- 1Department of General Practice, Central South University Xiangya School of Medicine Affiliated Haikou Hospital, Haikou, China
- 2Department of Geriatrics, Central South University Xiangya School of Medicine Affiliated Haikou Hospital, Haikou, China
- 3Department of Cardiovasology, Central South University Xiangya School of Medicine Affiliated Haikou Hospital, Haikou, China
Propose: Cytochrome P450 family 2 subfamily R member 1 (CYP2R1) variations can affect the activity of 25-hydroxylase, resulting in the deficiency of 25(OH)D, which leads to an increased incidence and mortality of coronary heart disease (CHD). The purpose is to assess the influence of CYP2R1 variants on CHD risk among the Chinese Han population.
Methods: A total of 508 CHD patients and 510 healthy controls were enrolled. The MassARRAY platform completed genotyping of CYP2R1 variants. Odds ratios (ORs) with 95% confidence intervals (CI) were calculated using logistic regression analysis.
Results: Rs6486205 (OR = 1.25, 95% CI: 1.05–1.50, p = 0.014), rs10741657 (OR = 1.29, 95% CI: 1.08–1.54, p = 0.005), and rs2060793 (OR = 1.27, 95% CI: 1.06–1.51, p = 0.009) were associated with the increased susceptibility to CHD in the whole subjects. Interestingly, the relationships between these variants and CHD risk were observed in the subjects with age >60 years, males or non-smoker. Additionally, the haplotypes Ars10741657Ars2060793 and Grs10741657Grs2060793 had the higher risk of CHD, and the combination (rs6486205 and rs10741657) was the best multi-locus model.
Conclusion: Our study suggested the contribution of CYP2R1 polymorphisms to the increased CHD predisposition in the Chinese Han population. Furthermore, the risk association was related to confounding factors for CHD, including age, sex, and smoking. These findings might help to strengthen the understanding of the CYP2R1 gene in the occurrence of CHD.
Introduction
Cardiovascular diseases (CVDs) cause 17.9 million deaths every year, accounting for a 31% global death toll, and approximately 85% of all CVD deaths are caused by coronary heart disease (CHD) and stroke (1). According to the 2018 China CVD report, there were approximately 290 million CVD patients in China, of which 11 million suffered from CHD (2). The incidence of CHD in women is lower than that of men, but the outcomes of CHD in females are worse than that of males (3). Coronary heart disease is one of the most common CVDs, characterized by remodeling and narrowing of coronary arteries (4). Coronary heart disease is a complex multifactorial disease. Previous studies have identified various risk factors for CHD, including smoking, drinking, hypertension, diabetes, dyslipidemia, and dietary factors (5, 6). To date, some genome-wide association studies have reported many susceptibility genes to CHD predisposition (7, 8), suggesting that genetic variants may have a central role in the occurrence of CHD.
The human cytochrome P450 family 2 subfamily R member 1 (CYP2R1) gene, located at 11p15.2, encodes a member of the CYP450 enzyme superfamily. CYP2R1 enzyme, produced in hepatic microsomes, is a physiologically important vitamin D hydroxylase that can convert vitamin D into 25-hydroxyvitamin D [25(OH)D] (9). Mutations in CYP2R1 were related to the insufficient levels of 25(OH)D in individuals (10). The decreased level of vitamin D in circulation was related to the higher relative risk of CVDs, and the deficiency of vitamin D increased the mortality rate of CVDs (11, 12). Serum 25(OH)D in patients with coronary artery disease was correlated with cardiac structure and function (13). A large observational study suggested a reverse J-shaped relationship between serum 25(OH)D levels and CVDs, with the highest risk at lower levels (14). In previous observational research, low 25(OH)D might have a higher CHD risk, and this relationship might vary by race (15). These studies supported the physiological importance of the CYP2R1 gene in the occurrence and development of CHD. Previously, genetic polymorphisms in CYP2R1 were associated with various CVD-related diseases, including myocardial infarction and stroke, type 2 diabetes, and hypertension (16–19). However, the contribution of CYP2R1 variants to CHD predisposition has not been reported among the Chinese Han population. These studies suggest that CYP2R1 has a significant role in the development of CHD, which led us to propose the hypothesis that CYP2R1 polymorphisms could be of importance in CHD susceptibility among the Chinese Han population.
CYP2R1 rs10741657 and rs2060793 are involved in the regulation of gene expression and activity of 25-hydroxylase (20, 21), and the function of rs6486205 has not been reported to date. Here, three single nucleotide polymorphisms (SNPs) in CYP2R1 (rs6486205, rs10741657, and rs2060793) were randomly selected and genotyped to assess the effect of single variants and combined SNPs on CHD predisposition among the Chinese Han population. Considering that age, sex, smoking, drinking, diabetes, and hypertension were confounding factors for CHD, stratification analysis was also performed to evaluate the contribution of CYP2R1 SNPs to CHD risk.
Materials and Methods
Study Participants
A total of 1,018 genetically unrelated participants comprised 508 CHD patients, and 510 healthy controls were enrolled from Haikou People's Hospital. Patients were diagnosed with angiographically documented CHD by severe coronary stenosis (≥50%) in the main coronary arteries or their major branches. Patients with concomitant cardiomyopathy, congenital or valvar heart disease, brain, renal, liver, and lung disease, and tumor were excluded. Controls were recruited at the health examination of the hospital. No chest symptoms or electrocardiogram abnormalities confirmed healthy individuals without CHD. The controls were free from cerebrovascular disease, CVDs, peripheral vascular disease, kidney disease, autoimmune diseases, and cancer. All participants were Chinese Han population. Demographic information and clinical data were collected by standardized questionnaires and medical records, including age, sex, cigarette smoking, drinking, hypertension and diabetic status, blood biochemical index, etc. The Ethics Committee of Haikou People's Hospital (2018-179) approved the study, and informed consent was gained from all subjects. The study was carried out in compliance with the declaration of Helsinki.
