
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 14 July 2021
Sec. Heart Failure and Transplantation
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.684919
This article is part of the Research TopicImproving Early Detection and Risk Prediction in Heart FailureView all 14 articles
Aim: The present study was established to investigate the use of the serum cystatin C/prealbumin (Cys-C/PAB) ratio as a predictive factor for long-term prognosis in patients with chronic heart failure.
Methods: We divided our retrospective cohort of 6,311 patients admitted to hospital due to an episode of heart failure (HF) into three groups according to the Cys-C/PAB ratio. The endpoints were cardiovascular and all-cause mortality. Median follow-up time were 3.3 years (2–8 years), during which 2,945 (46.7%) patients died.
Results: The Cys-C/PAB ratio was revealed to be an independent predictor of cardiovascular mortality (HR: 1.12, 95% CI: 1.15–1.23, P < 0.01) and all-cause mortality (HR: 1.19, 95% CI: 1.13–1.24, P < 0.01) by multivariable Cox analysis. Integrated discrimination improvement (IDI) showed that the Cys-C/PAB ratio in conjunction with the level of N-terminal pro-B-type natriuretic peptide (NT-proBNP) conferred a significant improvement in predicting individual risks of cardiovascular (P = 0.023) and all-cause (P = 0.028) mortality. For those with a high Cys-C/PAB ratio in combination with a high NT-proBNP level, the long-term cardiovascular mortality risk ratio was 8.6-times higher than for those with low values, and 7.51-times for all-cause mortality. Our study also showed that Cys-C/PAB and NT-proBNP in combination displayed higher value for the prediction of cardiovascular and all-cause in-hospital mortality in patients with HF.
Conclusions: The Cys-C/PAB ratio is valuable for predicting cardiovascular and all-cause mortality in patients with HF and offers additional information to that provided by NT-proBNP.
It is estimated that about 26 million adults worldwide suffer from heart failure (HF), a number that is expected to increase in the coming decades (1, 2). Despite advances in medical therapy, there has been only a modest improvement in the survival rate of HF in the 21st century, with both 1-year readmission and mortality rates ranging from 30 to 50% and the 5-year mortality reaching 50% (3, 4). Thus, there is an urgent need to establish an individualized therapeutic approach in HF patients, which has been boosted by the availability of biomarker and relative mechanisms-guided management in prognostication, diagnosis, and treatment (5, 6). The most commonly used indicator for HF diagnosis and prognosis is N-terminal pro-B-type natriuretic peptide (NT-proBNP) since it has a longer plasma half-life and less biological variation than those of BNP (7). In addition to NT-proBNP, several other biomarkers possess prognostic value in patients with HF; cystatin C (Cys-C), and prealbumin (PAB) have been extensively studied.
Cys-C, a small 13-kDa endogenous cysteine proteinase inhibitor, is constitutively produced by all nucleated cells. In the kidney, Cys-C is removed from circulation by glomerular filtration and subsequently reabsorbed and catabolized by the proximal convoluted tubules (8). Given this background, it has been proposed that the level of circulating Cys-C may be an appropriate early biomarker of renal impairment. It has also been shown that Cys-C is associated with remodeling of the heart extracellular matrix (9). In addition, it has been revealed that blood and/or myocardium from different animal models of cardiac injury exhibit excessive Cys-C levels (9, 10). Clinically, plasma Cys-C levels are correlated with diastolic dysfunction, left ventricular hypertrophy (LVH), and mortality in HF patients (11, 12). Several studies have reported that baseline Cys-C levels can play a prognostic role in rehospitalization and all-cause mortality in acute decompensated HF (13–15) and chronic heart failure (CHF) (16, 17).
PAB is a visceral protein that exhibits a rapid turnover and reflects the status of whole-body nitrogen metabolism. As a protein with a shorter half-life than that of albumin, PAB can be used as a more precise estimation of a patient's current inflammatory and nutritional status (18), which are associated with a higher mortality rate and longer hospitalization in HF patients (19–21). Additionally, in patients suffering from acute coronary syndrome, lower serum PAB levels (<17 mg/dl) at admission have been shown to be independently predictive of subsequent major adverse cardiac events while hospitalized (22). Moreover, two different studies have demonstrated that low PAB levels (<15 mg/dl) are linked to an increase in short-term mortality and readmission in HF patients (23, 24).
