
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 16 April 2021
Sec. Atherosclerosis and Vascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.619386
 Dongmei Wu1†
Dongmei Wu1† Qiuju Yang2†
Qiuju Yang2† Baohua Su3
Baohua Su3 Jia Hao1
Jia Hao1 Huirong Ma1
Huirong Ma1 Weilan Yuan4
Weilan Yuan4 Junhui Gao4
Junhui Gao4 Feifei Ding4
Feifei Ding4 Yue Xu4
Yue Xu4 Huifeng Wang1*
Huifeng Wang1* Jiangman Zhao4*
Jiangman Zhao4* Bingqiang Li2*
Bingqiang Li2*Background: Coronary artery disease (CAD) is the leading cause of death worldwide, which has a long asymptomatic period of atherosclerosis. Thus, it is crucial to develop efficient strategies or biomarkers to assess the risk of CAD in asymptomatic individuals.
Methods: A total of 356 consecutive CAD patients and 164 non-CAD controls diagnosed using coronary angiography were recruited. Blood lipids, other baseline characteristics, and clinical information were investigated in this study. In addition, low-density lipoprotein cholesterol (LDL-C) subfractions were classified and quantified using the Lipoprint system. Based on these data, we performed comprehensive analyses to investigate the risk factors for CAD development and to predict CAD risk.
Results: Triglyceride, LDLC-3, LDLC-4, LDLC-5, LDLC-6, and total small and dense LDL-C were significantly higher in the CAD patients than those in the controls, whereas LDLC-1 and high-density lipoprotein cholesterol (HDL-C) had significantly lower levels in the CAD patients. Logistic regression analysis identified male [odds ratio (OR) = 2.875, P < 0.001], older age (OR = 1.018, P = 0.025), BMI (OR = 1.157, P < 0.001), smoking (OR = 4.554, P < 0.001), drinking (OR = 2.128, P < 0.016), hypertension (OR = 4.453, P < 0.001), and diabetes mellitus (OR = 8.776, P < 0.001) as clinical risk factors for CAD development. Among blood lipids, LDLC-3 (OR = 1.565, P < 0.001), LDLC-4 (OR = 3.566, P < 0.001), and LDLC-5 (OR = 6.866, P < 0.001) were identified as risk factors. To predict CAD risk, six machine learning models were constructed. The XGboost model showed the highest AUC score (0.945121), which could distinguish CAD patients from the controls with a high accuracy. LDLC-4 played the most important role in model construction.
Conclusions: The established models showed good performance for CAD risk prediction, which can help screen high-risk CAD patients in asymptomatic population, so that further examination and prevention treatment might be taken before any sudden or serious event.
Ischemic heart disease (IHD) is the leading cause of death worldwide according to World Health Organization statistics, and its incidence has been increasing over decade (1). IHD pathophysiological mechanisms (2) are mainly involved in atherosclerosis, coronary microvascular dysfunction, inflammation, and vasospasm. Coronary artery disease (CAD) caused by atherosclerosis is the main cause of IHD. Atherosclerosis is a slowly progressing disease, which might be revealed by typical symptoms such as angina pectoris, or an acute event, including sudden death, without any preceding symptoms (3). Hence, it is crucial to screen population at high risk of CAD, and further medical tests, lifestyle changes, or preventive treatment should be proposed before potentially fatal events occur. So far, coronary angiography is the gold standard for CAD diagnosis, but it is an invasive method and is unpractical for universal screening.
Dyslipidemia is a well-studied risk factor for atherosclerosis (4). Numerous epidemiologic studies and randomized clinical trials have suggested that elevated low-density lipoprotein cholesterol (LDL-C) is a major cause and the target to be controlled to reduce atherosclerotic cardiovascular disease (ASCVD) risk (5–7). However, in fact, a large proportion of atherosclerosis and CAD patients have normal range of blood LDL-C level. LDL-C particles are heterogeneous and can be classified into seven subfractions according to their density and size (8). Increasing evidence indicates that small dense LDL-C (sdLDL-C) is more atherogenic than large buoyant LDL-C (9). SdLDL-C is composed of LDL-C subfraction 3 (LDLC-3) to LDLC-7, and large buoyant LDL-C (lbLDL-C) is composed of LDLC-1 and LDLC-2. A few scattered studies with small sample size have reported LDL-C subfractions' role on CAD. For example, Chaudhary et al. reported that lbLDL-C was negatively correlated with CAD severity (10). In addition, elevated LDLC-4 level was associated with severe CAD in their research, which recruited 179 consecutive patients with suspected CAD, and LDLC-4 was considered as an independent predictive factor for severe CAD based on the result of multivariate analysis (10). Yet there is still lack of substantial studies to elaborate the effects of detailed LDL-C subfractions (LDLC-1 to LDLC-7) on CAD development and its severity.
In this study, we described the blood lipid profile, including LDL-C subfractions, of CAD patients and controls. Then, risk factors of CAD development and severity were analyzed, and predicted models were constructed to assess CAD risk by machine learning method.
A total of 356 newly diagnosed CAD patients and 164 non-CAD controls were recruited (Table 1) from the Department of Cardiovascular Medicine, the General Hospital of Tisco Affilated to Shanxi Medical University, the First People's Hospital of Pingdingshan, and Mianxian Hospital. The inclusion criterion in this study was suspected patients who underwent coronary angiography. And the exclusion criteria were as follows: (1) having a history of severe cardiovascular diseases such as CAD and stroke, (2) having received lipid-lowering therapy, and (3) inability to understand the research aims of this study. When patients were admitted to the hospitals, essential and clinical information were collected, including age, gender, body mass index (BMI), smoking and drinking history, and disease history such as hypertension, diabetes mellitus, and so on. Hypertension is defined as a systolic blood pressure of 140 mm Hg or more or a diastolic blood pressure of 90 mm Hg or more, or taking antihypertensive medication. Diabetes mellitus was evaluated as follows: a previous diagnosis or typical clinical symptoms of diabetes mellitus with random plasma glucose ≥11.1 mmol/L and/or fasting plasma glucose ≥7.0 mmol/L and/or 2-h plasma glucose after 75-g oral glucose tolerance test ≥11.1 mmol/L.
