- 1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2China National Clinical Research Center for Neurological Diseases, Beijing, China
- 3Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China
- 4Beijing Key Laboratory of Translational Medicine for Cerebrovascular Disease, Beijing, China
- 5Department of Pharmacy, National Center of Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Proprotein convertase subtilisin/kexin type 9 (PCSK9), a pivotal protein in low-density lipoprotein cholesterol metabolism, has been validated to be an established target for cardiovascular (CV) risk reduction. Nevertheless, prospective studies concerning the associations between circulating PCSK9 and the risk of CV events and mortality have yielded, so far, inconsistent results. Herein, we conducted a meta-analysis to evaluate the association systemically.
Methods: Pertinent studies were identified from PubMed, EMBASE, and Cochrane Library database through July 2020. Longitudinal studies investigating the value of circulating PCSK9 for predicting major adverse cardiovascular events (MACEs) or stroke or all-cause mortally with risk estimates and 95% confidence intervals (CI) were included in the analyses. Dose-response meta-analysis was also applied to evaluate circulating PCSK9 and risk of MACEs in this study.
Results: A total of 22 eligible cohorts comprising 28,319 participants from 20 eligible articles were finally included in the study. The pooled relative risk (RR) of MACEs for one standard deviation increase in baseline PCSK9 was 1.120 (95% CI, 1.056–1.189). When categorizing subjects into tertiles, the pooled RR for the highest tertile of baseline PCSK9 was 1.252 (95% CI, 1.104–1.420) compared with the lowest category. This positive association between PCSK9 level and risk of MACEs persisted in sensitivity and most of the subgroup analyses. Twelve studies were included in dose-response meta-analysis, and a linear association between PCSK9 concentration and risk of MACEs was observed (x2 test for non-linearity = 0.31, P non-linearity = 0.575). No significant correlation was found either on stroke or all-cause mortality.
Conclusion: This meta-analysis added further evidence that high circulating PCSK9 concentration significantly associated with increased risk of MACEs, and a linear dose-response association was observed. However, available data did not suggest significant association either on stroke or all-cause mortality. Additional well-designed studies are warranted to further investigate the correlations between PCSK9 concentration and stroke and mortality.
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9), a circulating serine protease, has a fundamental role in low-density lipoprotein cholesterol (LDL-C) metabolism by enhancing the endosomal and lysosomal degradation of hepatic LDL-Receptor, thereby resulting in increased LDL-C concentration.
Over the past years, PCSK9 has been validated to be an established target for cholesterol-lowering therapies. Three randomized, double-blind, placebo-controlled cardiovascular (CV) outcome trials were completed and demonstrated that PCSK9 monoclonal antibodies significantly reduce plasma LDL-C level and major vascular events in subjects with high CV risk (1–3). The prespecified analyses designed to assess the effect of PCSK9 inhibitors on stroke demonstrated a reduction in risk of ischemic stroke (IS) without increasing hemorrhagic stroke, irrespective of baseline LDL-C and of prior IS history (4, 5). Moreover, emerging evidence has suggested that PCSK9 exerts pleiotropic effects beyond plasma LDL regulation, implying that PCSK9 might be a CV risk factor independent of LDL-C (6).
Circulating concentration of PCSK9 has attracted scientific interest as a biomarker for CV risk stratification. In recent years, mounting studies have explored the association between circulating PCSK9 and the risk of CV events; however, the results remained divergent. Werner et al. reported that elevated PCSK9 serum concentrations are correlated with CV events in patients with stable coronary artery disease (7). Nevertheless, Khoury et al. assessed the association and found that PCSK9 was inconsistently associated with CV events in two diabetes cohorts (8). In a large-scale primary prevention cohort, plasma levels of PCSK9 measured at baseline did not predict future CV events (9). Therefore, an updated meta-analysis concerning this topic was performed to improve statistical power and investigate the possible source of heterogeneity between published studies. Our meta-analysis differed from previously published meta-analyses by the inclusion of more recent studies, the inclusion of stroke as clinical outcome, exploring more potential aspects for heterogeneity sources. Accordingly, we conducted the current meta-analysis to add substantive new data and insights into the predictive ability of circulating PCSK9 level in terms of major adverse cardiovascular events (MACEs), stroke, and all-cause mortality from the eligible prospective studies.
Methods
Search Strategy and Selection Criteria
In accordance with recommendations of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group (10), we searched electronic databases (PubMed, Embase, and Cochrane) up to July 2020 using a combined MeSH heading and keyword search strategy; the query syntax of searching was shown in the Supplementary Materials (see search strategy). To avoid missing any relevant study, we also checked and manually searched the references of the included articles.
Study Selection
Studies were deemed eligible if they: (1) included participants of any age across different countries; (2) had PCSK9 levels in plasma or serum at baseline as exposure of interest; (3) had clinical outcomes including MACEs and/or stroke and/or all-cause mortality; (4) were prospective cohort studies or nested case-control studies performed within prospective cohort with a minimum follow-up of 1 year; (5)were full-text publications; (6) had a multivariable-adjusted relative risk (RR) or hazard ratio (HR) or odds ratio (OR) and the corresponding 95% confidence interval (CI) or provision of available information to calculate them. MACEs were defined as composite outcomes, including fatal and non-fatal coronary artery disease (CAD), fatal and non-fatal stroke, and heart failure. In order to better evaluate the causality between PCSK9 concentration and the clinical outcomes, we included only prospective cohort studies or prospective nested case-control studies.
Data Extraction and Quality Assessment
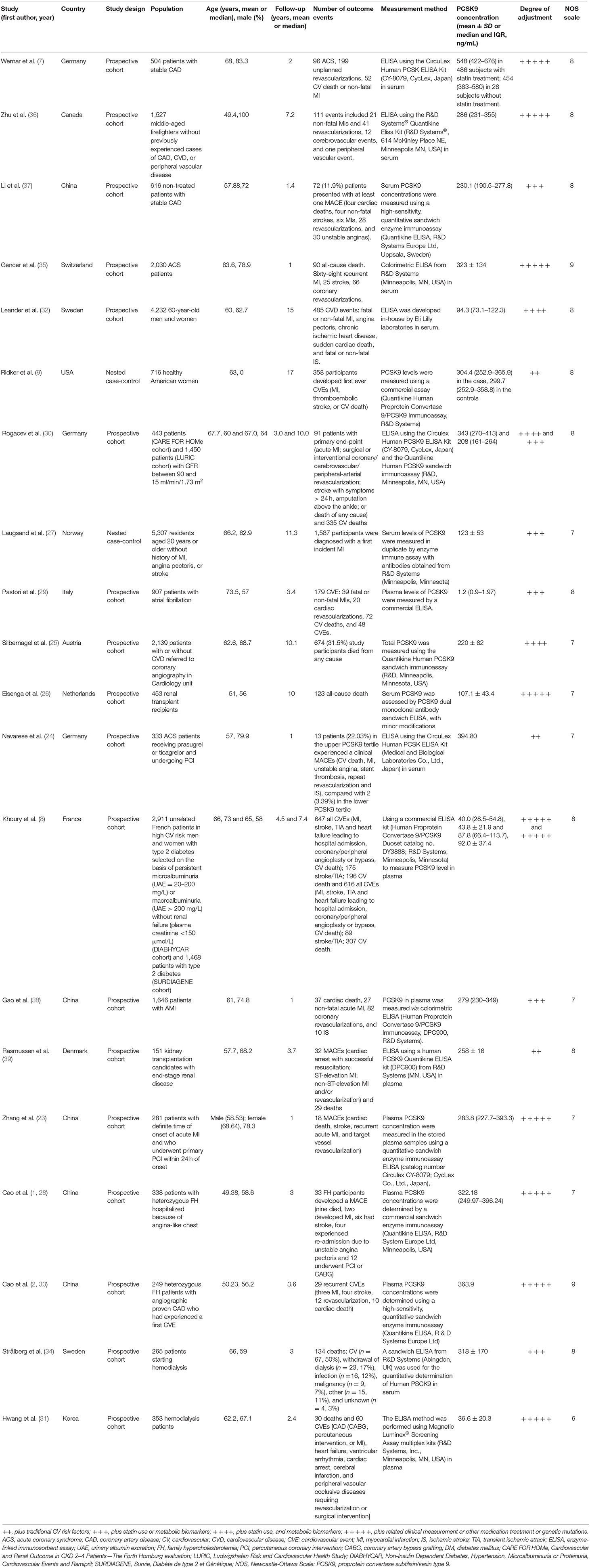
Two reviewers (YZ and WC) independently searched, selected studies and extracted data. The disagreement between the two reviewers was resolved by consensus. The following data were extracted from each study: the last name of the first author, year of publication, country of study, type and amount of participants, study type, the proportion of men, mean age, duration of follow-up, number of outcome events, CV risk status, the measurement method of PCSK9, mean or median concentration of PCSK9, sample source, adjusted confounding factors, statin use, the history of family hypercholesterolemia (FH), and the most fully adjusted HRs or RRs or ORs with 95% CIs of circulating PCSK9.
