- 1Department of Interventional Radiology and Vascular Surgery, Peking University First Hospital, Beijing, China
- 2Department of Vascular Surgery, The Central Hospital of Wuhan, Wuhan, China
- 3Department of Interventional Radiology and Vascular Surgery, Peking University Third Hospital, Beijing, China
- 4Department of Interventional Radiology and Vascular Surgery, Nanjing First Hospital, Nanjing, China
- 5Department of Vascular Surgery, Peking University People's Hospital, Beijing, China
- 6Department of Vascular Surgery, Tianjin Medical University General Hospital, Tianjin, China
- 7Department of Vascular Surgery, The Second Hospital of Tianjin Medical University, Tianjin, China
- 8Department of Vascular Surgery, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, China
- 9Department of Vascular Surgery, Ningbo No. 2 Hospital, Ningbo, China
- 10Department of Vascular Surgery, Renmin Hospital of Wuhan University, Hubei General Hospital, Wuhan, China
- 11Department of Vascular Surgery, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 12Peripheral Vascular Department, Beijing University of Chinese Medicine Dongfang Hospital, Beijing, China
- 13Department of Vascular Surgery, People's Liberation Army (PLA) Strategic Support Force Characteristic Medical Center, Beijing, China
- 14Department of Vascular Surgery, Jiangsu Province Hospital, Nanjing, China
Background: Several paclitaxel-coated balloons have been proved to provide better efficacy results than uncoated balloons in femoropopliteal lesions. But the efficacy and safety of FREEWAY balloons have not been investigated in Chinese patients. This study aimed to evaluate the efficacy and safety performance of FREEWAY paclitaxel-coated balloons vs. uncoated balloons in Chinese femoropopliteal artery lesions.
Methods: In this prospective multi-center randomized controlled FREEWAY-CHINA study, 311 patients with symptomatic lower limb ischemia (Rutherford category 2–5) and femoropopliteal lesions of 14 Chinese centers were randomly assigned in a 1:1 ratio to endovascular treatment with either FREEWAY paclitaxel-coated balloons or uncoated balloons (control). The primary endpoint was the 6-month clinically-driven target lesion revascularization (CD-TLR) rate. Secondary endpoints included the device and technical success rate, the ankle-brachial indexes (ABIs), Rutherford category change, the 6-month primary and secondary patency rates, severe adverse effects, and the 12-month CD-TLR rate.
Results: The two groups were comparable in terms of their demographic and lesion characteristics. Patients' mean age was 70 years, and 70% were men. The mean lesion length was 71 mm. The 6-month CD-TLR rate was 2.6% in the FREEWAY group and 11.7% in the control group (P = 0.001). The 12-month CD-TLR rate was 2.7% in the FREEWAY group and 13.2% in the control group (P = 0.0005). Other endpoints, including patency rates, major adverse events, and ABI or Rutherford change, did not differ between the two groups.
Conclusion: The FREEWAY balloon resulted in an effective decrease in CD-TLR rates and had similar safety results compared to the uncoated balloon in Chinese femoropopliteal artery patients at the 12-month follow-up appointment.
Introduction
Peripheral artery disease (PAD) is the third leading cause of atherosclerotic cardiovascular morbidity and has become a global problem (1). Endovascular treatment has been increasingly performed in clinical practice worldwide and is recommended for more complex lesions (2–6). Percutaneous transluminal angioplasty (PTA) has an initial effect in restoring blood flow but is limited by vessel recoil, remodeling, and intimal hyperplasia (7). Stents have improved patency and technical success, but the patency rates of 12 months in lesions longer than 10 cm remain poor, ranging from 50 to 65% (8–10). Concerns about stent fractures and intractable in-stent restenosis lesions also exist (11, 12).
The drug-coated devices, including drug-coated balloon (DCB) and drug-eluting stents (DES), are emerging therapeutic methods that multiple randomized controlled trials have shown promising results at reducing restenosis, target lesion revascularization, and late lumen loss (13–19). Although paclitaxel-related mortality and amputation raised concerns in recent years (20, 21), paclitaxel-coated devices are not associated with increased mortality in multiple researches (22–25). DCBs vary in terms of the materials used to make the balloons, the coating techniques, the choice of the coating drugs, and the drug's release patterns at the site (26). Additionally, patients of different races or ethnicities may react differently to the same therapy (27). The efficacy and safety of the new DCB-FREEWAY balloon in China in the context of precise medicine needed to be examined. Thus, the FREEWAY-CHINA study (a prospective, multi-center, randomized controlled trial on the FREEWAY paclitaxel-coated balloon's safety and efficacy vs. the conventional uncoated balloon in the treatment of femoropopliteal artery lesions in China) sought to investigate the performance of the FREEWAY DCB in Chinese femoropopliteal patients.
Materials and Methods
Study Design
The FREEWAY-CHINA study is a prospective, multi-center, randomized, controlled, superiority trial conducted in 14 hospitals in China (see Supplementary Appendix for details). This study aimed to evaluate the safety and efficacy of the FREEWAY paclitaxel-coated balloon (EUROCOR GmbH, Bonn, Germany) in de novo and restenotic femoropopliteal artery lesions by comparing a FREEWAY DCB to an uncoated balloon (JOKER balloon, EUROCOR GmbH, Bonn, Germany). The coating was paclitaxel (3 mg/mm2), and the shellac was applied as excipient using a micro-pipetting procedure. Shellac is a natural resin composed of shellolic and alleuritic acid used to coat gastric-resistant tablets and is European Medicines Agency (EMA) and US Food and Drug Administration (FDA) approved and generally recognized as safe. Patients with successful pre-dilation were randomly assigned using a 1:1 ratio to either the FREEWAY DCB treatment group or the uncoated balloon control group by a central randomization computer system. Patients with unsuccessful pre-dilation were considered screening failures and were not included in the study.
