- 1Division of Cardiology, Department of Internal Medicine, The University of Texas Health Science Center at Houston McGovern Medical School, Houston, TX, United States
- 2Division of Cardiology, Department of Internal Medicine, Baylor College of Medicine, Houston, TX, United States
- 3Division of Cardiology, Department of Internal Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 4Division of Biostatistics, University of Texas MD Anderson Cancer Center, Houston, TX, United States
Background: Pericardiocentesis is an important diagnostic and therapeutic tool for cancer-associated pericardial effusion. Limited safety and outcomes data exists regarding the management of malignancy-related pericardial effusion in patients with thrombocytopenia.
Objectives: Our study aimed to analyze prognostic factors and overall survival (OS) after pericardiocentesis in thrombocytopenic cancer patients.
Methods and Results: A retrospective review of 136 thrombocytopenic cancer patients who underwent primary percutaneous pericardiocentesis was performed. Degree of thrombocytopenia was classified by platelet count recorded on day of pericardiocentesis: 75–149 × 103 cells/μL (41%); 50–74 × 103 cells/μL (10%); 25–49 × 103 cells/μL (24%); <25 × 103 cells/μL (25%). Median OS was 2.6 months and median follow-up was 37.4 months. Kaplan-Meier survival analysis showed significant OS differences among thrombocytopenia severity groups (p = 0.023), and worse OS with platelets <100 vs. ≥100 × 103 cells/μL (p = 0.031). By univariate analysis, thrombocytopenia severity was associated with increased risk of death (HR 0.993; 95% CI 0.989–0.997; p = 0.002). Poor prognostic factors for OS were advanced cancer, malignant effusion, elevated international normalized ratio (INR), quantity of platelet transfusions, and platelet transfusion resistance. However, thrombocytopenia severity became insignificant for OS (p = 0.802), after adjusting for advanced cancer and INR.
Conclusions: For patients with malignancy-related large pericardial effusion and thrombocytopenia, pericardiocentesis is a feasible intervention and should be considered due to low complication rates. There is no absolute contraindication to pericardiocentesis in case of hemodynamic instability, even with severe thrombocytopenia.
Introduction
Cancer causes pericardial disease by direct structural infiltration or indirectly via chemotherapy, radiotherapy, immunotherapy, or opportunistic infections (1, 2). Pericardial effusion (PE) is associated with malignancy in up to 20% of cases in autopsy studies, and 33% of patients with symptomatic PE have concomitant cancer in a large retrospective review (3–5). Pericardiocentesis is an important diagnostic and therapeutic tool for the cardio-oncologist as large PE manifesting with tachycardia, dyspnea, chest pain, cardiac tamponade, or cardiogenic shock are common (6). According to the European Society of Cardiology 2015 guidelines, in cardiac tamponade with underlying malignancy requiring therapeutic pericardiocentesis, extended pericardial drainage is indicated (class IB level recommendation) (7).
Limited data and safety outcomes exist regarding the management of malignancy-related PE in patients with thrombocytopenia (platelet count <150 × 103 cells/μL). In thrombocytopenic patients, platelet count has an imprecise association with increased risk of bleeding. Prior study found increased risk of bleeding in those with platelet counts ≤5,000 cells/μL compared to those with platelet counts ≥81,000 cells/μL, although there was otherwise no clear correlation of decreased bleeding risk with increased platelet counts (8). There is no platelet count threshold at which the risk of bleeding cannot be accounted for (9), and hemorrhagic complications directly impact survivorship among patients with malignancy (10). Thrombocytopenia often carries prohibitive surgical risk and is a relative contraindication for percutaneous pericardiocentesis (11). Traditional approach included attempts to correct thrombocytopenia with prophylactic platelet transfusion with a platelet count goal >50 × 103 cells/μL (12).
Our study analyzed the prognostic factors and overall survival (OS) of pericardiocentesis in thrombocytopenic patients with diagnosis of malignancy and attempted to determine the utility of platelet count and hemostatic evaluations in predicting bleeding risk, hypocoaguable state, and mortality among thrombocytopenic cancer patients undergoing pericardiocentesis. To our knowledge, this was the first retrospective survivorship analysis of this particular patient population after primary percutaneous pericardiocentesis.
