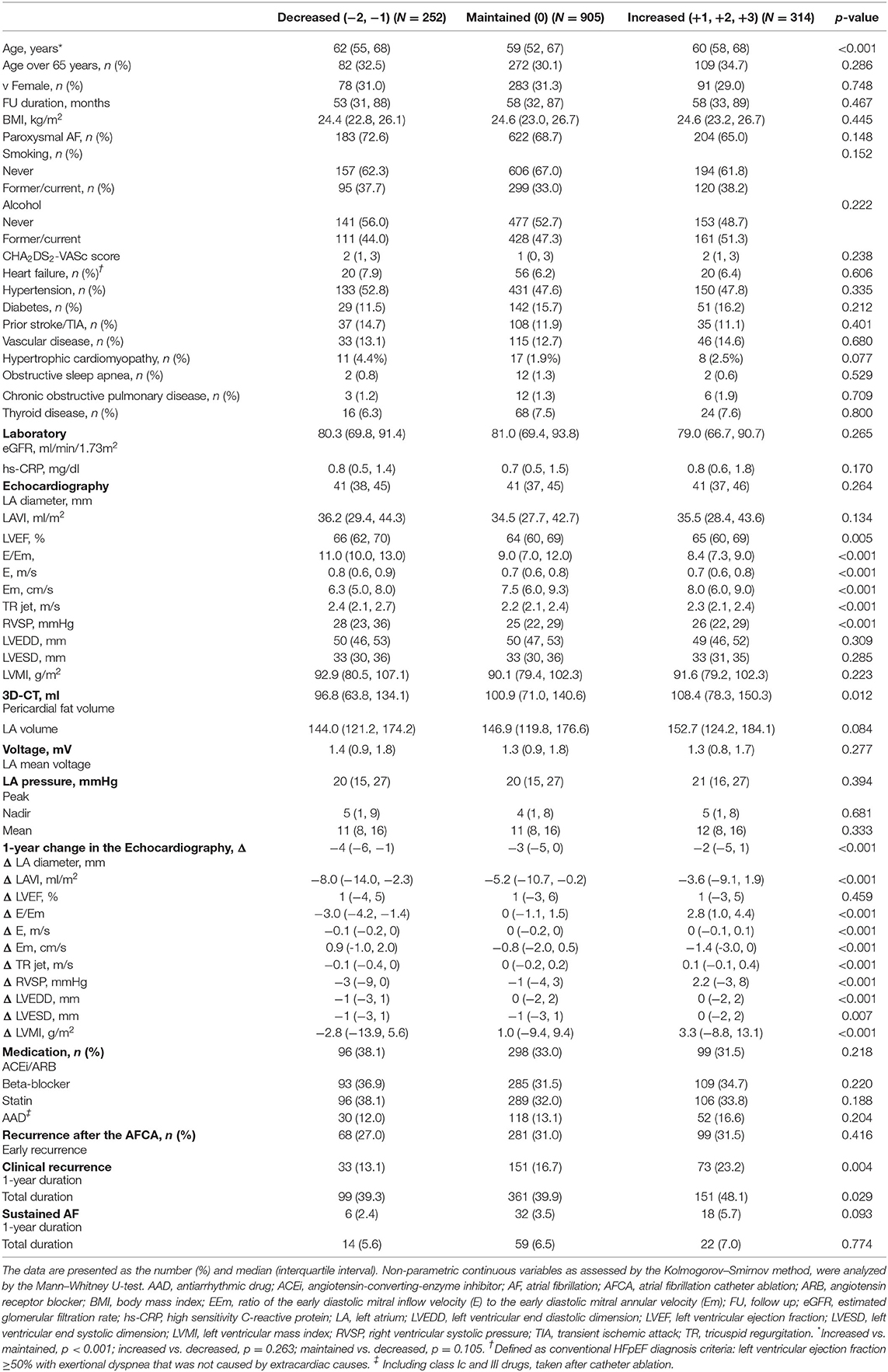
Table 2. Baseline characteristics according to the change in the H2FPEF scores in AF patients with a normal LVEF, 1-year after the atrial fibrillation catheter ablation (AFCA).
