
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 13 May 2021
Sec. Cardiovascular Therapeutics
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.633878
This article is part of the Research Topic What do we know about COVID-19 implications for cardiovascular disease? View all 109 articles
 Guido Iaccarino1*†‡
Guido Iaccarino1*†‡ Guido Grassi2‡
Guido Grassi2‡ Claudio Borghi3‡
Claudio Borghi3‡ Davide Grassi4
Davide Grassi4 Costantino Mancusi1
Costantino Mancusi1 Maria Lorenza Muiesan5
Maria Lorenza Muiesan5 Massimo Salvetti5
Massimo Salvetti5 Massimo Volpe6,7‡
Massimo Volpe6,7‡ Claudio Ferri4† on behalf of the SARS-RAS Investigators
Claudio Ferri4† on behalf of the SARS-RAS InvestigatorsObjective: Altered coagulation parameters in COVID-19 patients is associated with a poor prognosis. We tested whether COVID-19 patients on chronic oral anticoagulants (cOACs) for thromboembolism prophylaxis could receive protection from developing more severe phenotypes of the disease.
Approach and Results: We searched the database of the SARS-RAS study (Clinicaltrials.gov: NCT04331574), a cross-sectional observational multicenter nationwide survey in Italy designed by the Italian Society of Hypertension. The database counts 2,377 charts of Italian COVID-19 patients in 26 hospitals. We calculated the Charlson comorbidity index (CCI), which is associated with death in COVID-19 patients. In our population (n = 2,377, age 68.2 ± 0.4 years, CCI: 3.04 ± 0.04), we confirm that CCI is associated with increased mortality [OR: 1.756 (1.628-1.894)], admission to intensive care units [ICU; OR: 1.074 (1.017-1.134)], and combined hard events [CHE; OR: 1.277 (1.215-1.342)]. One hundred twenty-five patients were on cOACs (age: 79.3 ± 0.9 years, CCI: 4.35 ± 0.13); despite the higher CCI, cOACs patients presented with a lower risk of admissions to the ICU [OR 0.469 (0.250-0.880)] but not of death [OR: 1.306 (0.78-2.188)] or CHE [OR: 0.843 (0.541-1.312)]. In multivariable logistic regression, cOACs confirmed their protective effect on ICU admission and CHE. The CCI remains the most important risk factor for ICU admission, death, and CHE.
Conclusions: Our data support a mechanism for the continuation of cOAC therapy after hospital admission for those patients who are on chronic treatment. Our preliminary results suggest the prophylactic use of direct cOACs in patients with elevated CCI score at the time of the COVID-19 pandemic even in absence of other risks of thromboembolism.
The current epidemic of COVID-19 has put the world population and health care systems under enormous stress, acting as an accelerator for death in the older population and anticipating the failure of hospitalocentric administration of health care in light of the increased request for hospital admissions. The scientific community has to, therefore, identify how to protect the high-risk population from the development of critical conditions that would increase the request for high-intensity care. It is now clear from the available literature that older and multimorbid patients are at risk of worse outcomes of COVID-19 (1, 2). Emerging evidence, though, proposes that the more severe outcomes of COVID-19 are also associated with alteration in coagulation patterns. The evidence that altered coagulation parameters in COVID-19 patients is associated with poor prognosis (3, 4) and has led us to hypothesize that the virus can cause an endothelial disease with systemic manifestation due to increased thrombosis (5). Low-molecular-weight heparin anticoagulation in the intensive care unit is associated with better prognosis in severe COVID-19 patients (6). Indeed, in this scenario, anticoagulant treatments are indicated by the majority as pivotal for the management of COVID-19 (7, 8). We explore the possibility that COVID-19 patients on chronic oral anticoagulants (cOACs) for a concomitant condition (i.e., atrial fibrillation, mechanic valvular replacement, pulmonary thromboembolism prophylaxis) before admission receive protection from more severe outcomes.