The CAD patients were diagnosed by coronary angiography, which were divided into two groups including non-obstructive CAD and significant CAD. Significant CAD is defined as coronary artery stenosis ≥50% in at least one main vessel or its major branches. Non-obstructive CAD was defined as visible plaque resulting in <50% luminal stenosis. The Gensini score method was used to evaluate the severity of CAD (11, 12). Gensini score of CAD patients was calculated according to coronary angiography result (12). The controls were diagnosed by coronary angiography without any luminal stenosis or plaque in main vessels and branches.
Blood samples were collected after overnight fasting from all participants before taking coronary angiography and concomitant medications. Then, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), LDL-C, serum creatinine (SCr), and other routine detection indexes were tested in the Department of Clinical Laboratory. After having been immediately separated, plasma was subjected to 800×g centrifugation for 10 min at 4°C. LDL-C subfractions were then classified and quantified by a well-established method, namely, Quantimetrix Lipoprint LDL System (8) (Quantimetrix Corporation, Redondo Beach, CA, USA) according to the manufacturer's instructions. Briefly, the plasma mixed with liquid loading gel was added to the top of precast 3% polyacrylamide gel tubes. After 30 min of photopolymerization at room temperature, samples were electrophoresed for 1 h. Then, densitometry was performed at 610 nm.
Statistical analyses were performed using SPSS 19.0 (IBM, NY, USA). Differences of categorical variables in distribution between groups were assessed with χ2 or the Fisher exact test, as appropriate. Differences of continuous variables among groups were compared by Mann–Whitney U or Kruskal–Wallis H test. Correlation analysis was conducted by the Pearson correlation method. Graphical plots were generated using GraphPad Prism 6.0 software (La Jolla, CA, USA) and R Project. Logistic regression (LR) analysis was performed to investigate the risk factors of CAD risk by SPSS software. P < 0.05 was considered statistically significant. We used Python version 3.7 as the basic language of the whole model and call numpy, panda, sklearn, xgboost, and Matplotlib libraries to process and model the data. After preprocessing the data with numpy and panda, xgboost was used to analyze the importance of features according to the total gain and to select the features with higher importance. StratifiedKFold was used to divide samples into the training set and the test set. The training set was trained by k-nearest neighbors classifier, LR, the support vector machine, the decision tree (DT) model, the multilayer perceptron network, and the XGBoost model. After the training, each model was evaluated in the test set, and then the accuracy, precision, recall, F1 value, and AUC score of each model were calculated, respectively. The receiver operating characteristic (ROC) curve and precision recall curve (PRC) of each model were drawn to present the judgment ability of the model.
Among 356 CAD patients, 57 and 299 patients were divided into non-obstructive and significant CAD patients, respectively (Table 1). The distribution of coronary artery stenosis severity and location are shown in Figure 1A, from which we can see left anterior descending arteries develop the most frequent stenosis (67.7%) comparing to other vessels. The peak of severity distribution is 76–90%. We also calculated Gensini score of 356 CAD patients, which is shown in Figure 1B.
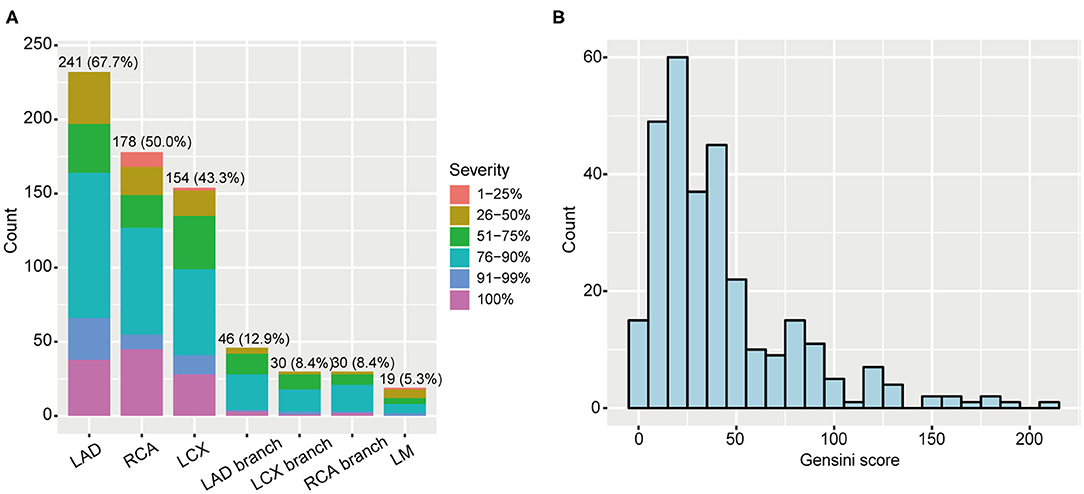
Figure 1. Coronary artery stenosis severity of 356 CAD patients. (A) Distribution of stenosis severity and locations. (B) Gensini score distribution in CAD patients. CAD, coronary artery disease; LAD, left anterior descending artery; RCA, right coronary artery; LCX, left circumflex artery; LM, left main.
Table 1 shows the clinical information of 164 controls and 356 first-visit CAD patients. We found that controls and non-obstructive CAD patients had comparative gender ratio, but significant CAD patients had significantly higher ratio of male patients (74.58%). Besides, we also found CAD patients had advantageous distribution in hypertension (P < 0.0001), diabetes mellitus (P < 0.0001), and smoking (P < 0.0001) and drinking history (P = 0.028) and had elder age (P = 0.004) and higher BMI (P < 0.0001) than those in controls.