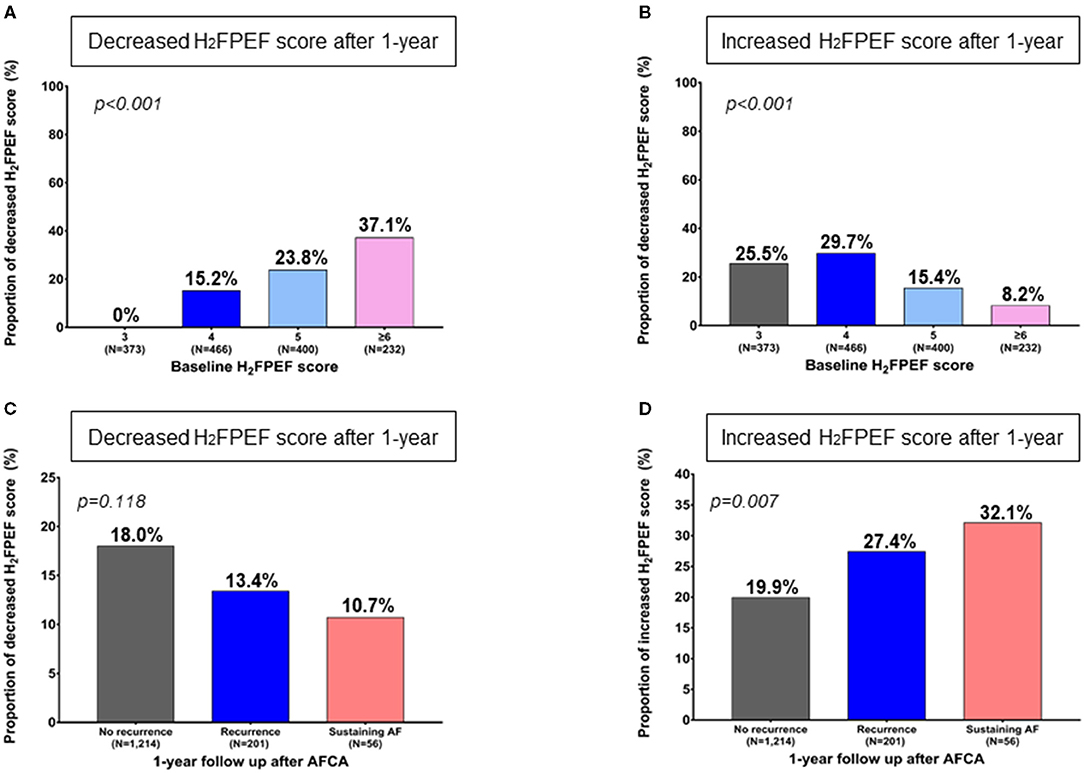
Figure 2. Change in the 1-year H2FPEF scores after the AFCA, according to the baseline H2FPEF scores (A, B) and rhythm outcomes within a year C, D). AF, atrial fibrillation; AFCA, atrial fibrillation catheter ablation.