Materials and Methods
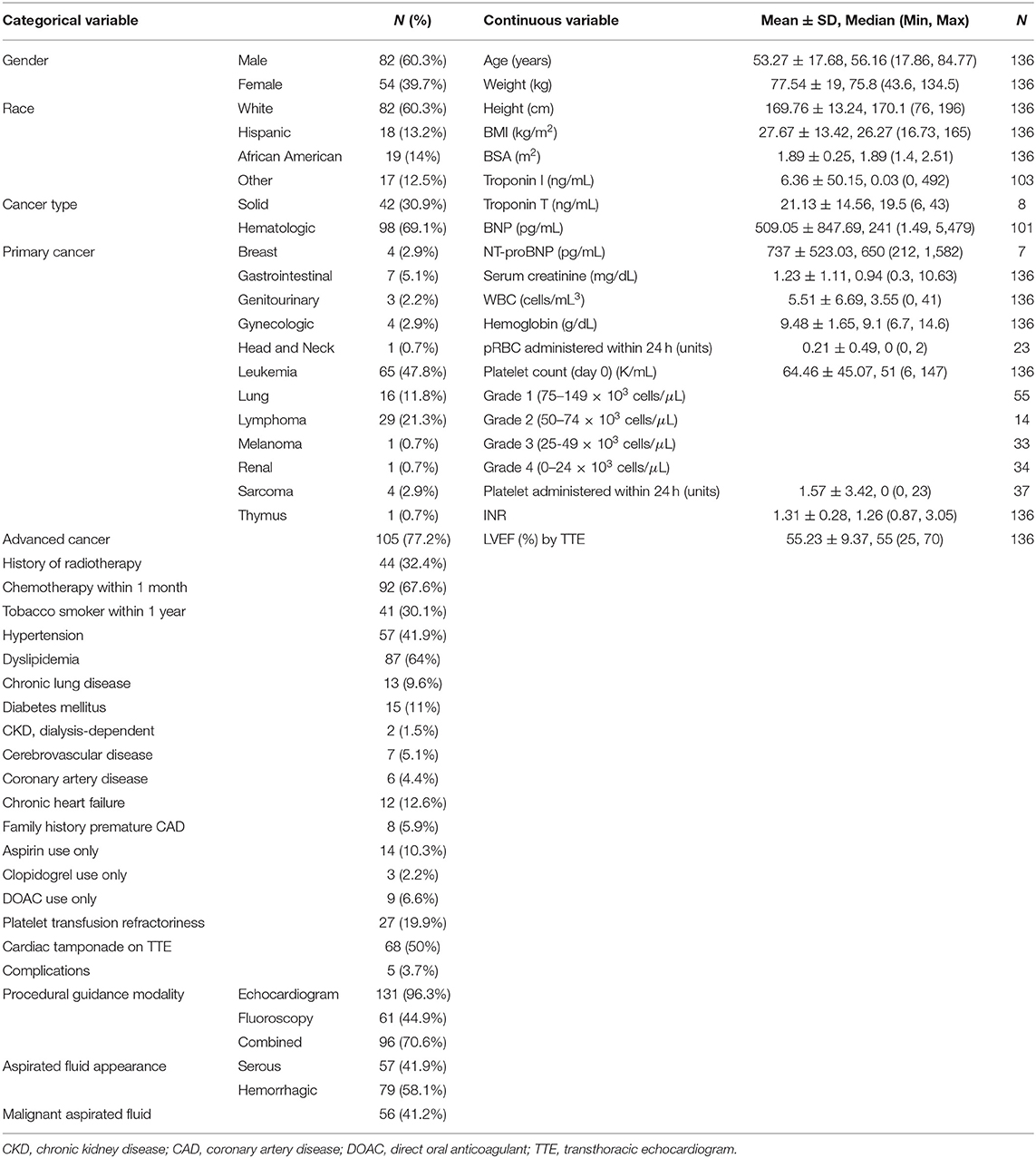
The Institutional Review Board of The University of Texas MD Anderson Cancer Center (MDACC) approved this study with a waiver for written informed consent. In December 2018, a retrospective review of the MDACC cardiac catherization laboratory registry for cancer patients with platelet counts <150 × 103 cells/μL who underwent primary percutaneous pericardiocentesis between October 1, 2009 to November 30, 2018 was performed. In total, 136 patients met the criteria above and were included in this study. Severity of thrombocytopenia was classified based on platelet count recorded for each patient on the day of pericardiocentesis based on NCI-CTCAE version 5 criteria (13): grade 1 (75–149 × 103 cells/μL), grade 2 (50–74 × 103 cells/μL), grade 3 (25–49 × 103 cells/μL), and grade 4 (<25 × 103 cells/μL). Recorded data included patient demographics, cancer history, and serological test results obtained 24 h peri-procedurally (Table 1) and echocardiographic data with evidence of increased pericardial pressure or cardiac tamponade physiology. Patients then underwent percutaneous pericardiocentesis with an indwelling pigtail catheter placement (5F Cook pericardial drain) preferably for 3–5 days. The catheter was removed if fluid drainage dropped below 25–50 mL with no residual effusion seen by follow-up echocardiography.
