- 1Department of Cardiology, Affiliated Hospital of Jiaxing University, Zhejiang, China
- 2Peking University People's Hospital, Beijing, China
- 3China-Japan Friendship Hospital, Beijing, China
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) share a target receptor with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The use of ACEIs/ARBs may cause angiotensin-converting enzyme 2 receptor upregulation, facilitating the entry of SARS-CoV-2 into host cells. There is concern that the use of ACEIs/ARBs could increase the risks of severe COVID-19 and mortality. The impact of discontinuing these drugs in patients with COVID-19 remains uncertain. We aimed to assess the association between the use of ACEIs/ARBs and the risks of mortality and severe disease in patients with COVID-19. A systematic search was performed in PubMed, EMBASE, Cochrane Library, and MedRxiv.org from December 1, 2019, to June 20, 2020. We also identified additional citations by manually searching the reference lists of eligible articles. Forty-two observational studies including 63,893 participants were included. We found that the use of ACEIs/ARBs was not significantly associated with a reduction in the relative risk of all-cause mortality [odds ratio (OR) = 0.87, 95% confidence interval (95% CI) = 0.75–1.00; I2 = 57%, p = 0.05]. We found no significant reduction in the risk of severe disease in the ACEI subgroup (OR = 0.95, 95% CI = 0.88–1.02, I2 = 50%, p = 0.18), the ARB subgroup (OR = 1.03, 95% CI = 0.94–1.13, I2 = 62%, p = 0.48), or the ACEI/ARB subgroup (OR = 0.83, 95% CI = 0.65–1.08, I2 = 67%, p = 0.16). Moreover, seven studies showed no significant difference in the duration of hospitalization between the two groups (mean difference = 0.33, 95% CI = −1.75 to 2.40, p = 0.76). In conclusion, the use of ACEIs/ARBs appears to not have a significant effect on mortality, disease severity, or duration of hospitalization in COVID-19 patients. On the basis of the findings of this meta-analysis, there is no support for the cessation of treatment with ACEIs or ARBs in patients with COVID-19.
Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has initiated a global epidemic. SARS-CoV-2 uses the receptor angiotensin-converting enzyme 2 (ACE2) to gain entry into target cells (1–3). ACE2 is part of the renin–angiotensin system (RAS). Because RAS inhibitors, such as angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), increase the levels of ACE2, the protein that facilitates the entry of SARS-CoV-2 into cells, there are concerns that these drugs could increase the risks of severe COVID-19 and mortality (4). Evidence that ACEIs and ARBs might upregulate ACE2 in several organs, including the lungs and heart (5), supported the hypothesis widely reported by the press that their use might increase susceptibility to infection with SARS-CoV-2 and that their discontinuation might therefore be an appropriate preventive measure (6). Based on these facts and observations, the hypothesis has been developed that their use may affect human susceptibility to infection with SARS-CoV-2.
However, in animal models, ACEIs and ARBs are protective against acute lung injury, and pretreatment with ACEIs or ARBs may reduce the extent of experimentally induced lung injury and improve outcomes, an effect mediated by inhibition of the RAS (7).
Activation of the RAS can cause widespread endothelial dysfunction and varying degrees of injury to multiple organs (heart, kidney, and lung) (8). Thus, researchers have hypothesized that ACEIs/ARBs could theoretically be beneficial and reduce the risk of severe disease in patients with COVID-19.
These possibilities pose a dilemma for cardiologists in terms of whether they should recommend discontinuing treatment with ACEIs/ARBs. Therefore, we performed a large-scale meta-analysis to estimate the associations between ACEIs/ARBs use and the risk of severe COVID-19 and prolonged hospitalization due to COVID-19 (9).
Methods
Literature Search
The present analysis was conducted in accordance with published PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and MOOSE (Meta-Analysis of Observational Studies in Epidemiology) guidelines (10). The meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO identifier: CRD42020183921). Electronic searches were conducted in PubMed, EMBASE, Cochrane Library, and MedRxiv.org from December 1, 2019, to June 20, 2020. As of the date the searches were performed, no randomized controlled clinical trials had been published; therefore, only observational studies were included. We also identified additional citations by manually searching the reference lists of eligible studies.
We used the following medical subject headings and keywords to search for articles related to COVID-19: COVID-19, severe acute respiratory syndrome coronavirus 2, 2019-nCoV, and SARS-CoV-2; and the following search terms related to ACEIs/ARBs: renin–angiotensin system, angiotensin-converting enzyme inhibitor, and angiotensin II receptor blockers (Supplementary Table 1).