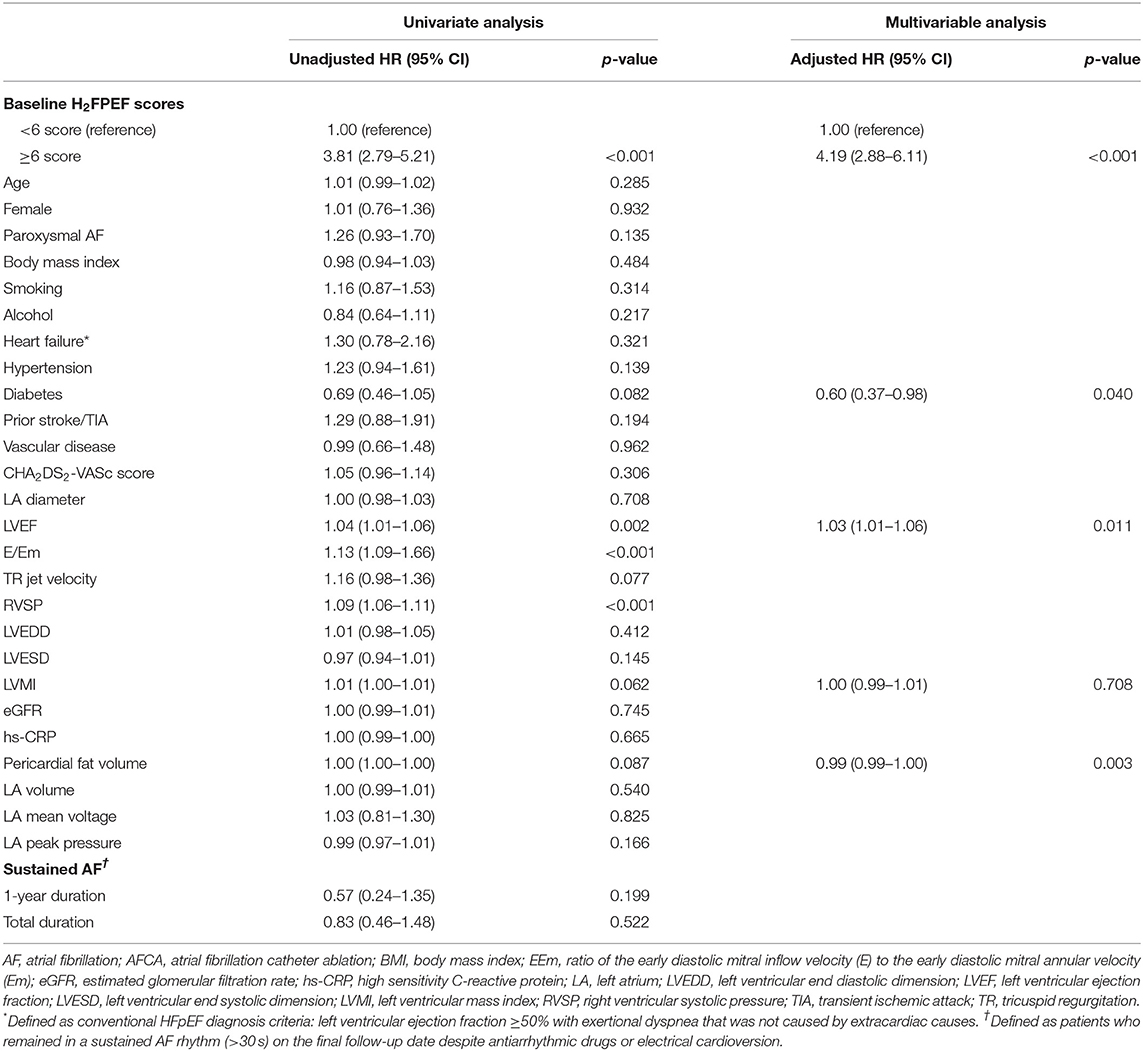
Table 3. Logistic regression analysis for predictors of decreased H2FPEF scores, 1-year after the AFCA.
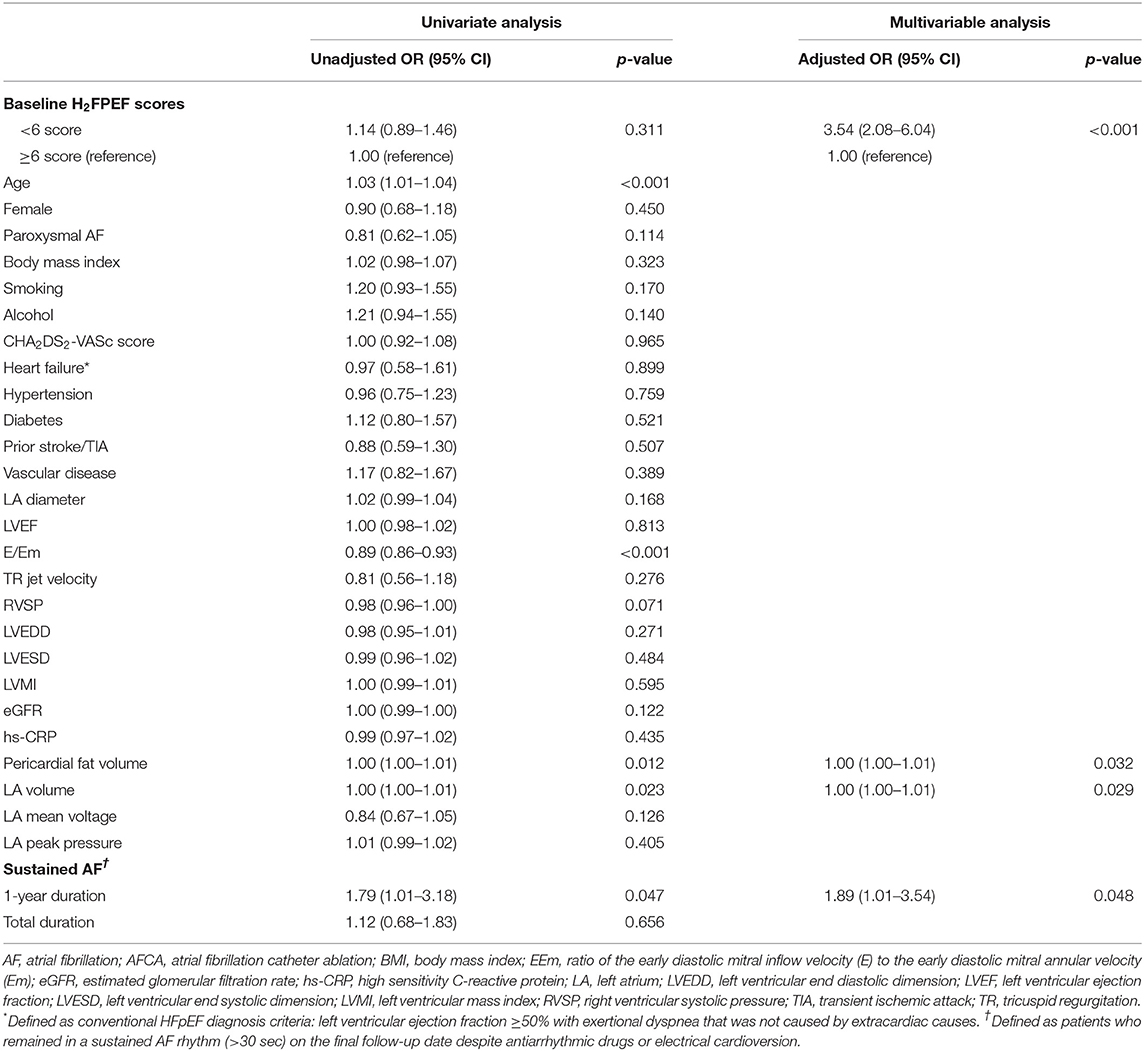
Table 4. Logistic regression analysis for predictors of increased H2FPEF scores, 1-year after the AFCA.