Using the Newcastle-Ottawa Scale (NOS), the quality of the included studies was assessed (11). Each study was evaluated on three broad criteria: (1) subject selection; (2) comparability of the subjects; and (3) ascertainment of the outcome or exposure. Two reviewers independently evaluated the quality of each study. Disagreements were resolved through discussion to reach a consensus. A star system ranging between zero to nine stars is used to allow a semi-quantitative assessment of study quality. Studies which scored seven points or more were considered high quality.
Statistical Analysis
The risk estimates of the association between PCSK9 and the outcomes of interest in each study were reported as a HR, RR, or OR with 95% CI. Risk estimates adjusted for the maximum number of covariates were pooled across studies for inclusion in the meta-analyses. In this meta-analysis, all associations were estimated as RRs and 95% CIs. HRs were approximately considered as RRs, which have been commonly used in previous studies (12). ORs were transformed into RRs using the formula RR = OR/[(1−P0) + (P0 × OR)] where P0 is the incidence of the outcome of interest in the non-exposed group, and OR was considered equivalent to the RR in cohort studies if the value of P0 was small (13). Both continuous (per one unit or one standard derivation (SD) increase) and categorical (tertiles or quartiles) variables of circulating PCSK9 were used in the included literature. In order to acquire a consistent comparison of the results, we transformed the RR of each study to standard risk estimates for 1-SD increase in PCSK9 levels, as well as for the highest tertile vs. lowest one for PCSK9 distribution using methods described previously (14, 15); briefly, these transformed estimates were calculated by multiplying the log RR and the upper and lower CIs for 1-SD increase with a scaling factor (2.18 for tertiles, and 2.54 for quartiles). The scaling methods assume that the PCSK9 is log-normally distributed and a log-linear association with the outcome. For normally transformed PCSK9, RR reported per unit were first converted to 1-SD increase, using the study-specific SD and then to tertiles.
Heterogeneity of RRs was evaluated by calculating the Cochrane Q statistic (P < 0.10 was deemed to be statistically significant) and the I2 statistic (low heterogeneity, I2 ≤ 50%; moderate heterogeneity, 50% < I2 < 75%; high heterogeneity, I2 ≥ 75%) (16). We pooled the RRs of the outcomes of interest using the random effects model (I2 > 50%, the DerSimonian-Laird method) or fixed effects model (I2 ≤ 50%, the Mantel-Haenszel method) as appropriate. To test the robustness of the pooled results, sensitivity analyses were conducted by leave-one-out method in each turn to investigate the influence of every single study on the overall risk estimate (17), and by excluding two nested case-control studies.
To assess the potential sources of heterogeneity, subgroup analyses which sorted by published year, mean age at baseline, sample size, CV risk, percentage of the history of FH, sample source, percentage of statin use, PCSK9 level at baseline, the assays for PCSK9 measurement, and degree of adjustment were performed. High CV risk cohort referred to participants in the studies with established CVD or known CVD risk factors (such as chronic renal disease, atrial fibrillation, type 2 diabetes, familial hypercholesterolemia, and hemodialysis) and low CV risk cohort to apparently healthy participants at baseline. Mean/median level of PCSK9 at baseline for included studies were extracted, and 258 ng/mL, the median for the 21 cohorts, was used as the cut-off point. A univariate meta-regression with restricted maximum likelihood was performed to measure if pooled RR significantly differed between each stratum analyzed.
Additionally, a dose-response meta-analysis was further conducted to determine a potential curvilinear (non-linear) or linear association between circulating PCSK9 and risk of MACEs. We used the two-stage generalized least-squares trend (GLST) estimation method proposed by Greenland and Longnecker to estimate the study-specific slope lines first and then derive an overall average slope (18, 19). This method requires the cases and cohort size/control subjects of each category and the risk estimate with its variance estimate for at least three quantitative exposure categories to be known. We excluded the studies without the aforementioned values required for the dose-response meta-analysis or without sufficient data for deriving them. The dosage value assigned to each stratum of PCSK9 was the median or mean in each category provided by the original article. In terms of the studies not containing median/mean, the midpoint was used for closed category and the same amplitude as the adjacent category for the open-ended highest or lowest category. A restricted cubic spline with three knots (two spline transformations) was first created, and then a P for non-linearity was calculated to detect potential departure from a linear trend by testing the coefficient of the second spline equal to zero. In the presence of substantial linear trends (Pnon−linearity > 0.05), a linear model was conducted to achieve the association between circulating PCSK9 and the risk of MACEs by using the method of two-stage GLST (19).
The possibility of publication bias of the outcome of MACEs was assessed graphically by funnel plots and quantitatively by Begg's rank correlation test and Egger's linear regression test (20, 21). Where asymmetry of the plot was found, a contour-enhanced funnel with the trim and fill method was further applied to differentiate asymmetry due to publication bias from that due to other factors (22). Statistical analysis was performed with STATA package, version 15.1 for Mac (StataCorp, College Station, TX, USA). P < 0.05 was considered statistically significant, except where otherwise specified.
