- Department of Vascular, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
Background: Carotid artery stenosis has long been a critical cause of stroke and death, and it can seriously affect the life quality. Transcarotid artery revascularization (TCAR) and carotid endarterectomy (CEA) are both feasible therapies for this disease. This systematic review and meta-analysis aim to evaluate if the efficacy of the two approaches is comparable.
Methods: Clinical studies up to March 2021 were searched through PubMed, Embase, and Scopus from a computer. The screening process was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. Newcastle-Ottawa Scale (NOS) was used for methodological quality assessment of works of literature meeting the inclusion criteria, and Review Manager 5.4 was used for data synthesis. The I2 statistic was performed to measure the heterogeneity, and M-H/I-V fixed or random model was utilized depending on the I2 value. The evidence evaluation was accomplished based on grades of recommendation, assessment, development, and evaluation (GRADE) online tool.
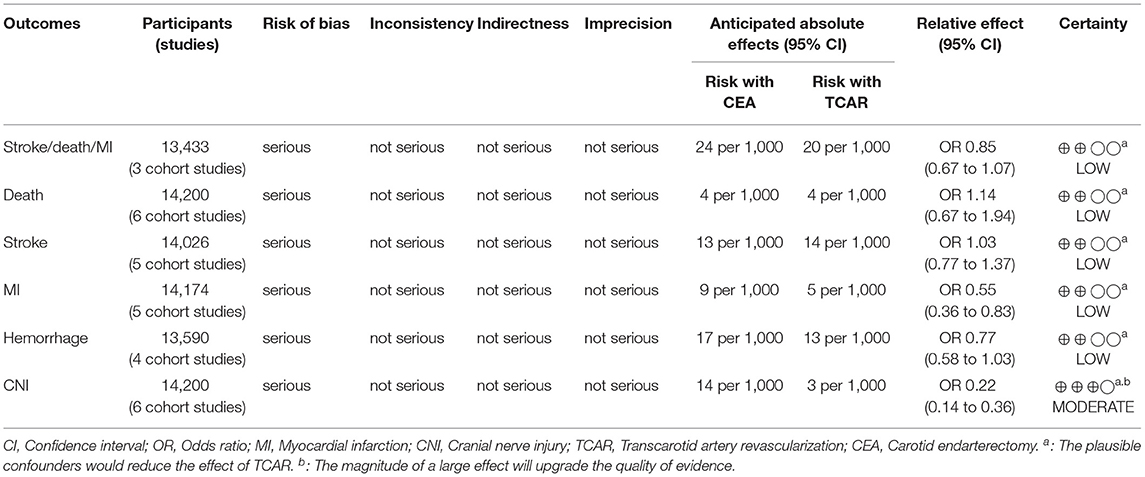
Results: A total of 14,200 subjects (six comparative studies) were finally included in this pooled study. There is no statistical discrepancy between the two treatments on reducing stroke/death/myocardial infarction (odds ratio [OR] 0.85, 95% CI 0.67–1.07), stroke (OR 1.03, 95% CI 0.77–1.37), or death (OR 1.14, 95% CI 0.67–1.94). Besides, TCAR is associated with a lower incidence of myocardial infarction (P = 0.004), cranial nerve injury (P < 0.00001), and shorter procedure time (P < 0.00001) than CEA among the overall cohort.
Conclusions: TCAR is a rapidly developing treatment that reaches a comparable prognosis to CEA and significantly reduces the risk of myocardial infarction under the well-matched condition, which is a dependable choice for patients with carotid stenosis.
Introduction
One of the pivotal causes of stroke is carotid stenosis (CS) (1). About 30% of ischemic stroke is triggered by extracranial CS, and atherosclerosis occupies 90% of adverse lesions leading to ischemia (2). At present, a long-term global survey indicated that stroke, as a severe disease threatening human life, has become the second leading cause of death and the third of disability (3). Elderly people are particularly more likely to be subject to stroke (4), due to complex comorbidities or vasculopathy (5). As a consequence, early intervention is necessary for CS to prevent stroke and maintain life quality of patients.
Several randomized controlled trials have demonstrated the safety and efficacy of transfemoral carotid artery stenting (TF-CAS) and carotid endarterectomy (CEA) for symptomatic or asymptomatic CS (6–9). However, TF-CAS has commonly been considered as an alternative to CEA, which is seen as the gold standard for treating CS (10–12). Even though TF-CAS has reached equivalent effects on late outcomes as CEA (13) and has been more favorable for patients with higher risks at anatomy or clinical picture (14). However, during the perioperative period, TF-CAS is associated with a greater risk of neurological events, which has been documented in quite a few studies (15–17). The critical reasons resulting in the failure for TF-CAS to optimize outcomes include inconvenient manipulation for the guidewire to pass through the aortic arch, and plaque fracture or thrombus embolizing intracranial arteries when carrying protected device through carotid lesion (18). At this stage, a newer technique (transcarotid artery revascularization, TCAR) has been valued and developed rapidly, which consists of direct manipulation to the lesion and minimized incision with short-path stenting (19). Moreover, as an assisted neuroprotection device, the flow reversal system significantly improves the efficacy of TCAR, which is extracorporeal arteriovenous access and filters bloodstream into the brain (20, 21).
Recently, a meta-analysis has demonstrated the short-term and long-term efficacy and safety of TCAR (22). Moreover, two prospective, single-arm, multicenter studies (ROADSTER and ROADSTER2) have also shown that TCAR was associated with satisfactory outcomes, such as the rates of freedom from stroke, transient ischemic attack, and death in the perioperative period after the procedure (23, 24). In the context of such favorable postoperative prognoses of TCAR, comparative studies were performed compared to conventional therapies (25–27), and this emerging technique also partly presented superiority over the transfemoral procedure. A 2019 meta-analysis found that the transcarotid approach reduced the risk of stroke contrasted with TF-CAS (28). In addition, high-volume, multicenter research using the propensity score matching (PSM) suggested TCAR with dynamic flow reversal had significantly mitigated the rate of stroke/death than TF-CAS (29). Being the first-class choice for CS, it is inevitable for CEA to be a reference standard measuring this novel technique. However, few randomized controlled trials comparing TCAR and CEA were conducted, which means it is still a lack of high-level evidence that TCAR may be comparable to CEA for CS. Although a 2020 meta-analysis comprised of non-randomized studies indicated that the two procedures were equivalent on stroke/death/myocardial infarction (MI), the less power was limited to the relatively small sample size of the TCAR arm (30). In addition, the comparative effects based on different symptom status remain uncertain. Given these margins in current meta-analyses, a complementary study was carried out by us, which aims to explore the efficacy of TCAR compared to CEA in a larger sample size, especially for symptomatic patients.