We designed a cross-sectional, multicenter, observational study involving 26 hospitals approached through the Italian Society of Hypertension network in 14 regions of Italy to achieve a nationally representative population sample (SARS-RAS Study) (9). The study is based on an online questionnaire to be filled in with data collected from the hospital charts of COVID-19 patients (see Supplementary Material). The patient cohort included 2,377 patients aged 18-101 years who were referred to the hospital for symptoms of COVID-19. All patients included in the questionnaire were diagnosed with COVID-19 according to World Health Organization interim guidance (10). The observation period started March 9 and ended May 9, 2020. The study was performed following article 89 of General Data Protection and Regulation (https://gdpr-info.eu). The SARS-RAS study is registered on Clinicaltrials.gov with the accession number NCT04331574. The online questionnaire was distributed among the centers to collect reviewed epidemiological, clinical, and outcomes data from hospital emergency rooms and regular and intensive care wards. Each center designated one or more physicians who were tasked with the acquisition and review of the requested information. Patients were pseudonymized by assigning a deidentified identification code. The questionnaire collected information regarding the center and the age, sex, nationality (Italian or other), and city of origin of the patient. From the anamnesis, we collected whether the patient had a known diagnosis of hypertension, coronary artery disease (history of myocardial infarction, PCI, or CABG), heart failure (based on clinical history), atrial fibrillation, diabetes, chronic kidney disease (anamnestic estimated glomerular filtration rate below 60 ml/min/kg), chronic obstructive pulmonary disease (according to GOLD 2019), obesity (body mass index > 30 kg/m2), history of blood and solid tumors, liver disease, or other comorbidities; we annotated the presence of prescribed antihypertensive, anticoagulant, and antidiabetic therapy.
The severity of the disease was classified according to the Chinese Center for Disease Control (11) into three groups: asymptomatic or with light symptoms, moderate symptoms, and severe intensity.
We collected also the outcomes (hospital dismission or exitus). All patients for which the course of the disease was in an active state were classified as such (10).
For each patient, we calculated the Charlson comorbidity index (CCI) based on the available data and according to the original description of the score (12). Descriptive analyses of the variables were expressed as mean and standard errors or frequencies expressed in absolute numbers and percentages. Continuous variables were analyzed by ANOVA; categorical data were compared using the χ2 test. Regression analyses, odds ratio, and confidence intervals were tested on the interest variables grouped by outcomes; multivariable regression analyses were performed on the significant and clinically relevant continuous and categorical variables.
We collected charts of 2,569 patients. We excluded from the analysis 192 patients for incomplete or discordant data. The analysis was then performed on 2,377 patients. The clinical features of our population are indicated in Table 1. Women were less frequently affected than men, and the mean age indicates that the disease was prevalent among the senior population (Figure 1). We counted 285 deaths and more than 400 patients admitted to intensive care units (ICUs) (Figure 2). We confirm that CCI is associated with increased mortality [Table 2, OR: 1.756 (1.628-1.894)], admission to ICUs [OR: 1.074 (1.017-1.134)], and combined hard events [CHE, OR: 1.277 (1.215-1.342)]. One hundred twenty-five patients were on cOACs for thromboembolic prevention for atrial fibrillation and venous thromboembolism for at least 6 months before the diagnosis of COVID-19. Compared with non-cOACs, cOACs patients were older, included more women, and had a larger CCI (Table 1). Despite the larger CCI, cOACs patients were less likely to be referred to the ICU [Table 2, OR 0.469 (0.250-0.880)], but with a similar risk of death [OR: 1.306 (0.78-2.188)] or CHE [OR: 0.843 (0.541-1.312)]. To ascertain the role of age, multimorbidity (combined in the CCI score), sex, and cOACs on the outcome, we performed a multivariable logistic regression analysis on ICU admission, death, and CHE. cOACs confirmed the protective effect on admission to the ICU and CHE (Figures 3A,C) but not on death (Figure 3B). Sex and CCI remain significant risk factors for ICU access and CHE in COVID-19 patients (Figures 3A-C). In particular, CCI represents the most important risk factor for death in COVID-19 patients.