Table 1 shows the abnormal ratio of blood lipids among controls and non-obstructive and significant CAD patients. We found that the abnormal ratios of total LDL-C were only 10.53 and 13.38% in the two groups of CAD patients, which verified that the blood LDL-C levels in a large proportion of CAD patients were in normal ranges. Besides, after comparing with other LDL-C subfractions, LDLC-3 and LDLC-4 have a higher positive rate in non-obstructive (84.21, 84.21%) and significant CAD (74.25, 82.94%) patients.
We also compared blood lipid levels among controls and non-obstructive and significant CAD patients, and the results are shown in Figures 2, 3. Surprisingly, there is no significant difference of LDL-C (Figure 2D) between controls and CAD patients. TG is significantly higher (P < 0.0001, Figure 2B) and HDL-C (P < 0.0001, Figure 2C) is lower in CAD patients than in controls. Figure 3 shows that LDLC-3, LDLC-4, LDLC-5, LDLC-6, and sdLDL-C are significantly higher in CAD patients (P < 0.0001), but no significant difference is found between non-obstructive and significant CAD patients. LDLC-1 is higher in controls than those in the two groups of CAD patients, which indicated that LDLC-1 played a protective role in CAD development.
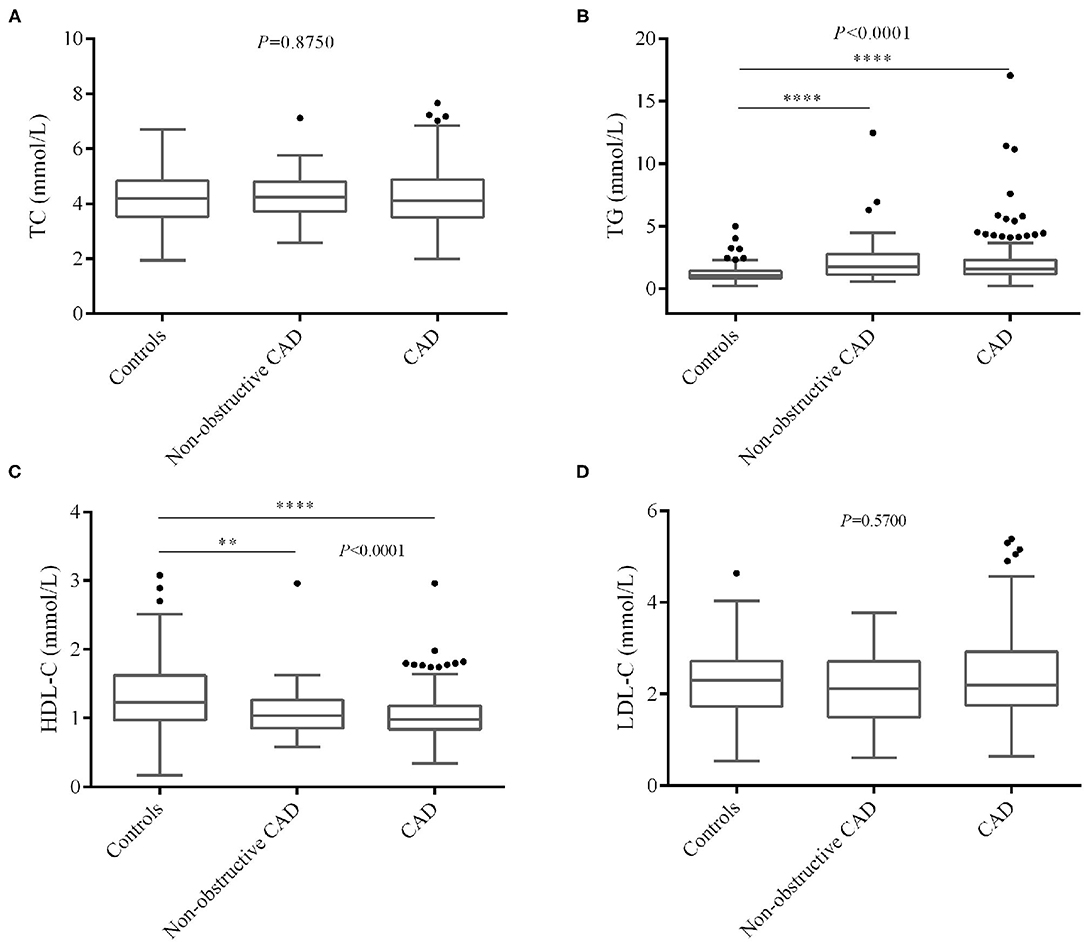
Figure 2. Comparison of blood lipids level among controls and non-obstructive and significant CAD patients. (A) TC, (B) TG, (C) HDL-C, (D) LDL-C. CAD, coronary artery disease; TC, total cholesterol; TG, triglyceride, HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
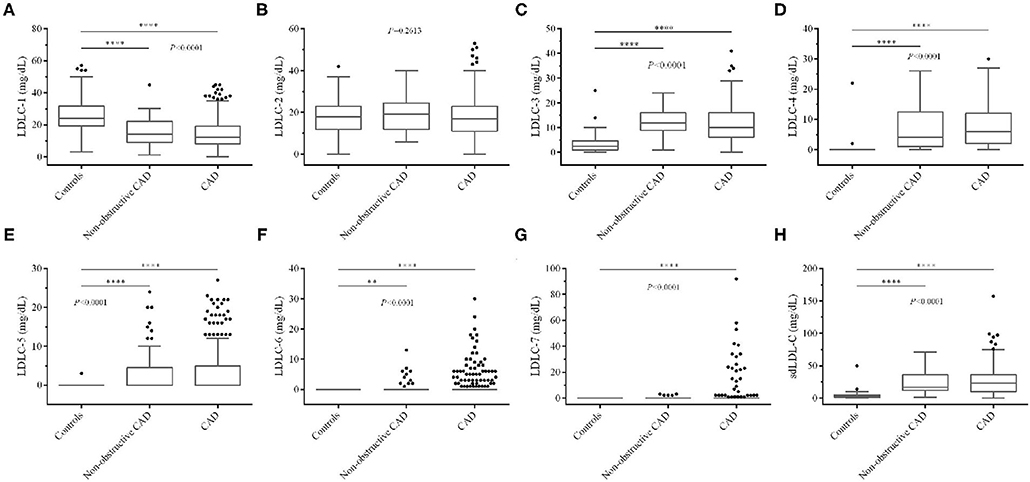