Eligibility Criteria
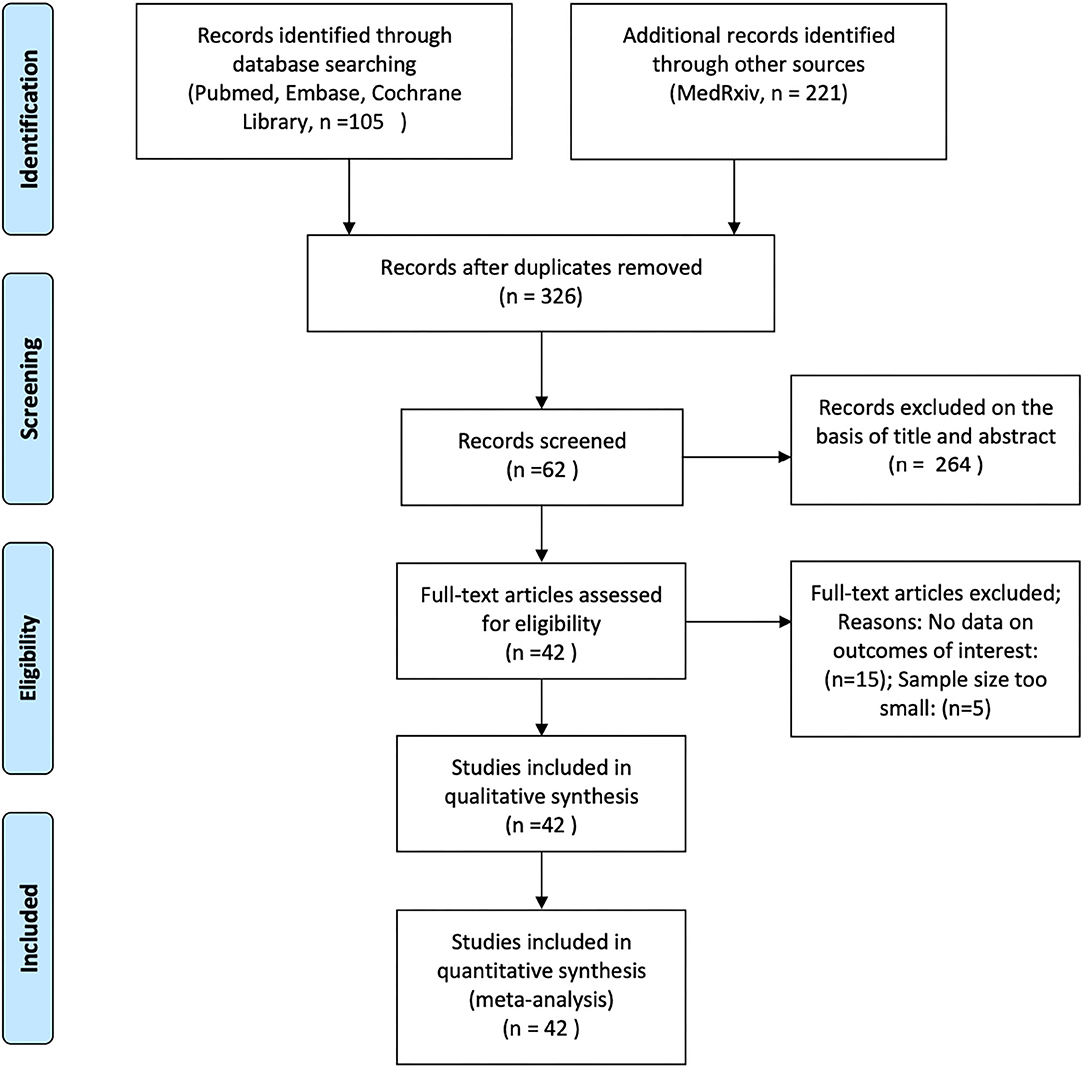
Two of the authors (ZA and ZW) independently analyzed the titles and abstracts of all articles retrieved from these searches to ascertain whether they met the inclusion criteria. We assessed the full texts of the initially eligible articles based on the PICOTS (Population, Intervention, Comparator, Outcome, Timing and Setting) framework, and articles were selected according to the following criteria: (1) articles reporting observational studies, including cohort studies and case-control studies; (2) articles that analyzed the effects of ACEIs/ARBs on COVID-19 in adult patients with hypertension; (3) articles that contained data on mortality, disease severity, and hospitalization durations in COVID-19 patients; and (4) articles that enrolled at least 50 patients (Figure 1).
Data Extraction
Two investigators (ZA and ZW) independently extracted the relevant data with a predetermined data collection table. Any discrepancies were settled by consensus or consultation with a third investigator (DXC). All the included data were aggregate data, and no patient-level data were available.
Quality Assessment
Study quality was assessed with the Newcastle–Ottawa Scale (NOS, maximum 9 points), which rates studies based on three parameters: the selection of groups, the comparability, and the ascertainment of outcome and exposure. The NOS can be used to evaluate the overall risk of bias in non-randomized studies.
Outcomes of Interest
Data for all-cause mortality, severity, and hospitalization duration in COVID patients were collected. Severe cases of COVID-19 were generally characterized by dyspnea, a respiratory rate >30 breaths/min, a blood oxygen saturation level <93% on room air, a PaO2/FIO2 ratio <300, and/or infiltration of >50% of the lung within 24–48 h, or according to the criteria defined in each included study (11).
Statistical Analysis
The adjusted odds ratios (ORs) and hazard ratios for all-cause mortality, severe disease, and prolonged hospitalization duration in COVID patients were reported in these studies. Both adjusted and unadjusted ORs were initially considered in the analysis. We pooled the adjusted ORs, which were derived from multivariate analyses. We used the I2 statistic to assess the heterogeneity of the summary estimates, and a value >50% was considered evidence of significant heterogeneity (12). A random-effects model was used because the I2 statistic was >50%. To assess publication bias, we constructed a funnel plot and adopted the Begg rank correlation method (p < 0.05 indicated significant bias). We used Stata version 14.0 (Stata Corp., College Station, TX, USA) for all calculations. We used RevMan 5.3 (Nordic Cochrane Centre, Cochrane Collaboration) to generate forest plots to show the results for the individual studies and the pooled analysis.
Results
Characteristics and Quality of Included Studies
Among the 42 studies included, 14 were performed in Europe, 7 in the United States, 4 in Korea, 1 in Iran, and 16 in China. All studies were published within the past 6 months, and all were observational studies. We confirmed that all observational studies had adequate inclusion and exclusion criteria and an appropriate justification for the selection of the cohort. We collected and sorted the data on intervention measures and examination results obtained from the electronic medical records in all studies.
We summarized the baseline characteristics in each study in Table 1 (8, 13–43, 45–48, 50, 52–56). The identified studies included 63,893 patients with COVID-19. Of these, 20,686 were taking ACEIs/ARBs. Thirty-five studies adjusted their analyses for comorbidities (including diabetes mellitus, cardiovascular disease, and chronic kidney disease). Seven studies did not describe the controls; however, the remaining studies described the controls as patients who had COVID-19 but had not been exposed to ACEIs/ARBs. Seven studies compared the hospitalization durations (days) between the ACEI/ARB and non-ACEI/ARB groups. Twenty-nine studies (69.04%) described mortality in the study populations.
