- 1Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2School of Medicine, Guangdong Provincial People's Hospital, South China University of Technology, Guangzhou, China
- 3The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
- 4Fujian Provincial Key Laboratory of Cardiovascular Disease, Department of Cardiology, Fujian Provincial Center for Geriatrics, Fujian Cardiovascular Institute, Fujian Provincial Hospital, Provincial Clinical Medicine College of Fujian Medical University, Fuzhou, China
- 5Department of Biostatistics, School of Public Health, Southern Medical University, Guangzhou, China
Aims: Very few of the risk scores to predict infection in ST-segment elevation myocardial infarction (STEMI) patients undergoing percutaneous coronary intervention (PCI) have been validated, and reports on their differences. We aimed to validate and compare the discriminatory value of different risk scores for infection.
Methods: A total of 2,260 eligible patients with STEMI undergoing PCI from January 2010 to May 2018 were enrolled. Six risk scores were investigated: age, serum creatinine, or glomerular filtration rate, and ejection fraction (ACEF or AGEF) score; Canada Acute Coronary Syndrome (CACS) risk score; CHADS2 score; Global Registry for Acute Coronary Events (GRACE) score; and Mehran score conceived for contrast induced nephropathy. The primary endpoint was infection during hospitalization.
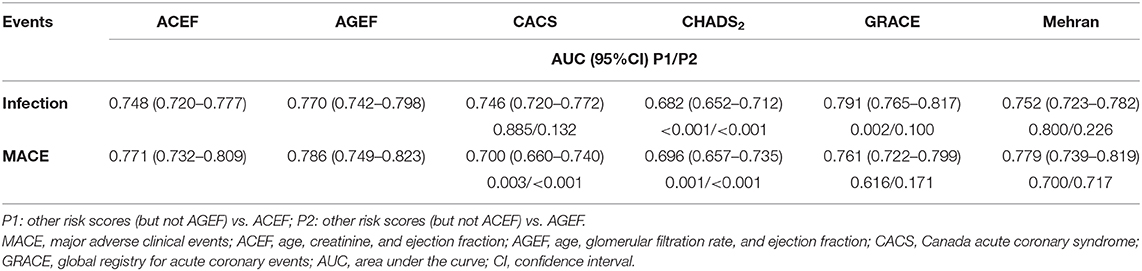
Results: Except CHADS2 score (AUC, 0.682; 95%CI, 0.652–0.712), the other risk scores showed good discrimination for predicting infection. All risk scores but CACS risk score (calibration slope, 0.77; 95%CI, 0.18–1.35) showed best calibration for infection. The risks scores also showed good discrimination for in-hospital major adverse clinical events (MACE) (AUC range, 0.700–0.786), except for CHADS2 score. All six risk scores showed best calibration for in-hospital MACE. Subgroup analysis demonstrated similar results.
Conclusions: The ACEF, AGEF, CACS, GRACE, and Mehran scores showed a good discrimination and calibration for predicting infection and MACE.
Introduction
Infection complicating the course of ST-segment elevation myocardial infarction (STEMI) is uncommon, with a reported incidence of 2.4%; nonetheless, it is associated with markedly worse 90-day clinical outcomes and longer hospital stay (1). Since a substantial proportion of infections are considered preventable, identifying patients at risk of infection is essential to estimate patients' prognosis, aid in clinical decision making, and ensure quality control. However, current data on infection prediction in patients with STEMI undergoing percutaneous coronary intervention (PCI) are limited.
Some commonly used risk scores in clinical practice, such as the age, serum Creatinine (sCr), or Glomerular filtration rate, and Ejection Fraction (ACEF or AGEF) score, Canada Acute Coronary Syndrome (CACS) score, CHADS2 score, Global Registry for Acute Coronary Events (GRACE) score and Mehran score (conceived for contrast induced nephropathy) have been reported to predict several clinical outcomes in patients with STEMI who have undergone PCI (2–7), and all scores include some risk factors of infection (age, heart failure, diabetes mellitus and so on). Additionally, our recent studies showed that the ACEF/AGEF, and CACS scores performed well in predicting infection in STEMI patients (8, 9). However, the use of other risk scores for infection prediction in such patients has not been fully examined and validated in large-sample studies. Thus, the present study aimed to validate and compare the performance of six risk scores for infection in patients with STEMI undergoing PCI.