Ethics
The study involving human participants was reviewed and approved by the Ethical Committees of the Peking University First Hospital and other participating centers. Patients provided written informed consent before enrolment. The trial was conducted following the Declaration of Helsinki and the provisions for the conduct of clinical trials of medical devices issued by the China Food and Drug Administration.
Patient Population
Patients eligible for inclusion in the trial had intermittent claudication or critical limb ischemia (Rutherford category 2–5) and an angiographically significant atherosclerotic lesion (diameter of stenosis ≥ 70%) in the superficial femoral or popliteal artery (or both). The total treated lesion length had to be 15 cm or less, and the reference diameter of the target vessel had to be 3–8 mm. Successful guidewire crossing and pre-dilation were acquired. Patent or successfully recanalized inflow vessels and at least one outflow vessel was needed.
Patients were excluded from the study if they met any of the following exclusion criteria: had severe calcification of the target vessel; had previously undergone surgery of the target femoropopliteal segment; had acute or subacute thrombosis or embolization of the target vessel; and/or had undergone adjuvant therapy (e.g., laser, directional or rotational atherectomy, cryoplasty, or scoring/cutting balloon). The complete inclusion and exclusion criteria are set out in the Supplementary Appendix.
Procedure and Medication
The target lesion was pre-dilated with a balloon/vessel ratio of 0.9:1–1:1 after successful lesion crossing during the operation. A FREEWAY DCB or a Joker PTA balloon was used according to the results of the randomization. For DCB sizing, the nominal balloon diameter had to match the reference vessel diameter distal to the target lesion. To secure full lesion coverage, the DCB or uncoated balloon's length was required to cover at least 1 cm proximal and distal to the lesion. DCB inflation time should be at least 180 s for the first dilation. In case of residual stenosis of >30% after the first balloon dilation, the same type of balloon was used for the second dilation for > 120 s. A new FREEWAY DCB had to be used in the FREEWAY group; however, the same Joker uncoated balloon could be used in the control group. After the second dilation, no treatment was needed if the residual stenosis was >30% but <50%. Laser-cut self-expanding nitinol stents had to be implanted when residual stenosis was >50% or D-type dissection was found after the second dilation, and covered stents were not allowed. If two or more DCBs were needed, the overlap had to be at least 0.5 cm. Each lesion was covered by 2 DCBs at most. A representative case is shown in Figure 1.
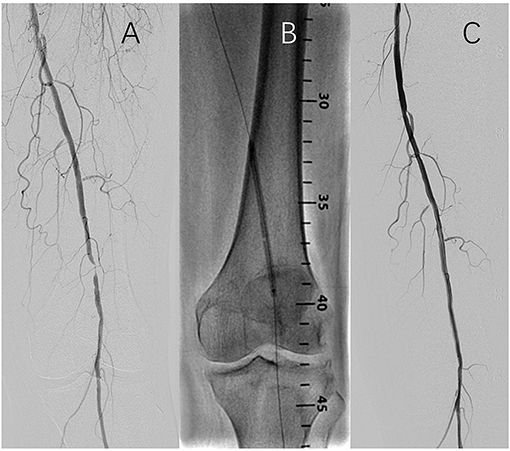
Figure 1. Representitive case of the endovascular procedure. (A) Baseline angiography; (B) Balloon inflation; (C) Completion angiography.
Clopidogrel was given for at least 3 days (75 mg/day) before or immediately after the intervention (300 mg). Heparin (3,000–5,000 units) was given intravenously during the procedure. After the procedure, clopidogrel (75 mg/day) was recommended for 4 weeks. Patients were given aspirin (100 mg daily/long-term) before and after endovascular treatment.
Follow-Up
A 30-day follow-up appointment was conducted by phone or in-house to evaluate patients' clinical status, medication compliance, and adverse events. Concerning patients' in-house follow-up clinical status, the calculation of ankle-brachial indexes (ABIs) at rest, duplex ultrasonography, and medicine compliance were performed after the procedure and at 6 and 12 months.
Endpoints and Definitions
The primary endpoint was the clinically-driven target lesion revascularization (CD-TLR) rate at the 6-month follow-up appointment. The term “clinically driven” was defined as an increase in the Rutherford grade and a Doppler ultrasound scan showing diameter stenosis of ≥ 70%. Revascularization included arterial endarterectomy, bypass grafting, or an endovascular procedure.
The secondary endpoints included: (1) Device success rate, which was defined as the percentage of successful balloon placement and expansion without rupture and successful withdrawal; (2) Technical success rate, which was defined as a percentage of residual stenosis of ≤ 30% after target lesion treatment; (3) an ABI increased by more than 0.1 (the ABIs at discharge and the follow-up appointment compared to the ABI at baseline); (4) The primary and secondary patency rates at the 6-month follow-up appointment. Patency was defined as a peak systolic velocity of ≤ 2.4 m/s, as evaluated by duplex ultrasonography; (5) Major adverse events including all-cause death, revascularization, or major amputation; (6) Any changes in Rutherford classification from preoperative to discharge, 30 days, 6 months, and 12 months; (7) The incidence of CD-TLR at 12 months after the procedure; and (8) Device-associated severe adverse effects anytime in the process of the trial. Severe adverse events were defined as definite, probable, or possible device-associated events that required patient hospitalization, a prolonged hospitalization time, caused disability or death, affected workability, or led to congenital malformations.
