- 1Department of Pharmacy, Renmin Hospital, Wuhan University, Wuhan, China
- 2School of Pharmaceutical Sciences, Wuhan University, Wuhan, China
- 3Department of Pharmacy, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 4School of Medicine, Tongji University, Shanghai, China
- 5Shanghai Anticoagulation Pharmacist Alliance, Shanghai Pharmaceutical Association, Shanghai, China
- 6Chinese Society of Cardiothoracic and Vascular Anesthesiology, Beijing, China
Background: In the clinical setting, the economic benefits of direct oral anticoagulants (DOACs) in elderly patients with atrial fibrillation (AF) remain unclear. This study aimed to estimate and compare the cost-effectiveness of DOACs (dabigatran, rivaroxaban, apixaban, and edoxaban) and vitamin K antagonists (VKAs; warfarin) in preventing stroke among AF patients aged >75 years in real-world practice.
Methods: A Markov model with a 10-year span was constructed to estimate the long-term clinical and economic outcomes among AF patients aged >75 years treated with DOACs and warfarin. The study was populated with a hypothetical cohort of 10,000 AF patients aged >75 years. Probabilities of clinical outcomes were obtained from the pooled observational studies (OSs), comparing DOACs (dabigatran, rivaroxaban, apixaban, and edoxaban) with VKAs. Other model inputs, including the utilities and the costs, were all estimated from public sources and the published literature. The costs, quality-adjusted life-years (QAYLs), and incremental cost-effectiveness ratios (ICER) were estimated for each treatment strategy. Subgroup analyses of individual DOACs and the scenario analysis were performed. Uncertainty was evaluated by deterministic sensitivity analysis and probabilistic sensitivity analysis (PSA).
Results: Compared to warfarin, DOACs were associated with a gain of 0.36 QALY at an additional cost of $15,234.65, resulting in an ICER of $42,318.47 per QALY. Sensitivity analysis revealed that the ICER was sensitive to the cost of DOACs. Direct oral anticoagulants also shifted from dominating to dominated status When their annual costs of DOACs were over $3,802.84 or the risk ratio of death compared to warfarin was over 1.077%/year. Probabilistic sensitivity analysis (PSA) suggested that DOACs had a 53.83 and 90.7% probability of being cost-effective when the willingness-to-pay threshold was set at $50,000 and $100,000, respectively. Among all the four individual DOACs, edoxaban treatment was revealed as the preferred treatment strategy for the AF patients aged over 75 years by yielding the most significant health gain with the relatively low total cost.
Conclusions: Despite the high risk for major bleeding in elderly patients with AF, DOACs are more cost-effective treatment options than warfarin in real-world practice. Edoxaban was the preferred treatment strategy among four kinds of DOACs for AF patients aged over 75 years. Furthermore, beyond their safety profiles, the treatment benefits of DOACs assumed greater relevance and importance in older adults.
Introduction
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia in adults, affecting approximately 33 million individuals worldwide (1). Epidemiological studies have shown that advancing age is a major risk factor for AF, with its prevalence almost doubling with every 10-year increase in age (from 0.1% among patients aged <35 years to 14% among those aged >75 years) (2–4). Age is also a non-modifiable risk factor for ischemic stroke in patients with AF. More than 50% of ischemic stroke cases diagnosed in patients with AF occur in those aged >80 years (5). Given the high mortality and disability rates associated with AF-induced ischemic stroke, it is considered major public health, social, and economic burden in the elderly population (6).
Traditionally, stroke in AF has been prevented by using vitamin K antagonists (VKAs), such as warfarin. Owing to their efficacy profiles, which have been recently reported by several large, randomized controlled trials (RCTs), direct oral anticoagulants (DOACs), such as dabigatran, rivaroxaban, apixaban, and edoxaban, are viable alternatives for VKAs and have been established as cornerstones in AF management for the prevention of stroke (7, 8). Based on the results of a meta-analysis of RCTs, DOACs have been reported to be more cost-effective than VKAs (9–11). However, since no RCT was designed to focus on the elderly population specifically, there is little clarity regarding the health and economic benefits of DOACs in the said population (12). Although subgroup analyses of RCTs have usually been conducted for the elderly population, there remain many uncertainties about the relevance of the results of these RCTs in the real-world setting, given that the elderly population is known in real practice to be at high risk for bleeding (13). Furthermore, given the poor prognosis and heavy burden of major bleeding events in elderly patients with AF, it remains unknown whether the benefits of DOACs will be offset by the high incidence rates of intracranial bleeding in real clinical settings. Therefore, using data from a comprehensive meta-analysis of high-quality OSs and RCTs, we performed a cost-effectiveness evaluation comparing DOACs and VKAs to assess the expected costs and benefits of using the former in the elderly population with AF in real-world settings.
Methods
Model
A decision-analysis Markov model with a 1-year cycle and a 10-year horizon was constructed to compare the cost-effectiveness of DOACs (including dabigatran, apixaban, rivaroxaban, and edoxaban) and VKAs in elderly AF patients in real-world settings. A simplified schematic represents the model structure in Figure 1. Eight mutually exclusive health states were included in the Markov model: AF without complications, major ischemic stroke, minor stroke, major intracranial hemorrhage (ICH) on aspirin, minor ICH on aspirin, stroke and ICH on aspirin, myocardial infarction (MI), and death. The following assumptions were made to reflect the approximate progression of AF in older age: The starting age of the patient cohort was set at 75 years, and all were assumed to begin in the state of AF without complications. In each cycle, patients may either remain in their current health state or transition to the next due to a clinical event. Four types of stroke (reversible, major, minor, and fatal) and three types of ICH (major, minor, and fatal) were included in the model. To reflect the treatment pattern of AF and to approximate the AF disease progression in real-world practice, we made the following assumptions in the model. Due to the high case fatality rate in the hospitalized stroke, which reflects the immediate severity of the condition, transition to death was assumed after two major neurological events (stroke or ICH) (14, 15). Patients with two minor neurological were supposed to proceed to the major event state (15). After any ICH, patients were assumed to discontinue the therapy of anticoagulants and switch to aspirin in case of recurrent ICH. This estimate of drug switch is widely used in previously published cost-effectiveness analyses of anticoagulants (15–17). Considering that the risk of MI is usually greatly reduced with anticoagulant therapy, no MI event was considered after any neurological events. TreeAge Pro Suite TM software 2019 (Williamstown, MA. USA) was used for the model construction and analysis.