Genotyping
Peripheral blood samples (5 ml) were gathered in ethylenediaminetetraacetic acid tubes. Genomic DNA was isolated using commercial GoldMag DNA extraction kits (GoldMag, Xi′an, China). Three candidate SNPs in CYP2R1 (rs6486205, rs10741657, and rs2060793) were randomly selected based on the minor allele frequency >0.05 from 1,000 Genomes Project database, Hardy–Weinberg equilibrium (HWE) >0.05, and the call rate >95%. Genotyping of CYP2R1 polymorphisms was performed by the Agena MassARRAY platform (Agena, San Diego, CA, USA). Primers design and data management were carried out by supporting software. The primers were listed in Supplementary Table 1. Approximately 10% of the samples were randomly re-genotyped for quality control, and the concordance rate was 100%.
Data Analysis
The distribution of characteristics between CHD patients and healthy controls were compared by χ2-test and sample t-test or Mann–Whitney U-test. The goodness of fit χ2-test analyzed HWE in controls and cases. Multiple genetic models were used to assess the contribution of CYP2R1 variants to CHD susceptibility. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using logistic regression analysis. We used Power and Sample Size Calculation software (http://sampsize.sourceforge.net/iface/s3.html#ccp) to calculate the power values. The Haploview v4.2 program constructed linkage disequilibrium and haplotype. Multifactor dimension reduction (MDR) was used to assess the best models for the SNP–SNP interaction and the gene–environment interaction on CHD risk. Analysis of variance was used to evaluate the association between genotypes of CYP2R1 variants and blood biochemical index. Statistical analysis was completed by SPSS 20.0 and PLINK 1.0.7software. Two-tailed p < 0.05 was considered statistically significant.
Results
Features of Participants
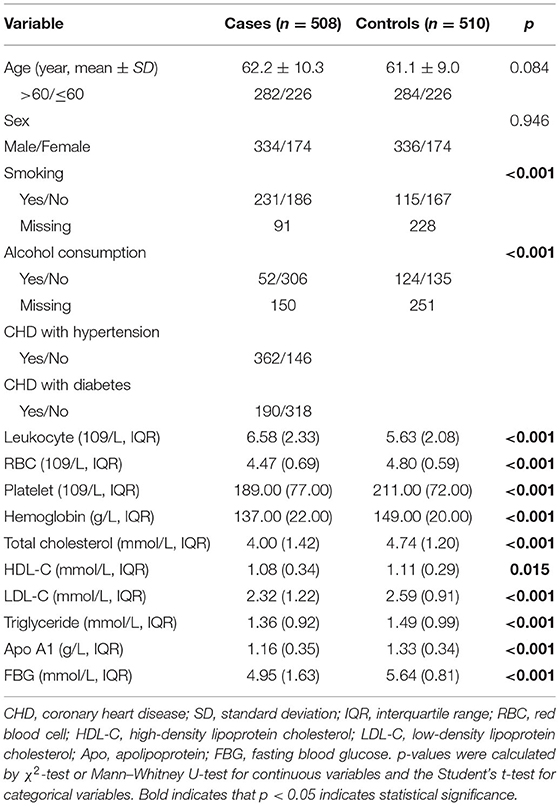
The appearances of participants are shown in Table 1. The study included 508 CHD patients (62.2 ± 10.3 years, 334 males and 174 females) and 510 healthy controls (61.1 ± 9.0 49 years, 336 males and 174 females). There was no significant difference in age and sex distribution (p = 0.084 and 0.946, respectively) between the two groups. However, significant differences in smoking, alcohol consumption, and the concentration of leukocyte, red blood cell, platelet, hemoglobin, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, triglyceride, Apo A1, and fasting blood glucose were found between the two groups (p < 0.05).
Association Between Cytochrome P450 Family 2 Subfamily R Member 1 Single Nucleotide Polymorphisms and Coronary Heart Disease Predisposition
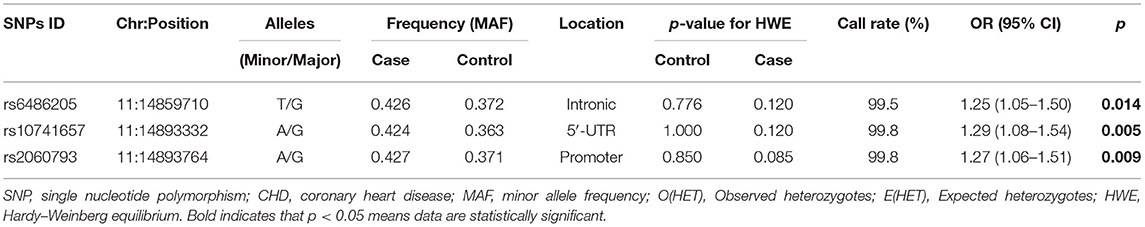
Three CYP2R1 SNPs were in line with HWE (all p > 0.05, Table 2). The frequencies of rs6486205-T, rs10741657-A, and rs2060793-A alleles were higher in the cases and were related to the higher risk of CHD (rs6486205, OR = 1.25, 95% CI: 1.05–1.50, p = 0.014; rs10741657, OR = 1.29, 95% CI: 1.08–1.54, p = 0.005; and rs2060793, OR = 1.27, 95% CI: 1.06–1.51, p = 0.009).