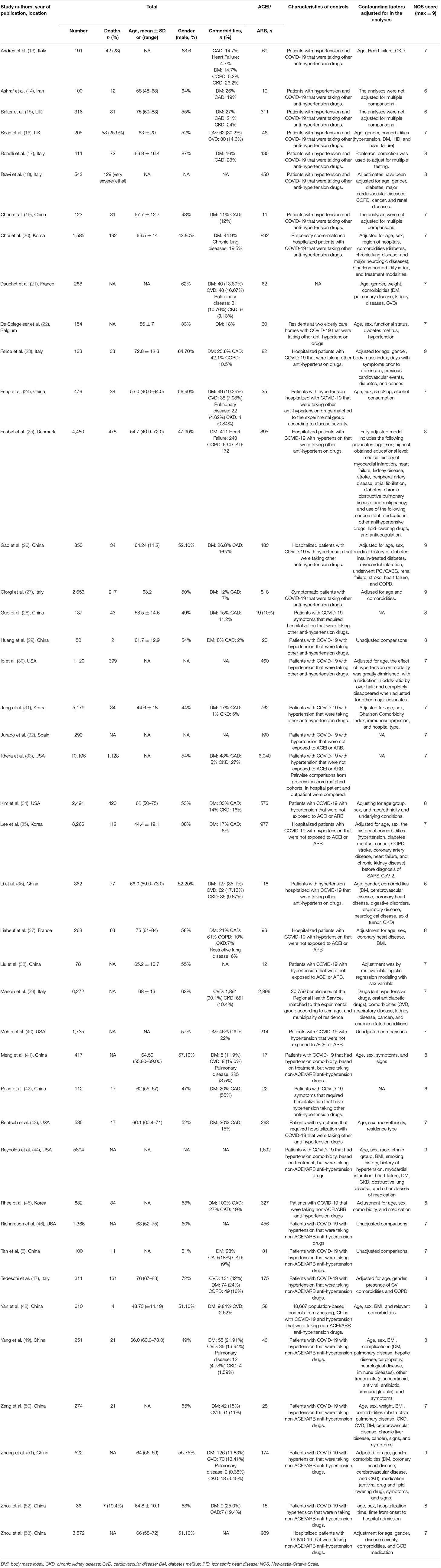
Table 1. Baseline characteristics of patients assessed in the studies included in the meta-analysis.
We carefully evaluated the quality of each study with the NOS. Thirty-eight studies (90.5%) had 7 points or more, and the remaining four studies had 6 points. Supplementary Table 2 shows the NOS scores of the included studies.
Outcome Measures
Effects of ACEIs/ARBs on All-Cause Mortality in Patients With COVID-19
Twenty-nine studies discussed the relationship between the use of ACEIs/ARBs and all-cause mortality in patients with COVID-19 (Figure 2). The use of ACEIs/ARBs was not significantly associated with a reduction in the relative risk of all-cause mortality [OR = 0.87, 95% confidence interval (95% CI) = 0.75–1.00; I2 = 57%, p = 0.05]. The control groups generally included patients with COVID-19 who had taken other antihypertensive treatments. Most studies calculated the OR after adjusting for age, sex, and other factors to reduce the influence of confounding factors. To determine whether there was a difference between data from articles published in peer-reviewed journals and those posted on preprint servers, a subgroup analysis was conducted. There was no significant difference in the results for all-cause mortality between the two subgroups.
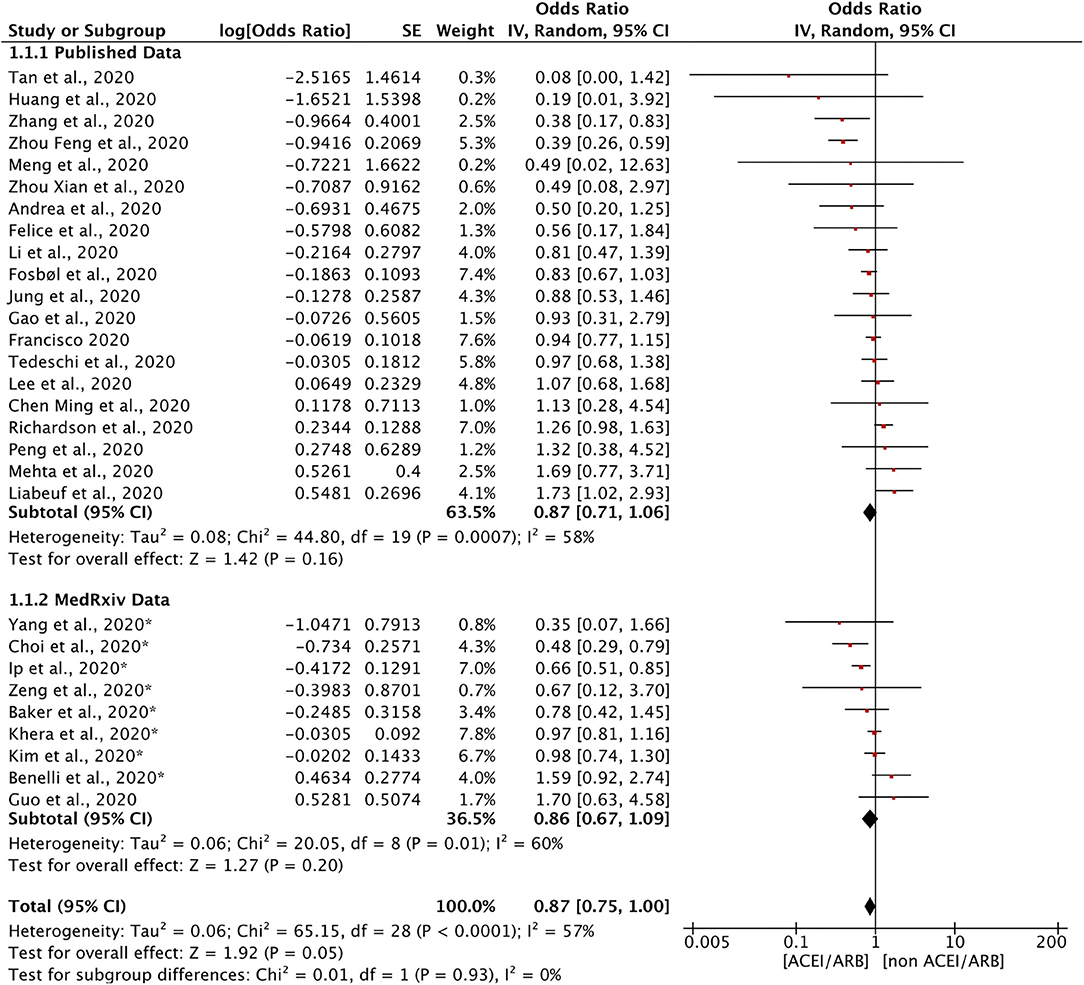
Figure 2. Forest plot showing the effects of ACEIs/ARBs on all-cause mortality in patients with COVID-19. SE, standard error; IV, inverse variance; df, degrees of freedom.