Results
Literature Search and Study Characteristics
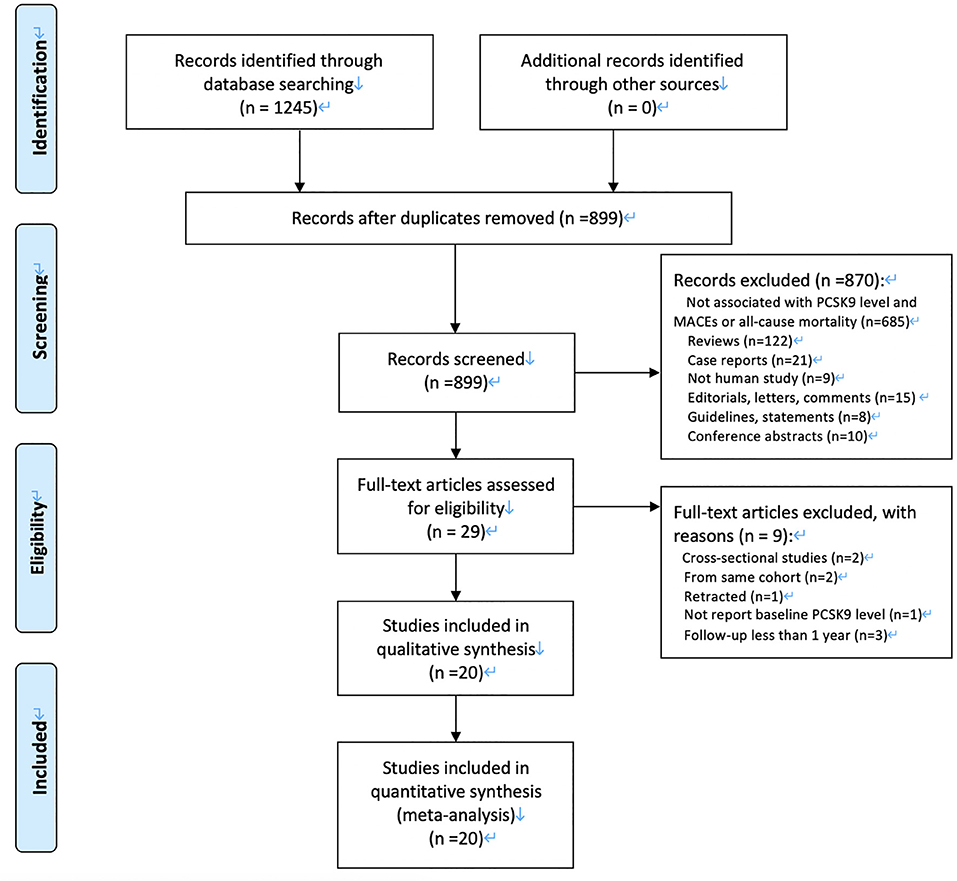
Our initial search returned 1,245 articles. After we screened titles and abstracts, 29 articles were qualified for full-text evaluation. After full-text review, nine studies were excluded, and 22 eligible cohorts from 20 eligible articles were finally included for meta-analysis (7–9, 23–39). Figure 1 demonstrates a flowchart for the study selection.
Table 1 summarizes the main characteristics of the included studies. Among the 20 articles, 19 articles were included in the analysis of MACEs, and six (8, 25, 26, 31, 34, 35) were in the all-cause mortality. The publication period of these articles ranged from 2014 to 2020, and the sample size of each study range from 151 to 5,307, with a total of 28,319 participants. Two studies were nested case-control studies, and the others were prospective cohort studies. According to quality assessment criteria, all but one studies were graded as high quality. A total of 12 studies reported risk estimates according to tertiles, two studies according to quartiles, and 16 studies according to continuous levels of PCSK9.
Association Between PCSK9 and MACEs
PCSK9 as a Continuous Variable
As shown in Figure 2A, RRs of the risk of MACEs for an increase in baseline PCSK9 by 1-SD varied from 0.89 to 2.26 across different cohorts, and a significantly positive association was found when pooling the risk estimate in a random-effect model (RR 1.120; 95% CI: 1.056–1.189; P < 0.001), with moderate heterogeneity across studies (I2 = 66.30%; Pheterogeneity < 0.001).
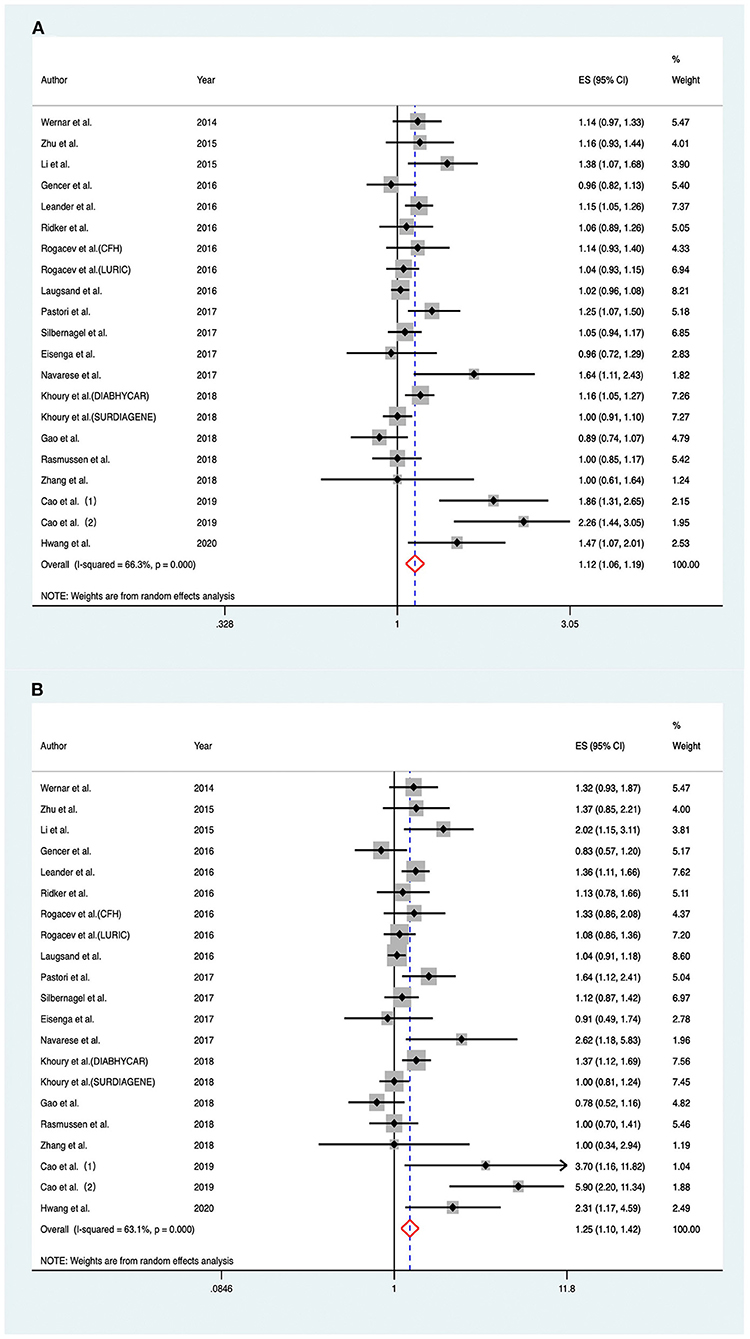
Figure 2. Associations between circulating proprotein convertase subtilisin/kexin type 9 and risk of major adverse cardiovascular events. (A) Per one standard derivation increase in baseline proprotein convertase subtilisin/kexin type 9 levels, (B) top vs. bottom tertile of baseline proprotein convertase subtilisin/kexin type 9. CFH indicates Cardiovascular and Renal Outcome in CKD 2–4 Patients—The Forth Homburg evaluation; LURIC, Ludwigshafen Risk and Cardiovascular Health Study; DIABHYCAR, Non-Insulin Dependent Diabetes, Hypertension, Microalbuminuria or Proteinuria, Cardiovascular Events and Ramipril; SURDIAGENE, Survie, Diabète de type 2 et Génétique; ES, effect size; CI, confidence intervals.