Figure 3. Comparison of LDL-C subfraction levels among controls and non-obstructive and significant CAD patients. (A) LDLC-1, (B) LDLC-2, (C) LDLC-3, (D) LDLC-4, (E) LDLC-5, (F) LDLC-6, (G) LDLC-7, (H) sdLDL-C. LDL-C, low-density lipoprotein cholesterol; CAD, coronary artery disease. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Pearson correlation analysis was performed among clinical factors and blood lipids, which is shown in Figure 4. Based on our analyses, LDL-C is strongly positively correlated with TC level (r = 0.82, P < 0.001). HDL-C is negatively correlated with TG (r = −0.31, P < 0.001), LDLC-3 (r = −0.18, P < 0.001), LDLC-4 (r = −0.22, P < 0.001), and sdLDL-C (r = −0.23, P < 0.001). A large number of studies have demonstrated that sdLDL-C is negatively correlated with HDL-C concentration (13–15). In our study, we found LDLC-1 is negatively correlated with LDLC-3 (r = −0.32, P < 0.001), LDLC-4 (r = −0.5, P < 0.001), LDLC-5 (r = −0.47, P < 0.001), LDLC-6 (r = −0.33, P < 0.001), LDLC-7 (−0.2, P < 0.001), sdLDL-C (r = −0.52, P < 0.001), and SCr (r = −0.11, P < 0.01). We also performed linear correlation analysis between Gensini score of CAD patients and aforementioned blood lipids, and HDL-C was negatively correlated with Gensini score (r = −0.178, P = 0.001). However, no significant correlation was found in other blood lipids.
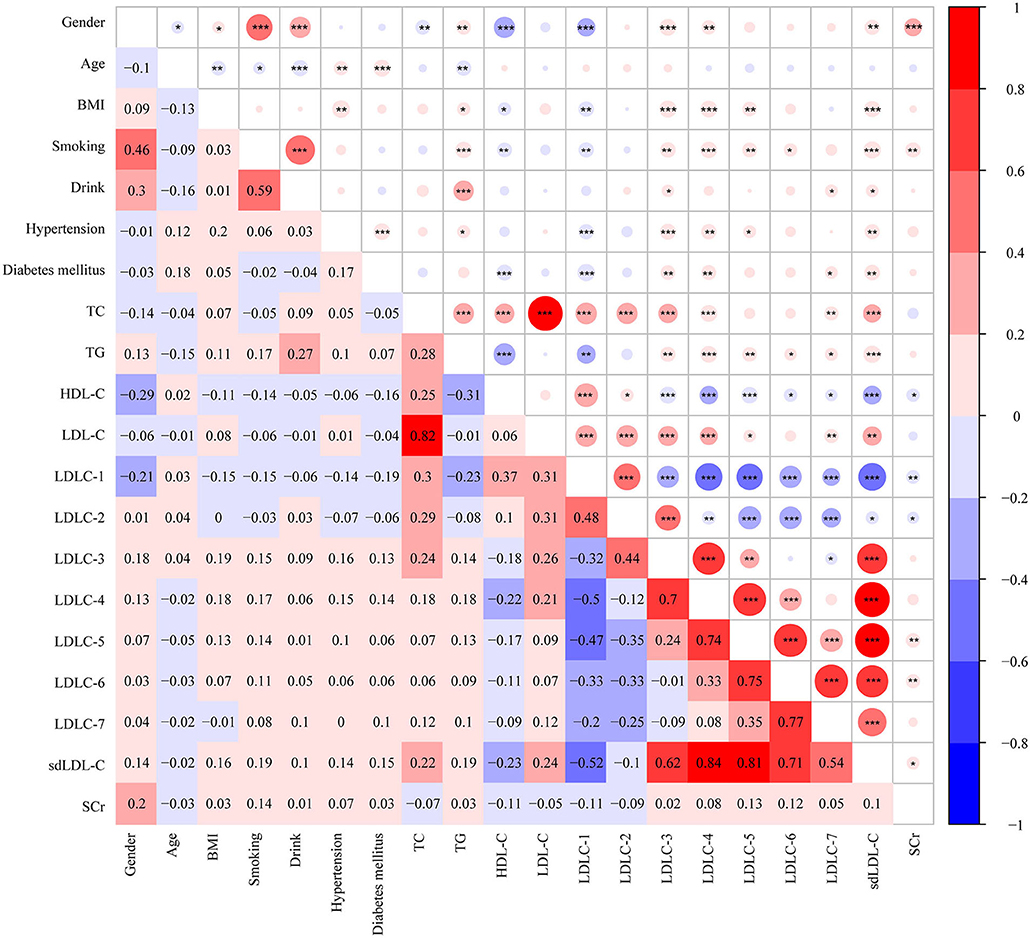
Figure 4. Heatmap showing the correlation among clinical characters and blood markers. The value in grids is Pearson correlation coefficient (Pearson r), which is also marked by colors. *P <0.05, **P < 0.01, ***P < 0.001. BMI, body weight index; TC, total cholesterol; TG, triglyceride, HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; sdLDL-C, small and dense LDL-C; SCr, serum creatinine.
