- 1Institute of Anesthesiology, Cantonal Hospital of Winterthur, Winterthur, Switzerland
- 2Department of Internal Medicine, Cantonal Hospital of Baden, Baden, Switzerland
- 3Center for Molecular Cardiology University of Zurich, Zurich, Switzerland
- 4Department of Internal Medicine, Cantonal Hospital of Baden, Baden, Switzerland
- 5Clinical Trials Unit (CTU) Bern, University of Bern, Bern, Switzerland
- 6Cardiovascular Research Institute Basel and Division of Cardiology, University Hospital Basel, University of Basel, Basel, Switzerland
- 7Labormedizinisches Zentrum Dr Risch, Vaduz, Liechtenstein
- 8Division of Laboratory Medicine, Cantonal Hospital Graubünden, Chur, Switzerland
- 9Department of Laboratory Medicine, Institute of Clinical Chemistry, Inselspital Bern University Hospital, University of Bern, Bern, Switzerland
- 10Private University Triesen, Triesen, Liechtenstein
- 11Population Health Research Institute, McMaster University, Hamilton, ON, Canada
Background: Omega-3 fatty acids are associated with a lower risk of cardiovascular disease (CVD) and with beneficial effects on CV risk factors. The albumin-creatinine ratio (ACR) is a risk factor for CVD, all-cause mortality and accelerated glomerular filtration rate (GFR) decline in the general population. We aimed to investigate the association between n-3 PUFAS and ACR in heathy individuals with preserved GFR.
Design and Methods: The present cross-sectional analysis is part of the GAPP study, a population-based cohort of healthy adults aged 25–41 years. Individuals with known CVD, diabetes, or a BMI >35 kg/m2 were excluded. eGFR was calculated according to the combined Creatinine/Cystatin C CKD-EPI formula. ACR was obtained from a fasting morning urine sample. The Omega-3 Index (relative amount of EPA and DHA of total fatty acids in %) was obtained from whole blood aliquots.
Results: Overall, 2001 participants (median age 37 years IQR 31; 40, 53% female) were included in this analysis. Median Omega-3 Index was 4.59 (IQR 4.06; 5.25) and median eGFR 111 ml/min/1.73 m2 (IQR 103; 118). Median ACR was 0.14 mg/mmol (IQR 0; 0.43). We found a significant inverse association of the Omega-3 Index with ACR (ratio 0.84, 95%CI 0.73–0.96; p = 0.011) which remained after comprehensive adjustment (ratio 0.86, 95%CI 0.74–1.00; p = 0.048). No association of the Omega-3 Index with eGFR was found. The adjusted difference in eGFR per 1-unit increase in Omega3-Index was −0.21 (95%CI −0.76; 0.35; p = 0.47).
Conclusions: A higher Omega-3 Index was significantly associated with lower ACR in this young and healthy population with preserved eGFR. Omega-3 fatty acids may exhibit cardio- and nephroprotective effects in healthy individuals through modulation of ACR.
Introduction
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) include the fish-derived eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) as well as alpha-linolenic acid (ALA) derived from plants. Their beneficial effect on cardiovascular disease and risk factors has been the focus of a multitude of experimental studies and clinical investigations. Of note, they are associated with anti-inflammatory as well as antithrombotic activity (1), they are likely to improve endothelial dysfunction (2) and positively influence cardiovascular risk factors like HbA1c (3), triglycerides (3), and blood pressure (4).
Chronic kidney disease (CKD) and albuminuria as an early marker for kidney damage are both well-established cardiovascular risk factors (5, 6). In animal models dietary supplementation with n-3 PUFAs slows the progression of kidney disease and reduces urine albumin excretion as well as renal inflammation and fibrosis (7, 8). In older community-dwelling individuals, higher plasma PUFAs were associated with a lower age-related decline in kidney function (9). Data form interventional trials showed the potential of n-3 PUFA supplementation to attenuate the progression of albuminuria in individuals with type 2 diabetes and coronary heart disease (10). Nevertheless, data from both observational and interventional trials lack in healthy individuals free of cardiovascular disease—where long-term prevention efforts may be particularly effective.
We addressed these issues by investigating the associations of whole blood n-3 PUFA levels (given as the Omega-3 Index) with renal function and albuminuria in a large population of young healthy adults.