Recording a successful pericardiocentesis required an accurate technique with meticulous hemostasis, equipment availability (7 and 12 cm Cook micropuncture kits), image guidance (when possible a “triple safety” approach consisting in ultrasound-guided needle advancement, fluoroscopy, and echocardiography), and proficiency in subxiphoid and apical approach. Percutaneous pericardiocentesis was performed by using the shortest distance to the pericardial cavity from the subxiphoid or intercostal space, and using the 5-F micropuncture kit (Micropuncture Introducer Kit, Silhouette Transitionless Push-Plus Design, Cook Medical, Bloomington, Indiana) in order to reduce the bleeding risk, with intercostal site entry (lateral) being the preferred approach. Based on body habitus, skin and breast anatomy, scarring from previous surgeries, mediastinal shift from underlying malignancy or abdominal distension, lateral approach expanded between 4 and 6th intercostal space and from midclavicular to midaxillary line. In procedures where only echocardiographic guidance was available, or in patients with concomitant pericardial and pleural effusion or ascites, upon accessing the pericarial space, position was confirmed with agitated saline injection, followed by advancement of micropuncture dilator and additional confirmation with “microbubbles,” and completed with the advancement of the multi-hole pigtail catheter under fluoroscopic guidance and suturing in place. In complex (unstable, challenging) patients where both echocardiographic and fluoroscopic guidance were available, to avoid incidental needle displacement and increase in procedural time, if fluid was serous, the pericardial space was secured advancing the micropuncture guidewire with fluoroscopic confirmation of the intrapericardial position prior to advancement. Fluid samples were sent to pathology and microbiology for analysis and results were documented.
Patient demographical characteristics were summarized using mean (SD) and median (minimum-maximum) for continuous variables and counts (%) for categorical variables. Overall survival (OS, time interval from procedure (pericardiocentesis) to death or last follow up) was calculated. Univariate and multivariate Cox proportional hazards regression analyses were conducted to identify variables that were associated with increased risk of death. Kaplan-Meier survival plots were generated and log-rank test was used to compare among subgroups in OS. Estimated median follow-up using reverse Kaplan-Meier method was used, considering the event of death as a censor, so that unobservable follow-up time of each subject was interpreted as follow-up time. A p < 0.05 indicated a statistical significance. SAS 9.4 (SAS Institute INC, Cary, NC) was used for data analysis.
Results
Our study included 136 patients with malignancy stratified by grade of severity of thrombocytopenia: 41% grade 1 (75–149 × 103 cells/μL); 10% grade 2 (50–74 × 103 cells/μL); 24% grade 3 (25–49 × 103 cells/μL); 25% grade 4 (<25 × 103 cells/μL) (Tables 1, 2). Of the 136 patients, 35 survived during the follow-up period. After pericardiocentesis, median OS using reverse Kaplan-Meier method was 2.6 months with a median follow-up of 21.4 months (95% confidence interval (CI) 0.2–106.8 months). Significant OS differences were observed across thrombocytopenia grades recorded on day 0 (p = 0.023, Figure 1). Evaluation of patients based on platelet counts <100 × 103 cells/μL or ≥100 × 103 cells/μL showed a statistical significance in OS (p = 0.031). However, there were more patients with platelet count ≥100 × 103 cells/μL without advanced cancer than with advanced cancer (54.84 vs. 22.86%, p = 0.0007).
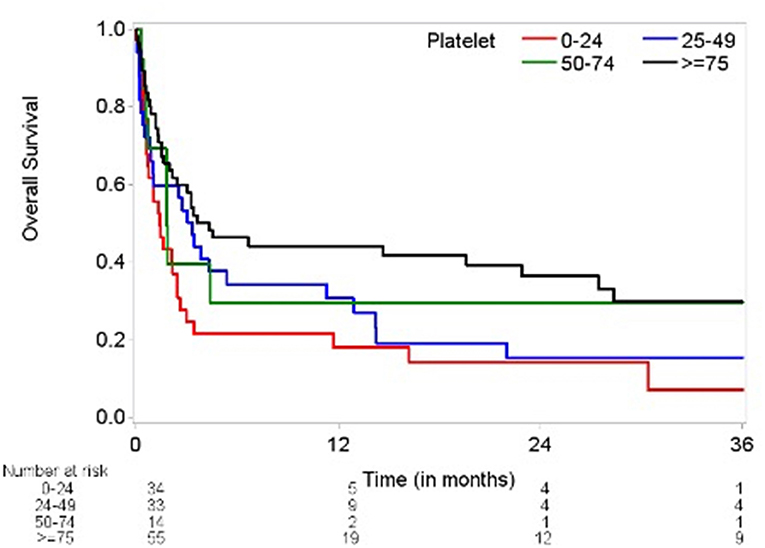
Figure 1. Kaplan-Meier plot of overall survival by thrombocytopenia severity (log-rank test p = 0.0234). Time (months) on the x-axis marks time elapsed from pericardiocentesis. Overall survival (percentage) on the y-axis. Number at risk delineates the remainder of surviving patients at each time point in each group based on degree of thrombocytopenia and platelet count.