Considering the aforementioned moderate heterogeneity between the included studies, we further conducted subgroup analyses based on potential clinical relevance (Figure 3A). The positive association between PCSK9 level and risk of MACEs persisted in most of the subgroup analyses. The association was much stronger in studies with a high percentage of FH (RR 2.038; 95% CI: 1.576–2.634; P < 0.001) than those with a low percentage (RR 1.085; 95% CI: 1.035–1.138; P = 0.001); the heterogeneity was also reduced, indicating that the source of heterogeneity appeared to be contributed by the medical history of FH. High baseline PCSK9 level was only significantly associated with increased MACEs in studies with a higher degree of cofounder adjustment (RR 1.149; 95% CI: 1.057–1.248; P = 0.001) but not in those with a lower degree (RR 1.085; 95% CI: 0.994–1.189; P = 0.067).

Figure 3. Subgroup analyses for circulating proprotein convertase subtilisin/kexin type 9 and the risk of major adverse cardiovascular events. (A) Per one standard derivation increase in baseline PCSK9 levels, (B) top vs. bottom tertile of baseline PCSK9. P†, for heterogeneity within each subgroup with Q-test. P‡, for difference between subgroups with meta-regression analysis. FH, family hypercholesterolemia; PCSK9, Proprotein convertase subtilisin/kexin type 9; CV, cardiovascular; RR, relative risk; CI: confidence intervals.
For sensitivity analysis, exclusion of any single study did not immensely alter the combined risk estimate (Supplementary Figure 1A). After excluding two nested case-control studies, the combined RR did not substantial change (RR 1.139; 95% CI: 1.064–1.220; P < 0.001; I2 = 66.90%; Pheterogeneity < 0.001).
PCSK9 as a Category Variable
Considering the fact that most of the included articles reported circulating PCSK9 as tertiles, we also compared individuals within the top tertile with the bottom tertile of circulating PCSK9 levels at baseline. Overall, there was a significant association between the highest PCSK9 tertile and the risk of MACEs (RR, 1.252; 95% CI: 1.104–1.420) (Figure 2B), with moderate heterogeneity across studies (I2 = 63.10%; Pheterogeneity < 0.001).
Subgroup and sensitivity analyses for category PCSK9 achieved similar results to the analyses mentioned above for PSCK9 per 1-SD increase (Figure 3B, Supplementary Figure 1B); Yet, when pooling the risk estimate for cohorts using ELISA (CycLex, Japan) independently, the correlation between baseline PCSK9 and MACEs lost significance (RR, 1.302; 95% CI: 0.998–1.697).
Dose–Response Meta-Analysis
Among the 19 articles concerning PCSK9 and MACEs, seven articles were excluded because of lack of cases or cohort size or the risk estimate of each category, and 12 articles were finally involved in the dose-response meta-analysis (7–9, 25, 27–29, 31–33, 35, 38). Using a restricted cubic spline model, no significantly curvilinear (non-linear) association was observed through a test for non-linearity (x2 test for non-linearity = 0.31, P non-linearity = 0.575). The linear dose–response curve demonstrated that the risk of MACEs increased slightly with elevation of PCSK9 concentration (Figure 4).
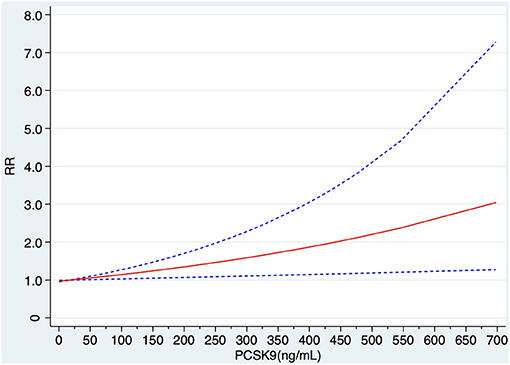
Figure 4. Linear dose–response of relationship between circulating PCSK9 concentration and risk MACEs. RR, relative risk.
Association Between PCSK9 and Stroke
In total, only two studies (8, 28), including three cohorts reported results on stroke (Supplementary Figure 2). Baseline PCSK9 could not significantly predict stroke when combining risk estimate by random effect models both for per 1-SD increase (RR, 1.022; 95% CI: 0.771–1.354; I2 = 57.6%, Pheterogeneity = 0.095) and for the highest tertile vs. the lowest tertile (RR, 1.051; 95% CI: 0.567–1.918; I2 = 63.1%, Pheterogeneity < 0.001).
Association Between PCSK9 and All-Cause Mortality
The association between PCSK9 levels and risk of all-cause mortality was investigated in six studies (8, 25, 26, 31, 34, 35). The pooled RR of all-cause mortality in fixed-effect model for 1-SD increase in baseline PCSK9 was (RR 1.007; 95% CI: 0.950–1.068; I2 = 12.60%, Pheterogeneity = 0.334) (Figure 5A). For subjects distributed in the highest tertile of baseline PCSK9, the pooled RR was (RR 1.036; 95% CI: 0.909–1.181; I2 = 27.00%, Pheterogeneity = 0.222) (Figure 5B).
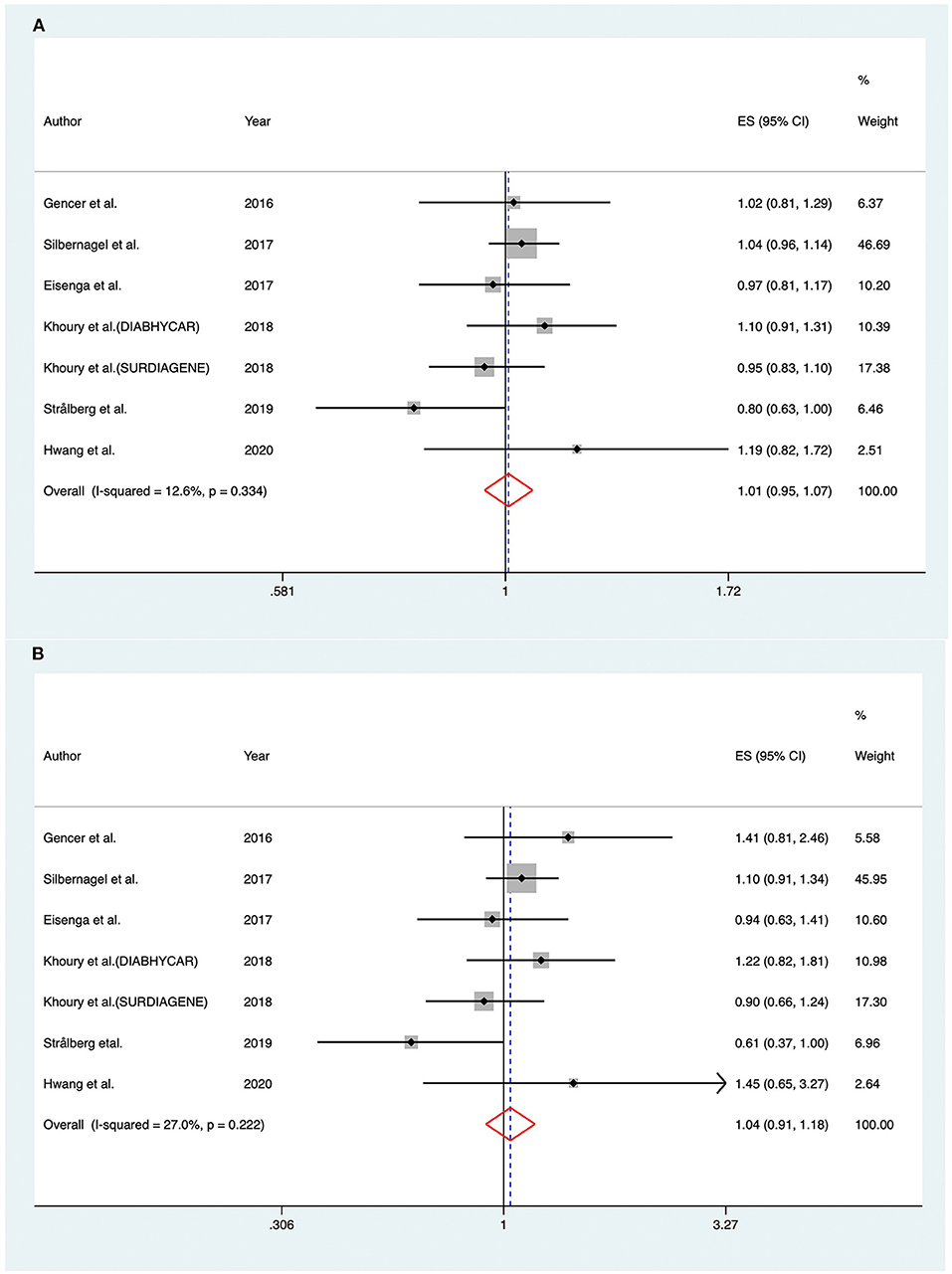
Figure 5. Associations between circulating proprotein convertase subtilisin/kexin type 9 and risk of all-cause mortality. (A) Per one standard derivation increase in baseline proprotein convertase subtilisin/kexin type 9 levels, (B) top vs. bottom tertile of baseline proprotein convertase subtilisin/kexin type 9. DIABHYCAR indicates Non-Insulin Dependent Diabetes, Hypertension, Microalbuminuria or Proteinuria, Cardiovascular Events, and Ramipril; SURDIAGENE, Survie, Diabète de type 2 et Génétique; ES, effect size; CI, confidence intervals.
Small-Study Effect and Publication Bias