Effects of ACEIs and ARBs on the Severity of COVID-19
Twenty-five retrospective studies evaluated the effects of ACEIs and ARBs on the severity of COVID-19 (Figure 3). We found no significant reduction in disease severity in the ACEI subgroup (OR = 0.95, 95% CI = 0.88–1.02, I2 = 50%, p = 0.18), in the ARB subgroup (OR = 1.03, 95% CI = 0.94–1.13, I2 = 62%, p = 0.48), or in the ACEI/ARB subgroup (OR = 0.83, 95% CI = 0.65–1.08, I2 = 67%, p = 0.16). Our meta-analysis demonstrated that there was no significant reduction in disease severity in patients taking ACEIs/ARBs (OR = 0.97, 95% CI = 0.92–1.03, I2 = 58%, p = 0.38). These findings indicate that ACEIs and ARBs might not have either a protective or adverse effect on disease severity.
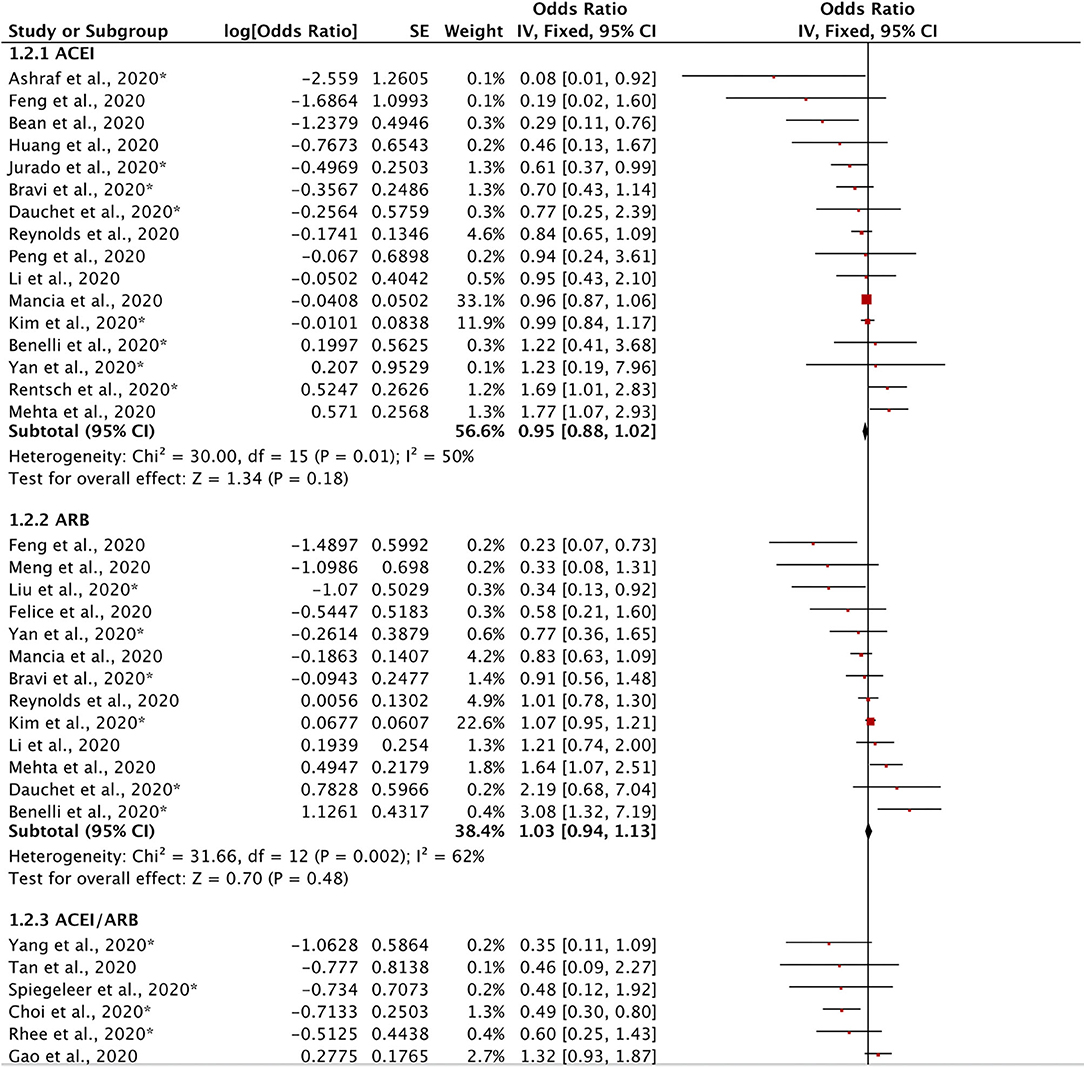
Figure 3. Forest plot showing the effects of ACEIs and ARBs on the severity of COVID-19. SE, standard error; IV, inverse variance; df, degrees of freedom.
