
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cardiovasc. Med. , 05 May 2021
Sec. Heart Valve Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.675339
This article is part of the Research Topic Rheumatic Fever: 21st Century clinical and experimental insights View all 19 articles
 Rukshan A. M. Rafeek1
Rukshan A. M. Rafeek1 Suchandan Sikder1,2
Suchandan Sikder1,2 Adam S. Hamlin1
Adam S. Hamlin1 Nicholas M. Andronicos1
Nicholas M. Andronicos1 David J. McMillan1,3
David J. McMillan1,3 Kadaba S. Sriprakash1,4
Kadaba S. Sriprakash1,4 Natkunam Ketheesan1*
Natkunam Ketheesan1*The pathogenesis of Acute Rheumatic Fever/Rheumatic Heart Disease (ARF/RHD) and associated neurobehavioral complications including Sydenham's chorea (SC) is complex. Disease complications triggered by Group A streptococcal (GAS) infection are confined to human and determining the early events leading to pathology requires a robust animal model that reflects the hallmark features of the disease. However, modeling these conditions in a laboratory animal, of a uniquely human disease is challenging. Animal models including cattle, sheep, pig, dog, cat, guinea pigs rats and mice have been used extensively to dissect molecular mechanisms of the autoimmune inflammatory responses in ARF/RHD. Despite the characteristic limitations of some animal models, several rodent models have significantly contributed to better understanding of the fundamental mechanisms underpinning features of ARF/RHD. In the Lewis rat autoimmune valvulitis model the development of myocarditis and valvulitis with the infiltration of mononuclear cells along with generation of antibodies that cross-react with cardiac tissue proteins following exposure to GAS antigens were found to be similar to ARF/RHD. We have recently shown that Lewis rats injected with recombinant GAS antigens simultaneously developed cardiac and neurobehavioral changes. Since ARF/RHD is multifactorial in origin, an animal model which exhibit the characteristics of several of the cardinal diagnostic criteria observed in ARF/RHD, would be advantageous to determine the early immune responses to facilitate biomarker discovery as well as provide a suitable model to evaluate treatment options, safety and efficacy of vaccine candidates. This review focuses on some of the common small animals and their advantages and limitations.
The concept of comparative medicine developed based on the theory that animal species share physiological, anatomical and behavioral characteristics similar to human (1). This concept led to the use of different model organisms in all fields of biomedical research (2) and they continue to play a vital role in translational research for the advancement of human and animal health. The use of animal models to investigate human disease has its origins over 2,400 years ago. By the beginning of the twentieth century the use of animal models became more experimental rather than observational (1). Animals have contributed immensely in elucidating the disease mechanisms and the development of therapeutics including vaccines. An animal model, in which the immunopathological mechanisms or outcome of disease resembles those that occur in humans, is a logical adjunct to investigate human diseases. Thus, in this review we summarize the current animal models available to investigate the pathogenesis of Acute Rheumatic Fever (ARF), Rheumatic Heart Disease (RHD) and associated post-streptococcal autoimmune complications.
Post-streptococcal autoimmune disorders are complex immune mediated disease mostly affecting children and young adults following exposure to Group A streptococcal (GAS) infection. These includes ARF, RHD, Sydenham Chorea (SC) and possibly, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) (3–5). After 1–3 weeks of an untreated GAS infection, ~1–3% of individuals develop non-suppurative post streptococcal complications including ARF, which may lead to RHD and cardiac failure (6). ARF affects multiple organs and primarily involve the joints, skin, brain and the heart. Except for cardiac damage most other manifestations are transient. After the initial or repeated episodes of ARF, about 30–45% of patients develop RHD (5) which poses an important public health problems in low to middle-income countries, and First Nation Peoples of high-income countries. Indigenous Australians (Aboriginal and Torres Strait Islander people) and New Zealanders (Māori and Pacific Islander populations) have among the highest rates of ARF in the developed countries (5, 7). RHD is the most common acquired cause of cardiac damage (8) affecting children between the ages of 5 and 15 years old (9). A gender propensity for ARF has not been widely observed although some studies have found RHD to be prevalent among females (10). The epidemiology of ARF/RHD is highly diverse and is relatively rare where access to modern medical care is readily available. However, it has not been completely eradicated with annual incidence of ARF varying from <0.5/100,000 in developed countries to >100/100,000 in developing countries (11). It is estimated that annually, approximately half a million new ARF cases are diagnosed globally (11). On the other hand, the overall prevalence of RHD is highest in sub-Saharan Africa, South Asia and Oceania. In 2015, 33.4 million people were reported to be have RHD with ~297,300–337,300 deaths in RHD endemic regions. In non-endemic regions it was 221,600 cases (12).
Variety of host, bacterial, socioeconomic and environmental factors contribute to the prevalence and incidence of ARF/RHD (5). Environmental factors includes climatic factors, sanitation, poor hygiene, overcrowding and house hold conditions (5). In addition better living conditions led to decrease in the incidence of ARF/RHD (13, 14). Malnutrition and poverty are two other important factor among children contributing to repeated exposure to streptococcal and the spread of infection (13, 15). Poor healthcare system due to low socioeconomic status and inadequate awareness of the disease in the community leads to misdiagnosis or late diagnosis and treatment of GAS infection and ARF/RHD (5, 14, 16). In addition, a strong predisposition of genetic factors including genetic polymorphisms in many human leukocyte antigen (HLA) class II alleles in the development of ARF/RHD have also been described (17).
The pathophysiology of post-streptococcal complication is not fully understood, however antigenic mimicry between GAS antigens and host proteins is partly considered as factor that triggers autoimmunity. It may also be affected by several environmental, genetic and socioeconomic factors. Although an autoimmune process has long been considered to be responsible for the initiation of ARF/RHD, it is only in the last few decades that the mechanisms involved in the pathogenesis of this post streptococcal conditions have been unraveled partly due to experimentation on animal models. Studies have shown that molecular mimicry of streptococcal antigens enable the generation of antibodies that bind to both GAS antigens and cross-react with host tissue proteins including cardiac myosin, collagen I and IV, tropomycin, laminin, vimentin, and keratin (18).