It has been reported that increased Cys-C concentrations are significantly correlated with a higher risk of cardiovascular-related death in the long-term (median follow-up of 2.5 years) (15). A positive correlation has also been found between Cys-C levels and 5-year all-cause mortality in patients with CHF (25), however, an association between low PAB levels and long-term prognosis in HF patients has not yet been revealed. In the present study, we hypothesized that the Cys-C/PAB ratio would be a significant biomarker for the prediction of long-term outcome in HF patients, and combining this ratio with NT-proBNP would further improve risk stratification.
Retrospective clinical data were collected from HF patients hospitalized in the Department of Cardiology, Shengjing Hospital of China Medical University, Shenyang, China, between January 2013 and December 2018, with which we established a database. HF was diagnosed according to signs and symptoms, echocardiography, and the results of laboratory tests, as recommended by current guidelines. Heart failure with reduced ejection fraction (HFrEF) was defined as left ventricular ejection fraction (LVEF) <40%, HF with mid-range LVEF (HFmrEF) as 40% ≤ LVEF <50%, and HF with preserved LVEF (HFpEF) as LVEF ≥ 50% (26). In accordance with the cardiac function classification published by the New York Heart Association (NYHA), heart function was divided into four levels (I–IV). Patients displaying evidence of acute myocardial infarction, renal failure, severe anemia, or severe infection were excluded. The follow-up was assessed in December 2020. Patients' survival status was investigated using the population death information registration management system of the Disease Control and Prevention Center of Liaoning Province, and cardiac and non-cardiac mortality was determined in accordance with the International Classification of Diseases (ICD) code of death diagnosis. When information was not available in the system, it was obtained from medical records, patients' physicians, or patients' relatives via telephone. Efforts were made to determine the nature of death in each case. Median follow-up time were 3.3 years (2–8 years). This study was approved by the Shengjing Hospital of China Medical University Ethics Committee and carried out in accordance with the Declaration of Helsinki.
The investigators extracted comprehensive clinical data from electronic medical records. The obtained variables included patient demographics, past cardiac and non-cardiac history, physical examination results, laboratory test results, and echocardiography. The laboratory tests of fasting peripheral venous blood samples taken on the day of admission or on the next morning included the following: white blood cell (WBC) counts, platelet counts; and levels of albumin, prealbumin, hemoglobin, glycated hemoglobin, triglyceride, low-density lipoprotein (LDL-C), blood urea nitrogen (BUN), creatinine, uric acid, potassium ion (K+), serum sodium ion (Na+), troponin I (cTNI), and NT-proBNP. Venous blood samples were tested within 2 h of blood collection in all cases. A particle-enhanced immunonephelometric assay was employed using a Beckman AU 5800 analyzer (Beckman Coulter, Brea, CA, USA) to measure Cys-C and PAB levels. The reference ranges for serum Cys-C and PAB concentrations were 0.59-1.03 mg/L and 18-45 mg/dl, respectively. Left ventricular ejection fraction (LVEF) was determined by echocardiography using the biplane Simpson method within 3 days of admission (27).
Quantitative variables that normally distributed were compared using one-way analysis of variance and are expressed as the mean ± standard deviation (SD). Quantitative variables with a non-normal distribution were compared using the Mann–Whitney U-test and are expressed as the median (interquartile range, IQR). Categorical variables were compared using the chi-square test and are presented as counts and proportions (%). The association of variables with survival was assessed using univariate and multivariable Cox regression models and reported as the hazard ratio (HR) [95% confidence interval (CI)]. We used C statistics to quantify the ability of Cys-C/PAB ratio to identify patients who died, in addition, continuous net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were performed to assess the incremental prognostic value of Cys-C/PAB ratio (28). Patients were allocated to three groups according to tertiles of the Cys-C/PAB ratio and NT-proBNP level (low, medium, high). These two indexes were combined to compare and analyze the HR values. We assigned 0, 1, or 2 points to members of the tertiles of the Cys-C/PAB ratio and NT-proBNP level, with a maximum score of 4. Effects of the Cys-C/PAB ratio and NT-proBNP levels on survival were visualized using Kaplan-Meier curves, and the log-rank test was used to make comparisons. According to the AUROC curve, the Cys-C/PAB ratio in combination with the NT-proBNP level was predictive of cardiovascular and all-cause mortality in HF patients with different types of LVEF. All tests were two-sided, with P < 0.05 indicating statistical significance. The Medcalc, and SPSS version 23.0 and SAS9.4 software were used for statistical analysis.