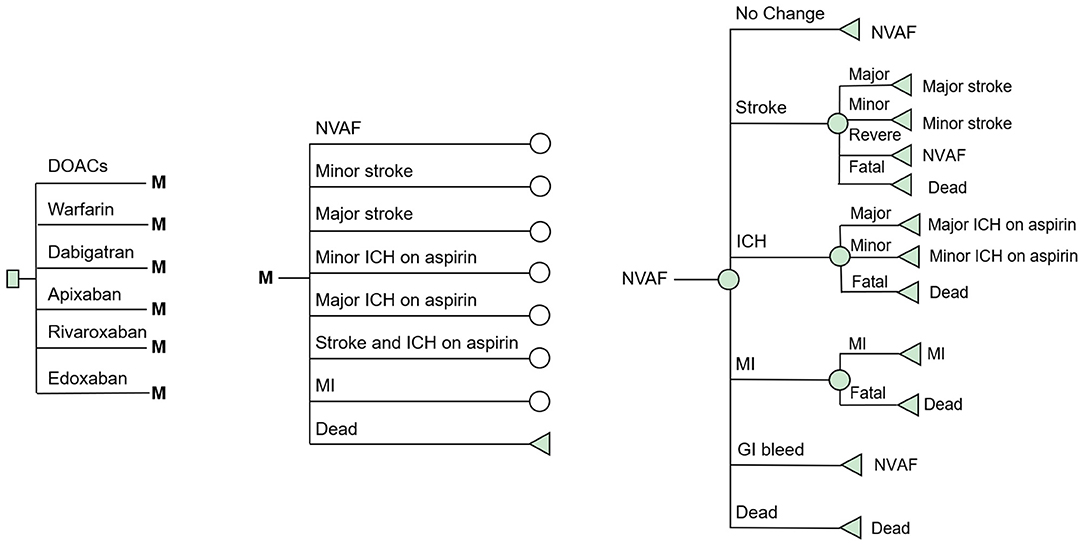
Figure 1. Schematic representation of the Markov model. AF, atrial fibrillation; Stroke, ischemic or hemorrhagic stroke; SE, systemic embolism; GI Bleeding, gastrointestinal bleeding; ICH, intracranial hemorrhage; MI, myocardial infarction.
Date and Sources
Treatment Effectiveness
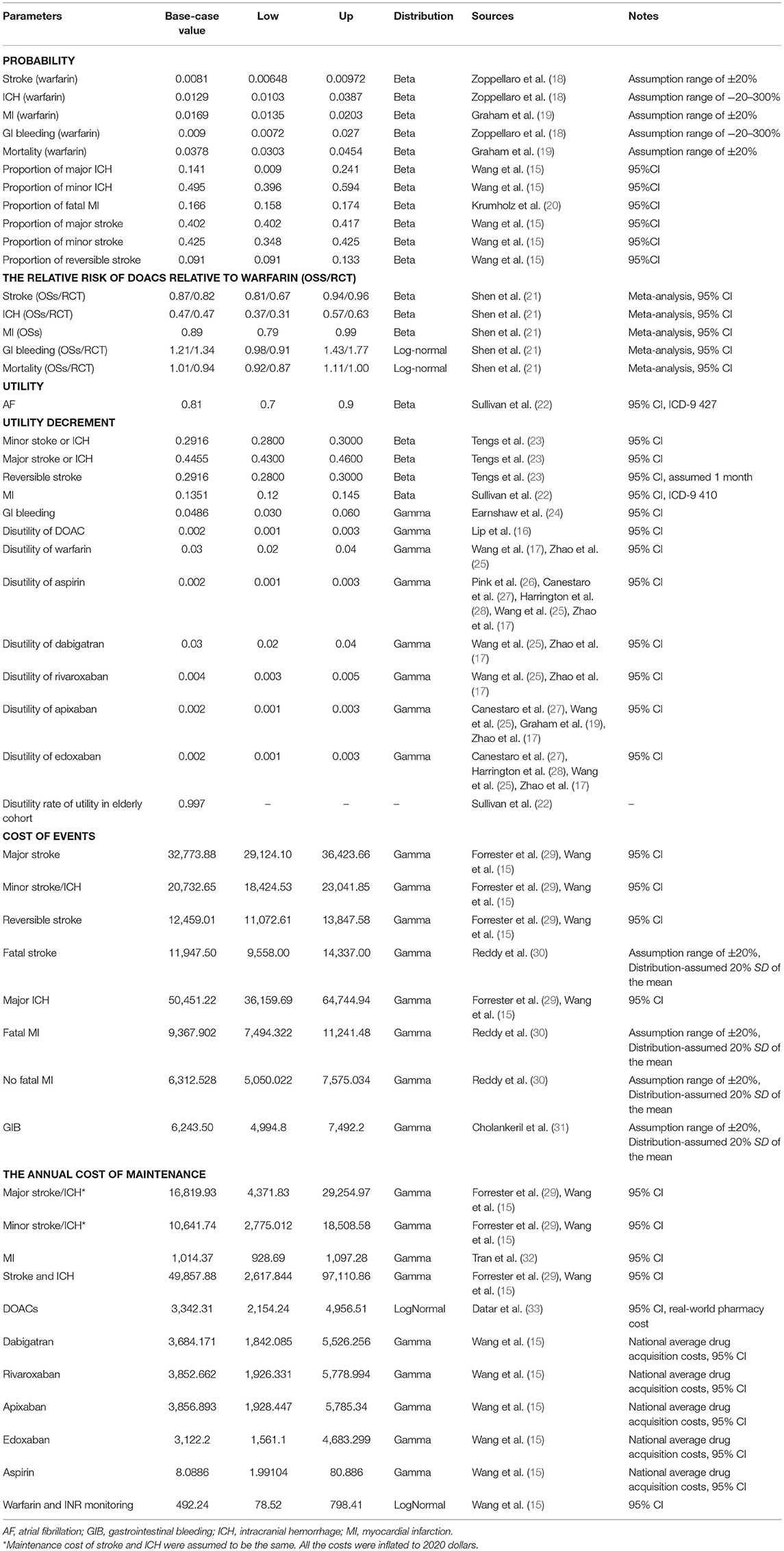
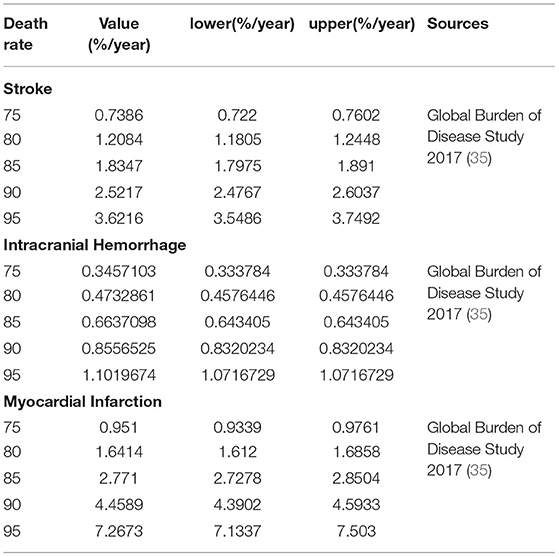
A targeted literature review was performed to identify the appropriate parameter inputs. The probabilities of the clinical outcomes associated with VKAs were obtained from the included OSs or RCTs, respectively (Table 1 and Supplementary Table 1). The comparative probabilities of clinical outcomes relative to DOACs were calculated based on the corresponding rates of VKAs and the pooled hazard ratios reported in our previous work (21) (Table 1 and Supplementary Table 1). The relative risks for clinical outcomes for aspirin compared to warfarin were obtained from a network meta-analysis reported previously (34). The age-dependent baseline death rates for stroke, ICH, and MI were derived from the Global Burden of Disease Study 2017 (35), which were assumed to be similar in DOACs and VKAs (Table 2). All clinical event rates were ultimately converted into probabilities per cycle and then input into the model.