Materials and Methods
Study Population
For this cross-sectional study, baseline data from the GAPP study (genetic and phenotypic determinants of blood pressure and other cardiovascular risk factors) were analyzed. The GAPP study is an ongoing large population-based cohort study, which investigates the determinants of BP and other cardiovascular risk factors in young and healthy adults, described elsewhere (11). In brief, from 2010 to 2013 all inhabitants of the Principality of Liechtenstein in the given age range were invited and 2,170 healthy adults aged 25–41 years agreed to participate. Main exclusion criteria were established cardiovascular disease, chronic kidney disease, a BMI higher than 35 kg/m2, obstructive sleep apnea, daily intake of non-steroidal anti-inflammatory medication and diabetes.
From a total of 2,170 patients enrolled in the GAPP study, we excluded participants on ACE-Inhibitors (n = 5), angiotensin-receptor blockers (n = 22), diuretics (n = 9) as well as the data sets with missing information on ethnicity (n = 136), creatinine or cystatin C values (n = 9). Because of multiple exclusion criteria in one individual, 169 individuals were excluded, resulting in a total of 2,001 participants for this analysis.
The study protocol was approved by the local ethics committee (Cantonal Ethics Commission of Zurich, Zurich, Switzerland). Informed written consent was obtained from every individual participant.
Blood Sampling and Whole Blood Fatty Acid Composition
At baseline, venous whole blood samples were collected from every participant after an overnight fast. The aliquoted cryotubes where immediately stored at −80°C (11), which has been shown to not alter the n-3 levels measured (12), and underwent no further freeze-thaw cycles. Whole blood fatty acids were analyzed according to the high sensitivity-Omega-3 Index methodology, initially described for erythrocyte samples (13). FA methyl esters were generated from whole blood by acid transesterification and analyzed by gas chromatography using a GC2010 Gas Chromatograph (Shimadzu, Duisburg, Germany) equipped with a SP2560, 100-m column (Supelco, Bellefonte, Pennsylvania, USA) hydrogen as carrier gas. FAs were identified by comparison with a standard mixture of FAs characteristic of erythrocytes. Results are given as relative amounts of EPA (C20:5n3) and DHA (C22:6n3), expressed as a percentage of a total of 26 identified FAs, referred to as Omega-3 Index. A validated correction factor was applied for whole blood aliquots (14, 15). Where mentioned, ALA (C18:3n3) is given as percentage of a total of 26 identified FAs. The coefficient of variation for FA levels was 5%. Analyses were quality controlled according to DIN ISO 15189.
Assessment of Kidney Function, Albuminuria and Other Biomarkers
Plasma creatinine, Cystatin C and high-sensitivity C-reactive protein (hs-CRP) were assayed from fresh samples immediately after blood draw on a Roche Cobas analyzer (F. Hoffmann-La Roche, Basel, Switzerland). Estimated glomerular filtration rate (eGFR) given as mL/ min /1.73m2 was computed using the CKD-EPI Creatinine-Cystatin Equation (2012) as published by Inker et al. (16). Urine albumin and urine creatinine were obtained from fasting morning urine samples. Albuminuria was quantified with the ACR and given in mg/mmol. Hemoglobin A1c (HbA1c) was measured using HPLC.
Other Study Variables
For all participants, a detailed assessment of personal, medical, lifestyle and nutritional factors was performed. Smoking status was self-assessed and categorized into current, past and never smokers. Moderate and vigorous physical activity was assessed using the validated international physical activity questionnaire (17). Regular physical activity was defined as moderate or vigorous physical activity of at least 75 or 150 min/week, respectively. Alcohol consumption and frequency of fruit, vegetable and fish consumption were obtained with the Swiss health survey questionnaire from 2007. Healthy diet was defined as at least five servings of fruits or vegetables per day. Highest educational status was reported. Weight and height were directly measured in a standardized way. BMI was calculated by dividing weight in kg by height in m2.
Statistical Analysis
Data were tested against a predefined hypothesis, assuming an inverse association of Omega-3 Index with eGFR and ACR. Baseline characteristics were presented overall as well as stratified according to quartiles of Omega-3 Index in % (medians with upper and lower quartiles and p-values from Wilcoxon-Mann-Whitney or chi-squared tests). To assess the relationship of n-3 PUFAs with eGFR we fit simple and multivariable linear regression models with eGFR as the dependent variable.
To account for the skewed distribution and excess of zeros in ACR, we fit a generalized linear model using the Tweedie distribution and a log-link. Model coefficients were back-transformed by exponentiation and expressed as ratio. Six outlier observations with very high ACR (ranging from 79.77 to 104.22, see Supplementary Table 3) were excluded from the analysis as values were highly pathological and atypical for this set of healthy subjects.
For all fitted linear models, we assessed model assumptions (linearity, normality of residuals, and presence of influential values); all assumptions held true.