Rhythm Outcomes After Atrial Fibrillation Catheter Ablation and the H2FPEF Score
Because we evaluated the H2FPEF score before and 1 year after the procedure, we compared the 1-year and long-term clinical recurrence rates of AF separately, depending on the baseline H2FPEF scores and ΔH2FPEF scores[1−yr]. In contrast, the baseline H2FPEF scores did not affect the 1-year rhythm outcome (log rank, p = 0.82; Figure 3A), and the clinical recurrence of AF was significantly higher in the patients with an increased ΔH2FPEF scores[1−yr] (log rank, p = 0.003; Figure 3B). In the multivariate Cox regression analysis, increased ΔH2FPEF scores[1−yr] [HR, 2.34 (1.36–4.03), p = 0.002] and persistent AF [HR, 1.43 (1.01–2.03), p = 0.043] were independently associated with an AF recurrence within a year (Supplementary Table 2). During the median follow-up of 56 (32, 87) months, increased ΔH2FPEF scores[1−yr] [HR, 1.41 (1.01–1.98), p = 0.045], the LA diameter [HR, 1.03 (1.01–1.06), p = 0.002], and the LA voltage [HR, 0.59 (0.48–0.73), p < 0.001] were independently associated with a long-term AF recurrence (Table 5). The rhythm outcomes in the overall duration were consistent with the 1-year rhythm outcome depending on the baseline H2FPEF score (log rank, p = 0.57; Figure 3C) or ΔH2FPEF score[1−yr] (log rank, p = 0.01; Figure 3D). In the subgroup of patients with baseline H2FPEF scores of ≥5, the risk of an AF recurrence was significantly higher in the patients with increased ΔH2FPEF scores[1−yr] than in those with reduced ΔH2FPEF scores[1−yr] (Supplementary Figure 1).
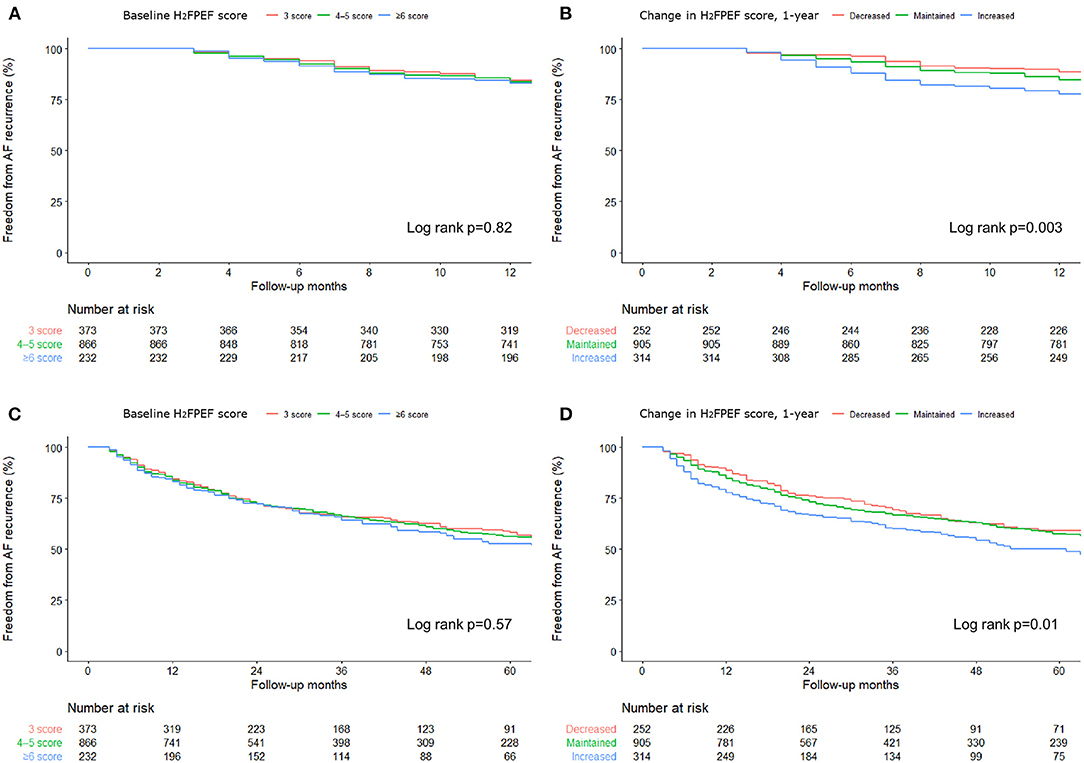
Figure 3. Rate of the freedom from an AF recurrence over 1-year (A, B) and during the long-term (C, D) after the AFCA based on the baseline H2FPEF scores and changes in the 1-year H2FPEF scores.