Materials and Methods
This systematic review and meta-analysis have reported items according to the PRISMA statement to complete a specific project mainly, such as article retrieval (31), data synthesis, and integrated analysis.
Search Strategy
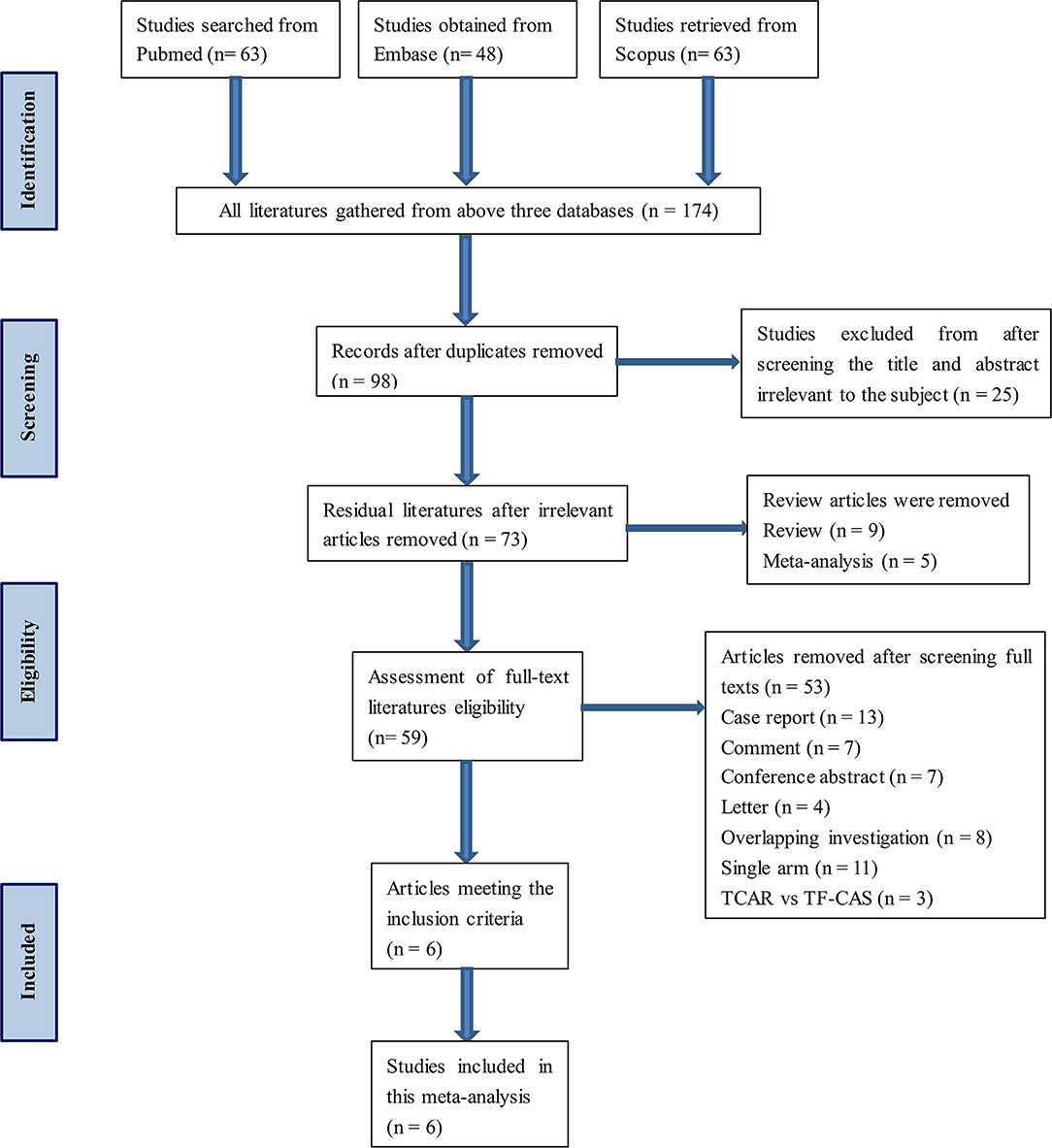
Databases, namely, PubMed, Embase, and Scopus, were searched and no literature was added from other channels. The keywords contained TCAR, transcervical, CEA, and CS. This process was carried out by two independent investigators. All the selected works of literature meet the inclusion criteria. Besides, any disagreement during the inclusion process would be reviewed and resolved by the third researcher. A detailed flow diagram of articles screening is presented in Figure 1. Search strategy within each database can be found in Supplementary Table 1.
Selection Criteria
The study to be included needs to meet all the following criteria: (1) the type of research was comparative analysis, (2) perioperative medication was presented in the study, (3) TCAR vs. CEA, and (4) relevant endpoints of interest. And works of literature that satisfied any of the following criteria were excluded: (1) non-clinical trial, (2) the use of flow reversal system in TCAR group was not described, (3) symptomatic status was not reported in each cohort, (4) conference abstract or case report, (5) TCAR vs. TF-CAS, and (6) overlapping investigation (data of patients from the same available sites may be repeatedly used in multiple studies).