The funnel plot for the correlation between PCSK9 and MACEs showed asymmetry (small-study effect) at its bottom (Figure 6), which was confirmed by Begg's and Egger's test (P = 0.020, 0.016, respectively).
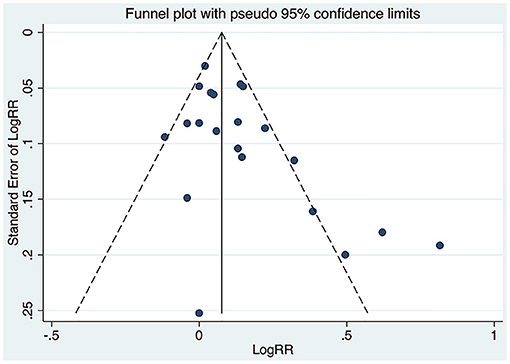
Figure 6. Funnel plot of the association between circulating proprotein convertase subtilisin/kexin type 9 concentration and major adverse cardiovascular events. RR, relative risk.
Whereas, the contour-enhanced funnel plot with four filled studies estimated from the trim-and-fill method plotted (Figure 7) demonstrated that the “missing” studies were expected to lie in areas of high statistical significance, indicating that the small-study effect may not be due to publication bias.
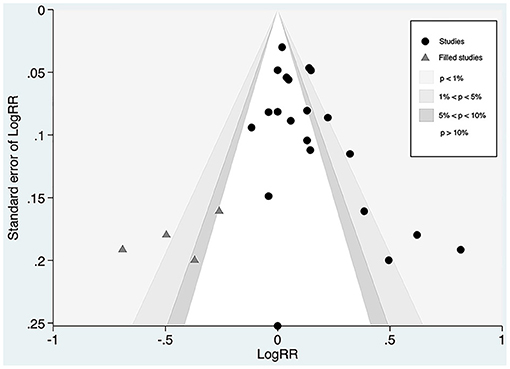
Figure 7. Contour-funnel plot of circulating proprotein convertase subtilisin/kexin type 9 concentration and major adverse cardiovascular events with “filled” studies estimated from the trim-and-fill method plotted. RR, relative risk.
Discussion
Our study is an updated meta-analysis investigating the predictive role of circulating PCSK9 with clinical outcomes. In the present meta-analysis, 20 published articles, namely, 19 studies on MACEs, three studies on stroke, and six studies on all-cause mortality, involving a total of 28,319 participants were included. The result indicated that the PCSK9 level is an independent predictive marker for MACEs with a 25% increased risk while compared with the lowest tertile, and per unit of SD change in baseline PCSK9 corresponds to an increase of 12%. A dose-response meta-analysis between circulating PCSK9 concentration and MACEs risk was conducted further, and a linear dose-response relationship was observed. However, a significant association either with stroke or all-cause mortality was not suggested in the study.
Since substantial heterogeneity was observed across studies for MACEs, subgroup and meta-regression analysis were employed to get more reliable pooled risk estimates. Despite consistent results found in most of the subgroup analyses, heterogeneity could be partially explained by the percentage of FH history in the study (i.e., more robust association in studies with a high percentage of FH history).
The association between circulating PCSK9 level and CV risk has been investigated in three previous meta-analyses. In line with the findings reported by Vlachopoulos et al., Qiu et al., and Xiao et al., circulating PCSK9 is associated with increased CV risk when compared the highest with the lowest category (40–42). PCSK9 increases LDL concentration by enhancing LDLR degradation and preventing LDLR recirculation back to the cell membrane (43). Apart from regulating cholesterol metabolism by directly targeting LDLR, experimental studies suggested that PCSK9 could affect vascular biology and accelerate the progression of atherosclerosis via other mechanisms (44). Increased expression of PCSK9 is related to oxidized LDL-induced apoptosis in endothelial cell, which may give rise to subsequent endothelial dysfunction and pathogenesis of atherosclerosis (45). It is noteworthy that PCSK9 is also expressed in atherosclerotic plaque; PCSK9 released by vascular smooth muscle cells reduces LDLR expression and thus prevents the uptake of LDL cholesterol, which is associated with lipid accumulation, oxidation, and plaque formation (46). Furthermore, some studies have demonstrated that the development of atherosclerosis by PCSK9 also correlates with platelet activation, blood pressure regulation and glucose metabolism (29, 47, 48). In view of the aforementioned functional diversity of PCSK9, it is rational to consider its circulating level as a potential atherogenic risk marker for CV events.
Incongruent results were yielded in previous meta-analyses when stratifying participants according to CV risk (40, 41); Vlachopoulos et al. found that high concentration of PCSK9 associated with increased risk of CV events in the general population but not in the high-risk population, while similar significant associations were observed both in low- and high-CV risk subgroups by Qiu et al. We pooled more recent articles, mostly focusing on high-CV risk patients in the pooling analysis, which reinforced the significant correlation regardless of the degree of CV risk. Additionally, CV outcome trials have already been conducted and shown that PCSK9 inhibitors effectively reduce LDL-C and MACEs in high-risk patients with atherosclerotic CVD (1, 3). However, to date, no studies have accessed whether PCSK9 inhibitors could be used for CV prevention in the general population. Moreover, several longitudinal studies suggested that higher PCSK9 concentration was associated with the development of carotid atherosclerosis in populations free of cardiovascular disease (CVD) at baseline (49, 50). Hence, it may be worth investigating the potential role of PCSK9 inhibitors for primary CV prevention.