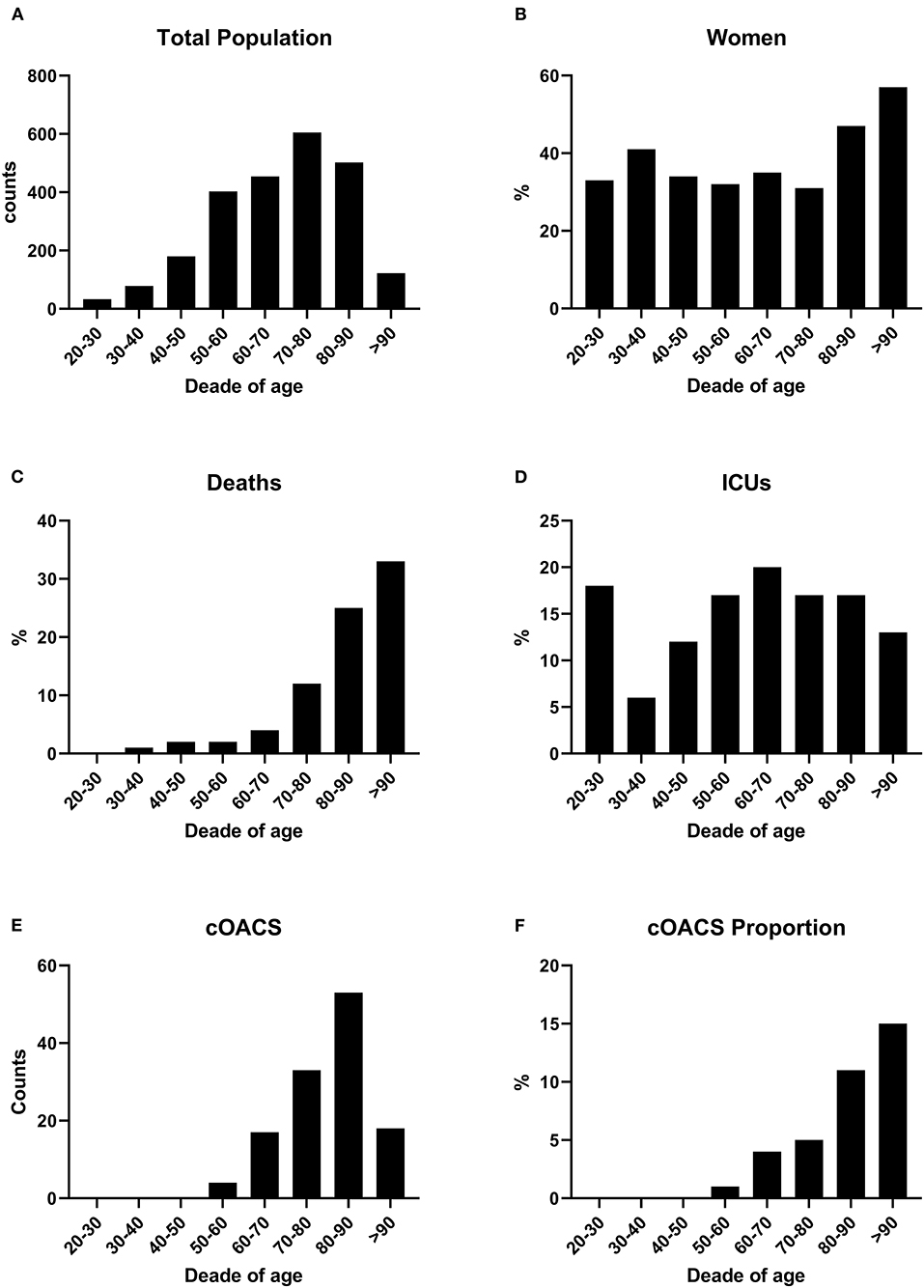
Figure 1. (A) COVID-19 patients were grouped by age decades to show the impact of the disease according to age. Patient numbers increased by age decades. (B) Women were stably below 40% of total cases up to the age of 80 years. (C) Death rates increased with age. (D) ICU admissions were stable along all ages. (E) cOACs were administered increasingly with age. (F) The percentage of patients on cOACs increased with age.