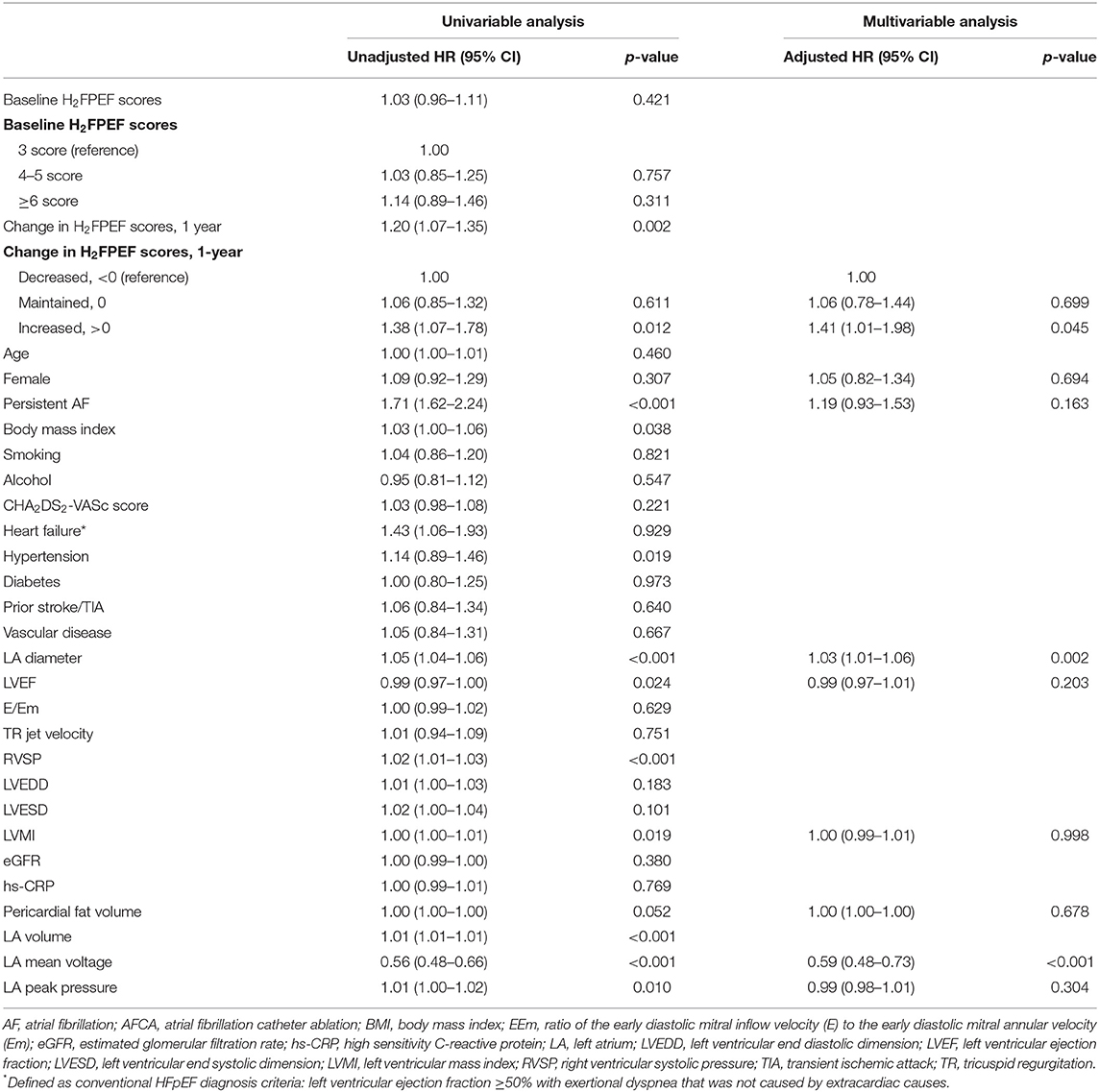
Table 5. Cox regression analysis of the predictors of an AF recurrence after the AFCA, during the long-term follow up.