Table 2 shows the results of LR analysis between the subject's factors and CAD risk. For clinical factors, male [odds ratio (OR) = 2.875, P < 0.001], older age (OR = 1.018, P = 0.025), BMI (OR = 1.157, P < 0.001), smoking (OR = 4.554, P < 0.001), drink (OR = 2.128, P = 0.016), hypertension (OR = 4.453, P < 0.001), and diabetes mellitus (OR = 8.776, P < 0.001) are identified as CAD risk factors. For blood lipids, LDLC-3 (OR = 1.565, P < 0.001), LDLC-4 (OR = 3.566, P < 0.001), and LDLC-5 (OR = 6.866, P < 0.001) are identified as risk factors for CAD development.
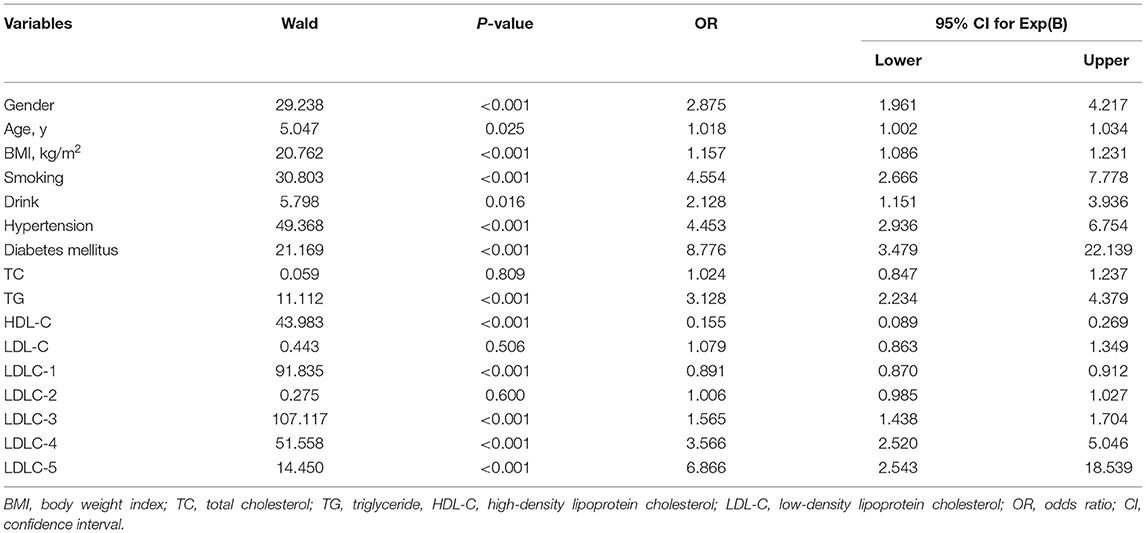
Table 2. Logistic regression analysis for independent association between subjects' variables and the risk of CAD.
One hundred sixty-four controls and 356 CAD patients were grouped, and six models were established to predict CAD risk by machine learning algorithms. The following features were selected according to their importance including LDLC-4, HDL-C, BMI, smoking history, LDLC-3, LDLC-1, hypertension history, age, TG, LDLC-2, SCr, LDL-C, TC, sdLDL-C, diabetes mellitus history, gender, and drinking history, which are shown in Figure 5A. Among all features, LDLC-4 played the most important role in model construction. Table 3 shows the performance of six predictive models. To be specific, LR model is better in accuracy, precision, and F1_score, with the values of 0.880769, 0.920108, and 0.911995, respectively. The DT model gets the highest recall (0.929812). Figure 5B shows the ROC curves of six models, and XGboost has the highest AUC score (0.945121).
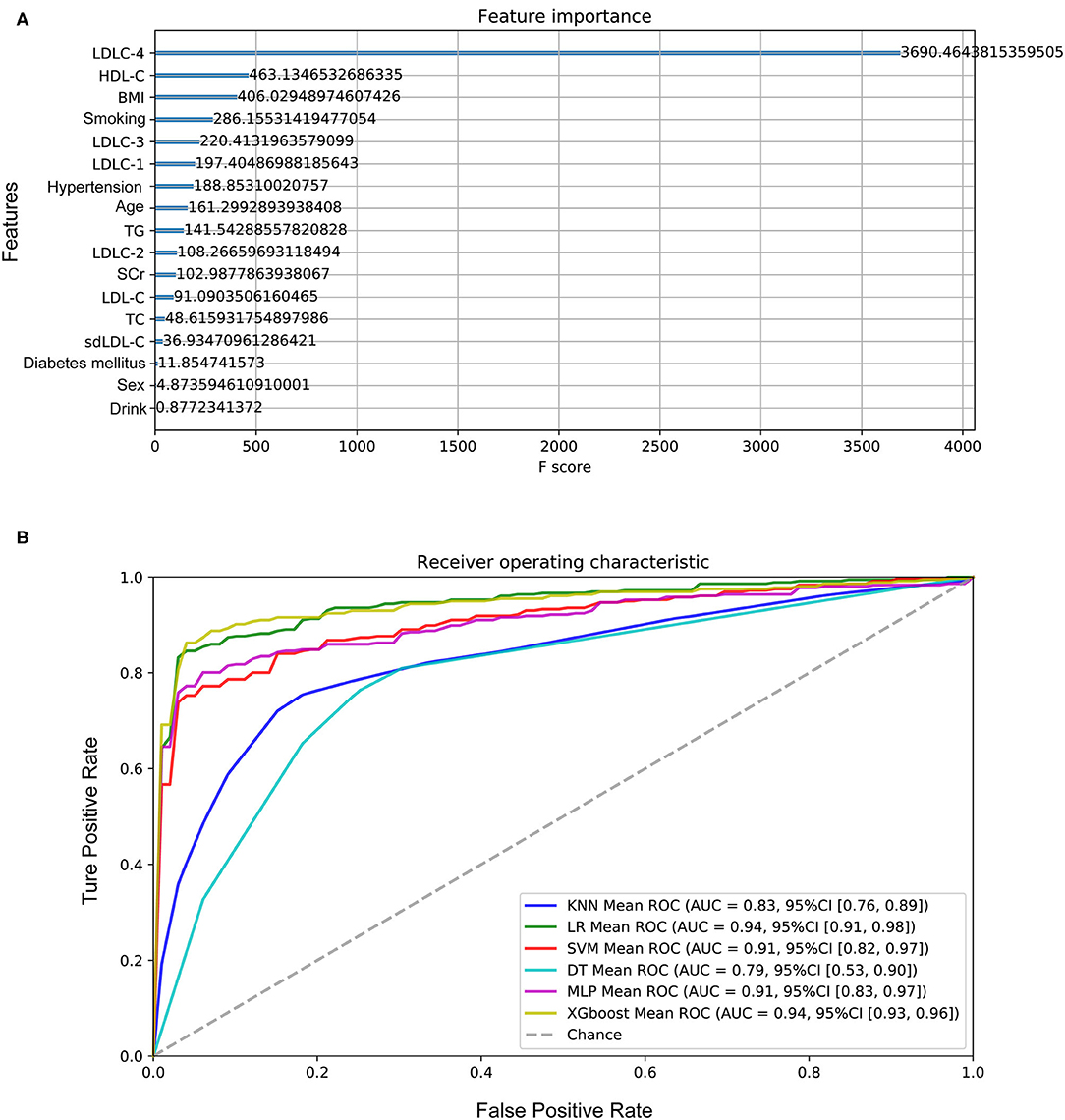
Figure 5. Performance of the models to predict CAD development. (A) The importance of features in model construction, including clinical features and laboratory indexes. (B) The ROC curves of six models. CAD, coronary artery disease; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BMI, body weight index; TG, triglyceride; SCr, serum creatinine; TC, total cholesterol; sdLDL-C, small and dense LDL-C.
To further investigate whether CAD severity could be distinguished, we applied similar strategies to establish models between non-obstructive CAD and significant CAD patients. However, the AUC score of the most optimal model in the test set was lower than 0.75, which is not practicable for CAD severity predicted.
In this study, we recruited 356 consecutive CAD patients and a group of controls, which were diagnosed with coronary angiogram. First, we analyzed the correlation between blood lipid profile and CAD risk and found LDLC-3 to LDLC-6, as well as TG, were significantly higher in CAD patients. Total LDL-C showed no significant difference between controls and CAD patients. Several studies reported that LDL-C concentration is not always high in patients with acute coronary syndrome (16–18). LDL-C consists of heterogeneous mixture of particles with different density and size, and sdLDL-C is more atherogenic than lbLDL-C (19, 20). The mechanisms behind this phenomenon may include easier transfer from the vessel lumen into the subintimal space (21), higher affinity to proteoglycans (22), decreased binding ability to LDL-C receptors (23), and increased plasma residence time (24), compared with lbLDL-C.