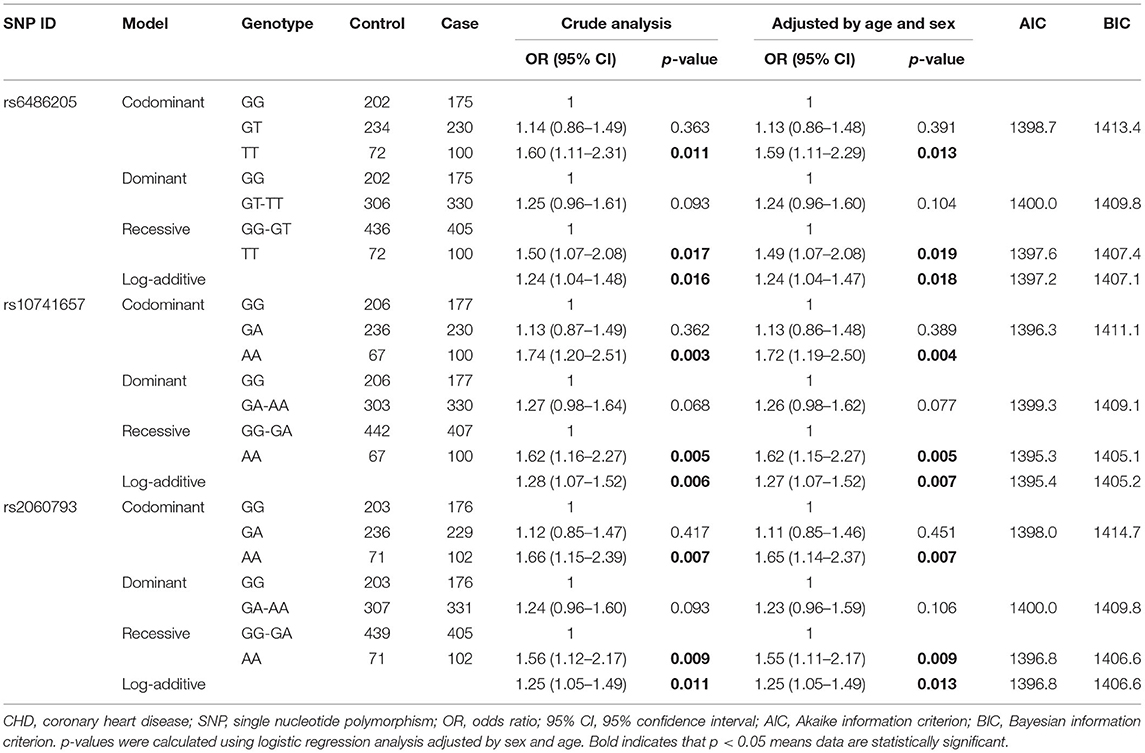
Multiple genetic models analysis also displayed that rs6486205, rs10741657, and rs2060793 were associated with increased susceptibility to CHD (Table 3). Concretely, the risk association between rs6486205 and CHD occurrence was found under codominant (OR = 1.59, 95% CI: 1.11–2.29, p = 0.013, power = 79.74%), recessive (OR = 1.49, 95% CI: 1.07–2.08, p = 0.019, power = 65.92%), and additive (OR = 1.24, 95% CI: 1.04–1.47, p = 0.018). Rs10741657 increased the risk of CHD (AA vs. GG, OR = 1.72, 95% CI: 1.19–2.50, p = 0.004, power = 89.76%; AA vs. GG-GA, OR = 1.62, 95% CI: 1.15–2.27, p = 0.005, power = 80.90%; and additive, OR = 1.27, 95% CI: 1.07–1.52, p = 0.007). In addition, the higher CHD risk was observed for rs2060793 under the codominant (OR = 1.65, 95% CI: 1.14–2.37, p = 0.007, power = 85.40%), recessive (OR = 1.55, 95% CI: 1.11–2.17, p = 0.009, power = 74.00%), and additive models (OR = 1.25, 95% CI: 1.05–1.49, p = 0.013).
Stratification Analysis for the Contribution of Cytochrome P450 Family 2 Subfamily R Member 1 Single Nucleotide Polymorphisms to Coronary Heart Disease Risk
Considering that age, sex, smoking, drinking, diabetes, and hypertension were confounding factors for CHD, stratification analyses were carried out to estimate the relation between CYP2R1 SNPs and CHD risk.
Stratified by age, rs6486205, rs10741657, and rs2060793 increased the risk of CHD in the subjects with age >60 years. Significant results were shown under the allele, codominant, recessive, and additive models, as shown in Table 4. In sex stratification, the association between CYP2R1 SNPs (rs6486205, rs10741657, and rs2060793) and CHD risk was observed in males but not in females (Table 4). For rs6486205, T allele and TT genotype carriers had a higher risk of CHD in males. For rs10741657, increased predisposition of CHD was found in the allele, codominant, dominant, and additive models. For rs2060793, A allele and AA genotype frequency distribution also differed between the cases and the controls among males.
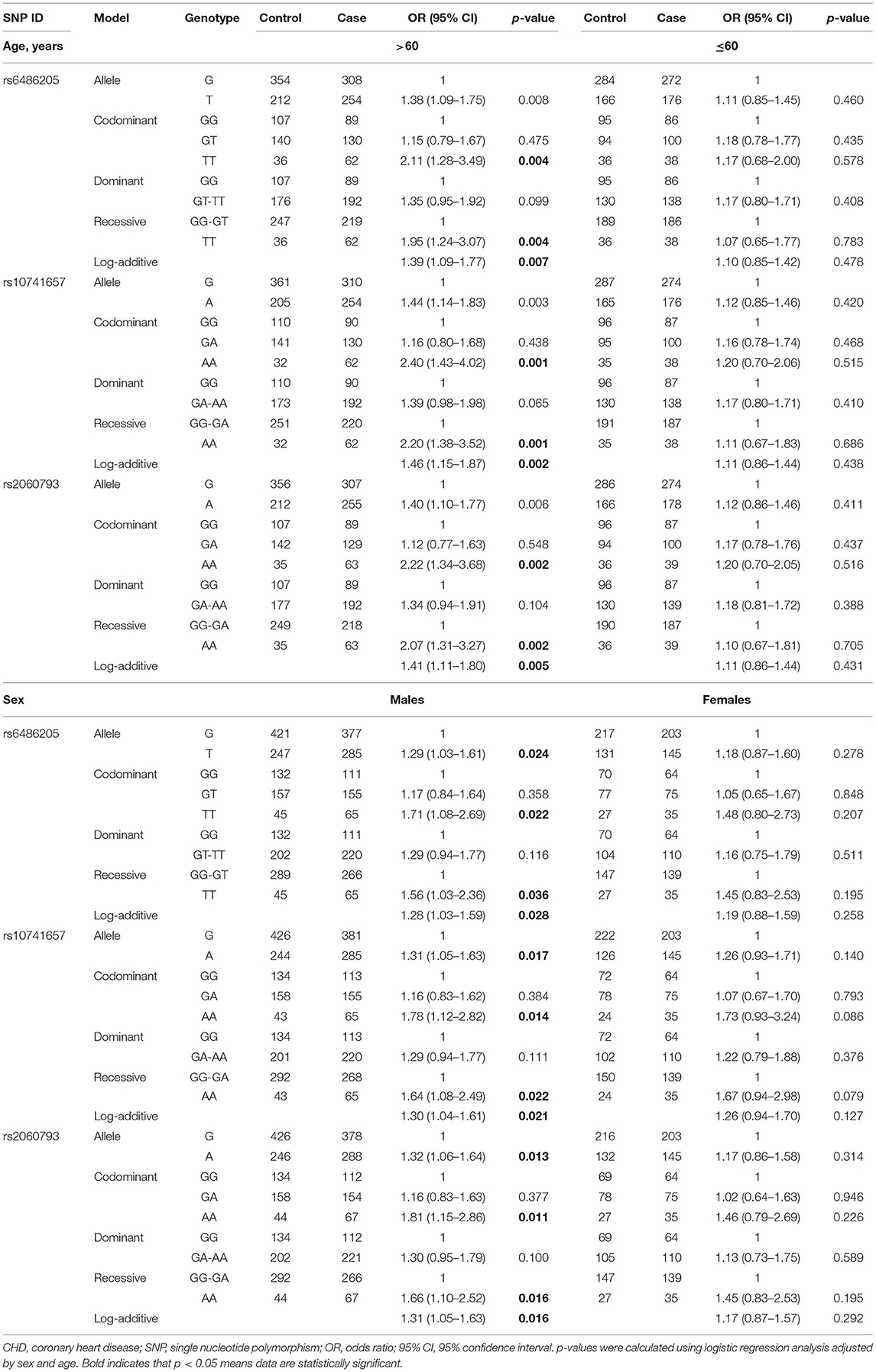
Table 4. Association between CYP2R1 polymorphisms and CHD risk according to stratification by age and sex.