Our cohort retrospectively included 7,563 HF patients hospitalized from January 2013 to December 2018. Those with acute myocardial infarction, severe anemia, renal failure, and severe infection were excluded from this study (Supplementary Figure 1), resulting in a final cohort of 6,311 patients consisting of 2,104 with a low ( ≤ 0.061) Cys-C/PAB ratio, 2,104 with a medium (>0.061 but ≤ 0.102) Cys-C/PAB ratio, and 2103 with a high (>0.102) Cys-C/PAB ratio. Baseline patient characteristics and the occurrence of risk factors according to tertiles of the Cys-C/PAB ratio are shown in Table 1.
The primary endpoint of all-cause mortality was reached in 2,945 (46.7%) HF patients during the follow-up, which included 2,071 (32.8%) cardiovascular-related deaths. Univariate Cox regression showed that the Cys-C/PAB ratio was significantly correlated with survival [HR: 1.34 (95% CI, 1.32–1.37), all-cause mortality; HR: 1.33 (95% CI, 1.30–1.37), cardiovascular mortality]. In the multivariable model, following adjustment for age, sex, NYHA class, CAD, Hypertension, Diabetes mellitus, Atrial fibrillation, Previous MI, COPD, SBP, DBP, Heart rate, WBC, Hemoglobin, Platelet, Albumin, LDL-C, Triglycerides, HbA1c,BUN, Creatinine, Uric acid, Potassium, Sodium, Troponin I, LVEDV, LVESV, LVEF. The Cys-C/PAB ratio remained a significant predictive factor of all-cause [adjusted HR: 1.19 (95% CI, 1.16–1.23) and cardiovascular (adjusted HR: 1.19 (95% CI, 1.13–1.24)] mortality (Table 2). Our findings also revealed significant associations between NT-proBNP levels and cardiovascular and all-cause mortality (Table 2).
The AUROC and integrated discrimination improvement revealed that the Cys-C/PAB ratio significantly improved the prediction of the individual risk of cardiovascular and all-cause mortality when added to NT-proBNP levels (Table 3). Patients were stratified into nine groups according to their tertiles of the Cys-C/PAB ratio and NT-proBNP levels with a view to analyzing the potential additive prognostic value of the former. We observed graduated increases in risk for those in the higher tertiles with respect to both markers. The risk ratio for long-term all-cause mortality for patients with a high Cys-C/PAB ratio in combination with high NT-proBNP levels was 7.51-times higher than for those with low levels, and 8.6-times higher for cardiovascular mortality (Figure 1). We assigned 0, 1, or 2 points to members of the tertiles of the Cys-C/PAB ratio and NT-proBNP level, with a maximum score of 4. Figure 2 shows the Kaplan–Meier survival curves and cardiovascular and all-cause mortality according to Cys-C/PAB ratio and scores of Cys-C/PAB ratio combined with NT-proBNP.
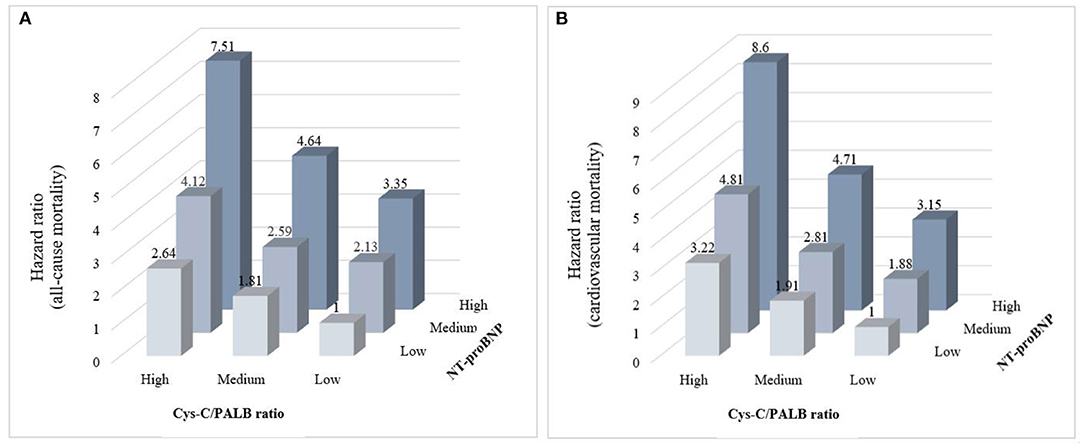
Figure 1. Relative risk stratified by combined tertiles of Cys-C/PAB ratio and NT-proBNP for all-cause mortality (A) and cardiovascular mortality (B).