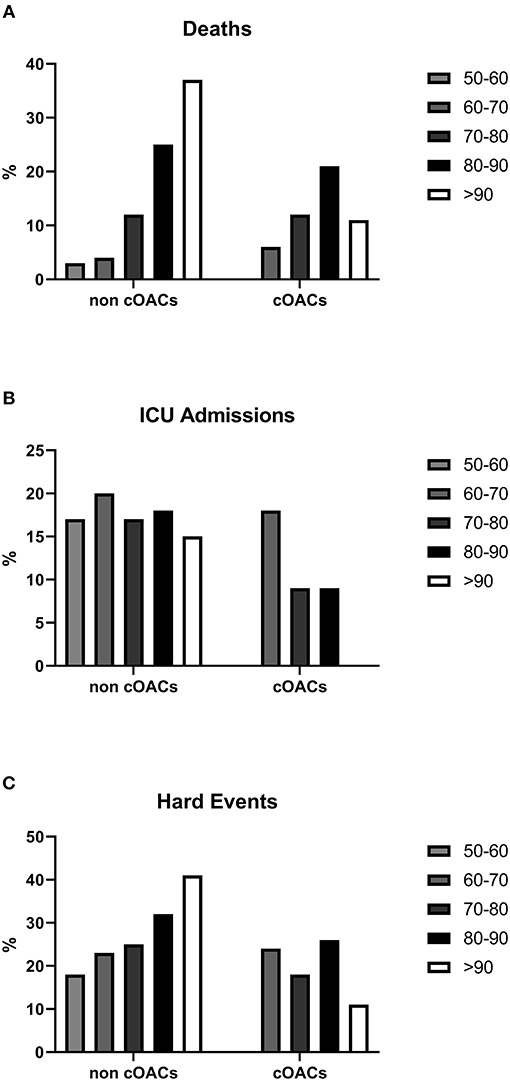
Figure 2. (A) Death rates in non-cOACs and cOACs COVID-19 patients. (B) ICU admission rates were lower in non-cOACs than cOACs COVID-19 patients. (C) CHE (death and ICU admission) rates were similar in non-cOACs and cOACs COVID-19 patients.
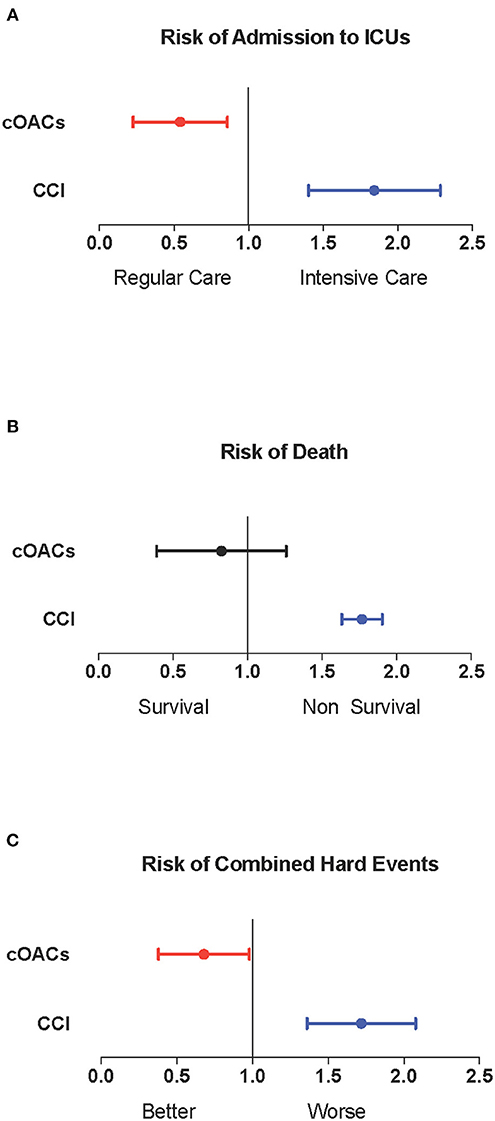
Figure 3. (A) Odds ratio and confidence intervals for admission to intensive care units; cOACs: T = −0.825, p < 0.01; CCI: T = 0.085, p < 0.003; male sex: T = 0.602; p < 0.001. (B) Odds ratio and confidence intervals for deaths; CCI: T = 0.567, p < 0.001. (C) Odds ratio and confidence intervals for combined hard events (deaths and ICU admissions); cOACs: T = −0.455; p < 0.05; male sex: T = 0.527; p < 0.001; CCI:T = 0.257; p < 0.001. CCI, Charlson comorbidity index; cOACs, chronic oral anticoagulants; ICU, intensive care units.
Our study shows that cOACs modify the risk of admission to the ICU and CHE even independently from the major determinants of outcomes in COVID-19, which are age, comorbidity, and sex. This result is well in agreement with the proposed empiric use of anticoagulants for the treatment of severely ill COVID-19 patients (13). So far, this use is based mainly on evidence gathered on a small number of patients of the subgroup analysis from a single retrospective, poorly controlled study (4). The largest number of patients, the multicenter design, and the possibility to perform a multivariable statistic approach confer our study larger statistical soundness.