Stratified by smoking (Table 5), rs2060793 A allele had a higher risk of CHD among smokers. Interestingly, three CYP2R1 SNPs increased the susceptibility to CHD in non-smokers under the allele, codominant, dominant, and additive models. However, there was no significant association in drinking-stratified analysis, as shown in Supplementary Table 2.
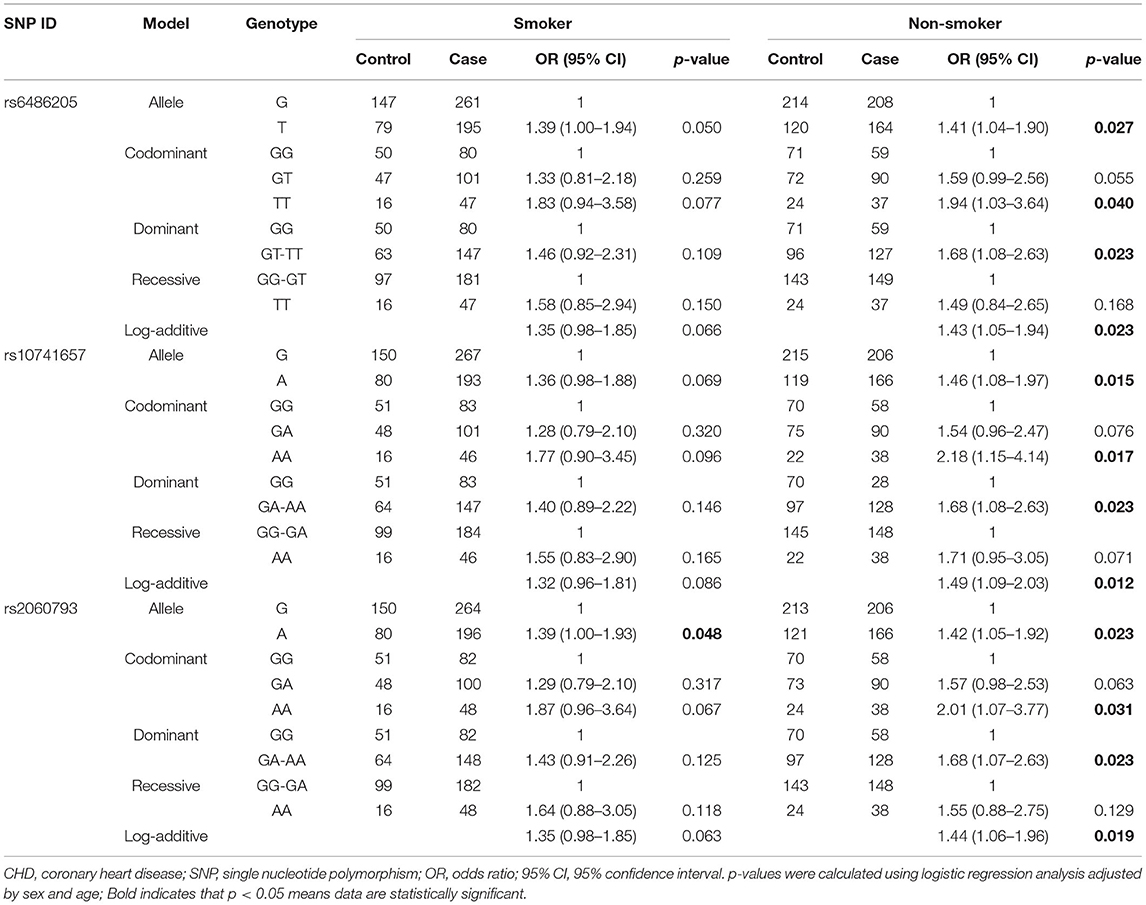
Table 5. Association between CYP2R1 polymorphisms and CHD risk according to stratification by smoking.
Furthermore, the combined effect of CYP2R1 SNPs on CHD patients with diabetes or hypertension was also assessed. However, CYP2R1 SNPs were not significantly related to diabetes or hypertension in CHD patients (Supplementary Table 3).