In addition, we evaluated the association of Omega-3, ALA, EPA and DHA with different eGFR quantiles to study the potential influence on people with high or low eGFR. We performed joint multivariate quantile regression using the method by Yang and Tokdar (18) implemented in the R package “qrjoint.” This exploratory analysis is presented in the Supplementary Material.
Results
Study Population
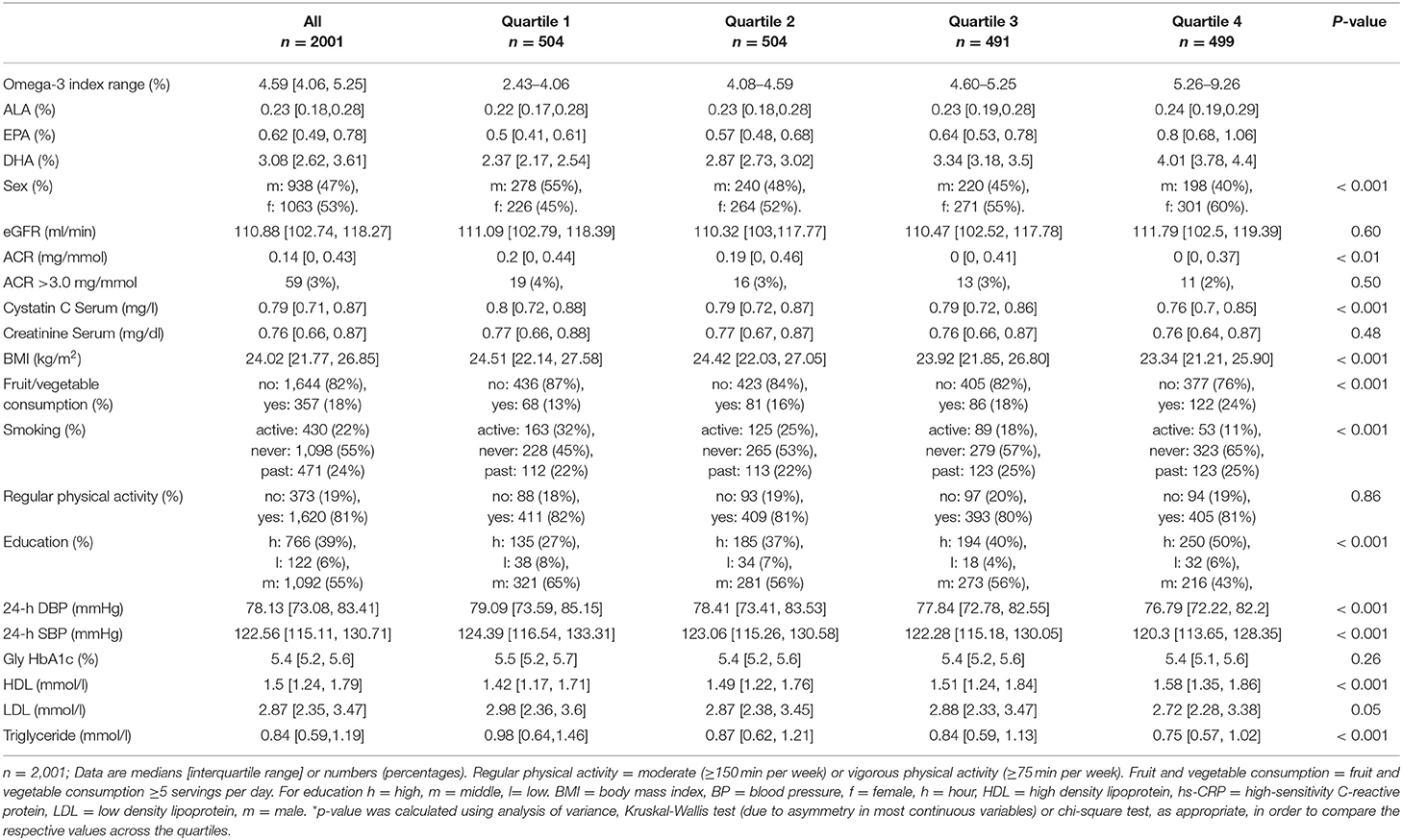
Baseline characteristics for the whole population and stratified by quartiles of the Omega-3 Index are shown in Table 1. Median age was 37 years (IQR 31; 40) and 53% were female. Median Omega-3 Index was 4.59 (IQR 4.06; 5.25). Median eGFR was 111 ml/min/1.73 m2 (IQR 103; 118). Median ACR was 0.14 mg/mmol (IQR 0; 0.43).