Statistical Analysis
This study was a superiority trial. It was assumed that the CD-TLR rate was 8% in the experimental group 6 months after the use of DCB, while the uncoated balloon's CD-TLR rate was 20% in the control group. The FREEWAY and control groups were allocated according to the ratio of 1:1, and each group had to comprise 131 patients (to achieve at least 80% power). Assuming a 15% attrition rate, a cohort of 312 patients was needed for the analysis.
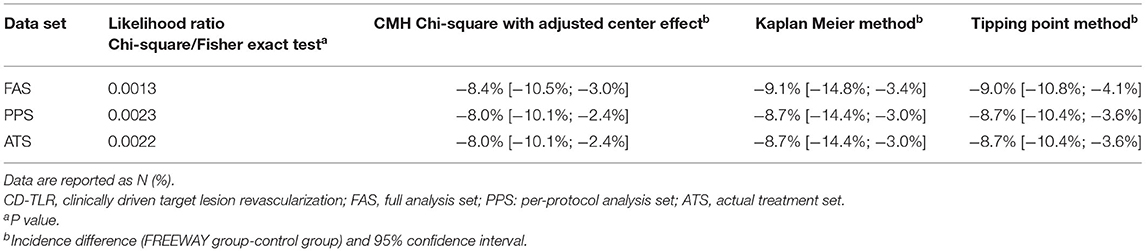
The outcomes were analyzed using the full analysis set (FAS) according to the intention-to-treat principle. Categorical data were described by frequency and ratio. Continuous data were described as mean ± standard deviation, maximum, minimum, median, 25th, and 75th quantile. Chi-square tests were used to compare the categorical data between the groups. An independent t-test was used to compare the continuous data of normal distribution. A Wilcoxon rank-sum test was used to compare the continuous data of non-normal distribution. For the 6-month CD-TLR rates, the likelihood ratio Chi-square/Fisher exact test, the Cochran–Mantel–Haenszel (CMH) Chi-square test with an adjusted center effect, the Kaplan Meier method, and the Tipping Point Method were used. A P value of 95% confidence interval (CI) was given on the FAS, per-protocol set (PPS), and actual treatment set (ATS). For the other 6-month and 12-month event rates, the likelihood ratio Chi-square/Fisher exact test was used. Mortality and adverse effects analyses were performed on the safety set (SS). All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NY, USA) at a bilateral 0.05 significance level.
Results
Patient and Procedural Characteristics
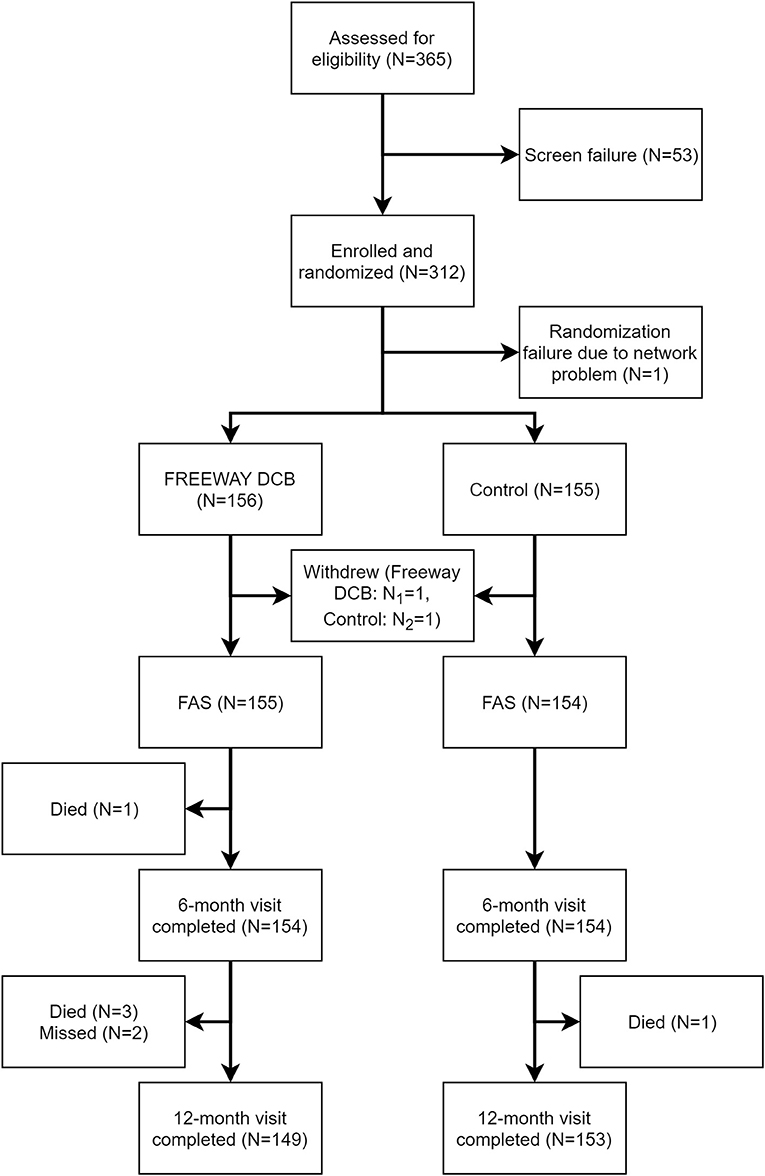