Haplotype and Multifactor Dimension Reduction Analysis for the Association Between Cytochrome P450 Family 2 Subfamily R Member 1 Single Nucleotide Polymorphisms and Coronary Heart Disease Risk
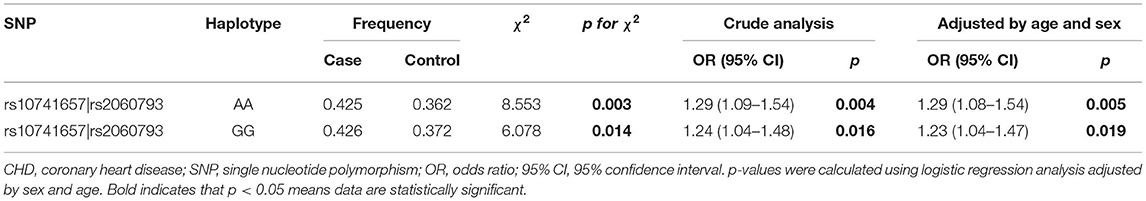
Linkage disequilibrium analysis displayed that two SNPs (rs10741657 and rs2060793) in CYP2R1 had strong linkage (Figure 1). Furthermore, the haplotypes Ars10741657Ars2060793 (OR = 1.29, 95% CI: 1.08–1.54, p = 0.005) and Grs10741657Grs2060793 (OR = 1.23, 95% CI: 1.04–1.47, p = 0.019) increased the predisposition of CHD (Table 6).
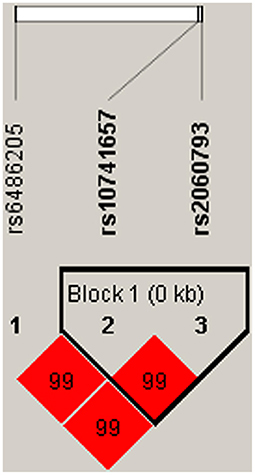
Figure 1. Haplotype block map for three SNPs in CYP2R1. A block was comprised of rs10741657 and rs2060793. Number in diamond represents D′-value.
Multifactor dimension reduction analysis of SNP–SNP interaction was performed to assess SNP interaction and its relation to CHD risk (Table 7). Rs10741657 was the best single-locus model (testing accuracy = 0.5079), and the two-locus model (rs6486205 and rs10741657) was the best combination in the multi-locus model (testing accuracy = 0.5049). Supplementary Figure 1 revealed the additive effect between CYP2R1 rs6486205-TT, rs10741657-AA, and rs2060793-AA on conferring risk toward CHD occurrence. Multifactor dimension reduction analysis of gene–environment interaction suggested that drinking was found to be the most important environmental factor affecting CHD susceptibility. In addition, the gene–environment interaction model, composed of rs6486205, smoking, drinking, and age, showed higher testing-balanced accuracy (0.6132) and cross-validation consistency (7/10), indicating that this interaction model was a candidate gene–environment model in our population. The result of the dendrogram (Supplementary Figure 2) exhibited a strong synergy effect of gene–environment interaction on CHD risk.
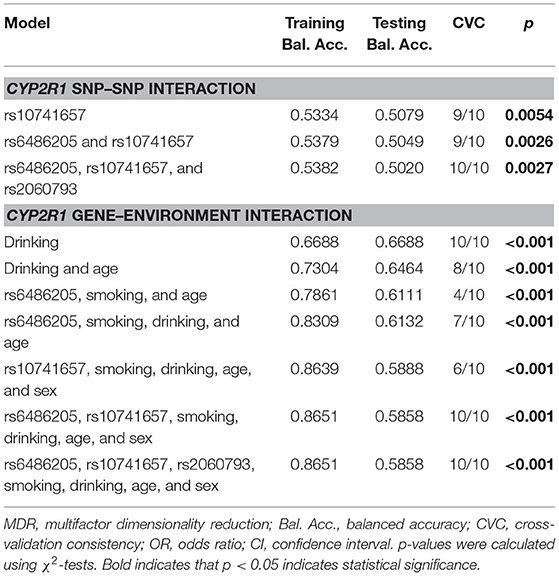
Table 7. MDR analysis for CYP2R1 SNP–SNP interaction and CYP2R1 gene–environment interaction with CHD risk.
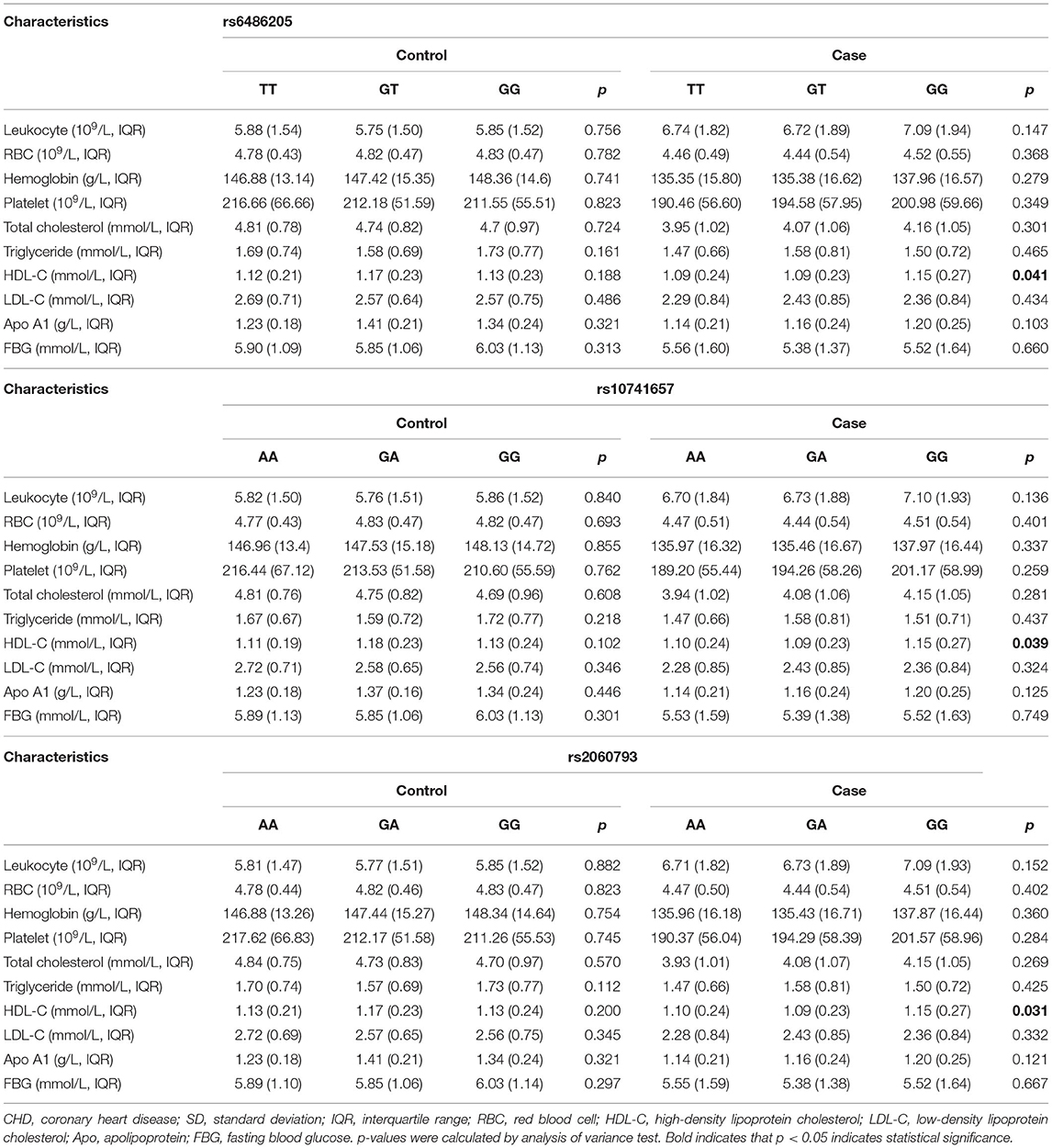
Association Between Genotypes of Cytochrome P450 Family 2 Subfamily R Member 1 Variants and Blood Biochemical Index
Next, the association between CYP2R1 SNPs and blood biochemical index in healthy control and CHD patients was assessed, as displayed in Table 8. We found that the genotypes of rs6486205 (p = 0.041), rs10741657 (p = 0.039), and rs2060793 (p = 0.031) were associated with serum concentration of HDL-C.