Health State Utilities and Cost
Health utility values, scored from 0 (death) to 1 (perfect health), were obtained from previous studies to describe the quality of life for each health state. The utility of 0.81 was reported for AF patients with an average age of 67 (22). Thus, the adjusted weight of 0.997 (age group >70 compared to 60–69) was further applied as a multiplying factor to calculate the baseline utilities of the cohort aged >75 years approximately (22). A permanent annual disutility of −0.2916, −0.4455, and −0.1351 were assumed for minor, major neurological events and MI. −0.0486 and −0.2916 were assessed as one-time dis-utilities for gastrointestinal bleeding (GIB) and the reversible stroke (23, 24). Anticoagulant therapies were also assumed to cause slight derogation of the health utilities, which were assessed to be from −0.002 to −0.03 for DOACs, warfarin, and Aspirin, respectively (Table 1) (16, 17, 25–28).
From the perspective of the US private payer, the model incorporated only direct healthcare costs for the therapies and treatments of the associated acute clinical events and the costs for long-term maintenance after experiencing the first non-fatal events. The costs of DOACs, warfarin, and aspirin were obtained from real-world pharmacy costs or the 2018 National Average Drug Acquisition cost in the US (Table 1). The clinical event costs were all obtained from the published literature and were inflated to reflect the 2020 value of the US dollar. Maintenance costs for major and minor neurological events were assumed to be similar, respectively. Annual maintenance costs for MI were derived from the average costs incurred during the 2–5-year period after diagnosis (32). All cost and utility inputs were discounted at 3 and 2% per annum to account for the effects of inflation and increasing economic valuation of health gains over time.
Base-Case Analysis
The base-case analyses were conducted incorporating data of RCTs and OSs, respectively. The main outcomes of this analysis were the incremental costs, quality-adjusted life-years (QALYs) gained, and the incremental cost-effectiveness ratio (ICER). Cost-effectiveness was evaluated using the United States' conservative willingness-to-pay threshold of $50,000 and $100,000 per QALY (36). It was also assessed annually to determine the point at which the treatment options had achieved acceptable levels.
Scenario and Sensitivity Analyses
Scenario, One-way sensitivity analysis, and the probabilistic sensitivity analysis (PSA) were performed to assess the impact of parameter uncertainty on the results. In one-way sensitivity analysis, the model parameters were varied over their 95% confidence intervals (CI). If the CI was not available, a variation of ±5% from the mean was assumed for the parameters of utility and ±20% for the parameters of probability and cost. In scenario analysis, the variations in the time horizons and the cost reduction of DOACs after patent expiry were conducted. In PSA, beta distributions were assumed for the clinical outcomes and health utilities, while gamma or log-normal distributions were assumed for the drug and healthcare costs. Hazard or risk ratios of DOACs compared to VKAs were assigned to beta or log-normal distributions. A 10,000-subject Monte Carlo simulation was conducted based on the variable distributions, and all the parameter inputs were allowed to vary stochastically in PSA. The PSA results are presented graphically as scatterplots.
Results
Base-Case Analysis
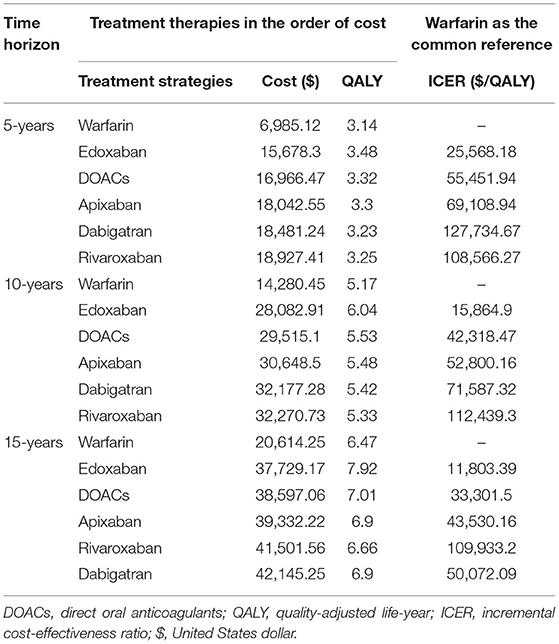
Over a 10-year projected time of a cohort with 10,000 patients, treatment with DOACs rather than with warfarin was predicted to result in fewer incidences of strokes, ICH, MI, and death, according to the simulation based on the real-world evidence (Table 3). Patients treated with warfarin were predicted to obtain 5.17 QALYs at the cost of $14,280.35, while treatment with DOACs resulted in 5.53 QALYs at the cost of $29,515.10. Therefore, the DOACs' additional benefit in reducing the number of total clinical events was associated with a gain of 0.36 QALYs at an additional cost of $15,234.65, resulting in an ICER of $42,318.47 per QALY. In the simulation incorporating the evidence of RCTs, a further reduction of ICH and death was predicted in both DOACs and warfarin but with increased stroke events. It is broadly consistent with the real practice because that regular follow-up of random control trials could help discover the asymptomatic stroke event but is more likely to screen out the patients with high bleeding risk by the rigorous inclusion and exclusion criteria. A slight rise of QALYs was predicted in RCTs than in OSs but with the increased ICER of $47,544.19 per QALY. It might due to the relatively higher drug cost of stroke compared to ICH, after which the patient is more likely to discontinue the therapy of anticoagulants and switch to aspirin (Table 3).