Participants in the higher Omega-3 Index quartiles had significantly lower Cystatin C (p < 0.001). Further, a higher Omega-3was found in women than men and associated with a lower BMI, less smoking (p-value for all < 0.001) and a lower 24-h blood pressure profile (systolic p < 0.0001; diastolic p < 0.001). Additionally, individuals with a higher Omega-3 Index had higher HDL levels (p < 0.001), lower triglycerides (p < 0.001) and consumed significantly more fruits and vegetables (p < 0.001) as well as fish (p < 0.001). Groups with a higher Omega-3 Index also had a higher educational status (p < 0.001).
Inverse Association of Omega-3 Index With ACR
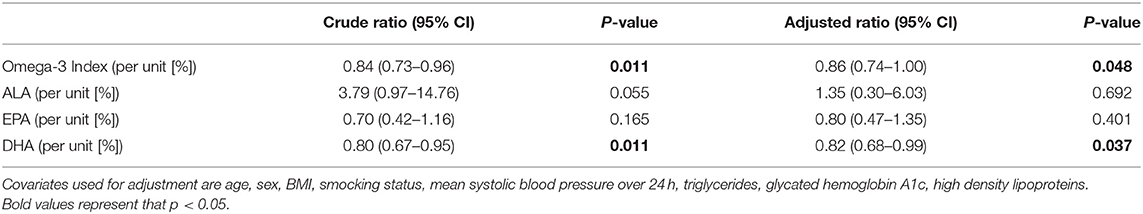
Omega-3 Index was inversely associated with ACR before and after adjustment for potential confounders (Table 2). A 1-unit increase in the Omega-3 Index was associated with a 14% lower ACR (adjusted ratio 0.86, CI 0.74–1.00; p = 0.048). Analyses of individual fatty acids showed a significant association for DHA with a ratio of (0.82, CI 0.68–0.99, p = 0.037) but not for ALA (p = 0.69) or EPA (p = 0.40), as presented in Table 2.
No Association of Omega-3 Index With eGFR
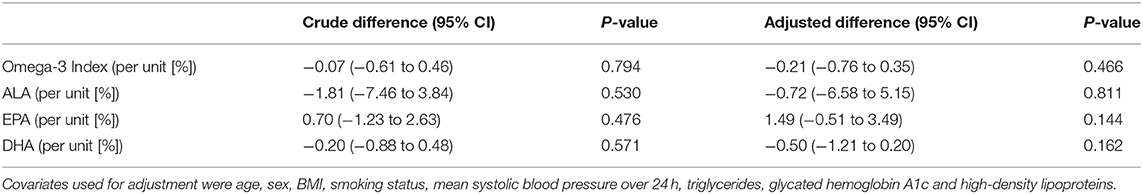
In linear regression analyses, no association between the Omega-3 Index and eGFR was found (Table 3); the adjusted difference in eGFR was −0.21 ml/min (95%-CI −0.76;0.35; p = 0.466) per 1-unit increase in Omega-3 Index. Additional analyses for individual fatty acids (ALA, EPA and DHA) also showed no significant association with eGFR (Table 3). Moreover, no significant association was found in non-linear additive models (Supplementary Material).
Discussion
Our main findings collectively indicate that n-3 FA intake, as reliably reflected by the Omega-3 Index, is inversely associated with ACR in young and healthy individuals with preserved eGFR. Thus, Omega-3 fatty acids may exhibit cardio- and nephroprotective effects in healthy individuals through modulation of the ACR.
Albuminuria is a well-established early marker not only for kidney damage but also as a cardiovascular risk factor. Both prospective and epidemiologic data support albuminuria as a predictor for cardiovascular morbidity and mortality as well as all-cause mortality (6)—not only in individuals already suffering from hypertension or diabetes, but also in the general population. Even a minimal increase in ACR is associated with an increased risk of kidney failure and cardiovascular disease (19). Moreover, an increased ACR predicts incident hypertension and cardiovascular disease mortality at an 11-year follow-up, highlighting its potential as a target for preventive interventions (20).
In CKD treatment, the reduction of albuminuria remains a central pillar. Concerning the effects of n-3 PUFAs, longitudinal, interventional studies focusing on healthy adults with preserved kidney function, where prevention measures could be of particular importance are not available (21). From an interventional perspective, Elajami et al. (10) showed, that EPA and DHA supplementation was able to attenuate the progression of established albuminuria in patients with type 2 diabetes mellitus and coronary artery disease, most of whom were already under treatment with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers.