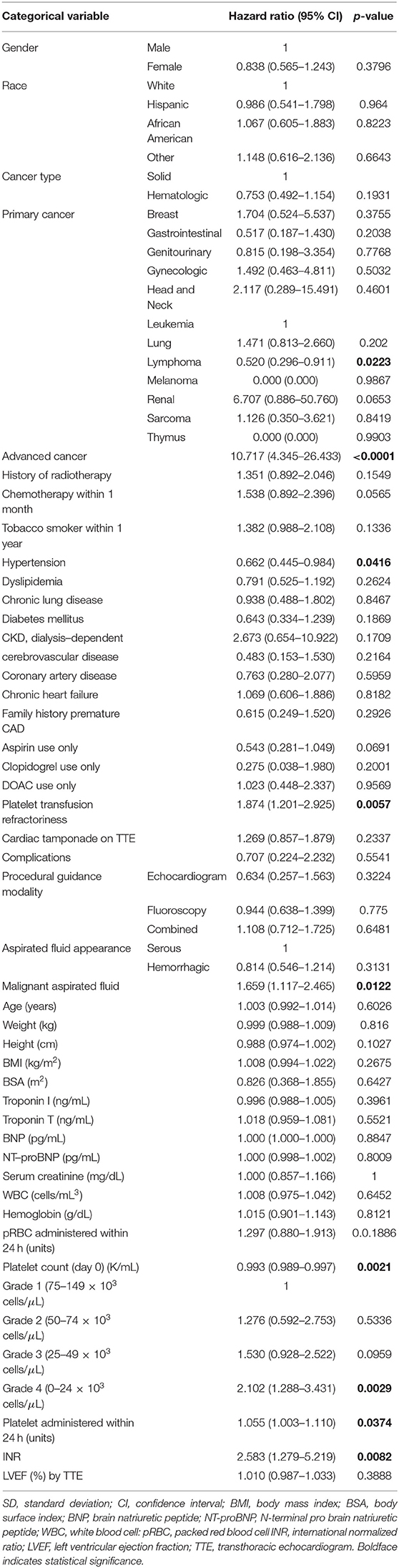
Variables showing significant association with OS based on univariate Cox models include elevated INR, platelet count on day of procedure, thrombocytopenia severity grade on day of procedure, platelet transfusion within 24 h of procedure, advanced cancer status, malignant fluid composition, and platelet resistance. Factors that did not show significant associations with OS included hemoglobin level, quantity of red blood cell transfusions, anticoagulant therapy, age, race, gender, cardiac tamponade, heart failure, prior radiotherapy, or recent chemotherapy.
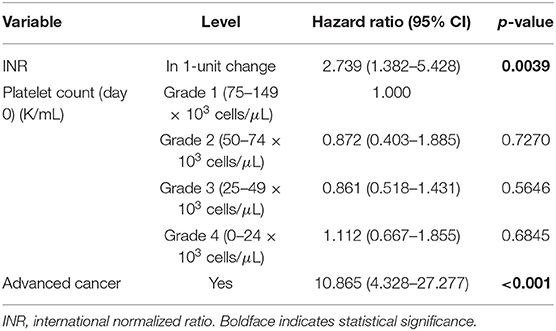
The increased recorded platelet count as a continuous variable on procedure day was significantly associated with decreased risk of death (hazard ratio (HR) 0.993; 95% CI 0.989–0.997; p = 0.002). Thrombocytopenia grade 4 (HR 2.10; 95% CI 1.29–3.43; p = 0.003) comparing to grade 1 was associated with increased risk of death. Poor prognostic factors for OS were advanced cancer, malignant effusion, elevated INR, quantity of platelet transfusions, and platelet transfusion resistance. Adjusting for INR (HR 2.739; 95% CI 1.382–5.428; p = 0.004) and advanced cancer status (HR 10.865; 95% CI 4.328–27.277; p < 0.0001), thrombocytopenia severity grade on day of procedure (p = 0.802) became insignificant (Table 3). Based on the current data, the majority of patients had advanced cancer [105 (77%) with advanced cancer vs. 31 (23%) with non-advanced cancer] and the majority of patients with higher thrombocytopenia grade had advanced cancer (85–88% with advanced cancer for grades 2, 3, and 4). Including 105 patients with advanced cancer, thrombocytopenia grade was not significantly associated with OS in a univariate Cox model (p = 0.736) and in a multivariate Cox model (p = 0.887), adjusting for INR (HR 2.588; 95% CI 1.261–5.311; p = 0.010). Marginally significant association was observed in platelet count (as a continuous variable) in a univariate Cox model (HR 0.981; 95% CI 0.960–1.003; p = 0.087) and a multivariate Cox model (HR 0.980; 95% CI 0.958–1.002; p = 0.077), adjusting for INR (HR 65.396; 95% CI 0.986–4335.876; p = 0.051) including patients with non-advanced cancer. However, this multivariate Cox model included 5 events which are not large enough number of events to provide reliable HR estimates.
Pericardiocentesis was performed via subxiphoid (16, 12%) and left apical (120, 88%) approaches. One patient with grade 1 thrombocytopenia developed a hematoma at the pericardial drain site. In addition to the hematoma, other periprocedural issues involved shoulder pain (1 patient), and transient pericarditis (3 patients). Of these 5 patients, the 3 patients with pericarditis survived past 2 months. Three out of 6 (50%) patients who died within 60 days all suffered from advanced malignancy and coagulopathy with elevated INR. Other than the one patient with hematoma, there were no significant periprocedural bleeding complications, regardless of platelet count.
Pericardial window was performed in 6 patients, four of whom survived past 1 month. Platelet counts on day 0 for patients undergoing pericardial window ranged from 12 × 103 to 106 × 103 cells/μL. The two patients who did not survive had additional neutropenia and one elevated international normalized ratio (INR) level. All had advanced cancer staging (4 leukemia, 1 lymphoma, 1 lung cancer) with recurrent PEs after subsequent pericardiocentesis.