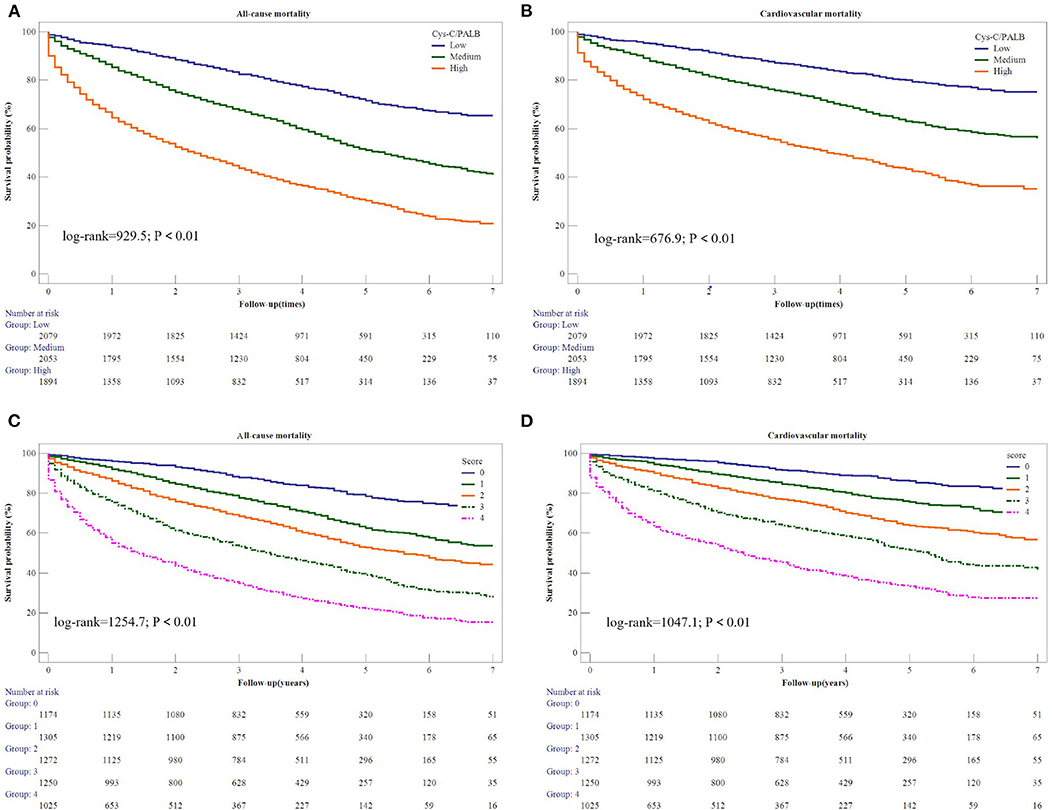
Figure 2. Kaplan-Meier estimates of all-cause mortality (A) and cardiovascular mortality (B) based on Cys-C/PAB ratio. Kaplan-Meier estimates of all-cause mortality (C) and cardiovascular mortality (D) based on scores of Cys-C/PAB ratio and NT-proBNP.
The AUC values for the Cys-C/PAB ratio in combination with NT-proBNP levels for predicting in-hospital, 1-, 3-, 5-, and 8-year all-cause mortality were 0.785, 0.768, 0.742, 0.740, and 0.743, respectively (Table 4), while those for predicting cardiovascular mortality were 0.785, 0.766, 0.727, 0.718, and 0.715, respectively. In the subgroup analysis, we found that the Cys-C/PAB ratio in combination with NT-proBNP levels probably had a greater ability to discriminate the risk of mortality for patients with HFpEF than for patients with HFrEF (Table 4).