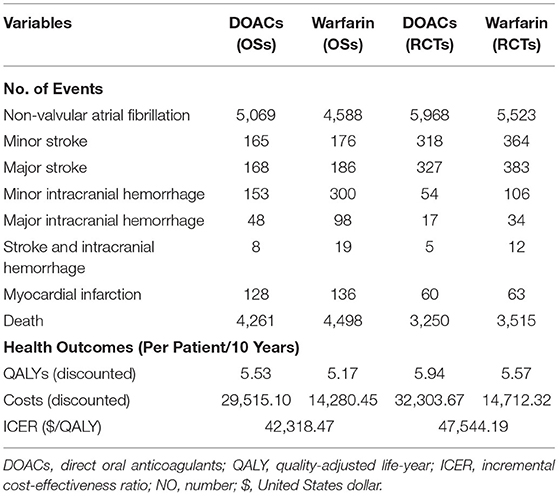
Table 3. Projected clinical events, costs, health benefits, and incremental ICER for base-case analysis over a 10-years life horizon in OSs and RCTs.
The total costs and QALYs of four individual DOACs (rivaroxaban, edoxaban, apixaban, and dabigatran) were also predicted. It indicated that the costs were lowest for warfarin ($14,280.45) and highest for rivaroxaban ($32,270.73). Edoxaban had the highest QALYs (6.04 QALYs), followed by apixaban, dabigatran, rivaroxaban, and warfarin. Compared to warfarin, the ICERs were $112,439.3, $71,587.32, $52,800.16, and $15,864.9 for rivaroxaban, dabigatran, apixaban, and edoxaban, indicating that edoxaban was the preferred therapy for stroke prevention in the elderly with AF.
Scenario and One-Way Sensitivity Analysis
Variations in the time horizons were conducted in scenario analyses (Table 4). At 5-, 10-, and 15-year follow-ups, the ICER per QALY gained decreased from $55,451.94 to $33,301.50, indicating that long-term use of DOACs may provide additional benefits. The decrease of ICER was also predicted for dabigatran, edoxaban, and apixaban with the extension of the time horizon. However, a slight increase in cost-effectiveness estimates was obtained with the long-term use of rivaroxaban. This finding could be explained by the higher drug cost and moderate intensity in reducing the clinical events of rivaroxaban, leading to the lower health gain but increased overall costs.
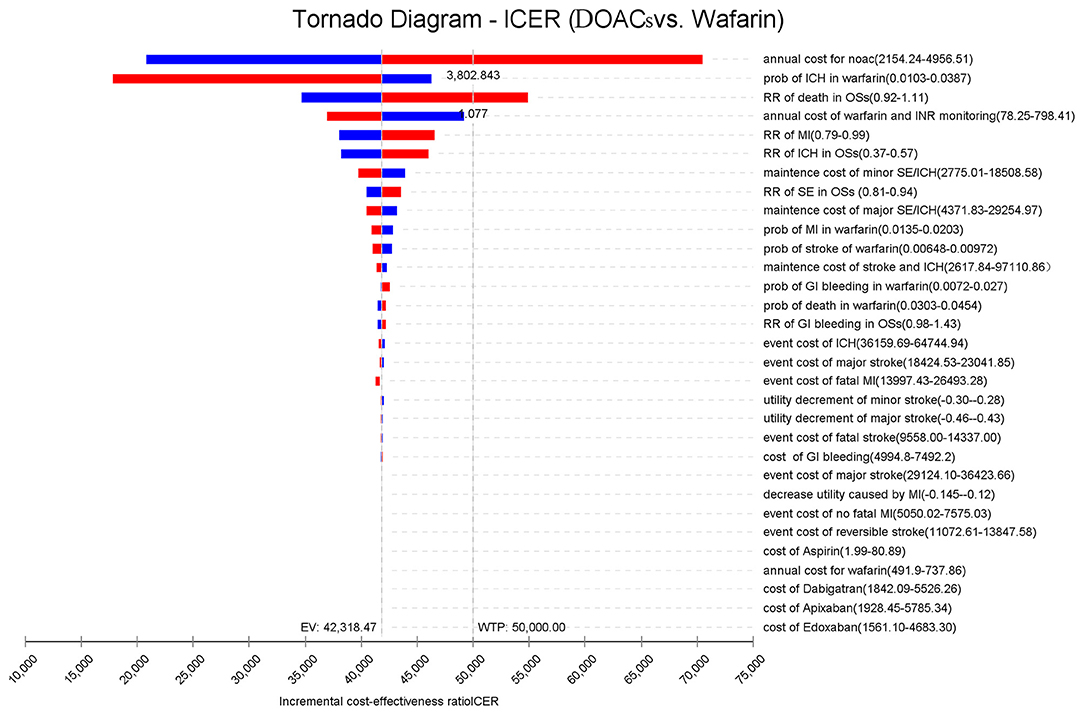
Figure 2 presents the sensitivity analyses of the vital parameter inputs that had the most significant impact on the ICERs, in the order of their respective influences. It was found that the cost of DOACs had an immense impact on the ICER, followed by the probability of ICH, the risk ratio of death, cost of warfarin and INR monitoring, and the risk ratio of MI. When the annual costs of DOACs were over $3,802.84 or the risk ratio of death was over 1.077%/year, the ICERs shifted from dominating to dominated status. As for the other vital parameters, the study found that varying the inputs across their plausible ranges resulted in changes in the ICER values. However, this had no significant effect on the results because DOACs was still more cost-effective than warfarin.