Further studies have demonstrated that human collagen IV, one of the major components of the basal membrane, a layer of extracellular matrix secreted by epithelial cells, can also be involved in the pathogenesis of ARF/RHD by acting as an autoantigen after forming a complex with GAS antigens (19). Several studies have demonstrated that GAS strains are capable of binding and aggregating to human collagen (6, 20–22). Collagen IV binds to cells and other molecules via an N- terminal Cyanogen Bromide fragment 3 (CB3) (19). GAS binds to CB3 of collagen via the octapeptide (AXYLZZLN) epitope of M protein and aggregate to form an antigenic complex with human collagen IV (19). The octapeptide region of M protein, which interact with collagen, is designated as PARF (peptide associated with rheumatic fever). The autoantigenicity of the M protein-collagen complex induces ARF/RHD. Higher levels of anti-collagen antibodies were found in the sera of ARF patients than healthy controls (22). In addition studies showed that injection of mice with GAS proteins also induce a collagen autoantibody response. However, these antibodies did not cross-react with the respective M protein. This observation leads to the understanding that the collagen autoimmunity caused by PARF motif of M protein does not depend on molecular mimicry (22).
Post streptococcal autoimmune complications including ARF/RHD is uniquely a human condition and humans are the only host and reservoir for GAS. Thus, modeling post-streptococcal autoimmune complications in animal is challenging. However, animals are the only experimental models used to investigate the characteristic signs, pathogenesis and pathophysiology specific to ARF/RHD. Animals including guinea pigs, rabbits, pigs, sheep, goats, cattle, cats, dogs, and non-human primates have been used as experimental model to understand the disease mechanism of ARF/RHD and to investigate the rheumatogenic potential of GAS M proteins (23–36). In the last two decades these animals were replaced by mice and rats due to lower costs, ease of handling and observation of pathological, immunological and functional changes comparable to ARF/RHD patients (Table 1).
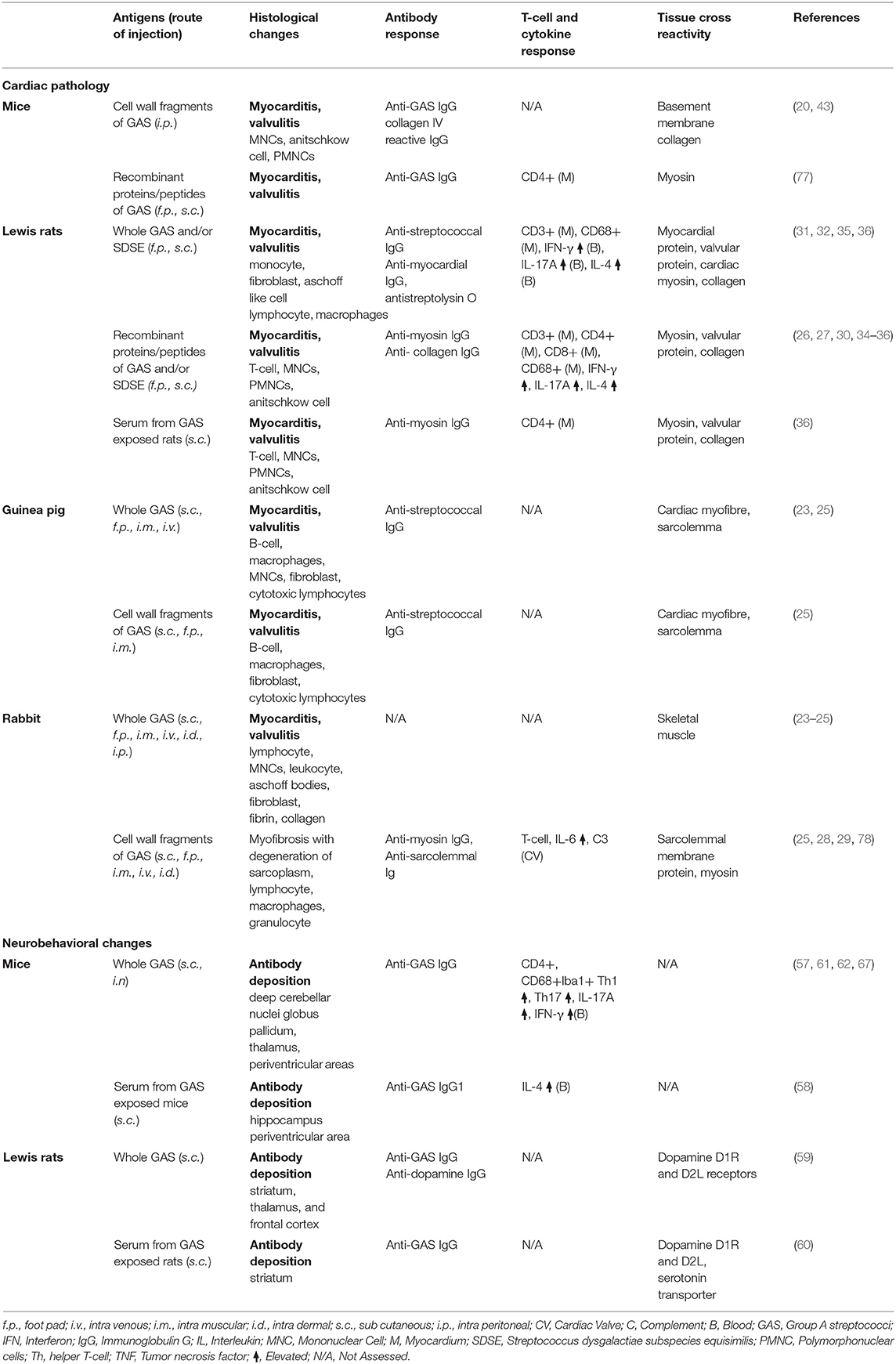
Table 1. Immunopathological changes in small animals and rodents investigated as experimental model for post streptococcal complications.