Data Extraction and Endpoints
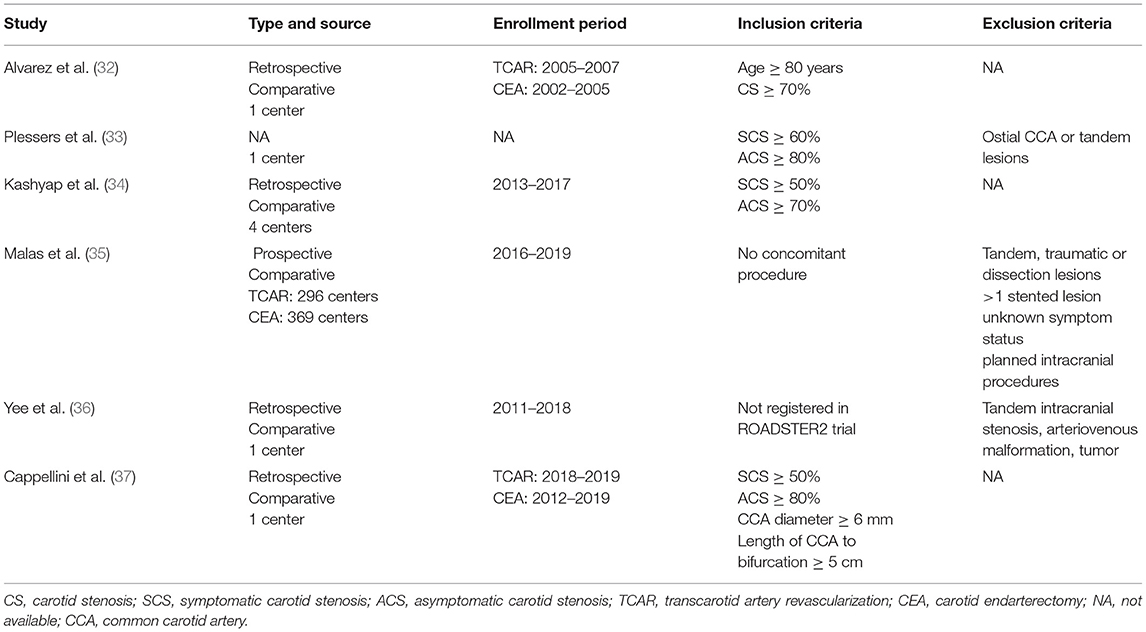
Detailed contents, such as publication time, study type, enrollment period, and inclusion and exclusion criteria, in each eligible literature, were collected (as shown in Table 1). The primary endpoint was postoperative stroke/death/MI. Death, stroke, MI, hemorrhage, cranial nerve injury (CNI), and procedure time were classified as secondary outcomes within 30 days after the procedure. Clinical endpoints concerning overall, symptomatic, or asymptomatic cohort were also extracted respectively.
Quality Assessment and Risk of Bias
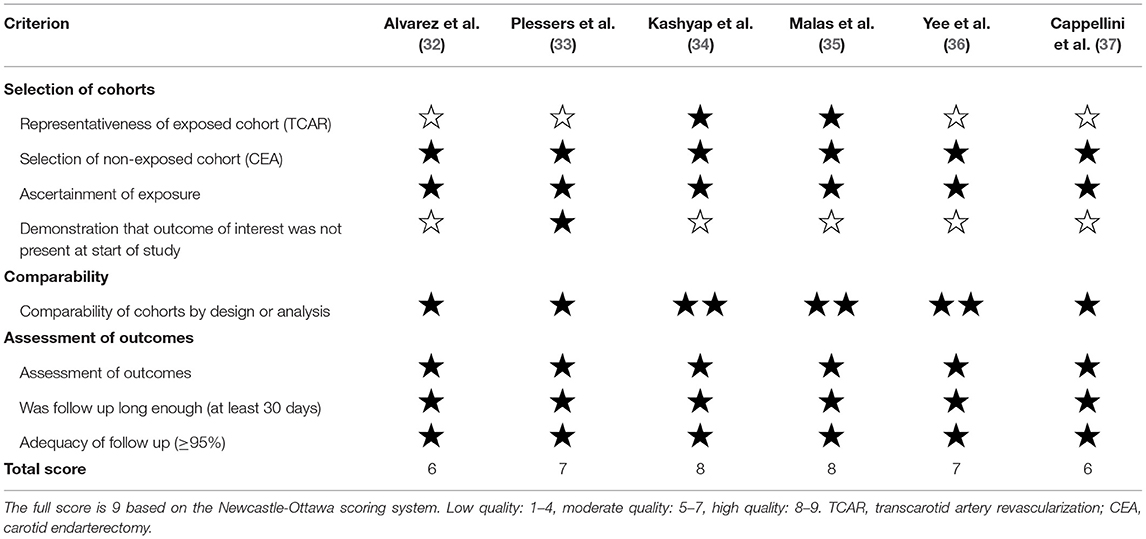
Newcastle-Ottawa Scale was used for quality assessment of each comparative study (38), which included three aspects: selection, comparability, and outcomes. According to the scoring system, the full score is 9. When the study scored 1–4 is classified as low quality articles methodologically, 5–7 as moderate quality, and 8–9 as high. The risk of bias and certainty of findings were described according to the grading system (39) designed by the grades of recommendation, assessment, development, and evaluation (GRADE) working group.
Data Synthesis and Heterogeneity
Review manager 5.4 was used for statistical analysis. Odds ratio (OR) and mean difference in 95% CI were selected as the effect size to reflect prognosis undergoing different treatment. P < 0.05 indicated that results were statistically significant. Fixed effects model was the preference unless any heterogeneity determined by I2 statistic was found, if so, random-effects model was carried out to adjust the ORs. According to the Cochrane handbook, the heterogeneous level was sorted as low, moderate, substantial, and considerable corresponding to I2 <40%, 30–60%, 50–90%, and 75–100%, respectively (40). Sensitivity analyses were performed to identify the stability of results (41).
Definitions
1. Symptomatic status was defined as having a transient ischemic attack, amaurosis fugax, or stroke in the previous 180 days.
2. Stroke was defined as ipsilateral or contralateral, cortical or vertebrobasilar, and ischemic or hemorrhagic strokes. If the symptoms last less than 24 h would be considered as a transient ischemic attack.
3. Myocardial infarction was defined as acute clinical symptoms plus troponin significantly increased or electrocardiogram sharp changed.