Belonging to lbLDL-C, LDLC-1 level was relatively lower in CAD patients compared with that in controls, which strengthen the hypothesis that lbLDL-C is a protective factor against CAD development. Chaudhary et al. reported that lbLDL-C was negatively correlated with CAD severity (10). In addition, some other studies also focused on the effects of lbLDL-C on metabolic diseases. For example, Srisawasdi and colleagues' study proposed the ratio of sdLDL-C to lbLDL-C as an excellent biomarker for evaluating lipid metabolic status in patients with metabolic syndrome (25). Chen et al. reported that sdLDL-C/lbLDL-C ratio was associated with glucose metabolic status in pregnancy (26). Interestingly, we also found LDLC-1 was negatively correlated with LDLC-3 to LDLC-7. LDL-C is routinely considered as a major cause of ASCVD risk. However, in the present study, no significant difference was observed in LDL-C levels between controls and CAD groups, and LDL-C was not identified as a significant risk factor for CAD development by LR analysis. A possible explanation could be that the effects of total LDL-C are neutralized by sdLDL-C and lbLDL-C. The possible factors impacting on lipoprotein cholesterol size are genetic background (27, 28) and dietary habits (29–31), but the current evidences were weak. It has great clinical significance on lipid-lowering therapy to explore the mechanisms affecting the size of LDL-C particles.
Silent ischemia occurs in about 20–40% of patients with unstable and stable coronary syndromes (32), and the first clinical manifestation in some cases is sudden events. Thus, we investigated the feasibility of predicting CAD risk by machine learning method. Among six models, XGboost (AUC score = 0.945121) and LR (AUC score = 0.944622) models presented excellent performance for predicting CAD development. Previous researchers (3, 33) had made a lot of efforts to assess cardiovascular risk based on known multiple risk factors. The dominant strategy was calculating the total risk score of a person by summing the risk imparted by each of the major risk factors, for example, Framingham risk score and its improved version (34, 35). By comparison, our study has some advantages. First, we employed a machine learning method, which has superiority on algorithms compared with accumulated risk scores. It could analyze diverse data types (e.g., demographic data, clinical data, laboratory data) and incorporate these features according to their weightiness of information gain into predicted models for disease risk, diagnosis, prognosis, and appropriate treatment (36). In addition, we not only included classical risk factors, but also explored and selected novel marker LDL-C subfractions. Despite that the recent study published by Sánchez-Cabo et al. (37) also used machine learning to cardiovascular risk definition, sdLDL-C or LDL-C subfractions were not considered. Our results showed that LDLC-4 played the most important role on models' establishment, which made a great contribution for better performance of our models than other studies.