There is increasing evidence, that Omega-3 fatty acids are crucial in resolving inflammatory responses as substrates for the endogenic production of specialized pro-resolving mediators (resolvins, protectins, and maresins)—stopping inflammation and giving way to tissue reparation (22, 23). Priante et al. (24) provided a number of mechanism how n-3 PUFAs counteract renal inflammation and fibrosis. Further, n-3 PUFA supplementation affects cell junctions and protects from distal tubular cell damage in rat kidneys, thus directly and indirectly affecting albuminuria (25, 26). There is increasing evidence for differing individual effects of EPA and DHA on cardiometabolic risk factors (27). In our collective, the inverse association of n-3 PUFAs with ACR was mainly driven by DHA. Accordingly, several mechanistic studies provided evidence for DHA suppressing inflammation pathways in the kidney—of note, oxygenated metabolites were decreased more effectively by DHA than EPA (28–30).
The observed inverse association of Omega-3 fatty acids with ACR is concordant with a multitude of well-established beneficial effects of n-3 fatty acids on the cardiovascular system (31). Hypertension, oxidative stress, dyslipidemia, endothelial dysfunction and thrombogenesis are all intermediaries in the pathogenesis of both kidney disorders and cardiovascular disease—and all are positively modifiable through n-3 PUFAs (21, 32, 33). As already shown earlier by our group, omega-3 fatty acids are inversely associated with blood pressure in the same, mostly normotensive population, which may be a driver for kidney damage in young and healthy individuals (4). Further, participants with more favorable fatty acid profile at baseline have significantly lower triglyceride and higher HDL levels. The decreased risk of ischemic events through n-3 PUFA mediated triglyceride level modification was impressively demonstrated in the REDUCE-IT trial, where very high dosages of n-3 PUFAS were administrated (34). In contrast, the median Omega-3 Index of 4.6% in our population is highly comparable to data from other Western countries with an average Index of 4–5% (35–37), but still well-below the 8% recommended for optimal cardioprotection (14) or countries with high fish consumption like Japan (Omega-3 Index between 8 and 10%) (38). This suggests, that in the measured range of our population, no specific lower n-3 threshold exists. As demonstrated in the JELIS trial for major coronary event, n-3 PUFA is likely to be a continuous beneficial variable, where no ceiling effect was observed (39). Both, more favorable lipid profiles as well as lower blood pressure might mediate the observed effects. Further, participants with a higher Omega-3 Index are better educated, eat healthier and do more exercise. Nevertheless, our results remained significant also after adjustment for potential confounders.
Concerning the effect of n-3 PUFAs on eGFR, Miller et al. (40) suggest in their metanalysis that n-3 PUFA supplementation reduces urine protein excretion but not decline in GFR. While also in our population no association of Omega-3 Index with eGFR could be found, one should consider several factors when interpreting these results: First, the CKD-EPI formula, as all other formulas calculating the glomerular filtration rate, remains an estimation. Especially in patients with preserved eGFR, this estimation becomes less accurate and may not detect fine differences between individuals (41). Further, a quantifiable reduction in eGFR is a sign of significant and advanced kidney damage—not to be expected in this cohort of generally young and healthy adults. However, Cystatin C as a probably superior predictor of GFR in individuals with normal and mildly impaired kidney function (42) significantly differed across Omega-3 Index quartiles at baseline. This could also be explained with effects, in contrast to ACR findings, only grasping with higher n-3 PUFA levels—reflected in the drop of Cystatin C in the highest Omega-3 Index quartile. Interestingly, an analysis of the serum n-3 PUFA, creatinine and Cystatin C levels in 549 Japanese community-dwellers by Higashiyama et al. (43) found an association of n-3 PUFA fatty acids with Cystatin C based but not creatinine based eGFR estimation. Whether higher Omega-3 fatty acid levels may have a preventive effect on physiologic (accelerated) decline in kidney function is to be determined in longitudinal studies.
The main strengths of our study include the availability of both creatinine and cystatin C for the computation of the eGFR, which permitted us to implement the internationally recognized combined CKD-EPI formula (16). Additionally, we utilized the still novel Omega-3 Index as a marker for n-3 PUFA intake. This method is proven to have a reduced biologic and analytic variability compared to plasma levels and thus reliably reflects and individual's long-term omega-3 status and tissue content (12). It is robust and resistant to short-term variation of n-3 PUFA intake and obviously superior to often unreliable nutritional questionnaires (13). Together with definition and homogeneity of our large and well-characterized study cohort composed of the young and healthy population of Liechtenstein with access to state-of-the-art preventive and medical service, it allows comparability to other Western societies. Further, we were careful to exclude individuals with known renal insufficiency, on ARBs as well as ACEs or suffering from any major illness including diabetes—thus allowing to minimize residual confounding.