Effect of ACEIs/ARBs on the Duration of Hospitalization for COVID-19 Treatment
Seven included studies discussed the effects of ACEIs/ARBs on the duration of hospitalization required for the treatment of COVID-19. A meta-analysis of these studies showed that ACEIs/ARBs had no obvious effect on hospitalization duration (mean difference = 0.33, 95% CI = −1.75 to 2.40, p = 0.76). Because of the obvious limitation of the small number of included studies, we described these results qualitatively. In general, ACEIs/ARBs did not significantly shorten or prolong the hospitalization duration for patients with COVID-19 (Figure 4).
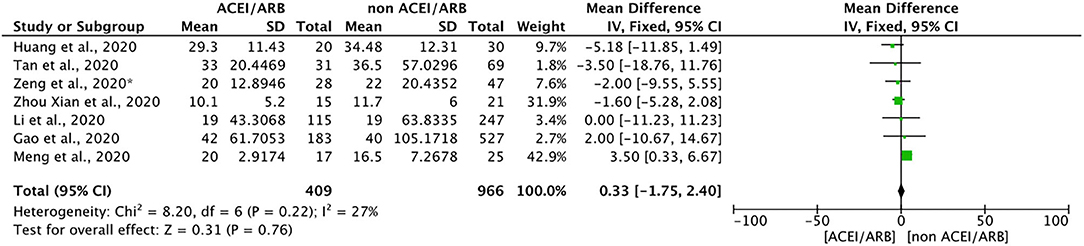
Figure 4. Forest plot showing the effects of ACEIs/ARBs on hospitalization duration in patients with COVID-19. SE, standard error; IV, inverse variance; df, degrees of freedom.
Sensitivity Analysis and Publication Bias
We performed a sensitivity analysis on the overall meta-analysis results. We performed sensitivity analyses for the effects of ACEIs/ARBs on the risks of mortality and severe disease by sequentially omitting one study at a time and investigating any changes in the findings. The results for all-cause mortality did not change significantly after excluding studies with low NOS scores, such as Li et al. (36). This finding indicated that the results were robust and reliable. Similarly, the pooled ORs for severe disease did not significantly change when we omitted studies one at a time.
We also evaluated publication bias with funnel plots. A visual inspection did not reveal any clear asymmetry (Supplementary Figures 1–3). Therefore, no significant publication bias was found among the included observational studies.
Discussion
In the present meta-analysis, we found no significant association between the use of ACEIs/ARBs and the risks of mortality and severe disease in patients with COVID-19 after adjusting for baseline demographics and comorbidities (16, 36, 41, 44, 49, 57–59).
The concerns about the use of ACEIs or ARBs in patients with COVID-19 have mainly stemmed from arguments based on biologic plausibility, particularly the observation that ACEIs and ARBs have the potential to upregulate ACE2 receptors (which seem to be the mediators of the entry of SARS-CoV-2 into host cells) (60). However, it is also biologically plausible that ARBs may have beneficial effects in patients with COVID-19, although the findings have not been consistent across animal and human models (7). Therefore, ACE2 may act as a double-edged sword, depending on the phase of the disease. On the one hand, increased baseline ACE2 expression could potentially increase susceptibility of infection, making ACEI/ARB use a modifiable risk factor. On the other hand, once infected, the downregulation of ACE2 may be a hallmark of COVID-19 progression. Consequently, upregulation by preferentially blocking the RAS and replacing ACE2 in the acute respiratory syndrome phase may be beneficial. Our analysis supports that in the context of the current COVID-19 epidemic, the use of ACEIs/ARBs should not be restricted.
Several researchers found that the use of ACEIs/ARBs could worsen the prognosis of COVID-19 among patients with hypertension by promoting the expression of ACE2 (2–4, 61). These observational studies accounted for confounding factors, which is important because the factors that might indicate treatment with ACEIs or ARBs, such as comorbid cardiovascular conditions or diabetes, might also contribute to the development of severe COVID-19. We suspect that most of the patients taking RAS inhibitors had multiple comorbidities and cardiovascular risk factors, leading to a worse prognosis. Additionally, some of these studies' analyses were crude estimates that were not adjusted for confounding factors associated with hypertension, such as older age and cardiovascular disease. The adjustment of analyses is crucial for controlling for confounding factors, reducing bias, and increasing the reliability of the conclusions.
There have been three previous systematic reviews examining the effects of ACEI/ARB use in COVID-19 patients. Zhang et al. (51) found that ACEI/ARB exposure was not associated with a higher risk of severe disease or mortality. However, only 12 studies with unadjusted estimates were considered. Guo et al. (62) showed that ACEI/ARB use was associated with lower mortality in COVID-19 patients, although only six studies were included. Mackey et al. (63) conducted a narrative synthesis of 14 studies and concluded that there was no evidence of an association between ACEI/ARB use and severe COVID-19. In addition, Calderia et al. (64) and Barochiner and Martínez (65) drew similar conclusions, indicating that the use of ACEIs/ARBs does not increase the risk of severe COVID-19 or mortality; indeed, they suggested that the use of ACEIs/ARBs may have a protective effect. Our analysis included 48 studies and evaluated three outcomes. Additionally, to our knowledge, our review is the first to pool 35 adjusted effect estimates for mortality and severe COVID-19.