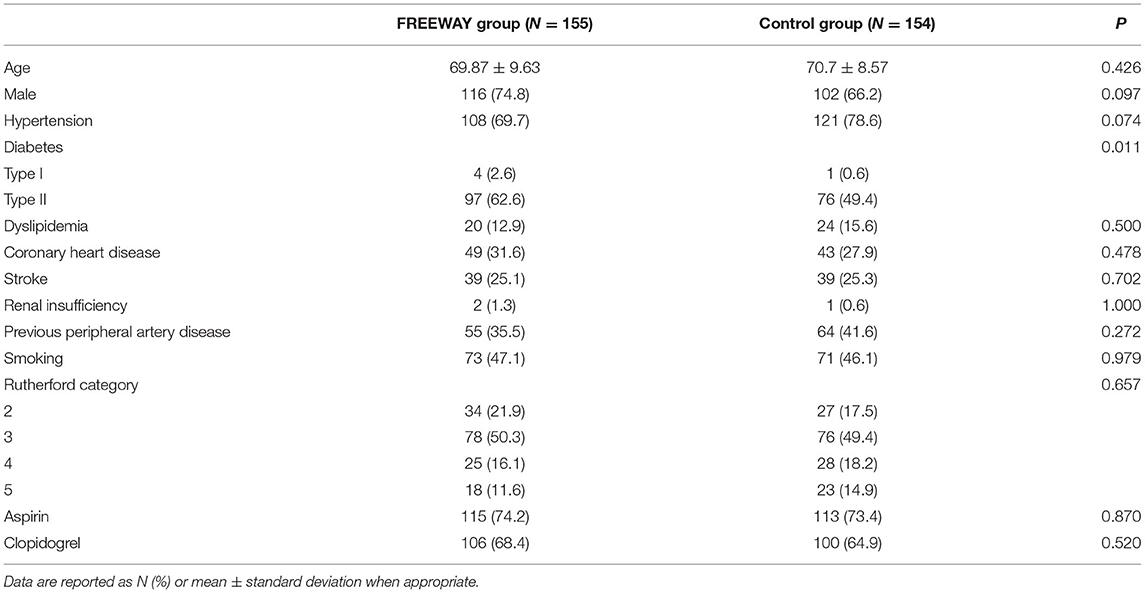
From July 9, 2015, to May 10, 2018, 311 patients (FREEWAY group:156, control group: 155) were enrolled at 14 centers in China, and the follow-up appointments were completed on May 4, 2019. Figure 2 shows the patient flowchart. The average age of patients was 69.87 ± 9.63 years old in the FREEWAY group and 70.70 ± 8.57 years old in the control group (P = 0.43). The proportion of males was 74.8% in the FREEWAY group and 66.2% in the control group (P = 0.10). The FREEWAY group had a higher proportion of diabetes (FREEWAY group: Type I 2.6%, Type II 62.6%, control group: Type I 0.6%, Type II 49.4%, P = 0.011). There was no significant difference between the two groups for the other risk factors, including hypertension, coronary heart disease, stroke, smoking, and renal insufficiency (see Table 1).
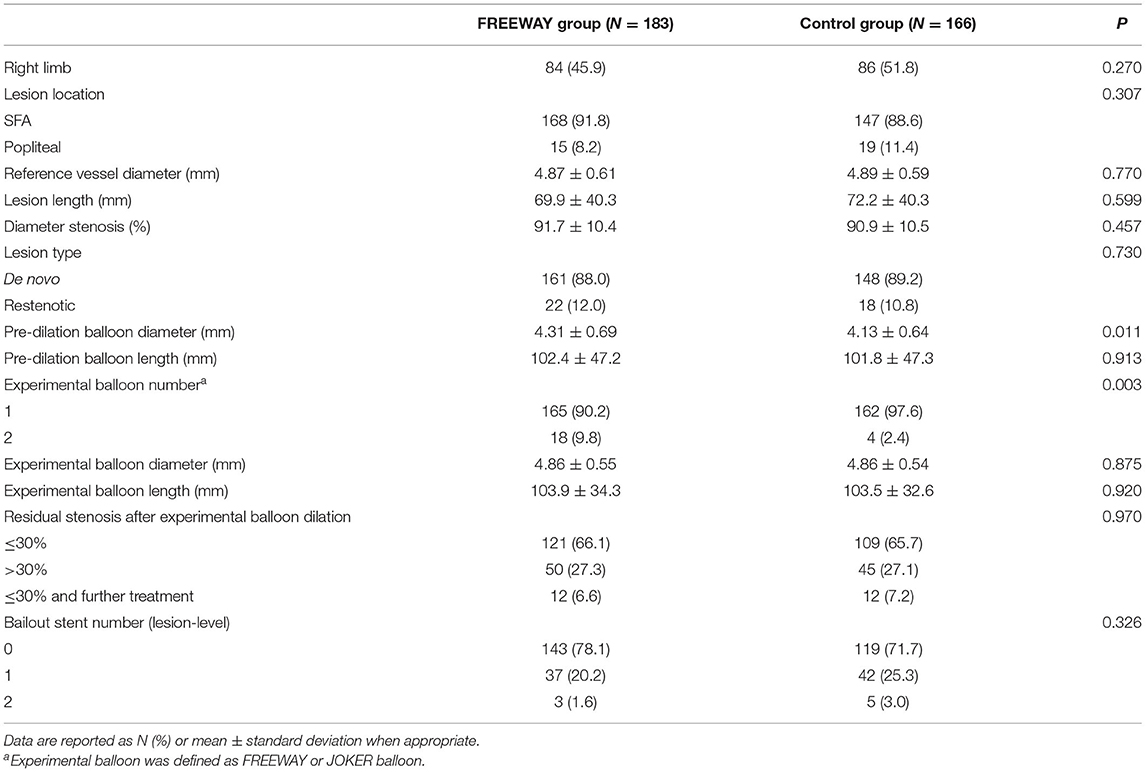
There were more cases of multiple lesions in the FREEWAY group (FREEWAY group: 1 lesion 83.9%, 2 lesions 14.2%, 3 lesions 1.9%, control group: 1 lesion 92.2%, 2 lesions 7.8%, 3 lesions 0%, P =0.03). There was no significant difference in reference vessel diameter, lesion length, and stenosis rate between the two groups. The pre-dilation balloon diameter was larger in the FREEWAY group than the control group (4.31 ± 0.69 mm vs. 4.13 ± 0.64 mm, P = 0.011). Second balloon dilation occurred in a higher proportion in the FREEWAY group (9.8 vs. 2.4%, P = 0.003). Bailout stenting after the second dilation was less frequent in the FREEWAY group than the control group(64.5 vs. 82.5%, P = 0.026); however, the overall bailout stent rate by patient-level did not differ between the groups (25.8 vs. 30.5%, P = 0.357). Details of the angiographic and procedural characteristics are shown in Table 2.