Despite the novelties in the present study, there are some limitations worthy of statement. First, numerous patients were excluded, such as patients who received daily lipid-lowering drugs before recruitment, which might limit the generalizability of our finding to wider population. Clinically, many patients with dyslipidemia take cholesterol-reducing medications to prevent atherosclerosis, but some of them still develop CAD. Thus, the efficacy of various lipid-lowering drugs on LDL-C subfractions, especially LDLC-3 and LDLC-4, is worthy of study. Second, in this study, no significant difference was found in blood lipid profile between non-obstructive and significant CAD patients, which is not consistent with the previous study (10). As our study is a cross-sectional study, the blood lipid profile was tested only when participants were recruited. However, CAD development is a long-period progress; both lipid levels and the duration time of dyslipidemia are important factors for atherosclerosis. Thus, this inconsistency might be caused by the lack of continuous monitoring of blood lipid profile changes. Finally, it needs to be mentioned that not only our study but also previous studies came to the conclusion based on a small size of samples. It is necessary to recruit a large cohort of samples to validate these results and perform a longitudinal study with long-time follow-up to give more robust evidence.
In this study, we proved that LDL-C was insufficient to be a risk factor for CAD development. LDL-C subfractions have shown their importance to be included in the clinical test for screening population with dyslipidemia or high-risk CAD. The established models presented good performance for CAD risk prediction, and LDLC-4 played the most important role in these models, which can help screen high-risk CAD patients in asymptomatic population so that further examination and prevention treatment can be taken before sudden or serious events.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethical Committee (the General Hospital of Tisco Affilated to Shanxi Medical University, the First People's Hospital of Pingdingshan and Mianxian Hospital). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
DW funded this study. DW, QY, and BL designed project. BS, JH, HM, and HW collected samples and clinical data. WY and JZ performed experiments and analyzed data. DW and JZ wrote the manuscript. BL, HW, JG, FD, and YX revised the manuscript. All authors read and approved the final manuscript.
This study was funded by Research Project of Shanxi Health and Family Planning Commission (No. 2017142) and Major Projects of Special Development Funds in Zhangjiang National Independent Innovation Demonstration Zone, Shanghai (ZJ2017-ZD-012).
WY, JG, FD, YX, and JZ were employed by the company Shanghai Biotecan Pharmaceuticals Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.619386/full#supplementary-material
CAD, coronary artery disease; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; SCr, serum creatinine; LR, logistic regression; MLP, the multilayer perceptron network; DT, the decision tree model; SVM, a support vector machine; KNN, k-nearest neighbors classifier; OR, odds ratio; AUC, area under curve.
1. National Center for Cardiovascular Diseases. Report on Cardiovascular Diseases in China 2018. Encyclopedia of China Publishing House. Beijing (2018). p. 123–30.
2. Severino P, D'Amato A, Pucci M, Infusino F, Adamo F, Birtolo LI, et al. Ischemic heart disease pathophysiology paradigms overview: from plaque activation to microvascular dysfunction. Int J Mol Sci. (2020) 21:8118. doi: 10.3390/ijms21218118
3. Degrell P, Sorbets E, Feldman LJ, Steg PG, Ducrocq G. Screening for coronary artery disease in asymptomatic individuals: why and how? Arch Cardiovasc Dis. (2015) 108:675–82. doi: 10.1016/j.acvd.2015.10.001
4. Kullo IJ, Ballantyne CM. Conditional risk factors for atherosclerosis. Mayo Clin Proc. (2005) 80:219–30. doi: 10.4065/80.2.219
5. Expert Panel on Detection, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
6. Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). Eur Heart J. (2007) 28:2375–414. doi: 10.1093/eurheartj/ehm316
7. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. (2016) 37:2999–3058. doi: 10.1093/eurheartj/ehw272
8. Hoefner DM, Hodel SD, O'Brien JF, Branum EL, Sun D, Meissner I, et al. Development of a rapid, quantitative method for LDL subfractionation with use of the Quantimetrix Lipoprint LDL system. Clin Chem. (2001) 47:266–74.
9. Hidenori Arai YK, Watanabe M, Sawamura T, Ito Y, Minagawa A, Okamura T, et al. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban japanese cohort: the suita study. J Atheroscler Thromb. (2013) 20: 195–203. doi: 10.5551/jat.14936
10. Chaudhary R, Mathew D, Bliden K, Tantry US, Sharma T, Gesheff MG, et al. Low-density lipoprotein 4: a novel predictor of coronary artery disease severity. Curr Med Res Opin. (2017) 33:1979–84. doi: 10.1080/03007995.2017.1365052
11. Yang B, Yan S, Yan J, Li Y, Khurwolah MR, Wang L, et al. A study of the association of rs12040273 with susceptibility and severity of coronary artery disease in a Chinese Han population. BMC Cardiovasc Disord. (2018) 18:10. doi: 10.1186/s12872-018-0743-2
12. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. (1983) 51:606.