Pre-procedural platelet transfusions were administered for 36 patients (26%), 27 of whom were determined to have platelet transfusion refractoriness, defined as post-transfusion platelet count increment < 10 × 103 cells/μL within 24 h after platelet transfusion.
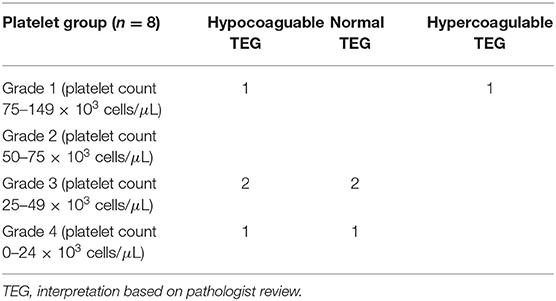
Thromboelastography (TEG) was performed in 8 patients prior to pericardiocentesis, among patients in all four grades of thrombocytopenia. TEG results revealed hypocoagulability in 4 patients (2 with grade 1, 2 with grade 3, 1 with grade 4); 3 TEGs revealed normal clotting function (2 with grade 3, 1 with grade 4), and 1 revealed hypercoagulability (grade 1) (Table 4). Five of the patients with performed TEGs presented in cardiac tamponade. Only one patient with TEG evaluation received pre-procedural platelet transfusion (patient had hypocoaguable TEG result, with grade I thrombocytopenia).
Discussion
Patients with PE and underlying malignancy typically present less acutely without hemodynamic compromise, and face decreased OS compared to those without malignancy (1). The only serological marker shown to have a statistically significant negative influence on OS was elevated INR, indicating underlying coagulopathy may worsen overall prognosis. INR was elevated for several non-specific reasons in these patients, however, including anticoagulation, liver dysfunction, malnutrition, vitamin K deficiency. Patients with elevated INR tended to be very ill, and the severity of their disease likely contributed to the correlation with trends for worse OS. Similarly, advanced cancer status was heavily correlated with degree of thrombocytopenia, and likely a confounding variable for OS as highlighted in Table 3. After multivariate analysis, thrombocytopenia severity was not significantly associated with OS when advanced cancer and elevated INR were also accounted for.
Worse OS was associated with advanced carcinoma stage and malignant etiology of effusion. However, chemotherapy, radiotherapy, or concomitant infection did not show statistical significance in respective effects on OS. At 1 year post-pericardiocentesis, increased mortality was noted in patients with thrombocytopenia grades 2, 3, and 4 that also correlated with cancer severity and was attributed to natural progression of cancer.
Approach to the pericardial space has evolved; the preferred approach in thrombocytopenic patients is lateral with intercostal site entry between 4 and 6th intercostal spaces and from midclavicular to midaxillary line, unless there are adhesions between the left ventricular apex and pericardial sac or technical barriers to access (skin infections, scars from previous interventions, implants, additional pleural effusion) or the access to the pericardial space is through reduced amount of tissue and avoids hepatic structure or the loculated pocket is accessible only subxiphoid (14). A large study at our institution of pericardiocentesis in malignant PE yielded procedural site selection rates of subxiphoid approach in 63% and lateral intercostal approach in 37% of patients, with low complication rates (12).
TEG, a hemostatic blood test which dynamically evaluates platelet function and clotting efficacy of whole blood (Figure 2), can be a helpful tool to determine bleeding risk in thrombocytopenic cancer patients prior to pericardiocentesis in stable patients (15, 16). In thrombocytopenic patients, platelet function rather than platelet count often correlates with bleeding, and hemostasis appears to be affected more than platelet adhesion (17, 18). TEG results in thrombocytopenic cancer patients with PE may provide a more comprehensive risk stratification before pericardiocentesis, and may help determine the appropriate blood product administration when hemorrhagic complications arise, approach already established for the coronary procedures (19).