The results of the latest clinical trial, BRACE CORONA, have shown that the discontinuation of ACEIs/ARBs had no significant impact on the average survival duration or hospitalization duration (66). Currently, the European Society of Hypertension recommends continuing treatment with ACEIs/ARBs in patients with hypertension and COVID-19. These conclusions are consistent with those of our meta-analysis (67). We believe that the benefits of continuing treatment with ACEIs/ARBs outweigh the potential risks. Future well-designed randomized controlled trials and studies exploring the underlying mechanisms are needed to improve the level of evidence and determine whether the use of ACEIs/ARBs has an effect on the prognosis of patients with COVID-19.
Limitation
First, although most of the available studies included in this meta-analyses reported adjusted estimates, some of the studies did not adjust the models, leading to an increased risk of bias in the pooled effect measures. Second, the majority of the included studies were observational in nature; thus, causality cannot be concluded because of the methodological limitations of this design. Third, heterogeneity was high in most of the evaluated outcomes. Possible reasons for the heterogeneity were the sample sizes, differences in outcome definitions, heterogeneous population, etc. Finally, the inconsistency of reporting the discontinuation of ACEIs or ARBs during hospitalization across studies could have influenced the pooled estimates.
Conclusion
This meta-analysis suggested that ACEI/ARB use was not significantly associated with all-cause mortality in patients with hypertension who contracted COVID-19. In addition, ACEIs/ARBs had no significant effect on disease severity or the duration of hospitalization in COVID-19 patients with hypertension. This study provides additional evidence in favor of continuing antihypertension therapy after contracting COVID-19 unless the drugs cannot be tolerated because of hemodynamic instability.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
Z-YA: analysis and interpretation of data, drafting the article, and reviewing and editing the article. X-CD, Z-YW, and Z-ZW: conception and design of the study, acquisition of data, and final approval of the version to be published. Z-YA and Y-RW: drafting the manuscript and checking the methodology and making the data curation. All authors: contributed to the article and approved the submitted version.
Funding
The study was supported by the Zhejiang Provincial Science Foundation of China under Grant No. LY20H020006 and the Zhejiang Provincial Basic Public Welfare Research Program of China under Grant No. LGF19H020007.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.609857/full#supplementary-material
References
1. Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens Res. (2020) 43:985–6. doi: 10.1038/s41440-020-0455-8
2. Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 Spike glycoprotein. Cell. (2020) 181:281–92.e6. doi: 10.1016/j.cell.2020.02.058
3. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80.e8. doi: 10.1016/j.cell.2020.02.052
4. Lopes RD, Macedo AVS, de Barros ESPGM, Moll-Bernardes RJ, Feldman A, D'Andréa Saba Arruda G, et al. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–The BRACE CORONA Trial. Am Heart J. (2020) 226:49–59. doi: 10.1016/j.ahj.2020.05.002
5. Kreutz R, Algharably EAE, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res. (2020) 116:1688–99. doi: 10.1093/cvr/cvaa097
6. Esler M, Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. (2020) 38:781–2. doi: 10.1097/HJH.0000000000002450
7. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. (2020) 382:1653–9. doi: 10.1056/NEJMsr2005760
8. Tan ND, Qiu Y, Xing XB, Ghosh S, Chen MH, Mao R. Associations between angiotensin-converting enzyme inhibitors and angiotensin ii receptor blocker use, gastrointestinal symptoms, and mortality among patients with COVID-19. Gastroenterology. (2020) 159:1170–2.e1. doi: 10.1053/j.gastro.2020.05.034
9. Ingraham NE, Barakat AG, Reilkoff R, Bezdicek T, Schacker T, Chipman JG, et al. Understanding the renin-angiotensin-aldosterone-SARS-CoV-axis: a comprehensive review. Eur Respir J. (2020) 56:2000912. doi: 10.1183/13993003.00912-2020
10. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
11. Organization WH. Clinical Management of Severe Acute Respiratory Infection when Novel Coronavirus (nCoV) Infection is Suspected. Available online at: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf
12. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statist Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
13. Andrea C, Francesco M, Antonio N, Evgeny F, Marzia S, Fabio C, et al. Renin-angiotensin-aldosterone system inhibitors and outcome in patients with SARS-CoV-2 pneumonia: a case series study. Hypertension. (2020) 76:e10–2. doi: 10.1161/HYP.0000000000000185
14. Ashraf MA, Shokouhi N, Shirali E, Davari-tanha F, Memar O, Kamalipour A, et al. COVID-19 in Iran, a comprehensive investigation from exposure to treatment outcomes. medRxiv [Preprint]. (2020). doi: 10.21203/rs.3.rs-26339/v1