4. Hemorrhage was defined as hematoma requiring surgery or intervention.
Results
Characteristics of the Included Studies
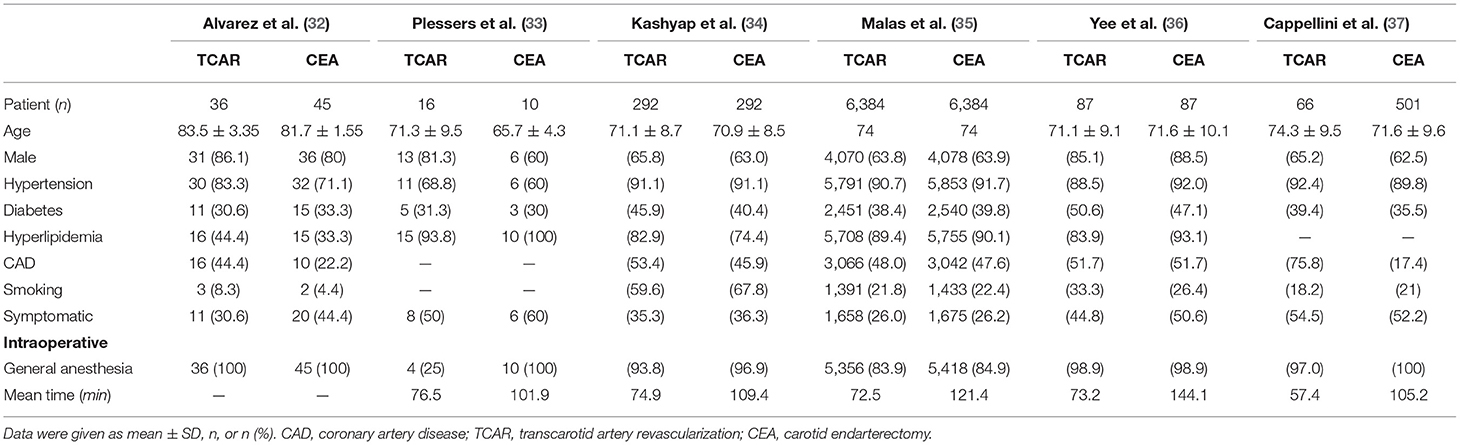
A total of 174 articles were obtained through the retrieval flow, thereafter removing duplicates and screening abstracts or full texts, the remaining six comparative cohort research studies were finally selected in this meta-analysis (32–37). Among all excluded studies, an overlapping investigation might occur in eight trials. TCAR group and CEA group were individually comprised of 6,881 and 7,319 patients with CS. The average age of patients in the TCAR cohort was slightly older than the CEA group, but no statistical significance was shown. The prevalence distributions of hypertension, diabetes, hyperlipidemia, and coronary artery disease were not discrepant in each study except for Cappellini et al. (37) containing more patients with coronary artery disease in the TCAR arm. Most subjects treated by the transcarotid approach were given general anesthesia, but Plessers et al. (33) reported that only 25% was received. Five included articles indicated that procedure time could be shortened by transcarotid way, which was significantly less than endarterectomy. Only Yee et al. documented intra-operative blood loss and the outcome favored TCAR. Dynamic flow reversal system had to be attached to TCAR technique in principle if there is no severe intolerance or contraindication. More preoperative and intra-operative information are shown in Table 2.
According to our independent adjudication, four of six works of literature were moderate quality and the other two were high quality by the NOS scale (Table 3). In terms of comparability of cohorts, Kashyap et al. (34), Malas et al. (35), and Yee et al. (36) have adjusted critical confounders containing age, gender, symptom status, hyperlipidemia, diabetes, and hypertension. Either age and gender or common comorbidities were balanced against between two arms in the remaining articles (32, 33, 37). Moreover, being a feasible and recommended method, PSM improves intergroup comparability and was also applied in the two works of literature by Kashyap et al. (34) and Malas et al. (35), respectively.
Outcomes of Data Synthesis
Stroke/Death/MI
Three research studies have reported concerning data based on mentioned definitions (6,712 TCARs among 13,433 patients). The composite incidence in TCAR and CEA group was 2.0% and 2.4% individually without statistical significance (OR 0.85, 95% CI 0.67–1.07, P = 0.17). No heterogeneity was detected (I2 = 0%; Supplementary Figure 1A).
Death
Data were extracted from six studies (6,881 TCARs among 14,200 patients). Slightly higher mortality in the TCAR cohort was 0.4% compared to 0.3% of that for CEA within 30 days after the procedure. However, there is no significant difference between the two approaches and no heterogeneity in pooled analysis (OR 1.14, 95% CI 0.67–1.94, P = 0.63, I2 = 0%; Supplementary Figure 1B).
Stroke
Although all articles have reported postoperative stroke, Yee et al. was excluded from data synthesis, because the definition may include transient ischemic attack. A total of 6,794 and 7,232 patients underwent TCAR and CEA respectively, 1.4% compared to 1.3% of stroke rates, which is a non-heterogeneous result with no statistical discrepancy (OR 1.03, 95% CI 0.77–1.37, P = 0.84, I2 = 0%; Supplementary Figure 1C).
Myocardial Infarction
Postoperative incidence of MI was available from five works of literature (6,865 TCARs among 14,174 patients). The significant difference indicated the MI rates in the TCAR arm was 0.5% contrasted with 0.9% in controls (OR 0.55, 95% CI 0.36–0.83, P = 0.004, I2 = 0%; Supplementary Figure 1D).
Hemorrhage
Four studies have reported this endpoint (6,573 TCARs among 13,590 patients). TCAR was not differed from CEA statistically in terms of reducing the risk of hemorrhage and no heterogeneity was found (OR 0.77, 95% CI 0.58–1.03, P = 0.08, I2 = 0%), however, in fact, the transcarotid approach had a lower 0.4% of risk degree (CEA: 1.7%, TCAR: 1.3%; Supplementary Figure 1E).
Cranial Nerve Injury
Data regarding CNI were available from all research studies (6,881 TCARs among 14,200 patients). This non-heterogeneous result favored that TCAR was strongly associated with a much lower risk of CNI after the procedure than CEA (0.3 vs. 1.4%, OR 0.22, 95% CI 0.14–0.36, I2 = 0%), which was as same as all the included articles (Supplementary Figure 1F).
Procedure Time
The finding based on five studies suggested that TCAR had significantly shortened operative time compared to CEA (6,867 TCARs among 14,475 patients), though the heterogeneity was considerable (Supplementary Figure 1G).
Subgroup Analysis
Detailed information of pooled analysis based on symptomatic or asymptomatic status is recorded in Table 4, and corresponding forest plots are shown in Supplementary Figures 2, 3 individually.