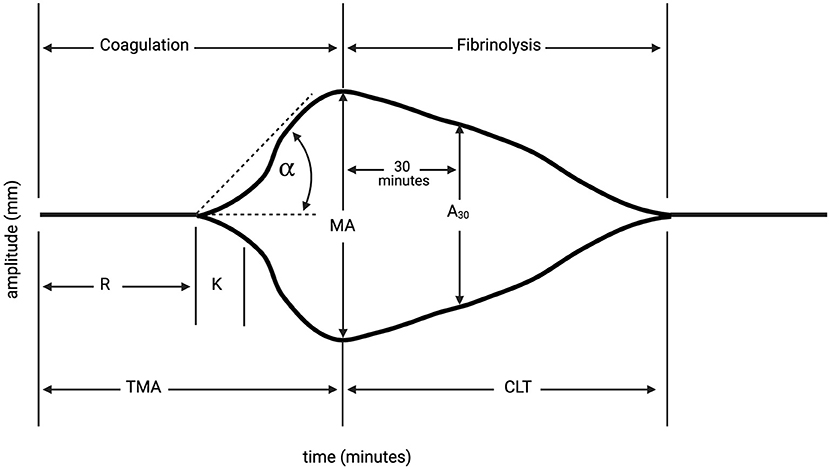
Figure 2. Thromboelastography (TEG). TEG is a hemostatic blood test which dynamically evaluates platelet function and clotting efficacy of whole blood. Reaction time (R) is latency from start of test to initial fibrin formation, and is dependent on clotting factors (normal: 4–8 min). Kinetics (K) is the duration taken to achieve clot strength of amplitude 20 millimeters (mm) (normal: 1–4 min). Alpha (α) measures the speed of fibrin cross-linking, dependent on fibrinogen (normal: 47–74°). Maximum amplitude measures ultimate strength and stability of fibrin clot (mm) (normal: 55–73 mm). Time to reach maximum amplitude (TMA); percentage decrease in amplitude 30-min post-MA (A30); Clot lysis time (CLT). At our institution, hypocoagulability on TEG is defined as a prolonged reaction time (R-time, minutes to clot formation), low alpha angle (measures clot kinetics), and low maximum amplitude (MA, millimeters, measures clot strength). Created with Biorender.com.
In terms of intervention modality, an initial surgical approach with pericardial window could potentially provide superior results compared to percutaneous procedures due to decreased PE recurrence rates (1). When balancing the increased safety from combining echocardiographic and/or fluoroscopic guidance during percutaneous pericardiocentesis with the increased bleeding risk in thrombocytopenic patients with open surgical procedures, the clinical decision has gradually inclined toward the less-invasive approach without any apparent impact on long-term outcomes. Complication rates in this study were consistent with the low incidences reported in prior image-guided studies in non-thrombocytopenic patients, of anywhere between 4 and 20% (7, 20–23). The decreased rate of complication we assume is due to using micropuncture technique and both echocardiographic and fluoroscopic guidance, and in comlex cases even triple-guidance with additional ultrasound-guided access.
In thrombocytopenia grades 3 and 4, mortality rate only increased after 1 year and only one patient with grade 4 thrombocytopenia had peri-procedural complications. In patients with extreme thrombocytopenia we found value in having a detailed discussion with the patient and family reflecting the lack of a surgical rescue option if certain complications occur, therefore a “no plan B situations” explanation is of paramount importance before the procedure. Especially in cases of hemodynamic instability, there are no absolute contraindications for pericardiocentesis in that it may be a necessary life-saving procedure, even in patients with severe thrombocytopenia and coagulopathy (24).
In cardiology and medicine, it is imperative to consider the ratio of risk to benefit in considering interventional diagnostic and therapeutic procedures. This study found that the greater the severity of thrombocytopenia, the greater the risk for intervention. In the case of pericardiocentesis with thrombocytopenia, the procedural risk increases as the platelet count decreases. More severe thrombocytopenia may be associated with more platelet transfusion refractoriness, less surgical back-up available, and lower overall survival. However, in patients with advanced malignancy, it is sometimes pertinent to proceed with higher risk procedures to achieve desired benefit due to the severity of disease and need for intervention. It is of utmost importance, therefore, for the cardio-oncology team to weigh risk and benefits and have the discussion with patients when performing diagnostic and therapeutic interventions such as pericardiocentesis (Figure 3). The low rate of periprocedural complications in our study may well be attributed to the consideration of these risks and benefits, and careful appropriate procedural technique.
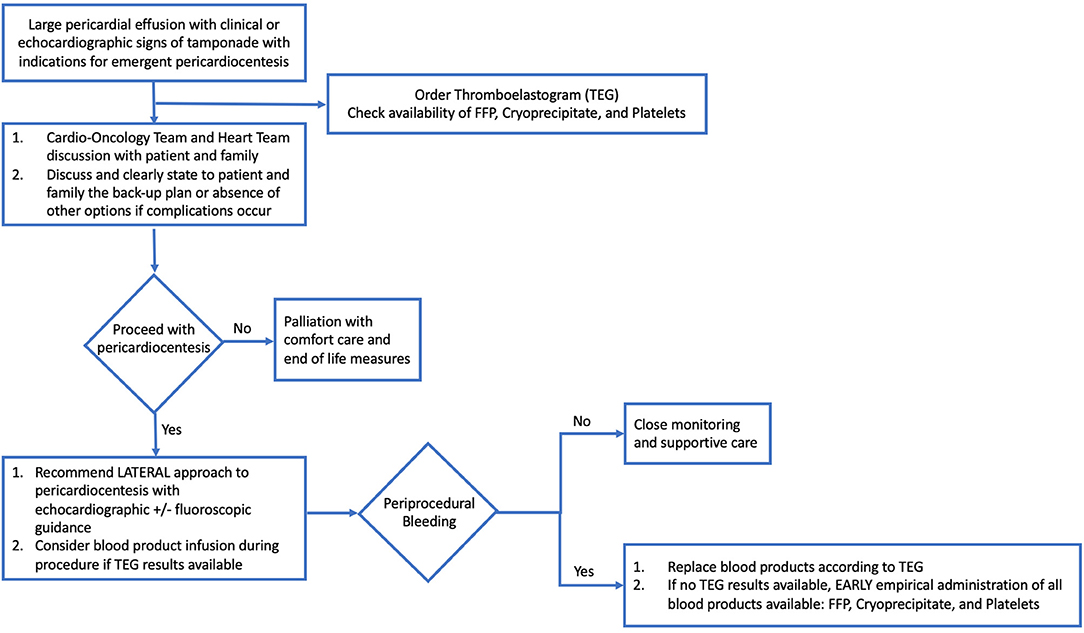
Figure 3. Flow-Chart: Approach to large pericardial effusion in patient with malignancy. Hemodynamically significant pericardial effusion in a patient with malignancy is a complex clinical scenario, one which requires appropriate clinical coordination, laboratory evaluation, and preparation. Important factors include a multidisciplinary discussion with Cardio-Oncology Team, Heart Team, patient and family members to discuss risks, benefits, and back-up options for complications, as well as consideration of palliation and comfort care measures. If proceeding with pericardiocentesis, recommended procedural approach is lateral and with echocardiographic ± fluoroscopic guidance, plus consideration of blood product administration based on laboratory results and thromboelastogram results. In the setting of periprocedural bleeding, early replacement of blood products according to TEG results or empiric administration of all blood products available is of paramount importance, in addition to achieving hemostasis and ongoing supportive care.