Limitations include the cross-sectional design not allowing to infer causal relationship or to determine the directionality of the observed effects. Also, residual confounding despite extensive adjustment cannot be completely ruled out. Our findings are also limited to the observed population (white, affluent young and healthy) with its specific characteristics including usually healthy nutritional habits. Further, the use of single spot-urine after an overnight fast, rather than repeated measurements or the gold-standard of 24-h collection may have influenced our results for albuminuria. Fourth, none of the results were adjusted for multiple testing, given a clear, predefined hypothesis and to avoid potential over-adjustment because of significant correlations between individual fatty acids. Lastly, we do not have data on the source of n-3 PUFAs or the number of individuals taking supplements. The latter is likely to be very low given the relatively low levels of the n-3 concentrations measured, as also shown in a study by Shen et al. (44) linking measured Omega-3 Index levels to self-reported fatty acid intake. Overall, detailed information on the n-3 PUFA source is probably not of relevance to the association, with the Omega-3 Index representing an active biologic analog (independent of data on the form and frequency of intake).
In conclusion, a higher Omega-3 Index was significantly associated with a lower ACR in this cohort of young healthy individuals with preserved GFR. In addition to cardiovascular preventive properties, Omega-3 fatty acids may have a protective effect on early kidney injury. The supplementation and/or higher intake of n-3 PUFAs may provide a well-tolerated, cost-effective approach for the prevention of chronic kidney disease—another established cardiovascular risk factor with its own substantial burden of disease and socioeconomic footprint.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Cantonal Ethics Commission of Zurich, Zurich, Switzerland. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MF, DC, and JB contributed to the conception or design of the work. All authors contributed to the acquisition, analysis, or interpretation of data for the work. MF drafted the manuscript. SA, SR, MRe, DC, and JB critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
The GAPP study was supported by the Liechtenstein Government, the Swiss Heart Foundation, the Swiss Society of Hypertension, the University of Basel, the University Hospital Basel, the Hanela Foundation, Schiller AG and Novartis. The Swiss National Science Foundation supported the current sub-study (grant no. 310030_144152/1 to JB) as well as the Swiss Heart Foundation, the Foundation Kardio, Baden, Switzerland and the Cantonal Hospital of Baden. DC holds a McMaster University Department of Medicine Mid-Career Research Award.
Conflict of Interest
JB has received research and educational grants and honoraria from Amgen, Bayer HealthCare, Boehringer Ingelheim, Daiichi Sankyo, MARS, Inc. and Novartis outside this study. DC received honoraria from Servier, Canada, outside of this study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.622619/full#supplementary-material
References
1. Holy EW, Forestier M, Richter EK, Akhmedov A, Leiber F, Camici GG, et al. Dietary α-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler Thromb Vasc Biol. (2011) 31:1772–80. doi: 10.1161/ATVBAHA.111.226118
2. Wang Q, Liang X, Wang L, Lu X, Huang J, Cao J, et al. Effect of omega-3 fatty acids supplementation on endothelial function: a meta-analysis of randomized controlled trials. Atherosclerosis. (2012) 221:536–43. doi: 10.1016/j.atherosclerosis.2012.01.006
3. Nestel P, Clifton P, Colquhoun D, Noakes M, Mori TA, Sullivan D, et al. Indications for Omega-3 long chain polyunsaturated fatty acid in the prevention and treatment of cardiovascular disease. Heart Lung Circ. (2015) 24:769–79. doi: 10.1016/j.hlc.2015.03.020
4. Filipovic MG, Aeschbacher S, Reiner MF, Stivala S, Gobbato S, Bonetti N, et al. Whole blood omega-3 fatty acid concentrations are inversely associated with blood pressure in young, healthy adults. J Hypertens. (2018) 36:1548–54. doi: 10.1097/HJH.0000000000001728
5. Pun PH. The interplay between chronic kidney disease, sudden cardiac death, and ventricular arrhythmias. Adv Chronic Kidney Dis. (2014) 21:480–8. doi: 10.1053/j.ackd.2014.06.007
6. Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. (2015) 3:514–25. doi: 10.1016/S2213-8587(15)00040-6
7. Baggio B, Musacchio E, Priante G. Polyunsaturated fatty acids and renal fibrosis: pathophysiologic link and potential clinical implications. J Nephrol. (2005) 18:362–7.