13. King RI, Florkowski CM, Yeo J, Walmsley TA, Shand BI, Scott RS, et al. What is the best predictor of the atherogenic LDL subclass phenotype 'pattern B' in patients with type 2 diabetes mellitus? Ann Clin Biochem. (2011) 48:166–9. doi: 10.1258/acb.2010.010185
14. Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PW, et al. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. (2006) 113:20–9. doi: 10.1161/CIRCULATIONAHA.105.567107
15. Rizzo M, Kotur-Stevuljevic J, Berneis K, Spinas G, Rini GB, Jelic-Ivanovic Z, et al. Atherogenic dyslipidemia and oxidative stress: a new look. Transl Res. (2009) 153:217–23. doi: 10.1016/j.trsl.2009.01.008
16. Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol. (2009) 54:293–302. doi: 10.1016/j.jacc.2009.04.033
17. Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation. (2004) 110:1061–8. doi: 10.1161/01.CIR.0000140261.58966.A4
18. Tashiro J, Miyazaki O, Nakamura Y, Miyazaki A, Fukamachi I, Bujo H, et al. Plasma pre beta1-HDL level is elevated in unstable angina pectoris. Atherosclerosis. (2009) 204:595–600. doi: 10.1016/j.atherosclerosis.2008.10.015
19. Grammer TB, Kleber ME, Marz W, Silbernagel G, Siekmeier R, Wieland H, et al. Low-density lipoprotein particle diameter and mortality: the Ludwigshafen Risk and Cardiovascular Health Study. Eur Heart J. (2015) 36:31–8. doi: 10.1093/eurheartj/ehu055
20. Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid Med Cell Longev. (2017) 2017:1273042. doi: 10.1155/2017/1273042
21. Bjornheden T, Babyi A, Bondjers G, Wiklund O. Accumulation of lipoprotein fractions and subfractions in the arterial wall, determined in an in vitro perfusion system. Atherosclerosis. (1996) 123:43–56. doi: 10.1016/0021-9150(95)05770-6
22. Hurt E, Camejo G. Effect of arterial proteoglycans on the interaction of LDL with human monocyte-derived macrophages. Atherosclerosis. (1987) 67:115–26. doi: 10.1016/0021-9150(87)90272-3
23. Campos H, Arnold KS, Balestra ME, Innerarity TL, Krauss RM. Differences in receptor binding of LDL subfractions. Arterioscler Thromb Vasc Biol. (1996) 16:794–801. doi: 10.1161/01.atv.16.6.794
24. Miller BD, Alderman EL, Haskell WL, Fair JM, Krauss RM. Predominance of dense low-density lipoprotein particles predicts angiographic benefit of therapy in the Stanford Coronary Risk Intervention Project. Circulation. (1996) 94:2146–53. doi: 10.1161/01.cir.94.9.2146
25. Srisawasdi P, Vanavanan S, Rochanawutanon M, Kruthkul K, Kotani K, Kroll MH. Small-dense LDL/large-buoyant LDL ratio associates with the metabolic syndrome. Clin Biochem. (2015) 48:495–502. doi: 10.1016/j.clinbiochem.2015.01.011
26. Chen Y, Du M, Xu J, Chen D. The small dense LDL particle/large buoyant LDL particle ratio is associated with glucose metabolic status in pregnancy. Lipids Health Dis. (2017) 16:244. doi: 10.1186/s12944-017-0627-y
27. Alena Stančáková JP, Soininen P, Kangas AJ, Bonnycastle LL, Morken MA, Collins FS, et al. Effects of 34 risk loci for type 2 diabetes or hyperglycemia on lipoprotein subclasses and their composition in 6,580 nondiabetic Finnish men Diabetes. (2011) 60:1608–16. doi: 10.2337/db10-1655/-/DC1
28. Kim M, Yoo HJ, Kim M, Kim J, Baek SH, Song M, et al. EPHA6 rs4857055 C > T polymorphism associates with hypertension through triglyceride and LDL particle size in the Korean population. Lipids Health Dis. (2017) 16:230. doi: 10.1186/s12944-017-0620-5
29. Manninen SM, Lankinen MA, de Mello VD, Laaksonen DE, Schwab US, Erkkila AT. Intake of fatty fish alters the size and the concentration of lipid components of HDL particles and camelina sativa oil decreases IDL particle concentration in subjects with impaired glucose metabolism. Mol Nutr Food Res. (2018) 62:e1701042. doi: 10.1002/mnfr.201701042
30. Rundblad A, Holven KB, Ottestad I, Myhrstad MC, Ulven SM. High-quality fish oil has a more favourable effect than oxidised fish oil on intermediate-density lipoprotein and LDL subclasses: a randomised controlled trial. Br J Nutr. (2017) 117:1291–8. doi: 10.1017/S0007114517001167
31. Nathalie Bergeron SC, Williams PT, King SM, Krauss RM. Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: a randomized controlled trial. Am J Clin Nutr. (2019) 110:24–33. doi: 10.1093/ajcn/nqz035
32. Park HB, Jeong H, Lee JH, Suh Y, Hwang ES, Cho YH, et al. Predictors of severe or moderate coronary artery disease in asymptomatic individuals with extremely low coronary calcium scores. Yonsei Med J. (2019) 60:619–25. doi: 10.3349/ymj.2019.60.7.619
33. Grundy RPSM, Greenland P, Smith Jr S, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology Circulation. (1999) 100:1481–92.
34. Ankle Brachial Index C, Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. (2008) 300:197–208. doi: 10.1001/jama.300.2.197
35. Ko DT, Sivaswamy A, Sud M, Kotrri G, Azizi P, Koh M, et al. Calibration and discrimination of the Framingham Risk Score and the Pooled Cohort Equations. CMAJ. (2020) 192:E442–E9. doi: 10.1503/cmaj.190848
36. Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. (2019) 20:e262–e73. doi: 10.1016/s1470-2045(19)30149-4
Keywords: coronary artery disease, LDL-C subfractions, machine learning, coronary angiography, risk factors
Citation: Wu D, Yang Q, Su B, Hao J, Ma H, Yuan W, Gao J, Ding F, Xu Y, Wang H, Zhao J and Li B (2021) Low-Density Lipoprotein Cholesterol 4: The Notable Risk Factor of Coronary Artery Disease Development. Front. Cardiovasc. Med. 8:619386. doi: 10.3389/fcvm.2021.619386
Received: 23 October 2020; Accepted: 23 February 2021;
Published: 16 April 2021.
Edited by:
Johannes A. Schmid, Medical University of Vienna, AustriaReviewed by:
Cécile Oury, University of Liège, BelgiumCopyright © 2021 Wu, Yang, Su, Hao, Ma, Yuan, Gao, Ding, Xu, Wang, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingqiang Li, YmluZ3FpYW5nLjA0MDhAMTYzLmNvbQ==; Jiangman Zhao, emhhb2ppYW5nbWFuODZAMTYzLmNvbQ==; Huifeng Wang, d2hmMTc3MDFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.