15. Baker KF, Hanrath AT, Schim van der Loeff I, Tee SA, Capstick R, Marchitelli G, et al. COVID-19 management in a UK NHS Foundation trust with a high consequence infectious diseases centre: a detailed descriptive analysis. Med Sci (Basel). (2021) 9:6. doi: 10.3390/medsci9010006
16. Bean DM, Kraljevic Z, Searle T, Bendayan R, Kevin O, Pickles A, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur J Heart Fail. (2020) 22:967–74. doi: 10.1002/ejhf.1924
17. Gili T, Benelli G, Buscarini E, Canetta C, La Piana G, Merli G, et al. SARS-COV-2 comorbidity network and outcome in hospitalized patients in Crema, Italy. PLoS One. (2021) 16:e0248498. doi: 10.1371/journal.pone.0248498
18. Bravi F, Flacco ME, Carradori T, Volta CA, Cosenza G, De Togni A, et al. Predictors of severe or lethal COVID-19, including Angiotensin Converting Enzyme inhibitors and Angiotensin II Receptor Blockers, in a sample of infected Italian citizens. PLoS One. (2020) 15:e0235248. doi: 10.1371/journal.pone.0235248
19. Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C, et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. (2020) 43:1399–407. doi: 10.2337/dc20-0660
20. Choi HK, Koo H-J, Seok H, Jeon JH, Choi WS, Kim DJ, et al. ARB/ACEI use and severe COVID-19: a nationwide case-control study. medRxiv [Preprint]. (2020). doi: 10.1101/2020.06.12.20129916
21. Dauchet L, Lambert M, Gauthier V, Poissy J, Faure K, Facon A, et al. ACE inhibitors, AT1 receptor blockers and COVID-19: clinical epidemiology evidences for a continuation of treatments. the ACER-COVID study. medRxiv [Preprint]. (2020). doi: 10.1101/2020.04.28.20078071
22. De Spiegeleer A, Bronselaer A, Teo JT, Byttebier G, De Tré G, Belmans L, et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J Am Med Dir Assoc. (2020) 21:909–14.e2. doi: 10.1016/j.jamda.2020.06.018
23. Felice C, Nardin C, Di Tanna GL, Grossi U, Bernardi E, Scaldaferri L, et al. Use of RAAS inhibitors and risk of clinical deterioration in COVID-19: results from an Italian cohort of 133 hypertensives. Am J Hypertens. (2020) 33:944–8. doi: 10.1093/ajh/hpaa096
24. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. (2020) 201:1380–8. doi: 10.1164/rccm.202002-0445OC
25. Fosbol EL, Butt JH, Ostergaard L, Andersson C, Selmer C, Kragholm K, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. (2020) 324:168–77. doi: 10.1001/jama.2020.11301
26. Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. (2020) 41:2058–66. doi: 10.1093/eurheartj/ehaa433
27. Giorgi Rossi P, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R. Reggio emilia COVID-19 working group. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS One. (2020) 15:e0238281. doi: 10.1371/journal.pone.0238281
28. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease (2019). (COVID-19). JAMA Cardiol. (2020) 5:811–8. doi: 10.1001/jamacardio.2020.1017
29. Huang Z, Cao J, Yao Y, Jin X, Luo Z, Xue Y, et al. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med. (2020) 8:430. doi: 10.21037/atm.2020.03.229
30. Ip A, Parikh K, Parrillo JE, Mathura S, Hansen E, Sawczuk IS. hypertension and renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. medRxiv [Preprint]. (2020). doi: 10.1101/2020.04.24.20077388
31. Jung SY, Choi JC, You SH, Kim WY. Association of renin-angiotensin-aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis. (2020) 71:2121–8. doi: 10.1093/cid/ciaa624
32. Jurado A, Martín MC, Abad-Molina C, Orduña A, Martínez A, Ocaña E, et al. COVID-19: age, Interleukin-6, C-reactive protein, and lymphocytes as key clues from a multicentre retrospective study. Immun Ageing. (2020) 17:22. doi: 10.1186/s12979-020-00194-w
33. Khera R, Clark C, Lu Y, Guo Y, Ren S, Truax B, et al. Association of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with coronavirus disease-19. J Am Heart Assoc. (2021) 24:e018086. doi: 10.1161/JAHA.120.018086
34. Kim L, Garg S, O'Halloran A, Whitaker M, Pham H, Anderson EJ, et al. Interim analysis of risk factors for severe outcomes among a cohort of hospitalized adults identified through the U.S. Coronavirus Disease. 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). medRxiv [Preprint]. (2020). doi: 10.1101/2020.05.18.20103390
35. Lee H-Y, Ahn J, Kang CK, Won S-H, Park J-H, Kang CH, et al. Association of Angiotensin II receptor association of Angiotensin II Receptor blockers and angiotensin-converting enzyme inhibitors on COVID-19-related outcome. Lancet. (2020). doi: 10.2139/ssrn.3569837. [Epub ahead of print].
36. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease (2019). (COVID-19) infection in Wuhan, China. JAMA Cardiol. (2020) 5:825–30. doi: 10.1001/jamacardio.2020.1624
37. Liabeuf S, Moragny J, Bennis Y, Batteux B, Brochot E, Schmit JL, et al. Association between renin-angiotensin system inhibitors and COVID-19 complications. Eur Heart J Cardiovasc Pharmacother. (2020) doi: 10.1093/ehjcvp/pvaa062. [Epub ahead of print].