The early experiments on rheumatic myocarditis were carried out in rabbits based on the hypothesis that ARF/RHD was caused either by direct streptococcal infection or by direct damage to heart tissues by streptococcal toxins. However, none of the rabbits showed similar pathology to rheumatic myocarditis in these studies (23). A study by Gross et al. as early as in 1929 examined the development of rheumatic myocarditis induced by live and killed streptococci isolated from patients with ARF/RHD in seven different animals including rabbits, guinea pigs, dogs, cats, swine, sheep, and calves. These studies failed to induce myocarditis in any of these animals (23). However, some rabbits showed accumulation of lymphocytes and mononuclear cells in their myocardium, low-grade pericarditis with mononuclear cells, acute focal interstitial myocarditis and large, irregular, thrombotic mass on the posterior cusp of the mitral valve. Similarly, guinea pigs showed focal interstitial accumulations of lymphocytes and large mononuclear cells in the myocardium, whereas dogs and cats had no gross or microscopic pathological cardiac lesions. Only one of the pigs in the study developed transient arthritis which disappeared after only a few days. The only positive pathological finding in sheep was a few interstitial foci of lymphocytes and mononuclear cells in the myocardium of the left ventricle (23).
To investigate the role of cellular immune response in RHD, Yang et al. (25) injected Guinea pigs with heat killed GAS and/or GAS M protein. Animals developed valvulitis and myocarditis with infiltration of T and B cells, macrophages and fibroblast into the myocardium and mitral valve (25). Myocardial and endothelial damage due to infiltration of granulocytes, macrophage and lymphocytes were observed in New Zealand White Rabbits injected with GAS M proteins (28, 29, 37) (Table 1). In addition, GAS pharyngeal spray on non-human primate (rhesus monkey, Macaca mulatta) induced typical RHD lesions as well as evidence of myocarditis and valvulitis along with infiltration of lymphocytes, histiocytes, Anitshkow cells, and plasma cells (38). Later, subcutaneous injection of GAS membrane antigens to rhesus monkeys' showed similar histological changes with endocardial and sub endocardial infiltration of mononuclear cells (39). Despite numerous attempts, relevant animal model for ARF/RHD still remains elusive (Table 1).
Rodent models due to ease of handling, small body size, large litter sigs, short life span and cost are considered ideal for biomedical research (1). The Swiss-Webster mice were the first rodent model of ARF/RHD (32). These mice developed cardiac lesions similar to ARF when infected with GAS cell wall fragments. MRL+/+ mice injected with N-terminal peptides of GAS M5 protein developed myocarditis (40). Moreover, myocarditis and CD4+ lymphocyte infiltration was detected in BALB/c (41, 42), Swiss mice (43), A/J mouse (44) and DBA/2 (45) mouse strains following the injection of GAS antigens and/or cardiac myosin. A more robust animal model for ARF/RHD was developed by immunizing Lewis rats with GAS M protein (26, 46). Upon injection of GAS antigens, or cardiac myosin, animals developed myocarditis and/or valvulitis similar to patients with ARF/RHD with antibody and T-cell responses that cross-reacted with host cardiac proteins.
Lewis rats were used to scrutinize myocarditis by injection of cardiac myosin. Marked cellular infiltration consisting of mononuclear cells, neutrophils, fibroblasts, and multinucleated giant cells were observed in the experimental allergic myocarditis (EAM) (47). Quinn et al. in 2001 developed the Lewis rat autoimmune valvulitis (RAV) model following exposure to streptococcal antigens to investigate the pathogenesis of ARF/RHD (26). This Lewis rat model has become the dominant animal model used to investigate the pathogenesis of ARF/RHD and to determine the safety of experimental GAS vaccine candidates (27, 30, 33–36).
Lewis rats immunized with recombinant M6 (rM6) protein demonstrated valvulitis and focal myocarditis, which were histologically similar to pathological lesions observed in patients with RHD (26). Later studies by Gorton et al. (30) reported valvulitis and myocarditis with infiltration of CD4+ cells, CD68+ macrophages and Anitschkow cells in the myocardium and mitral, aortic and tricuspid valves of Lewis rats following injection with recombinant M5 proteins (Table 1). Antibody and T cell responses to recombinant GAS M protein and the subsequent interactions with cardiac tissue have been predominantly investigated using a RAV model (Figure 1B) (30–34, 48). Furthermore, studies on Lewis rats indicated the role of infiltrating CD4+ cells and macrophages in the disease process. In addition to these histological changes, Lewis rats also demonstrated electrocardiographic and echocardiographic changes following exposure to killed GAS and recombinant GAS M proteins induce cardiac functional abnormalities comparable to patients with ARF (Figure 1D) (35, 36).