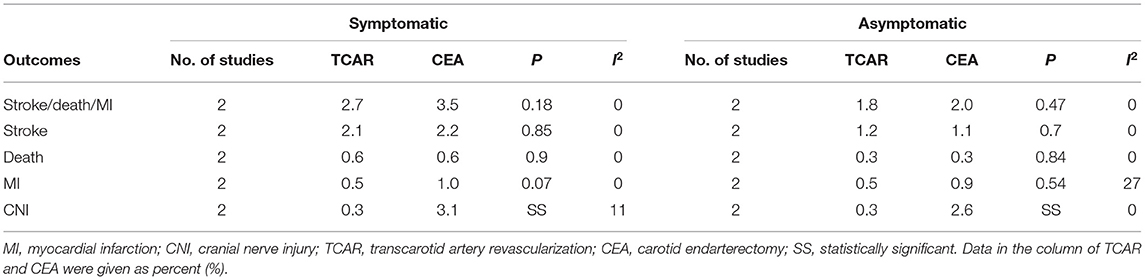
Table 4. Subgroup analysis of symptomatic or asymptomatic status for carotid stenosis treated by TCAR or CEA.
Symptomatic Status
Pre-designed subgroup analyses were performed to study the comparative effects between both approaches in different symptom status. Kashyap et al. and Malas et al. have divided subjects into two arms according to the mentioned definition. Among the symptomatic cohort, no statistical significance was found between the two groups in terms of the primary endpoint, stroke, death, or MI. However, TCAR was correlated to the decline of CNI and statistically superior to CEA under the random-effects model (OR 0.12, 95% CI 0.04–0.32, P < 0.0001, I2 = 11%).
Asymptomatic Status
The investigation concerning asymptomatic patients has also presented that no significant discrepancies were detected on stroke, death, or MI.
Although the incidence of postoperative MI in TCAR troop was similar to that in CEA, a little heterogeneity was shown in the plot (OR 0.71, 95% CI 0.24–2.10, P = 0.54, I2 = 27%). Besides, the non-heterogeneous result indicated that the higher CNI rate still occurred in the CEA cohort, with statistical significance.
Sensitivity Analysis
All studies at each endpoint would be single removed to verify the stability of the results. Severe instabilities containing reversed statistical significance or obvious changes in effect estimates were shown below.
Myocardial Infarction
After eliminating the research of Malas et al., in spite of heterogeneity was also not detected, the statistical significance favored TCAR disappeared, which means TCAR has no longer superior to CEA in terms of MI for the overall cohort (OR 0.70, 95% CI 0.17–2.90, P = 0.63, I2 = 0%).
Hemorrhage
Even though removing the study by Malas et al. will lead to a decline of the OR from 0.77 to 0.38, the non-significant difference still exists between the two therapies for entire subjects (95% CI 0.08–1.83, P = 0.23, I2 = 0%).
Discussion
About one-third of healthy looking elder people suffered from atherosclerotic CS (42). With the development of plaque burden, the lumen patency to the brain is directly affected, in addition, the fractured fragments or thrombus may severely obstruct the neurological arteries and cause ischemic stroke. As the two effective therapies used for CS frequently, TF-CAS and CEA are unavoidable topics, however, the latter has most likely to be recommended due to the lower risk of stroke (43). Nowadays, a novel technique composed of carotid stenting and specially designed device was seen as a third strategy for treating CS, which received positive comment and efficacy, and distinctly reduced adverse events within the perioperative period (44–46). Therefore, it is necessary to study the prognosis of patients who underwent TCAR compared to CEA in a larger sample size and to explore whether there are differences related to symptom status.
From our findings, of all the patients in the TCAR cohort, in terms of stroke/death/MI, death, stroke, or hemorrhage, which is 2.0, 0.4, 1.4, and 1.3% respectively, is similar to the CEA cohort. In addition, the rates of adverse events are equivalent to the results reported in the current literature (23, 29, 47). Only a few included studies have presented a slightly higher stroke rate for TCAR compared to CEA, though this may due to patient selection or the relatively small sample size (34). The reduction of neurological risks from TF-CAS to TCAR, to a great extent, is associated with the use of flow reversal, which is a protected device driving thrombus to femoral vein based on arterio-venous pressure, meanwhile, directly carotid puncture avoiding manipulation in the aortic arch is another beneficial factor (48, 49).
It is worth noting that patients who underwent CEA were mostly like to be subject to MI than the TCAR group, which is identical to several published studies (35, 50). Considering the adjustment by PSM, even the prior health condition of subjects was comparable between two groups, the edge of TCAR over CEA is not difficult to explain. This situation maybe resulted from not only shorter procedure time and small transcervical incision, which reduces intra-operative blood loss and declines the burden on cardiopulmonary function, but also less use of general anesthesia and more active postoperative medications in the TCAR arm compared to CEA. On the other hand, in view of patients treated by TCAR could be discharged hospital generally earlier than CEA, potentially, which is an inconvenient plight for whole centers to capture the changes of ST-segment through electrocardiograph timely when asymptomatic MI happened, and imaging examination may be ignored due to the subjects were freedom from symptoms in the follow-up stage. Surprisingly, through the sensitivity analysis of MI for the overall cohorts, after removing the well-matched study with a large sample size by Malas et al., the statistical difference in favor of TCAR is lost. This study occupies 92.3% of total weight and suggests the MI rate among TCAR arm notably lower than CEA arm, however, the pooled data from residual studies showed equivalent efficacy, even the physical function was worse in the TCAR group. In other words, there is no statistical importance but a clinical value. Within a certain risk range, for patients under similar challenges from both procedures, TCAR might be more beneficial and safer. Despite no significant difference in MI was found in either symptomatic or asymptomatic cohort, which is possibly limited to the nature of PSM (51) where a part of patients who underwent CEA was excluded, hence the statistical significance was not revealed.
Symptomatic patients tend to be more sensitive to cerebral ischemia and also benefit apparently on postprocedure recovery of physical function from carotid surgery (52, 53). As shown in this analysis, TCAR has reached comparable performance to CEA on preventing stroke for symptomatic carotid stenosis (SCS), without significance, but the OR slightly supported the former. For SCS, being the reliable treatment demonstrated by the NASCET trial (54), however, accompanied by increasing age, CEA had been proven associated with higher perioperative risks, especially stroke or death (55). Besides, a current study comparing CEA with TCAR suggested that TCAR has reduced adverse events for symptomatic patients aged over 80 (56), this mostly owing to the active anesthesia management that maintains the steady state of hemodynamics, but also the appropriate lesions meeting the specific anatomy criteria, such as common carotid artery diameter ≥6 mm, clavicle to bifurcation ≥5 cm, and no atherosclerotic plaque at the access site, which optimize patient selection and bring the CEA-risk better prognoses through diminishing procedure threshold.