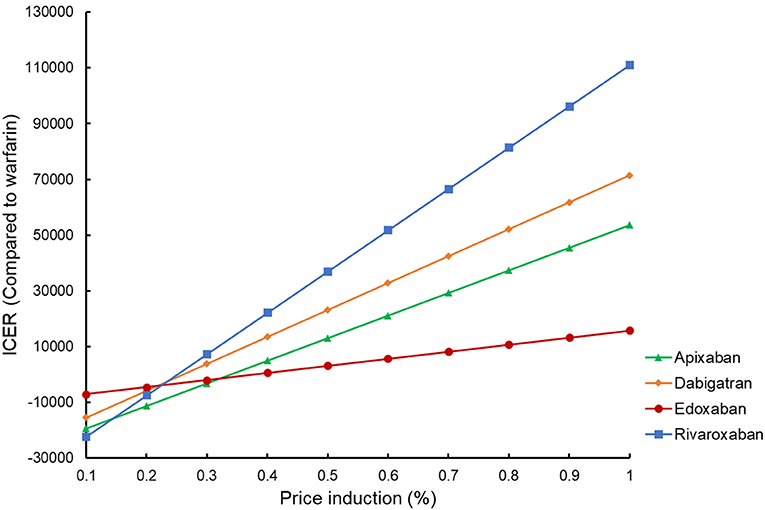
By reducing the price of four DOACs from 10 to 90%, the potential impact of DOACs patent expiry on the outcomes was assessed (Figure 3). Rivaroxaban, dabigatran, and apixaban would become cost-effective compared to warfarin if their prices were cut down to 58.7, 77.7, and 95.5%, using a WTP threshold of $50,000/QALY. When $100,000/QALY was applied as a WTP threshold, apixaban and dabigatran would become cost-effective for stroke prevention in the elderly with AF, and about 92.5% of price reduction should be considered for rivaroxaban being undominated. Considering that the price of a given drug might drop by about 9–42% after the patent expiry in the competitive market (37), these four DOACs would probably become the preferred therapy for stroke prevention in the elderly population compared to warfarin in the further. In addition to the drug cost, deterministic sensitivity analysis showed that ICH event rate was another critical drive of the outcomes in the cost-effectiveness analysis between DOACs and warfarin. Using a WTP threshold of $50,000/QALY, dabigatran, rivaroxaban, and apixaban would be cost-effective compared to warfarin if the ICH rate dropped to <2.09, 3.33, and 1.47% per year, respectively. If there was a lower incidence of death and MI (<3.32 and 2.0% per year) or if the annual cost of warfarin exceeded $690.09, apixaban would shift to dominating status either.
Probabilistic Sensitivity Analysis
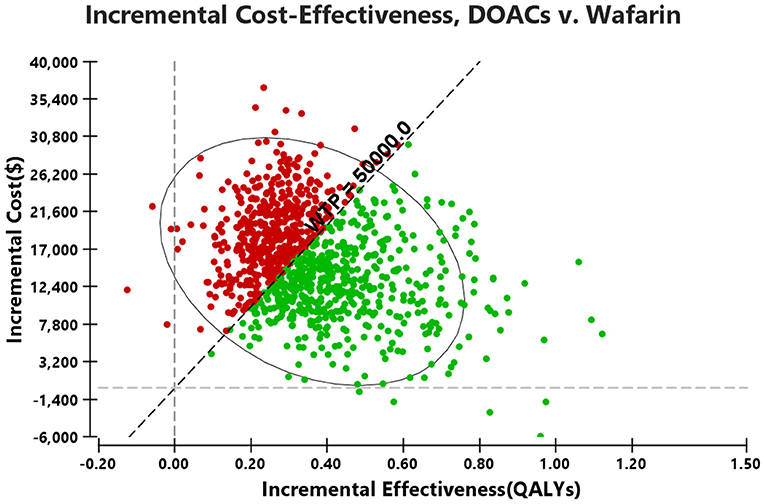
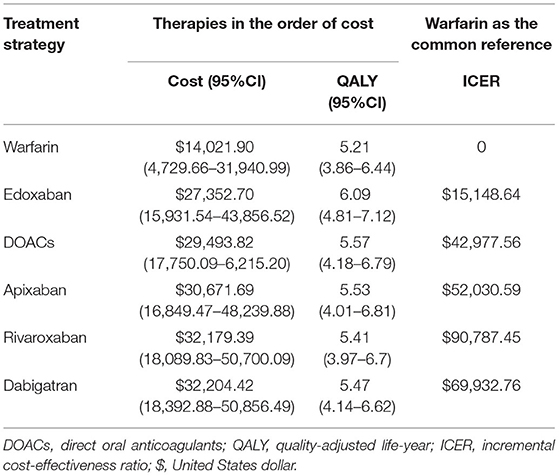
Probabilistic sensitivity analysis simulations (Figure 4) demonstrated that over a 10-year life horizon, though requiring minimal additional costs, DOACs were more effective than warfarin. Treatment with DOACs resulted in a total cost of $29,493.82 (95% CI, 17,750.09–6,215.20), while the total cost of warfarin was estimated to be $14,021.90 (95% CI, 4,729.66–31,940.99). Over a 10-year horizon, DOACs gained an average QALY of 5.57 (95% CI, 4.18–6.79) vs. 5.21 (95% CI, 3.86–6.44) for warfarin (Table 5). The mean costs, QALYs, and ICERs of four DOACs were also estimated, and all the results were largely consistent with the base-case analysis (Table 5).