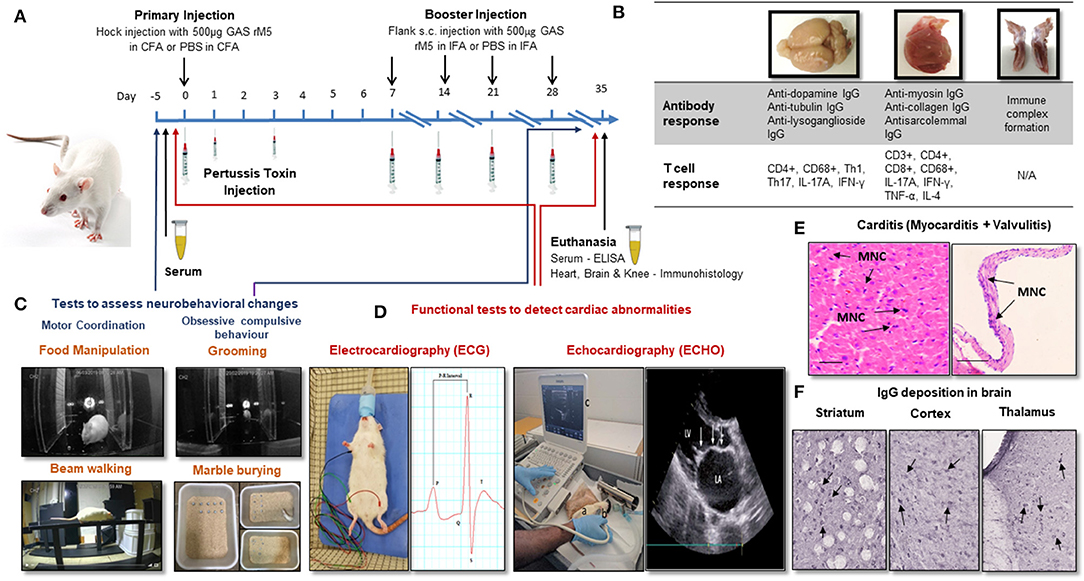
Figure 1. Procedures for the induction of carditis and neurobehavioral changes in Lewis rats. (A) Carditis, valvulitis and neurobehavioral changes can be induced by injection of Lewis rats with GAS antigens followed by Bordetella pertussis toxin injection and booster injection of GAS antigens. (B) Antibody and T cell response can be assessed in brain, heart and knee joints following injection of GAS antigens. (C) Standard behavioral tests to assess neurobehavioral changes following exposure to GAS antigens. (D) ECG and ECHO changes will demonstrate impairment cardiac function. (E) Characteristic mononuclear cell infiltration in the myocardium and valvular tissue (arrows) can be demonstrated in the histological sections of cardiac tissue from rats injected with GAS antigens. (F) IgG deposition can be demonstrated in sections of brain following incubation with sera from rats exposed to GAS antigens. CFA, Complete Freund's Adjuvant; IFA, Complete Freund's Adjuvant; PBS, Phosphate Buffered Saline; GAS rM5, Recombinant M5 protein of Group A streptococcus; ELISA, Enzyme Linked Immunosorbent Assay; Th, Helper T-cell; TNF, Tumor Necrosis Factor; IFN, Interferon; IgG, immunoglobulin G; IL, interleukin; MNC, Mononuclear Cell.
The hallmark features of ARF/RHD includes lesions in myocardium and valves (Figure 1E). In Lewis rats repeat injection with whole-killed GAS or recombinant GAS M proteins induced tissue cross-reactive antibodies and T cells (26, 27, 30, 31, 33, 34, 46, 48). Moreover, the involvement of Th-17 cells and associated regulators observed in the pathological process may potentially be considered as biomarkers for RHD (Figure 1B) (49, 50). In a separate experiment, in response to different streptococcal antigens, including both GAS and Streptococcus dysgalactiae subsp. equisimilis (SDSE/GGS), Lewis rats developed typical histological lesions with infiltration of inflammatory cells into cardiac tissue providing experimental evidence that streptococci other than GAS could trigger and/or exacerbate post-streptococcal carditis (Table 1). Lewis rats were also used to assess the preclinical immunogenicity and safety of a GAS M protein-based vaccine candidate (51, 52). Therefore, the Lewis rat model is not only useful in elucidating the pathophysiological mechanisms in ARF/RHD, but also provides an opportunity to identify, validate streptococcal epitopes that are truly pathogenic to ARF/RHD. It also enables the assessment of safety and efficacy of GAS antigen based prototype vaccine candidates (51, 52).
The two major neurobehavioral complications associated with post GAS infections are Sydenham chorea (SC) and pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS) (53). SC is a neurological movement disorder described in ARF and is one of the major criterions for the diagnosis ARF (18). PANDAS is a sudden onset of obsessive-compulsive disorder (OCD) associated with GAS infection and not known to be clinically associated with ARF (54). The complex immunopathological mechanisms that mediated immune damage following GAS infections that leads to SC and PANDAS remain unclear (55). However, it has been shown that antibodies against GAS cross-react with neurotransmitter receptors (D1 and D2 dopamine receptors), signaling kinases and ion channels, located primarily in the basal ganglia of the brain in susceptible hosts due to molecular mimicry (56).
In the past many studies have been carried out to develop an animal model to investigate the post streptococcal neurobehavioral disorders (Table 1) (57–62). Initial experiments were carried out by infusion of serum from patients with suspected streptococcal related neuropsychiatric disorders directly in to the striatum of rats. However, not all such studies succeeded in modeling these stereotypic behaviors in mice and rats (63–66). In 2004, Hoffman et al. (57) injected female SJL/J mice with purified GAS M6 protein along with Freund's adjuvant and observed that a group of mice developed motor and behavioral problem. These investigators conducted another study by the passive transfer of sera from mice injected with GAS to naïve mice, which also developed in similar neurological and behavioral changes (58). In both these studies immunological analysis of the brain tissue showed anti streptococcal antibody deposition in deep cerebella nuclei and hippocampus.
A recent study by Brimberg et al. (59) observed neurobehavioral and immunological changes akin to SC and PANDAS in male Lewis rats following exposure to GAS antigens. Behavioral changes included impairment in handling food, traversing the narrow beam and obsessive-compulsive behavior (Figure 1C) (59). Lewis rats developed behavioral and neurological conditions similar to SC and PANDAS after passive transfer of serum from rats exposed to GAS infection (60). These studies showed elevated levels of antibodies against GAS M protein and cross-reactive antibodies against brain in the peripheral blood and brain, similar to antibodies present in SC and PANDAS patients (59, 60). Antibodies derived from GAS exposed animals have shown strong reactivity with D1 and D2 dopamine receptors and activated calcium/calmodulin-dependent protein kinase II signaling in brain tissue (59, 60). Similarly, in vitro studies demonstrated that monoclonal antibodies against N-acetyl-β-D-glucosamine and lysoganglioside GM1 induced the activity of calcium/calmodulin-dependent protein kinase II, which is potentially implicated as an important mediator of learning and behavior (56). Recent studies in C57BL/6, C57BL/6J, or SJL/J female mice following intranasal GAS challenge have demonstrated a breakdown in the Blood Brain Barrier (BBB) enabling the migration of GAS specific Th17 cells from nasal-associated lymphoid tissue to the brain, with the microglial activation and IgG deposition in the striatum (62, 67). Elevated levels of pro inflammatory cytokines including IL17A+ IFN-γ+ due to GAS autoimmunity disrupts the BBB to allow circulating autoantibodies and Th17 and Th1 cells to enter the brain, which targets neurons and trigger neurobehavioral changes (Table 1) (67, 68). In addition genetically modified mice lacking Th17 lymphocytes (SJL/J, RORγt+/GFP and RORγtGFP/GFP mice) have shown reduced BBB leakage, microglial activation, and antibody infiltration into the brain following intranasal challenge with GAS (Figure 1B). This demonstrates the importance of Th17 lymphocytes in BBB leakage and infiltration of autoantibodies into the brain tissue (67). Thus, rodent models are very useful for assessing the disease mechanisms associated with central nervous system to precisely determine sequential events following infection with GAS.