Although the management of asymptomatic carotid stenosis (ACS) has always been controversial (57), CEA has still been recommended by guidelines due to the considerable prognosis resulted from multi-center randomized trials (58, 59). According to our findings, TCAR is identical to CEA for ACS in terms of reducing stroke, death, or MI, except for CNI, which is irrespective of symptom status but related to the nature of CEA. Malas et al. have indicated the two therapies were statistically equivalent on postoperative death after matching the baselines (35), and similar results were also reported by other two literature (44, 50). From the incidence perspective in this meta-analysis, the TCAR cohort had shown slightly higher mortality, but no statistical difference was detected. In summary, it is reasonable to speculate that the TCAR with flow reversal has non-inferiority to CEA for ACS.
In this pooled study, the certainty of evidence was assessed as moderate and low due to the risk of bias (Table 5). The confounding bias of included studies was generally limited to the inherent traits of non-randomized trial downgrading the level of evidence, moreover, various stenotic degrees, lesion length, and anesthesia details will decline the applicability of findings. However, given the higher prevalence of medical comorbidities in the TCAR arm will negatively affect the effects, in order to reflect the authentic situation, the certainty needed to be upgraded. Meanwhile, according to the Grade scoring tool, consistent evidence concerning CNI can also be upgraded due to the magnitude of the large effect. Additionally, here the funding information or commercial support potentially led to reporting bias was investigated in this analysis. The research of Plessers et al. was supported by scientific funding from Belgium (33). Although these three studies have reported no funding obtained (34–36), at least one author in each article has ever received sponsorship or served as a consultant in the Silk Road Medical, which is an institution responsible for researching and developing TCAR with flow reversal system. The residual two literature have none of the above situations (32, 37).
As a result of a randomized trial comparing TCAR with CEA is still lacking (60), no powerful evidence could be found to support our findings. Even so, this meta-analysis has demonstrated that the perioperative efficacy of TCAR is similar to CEA under a larger sample size and also compared the two therapies in different symptom status, which are steps forward building on the foundation of the previous study (30).
Limitations
Firstly, no randomized controlled trial could be obtained during searching databases, only several cohort studies met pre-specified inclusion criteria, which have increased the risk of bias and weakened evidence. Besides, the definition of stroke in the research of Yee et al. (36) includes transient ischemic attack, hence, after eliminating it, the decline of sample size would affect OR. No economic data were available so that could not be analyzed in this study to guide clinical choice. Indeed, PSM may lead to selection bias, and low-risk patients who underwent CEA were excluded, however, it vastly balanced the confounders between two cohorts and improved comparability. Although a pooled analysis was not performed due to absent data, prior investigation presented the learning curve of TCAR is short, even in the novice stage, the procedures can be completed with lower stroke or death (61). Lastly, considering the lack of patient-level details of anesthesia, which type of CS undergoing TCAR can obtain a better prognosis from local or general anesthesia remains unknown in this meta-analysis.
Conclusions
From this meta-analysis, TCAR has achieved comparable efficacy to CEA on preventing stroke/death/MI, stroke, death, and reached better in terms of CNI and operation time, irrespective of symptom status. Under the well-matched condition, TCAR can more likely reduce MI rate than CEA. More high-volume, prospective and long-term comparative studies are needed to testify our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
JG: data collection and synthesis analysis. JG and ZC: quality assessment of included literatures. ZC, LK, HZ, and YY: manuscript reversion. JG, ZC, LK, HZ, and YY: final approval of the article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.695295/full#supplementary-material
References
1. Petty GW, Brown RD, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. (2013) 30:2513–6. doi: 10.1161/01.STR.30.12.2513
2. Hankey GJ. Secondary stroke prevention. Lancet Neurol. (2014) 13:178–94. doi: 10.1016/S1474-4422(13)70255-2
3. Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
4. Spence JD, Azarpazhooh MR, Larsson SC, Bogiatzi C, Hankey GJ. Stroke prevention in older adults: recent advances. Stroke. (2020) 51:3770–7. doi: 10.1161/STROKEAHA.120.031707
6. Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. (2010) 363:11–23. doi: 10.1056/NEJMoa0912321
7. European Carotid Surgery Trialists' Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. (1998) 351:1379–87. doi: 10.1016/S0140-6736(97)09292-1
8. Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, et al. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med. (2016) 374:1011–20. doi: 10.1056/NEJMoa1515706
9. Bonati LH, Dobson J, Featherstone RL, Ederle J, van der Worp HB, de Borst GJ, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet. (2015) 385:529–38. doi: 10.1016/S0140-6736(14)61184-3
10. Mantese VA, Timaran CH, Chiu D, Begg RJ, Brott TG, CREST Investigators. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): stenting versus carotid endarterectomy for carotid disease. Stroke. (2010) 41:S31–4. doi: 10.1161/STROKEAHA.110.595330
11. Moresoli P, Habib B, Reynier P, Secrest MH, Eisenberg MJ, Filion KB. Carotid stenting versus endarterectomy for asymptomatic carotid artery stenosis: a systematic review and meta-analysis. Stroke. (2017) 48:2150–7. doi: 10.1161/STROKEAHA.117.016824