Endpoint Evaluation
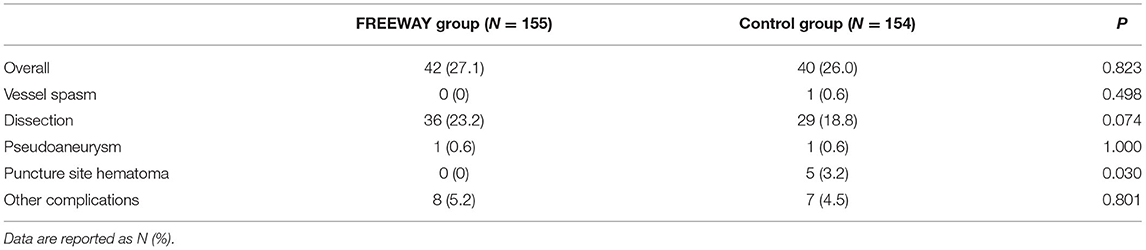
The overall complication rate was similar between the 2 groups (27.1 vs. 26.0%, P = 0.823), most of which were dissections (23.2 vs. 18.8%, P = 0.343). Hematomas at the puncture occurred more frequently in the control group than the FREEWAY group (0 vs. 3.2%, P = 0.030). Other complications did not differ between the two groups (see Table 3). The device success rates were 100% in both groups, and the technical success rate did not differ between the groups (FREEWAY group 96.2%, control group 98.2%, P = 0.343).
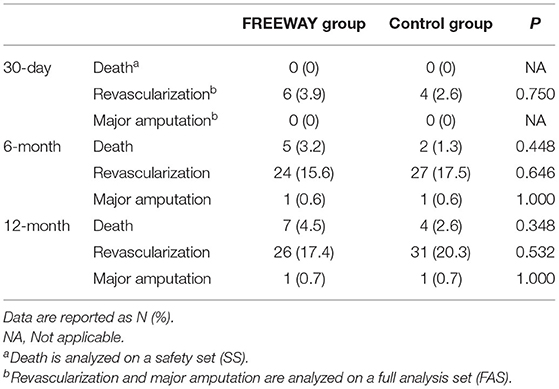
The 6-month CD-TLR rate was 2.6% in the FREEWAY group and 11.7% in the control group on FAS (P = 0.001). The 95% CI of the incidence difference of 6-month CD-TLR (FREEWAY group vs. control group) was <0% when analyzed using various FAS, PPS, and ATS, which proved the superiority of the FREEWAY balloon (see Table 4). The 6-month primary patency rate of the FREEWAY group was 70.1%, and that of the control group was 62.3%; thus, the rate was slightly higher in the FREEWAY group, but the difference was not significant (P = 0.115). The 6-month secondary patency rates were similar between the 2 groups (71.4% in the FREEWAY group and 71.0% in the control group, P = 0.786). The 12-month CD-TLR rate was 2.7% in the FREEWAY group and 13.2% in the control group (P = 0.0005). The rates of major adverse events including all-cause death, revascularization, and major amputation occurred in similar proportions in both groups at 1, 6, and 12 months (see Table 5). Additionally, the device-associated severe adverse effects did not differ between the FREEWAY and control groups (6.4 vs. 9.7%, P = 0.288).
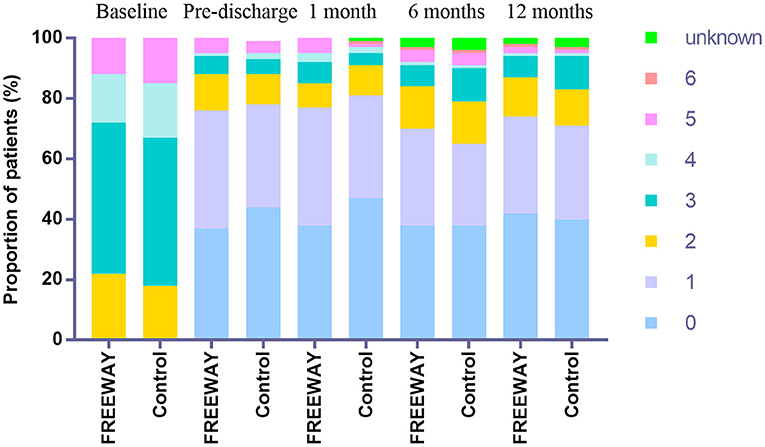
The ABI elevation of the target limb by at least 0.1 compared with the baseline was 80.5% in the FREEWAY group and 78.2% in the control group before discharge (P = 0.651). At the 6-month follow-up time, the ABI elevation of the target limb by at least 0.1 compared with the baseline was 68.8% in the FREEWAY group and 59.5% in the control group (P = 0.141). The percentage of the decline in the Rutherford classification categories was similar between the two groups at 6 (FREEWAY group 81.8%, control group 79.2%, P = 0.844) and 12 months (FREEWAY group 86.6%, control group 84.1%, P = 0.856). The distribution of Rutherford categories at the baseline and follow-up times are shown in Figure 3.