It is known that FH is a special group of the population who has genetic mutations resulting in persistent lifelong extremely raised LDL-C levels, premature CAD and systemic atherosclerosis (51). To the best of our knowledge, the present study is the first circulating PCSK9 meta-analysis to include studies with a high percentage of FH participants. As a special part involved in a high CV risk group, a much stronger association was found in participants with FH (as mentioned above), which might partially attribute to lifelong exposure of elevated LDL-C and substantially increased risk of early atherosclerosis among these participants (47, 52). By removing two studies specifically focusing on FH participants from the meta-analysis of MACEs (28, 33), heterogeneity mildly reduced (I2 = 48.60%, P = 0.009, Pfor interaction = 0.001). The reduced heterogeneity might also indicate the heterogeneous nature of FH population, as reported in previous studies that the predictive value of some traditional risk factors for future MACEs was different from the general population (53, 54) Of note, although PCSK9 showed the prognostic value in FH patients, further steps are still needed to confirm it in large cohorts and different ethnic population.
It has been well-elucidated that PCSK9 antibodies significantly decrease the risk of stroke in randomized trials of therapeutic PCSK9-inhibition as comparable to the effect on MACEs. Nevertheless, it remains controversial whether PCSK9 variants associates with risk of stroke (55–57). A mendelian randomization study involving 10307 IS cases and 19,326 controls of European ancestry showed a weaker effect of PCSK9 on IS risk than on coronary heart disease (CHD) risk (58). These findings indicated that the impact of PCSK9 on the risks of IS might be of more complexity; unlike homogenous phenotype in CHD, IS involves etiological heterogeneity with different subtypes (such as large artery atherosclerosis, cardioembolic embolism, and small vessel disease) (59). It was shown that the effect of life-long lower genetically determined LDL-C and PCSK9 on different etiologically distinct IS subtypes varied materially (58, 60, 61). Moreover, in the exploration of canonical pathways of the diseases, IS are linked to natural killer cell signaling pathway rather than to lipid pathways as CHD does (62, 63). We analyzed circulating PCSK9 to elucidate the relationship between PCSK9 and stroke further. However, limited numbers of studies comprising stroke as the outcome of interest included in our meta-analysis might diminish the statistical power to detect the association for stroke, and thus the result should be viewed cautiously. Large-scale and well-designed prospective population-based studies are required to investigate further whether an increased level of PCSK9 will have predictive value for stroke and its subtypes.
PCSK9 has generally been measured by ELISA immunoassay, while the concentrations varied in a wide range (40–800 ng/ml) among different ELISA techniques (64). Studies making the head-to-head comparison of the methods to investigate the differences are scarce (64). Hence, the wide variability of results would substantially limit the utility of PCSK9 measurement in clinical practice. Moreover, it should be noted that PCSK9 circulates as mature and furin-cleaved forms in the blood. Previous studies revealed that furin-cleaved PCSK9 was inactive to regulate serum LDL-C or less activity than mature form (65–67). Nevertheless, most commercial ELISA techniques used in published studies measured the total amount of PCSK9 and could not distinguish between furin-cleaved and mature forms. The correlation between PCSK9 and CV risk might be strengthened if only the mature form was measured. Further steps are still needed to standardize, assess the agreement of different assays, and improve specificity for the total and active form of PCSK9 before using it as a CV biomarker in extensive clinical practice.
In our view, the current meta-analysis has several strengths. First, it is the most comprehensive meta-analysis on this topic to date with a relatively large number of cases and participants. Finally, the risk estimates from the fully adjusted models for each study were applied in our analyses to reduce the potential of confounding. Despite these strengths, limitations of this meta-analysis should be noted. Firstly, the pooled result of PCSK9 and MACEs showed substantial heterogeneity among the included studies, which may affect the interpretation of the results. Although we conducted on stratified and sensitivity analyses to identify the sources of heterogeneity, the heterogeneity could not be fully explained. Furthermore, meta-regression techniques are limited used in the present analysis given the lack of information for many continuous factors, such as baseline LDL, high-sensitivity C-reactive protein, and the result should therefore be viewed with caution. Secondly, original studies included in the study reported the risk estimates calculated by different multivariable models, and the pooled association lost significance in the subgroup with a lower degree of confounder adjustment. On these grounds, the combined result might potentially be influenced. Thirdly, most of the included studies used the combined CV events as the outcome of interest, making it difficult to identify the risk of specific CV events including stroke and different stroke subtypes; the statistical power might be compromised, and therefore, advanced studies focusing specific CV outcomes are warranted in future research. Finally, statistical tests for detecting publication bias in pooling the effect estimates of PCSK9 and all-cause mortality and stroke may be potentially unreliable due to less than the recommended minimum number of 10 studies analyzed (68).
Conclusion
This meta-analysis provided further evidence that high circulating PCSK9 concentration is associated with increased risk of MACEs with a linear dose-response relationship. However, available data did not suggest a significant correlation either on stroke or all-cause mortality. Our finding suggested that measurement of PCSK9 level may have the potential to improve risk stratification for medical decision and also support the result of the beneficial clinical role of PCSK9 inhibitors. To further investigate the correlations between PCSK9 concentration and stroke and mortality, additional well-designed multicenter studies with standardized methodologies are warranted.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YZ had the idea for the study, did the statistical analysis with guidance from ML and WC, and drafted the manuscript. YZ and YW contributed to the study designed. YZ and WC participated in the search, data collection, and extraction. ML and WC did the major revision. All authors read and approved the final manuscript.
Funding
This work was supported by grants from National Key R&D Program of China (2018YFC1312903), grants from National Science and Technology Major Project (2017ZX09304018), grants from Beijing Municipal Science & Technology Commission (D171100003017002 and Z181100001818001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.617249/full#supplementary-material
References
1. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. (2017) 376:1713–22. doi: 10.1056/NEJMoa1615664
2. Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. (2017) 376:1527–39. doi: 10.1056/NEJMoa1701488
3. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. (2018) 379:2097–107. doi: 10.1056/NEJMoa1801174
4. Giugliano RP, Pedersen TR, Saver JL, Sever PS, Keech AC, Bohula EA, et al. Stroke prevention with the PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitor evolocumab added to statin in high-risk patients with stable atherosclerosis. Stroke. (2020) 51:1546–54. doi: 10.1161/STROKEAHA.119.027759
5. Jukema JW, Zijlstra LE, Bhatt DL, Bittner VA, Diaz R, Drexel H, et al. Effect of alirocumab on stroke in odyssey outcomes. Circulation. (2019) 140:2054–62. doi: 10.1161/CIRCULATIONAHA.119.043826
6. Cariou B, Ding Z, Mehta JL. PCSK9 and atherosclerosis: beyond LDL-cholesterol lowering. Atherosclerosis. (2016) 253:275–7. doi: 10.1016/j.atherosclerosis.2016.08.007
7. Werner C, Hoffmann MM, Winkler K, Böhm M, Laufs U. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vascul Pharmacol. (2014) 62:94–102. doi: 10.1016/j.vph.2014.03.004
8. El Khoury P, Roussel R, Fumeron F, Abou-Khalil Y, Velho G, Mohammedi K, et al. Plasma proprotein-convertase-subtilisin/kexin type 9 (PCSK9) and cardiovascular events in type 2 diabetes. Diabetes Obes Metab. (2018) 20:943–53. doi: 10.1111/dom.13181
9. Ridker PM, Rifai N, Bradwin G, Rose L. Plasma proprotein convertase subtilisin/kexin type 9 levels and the risk of first cardiovascular events. Eur Heart J. (2016) 37:554–60. doi: 10.1093/eurheartj/ehv568
10. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. J Am Med Assoc. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
11. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. (2012). Available online at: http//www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed November 21, 2012).