Among the 6,311 patients hospitalized due to HF, 2,945 (46.7%) died during the follow-up period (Median 3.3 years, range 2–8 years). Our findings show that cardiovascular and all-cause mortality in patients with HF could be predicted independently by both high Cys-C levels and low PAB levels. Thus, it is unsurprising that a higher Cys-C/PAB ratio provided great value for predicting all-cause and cardiovascular mortality. When this ratio was combined with NT-proBNP, even better prognostic prediction was achieved in HF patients in the long term. Our results show that the risk ratio of long-term all-cause mortality for patients with a high Cys-C/PAB ratio in combination with high NT-proBNP levels was 7.51-times higher than for those with low levels, and 8.6-times for cardiovascular mortality. This study also shows high value of the Cys-C/PAB ratio in combination with NT-proBNP levels for predicting in-hospital or long-term all-cause and cardiovascular mortality in HF patient.
HF is a complex syndrome involving different pathophysiological pathways (e.g., remodeling, myocardial injury, fibrosis, and inflammation), the components of which can be reflected by various biomarkers, including natriuretic peptides, PAB, galectin-3, soluble suppressor of tumorgenicity 2 (ST-2), Cys-C, interleukin-6 (IL-6), highly sensitive troponin, and procalcitonin (21, 29). These pathways may provide biomarkers that could act as a clinical bridge between HF and potential treatment strategies (5). In vitro, cardiomyocytes and fibroblasts release an excess of Cys-C upon exposure to oxidative stress, which has also been confirmed by in vivo studies (9), and Cys-C can in turn promote cardiomyocyte injury and autophagy (30, 31) under oxidative stress. In addition, an increase in Cys-C may be positively associated with osteopontin, a profibrotic matricellular protein associated with myocardial fibrosis in HF patients (32). It may also inhibit the degradation of tissue inhibitor of metalloproteinases-1 (TIMP-1) and osteopontin, promoting myocardial fibrosis and aggravating ventricular remodeling, which leads to a vicious cycle (33). Studies have also demonstrated that Cys-C can selectively inhibit the activity of cystine protease, reduce elastic fiber degradation in cardiomyocytes, and increase the destruction of myocardial collagen fibers, disturbing cardiac structure and function (34, 35). These mechanisms may explain why clinical studies have shown a significant association between Cys-C and diastolic dysfunction and left ventricular hypertrophy (LVH) in HF patients, in addition to other indicators of renal function such as eGFR and serum creatinine (12, 36).
Malnutrition is commonly found in patients suffering from chronic conditions, including HF. Its prevalence in HF patients has been reported to vary from 20 to 70% (37). Albumin, a well-established prognostic factor in patients with HF, is a biomarker reflecting nutritional status, while with a half-life up to 17 days (38), it is insensitive to changes of nutritional status. PAB, known as a transthyroxine protein, is a complex molecule of a non-glycosylated protein and a retinol-binding protein. The reduced sensitivity to hydration status, small pool, and short half-life (2 days) promotes its use in detecting early deficits in nutritional status (39). Thus, PAB is now accepted as a more accurate biomarker of nutritional status, with a higher sensitivity than albumin to changes in nutrition. PAB is an acute-phase protein whose levels are decreased under inflammatory conditions due to cytokine stimulation (40). Therefore, in addition to being a nutritional marker, PAB may be an inflammatory reactant during the acute stage (23, 41). Franco et al. showed a significant correlation of high CRP levels with low PAB levels in patients with acute HF, indicating that systemic inflammation may also occur in patients suffering from protein malnutrition (23), contributing to a worse outcome.
Regarding the future application of prognostic biomarkers, guidance for therapy and rehabilitation is potentially the most valuable use. It should be borne in mind that changes in biomarkers themselves are not as important as determinants of the outcome; instead, their cause and the clinical context during which these changes develop are most important. As suggested by D'Elia et al. (42), biomarkers may act as a clinical bridge between the pathophysiological changes in HF and potential treatment strategies. As mentioned above, there are associations between Cys-C and ventricular remodeling and myocardial fibrosis, and therapeutic strategies aimed at reducing these processes may achieve certain cardioprotective results in patients with HF. In fact, Lopez et al. showed that treatment with various diuretics that act at the ascending limb of the loop of Henle may have different long-term effects on myocardial fibrosis in patients suffering from chronic failure. Moreover, patients treated with torasemide, but not those treated with furosemide, displayed decreased type I collagen synthesis and decreased accumulation of myocardial collagen (43). This indicates the need for more sophisticated mechanisms concerning biomarkers and HF pathophysiological changes in order to perform individualized therapy and improve prognosis in HF patients. Since low PAB levels are representative of malnutrition or inflammation, we should clinically assess the intake or absorption of nutrients by taking patient history or providing adequate feeding. If malnutrition is ruled out, treatment of the disease causing the inflammation and excessive cytokine production should be considered (44).