Failed Rhythm Control and the ΔH2FPEF Score[1-yr]
Among the 1,471 patients, 257 (17.5%) had an AF recurrence within a year, and 201 (13.7%) had sinus rhythm restored after using antiarrhythmic drugs, but 56 (3.8%) patients had sustained AF under antiarrhythmic drugs even after cardioversion. The proportion of patients with reduced ΔH2FPEF scores[1−yr] tended to be higher without a statistical significance among those with no recurrence (p = 0.118, Figure 2C). However, the proportion of patients with increased ΔH2FPEF scores[1−yr] was significantly higher in the group with sustained AF after a recurrence than in those with sinus rhythm maintained at a year after the AFCA (p = 0.007, Figure 2D).
Discussion
Main Findings
In this study, we observed a change in the H2FPEF score 1 year after the AFCA in AF patients with a normal LV systolic function. The H2FPEF score[1−yr] decreased in 17% of the patients but increased in 21% a year after the AFCA. A high baseline H2FPEF score, which is related to LV diastolic dysfunction, was independently associated with a reduced ΔH2FPEF score[1−yr]. On the other hand, low baseline scores or sustained AF after a recurrence were significantly associated with an increase in the ΔH2FPEF scores[1−yr] after the AFCA. Patients with an increased ΔH2FPEF score[1−yr] had higher rates of recurrence within a year or longer. Therefore, AFCA improved the H2FPEF scores[1−yr] in patients with baseline LV diastolic dysfunction; however, patients with a poor rhythm control and sustained AF despite AFCA had a significant increase in the ΔH2FPEF scores[1−yr].
Atrial Fibrillation and the Ventricular Diastolic Function
AF and LV diastolic dysfunction are closely related and have important features in common, such as age, obesity, hypertension, and diabetes (19). LV diastolic dysfunction has deteriorative effects on the atrial function and structure, which contributes to the development, progression, and maintenance of AF (20). Consequently, AF has an influence on the LV function and LA remodeling (21), and can lead to increasingly sustained AF episodes. Therefore, these two conditions have pathophysiological effects on the occurrence and aggravation of each, and coexistence is associated with a poor prognosis (2, 22, 23). Furthermore, as the AF burden increases chronically, the right ventricular function progressively worsens (24). Reddy et al. (10) proposed a novel risk score, the H2FPEF score, which includes all of the abovementioned factors, to diagnose HFpEF patients. We adopted this score to evaluate the prognostic utility in AF patients with a normal LVEF after AFCA.
Effects of Atrial Fibrillation Catheter Ablation on the Left Venticular Diastolic Function
The data to support the clinical benefits of catheter ablation in symptomatic AF patients with HFrEF are strong and compatible with those from previous clinical trials and meta-analyses (6, 25). However, AF is more potently associated with HFpEF (2) with the prevalence of HFpEF increasing by almost half in AF patients (26). The benefits of AFCA in symptomatic AF patients with HFpEF have been investigated in previous studies (7, 8), but the effects seem less favorable than those in HFrEF patients. Machino-Ohtsuka et al. (7) showed that the longstanding persistent-type AF and a lack of hypertension were factors associated with an improvement in the LV systolic and diastolic indices when sinus rhythm was maintained. Black-Maier et al. (8) showed equivalent rhythm outcomes, all-cause hospitalization, and cardiovascular hospitalization in patients with both HFpEF and HFrEF after AFCA over a median follow-up of 10 months. However, previous studies adopted conventional diagnostic approaches that mainly consisted of symptoms and the LVEF for an HFpEF diagnosis, which show a heterogeneity in terms of the inclusion (9). Thus, we used the H2FPEF scores that were newly developed for identifying HFpEF patients and which were superior to the previous diagnostic algorithms. Although the baseline H2FPEF scores do not have prognostic value for rhythm outcomes after AFCA, which is consistent with a recent study (27), increased ΔH2FPEF scores[1−yr] were independently associated with the rhythm outcomes in this study. Although the cause–result relationship was unclear, sustained AF despite AFCA had a significant correlation with an increased ΔH2FPEF score[1−yr].