Limitations
Study limitations included a small sample size and the retrospective nature of data collection. A process of randomization of patients to pericardiocentesis, pericardial window, or medical therapy alone is challenging to imagine, more so to execute. Furthermore, the entry site during pericardiocentesis was ultimately dependent on patient's anatomy, ability to lie flat and interventionalists level of comfort with the approach. The use of TEG in a very small number of patients limits the ability to draw strong inferences from its interpretation. Future analysis of TEG in this patient population could be a helpful risk stratification tool. Finally, determining the utility of peri-procedural platelet transfusion is difficult since certain malignancies and their treatment can add complexity to an already coagulopathic clinical challenge.
Conclusions
In a high-risk patient population with cancer-related large pericardial effusion and thrombocytopenia, pericardiocentesis is a feasible intervention with low complication rates when appropriate equipment and technique are used. Furthermore, there is no absolute contraindication to pericardiocentesis in cases of hemodynamic instability, even with severe thrombocytopenia. The grade of thrombocytopenia reflects disease severity; however, no significant association was observed with respect to OS when adjusting for advanced cancer status and INR. Further studies will be needed to refine the role of grade of thrombocytopenia in non-advanced cancer patients, and platelet transfusions and platelet function tests in multivariate analysis in this patient population.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Institutional Review Board of the University of Texas MD Anderson Cancer Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
NW and ML wrote the manuscript. NP, JL-M, MC, KM, and CI conceived the study, performed data analysis, and reviewed and approved the final manuscript product. NW, ML, CG, AS, TD, DB, and JS performed data collection and analysis and reviewed, edited, and approved the final manuscript product. All authors contributed to the article and approved the submitted version.
Funding
The statistical analysis work was supported in part by the Cancer Center support Grant (NCI Grant P30 CA016672).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
PE, pericardial effusion; PLADO, prophylactic platelet dose on transfusion outcomes trial; OS, overall survival; MDACC, MD Anderson Cancer Center; CI, confidence interval; CS, cumulative survival; TEG, Thromboelastography; INR, international normalized ratio; HR, hazard ratio; MA, maximum amplitude.
References
1. Vaitkus PT, Herrmann HC, LeWinter MM. Treatment of malignant pericardial effusion. JAMA. (1994) 272:59–64. doi: 10.1001/jama.272.1.59
2. Ghosh AK, Crake T, Manisty C, Westwood M. Pericardial disease in cancer patients. Curr Treat Options Cardiovasc Med. (2018) 20:60. doi: 10.1007/s11936-018-0654-7
3. Burazor I, Aviel-Ronen S, Imazio M, Markel G, Grossman Y, Yosepovich A, et al. Primary malignancies of the heart and pericardium. Clin Cardiol. (2014) 37:582–88. doi: 10.1002/clc.22295
4. Imazio M, Demichelis B, Parrini I, Favro E, Beqaraj F, Cecchi E, et al. Relation of acute pericardial disease to malignancy. Am J Cardiol. (2005) 95:1393–94. doi: 10.1016/j.amjcard.2005.01.094
5. Ben-Horin S, Bank I, Guetta V, Livneh A. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: a study in 173 consecutive patients undergoing pericardiocentesis. Medicine. (2006) 85:49. doi: 10.1097/01.md.0000199556.69588.8e
6. Atar S, Chiu J, Forrester JS, Siegel RJ. Bloody pericardial effusion in patients with cardiac tamponade: is the cause cancerous, tuberculous, or iatrogenic in the 1990s? Chest. (1999) 116:1564. doi: 10.1378/chest.116.6.1564
7. Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2015). 36:2921–64. doi: 10.5603/KP.2015.0228
8. Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. (2010) 362:600–13. doi: 10.1056/NEJMoa0904084
9. Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. (2002) 3:425–30. doi: 10.1016/S1470-2045(02)00789-1
10. Pereira JT. Phan. management of bleeding in patients with advanced cancer. Oncologist. (2004) 9:561–70. doi: 10.1634/theoncologist.9-5-561
11. Iliescu C, Khair T, Marmagkiolis K, Iliescu G, Durand JB. Echocardiography and fluoroscopy-guided pericardiocentesis for cancer patients with cardiac tamponade and thrombocytopenia. J Am Coll Cardiol. (2016) 68:771–73. doi: 10.1016/j.jacc.2016.05.068
12. El Haddad D, Iliescu C, Yusuf SW, William WNJ, Khair TH, Song J, et al. Outcomes of cancer patients undergoing percutaneous pericardiocentesis for pericardial effusion. J Am Coll Cardiol. (2015) 66:1119. doi: 10.1016/j.jacc.2015.06.1332
13. National Institutes of Health - National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Bethesda, MD: U.S. Department of Health and Human Services (2017).