There are a few limitations of this study. Firstly, the single-center retrospective nature of this work confers an inherent limitation associated with retrospective investigation, preventing us from ruling out the effects of residual or unmeasured confounding factors. Retrospective studies are also inherently associated with a risk of selection bias. Secondly, the Cys-C, NT-proBNP and PAB levels were measured at baseline in our study without dynamic monitoring, limiting accuracy. Thirdly, HF is a highly complex syndrome orchestrated by many different pathophysiological pathways. As such, Cys-C, PAB, and NT-proBNP may not provide sufficient prognostic information; thus, further biomarkers reflecting different pathophysiological processes may be required to provide greater prognostic value than their isolated use.
Our findings reinforce the assumption that the Cys-C/PAB ratio is a valuable predictive factor for cardiovascular and all-cause mortality in patients with HF. Moreover, this ratio provided additional prognostic information alongside NT-proBNP for HF patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The study was approved by the Shengjing Hospital of China Medical University Ethics Committee and carried out in accordance with the Declaration of Helsinki. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
CW and ZS conceived and designed the study and wrote the paper. CW, SH, and YL extracted and sorted clinical data. ZL and FT analyzed the data. All authors contributed to the article and approved the submitted version.
The present study was financially supported by a grant from the Science and Technology Program of Liaoning Province (No. 2018225003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.684919/full#supplementary-material
1. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2015) 385:117–71. doi: 10.1016/S0140-6736(14)61682-2
2. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, et al. Heart failure: preventing disease and death worldwide. Esc Heart Fail. (2014) 1:4–25. doi: 10.1002/ehf2.12005
3. Yancy CW, Jessup M, Bozkurt B, Jessup M, Bozkurt B, Butler J, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. (2017) 70:776–803. doi: 10.1016/j.jacc.2017.04.025
4. Taylor CJ, Ordóñez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: population based cohort study. BMJ. (2019) 364:l223. doi: 10.1136/bmj.l223
5. Sarhene M, Wang Y, Wei J, Huang Y, Li M, Li L, et al. Biomarkers in heart failure: the past, current and future. Heart Fail Rev. (2019) 24:867–903. doi: 10.1007/s10741-019-09807-z
6. Dungen HD, Petroni R, Correale M, Coiro S, Monitillo F, Triggiani M, et al. A new educational program in heart failure drug development: the Brescia international master program. J Cardiovasc Med. (2018) 19:411–21. doi: 10.2459/JCM.0000000000000669
7. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2016) 18:891–975. doi: 10.1093/eurheartj/ehw128
8. Inker LA, Okparavero A. Cystatin C as a marker of glomerular filtration rate: prospects and limitations. Curr Opi Nephrol Hypertens. (2011) 20:631–9. doi: 10.1097/MNH.0b013e32834b8850
9. Xie L, Terrand J, Xu B, Tsaprailis G, Boyer J, Chen QM. Cystatin C increases in cardiac injury: a role an extracellular matrix protein modulation. Cardiovasc Res. (2010) 87: 628-35. doi: 10.1093/cvr/cvq138
10. Cheng XW, Obata K, Kuzuya M, Izawa H, Nakamura K, Asai E, et al. Elastolytic cathepsin induction/activation system exists in myocardium and is upregulated in hypertensive heart failure. Hypertension. (2006) 48:979–87. doi: 10.1161/01.HYP.0000242331.99369.2f
11. Li X, Zhu H, Li P, Xin Q, Liu J, Zhang W, et al. Serum cystatin C concentration as an independent marker for hypertensive left ven- tricular hypertrophy. J Geriatr Cardiol. (2013) 10: 286–90. doi: 10.3969/j.issn.1671-5411.2013.03.001