Post streptococcal autoimmune sequelae is a multisystem disorder affecting multiple organs including heart, brain, joints, connective tissues and skin (5). The immunopathology due to autoimmune response defers between organs. In the heart it is due to the pathological process initiated by the cross-reactive anti-GAS antibodies and T cells against host proteins (69). In the brain the disease is associated with IgG deposition (Figure 1F) (70). Whereas, in joints the pathogenesis is due to the immune complexes that bind to the synovial membrane and/or collagen in joints (5), and erythema marginatum might be due to cross-reactivity of anti-GAS antibody with keratin (71) and subcutaneous nodules might be due to a delayed hypersensitivity against GAS antigens (5). ARF patients can develop a combination of clinical symptoms that can lead to serious consequences. Approximately 30% of the patients with ARF can suffer from both cardiac and neurobehavioral complications (3). Moreover, due to the heterogeneity of ARF/RHD, an animal model might reflect a specific phenotype of the diverse complications from those observed in human disease. Therefore, an animal model which can reflect both cardiac and neurobehavioral conditions would be a remarkable advancement in ARF/RHD research, not only to investigate the pathophysiology but also to assess the safety and efficacy of vaccine candidates and treatment modalities. Furthermore, in compliance with more stringent animal welfare considerations (e.g., 3Rs rules', for replacement, reduction and refinement) determining different aspects of a disease in a single animal will minimize the number of animals needed for research (Figure 1). Recently we have achieved this goal by modeling both cardiac and neurobehavioral changes in Lewis rats and rats injected with GAS shown impairments in fine motor control, gait and balance and obsessive-compulsive behavior similar to SC and PANDAS together with functional and immunological changes previously observed in the RAV model (72). Moreover, post-streptococcal complications including RHD and neurobehavioral changes such as SC are prominent in females (10, 73–75), thus most of the studies on RHD have been conducted in female mice or rats. However, neurobehavioral studies described in the literature have either been conducted on male or female mice but solely on male rats. Our recent observations demonstrated that there were no significant difference in using both genders of Lewis rats to simultaneously model carditis and neurobehavioral changes (72). To further validate multiple complications associated with ARF/RHD, more studies are warranted on the Lewis rat model.
While significant advances in animal models of ARF/RHD have been made in the last decade, there is still a paucity in pre-clinical studies on other complications associated with ARF/RHD including neurobehavioral changes, arthritis and skin manifestations. Arthritis is observed in ~50–70% of patients with ARF and is a major Jones Criterion for the diagnosis of ARF. However, none of the animal studies have investigated GAS induced autoimmune process in subcutaneous tissue and joint tissue in any of these models.
Laboratory models are important to determine the early events leading to chronic disease. In particular when clinical studies are not possible during the early stages. In a credible animal model symptoms of physiological, anatomical and behavioral conditions must be comparable to those observed in human disease. In addition, an animal model should be reliable and the changes observed must be reproducible across laboratories. An animal model of ARF/RHD and associated neurobehavioral complications should possess functional and pathological changes encompassing motor deficits as well as compulsive and stereotyped behaviors similar to SC. Since genotypes, sex and age difference affects the development of autoimmune complication; selection of an appropriate animal model is important to investigate the pathogenesis of ARF/RHD and associated complications. Several animal models have been tested to investigate the onset, and progression of ARF/RHD. The Lewis rat model characterized by us and others, is a reliable model to investigate early events that lead to cardiac valvular pathology. Together with advances in novel imaging technologies and integrated computational approaches our model will provide the means to address these challenges (76). Importantly, comparison of experimental results with clinical observations to extrapolate the sequential event that follow infection with GAS leading to autoimmune complications requires prudence and caution.
RAMR, SS, ASH, NMA, KSS, DJM, and NK wrote the main manuscript. All authors have read and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
RAMR is recipient of International Postgraduate Research Award (IPRA) from University of New England.
2. Hackerman N. Use of Laboratory Animals in Biomedical and Behavioral Research. Washington, DC: National Academies Press (1988).
3. Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. (2005) 366:155–68. doi: 10.1016/S0140-6736(05)66874-2
4. Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. (2005) 5:685–94. doi: 10.1016/S1473-3099(05)70267-X
5. Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. (2016) 2:15084. doi: 10.1038/nrdp.2015.84
6. Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, et al. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin Microbiol Rev. (2014) 27:264–301. doi: 10.1128/CMR.00101-13
7. Jaine R, Baker M, Venugopal K. Epidemiology of acute rheumatic fever in New Zealand 1996-2005. J Paediatr Child Health. (2008) 44:564–71. doi: 10.1111/j.1440-1754.2008.01384.x
8. Roberts KV, Maguire GP, Brown A, Atkinson DN, Remenyi B, Wheaton G, et al. Rheumatic heart disease in indigenous children in northern Australia: differences in prevalence and the challenges of screening. Med J Aust. (2015) 203:221.e1–7. doi: 10.5694/mja15.00139
9. Cunningham MW. Post-streptococcal Autoimmune Sequelae: Rheumatic Fever and Beyond. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma: University of Oklahoma Health Sciences Center (2016).
10. Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation. (2013) 128:492–501. doi: 10.1161/CIRCULATIONAHA.113.001477
11. Szczygielska I, Hernik E, Kolodziejczyk B, Gazda A, Maslinska M, Gietka P. Rheumatic fever - new diagnostic criteria. Reumatologia. (2018) 56:37–41. doi: 10.5114/reum.2018.74748
12. Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. (2017) 377:713–22. doi: 10.1056/NEJMoa1603693
13. Kaplan EL. Epidemiological approaches to understanding the pathogenesis of rheumatic fever. Int J Epidemiol. (1985) 14:499–501. doi: 10.1093/ije/14.4.499
14. Kumar R. Controlling rheumatic heart disease in developing countries. World Health Forum. (1995) 16:47–51.
15. Kaplan EL. The group A streptococcal upper respiratory tract carrier state: an enigma. J Pediatr. (1980) 97:337–45. doi: 10.1016/S0022-3476(80)80178-8
16. Kaplan EL. Recent epidemiology of group A streptococcal infections in North America and abroad: an overview. Pediatrics. (1996) 97:945–8.
17. Guilherme L, Weidebach W, Kiss MH, Snitcowsky R, Kalil J. Association of human leukocyte class II antigens with rheumatic fever or rheumatic heart disease in a Brazilian population. Circulation. (1991) 83:1995–8. doi: 10.1161/01.CIR.83.6.1995
18. Cunningham MW. Molecular mimicry, autoimmunity, and infection: the cross-reactive antigens of group A Streptococci and their sequelae. Microbiol Spectr. (2019) 7. doi: 10.1128/microbiolspec.GPP3-0045-2018
19. Dinkla K, Talay SR, Morgelin M, Graham RM, Rohde M, Nitsche-Schmitz DP, et al. Crucial role of the CB3-region of collagen IV in PARF-induced acute rheumatic fever. PLoS ONE. (2009) 4:e4666. doi: 10.1371/journal.pone.0004666
20. Dinkla K, Rohde M, Jansen WT, Kaplan EL, Chhatwal GS, Talay SR. Rheumatic fever-associated Streptococcus pyogenes isolates aggregate collagen. J Clin Invest. (2003) 111:1905–12. doi: 10.1172/JCI17247
21. Tandon R, Sharma M, Chandrashekhar Y, Kotb M, Yacoub MH, Narula J. Revisiting the pathogenesis of rheumatic fever and carditis. Nat Rev Cardiol. (2013) 10:171–7. doi: 10.1038/nrcardio.2012.197
22. Dinkla K, Nitsche-Schmitz DP, Barroso V, Reissmann S, Johansson HM, Frick IM, et al. Identification of a streptococcal octapeptide motif involved in acute rheumatic fever. J Biol Chem. (2007) 282:18686–93. doi: 10.1074/jbc.M701047200
23. Gross L, Loewe L, Eliasoph B. Attempts to reproduce rheumatic fever in animals. J Exp Med. (1929) 50:41–65. doi: 10.1084/jem.50.1.41
24. Baker BM, Thomas CB, Penick RM. Experimental carditis. Changes in the myocardium and pericardium of rabbits sensitized to streptococci. J Clin Invest. (1935) 14:465–73. doi: 10.1172/JCI100697
25. Yang LC, Soprey PR, Wittner MK, Fox EN. Streptococcal-induced cell-mediated-immune destruction of cardiac myofibers in vitro. J Exp Med. (1977) 146:344–60. doi: 10.1084/jem.146.2.344
26. Quinn A, Kosanke S, Fischetti VA, Factor SM, Cunningham MW. Induction of autoimmune valvular heart disease by recombinant streptococcal m protein. Infect Immun. (2001) 69:4072–8. doi: 10.1128/IAI.69.6.4072-4078.2001
27. Lymbury RS, Olive C, Powell KA, Good MF, Hirst RG, LaBrooy JT, et al. Induction of autoimmune valvulitis in Lewis rats following immunization with peptides from the conserved region of group A streptococcal M protein. J Autoimmun. (2003) 20:211–7. doi: 10.1016/S0896-8411(03)00026-X
28. Burova LA, Nagornev VA, Pigarevsky PV, Gladilina MM, Molchanova IV, Gavrilova EA, et al. Induction of myocarditis in rabbits injected with group A streptococci. Indian J Med Res. (2004) 119 (Suppl.):183–5.
29. Burova LA, Nagornev VA, Pigarevsky PV, Gladilina MM, Gavrilova EA, Seliverstova VG, et al. Myocardial tissue damage in rabbits injected with group A streptococci, types M1 and M22. Role of bacterial immunoglobulin G-binding surface proteins. APMIS. (2005) 113:21–30. doi: 10.1111/j.1600-0463.2005.apm1130104.x
30. Gorton D, Govan B, Olive C, Ketheesan N. B- and T-cell responses in group a streptococcus M-protein- or peptide-induced experimental carditis. Infect Immun. (2009) 77:2177–83. doi: 10.1128/IAI.01514-08
31. Huang J, Xie X, Lin ZF, Luo MQ, Yu BY, Gu JR. Induction of myocarditis lesions in Lewis rats by formalin-killed cells of group A Streptococcus. J Int Med Res. (2009) 37:175–81. doi: 10.1177/147323000903700121
32. Xie X, Zhou H, Huang J, Huang H, Feng Z, Mei K, et al. An animal model of chronic rheumatic valvulitis induced by formalin-killed streptococci. Rheumatol Int. (2010) 30:1621–5. doi: 10.1007/s00296-009-1246-3
33. Kirvan CA, Galvin JE, Hilt S, Kosanke S, Cunningham MW. Identification of streptococcal m-protein cardiopathogenic epitopes in experimental autoimmune valvulitis. J Cardiovasc Trans Res. (2014) 7:172–81. doi: 10.1007/s12265-013-9526-4
34. Gorton D, Sikder S, Williams NL, Chilton L, Rush CM, Govan BL, et al. Repeat exposure to group A streptococcal M protein exacerbates cardiac damage in a rat model of rheumatic heart disease. Autoimmunity. (2016) 49:563–70. doi: 10.1080/08916934.2016.1217999
35. Sikder S, Williams NL, Sorenson AE, Alim MA, Vidgen ME, Moreland NJ, et al. Group G Streptococcus induces an autoimmune carditis mediated by interleukin 17A and interferon gamma in the Lewis rat model of rheumatic heart disease. J Infect Dis. (2018) 218:324–35. doi: 10.1093/infdis/jix637
36. Sikder S, Price G, Alim MA, Gautam A, Scott Simpson R, Margaret Rush C, et al. Group A streptococcal M-protein specific antibodies and T-cells drive the pathology observed in the rat autoimmune valvulitis model. Autoimmunity. (2019) 52:78–87. doi: 10.1080/08916934.2019.1605356
37. Sargent SJ, Beachey EH, Corbett CE, Dale JB. Sequence of protective epitopes of streptococcal M proteins shared with cardiac sarcolemmal membranes. J Immunol. (1987) 139:1285–90.