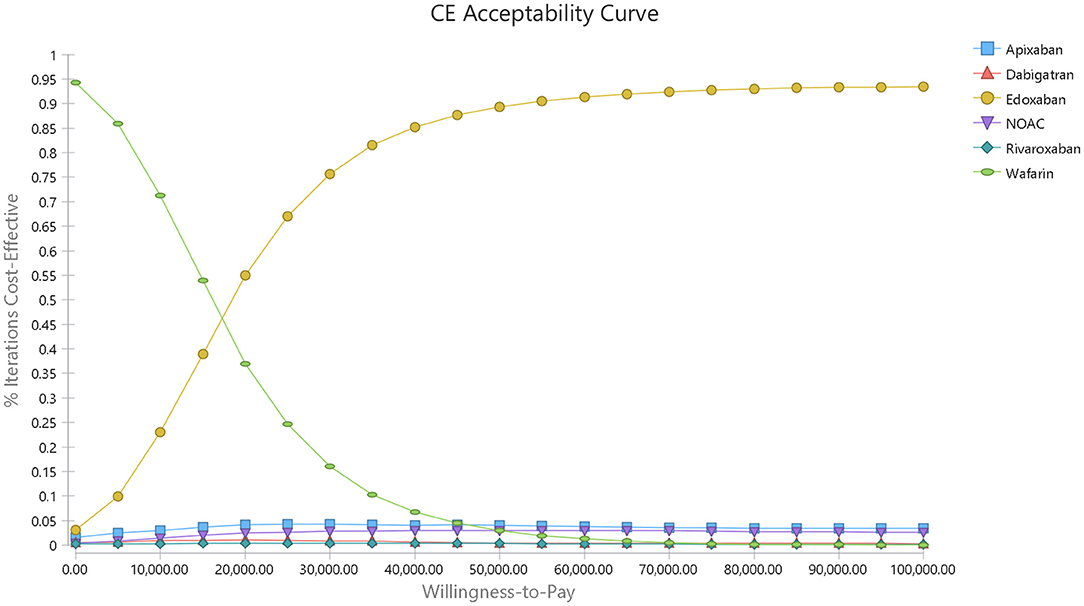
A cost-effectiveness acceptability curve was plotted to demonstrate the proportion of simulations that were cost-effective at willingness-to-pay values (Figure 5). There was a 53.83 and 90.7% probability that DOACs were more cost-effective than warfarin when the willingness-to-pay threshold was set at $50,000 and $100,000/QALY, respectively. Compared to warfarin, dabigatran, apixaban, rivaroxaban, and edoxaban was cost-effective in 29.9, 44.27, 23.55, and 95.38% at the most conservative WTP of $50,000/QALY, and these probabilities arose to 59.37, 68.05, 52.91, and 99.40% correspondingly when the WTP of $100,000/QALY was applied (Supplementary Figure 1 and Figure 2).
Discussion
Main Findings and Interpretation
Based on a comprehensive overview of benefits and harms in real-life practice, this study provided a cost-effectiveness analysis and comparison of DOACs and warfarin in elderly AF patients from the perspective of the US private payer. The comparison of DOACs vs. warfarin in AF has been discussed frequently. Owing to their impressive efficacy and convincing safety profiles reported by RCTs, the economic viability of DOACs has been demonstrated in the US (9, 28), European countries (10, 11), and in some Asian countries (17, 38, 39). Despite their much higher costs, DOACs are generally more cost-effective than warfarin. However, the benefits of DOACs in the elderly population remain unclear as the elderly are generally perceived to have a high incidence of ICH, leading to a significant loss in quality of life and increased medical costs. In our previous work, a comprehensive systematic review and meta-analysis involving 27 high-quality observational studies (OSs) (519,267 patients) and three RCTs (28,152 patients) were conducted to estimate and compare the benefits and harms of DOACs and VKAs in the cohort aged >75 years (21). Compared with VKAs, DOACs significantly reduced the risk of stroke/SE and ICH without increasing the risk of GIB and all-cause mortality. In this study, based on the efficacy profiles derived from our previous work, we further conducted a comparative cost-effectiveness evaluation of DOACs (dabigatran, rivaroxaban, apixaban, and edoxaban) and VKAs in AF patients aged >75 years to thoroughly assess the expected costs and benefits of using DOACs in the elderly population. Major findings were as follows: (I) DOACs are more cost-effective treatment options than warfarin in real-world practice; (II) among all the four individual DOACs, edoxaban was the preferred treatment strategy by yielding the most significant health gain with the relatively low total cost.
Comparison With Previous Studies
Until now, only one study has assessed the cost-effectiveness of DOACs in elderly AF patients (17). Although DOACs were generally more cost-effective than warfarin, it was concluded that the benefits appeared to be partially offset in elderly patients due to the increased bleeding risk. If the real-world risks of bleeding and ICH were factored in 4.8/1,000 person-years (40), the cost-effectiveness of DOACs relative to warfarin might end up being negated. However, only OSs on dabigatran and rivaroxaban were included in the above-mentioned study, resulting in underestimating the protective benefits of apixaban and edoxaban. In the present study, a cost-effectiveness evaluation was built upon a comprehensive meta-analysis of OSs that included all the available DOACs. Despite the high risk for major bleeding (about 13/1,000 person-years), which might be associated with the relatively loose inclusion and exclusion in OSs than in RCTs, it was concluded that DOACs was still more cost-effective than VKAs for elderly AF patients, In the sensitivity analysis, we further tripled the incidence of ICH and GIB. The ICER values did not increase over the cost-effectiveness threshold despite the significant increase in major bleeding risk, indicating that the treatment benefits of DOACs were of greater relevance and importance in older adults beyond the safety profile. In previous work, although all four individual DOACs were cost-effective compared to warfarin from the perspective of the private patient in Singapore, apixaban was found to be the preferred treatment strategy for AF patients aged over 75 years. While because of the relatively lower risk of all-cause death and MI, and the good protection effect in stroke prevention for elderly patients in real practice, edoxaban was revealed to be the optimal therapeutic strategy among all DOACs by yielding the highest health gain and comparatively lower total cost in the present study.
Pivotal Factors for Cost-Effectiveness Analysis
In sensitivity analysis, drug cost was found to be one of the crucial factors in cost-effectiveness analysis. Since drug price reduction always happened for experiencing the generic entry after the patent protection, the potential affordability of DOACs compared to warfarin after patent expiry was further assessed in scenario analysis. Using a most conservative WTP threshold of $50,000/QALY, rivaroxaban, apixaban, and edoxaban, the three dominated DOACs with the current prices, could become cost-effective only by a slight to a moderate price reduction <50%). As the price of a given drug might drop by about 9–42% after the patent expiry (37), it is plausible to suppose that all the four DOACs would become the preferred therapy in comparison with warfarin for the stroke prevention in the elderly population in the further.