12. Cui L, Han Y, Zhang S, Liu X, Zhang J. Safety of stenting and endarterectomy for asymptomatic carotid artery stenosis: a meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg. (2018) 55:614–24. doi: 10.1016/j.ejvs.2018.02.020
13. Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. (2016) 374:1021–31. doi: 10.1056/NEJMoa1505215
14. Mas JL, Arquizan C, Calvet D, Viguier A, Albucher JF, Piquet P, et al. Long-term follow-up study of endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis trial. Stroke. (2014) 45:2750–6. doi: 10.1161/STROKEAHA.114.005671
15. Lacroix V, Hammer F, Astarci P, Duprez T, Grandin C, Cosnard G, et al. Ischemic cerebral lesions after carotid surgery and carotid stenting. Eur J Vasc Endovasc Surg. (2007) 33:430–5. doi: 10.1016/j.ejvs.2006.11.012
16. Arquizan C, Trinquart L, Touboul PJ, Long A, Feasson S, Terriat B, et al. Restenosis is more frequent after carotid stenting than after endarterectomy: the EVA-3S study. Stroke. (2011) 42:1015–20. doi: 10.1161/STROKEAHA.110.589309
17. Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. (2008) 7:893–902. doi: 10.1016/S1474-4422(08)70196-0
18. Paraskevas KI, Mikhailidis DP, Veith FJ. Mechanisms to explain the poor results of carotid artery stenting (CAS) in symptomatic patients to date and options to improve CAS outcomes. J Vasc Surg. (2010) 52:1367–75. doi: 10.1016/j.jvs.2010.04.019
19. Alpaslan A, Wintermark M, Pintér L, Macdonald S, Ruedy R, Kolvenbach R. Transcarotid artery revascularization with flow reversal. J Endovasc Ther. (2017) 24:265–70. doi: 10.1177/1526602817693607
20. Pinter L, Ribo M, Loh C, Lane B, Roberts T, Chou TM, et al. Safety and feasibility of a novel transcervical access neuroprotection system for carotid artery stenting in the PROOF study. J Vasc Surg. (2011) 54:1317–23. doi: 10.1016/j.jvs.2011.04.040
21. Malas MB, Leal J, Kashyap V, Cambria RP, Kwolek CJ, Criado E. Technical aspects of transcarotid artery revascularization using the ENROUTE transcarotid neuroprotection and stent system. J Vasc Surg. (2017) 65:916–20. doi: 10.1016/j.jvs.2016.11.042
22. Galyfos GC, Tsoutsas I, Konstantopoulos T, Galanopoulos G, Sigala F, Filis K, et al. Early and late outcomes after transcarotid revascularisation for internal carotid artery stenosis: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. (2021) 13:e0205848. doi: 10.1016/j.jvs.2021.04.012
23. Kwolek CJ, Jaff MR, Leal JI, Hopkins LN, Shah RM, Hanover TM, et al. Results of the ROADSTER multicenter trial of transcarotid stenting with dynamic flow reversal. J Vasc Surg. (2015) 62:1227–34. doi: 10.1016/j.jvs.2015.04.460
24. Kashyap VS, Schneider PA, Foteh M, Motaganahalli R, Shah R, Eckstein HH, et al. Early outcomes in the ROADSTER 2 study of transcarotid artery revascularization in patients with significant carotid artery disease. Stroke. (2020) 51:2620–9. doi: 10.1161/STROKEAHA.120.030550
25. Leal I, Orgaz A, Flores Á, Gil J, Rodríguez R, Peinado J, et al. A diffusion-weighted magnetic resonance imaging-based study of transcervical carotid stenting with flow reversal versus transfemoral filter protection. J Vasc Surg. (2012) 56:1585–90. doi: 10.1016/j.jvs.2012.05.107
26. Malas MB, Dakour-Aridi H, Wang GJ, Kashyap VS, Motaganahalli RL, Eldrup-Jorgensen J, et al. Transcarotid artery revascularization versus transfemoral carotid artery stenting in the society for vascular surgery vascular quality initiative. J Vasc Surg. (2019) 69:92–103.e2. doi: 10.1016/j.jvs.2018.05.011
27. Liang P, Soden P, Wyers MC, Malas MB, Nolan BW, Wang GJ, et al. The role of transfemoral carotid artery stenting with proximal balloon occlusion embolic protection in the contemporary endovascular management of carotid artery stenosis. J Vasc Surg. (2020) 72:1701–10. doi: 10.1016/j.jvs.2020.02.036
28. Texakalidis P, Giannopoulos S, Kokkinidis DG, Charisis N, Kakkar A, Jabbour P, et al. Direct transcervical access vs the transfemoral approach for carotid artery stenting: a systematic review and meta-analysis. J Endovasc Ther. (2019) 26:219–27. doi: 10.1177/1526602819833370
29. Schermerhorn ML, Liang P, Eldrup-Jorgensen J, Cronenwett JL, Nolan BW, Kashyap VS, et al. Association of transcarotid artery revascularization vs transfemoral carotid artery stenting with stroke or death among patients with carotid artery stenosis. JAMA. (2019) 322:2313–22. doi: 10.1001/jama.2019.18441
30. Naazie IN, Cui CL, Osaghae I, Murad MH, Schermerhorn M, Malas MB, et al. Systematic review and meta-analysis of transcarotid artery revascularization with dynamic flow reversal versus transfemoral carotid artery stenting and carotid endarterectomy. Ann Vasc Surg. (2020) 69:426–36. doi: 10.1016/j.avsg.2020.05.070
31. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
32. Alvarez B, Ribo M, Maeso J, Quintana M, Alvarez-Sabin J, Matas M. Transcervical carotid stenting with flow reversal is safe in octogenarians: a preliminary safety study. J Vasc Surg. (2008) 47:96–100. doi: 10.1016/j.jvs.2007.09.032
33. Plessers M, Van Herzeele I, Hemelsoet D, Patel N, Chung EM, Vingerhoets G, et al. Transcervical carotid stenting with dynamic flow reversal demonstrates embolization rates comparable to carotid endarterectomy. J Endovasc Ther. (2016) 23:249–54. doi: 10.1177/1526602815626561
34. Kashyap VS, King AH, Foteh MI, Janko M, Jim J, Motaganahalli RL, et al. A multi-institutional analysis of transcarotid artery revascularization compared to carotid endarterectomy. J Vasc Surg. (2019) 70:123–9. doi: 10.1016/j.jvs.2018.09.060
35. Malas MB, Dakour-Aridi H, Kashyap VS, Eldrup-Jorgensen J, Wang GJ, Motaganahalli RL, et al. TransCarotid revascularization with dynamic flow reversal versus carotid endarterectomy in the vascular quality initiative surveillance project. Ann Surg. (2020). doi: 10.1097/SLA.0000000000004496. [Epub ahead of print].