Discussion
The randomized controlled FREEWAY-CHINA study showed that the FREEWAY paclitaxel-coated balloon was superior to the uncoated balloon in terms of the 6- and 12-month CD-TLR rates in Chinese patients. Other efficacy endpoints, including 6-month primary/secondary patency rates, clinical status, and ABI, did not differ between the two groups. Safety endpoints, of the FREEWAY balloon, including all-cause death, revascularization, major amputation, and the device-associated severe adverse effects, were also similar between the FREEWAY and control groups.
Several randomized controlled trials have shown that paclitaxel-coated balloons have superior patency rates and freedom from CD-TLR rates than uncoated balloons (15, 16, 28–30). DCBs were shown to have higher primary patency (82.2 vs. 52.4%, P < 0.001) and lower CD-TLR rates (2.4 vs. 20.6%, P < 0.001) at 12 months in the IN. PACT SFA trial (16). Primary patency rates of 12 months were also shown to be higher in the DCB group than in the uncoated balloon group in the LEVENT 2 trial (65.2 vs. 52.6%, P = 0.02) (15). A low-dose paclitaxel-coated DCB was non-inferior to a high-dose paclitaxel-coated DCB concerning primary patency over 12 months in femoropopliteal interventions (31). The FREEWAY balloons provided better 12-month primary patency and freedom from CD-TLR rates than uncoated balloons in in-stent restenosis in femoropopliteal arteries in a PACUBA trial (32). The CD-TLR rates were also lower in the FREEWAY group than the control group in the present study (2.6 vs. 11.7% at 6 months, P = 0.001; 2.7 vs. 13.2%, P = 0.0005). Thus, the FREEWAY balloon could be another effective treatment option for DCBs for femoropopliteal patients.
Multiple trials have compared DCBs and uncoated balloons; however, most of the efficacy and safety results were obtained from Caucasian participants. As ethnicity may affect the pharmacological response to therapies, uncertainties about the use of paclitaxel-coated balloons in Chinese patients remain (27). Previously, the AcoArt I Trial was the only published randomized controlled trial to compare DCBs and uncoated balloons in 200 Chinese patients (28). Similarly, the target lesion revascularization rates were 7.2% in the Acotec DCB group and 39.6% in the control group (p < 0.001). The 6-month LLL and restenosis rate was also lower in the FREEWAY group in the AcoArt I Trial. The FREEWAY-CHINA trial results provide further evidence of the efficacy of paclitaxel-coated balloons in treating femoropopliteal lesions in Chinese patients.
The debate about paclitaxel's safety has been fierce since the publication of a meta-analysis showing higher 2- and 5-year all-cause mortality rates and heightened risk of major amputation in the paclitaxel group than control (20, 21). Although an association between increased all-cause mortality with paclitaxel was not found in large cohort studies and patient-level meta-analysis of randomized trials, concerns remain (22, 24, 33–37). In the present study, in which the follow-up rate was 98.7% in the FREEWAY group and 100% in the control group in the SS, the 12-month all-cause mortality and major amputation rates did not differ (P = 0.348 and P = 1.000). Thus, the FREEWAY balloon is safe for Chinese patients.
This study had several limitations. First, the endpoint evaluation was not performed by independent core laboratories. Second, patients with severe calcified and long lesions were not enrolled in this study, limiting the extrapolation. Third, long-term mortality data need to be examined further to prove FREEWAY balloons' safety in Chinese patients.
Conclusions
In conclusion, in the multi-center, randomized controlled, FREEWAY-CHINA study, we demonstrated that the 6- and 12-month CD-TLR rates were lower in the FREEWAY group than the uncoated balloon group, and the safety results were similar between the FREEWAY group and the uncoated balloon group.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study involving human participants was reviewed and approved by Ethical Committees of the Peking University First Hospital, The Central Hospital of Wuhan, Peking University Third Hospital, Nanjing First Hospital, Peking University People's Hospital, Tianjin Medical University General Hospital, The Second Hospital of Tianjin Medical University, Shanghai Ninth People's Hospital, Ningbo No. 2 Hospital, Renmin Hospital of Wuhan University, Hubei General Hospital, Beijing Friendship Hospital, Beijing University of Chinese Medicine Dongfang Hospital, PLA Strategic Support Force Characteristic Medical Center and Jiangsu Province Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
BZ and MY contributed in writing the manuscript. YZ contributed in organizing, supervising, and revising. All the other authors contributed in organizing the study and revising the manuscript.
Funding
This study was funded by Shanghai Micro Medical Devices.