Strengths and Limitations
The main strength of this study was that using data from OSs, it compared the cost-effectiveness of all available DOACs (dabigatran, rivaroxaban, apixaban, and edoxaban) and warfarin in elderly AF patients. However, several intrinsic limitations should be addressed in this study. First, the rates of clinical events in this model were derived from OSs that had follow-up durations of up to 2.8 years. It was likely to produce a bias by extrapolating the event rates to a 10-year time horizon. Second, although medical costs are generally high for elderly patients, cost increases associated with age were not included in this study due to the lack of accurate cost information. Third, the results of this work were subject to several assumptions, such as the switch to aspirin after any ICH event, the transition to death after two major neurological events, and so on. These assumptions may help to approximate the AF disease progression in real-world practice but would probably produce conservative results to a certain extent. Fourth, specific treatment effects associated with the dosage of DOACs (including off-label and the low dosage), time-in-therapeutic ranges of warfarin, poor compliance in elderly patients, and the switch among different DOACs are not noted due to the lack of specific data in available OSs. In fact, because of the poor kidney function or some other risk of bleeding in the elderly, the reduced-dose DOACs were widely used in most populations. And the poor compliance in frail elderly patients complicates the management of antithrombotic therapy further. Time-in-therapeutic ranges of warfarin should also be noted, as they are closely related to the treatment effect of the warfarin. Thus, the results of our analysis may be conservative because of the deviations of drug administration in real practice.
Conclusions
For the elderly with AF, despite the high risk for major bleeding, DOACs are more cost-effective treatment options than warfarin in real-world practice. Edoxaban was the preferred treatment strategy among four kinds of DOACs for AF patients aged over 75 years. The treatment benefits of DOACs assumed greater relevance and importance in older adults beyond the safety profile.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
Z-CG is the guarantor of the entire manuscript. Z-CG and YW contributed to the study conception and design and critical revision of the manuscript for important intellectual content. CZ contributed to data acquisition, analysis, and interpretation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Research Funds of Shanghai Health and Family Planning commission (20184Y0022), Clinical Pharmacy Innovation Research Institute of Shanghai Jiao Tong University School of Medicine (CXYJY2019ZD001), Shanghai “Rising Stars of Medical Talent” Youth Development Program—Youth Medical Talents—Clinical Pharmacist Program [SHWJRS (2019)_072], and Teaching and Research Project in Faculty of Medical Sciences of Wuhan University (2018016).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dwight Keith for the constructive suggestions about language editing in our manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.675200/full#supplementary-material
References
1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. (2014) 129:837–47. doi: 10.1161/circulationaha.113.005119
2. Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. (2004) 110:1042–6. doi: 10.1161/01.Cir.0000140263.20897.42
3. Lip GYH, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. (2012) 142:1489–98. doi: 10.1378/chest.11-2888
4. Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging. (2013) 30:949–58. doi: 10.1007/s40266-013-0119-3
5. Björck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population-based study. Stroke. (2013) 44:3103–8. doi: 10.1161/strokeaha.113.002329
6. Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. (2013) 167:1807–24. doi: 10.1016/j.ijcard.2012.12.093
7. Alkhouli M, Noseworthy PA, Rihal CS, Holmes DR Jr. Stroke prevention in nonvalvular atrial fibrillation: a stakeholder perspective. J Am Coll Cardiol. (2018) 71:2790–801. doi: 10.1016/j.jacc.2018.04.013
8. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. (2018) 39:1330–93. doi: 10.1093/eurheartj/ehy136
9. Hernandez I, Smith KJ, Zhang Y. Cost-effectiveness of non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with atrial fibrillation at high risk of bleeding and normal kidney function. Thromb Res. (2017) 150:123–30. doi: 10.1016/j.thromres.2016.10.006
10. López-López JA, Sterne JAC, Thom HHZ, Higgins JPT, Hingorani AD, Okoli GN, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. (2017) 359:j5058. doi: 10.1136/bmj.j5058
11. Thom HHZ, Hollingworth W, Sofat R, Wang Z, Fang W, Bodalia PN, et al. Directly acting oral anticoagulants for the prevention of stroke in atrial fibrillation in England and Wales: cost-effectiveness model and value of information analysis. MDM Policy Pract. (2019) 4:2381468319866828. doi: 10.1177/2381468319866828
12. Schäfer A, Flierl U, Berliner D, Bauersachs J. Anticoagulants for stroke prevention in atrial fibrillation in elderly patients. Cardiovasc Drugs Ther. (2020) 34:555–68. doi: 10.1007/s10557-020-06981-3
13. Rivera-Caravaca JM, Esteve-Pastor MA, Marín F, Valdés M, Vicente V, Roldán V, et al. A propensity score matched comparison of clinical outcomes in atrial fibrillation patients taking vitamin K antagonists: comparing the “real-world” vs clinical trials. Mayo Clinic Proc. (2018) 93:1065–73. doi: 10.1016/j.mayocp.2018.01.028
14. Gabet A, Grimaud O, de Peretti C, Béjot Y, Olié V. Determinants of case fatality after hospitalization for stroke in France 2010 to 2015. Stroke. (2019) 50:305–12. doi: 10.1161/strokeaha.118.023495
15. Wang CY, Pham PN, Thai TN, Brown JD. Updating the cost effectiveness of oral anticoagulants for patients with atrial fibrillation based on varying stroke and bleed risk profiles. Pharmacoeconomics. (2020) 38:1333–43. doi: 10.1007/s40273-020-00960-0