12. Huerta A, Lo pez B, Ravassa S, San José G, Querejeta R, Beloqui Ó, et al. Association of Cystatin C with heart failure with preserved ejection fraction in elderly hypertensive patients: potential role of altered collagen metabolism. J Hypertens. (2016) 34:130–8. doi: 10.1097/HJH.0000000000000757
13. Lassus J, Harjola VP, Sund R, Siirilä-Waris K, Melin J, Peuhkurinen K, et al. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J. (2007) 28:1841–7. doi: 10.1093/eurheartj/ehl507
14. Lassus JP, Harjola VP, Peuhkurinen K, Sund R, Mebazaa A, Siirilä-Waris K, et al. Cystatin C, NT-proBNP, and inflammatory markers in acute heart failure: insights into the cardiorenal syndrome. Biomarkers. (2011) 16:302–10. doi: 10.3109/1354750X.2011.555822
15. Correa S, Morrow DA, Braunwald E, Davies RY, Goodrich EL, Murphy SA, et al. Cystatin C for risk stratification in patients after an acute coronary syndrome. J Am Heart Assoc. (2018) 7:e009077. doi: 10.1161/JAHA.118.009077
16. Damman K, van der Harst P, Smilde TD, Voors AA, Navis G, van Veldhuisen DJ, et al. Use of cystatin C levels in estimating renal function and prognosis in patients with chronic systolic heart failure. Heart. (2012) 98:319–24. doi: 10.1136/heartjnl-2011-300692
17. Dupont M, Wu Y, Hazen SL, Tang WH. Cystatin C identifies patients with stable chronic heart failure at increased risk for adverse cardiovascular events. Circ Heart Fail. (2012) 5:602–9. doi: 10.1161/CIRCHEARTFAILURE.112.966960
18. Ingenbleek Y, Young V. Transthyretin (prealbumin) in health and disease: nutritional implications. Annu Rev Nutr. (1994) 14:495–533. doi: 10.1146/annurev.nu.14.070194.002431
19. Agra Bermejo RM, Gonzalez R, Varela A, Gomez I, Kreidieh O, Conde P, et al. Nutritional status is related to heart failure severity and hospital readmissions in acute heart failure. Int J Cardiol. (2017) 230:108–14. doi: 10.1016/j.ijcard.2016.12.067
20. Iwakami N, Nagai T, Furukawa TA, Sugano Y, Honda S, Okada A, et al. Prognostic value of malnutrition assessed by Controlling Nutritional Status score for long-term mortality in patients with acute heart failure. Int J Cardiol. (2017) 230:529–36. doi: 10.1016/j.ijcard.2016.12.064
21. Namiuchi S, Sugie T, Sajj K, Takii T, Suda A, Kato A. The systemic inflammation-based Glasgow prognostic score as a prognostic factor in patients with acute heart failure. J Cardio Vasc Med. (2015) 16:409–15. doi: 10.2459/JCM.0000000000000184
22. Wang W, Wang CS, Ren D, Li T, Yao HC, Ma SJ. Low serum prealbumin levels on admission can independently predict in-hospital adverse cardiac events in patients with acute coronary syndrome. Medicine. (2018) 97:e11740. doi: 10.1097/MD.0000000000011740
23. Franco J, Formiga F, Trullas JC, Salamanca Bautista P, Conde A, Manzano L, et al. Impact of prealbumin on mortality and hospital readmission in patients with acute heart failure. Eur J Intern Med. (2017) 43:36–41. doi: 10.1016/j.ejim.2017.05.009
24. Lourenco P, Silva S, Frioes F, Alvelos M, Amorim M, Couto M, et al. Low prealbumin is strongly associated with adverse outcome in heart failure. Heart. (2014) 100:1780–5. doi: 10.1136/heartjnl-2014-305747
25. Wu X, Xu G, Zhang S. Association between Cystatin C and cardiac function and long-term prognosis in patients with chronic heart failure. Med Sci Monit. (2020) 26:e919422. doi: 10.12659/MSM.919422
26. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland John GF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2016) 18:891–975. doi: 10.1002/ejhf.592
27. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2016) 17:1321–60. doi: 10.1093/ehjci/jew082
28. Coiro S, Simonovic D, Deljanin-I M, Duarte K, Carluccio E, Cattadori Gai, et al. Prognostic value of dynamic changes in pulmonary congestion during exercise stress echocardiography in heart failure with preserved ejection fraction. Circ Heart Fail. (2020) 13:11–22. doi: 10.1161/CIRCHEARTFAILURE.119.006769
29. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, et al. Role of biomarkers for the prevention, assessment, and management of heart failure. Circulation. (2017) 135:e1054-91. doi: 10.1161/CIR.0000000000000490
30. Dandana A, Gammoudi I, Chalghoum A, Chahed H, Addad F, Ferchichi S, et al. Clinical utility of serum cystatin c in predicting coronary artery disease in patients without chronic kidney disease. J Clin Lab Anal. (2014) 28:191–7. doi: 10.1002/jcla.21665
31. Sugiyama H, Miyoshi T, Osawa K, Miki T, Koide Y, Nakamura K, et al. Serum cystatin C levels are associated with coronary artery calcification in women without chronic kidney disease. J Cardiol. (2017) 70:559–64. doi: 10.1016/j.jjcc.2017.05.001
32. Lo pez B, Gonza lez A, Lindner D, Westermann D, Ravassa S, Beaumont J, et al. Osteopontin-mediated myocardial fibrosis in heart failure: a role for lysyl oxidase? Cardiovasc Res. (2013) 99:111–20. doi: 10.1093/cvr/cvt100
33. Correale M, Monaco I, Brunetti ND, Di Biase M, Metra M, Nodari S, et al. Redefining biomarkers in heart failure. Heart Fail Rev. (2018) 23:237–53. doi: 10.1007/s10741-018-9683-2
34. Tousoulis D, Michalea S, Siasos G, Oikonomou E, Athanasiou D, Tourikis P, et al. Cystatin C serum levels and vascular function in heart failure. Int J Cardiol. (2014) 173:542–4. doi: 10.1016/j.ijcard.2014.03.083
35. Mcmurray MD, Trivax JE, Mccullough PA. Serum Cystatin C, renal filtration function, and left ventricular remodeling. Circ Heart Fail. (2009) 2:86–9. doi: 10.1161/CIRCHEARTFAILURE.109.856393
36. Xu CC, Fu GX, Liu QQ, Zhong Y. Association between cystatin C and heart failure with preserved ejection fraction in elderly Chinese patients. Z Gerontol Geriatr. (2018) 51:92–7. doi: 10.1007/s00391-016-1058-5
37. Gastelurrutia P, Lupón J, Domingo M, Ribas N, Noguero M, Martinez C, et al. Usefulness of body mass index to characterize nutritional status in patients with heart failure. Am J Cardiol. (2011) 108:1166–70. doi: 10.1016/j.amjcard.2011.06.020
38. Beck FK, Rosenthal TC. Prealbumin: a marker for nutritional evaluation. Am Fam Physician. (2002) 65:1575–8.
39. Kalantar-Zadeh K, Anker SD, Horwich TB, Fonarow GC. Nutritional and anti-inflammatory interventions in chronic heart failure. Am J Cardiol. (2008) 10:89E−103E. doi: 10.1016/j.amjcard.2008.03.007
40. Lourenço P, Araujo JP, Paulo C, Mascarenhas J, Frioes F, Azevedo A, et al. Higher C-reactive protein predicts worse prognosis in acute heart failure only in non-infected patients. Clin Cardiol. (2010) 33:708–14. doi: 10.1002/clc.20812
41. Ancion A, Allepaerts S, Oury C, Gori AS, Piérard L A, Lancellotti P. Serum albumin level and hospital mortality in acute non-ischemic heart failure. ESC Heart Fail. (2017) 4:138–45. doi: 10.1002/ehf2.12128
42. D'Elia E, Vaduganathan M, Gori M, Gavazzi A, Butler J, Senni M. Role of biomarkers in cardiac structure phenotyping in heart failure with preserved ejection fraction: critical appraisal and practical use. Eur J Heart Fail. (2015) 17:1231–9. doi: 10.1002/ejhf.430
43. Lopez B, Gonzalez A, Hermida N, Laviades C, Diez J. Myocardial fibrosis in chronic kidney disease: potential benefits of torasemide. Kidney Int. (2008) 74:S19–23. doi: 10.1038/ki.2008.512
Keywords: cystatin C, prealbumin, heart failure, long-term, mortality, prognosis
Citation: Wang C, Han S, Tong F, Li Y, Li Z and Sun Z (2021) Predictive Value of the Serum Cystatin C/Prealbumin Ratio in Combination With NT-proBNP Levels for Long-Term Prognosis in Chronic Heart Failure Patients: A Retrospective Cohort Study. Front. Cardiovasc. Med. 8:684919. doi: 10.3389/fcvm.2021.684919
Received: 24 March 2021; Accepted: 24 June 2021;
Published: 14 July 2021.
Edited by:
Katrina Poppe, The University of Auckland, New ZealandReviewed by:
Stefano Coiro, Hospital of Santa Maria della Misericordia in Perugia, ItalyCopyright © 2021 Wang, Han, Tong, Li, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijun Sun, sunzj@sj-hospital.org
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.