38. Liu Y, Huang F, Xu J, Yang P, Qin Y, Cao M, et al. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.20.20039586
39. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. (2020) 382:2431–40. doi: 10.1056/NEJMoa2006923
40. Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, et al. Association of use of angiotensin-converting enzyme inhibitors and Angiotensin II receptor blockers with testing positive for coronavirus disease 2019. (COVID-19). JAMA Cardiol. (2020) 5:1020–6. doi: 10.1001/jamacardio.2020.1855
41. Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microb Infect. (2020) 9:757–60. doi: 10.1080/22221751.2020.1746200
42. Peng YD, Meng K, Guan HQ, Leng L, Zhu RR, Wang BY, et al. Clinical characteristics outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. (2020) 48:450–5. doi: 10.3760/cma.j.cn112148-20200220-00105
43. Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54-75 years. medRxiv [Preprint]. (2020). doi: 10.1101/2020.04.09.20059964
44. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. (2020) 382:2441–8. doi: 10.1056/NEJMoa2008975
45. Rhee SY, Lee J, Nam H, Kyoung DS, Shin DW, Kim DJ. Effects of a DPP-4 inhibitor and RAS blockade on clinical outcomes of patients with diabetes and COVID-19. Diabetes Metab J. (2021) 45:251–9. doi: 10.4093/dmj.2020.0206
46. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. (2020) 324:782–93. doi: 10.1001/jama.2020.6775
47. Tedeschi S, Giannella M, Bartoletti M, Trapani F, Tadolini M, Borghi C, et al. Clinical impact of renin-angiotensin system inhibitors on in-hospital mortality of patients with hypertension hospitalized for coronavirus disease 2019. Clin Infect Dis. (2020) 71:899–901. doi: 10.1093/cid/ciaa492
48. Yan H, Valdes AM, Vijay A, Wang S, Liang L, Yang S, et al. Role of drugs affecting the renin-angiotensin-aldosterone system on susceptibility and severity of COVID-19: a large case-control study from Zheijang Province, China. medRxiv [Preprint]. (2020). doi: 10.1101/2020.04.24.20077875
49. Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, et al. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID-19 patients with hypertension: a single center retrospective study. Hypertension. (2020) 76:51–8. doi: 10.1161/HYPERTENSIONAHA.120.15143
50. Zeng Z, Sha T, Zhang Y, Wu F, Hu H, Li H, et al. Hypertension in patients hospitalized with COVID-19 in Wuhan, China: a single-center retrospective observational study. medRxiv [Preprint]. (2020). doi: 10.1101/2020.04.06.20054825
51. Zhang X, Yu J, Pan LY, Jiang HY. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol Res. (2020) 158:104927. doi: 10.1016/j.phrs.2020.104927
52. Zhou X, Zhu J, Xu T. Clinical characteristics of coronavirus disease 2019. (COVID-19) patients with hypertension on renin-angiotensin system inhibitors. Clin Exp Hypertens. (2020) 42:656–60. doi: 10.1080/10641963.2020.1764018
53. Zhou F, Liu YM, Xie J, Li H, Lei F, Yang H, et al. Comparative impacts of ACE (Angiotensin-Converting Enzyme) inhibitors versus Angiotensin II receptor blockers on the risk of COVID-19 mortality. Hypertension. (2020) 76:e15–7. doi: 10.1161/HYPERTENSIONAHA.120.15622
54. Regina J, Papadimitriou-Olivgeris M, Burger R, Le Pogam MA, Niemi T, Filippidis P, et al. Epidemiology, risk factors and clinical course of SARS-CoV-2 infected patients in a Swiss university hospital: An observational retrospective study. PLoS One. (2020) 15:e0240781. doi: 10.1371/journal.pone.0240781
55. Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. (2020) 201:1430–4. doi: 10.1164/rccm.202003-0736LE
56. Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, et al. Angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors usage is associated with improved inflammatory status and clinical outcomes in COVID-19 patients with hypertension. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.31.20038935
57. Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. (2020) 116:1666–87. doi: 10.1093/cvr/cvaa106
58. Sriram K, Insel PA. Risks of ACE inhibitor and ARB usage in COVID-19: evaluating the evidence. Clin Pharmacol Ther. (2020) 108:236–41. doi: 10.1002/cpt.1863
59. Rico-Mesa JS, White A, Anderson AS. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr Cardiol Rep. (2020) 22:31. doi: 10.1007/s11886-020-01291-4
60. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. (2020) 94:e00127–20. doi: 10.1128/JVI.00127-20
61. Guo J, Huang Z, Lin L, Lv J. Coronavirus Disease 2019. (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome Coronavirus 2 infection. J Am Heart Assoc. (2020) 9:e016219. doi: 10.1161/JAHA.120.016219
62. Guo X, Zhu Y, Hong Y. Decreased mortality of COVID-19 with renin-angiotensin-aldosterone system inhibitors therapy in patients with hypertension: a meta-analysis. Hypertension. (2020) 76:e13–4. doi: 10.1161/HYPERTENSIONAHA.120.15572
63. Mackey K, King VJ, Gurley S, Kiefer M, Liederbauer E, Vela K, et al. Risks and impact of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers on SARS-CoV-2 INFECTION IN ADULTS. Ann Intern Med. (2020) 173:195–203. doi: 10.7326/M20-1515
64. Caldeira D, Alves M, Gouveia EMR, Silvério António P, Cunha N, Nunes-Ferreira A, et al. Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers and the risk of COVID-19 infection or severe disease: systematic review and meta-analysis. Int J Cardiol Heart Vasc. (2020) 31:100627. doi: 10.1016/j.ijcha.2020.100627
65. Barochiner J, Martínez R. Use of inhibitors of the renin-angiotensin system in hypertensive patients and COVID-19 severity: A systematic review and meta-analysis. J Clin Pharm Ther. (2020) 45:1244–52. doi: 10.1111/jcpt.13246
66. Lopes RD, Macedo AVS, de Barros ESPGM, Moll-Bernardes RJ, Dos Santos TM, Mazza L, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and Angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. (2021) 325:254–64. doi: 10.1001/jama.2020.25864
Keywords: angiotensin receptor blockers, angiotensin converting enzyme inhibitors, coronavirus disease 2019, hypertension, death
Citation: Dai X-C, An Z-Y, Wang Z-Y, Wang Z-Z and Wang Y-R (2021) Associations Between the Use of Renin–Angiotensin System Inhibitors and the Risks of Severe COVID-19 and Mortality in COVID-19 Patients With Hypertension: A Meta-Analysis of Observational Studies. Front. Cardiovasc. Med. 8:609857. doi: 10.3389/fcvm.2021.609857
Received: 24 September 2020; Accepted: 22 February 2021;
Published: 26 April 2021.
Edited by:
Hendrik Tevaearai Stahel, Bern University Hospital, SwitzerlandReviewed by:
Michel Burnier, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandJane A. Leopold, Brigham and Women's Hospital and Harvard Medical School, United States
Copyright © 2021 Dai, An, Wang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Ce Dai, aWVsdHNfa3Jpc0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Xiao-Ce Dai
Xiao-Ce Dai Zhuo-Yu An
Zhuo-Yu An Zi-Yang Wang2
Zi-Yang Wang2