38. Vanace PW. Experimental streptococcal infection in the rhesus monkey. Ann N Y Acad Sci. (1960) 85:910–30. doi: 10.1111/j.1749-6632.1960.tb50011.x
39. Anand IS, Ganguly NK, Khanna AK, Chakravarti RN, Wahi PL. Pathogenesis of immune-mediated carditis in monkeys. Adv Myocardiol. (1983) 4:215–26. doi: 10.1007/978-1-4757-4441-5_19
40. Huber SA, Cunningham MW. Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell-dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsakieviral myocarditis. J Immunol. (1996) 156:3528–34.
41. Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. (1987) 139:3630–6.
42. Cunningham MW, Antone SM, Smart M, Liu R, Kosanke S. Molecular analysis of human cardiac myosin-cross-reactive B- and T-cell epitopes of the group A streptococcal M5 protein. Infect Immun. (1997) 65:3913–23. doi: 10.1128/IAI.65.9.3913-3923.1997
43. Ohanian SH, Schwab JH, Cromartie WJ. Relation of rheumatic-like cardiac lesions of the mouse to localization of group A streptococcal cell walls. J Exp Med. (1969) 129:37–49. doi: 10.1084/jem.129.1.37
44. Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. (1991) 147:2141–7.
45. Liao L, Sindhwani R, Rojkind M, Factor S, Leinwand L, Diamond B. Antibody-mediated autoimmune myocarditis depends on genetically determined target organ sensitivity. J Exp Med. (1995) 181:1123–31. doi: 10.1084/jem.181.3.1123
46. Galvin JE, Hemric ME, Kosanke SD, Factor SM, Quinn A, Cunningham MW. Induction of myocarditis and valvulitis in lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditis. Am J Pathol. (2002) 160:297–306. doi: 10.1016/S0002-9440(10)64373-8
47. Wegmann KW, Zhao W, Griffin AC, Hickey WF. Identification of myocarditogenic peptides derived from cardiac myosin capable of inducing experimental allergic myocarditis in the Lewis rat. The utility of a class II binding motif in selecting self-reactive peptides. J Immunol. (1994) 153:892–900.
48. Rush CM, Govan BL, Sikder S, Williams NL, Ketheesan N. Animal models to investigate the pathogenesis of rheumatic heart disease. Front Pediatr. (2014) 2:116. doi: 10.3389/fped.2014.00116
49. Wu XD, Zeng ZY, Gong DP, Wen JL, Huang F. Potential involvement of S1PR1/STAT3 signaling pathway in cardiac valve damage due to rheumatic heart disease. Biotech Histochem. (2019) 94:398–403. doi: 10.1080/10520295.2019.1574028
50. Li W, Zeng Z, Gui C, Zheng H, Huang W, Wei H, et al. Proteomic analysis of mitral valve in Lewis rat with acute rheumatic heart disease. Int J Clin Exp Pathol. (2015) 8:14151–60.
51. Batzloff MR, Fane A, Gorton D, Pandey M, Rivera-Hernandez T, Calcutt A, et al. Preclinical immunogenicity and safety of a Group A streptococcal M protein-based vaccine candidate. Hum Vaccines Immunother. (2016) 12:3089–96. doi: 10.1080/21645515.2016.1222999
52. McNeilly C, Cosh S, Vu T, Nichols J, Henningham A, Hofmann A, et al. Predicted coverage and immuno-safety of a recombinant C-repeat region based Streptococcus pyogenes vaccine candidate. PLoS ONE. (2016) 11:e0156639. doi: 10.1371/journal.pone.0156639
53. Punukollu M, Mushet N, Linney M, Hennessy C, Morton M. Neuropsychiatric manifestations of Sydenham's chorea: a systematic review. Dev Med Child Neurol. (2016) 58:16–28. doi: 10.1111/dmcn.12786
54. Macerollo A, Martino D. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): an evolving concept. Tremor Other Hyperkinet Mov. (2013) 3. doi: 10.5334/tohm.167
55. Cutforth T, DeMille MM, Agalliu I, Agalliu D. CNS autoimmune disease after Streptococcus pyogenes infections: animal models, cellular mechanisms and genetic factors. Future Neurol. (2016) 11:63–76. doi: 10.2217/fnl.16.4
56. Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. (2003) 9:914–20. doi: 10.1038/nm892
57. Hoffman KL, Hornig M, Yaddanapudi K, Jabado O, Lipkin WI. A murine model for neuropsychiatric disorders associated with group A beta-hemolytic streptococcal infection. J Neurosci. (2004) 24:1780–91. doi: 10.1523/JNEUROSCI.0887-03.2004
58. Yaddanapudi K, Hornig M, Serge R, De Miranda J, Baghban A, Villar G, et al. Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol Psychiatry. (2010) 15:712–26. doi: 10.1038/mp.2009.77
59. Brimberg L, Benhar I, Mascaro-Blanco A, Alvarez K, Lotan D, Winter C, et al. Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology. (2012) 37:2076–87. doi: 10.1038/npp.2012.56
60. Lotan D, Benhar I, Alvarez K, Mascaro-Blanco A, Brimberg L, Frenkel D, et al. Behavioral and neural effects of intra-striatal infusion of anti-streptococcal antibodies in rats. Brain Behav Immun. (2014) 38:249–62. doi: 10.1016/j.bbi.2014.02.009