COVID-19 patients are prone to venous thromboembolism (3) and present with abnormal coagulation parameters, such as D-dimer and APTT (7). The underlying mechanisms include possibly a direct action of the virus on coagulation-competent tissues and cells, such as endothelium (5) and platelets as well as the indirect immune activation and further potentiated hyper-coagulable state, which leads to the development of thromboembolic complications in patients (14). In this scenario, cOACS cannot prevent the infection and the development of COVID-19 but might provide protection toward the consequences of the hypercoagulability state caused by the disease.
Our study also proposes oral anticoagulant therapy as a strategic therapy, in particular, for the early treatment of patients before they become severely ill, as an alternative to the use of parental anticoagulants.
Overall, our data support the proposed use of anticoagulant therapy to prevent a mechanistic approach for the prophylactic use of direct OACs in patients with elevated CCI score at the time of the COVID-19 pandemic to reduce the risk of more severe clinical disease.
In contrast to our findings, a very small study conducted in the United Kingdom indicates a non-significant death reduction in patients treated with either warfarin or DOACs with a paradoxical—although, again, non-significant—increment in ICU admission in patients on OACs (15). Further, progression to an acute respiratory distress syndrome was increased by OACs in 192 hospitalized Italian patients (RR = 1.24, 95% CI 0.56–2.08). However, due to the small sample size, the influence of OACs on disease severity was again non-significant (p = 0.465) (16). Thus, our findings seem to represent the only data available in a large population indicating that preexisting OAC therapy can reduce ICU admission in hospitalized patients. Further data from prospective studies could help better our understanding of the prophylactic strategy to choose between different OACs.
The study has a cross-sectional observational design, which could affect the results. For this reason, our research was never intended to be conclusive but rather hypothesis generating. Finally, we cannot discriminate against the role of different anticoagulants because we did not collect the names of the active principles.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: doi: 10.6084/m9.figshare.12622208.
Ethical approval was not provided for this study on human participants because the study is performed following the article 89 of the General Data Protection and Regulation (https://gdpr-info.eu). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Arrigo F. G. Cicero1, Claudia Agabiti Rosei14, Michele Bevilacqua10, Valeria Bisogni9, Michele Bombelli22, Luca Bulfone16, Flaminia Canichella18, Giovanni Carpani22, Massimo Catanuso17, Carmine Savoia2, Damiano Rizzoni15, Giulia Chiarini15, Fernando Chiumiento21, Giuseppe Mulè4, Rosario Cianci6, Giuliano Tocci2, Franco Cipollini23, Antonio Concistrè6, Andrea Dalbeni10, Roberto Alberto De Blasi2, Riccardo Sarzani19, Carolina De Ciuceis14, Raffaella Dell'Oro22, Antonino Di Guardo17, Stefano Perlini5, Santo Di Lorenzo2, Monica Di Norcia25, Giacomo Pucci9, Roberto Ervo11, Elisabetta Eula20, Davide Fabbricatore14, Pietro Minuz10, Elvira Fanelli20, Claudio Letizia6, Cristiano Fava10, Enzo Grasso12, Stefano Carugo13, Alessandro Grimaldi25, Maddalena Illario3, Claudio Invernizzi22, Maria Lorenza Muiesan14, Elena Iraca1, Federica Liegi1, Francesca Magalini26, Franco Veglio20, Paolo Malerba15, Leonardo Sechi16, Alessandro Maloberti12, Costantino Mancusi3, Martina Mezzadri6, Francesco Fallo7, Giulia Molinari22, Roberta Mussinelli5, Anna Paini14, Paola Pellimassi2, Paolo Mulatero20, Ornella Piazza8, Davide Grassi25, Luigi Pietramala6, Roberto Pontremoli24, Fosca Quarti Tevano22, Franco Rabbia20, Monica Rocco2, Anna Sabena5, Francesco Salinaro5, Paola Schiavi19, Maria Chiara Sgariglia6, Francesco Spannella19, Cristina Giannattasio12, Sara Tedeschi1, Pierluigi Viale1 and the COVID19 Niguarda group.