Discussion
In the study, we explored the contribution of three CYP2R1 SNPs to CHD risk in the Chinese Han population. Our results showed that rs6486205, rs10741657, and rs2060793 increased the predisposition of CHD in the whole subjects. Interestingly, the relations between these SNPs and CHD risk were observed in the subjects with age >60 years, males, or non-smokers. Additionally, the haplotypes Ars10741657Ars2060793 and Grs10741657Grs2060793 had a higher risk of CHD, and the combination (rs6486205 and rs10741657) was the best multi-locus model. This is first to reveal the correlation between CYP2R1 variants and CHD susceptibility in the Chinese Han population, and these variants could serve as potential biomarkers of CHD susceptibility.
Variation of CYP2R1 can affect the activity of 25-hydroxylase, resulting in the deficiency of 25(OH)D, which in turn leads to an increasing incidence and mortality of CVDs (22). Rs10741657, located in the non-coding region 5′-untranslated region, can regulate gene expression and activity of 25-hydroxylase (20). CYP2R1 rs10741657 leads to the lowered synthesis of CYP2R1 for the variant G-allele (23), presumably resulting in lowered conversion rate of cholecalciferol into 25(OH)D (20, 24). Rs10741657 was reported to be associated with type 2 diabetes, ischemic stroke, and blood pressure (18, 19, 25). CYP2R1 rs2060793, in the promoter region, is involved in the regulation of gene transcription (21). Furthermore, rs2060793 was also reported to be associated with 25(OH)D concentrations (26). The association of rs2060793 with atrial fibrillation, gestational diabetes mellitus, and type 1 diabetes was reported (27–29). In the Egyptian population, rs10741657 and rs2060793 were related to 25(OH)D levels and might be novel genetic markers for CADs (30). Our study revealed that rs10741657 and rs2060793 increased the risk of CHD in the Chinese Han population, which was consistent with previous studies. We also found that rs6486205 contributed to CHD susceptibility. However, there was no study reporting rs6486205 and the relationship of rs6486205 to disease risk. Whether the SNPs identified are also recurrent in other diseases with CYP2R1 mutations is necessary to explore.
Aging is a risk factor for CHD, and the potential risk factors for CHD incidence are influenced by age-related changes (31). In our study, the relationship between CYP2R1 SNPs (rs6486205, rs10741657, and rs2060793) and the increased CHD risk was observed in the subjects with age >60 years. Moreover, sex difference was related to the adult mortality of CHD, which is greater mortality rates and risks in males than females (32). We also found the relationship between CYP2R1 SNPs (rs6486205, rs10741657, and rs2060793) and the increased CHD risk in males. These results suggested that the association might be age- and sex-dependent. Previously, smoking is a significant risk factor for CHD, but polygenic risk scores have a better predictive effect among non-smokers compared with smokers (23). Our results displayed that CYP2R1 SNPs contributed to the increased CHD predisposition in non-smokers. This is in line with previous evidence that genetic factors may have a more important role in CHD. Epidemiologic research has revealed that alcohol consumption is related to the risk of CHD incidence (33). Besides, diabetes and hypertension are the major risk factors for CHD incidence (34). However, no association was observed in drinkers and in CHD patients with diabetes or hypertension. Further studies are necessary to verify our results.
Coronary heart disease is a complex multifactorial disease. Multiple genetic and environmental risk factors contribute to CHD. We also investigated the association of combined SNPs in CYP2R1 with CHD risk. The results showed that the haplotypes Ars10741657Ars2060793 and Grs10741657Grs2060793 increased the predisposition of CHD. SNP–SNP interaction analysis displayed the accumulated effect of CYP2R1 variants on conferring CHD risk. Moreover, gene–environment interaction suggested that drinking was found to be the most important environmental factor affecting CHD susceptibility. In addition, the gene–environment interaction model composed rs6486205, smoking, drinking, and age, indicating that the combined effect of gene–environment interaction should be appreciated in the pathogenesis of CHD.
Previous studies have shown that HDL-C levels are considered independent risk factors for the development of CHD (35, 36). We found that the genotypes of rs6486205, rs10741657, and rs2060793 were associated with serum concentration of HDL-C, suggesting that CYP2R1 polymorphisms might play an important role in serum concentration of HDL-C. However, more functional studies are required.