Heart Failure With Preserved Ejection Fraction, a Good Candidate for Atrial Fibrillation Catheter Ablation
Among the patients with a normal LV systolic function, which subgroup is the most helpful based on the H2FPEF scores after AFCA? Those with the most significant decrease in the ΔH2FPEF scores[1−yr] were those with a baseline HFpEF score of ≥6, i.e., successful rhythm control by AFCA can significantly improve the LV diastolic function in patients with HFpEF. Furthermore, the absence of diabetes, a higher LVEF, and a lower pericardial fat volume were associated with decreased ΔH2FPEF scores[1−yr], which suggested that metabolic factors may have influenced the recovery of the LV diastolic function.
Limitations
This study had several limitations. First, since the current study was conducted in a single center and included a relatively small number of patients, the findings cannot be generalized to all patients with a normal LV systolic function. However, there was also an advantage of this single-center cohort in that the ablation and rhythm follow-up protocols were consistent. Second, although we performed a regular rhythm follow-up in all included patients, the exact AF burden could not be assessed by the Holter monitoring. Third, we excluded patients who did not have both baseline and 1-year follow-up echocardiograms with all parameters taken from the same institute, to calculate appropriate H2FPEF scores. Fourth, there was some discrepancy between the HF clinically judged by the CH2A2DS-VASc and H2FPEF scores because the clinical HF was classified mainly by the LV systolic function. Finally, because of the limited follow-up duration, we could not determine the long-term changes in the biventricular function and other clinical outcomes in this study. Future prospective and controlled studies are warranted.
Conclusion
The H2FPEF scores decreased in 17% and increased in 21% of the patients with a normal LV function at 1 year after the AFCA. AFCA has shown a tendency to improve the H2FPEF scores[1−yr] in the patients with an abnormal diastolic function. However, a poor rhythm control and sustained AF after the AFCA were significantly associated with an increase in the ΔH2FPEF score[1−yr]. An increased ΔH2FPEF score[1−yr] was an independent prognostic factor for poorer rhythm outcomes after the AFCA.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by The institutional review board of the Yonsei University Health system. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HN-P and MK designed the study, analyzed and interpreted the data, drafted the manuscript, and did the final approval of the manuscript submission. HTY, T-HK, J-SU, BYJ, and M-HL interpreted data and contributed to acquiring patients' clinical data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant (HI19C0114) from the Ministry of Health and Welfare and a grant (NRF-2020R1A2B01001695) from the Basic Science Research Program run by the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT and Future Planning (MSIP).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Mr. John Martin for his linguistic assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.699364/full#supplementary-material
Abbreviations
ΔH2FPEF score[1−yr], 1-year change in the H2FPEF score; SustainAF[1−yr], sustained AF after a recurrence within a year.
References
1. Braunwald E. Shattuck lecture - cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. New Eng J Med. (1997) 337:1360–9. doi: 10.1056/NEJM199711063371906
2. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al. Atrial fibrillation begets heart failure and vice versa temporal associations and differences in preserved versus reduced ejection fraction. Circulation. (2016) 133:484–92. doi: 10.1161/CIRCULATIONAHA.116.022835
3. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. (2015) 8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667
4. Kuck KH, Merkely B, Zahn R, Arentz T, Seidl K, Schluter M, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure the randomized AMICA trial. Circ Arrhythmia Elec. (2019) 12:e007731. doi: 10.1161/CIRCEP.119.007731
5. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (The CAMTAF Trial). Circ Arrhythmia Elec. (2014) 7:31–38. doi: 10.1161/CIRCEP.113.000806
6. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. New Engl J Med. (2018) 378:417–27. doi: 10.1056/NEJMoa1707855
7. Machino-Ohtsuka T, Seo Y, Ishizu T, Sugano A, Atsumi A, Yamamoto M, et al. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. (2013) 62:1857–65. doi: 10.1016/j.jacc.2013.07.020
8. Black-Maier E, Ren XR, Steinberg BA, Green CL, Barnett AS, Rosa NS, et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm. (2018) 15:651–7. doi: 10.1016/j.hrthm.2017.12.001