The SARS-RAS centers are the following:
1AO Policlinico Sant'Orsola-Malpighi, Bologna; 2AOU Sant'Andrea, Roma; 3AOU Federico II, Napoli; 4AOU Policlinico Paolo Giaccone, Palermo; 5AOU Policlinico San Matteo, Pavia; 6AOU Policlinico Umberto I, Roma; 7AOU Policlinico Universitario, Padova; 8AOU San Giovanni di Dio e Ruggi d'Aragona, PO “Dell'Olmo” Cava de' Tirreni; 9AOU Santa Maria, Terni; 10AOUI Verona, Italy; 11ASL 1 Imperiese, Ventimiglia; 12ASST Grande Ospedale Metropolitano Niguarda, Milano; 13ASST Santi Paolo e Carlo, Milano; 14ASST SPEDALI CIVILI BRESCIA; 15ASST SPEDALI CIVILI. PO Montichiari; 16ASUI Friuli Centrale, Udine; 17Centro Ipertensione Mascalucia, Catania; 18INMI Lazzaro Spallanzani, Roma; 19INRCA, Ancona, Italy; 20Ospedale “Le Molinette”, Torino; 21Ospedale di Eboli, Salerno; 22Ospedale San Gerardo, Monza; 23Ospedale San Jacopo, Pistoia; 24Ospedale San Martino, Genova; 25PO San Salvatore, L'Aquila; 26Ospedale Maggiore, Parma.
GI: study design, statistical analysis, and paper writing. GG, CB, MM, and MV: study design and paper editing. DG and MS: data collection and elaboration. CM: data collection and elaboration, statistical analysis, and paper writing. CF: study design and paper writing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.633878/full#supplementary-material
1. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. (2020) 323:2052–59. doi: 10.1001/jama.2020.6775
2. Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. (2020) 8:e35.
3. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. (2020) 18:1421–4. doi: 10.1111/jth.14830
4. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. (2020) 18:844–7. doi: 10.1111/jth.14768
5. Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? a comprehensive evaluation of clinical and basic evidence. J Clin Med. (2020) 9:1417. doi: 10.3390/jcm9051417
6. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. (2020) 18:1094–99. doi: 10.1111/jth.14817
7. Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. (2020) 189:846–7. doi: 10.1111/bjh.16727
8. Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. (2020) 50:72–81. doi: 10.1007/s11239-020-02138-z
9. Iaccarino G, Borghi C, Cicero AFG, Ferri C, Minuz P, Muiesan ML, et al. Renin-angiotensin system inhibition in cardiovascular patients at the time of COVID19: much ado for nothing? A Statement of Activity from the Directors of the Board and the Scientific Directors of the Italian Society of Hypertension. High Blood Press Cardiovasc Prev. (2020) 27:105–8. doi: 10.1007/s40292-020-00380-3
10. World Health Organization. Clinical Management Of Severe Acute Respiratory Infection When Novel Coronavirus (Ncov) Infection Is Suspected: Interim Guidance. World Health Organization (2020).
11. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
13. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. Right ventricular: COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75:2950–73. doi: 10.1016/j.jacc.2020.04.031
14. Page EM, Ariens RAS. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb Res. (2021) 200:1–8. doi: 10.1016/j.thromres.2021.01.005
15. Sivaloganathan H, Ladikou EE, Chevassut T. COVID-19 mortality in patients on anticoagulants and antiplatelet agents. Br J Haematol. (2020) 190:e192–5. doi: 10.1111/bjh.16968
Keywords: multimorbidity, atrial fibrillation, prophylaxis, death, intensive care admissions, COVID-19 outcomes, hypertension, thrombosys
Citation: Iaccarino G, Grassi G, Borghi C, Grassi D, Mancusi C, Muiesan ML, Salvetti M, Volpe M and Ferri C (2021) Preexisting Oral Anticoagulant Therapy Ameliorates Prognosis in Hospitalized COVID-19 Patients. Front. Cardiovasc. Med. 8:633878. doi: 10.3389/fcvm.2021.633878
Received: 26 November 2020; Accepted: 19 March 2021;
Published: 13 May 2021.
Edited by:
Mingxing Xie, Huazhong University of Science and Technology, ChinaReviewed by:
Antonio Cano, University of Valencia, SpainCopyright © 2021 Iaccarino, Grassi, Borghi, Grassi, Mancusi, Muiesan, Salvetti, Volpe and Ferri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guido Iaccarino, Z3VpYWNjYXJAdW5pbmEuaXQ=
‡ORCID: Guido Iaccarino orcid.org/0000-0002-8997-835X
Guido Grassi orcid.org/0000-0003-1922-6547
Claudio Borghi orcid.org/0000-0001-8039-8781
Massimo Volpe orcid.org/0000-0002-9642-8380
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.