36. Yee EJ, Wang SK, Timsina LR, Ruiz-Herrera S, Liao JL, Donde NN, et al. Propensity-matched outcomes of transcarotid artery revascularization versus carotid endarterectomy. J Surg Res. (2020) 252:22–9. doi: 10.1016/j.jss.2019.12.003
37. Cappellini CA, Zheng H, Lamb KM, Sooppan R, Coffey J, Luo RQ. Outcomes of transcarotid artery revascularization and carotid endarterectomy at a single institution. Ann Vasc Surg. (2020) 73:329–35. doi: 10.1016/j.avsg.2020.10.023
38. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
39. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
40. Deek JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, editors. Cochrane handbook for systematic reviews of interventions. London: Cochrane. (2019). doi: 10.1002/9781119536604.ch10
41. Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. (2010) 1:112–25. doi: 10.1002/jrsm.11
42. Fernández-Friera L, Peñalvo JL, Fernández-Ortiz A, Ibañez B, López-Melgar B, Laclaustra M, et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation. (2015) 131:2104–13. doi: 10.1161/CIRCULATIONAHA.114.014310
43. Naylor AR, Ricco JB, de Borst GJ, Debus S, de Haro J, Halliday A, et al. Editor's choice - management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. (2017) 55:3–81. doi: 10.1016/j.ejvs.2017.06.021
44. Schermerhorn ML, Liang P, Dakour-Aridi H, Kashyap VS, Wang GJ, Nolan BW, et al. In-hospital outcomes of transcarotid artery revascularization and carotid endarterectomy in the society for vascular surgery vascular quality initiative. J Vasc Surg. (2020) 71:87–95. doi: 10.1016/j.jvs.2018.11.029
45. Paraskevas KI. Transcarotid artery revascularization with flow reversal. JAMA Surg. (2020) 155:366. doi: 10.1001/jamasurg.2019.5439
46. Lackey AR, Erben Y, Franco JADR, Meschia JF, Lal BK. Transcarotid artery revascularization results in low rates of periprocedural neurologic events, myocardial infarction, and death. Curr Cardiol Rep. (2020) 22:3. doi: 10.1007/s11886-020-1256-z
47. Dakour-Aridi H, Schermerhorn ML, Husain F, Eldrup-Jorgensen J, Lane J, Malas MB. Outcomes of transcarotid artery revascularization with dynamic flow reversal in patients with contralateral carotid artery occlusion. J Vasc Surg. (2021) 73:524–32. doi: 10.1016/j.jvs.2020.04.529
48. Naazie IN, Magee GA, Mathlouthi A, Elsayed N, Dakour-Aridi H, Malas MB. Primary mechanism of stroke reduction in transcarotid artery revascularization is dynamic flow reversal. J Vasc Surg. (2020) 74:187–94. doi: 10.1016/j.jvs.2020.10.082
49. Conway AM, Nguyen Tran NT, Qato K, Ehidom C, Stoffels GJ, Giangola G, et al. Complexity of aortic arch anatomy affects the outcomes of transcarotid artery revascularization versus transfemoral carotid artery stenting. Ann Vasc Surg. (2020) 67:78–89. doi: 10.1016/j.avsg.2020.04.016
50. Dakour-Aridi H, Ramakrishnan G, Zarrintan S, Malas MB. Outcomes of transcarotid revascularization with dynamic flow reversal versus carotid endarterectomy in the TCAR surveillance project. Semin Vasc Surg. (2020) 33:24–30. doi: 10.1053/j.semvascsurg.2020.10.001
51. Reiffel JA. Propensity score matching: the 'devil is in the details' where more may be hidden than you know. Am J Med. (2020) 133:178–81. doi: 10.1016/j.amjmed.2019.08.055
52. Rothwell PM. Endarterectomy for symptomatic and asymptomatic carotid stenosis. Neurol Clin. (2008) 26:1079–97. doi: 10.1016/j.ncl.2008.09.013
53. Abbott AL, Paraskevas KI, Kakkos SK, Golledge J, Eckstein HH, Diaz-Sandoval LJ, et al. Systematic review of guidelines for the management of asymptomatic and symptomatic carotid stenosis. Stroke. (2015) 46:3288–301. doi: 10.1161/STROKEAHA.115.003390
54. Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. (1991) 325:445–53. doi: 10.1056/NEJM199108153250701
55. Schmid S, Tsantilas P, Knappich C, Kallmayer M, König T, Breitkreuz T, et al. Risk of inhospital stroke or death is associated with age but not sex in patients treated with carotid endarterectomy for asymptomatic or symptomatic stenosis in routine practice: secondary data analysis of the nationwide german statutory quality assurance database from 2009 to 2014. J Am Heart Assoc. (2017) 6:e004764. doi: 10.1161/JAHA.116.004764
56. Dakour-Aridi H, Kashyap VS, Wang GJ, Eldrup-Jorgensen J, Schermerhorn ML, Malas MB. The impact of age on in-hospital outcomes after transcarotid artery revascularization, transfemoral carotid artery stenting, and carotid endarterectomy. J Vasc Surg. (2020) 72:931–42.e2. doi: 10.1016/j.jvs.2019.11.037
57. Gaba K, Ringleb PA, Halliday A. Asymptomatic carotid stenosis: intervention or best medical therapy? Curr Neurol Neurosci Rep. (2018) 18:80. doi: 10.1007/s11910-018-0888-5
58. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. (1995) 273:1421–8. doi: 10.1001/jama.273.18.1421
59. Hobson RW, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis the veterans affairs cooperative study group. N Engl J Med. (1993) 328:221–7. doi: 10.1056/NEJM199301283280401
60. Coelho A, Prassaparo T, Mansilha A, Kappelle J, Naylor R, de Borst GJ. Critical appraisal on the quality of reporting on safety and efficacy of transcarotid artery stenting with flow reversal. Stroke. (2020) 51:2863–71. doi: 10.1161/STROKEAHA.120.030283
61. Kashyap VS, King AH, Liang P, Eldrup-Jorgensen J, Wang GJ, Malas MB, et al. Learning curve for surgeons adopting transcarotid artery revascularization based on the vascular quality initiative-transcarotid artery revascularization surveillance project. J Am Coll Surg. (2020) 230:113–20. doi: 10.1016/j.jamcollsurg.2019.09.020
Keywords: carotid endarterectomy (CEA), carotid stenosis, stroke, meta-analysis, transcarotid artery revascularization (TCAR)
Citation: Gao J, Chen Z, Kou L, Zhang H and Yang Y (2021) The Efficacy of Transcarotid Artery Revascularization With Flow Reversal System Compared to Carotid Endarterectomy: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 8:695295. doi: 10.3389/fcvm.2021.695295
Received: 13 May 2021; Accepted: 18 October 2021;
Published: 19 November 2021.
Edited by:
Diego Arroyo, Fribourg Cantonal Hospital, SwitzerlandReviewed by:
Kosmas Paraskevas, Central Clinic of Athens, GreeceQingsheng Lu, Second Military Medical University, China
Copyright © 2021 Gao, Chen, Kou, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaoguo Yang, eWFuZ3lhb2d1b0BjY211LmVkdS5jbg==
 Jianfeng Gao
Jianfeng Gao Yaoguo Yang
Yaoguo Yang