9. Vaduganathan M, Michel A, Hall K, Mulligan C, Nodari S, Shah SJ, et al. Spectrum of epidemiological and clinical findings in patients with heart failure with preserved ejection fraction stratified by study design: a systematic review. Eur J Heart Fail. (2016) 18:54–65. doi: 10.1002/ejhf.442
10. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. (2018) 138:861–70. doi: 10.1161/CIRCULATIONAHA.118.034646
11. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. (2017) 14:e275–444. doi: 10.1016/j.hrthm.2017.05.012
12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Card Img. (2015) 16:233–71. doi: 10.1093/ehjci/jev014
13. Rudski LG, Lai WW, Afilalo J, Hua LQ, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the american society of echocardiography endorsed by the European association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography. J Am Soc Echocardiog. (2010) 23:685–713. doi: 10.1016/j.echo.2010.05.010
14. Park MJ, Jung JI, Oh YS, Youn HJ. Assessment of the structural remodeling of the left atrium by 64-multislice cardiac CT: Comparative studies in controls and patients with atrial fibrillation. Int J of Cardiol. (2012) 159:181–6. doi: 10.1016/j.ijcard.2011.02.053
15. Shin SY, Yong HS, Lim HE, Na JO, Choi CU, Choi JI, et al. Total and interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillation. J Cardiovasc Electr. (2011) 22:647–55. doi: 10.1111/j.1540-8167.2010.01993.x
16. Park JH, Pak HN, Choi EJ, Jang JK, Kim SK, Choi DH, et al. The relationship between endocardial voltage and regional volume in electroanatomical remodeled left atria in patients with atrial fibrillation: comparison of three-dimensional computed tomographic images and voltage mapping. J Cardiovasc Electr. (2009) 20:1349–56. doi: 10.1111/j.1540-8167.2009.01557.x
17. Kim TH, Parke J, Uhm JS, Joung B, Lee MH, Pak HN. Pulmonary vein reconnection predicts good clinical outcome after second catheter ablation for atrial fibrillation. Europace. (2017) 19:961–7. doi: 10.1093/europace/euw128
18. Kim M, Yu HT, Kim J, Kim TH, Uhm JS, Joung B, et al. Atrial fibrillation and the risk of ischaemic strokes or intracranial haemorrhages: comparisons of the catheter ablation, medical therapy, and non-atrial fibrillation population. Europace. (2021) 23:529–38. doi: 10.1093/europace/euaa235
19. Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. (2009) 119:3070–7. doi: 10.1161/CIRCULATIONAHA.108.815944
20. Tsang TSM, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. (2002) 40:1636–44. doi: 10.1016/S0735-1097(02)02373-2
21. Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. (2009) 119:1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877
22. Letsas KP, Filippatos GS, Pappas LK, Mihas CC, Markou V, Alexanian IP, et al. Determinants of plasma NT-pro-BNP levels in patients with atrial fibrillation and preserved left ventricular ejection fraction. Clin Res Cardiol. (2009) 98:101–6. doi: 10.1007/s00392-008-0728-8
23. Linssen GCM, Rienstra M, Jaarsma T, Voors AA, van Gelder IC, Hillege HL, et al. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. (2011) 13:1111–20. doi: 10.1093/eurjhf/hfr066
24. Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. (2020) 76:1051–64. doi: 10.1016/j.jacc.2020.07.009
25. AlTurki A, Proietti R, Dawas A, Alturki H, Huynh T, Essebag V. Catheter ablation for atrial fibrillation in heart failure with reduced ejection fraction: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. (2019) 19:18. doi: 10.1186/s12872-019-0998-2
26. Vasan RS, Xanthakis V, Lyass A, Andersson C, Tsao C, Cheng S, et al. Epidemiology of left ventricular systolic dysfunction and heart failure in the framingham study: an echocardiographic study over 3 decades. JACC Cardiovasc Imaging. (2018) 11:1–11. doi: 10.1016/j.jcmg.2017.08.007
Keywords: atrial fibrillation, catheter ablation, left venticular diastolic dysfunction, recurrent event, risk score
Citation: Kim M, Yu HT, Kim T-H, Uhm J-S, Joung B, Lee M-H and Pak H-N (2021) One-Year Change in the H2FPEF Score After Catheter Ablation of Atrial Fibrillation in Patients With a Normal Left Ventricular Systolic Function. Front. Cardiovasc. Med. 8:699364. doi: 10.3389/fcvm.2021.699364
Received: 23 April 2021; Accepted: 02 July 2021;
Published: 03 August 2021.
Edited by:
Konstantinos Letsas, Evaggelismos General Hospital, GreeceReviewed by:
Giannis G. Baltogiannis, Vrije University Brussel, BelgiumDimitris Asvestas, Mitera Hospital, Greece
Copyright © 2021 Kim, Yu, Kim, Uhm, Joung, Lee and Pak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Nam Pak, aG5wYWsmI3gwMDA0MDt5dWhzLmFj
 Min Kim
Min Kim Hee Tae Yu2
Hee Tae Yu2 Jae-Sun Uhm
Jae-Sun Uhm Hui-Nam Pak
Hui-Nam Pak