Conflict of Interest
The authors declare that this study received funding from Shanghai Micro Medical Devices. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.686267/full#supplementary-material
References
1. Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. (2013) 382:1329–40. doi: 10.1016/S0140-6736(13)61249-0
2. Rowe VL, Lee W, Weaver FA, Etzioni D. Patterns of treatment for peripheral arterial disease in the United States: 1996-2005. J Vasc Surg. (2009) 49:910–7. doi: 10.1016/j.jvs.2008.11.054
3. Committee TS, Jaff MR, White CJ, Hiatt WR, Fowkes GR, Dormandy J, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the inter-society consensus for the management of peripheral arterial disease (TASC II). J Endovasc Ther. (2015) 22:663–77. doi: 10.1177/1526602815592206
4. Park YY, Joh JH, Han SA, Kim SH, Cho S, Park HC, et al. National trends for the treatment of peripheral arterial disease in Korea between 2004 and 2013. Ann Surg Treat Res. (2015) 89:319–24. doi: 10.4174/astr.2015.89.6.319
5. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2017) 135:e686–725. doi: 10.1161/CIR.0000000000000470
6. Wright MA, Steffens D, Huilgol RL. Vascular surgery trends in Australia: 2001-2015: less open surgery, less limb loss and more endovascular intervention. ANZ J Surg. (2019) 89:309–13. doi: 10.1111/ans.14878
7. Brahmbhatt A, Misra S. Techniques in vascular and interventional radiology drug delivery technologies in the superficial femoral artery. Tech Vasc Interv Radiol. (2016) 19:145–52. doi: 10.1053/j.tvir.2016.04.007
8. Bosiers M, Deloose K, Callaert J, Moreels N, Keirse K, Verbist J, et al. Results of the protege EverFlex 200-mm-long nitinol stent (ev3) in TASC C and D femoropopliteal lesions. J Vasc Surg. (2011) 54:1042–50. doi: 10.1016/j.jvs.2011.03.272
9. Lammer J, Zeller T, Hausegger KA, Schaefer PJ, Gschwendtner M, Mueller-Huelsbeck S, et al. Heparin-bonded covered stents versus bare-metal stents for complex femoropopliteal artery lesions: the randomized VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive surface [VIA] versus bare nitinol stent in the treatment of long lesions in superficial femoral artery occlusive disease). J Am Coll Cardiol. (2013) 62:1320–7. doi: 10.1016/j.jacc.2013.05.079
10. Liistro F, Grotti S, Porto I, Angioli P, Ricci L, Ducci K, et al. Drug-eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE-SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery). JACC Cardiovasc Interv. (2013) 6:1295–302. doi: 10.1016/j.jcin.2013.07.010
11. Rits J, van Herwaarden JA, Jahrome AK, Krievins D, Moll FL. The incidence of arterial stent fractures with exclusion of coronary, aortic, and non-arterial settings. Eur J Vasc Endovasc Surg. (2008) 36:339–45. doi: 10.1016/j.ejvs.2008.05.005
12. van den Berg JC. In-stent restenosis management: the best is yet to come. J Cardiovasc Surg. (2017) 58:508–17. doi: 10.23736/S0021-9509.17.09953-0
13. Fanelli F, Cannavale A, Corona M, Lucatelli P, Wlderk A, Salvatori FM. The “DEBELLUM”–lower limb multilevel treatment with drug eluting balloon–randomized trial: 1-year results. J Cardiovascular Surg. (2014) 55:207–16.
14. Scheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, et al. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. (2014) 7:10–9. doi: 10.1016/j.jcin.2013.05.022
15. Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. (2015) 373:145–53. doi: 10.1056/NEJMoa1406235
16. Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. (2015) 131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004
17. Tepe G, Schnorr B, Albrecht T, Brechtel K, Claussen CD, Scheller B, et al. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv. (2015) 8(1 Pt A):102–8. doi: 10.1016/j.jcin.2014.07.023
18. Bausback Y, Willfort-Ehringer A, Sievert H, Geist V, Lichtenberg M, Del Giudice C, et al. Six-month results from the initial randomized study of the ranger paclitaxel-coated balloon in the femoropopliteal segment. J Endovasc Ther. (2017) 24:459–67. doi: 10.1177/1526602817710770
19. Gray WA, Keirse K, Soga Y, Benko A, Babaev A, Yokoi Y, et al. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. Lancet. (2018) 392:1541–51. doi: 10.1016/S0140-6736(18)32262-1
20. Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. (2018) 7:e011245. doi: 10.1161/JAHA.118.011245
21. Katsanos K, Spiliopoulos S, Teichgräber U, Kitrou P, Del Giudice C, Björkman P, et al. Risk of major amputation following application of paclitaxel coated balloons in the lower limb arteries: a systematic review and meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg. (2021) 62:140–1. doi: 10.1016/j.ejvs.2021.05.027
22. Secemsky EA, Kundi H, Weinberg I, Jaff MR, Krawisz A, Parikh SA, et al. Association of survival with femoropopliteal artery revascularization with drug-coated devices. JAMA Cardiol. (2019) 4:332–40. doi: 10.1001/jamacardio.2019.0325