12. Angeles L. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. (1987) 9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298
13. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. J Am Med Assoc. (1998) 280:1690–1. doi: 10.1001/jama.280.19.1690
14. Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. (1998) 279:1477–82. doi: 10.1001/jama.279.18.1477
15. Chêne G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. (1996) 144:610–21. doi: 10.1093/oxfordjournals.aje.a008971
16. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
17. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed. Chichester: John Wiley & Sons (2019).
18. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
19. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and non-linear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. (2012) 175:66–73. doi: 10.1093/aje/kwr265
20. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
21. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
22. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. (2008) 61:991–6. doi: 10.1016/j.jclinepi.2007.11.010
23. Zhang Z, Wei TF, Zhao B, Yin Z, Shi QX, Liu PL, et al. Sex differences associated with circulating PCSK9 in patients presenting with acute myocardial infarction. Sci Rep. (2019) 9:1–8. doi: 10.1038/s41598-018-35773-x
24. Navarese EP, Kolodziejczak M, Winter MP, Alimohammadi A, Lang IM, Buffon A, et al. Association of PCSK9 with platelet reactivity in patients with acute coronary syndrome treated with prasugrel or ticagrelor: the PCSK9-REACT study. Int J Cardiol. (2017) 227:644–9. doi: 10.1016/j.ijcard.2016.10.084
25. Silbernagel G, Scharnagl H, Kleber ME, Stojakovic T, März W. Circulating proprotein convertase subtilisin-kexin type 9, all-cause mortality, and cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health study. Eur J Prev Cardiol. (2017) 24:1095–101. doi: 10.1177/2047487317693938
26. Eisenga MF, Zelle DM, Sloan JH, Gaillard CAJM, Bakker SJL, Dullaart RPF. High serum PCSK9 is associated with increased risk of new-onset diabetes after transplantation in renal transplant recipients. Diabetes Care. (2017) 40:894–901. doi: 10.2337/dc16-2258
27. Laugsand LE, Åsvold BO, Vatten LJ, Janszky I, Platou CG, Michelsen AE, et al. Circulating PCSK9 and risk of myocardial infarction: the HUNT study in Norway. JACC Basic Transl Sci. (2016) 1:568–75. doi: 10.1016/j.jacbts.2016.06.007
28. Cao YX, Jin JL, Sun D, Liu HH, Guo YL, Wu NQ, et al. Circulating PCSK9 and cardiovascular events in FH patients with standard lipid-lowering therapy. J Transl Med. (2019) 17:1–11. doi: 10.1186/s12967-019-2123-9
29. Pastori D, Nocella C, Farcomeni A, Bartimoccia S, Santulli M, Vasaturo F, et al. Relationship of PCSK9 and urinary thromboxane excretion to cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol. (2017) 70:1455–62. doi: 10.1016/j.jacc.2017.07.743
30. Rogacev KS, Heine GH, Silbernagel G, Kleber ME, Seiler S, Emrich I, et al. PCSK9 plasma concentrations are independent of GFR and do not predict cardiovascular events in patients with decreased GFR. PLoS ONE. (2016) 11:1–14. doi: 10.1371/journal.pone.0146920
31. Hwang HS, Kim JS, Kim YG, Lee S-Y, Ahn SY, Lee HJ, et al. Circulating PCSK9 level and risk of cardiovascular events and death in hemodialysis patients. J Clin Med. (2020) 9:244. doi: 10.3390/jcm9010244
32. Leander K, Mälarstig A, Van'T Hooft FM, Hyde C, Hellénius ML, Troutt JS, et al. Circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factors. Circulation. (2016) 133:1230–9. doi: 10.1161/CIRCULATIONAHA.115.018531
33. Cao YX, Liu HH, Jin JL, Sun D, Guo YL, Wu NQ, et al. Plasma proprotein convertase subtilisin/kexin type 9 concentration and recurrent cardiovascular events in patients with familial hypercholesterolemia. Eur J Prev Cardiol. (2019) 11:2047487319880985. doi: 10.1177/2047487319880985
34. Strålberg T, Nordenskjöld A, Cao Y, Kublickiene K, Nilsson E. Proprotein convertase subtilisin/kexin type 9 and mortality in patients starting hemodialysis. Eur J Clin Invest. (2019) 49:1–7. doi: 10.1111/eci.13113
35. Gencer B, Montecucco F, Nanchen D, Carbone F, Klingenberg R, Vuilleumier N, et al. Prognostic value of PCSK9 levels in patients with acute coronary syndromes. Eur Heart J. (2016) 37:546–53. doi: 10.1093/eurheartj/ehv637
36. Zhu YM, Anderson TJ, Sikdar K, Fung M, McQueen MJ, Lonn EM, et al. Association of proprotein convertase subtilisin/kexin type 9 (PCSK9) with cardiovascular risk in primary prevention. Arterioscler Thromb Vasc Biol. (2015) 35:2254–9. doi: 10.1161/ATVBAHA.115.306172
37. Li JJ, Li S, Zhang Y, Xu RX, Guo YL, Zhu CG, et al. Proprotein convertase subtilisin/kexin type 9, C-reactive protein, coronary severity, and outcomes in patients with stable coronary artery disease a prospective observational cohort study. Med. (2015) 94:1–8. doi: 10.1097/MD.0000000000002426
38. Gao Y, Qiu Y, Wu J, Diao W, Zhang H, Wang S, et al. Acute-phase plasma PCSK9 levels and recurrent cardiovascular events in a Chinese acute myocardial infarction cohort. Cardiol. (2018) 141:88–97. doi: 10.1159/000493785
39. Rasmussen LD, Bøttcher M, Ivarsen P, Jørgensen HS, Nyegaard M, Buttenschøn H, et al. Association between circulating proprotein convertase subtilisin/kexin type 9 levels and prognosis in patients with severe chronic kidney disease. Nephrol Dial Transplant. (2018) 35:632–9. doi: 10.1093/ndt/gfy257
40. Vlachopoulos C, Terentes-Printzios D, Georgiopoulos G, Skoumas I, Koutagiar I, Ioakeimidis N, et al. Prediction of cardiovascular events with levels of proprotein convertase subtilisin/kexin type 9: a systematic review and meta-analysis. Atherosclerosis. (2016) 252:50–60. doi: 10.1016/j.atherosclerosis.2016.07.922
41. Qiu C, Zhou Q, Li X, Zhang Z, Zeng P, Cao Z, et al. High circulating proprotein convertase subtilisin/Kexin type 9 concentration associates with cardiovascular risk. Med. (2017) 96:1–7. doi: 10.1097/MD.0000000000008848
42. Xiao Y, Peng C, Huang W, Zhang J, Gao Y, Kim JH, et al. Circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) concentration and risk of cardiovascular events—systematic review and meta-analysis of prospective studies. Circ J. (2017) 81:1150–7. doi: 10.1253/circj.CJ-16-1142