8. Hagiwara S, Makita Y, Gu L, Tanimoto M, Zhang M, Nakamura S, et al. Eicosapentaenoic acid ameliorates diabetic nephropathy of type 2 diabetic KKAy/Ta mice: involvement of MCP-1 suppression and decreased ERK1/2 and p38 phosphorylation. Nephrol Dial Transplant. (2006) 21:605–15. doi: 10.1093/ndt/gfi208
9. Lauretani F, Semba RD, Bandinelli S, Miller ER, Ruggiero C, Cherubini A, et al. Plasma polyunsaturated fatty acids and the decline of renal function. Clin. Chem. (2008) 54:475–81. doi: 10.1373/clinchem.2007.095521
10. Elajami TK, Alfaddagh A, Lakshminarayan D, Soliman M, Chandnani M, Welty FK. Eicosapentaenoic and docosahexaenoic acids attenuate progression of albuminuria in patients with type 2 diabetes mellitus and coronary artery disease. J Am Heart Assoc. (2017) 6:247. doi: 10.1161/JAHA.116.004740
11. Conen D, Schön T, Aeschbacher S, Paré G, Frehner W, Risch M, et al. Genetic and phenotypic determinants of blood pressure and other cardiovascular risk factors (GAPP). Swiss Med Wkly. (2013) 143:w13728. doi: 10.4414/smw.2013.13728
12. Schacky C von. The Omega-3 Index as a risk factor for cardiovascular diseases. Prostaglandins Other Lipid Mediat. (2011) 96:94–8. doi: 10.1016/j.prostaglandins.2011.06.008
13. Harris WS, Schacky C von. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. (2004) 39:212–20. doi: 10.1016/j.ypmed.2004.02.030
14. Harris WS. The omega-3 index: clinical utility for therapeutic intervention. Curr Cardiol Rep. (2010) 12:503–8. doi: 10.1007/s11886-010-0141-6
15. William S, Harris, Jason Polreis. Measurement of the Omega-3 Index in dried blood spots. Ann Clin Lab Res. (2016) 4:4. doi: 10.21767/2386-5180.1000137
16. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. (2012) 367:20–9. doi: 10.1056/NEJMoa1114248
17. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
19. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. (2010) 375:2073–81. doi: 10.1016/S0140-6736(10)60674-5
20. Sung K-C, Ryu S, Lee J-Y, Lee SH, Cheong E, Hyun Y-Y, et al. Urine albumin/creatinine ratio below 30 mg/g is a predictor of incident hypertension and cardiovascular mortality. J Am Heart Assoc. (2016) 5:e003245. doi: 10.1161/JAHA.116.003245
21. Lee CC, Adler AI. Recent findings on the effects of marine-derived n-3 polyunsaturated fatty acids on urinary albumin excretion and renal function. Curr Atheroscler Rep. (2012) 14:535–41. doi: 10.1007/s11883-012-0279-3
22. Doyle R, Godson C, Brennan E. Promoting resolution in kidney disease: are we nearly there yet? Curr Opin Nephrol Hypertens. (2020) 29:119–27. doi: 10.1097/MNH.0000000000000558
23. Mendivil CO. Dietary fish, fish nutrients, and immune function: a review. Front Nutr. (2020) 7:617652. doi: 10.3389/fnut.2020.617652
24. Priante G, Musacchio E, Valvason C, Clari G, Bordin L, Sartori L, Baggio B. Further insights about the beneficial effects of n-3 fatty acids in the early molecular events of renal fibrosis in vitro. J Nephrol. (2013) 26:652–9. doi: 10.5301/jn.5000193
25. Luetić M, Vitlov Uljević M, Mašek T, Benzon B, Vukojević K, Filipović N. PUFAs supplementation affects the renal expression of pannexin 1 and connexins in diabetic kidney of rats. Histochem Cell Biol. (2019) 153:165–75. doi: 10.1007/s00418-019-01838-9
26. Vitlov Uljević M, Starčević K, Mašek T, Bočina I, Restović I, Kević N, et al. Dietary DHA/EPA supplementation ameliorates diabetic nephropathy by protecting from distal tubular cell damage. Cell Tissue Res. (2019) 378:301–17. doi: 10.1007/s00441-019-03058-y
27. Innes JK, Calder PC. The differential effects of eicosapentaenoic acid and docosahexaenoic acid on cardiometabolic risk factors: a systematic review. Int J Mol Sci. (2018) 19:532. doi: 10.3390/ijms19020532
28. Li G, Chen Z, Bhat OM, Zhang Q, Abais-Battad JM, Conley SM, et al. NLRP3 inflammasome as a novel target for docosahexaenoic acid metabolites to abrogate glomerular injury. J Lipid Res. (2017) 58:1080–90. doi: 10.1194/jlr.M072587
29. Leng S, Winter T, Aukema HM. Dietary ALA, EPA and DHA have distinct effects on oxylipin profiles in female and male rat kidney, liver and serum. J Nutr Biochem. (2018) 57:228–37. doi: 10.1016/j.jnutbio.2018.04.002
30. Zhou L, Di Yao, Zhao S, Jiang Y, Lu W. Clinical characterization of serum docosahexaenoic acid and its relationship with inflammation factors in patients with diabetic nephropathy. Iran J Kidney Dis. (2018) 12:91–98.
31. Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. (2011) 58:2047–67. doi: 10.1016/j.jacc.2011.06.063
32. McLennan PL. Cardiac physiology and clinical efficacy of dietary fish oil clarified through cellular mechanisms of omega-3 polyunsaturated fatty acids. Eur J Appl Physiol. (2014) 114:1333–56. doi: 10.1007/s00421-014-2876-z
33. Reiner MF, Stivala S, Limacher A, Bonetti NR, Méan M, Egloff M, et al. Omega-3 fatty acids predict recurrent venous thromboembolism or total mortality in elderly patients with acute venous thromboembolism. J Thromb Haemost. (2017) 15:47–56. doi: 10.1111/jth.13553
34. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. (2019) 380:11–22. doi: 10.1056/NEJMoa1812792
35. Harris WS, Pottala JV, Varvel SA, Borowski JJ, Ward JN, McConnell JP. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: observations from 160,000 patients. Prostaglandins Leukot Essent Fatty Acids. (2013) 88:257–63. doi: 10.1016/j.plefa.2012.12.004
37. Ritz PP, Rogers MB, Zabinsky JS, Hedrick VE, Rockwell JA, Rimer EG, et al. Dietary and biological assessment of the Omega-3 status of collegiate athletes: a cross-sectional analysis. PLoS ONE. (2020) 15:e0228834. doi: 10.1371/journal.pone.0228834
38. Harris WS. Redefining target omega-3 index levels: the Japan Public Health Center Study. Atherosclerosis. (2018) 272:216–8. doi: 10.1016/j.atherosclerosis.2018.01.030
39. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. (2007) 369:1090–8. doi: 10.1016/S0140-6736(07)60527-3
40. Miller ER, Juraschek SP, Appel LJ, Madala M, Am Anderson C, Bleys J, Guallar E. The effect of n−3 long-chain polyunsaturated fatty acid supplementation on urine protein excretion and kidney function: meta-analysis of clinical trials. Am J Clin Nutr. (2009) 89:1937–45. doi: 10.3945/ajcn.2008.26867
41. MacIsaac RJ, Ekinci EI, Premaratne E, Lu ZX, Seah J-M, Li Y, et al. The Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation does not improve the underestimation of Glomerular Filtration Rate (GFR) in people with diabetes and preserved renal function. BMC Nephrol. (2015) 16:198. doi: 10.1186/s12882-015-0196-0
42. Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Performance of serum cystatin C versus serum creatinine as a marker of glomerular filtration rate as measured by inulin renal clearance. Clin Exp Nephrol. (2011) 15:868–76. doi: 10.1007/s10157-011-0525-y
43. Higashiyama A, Kubota Y, Marumo M, Konishi M, Yamashita Y, Nishimura K, et al. Association between serum long-chain n-3 and n-6 polyunsaturated fatty acid profiles and glomerular filtration rate assessed by serum creatinine and cystatin C levels in Japanese community-dwellers. J Epidemiol. (2015) 25:303–11. doi: 10.2188/jea.JE20140093
Keywords: omega-3-fatty acids, nutrition, prevention, population, kidney, albuminuria, albumin-creatinine ratio
Citation: Filipovic MG, Reiner MF, Rittirsch S, Irincheeva I, Aeschbacher S, Grossmann K, Risch M, Risch L, Limacher A, Conen D and Beer JH (2021) Blood Omega-3 Fatty Acids Are Inversely Associated With Albumin-Creatinine Ratio in Young and Healthy Adults (The Omega-Kid Study). Front. Cardiovasc. Med. 8:622619. doi: 10.3389/fcvm.2021.622619
Received: 28 October 2020; Accepted: 31 March 2021;
Published: 27 April 2021.
Edited by:
Philip Calder, University of Southampton, United KingdomReviewed by:
William Harris, Fatty Acid Research Institute, Inc., United StatesMatti Sakari Jauhiainen, Minerva Foundation Institute for Medical Research, Finland
Copyright © 2021 Filipovic, Reiner, Rittirsch, Irincheeva, Aeschbacher, Grossmann, Risch, Risch, Limacher, Conen and Beer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juerg H. Beer, aGFuc2p1ZXJnLmJlZXJAa3NiLmNo
 Mark G. Filipovic
Mark G. Filipovic Martin F. Reiner2,3
Martin F. Reiner2,3