14. Jung HO. Pericardial effusion and pericardiocentesis: role of echocardiography. Korean Circ J. (2012) 42:725–34. doi: 10.1016/j.compbiomed.2012.04.003
15. Abdelfattah K, Cripps MW. Thromboelastography and rotational thromboelastometry use in trauma. Int J Surg. (2016) 33:196–201. doi: 10.1016/j.ijsu.2015.09.036
16. Khurana S. Monitoring platelet glycoprotein IIb/IIIa-fibrin interaction with tissue factor-activated thromboelastography. J Lab Clin Med. (1997) 130:401–11. doi: 10.1016/S0022-2143(97)90040-8
17. Kroll MH, Feng S. Targeting shear stress-induced platelet activation: is lesion-specific antiplatelet therapy a realistic clinical goal? Expert Rev Cardiovasc Ther. (2005) 3:941–51. doi: 10.1586/14779072.3.5.941
18. Iliescu C, Durand JB, Kroll M. Cardiovascular interventions in thrombocytopenic cancer patients. Tex Heart Inst J. (2011)38:259–60.
19. Agha AM, Gill G, Balanescu DV, Donisan T, Palaskas N, Lopez-Mattei JL, et al. Identifying hemostatic thresholds in cancer patients undergoing coronary angiography based on platelet count and thromboelastography. Front Cardiovasc Med. (2020) 7:9. doi: 10.3389/fcvm.2020.00009
20. Tsang TS. Echocardiography-guided pericardiocentesis for effusions in patients with cancer revisited. J Am Coll Cardiol. (2015) 66:1129–31. doi: 10.1016/j.jacc.2015.07.027
21. Adler Y, Charron P. The 2015 ESC guidelines on the diagnosis and management of pericardial diseases. Eur Heart J. (2015) 36:2873–74. doi: 10.1093/eurheartj/ehv479
22. Gluer R, Murdoch D, Haqqani HM, Scalia GM, Walters DL. Pericardiocentesis – how to do it. Heart Lung Circ. (2015) 24:621–25. doi: 10.1016/j.hlc.2014.11.009
23. Kumar R, Sinha A, Lin MJ, Uchino R, Butryn T, O'Mara MS, et al. Complications of pericardiocentesis: a clinical synopsis. Int J Crit Illn Inj Sci. (2015) 5:206–12. doi: 10.4103/2229-5151.165007
Keywords: pericardial effusion, pericardiocentesis, thrombocytopenia, safety, cancer
Citation: Wilson NR, Lee MT, Gill CD, Serauto Canache A, Donisan T, Balanescu DV, Song J, Palaskas N, Lopez-Mattei J, Cilingiroglu M, Marmagkiolis K and Iliescu CA (2021) Prognostic Factors and Overall Survival After Pericardiocentesis in Patients With Cancer and Thrombocytopenia. Front. Cardiovasc. Med. 8:638943. doi: 10.3389/fcvm.2021.638943
Received: 07 December 2020; Accepted: 25 March 2021;
Published: 21 April 2021.
Edited by:
Reto Asmis, Wake Forest School of Medicine, United StatesReviewed by:
George Lazaros, Hippokration General Hospital, GreeceIrma Bisceglia, San Camillo-Forlanini Hospital, Italy
Copyright © 2021 Wilson, Lee, Gill, Serauto Canache, Donisan, Balanescu, Song, Palaskas, Lopez-Mattei, Cilingiroglu, Marmagkiolis and Iliescu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cezar A. Iliescu, Y2lsaWVzY3VAbWRhZGVyc29uLm9yZw==
 Nathaniel R. Wilson
Nathaniel R. Wilson Michelle T. Lee
Michelle T. Lee Clarence D. Gill3
Clarence D. Gill3 Teodora Donisan
Teodora Donisan Juhee Song
Juhee Song Nicolas Palaskas
Nicolas Palaskas Juan Lopez-Mattei
Juan Lopez-Mattei Mehmet Cilingiroglu
Mehmet Cilingiroglu Cezar A. Iliescu
Cezar A. Iliescu