43. Burke AC, Dron JS, Hegele RA, Huff MW. PCSK9: regulation and target for drug development for dyslipidemia. Annu Rev Pharmacol Toxicol. (2017) 57:223–44. doi: 10.1146/annurev-pharmtox-010716-104944
44. Urban D, Pöss J, Böhm M, Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol. (2013) 62:1401–8. doi: 10.1016/j.jacc.2013.07.056
45. Wu C-Y, Tang Z-H, Jiang L, Li X-F, Jiang Z-S, Liu L-S. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol Cell Biochem. (2012) 359:347–58. doi: 10.1007/s11010-011-1028-6
46. Ferri N, Tibolla G, Pirillo A, Cipollone F, Mezzetti A, Pacia S, et al. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis. (2012) 220:381–6. doi: 10.1016/j.atherosclerosis.2011.11.026
47. Sharotri V, Collier DM, Olson DR, Zhou R, Snyder PM. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9). J Biol Chem. (2012) 287:19266–74. doi: 10.1074/jbc.M112.363382
48. Mbikay M, Sirois F, Mayne J, Wang GS, Chen A, Dewpura T, et al. PCSK9-deficient mice exhibit impaired glucose tolerance and pancreatic islet abnormalities. FEBS Lett. (2010) 584:701–6. doi: 10.1016/j.febslet.2009.12.018
49. Xie W, Liu J, Wang W, Wang M, Qi Y, Zhao F, et al. Association between plasma PCSK9 levels and 10-year progression of carotid atherosclerosis beyond LDL-C: a cohort study. Int J Cardiol. (2016) 215:293–8. doi: 10.1016/j.ijcard.2016.04.103
50. Chan DC, Pang J, McQuillan BM, Hung J, Beilby JP, Barrett PHR, et al. Plasma proprotein convertase subtilisin kexin type 9 as a predictor of carotid atherosclerosis in asymptomatic adults. Heart Lung Circ. (2016) 25:520–5. doi: 10.1016/j.hlc.2015.10.017
51. Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. (2013) 34:3478–90a. doi: 10.1093/eurheartj/eht273
52. Khera AV, Won H-H, Peloso GM, Lawson KS, Bartz TM, Deng X, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. (2016) 67:2578–89. doi: 10.1016/j.jacc.2016.03.520
53. Sharifi M, Rakhit RD, Humphries SE, Nair D. Cardiovascular risk stratification in familial hypercholesterolaemia. Heart. (2016) 102:1003–8. doi: 10.1136/heartjnl-2015-308845
54. Neil HAW, Seagroatt V, Betteridge DJ, Cooper MB, Durrington PN, Miller JP, et al. Established and emerging coronary risk factors in patients with heterozygous familial hypercholesterolaemia. Heart. (2004) 90:1431–7. doi: 10.1136/hrt.2003.022764
55. Abboud S, Karhunen PJ, Lütjohann D, Goebeler S, Luoto T, Friedrichs S, et al. Proprotein convertase subtilisin/Kexin type 9 (PCSK9) gene is a risk factor of large-vessel atherosclerosis stroke. PLoS ONE. (2007) 2:10–3. doi: 10.1371/journal.pone.0001043
56. Slimani A, Harira Y, Trabelsi I, Jomaa W, Maatouk F, Hamda KB, et al. Effect of E670G polymorphism in PCSK9 gene on the risk and severity of coronary heart disease and ischemic stroke in a Tunisian cohort. J Mol Neurosci. (2014) 53:150–7. doi: 10.1007/s12031-014-0238-2
57. Han D, Ma J, Zhang X, Cai J, Li J, Tuerxun T, et al. Correlation of PCSK9 gene polymorphism with cerebral ischemic stroke in Xinjiang Han and Uygur populations. Med Sci Monit. (2014) 20:1758–67. doi: 10.12659/MSM.892091
58. Worrall BB, Hopewell JC, Malik R, Valde E. Differential effects of PCSK9 variants on risk of coronary disease and ischaemic stroke. Eur Heart J. (2017) 39:354–9. doi: 10.1093/eurheartj/ehx373
59. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
60. Hindy G, Engström G, Larsson SC, Traylor M, Markus HS, Melander O, et al. Role of blood lipids in the development of ischemic stroke and its subtypes: a mendelian randomization study. Stroke. (2018) 49:820–7. doi: 10.1161/STROKEAHA.117.019653
61. Valdes-Marquez E, Parish S, Clarke R, Stari T, Worrall BB, Hopewell JC. Relative effects of LDL-C on ischemic stroke and coronary disease: a Mendelian randomization study. Neurology. (2019) 92:E1176–87. doi: 10.1212/WNL.0000000000007091
62. CARDIoGRAMplusC4D Consortium, Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. (2013) 45:25–33. doi: 10.1038/ng.2480
63. Malik R, Traylor M, Pulit SL, Bevan S, Hopewell JC, Holliday EG, et al. Low-frequency and common genetic variation in ischemic stroke: the METASTROKE collaboration. Neurology. (2016) 86:1217–26. doi: 10.1212/WNL.0000000000002528
64. Malo J, Parajuli A, Walker SW. PCSK9: from molecular biology to clinical applications. Ann Clin Biochem. (2020) 57:7–25. doi: 10.1177/0004563219864379
65. Benjannet S, Rhainds D, Hamelin J, Nassoury N, Seidah NG. The proprotein convertase (PC) PCSK9 is inactivated by furin and/or PC5/6A: functional consequences of natural mutations and post-translational modifications. J Biol Chem. (2006) 281:30561–72. doi: 10.1074/jbc.M606495200
66. Han B, Eacho PI, Knierman MD, Troutt JS, Konrad RJ, Yu X, et al. Isolation and characterization of the circulating truncated form of PCSK9. J Lipid Res. (2014) 55:1505–14. doi: 10.1194/jlr.M049346
67. Essalmani R, Susan-Resiga D, Chamberland A, Abifadel M, Creemers JW, Boileau C, et al. In vivo evidence that furin from hepatocytes inactivates PCSK9. J Biol Chem. (2011) 286:4257–63. doi: 10.1074/jbc.M110.192104
Keywords: proprotein convertase subtilisin/kexin type 9, cardiovascular events, stroke, mortality, association
Citation: Zhou Y, Chen W, Lu M and Wang Y (2021) Association Between Circulating Proprotein Convertase Subtilisin/Kexin Type 9 and Major Adverse Cardiovascular Events, Stroke, and All-Cause Mortality: Systemic Review and Meta-Analysis. Front. Cardiovasc. Med. 8:617249. doi: 10.3389/fcvm.2021.617249
Received: 15 October 2020; Accepted: 05 February 2021;
Published: 02 March 2021.
Edited by:
Kristi Reynolds, Kaiser Permanente, United StatesReviewed by:
Lydia Bazzano, Tulane University School of Public Health and Tropical Medicine, United StatesKirsten Dorans, Tulane University, United States
Copyright © 2021 Zhou, Chen, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjun Wang, eW9uZ2p1bndhbmdAbmNyY25kLm9yZy5jbg==
 Yimo Zhou
Yimo Zhou Weiqi Chen1,2,3,4
Weiqi Chen1,2,3,4 Meng Lu
Meng Lu Yongjun Wang
Yongjun Wang