23. Böhme T, Noory E, Beschorner U, Jacques B, Bürgelin K, Macharzina R, et al. Evaluation of mortality following paclitaxel drug-coated balloon angioplasty of femoropopliteal lesions in the real world. JACC Cardiovasc Interv. (2020) 13:2052–61. doi: 10.1016/j.jcin.2020.04.050
24. Bertges DJ, Sedrakyan A, Sun T, Eslami MH, Schermerhorn M, Goodney PP, et al. Mortality after paclitaxel coated balloon angioplasty and stenting of superficial femoral and popliteal artery in the vascular quality initiative. Circ Cardiovasc Interv. (2020) 13:e008528. doi: 10.1161/CIRCINTERVENTIONS.119.008528
25. Kumins NH, King AH, Ambani RN, Thomas JP, Bose S, Shishehbor MH, et al. Paclitaxel-coated peripheral artery devices are not associated with increased mortality. J Vasc Surg. (2020) 72:968–76. doi: 10.1016/j.jvs.2019.10.100
26. Bukka M, Rednam PJ, Sinha M. Drug-eluting balloon: design, technology and clinical aspects. Biomed Mater. (2018) 13:032001. doi: 10.1088/1748-605X/aaa0aa
27. Zhang F, Finkelstein J. Inconsistency in race and ethnic classification in pharmacogenetics studies and its potential clinical implications. Pharmgenomics Pers Med. (2019) 12:107–23. doi: 10.2147/PGPM.S207449
28. Jia X, Zhang J, Zhuang B, Fu W, Wu D, Wang F, et al. Acotec drug-coated balloon catheter: randomized, multicenter, controlled clinical study in femoropopliteal arteries: evidence from the AcoArt I Trial. JACC Cardiovasc Interv. (2016) 9:1941–9. doi: 10.1016/j.jcin.2016.06.055
29. Schroeder H, Werner M, Meyer DR, Reimer P, Krüger K, Jaff MR, et al. Low-dose paclitaxel-coated versus uncoated percutaneous transluminal balloon angioplasty for femoropopliteal peripheral artery disease: one-year results of the ILLUMENATE European randomized clinical trial (Randomized Trial of a Novel Paclitaxel-Coated Percutaneous Angioplasty Balloon). Circulation. (2017) 135:2227–36. doi: 10.1161/CIRCULATIONAHA.116.026493
30. Steiner S, Willfort-Ehringer A, Sievert H, Geist V, Lichtenberg M, Del Giudice C, et al. 12-month results from the first-in-human randomized study of the ranger paclitaxel-coated balloon for femoropopliteal treatment. JACC Cardiovasc Interv. (2018) 11:934–41. doi: 10.1016/j.jcin.2018.01.276
31. Steiner S, Schmidt A, Zeller T, Tepe G, Thieme M, Maiwald L, et al. COMPARE: prospective, randomized, non-inferiority trial of high- vs. low-dose paclitaxel drug-coated balloons for femoropopliteal interventions. Eur Heart J. (2020) 41:2541–52. doi: 10.1093/eurheartj/ehaa049
32. Kinstner CM, Lammer J, Willfort-Ehringer A, Matzek W, Gschwandtner M, Javor D, et al. Paclitaxel-eluting balloon versus standard balloon angioplasty in in-stent restenosis of the superficial femoral and proximal popliteal artery: 1-year results of the PACUBA trial. JACC Cardiovasc Interv. (2016) 9:1386–92. doi: 10.1016/j.jcin.2016.04.012
33. Beckman JA, White CJ. Paclitaxel-coated balloons and eluting stents: is there a mortality risk in patients with peripheral artery disease? Circulation. (2019) 140:1342–51. doi: 10.1161/CIRCULATIONAHA.119.041099
34. Schneider PA, Laird JR, Doros G, Gao Q, Ansel G, Brodmann M, et al. Mortality not correlated with paclitaxel exposure: an independent patient-level meta-analysis of a drug-coated balloon. J Am Coll Cardiol. (2019) 73:2550–63. doi: 10.1016/j.jacc.2019.01.013
35. Dake MD, Ansel GM, Bosiers M, Holden A, Iida O, Jaff MR, et al. Paclitaxel-coated zilver PTX drug-eluting stent treatment does not result in increased long-term all-cause mortality compared to uncoated devices. Cardiovasc Intervent Radiol. (2020) 43:8–19. doi: 10.1007/s00270-019-02324-4
36. Freisinger E, Koeppe J, Gerss J, Goerlich D, Malyar NM, Marschall U, et al. Mortality after use of paclitaxel-based devices in peripheral arteries: a real-world safety analysis. Eur Heart J. (2020) 41:3732–9. doi: 10.1093/eurheartj/ehz698
Keywords: drug-coated balloon, femoropopliteal lesions, randomized controlled trials, target lesion revascularization, FREEWAY
Citation: Zhang B, Yang M, He T, Li X, Gu J, Zhang X, Dai X, Li X, Lu X, Lang D, Hu H, Chen X, Yang B, Gu H, Zhang X and Zou Y (2021) Twelve-Month Results From the First-in-China Prospective, Multi-Center, Randomized, Controlled Study of the FREEWAY Paclitaxel-Coated Balloon for Femoropopliteal Treatment. Front. Cardiovasc. Med. 8:686267. doi: 10.3389/fcvm.2021.686267
Received: 23 June 2021; Accepted: 19 August 2021;
Published: 10 September 2021.
Edited by:
Stavros Spiliopoulos, National and Kapodistrian University of Athens, GreeceReviewed by:
Ioannis Paraskevopoulos, University of Ioannina, GreecePanagiotis Kitrou, General University Hospital of Patras, Greece
Copyright © 2021 Zhang, Yang, He, Li, Gu, Zhang, Dai, Li, Lu, Lang, Hu, Chen, Yang, Gu, Zhang and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinghua Zou, cGt1em91eWh1YUAxNjMuY29t
†These authors have contributed equally to this work
 Bihui Zhang
Bihui Zhang Min Yang
Min Yang Tao He2
Tao He2 Xiangchen Dai
Xiangchen Dai