Several limitations should be acknowledged. First, based on hospital-based research, selection bias was inevitable. Here, age and sex were matched to reduce the bias. Second, the subjects were the Chinese Han population, so these results should be interpreted with caution. Further studies in other different ethnic populations are needed to confirm our finding. Third, only three variants in CYP2R1 were assessed, and the risk association of other CYP2R1 SNPs remains to be further investigated. Moreover, the potential impact of the SNPs on the protein function of CYP2R1 is unknown; therefore, additional studies will be required. Another, whether the SNPs identified are also recurrent in other diseases with CYP2R1 mutations is necessary to explore. Four, the clinical symptoms, such as the severity of CHD, stage of CHD, were not examined. In the future, we would like to enlarge the sample size and complete the clinical symptoms, such as the severity of disease, stage of the disease to evaluate the association between CYP2R1 SNPs and the clinical symptoms of CHD.
Conclusion
In conclusion, our research firstly suggested the contribution of CYP2R1 SNPs (rs6486205, rs10741657, and rs2060793) and haplotypes (Ars10741657Ars2060793 and Grs10741657Grs2060793) to the increased CHD predisposition among the Chinese Han population, and these variants could serve as potential biomarkers of CHD susceptibility. Furthermore, the risk association was related to confounding factors for CHD, including age, sex, and smoking. These findings might help to strengthen the understanding of the CYP2R1 gene in the occurrence of CHD. Our finding increased our knowledge regarding the effect of the CYP2R1 gene on the process of CHD and also provided some data for future explorations of the relationship between the CYP2R1 gene and CHD risk in different populations.
Data Availability Statement
The data presented in the study are deposited in the Zenodo repository: https://zenodo.org/record/4977934#.YMxSmWhKiUl.
Ethics Statement
The study was approved by the Ethics Committee of the Haikou City people's Hospital, and informed consent was gained from all subjects. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QW: writing and conceptualization. ZL and HC: methodology. TM and BP: data curation. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Hainan Provincial Health and Family Planning Industry Research Project (19A200118).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all participants and volunteers in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.652729/full#supplementary-material
References
1. Pahlavanzade B, Zayeri F, Baghfalaki T, Mozafari O, Khalili D, Azizi F, et al. Association of lipid markers with coronary heart disease and stroke mortality: a 15-year follow-up study. Iran J Basic Med Sci. (2019) 22:1325–30. doi: 10.22038/ijbms.2019.35617.8775
2. Ma LY, Chen WW, Gao RL, Liu LS, Zhu ML, Wang YJ, et al. China cardiovascular diseases report 2018: an updated summary. J Geriatr Cardiol. (2020) 17:1–8. doi: 10.11909/j.issn.1671-5411.2020.01.001
3. Khamis RY, Ammari T, Mikhail GW. Gender differences in coronary heart disease. Heart. (2016) 102:1142–9. doi: 10.1136/heartjnl-2014-306463
4. Smits PC, Pasterkamp G, Quarles van Ufford MA, Eefting FD, Stella PR, de Jaegere PP, et al. Coronary artery disease: arterial remodelling and clinical presentation. Heart. (1999) 82:461–4. doi: 10.1136/hrt.82.4.461
5. Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. (2011) 306:2120–7. doi: 10.1001/jama.2011.1654
6. Puddu PE, Piras P, Menotti A. Lifetime competing risks between coronary heart disease mortality and other causes of death during 50years of follow-up. Int J Cardiol. (2017) 228:359–63. doi: 10.1016/j.ijcard.2016.11.157
7. Zheng H, Zeng Z, Wen H, Wang P, Huang C, Huang P, et al. Application of genome-wide association studies in coronary artery disease. Curr Pharmaceut Des. (2019) 25:4274–86. doi: 10.2174/1381612825666191105125148
8. Byars SG, Inouye M. Genome-wide association studies and risk scores for coronary artery disease: sex biases. Adv Exp Med Biol. (2018) 1065:627–42. doi: 10.1007/978-3-319-77932-4_38
9. Shinkyo R, Sakaki T, Kamakura M, Ohta M, Inouye K. Metabolism of vitamin D by human microsomal CYP2R1. Biochem Biophys Res Commun. (2004) 324:451–7. doi: 10.1016/j.bbrc.2004.09.073
10. Al Mutair AN, Nasrat GH, Russell DW. Mutation of the CYP2R1 vitamin D 25-hydroxylase in a Saudi Arabian family with severe vitamin D deficiency. J Clin Endocrinol Metab. (2012) 97:E2022–5. doi: 10.1210/jc.2012-1340
11. Gholami F, Moradi G, Zareei B, Rasouli MA. The association between circulating 25-hydroxyvitamin D and cardiovascular diseases: a meta-analysis of prospective cohort studies. (2019) 19:248. doi: 10.1186/s12872-019-1236-7
12. Chien KL, Hsu HC, Chen PC, Lin HJ, Su TC, Chen MF, et al. Total 25-hydroxyvitamin D concentration as a predictor for all-cause death and cardiovascular event risk among ethnic Chinese adults: a cohort study in a Taiwan community. PLoS ONE. (2015) 10:e0123097. doi: 10.1371/journal.pone.0123097
13. Pekkanen MP, Ukkola O, Hedberg P, Piira OP, Lepojärvi S, Lumme J, et al. Serum 25-hydroxyvitamin D is associated with major cardiovascular risk factors and cardiac structure and function in patients with coronary artery disease. Nutr Metab Cardiovasc Dis. (2015) 25:471–8. doi: 10.1016/j.numecd.2015.02.005
14. Durup D, Jørgensen HL, Christensen J, Tjønneland A, Olsen A, Halkjær J, et al. A reverse J-shaped association between serum 25-hydroxyvitamin D and cardiovascular disease mortality: the CopD study. J Clin Endocrinol Metab. (2015) 100:2339–46. doi: 10.1210/jc.2014-4551