61. Macri S, Ceci C, Onori MP, Invernizzi RW, Bartolini E, Altabella L, et al. Mice repeatedly exposed to Group-A beta-Haemolytic Streptococcus show perseverative behaviors, impaired sensorimotor gating, and immune activation in rostral diencephalon. Sci Rep. (2015) 5:13257. doi: 10.1038/srep13257
62. Dileepan T, Smith ED, Knowland D, Hsu M, Platt M, Bittner-Eddy P, et al. Group A Streptococcus intranasal infection promotes CNS infiltration by streptococcal-specific Th17 cells. J Clin Invest. (2016) 126:303–17. doi: 10.1172/JCI80792
63. Hallett JJ, Harling-Berg CJ, Knopf PM, Stopa EG, Kiessling LS. Anti-striatal antibodies in Tourette syndrome cause neuronal dysfunction. J Neuroimmunol. (2000) 111:195–202. doi: 10.1016/S0165-5728(00)00320-9
64. Taylor JR, Morshed SA, Parveen S, Mercadante MT, Scahill L, Peterson BS, et al. An animal model of Tourette's syndrome. Am J Psychiatry. (2002) 159:657–60. doi: 10.1176/appi.ajp.159.4.657
65. Loiselle CR, Lee O, Moran TH, Singer HS. Striatal microinfusion of Tourette syndrome and PANDAS sera: failure to induce behavioral changes. Mov Disord. (2004) 19:390–6. doi: 10.1002/mds.10522
66. Singer HS, Mink JW, Loiselle CR, Burke KA, Ruchkina I, Morshed S, et al. Microinfusion of antineuronal antibodies into rodent striatum: failure to differentiate between elevated and low titers. J Neuroimmunol. (2005) 163:8–14. doi: 10.1016/j.jneuroim.2005.02.018
67. Platt MP, Bolding KA, Wayne CR, Chaudhry S, Cutforth T, Franks KM, et al. Th17 lymphocytes drive vascular and neuronal deficits in a mouse model of postinfectious autoimmune encephalitis. Proc Natl Acad Sci USA. (2020) 117:6708–16. doi: 10.1073/pnas.1911097117
68. Wang B, Dileepan T, Briscoe S, Hyland KA, Kang J, Khoruts A, et al. Induction of TGF-β1 and TGF-β1–dependent predominant Th17 differentiation by group A streptococcal infection. Proc Natl Acad Sci. (2010) 107:5937–42. doi: 10.1073/pnas.0904831107
69. Kaplan MH, Bolande R, Rakita L, Blair J. Presence of bound immunoglobulins and complement in the myocardium in acute rheumatic fever: association with cardiac failure. N Engl J Med. (1964) 271:637–45. doi: 10.1056/NEJM196409242711301
70. Greenfield JG, Wolfsohn J. The pathology of Sydenham's chorea. Lancet. (1922) 200:603–6. doi: 10.1016/S0140-6736(01)01044-3
71. Shikhman AR, Cunningham MW. Immunological mimicry between N-acetyl-beta-D-glucosamine and cytokeratin peptides. Evidence for a microbially driven anti-keratin antibody response. J Immunol. (1994) 152:4375–87.
72. Rafeek RAM, Lobbe CL, Wilkinson EC, Hamlin AS, Andronicos NM, McMillan DJ, et al. Group A Streptococcal antigen exposed rat model to investigate neurobehavioral and cardiac complications associated with post-streptococcal autoimmune sequelae. Animal Models Exp Med. (2021). doi: 10.1002/ame2.12164. [Epub ahead of print].
73. Berry J. Prevalence survey for chronic rheumatic heart disease and rheumatic fever in northern India. Br Heart J. (1972) 34:143. doi: 10.1136/hrt.34.2.143
74. Carapetis JR, Currie BJ. Rheumatic chorea in northern Australia: a clinical and epidemiological study. Arch Dis Child. (1999) 80:353–8. doi: 10.1136/adc.80.4.353
75. Martino D, Tanner A, Defazio G, Church A, Bhatia K, Giovannoni G, et al. Tracing Sydenham's chorea: historical documents from a British paediatric hospital. Arch Dis Child. (2005) 90:507–11. doi: 10.1136/adc.2004.057679
76. Bartoli-Leonard F, Aikawa E. Heart valve disease: challenges and new opportunities. Front Cardiovasc Med. (2020) 7:602271. doi: 10.3389/fcvm.2020.602271
77. Cunningham MW, McCormack JM, Fenderson PG, Ho MK, Beachey EH, Dale JB. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J Immunol. (1989) 143:2677–83.
Keywords: animal model, acute rheumatic fever, rheumatic heart disease, sydenham chorea, lewis rats, autoimmunity, Group A streptococcus
Citation: Rafeek RAM, Sikder S, Hamlin AS, Andronicos NM, McMillan DJ, Sriprakash KS and Ketheesan N (2021) Requirements for a Robust Animal Model to Investigate the Disease Mechanism of Autoimmune Complications Associated With ARF/RHD. Front. Cardiovasc. Med. 8:675339. doi: 10.3389/fcvm.2021.675339
Received: 02 March 2021; Accepted: 09 April 2021;
Published: 05 May 2021.
Edited by:
Luiza Guilherme, University of São Paulo, BrazilReviewed by:
Francesca Bartoli-Leonard, Brigham and Women's Hospital and Harvard Medical School, United StatesCopyright © 2021 Rafeek, Sikder, Hamlin, Andronicos, McMillan, Sriprakash and Ketheesan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natkunam Ketheesan, bmtldGhlZXNAdW5lLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.