16. Lip GY, Kongnakorn T, Phatak H, Kuznik A, Lanitis T, Liu LZ, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther. (2014) 36:192.e20–210.e20. doi: 10.1016/j.clinthera.2013.12.011
17. Zhao YJ, Lin L, Zhou HJ, Tan KT, Chew AP, Foo CG, et al. Cost-effectiveness modelling of novel oral anticoagulants incorporating real-world elderly patients with atrial fibrillation. Int J Cardiol. (2016) 220:794–801. doi: 10.1016/j.ijcard.2016.06.087
18. Zoppellaro G, Zanella L, Denas G, Gennaro N, Ferroni E, Fedeli U, et al. Different safety profiles of oral anticoagulants in very elderly non-valvular atrial fibrillation patients. A retrospective propensity score matched cohort study. Int J Cardiol. (2018) 265:103–7. doi: 10.1016/j.ijcard.2018.04.117
19. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. (2015) 131:157–64. doi: 10.1161/circulationaha.114.012061
20. Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. (2009) 2:407–13. doi: 10.1161/circoutcomes.109.883256
21. Shen NN, Wu Y, Wang N, Kong LC, Zhang C, Wang JL, et al. Direct oral anticoagulants vs. vitamin-K antagonists in the elderly with atrial fibrillation: a systematic review comparing benefits and harms between observational studies and randomized controlled trials. Front Cardiovasc Med. (2020) 7:132. doi: 10.3389/fcvm.2020.00132
22. Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. (2006) 26:410–20. doi: 10.1177/0272989x06290495
23. Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics. (2003) 21:191–200. doi: 10.2165/00019053-200321030-00004
24. Earnshaw SR, Scheiman J, Fendrick AM, McDade C, Pignone M. Cost-utility of aspirin and proton pump inhibitors for primary prevention. Arch Intern Med. (2011) 171:218–25. doi: 10.1001/archinternmed.2010.525
25. Wang Y, Xie F, Kong MC, Lee LH, Ng HJ, Ko Y. Cost-effectiveness of dabigatran and rivaroxaban compared with warfarin for stroke prevention in patients with atrial fibrillation. Cardiovasc Drugs Ther. (2014) 28:575–85. doi: 10.1007/s10557-014-6558-1
26. Pink J, Lane S, Pirmohamed M, Hughes DA. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ. (2011) 343:d6333. doi: 10.1136/bmj.d6333
27. Canestaro WJ, Patrick AR, Avorn J, Ito K, Matlin OS, Brennan TA, et al. Cost-effectiveness of oral anticoagulants for treatment of atrial fibrillation. Circ Cardiovasc Qual Outcomes. (2013) 6:724–31. doi: 10.1161/circoutcomes.113.000661
28. Harrington AR, Armstrong EP, Nolan PE Jr, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. (2013) 44:1676–81. doi: 10.1161/strokeaha.111.000402
29. Forrester SH, Devine EB, Sullivan SD. Estimating the inpatient and outpatient costs of atrial fibrillation and associated adverse events. Value Health. (2014) 17:A109. doi: 10.1016/j.jval.2014.03.635
30. Reddy VY, Akehurst RL, Gavaghan MB, Amorosi SL, Holmes DR Jr. Cost-effectiveness of left atrial appendage closure for stroke reduction in atrial fibrillation: analysis of pooled, 5-year, long-term data. J Am Heart Assoc. (2019) 8:e011577. doi: 10.1161/jaha.118.011577
31. Cholankeril G, Hu M, Cholankeril R, Khan MA, Gadiparthi C, Yoo ER, et al. Inpatient outcomes for gastrointestinal bleeding associated with percutaneous coronary intervention. J Clin Gastroenterol. (2019) 53:120–6. doi: 10.1097/mcg.0000000000000971
32. Tran DT, Welsh RC, Ohinmaa A, Thanh NX, Kaul P. Resource use and burden of hospitalization, outpatient, physician, and drug costs in short- and long-term care after acute myocardial infarction. Can J Cardiol. (2018) 34:1298–306. doi: 10.1016/j.cjca.2018.05.022
33. Datar M, Crivera C, Rozjabek H, Abbass IM, Xu Y, Pasquale MK, et al. Comparison of real-world outcomes in patients with nonvalvular atrial fibrillation treated with direct oral anticoagulant agents or warfarin. Am J Health Syst Pharm. (2019) 76:275–85. doi: 10.1093/ajhp/zxy032
34. Roskell NS, Lip GY, Noack H, Clemens A, Plumb JM. Treatments for stroke prevention in atrial fibrillation: a network meta-analysis and indirect comparisons versus dabigatran etexilate. Thromb Haemost. (2010) 104:1106–15. doi: 10.1160/th10-10-0642
35. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2017 (GBD 2017) Results. Seattle, WA: Institute for Health Metrics and Evaluation (IHME) (2018). Available online at: http://ghdx.healthdata.org/gbd-results-tool (accessed August 28, 2019).
36. Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. (2008) 8:165–78. doi: 10.1586/14737167.8.2.165
37. Berndt ER, Dubois P. Impacts of patent expiry on daily cost of pharmaceutical treatments in eight OECD countries, 2004–2010. Int J Econ Bus. (2016) 23:125–47. doi: 10.1080/13571516.2015.1122969
38. Dilokthornsakul P, Nathisuwan S, Krittayaphong R, Chutinet A, Permsuwan U. Cost-effectiveness analysis of non-vitamin k antagonist oral anticoagulants versus warfarin in thai patients with non-valvular atrial fibrillation. Heart Lung Circ. (2020) 29:390–400. doi: 10.1016/j.hlc.2019.02.187
39. Liao CT, Lee MC, Chen ZC, Ku LE, Wang JD, Toh HS. Cost-effectiveness analysis of oral anticoagulants in stroke prevention among patients with atrial fibrillation in Taiwan. Acta Cardiol Sin. (2020) 36:50–61. doi: 10.6515/acs.202001_36(1)0.20190511a
Keywords: atrial fibrillation, elderly, direct oral anticoagulants, cost-effectiveness, real-world study, edoxaban, rivaroxaban, dabigatran
Citation: Wu Y, Zhang C and Gu Z-C (2021) Cost-Effectiveness Analysis of Direct Oral Anticoagulants Vs. Vitamin K Antagonists in the Elderly With Atrial Fibrillation: Insights From the Evidence in a Real-World Setting. Front. Cardiovasc. Med. 8:675200. doi: 10.3389/fcvm.2021.675200
Received: 02 March 2021; Accepted: 21 May 2021;
Published: 29 June 2021.
Edited by:
Kristi Reynolds, Kaiser Permanente, United StatesReviewed by:
Jaejin An, Kaiser Permanente Southern California, United StatesRuxu You, Huazhong University of Science and Technology, China
Copyright © 2021 Wu, Zhang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Wu, bWF5bW9vbkB3aHUuZWR1LmNu; Zhi-Chun Gu, Z3V6aGljaHVuMjEzQDE2My5jb20=
 Yue Wu
Yue Wu Chi Zhang
Chi Zhang Zhi-Chun Gu
Zhi-Chun Gu