15. Michos ED, Misialek JR, Selvin E, Folsom AR, Pankow JS, Post WS, et al. 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: the ARIC study. Atherosclerosis. (2015) 241:12–7. doi: 10.1016/j.atherosclerosis.2015.04.803
16. Sedky NK, Abdel Rahman MF, Hassanein SI, Gad MZ. Genetic variants of CYP2R1 are key regulators of serum vitamin D levels and incidence of myocardial infarction in middle-aged Egyptians. Curr Pharmaceut Biotechnol. (2018) 19:265–73. doi: 10.2174/1389201019666180528082737
17. Bertoccini L, Bailetti D, Buzzetti R, Cavallo MG, Copetti M, Cossu E, et al. Variability in genes regulating vitamin D metabolism is associated with vitamin D levels in type 2 diabetes. Oncotarget. (2018) 9:34911–8. doi: 10.18632/oncotarget.26178
18. Ye X, Jia J, Zhang N, Ding H, Zhan Y. Associations of genetic polymorphisms of the vitamin D pathway with blood pressure in a Han Chinese population. Clin Exp Hypertens. (2019) 41:460–5. doi: 10.1080/10641963.2018.1506469
19. Türkanoglu Özçelik A, Öner T, Can Demirdögen B, Bek VS, Demirkaya S, Adali O. Genetic polymorphisms of vitamin D3 metabolizing CYP24A1 and CYP2R1 enzymes in Turkish patients with ischemic stroke. Neurol Res. (2018) 40:364–71. doi: 10.1080/01616412.2018.1446281
20. Ramos-Lopez E, Brück P, Jansen T, Herwig J, Badenhoop K. CYP2R1 (vitamin D 25-hydroxylase) gene is associated with susceptibility to type 1 diabetes and vitamin D levels in Germans. Diabet Metab Res Rev. (2007) 23:631–6. doi: 10.1002/dmrr.719
21. Chanock S. Candidate genes and single nucleotide polymorphisms (SNPs) in the study of human disease. Dis Mark. (2001) 17:89–98. doi: 10.1155/2001/858760
22. Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. (2014) 349:g6330. doi: 10.1136/bmj.g6330
23. Hindy G, Wiberg F, Almgren P, Melander O, Orho-Melander M. Polygenic risk score for coronary heart disease modifies the elevated risk by cigarette smoking for disease incidence. Circ Genom Precis Med. (2018) 11:e001856. doi: 10.1161/CIRCGEN.117.001856
24. Kopp TI, Vogel U, Andersen V. Associations between common polymorphisms in CYP2R1 and GC, Vitamin D intake and risk of colorectal cancer in a prospective case-cohort study in Danes. PLoS ONE. (2020) 15:e0228635. doi: 10.1371/journal.pone.0228635
25. Lu L, Bennett DA. Association of vitamin D with risk of type 2 diabetes: a Mendelian randomisation study in European and Chinese adults. PLoS Med. (2018) 15:e1002566. doi: 10.1371/journal.pmed.1002566
26. Torugsa S, Nimitphong H, Warodomwichit D, Chailurkit LO, Srijaruskul K, Chanprasertyothin S, et al. The genetic determinants of circulating C3-epimers of 25-hydroxyvitamin D. J Clin Transl Endocrinol. (2018) 12:36–41. doi: 10.1016/j.jcte.2018.04.002
27. Chan YH, Yiu KH, Hai JJ, Chan PH, Lam TH, Cowling BJ, et al. Genetically deprived vitamin D exposure predisposes to atrial fibrillation. Europace. (2017) 19(Suppl_4):iv25–31. doi: 10.1093/europace/eux312
28. Wang Y, Wang O, Li W, Ma L, Ping F, Chen L, et al. Variants in vitamin D binding protein gene are associated with gestational diabetes mellitus. Medicine. (2015) 94:e1693. doi: 10.1097/MD.0000000000001693
29. Almeida JT, Rodrigues D, Guimarães J, Lemos MC. Vitamin D pathway genetic variation and type 1 diabetes: a case-control association study. Genes. (2020) 11:897. doi: 10.3390/genes11080897
30. Hassanein SI, Abu El, Maaty MA, Sleem HM, Gad MZ. Triangular relationship between single nucleotide polymorphisms in the CYP2R1 gene (rs10741657 and rs12794714), 25-hydroxyvitamin d levels, and coronary artery disease incidence. Biomarkers. (2014) 19:488–92. doi: 10.3109/1354750X.2014.939226
31. Abbott RD, Curb JD, Rodriguez BL, Masaki KH, Yano K, Schatz IJ, et al. Age-related changes in risk factor effects on the incidence of coronary heart disease. Ann Epidemiol. (2002) 12:173–81. doi: 10.1016/S1047-2797(01)00309-X
32. Barrett-Connor E. Gender differences and disparities in all-cause and coronary heart disease mortality: epidemiological aspects. Best Pract Res Clin Endocrinol Metab. (2013) 27:481–500. doi: 10.1016/j.beem.2013.05.013
33. Zhang Y, Yu Y, Yuan Y, Yu K, Yang H, Li X, et al. Association of drinking pattern with risk of coronary heart disease incidence in the middle-aged and older Chinese men: results from the Dongfeng-Tongji cohort. PLoS ONE. (2017) 12:e0178070. doi: 10.1371/journal.pone.0178070
34. Campbell DJ, Tam-Tham H, Dhaliwal KK, Manns BJ, Hemmelgarn BR, Sanmartin C, et al. Use of mixed methods research in research on coronary artery disease, diabetes mellitus, and hypertension: a scoping review. Circ Cardiovasc Qual Outcomes. (2017) 10:e003310. doi: 10.1161/CIRCOUTCOMES.116.003310
35. Arsenault BJ, Boekholdt SM, Kastelein JJ. Lipid parameters for measuring risk of cardiovascular disease. Nat Rev Cardiol. (2011) 8:197–206. doi: 10.1038/nrcardio.2010.223
Keywords: coronary heart disease, predisposition, CYP2R1 variants, haplotype, lifestyle
Citation: Wang Q, Lin Z, Chen H, Ma T and Pan B (2021) Effect of Cytochrome P450 Family 2 Subfamily R Member 1 Variants on the Predisposition of Coronary Heart Disease in the Chinese Han Population. Front. Cardiovasc. Med. 8:652729. doi: 10.3389/fcvm.2021.652729
Received: 22 January 2021; Accepted: 17 May 2021;
Published: 28 June 2021.
Edited by:
Xiang Xie, First Affiliated Hospital of Xinjiang Medical University, ChinaReviewed by:
Wenwang Rao, University of Macau, ChinaRaj Sewduth, VIB KU Leuven Center for Cancer Biology, Belgium
Copyright © 2021 Wang, Lin, Chen, Ma and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Wang, ODY3NjQ0MjdAcXEuY29t
 Qi Wang
Qi Wang Zhen Lin2
Zhen Lin2