Methods
Study Population
This observational cohort study prospectively enrolled a series of consecutive patients with STEMI undergoing PCI after symptoms onset in the department of cardiac care unit at Guangdong Provincial People's Hospital from January 2010 to May 2018. STEMI was defined as the manifestation of typical chest pain and concomitant symptoms for ≥ 30 min but <12 h, with ST-segment elevation ≥ 1 mm in ≥ 2 continuous leads or new or undetermined duration of left branch bundle block accompanying with ≥ 2 times increase in troponin I or T (10). Patients diagnosed with infection prior to admission were excluded. Patients with any of the following conditions were also excluded from the study: (a) tumors or chronic inflammatory diseases, (b) on hemodialysis at admission for chronic renal failure, (c) undergoing emergency cardiac surgery, (d) died within 24 h after admission, (e) readmission to hospital and (f) with missing variables that are needed to calculate the risk scores. The study protocol was approved by the ethics and research committee of our hospital and performed in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from the patients prior to study participation. This study was registered with the Chinese Clinical Trial Registry (ChiCTR 1900028278).
Procedure and Medications
Baseline demographic characteristics and relevant clinical information required for calculating the different scores of each patient were obtained from the hospital electronic database. All included patients had a routine chest X-ray, and ultrasonic cardiography was conducted after admission. Blood, urine, and sputum cultures were examined at the discretion of the physicians. All patients were treated in accordance with our institution's protocol and the European Society of Cardiology guidelines (10). A 24 h on-call interventional team performed PCI, following standard clinical practice and techniques. All eligible patients were administered aspirin (300 mg), and clopidogrel (300 or 600 mg) or ticagrelor (180 mg) before PCI. After the PCI procedure, the patients received dual antiplatelet therapy (aspirin, 100 mg/day combined with clopidogrel, 75 mg/day, or ticagrelor, 90 mg twice/day). According to clinical protocols that are based on interventional guidelines (10), prescription of anticoagulants, glycoprotein IIb/IIIa inhibitors, β blockers, or angiotensin-converting enzyme inhibitors/angiotensin receptor blockers was at the cardiologist's discretion.
Score Calculations
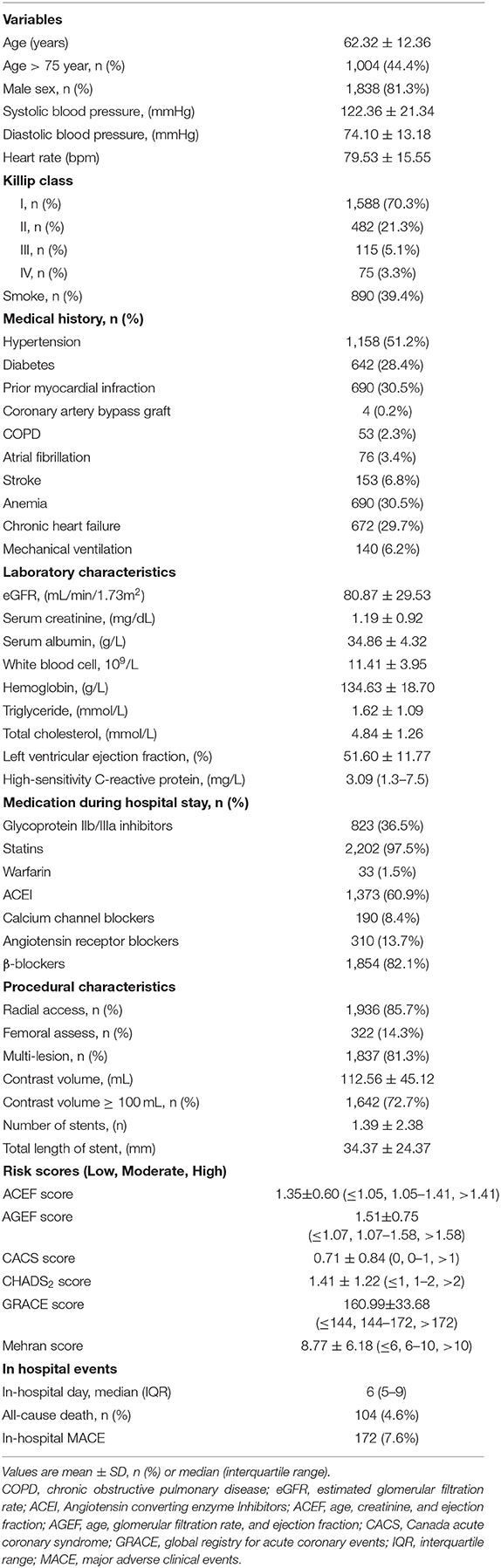
Score calculations were performed by experienced cardiologists who were blinded to the outcomes. The ACEF and AGEF scores were calculated within 24 h after admission using the following formula: ACEF = age/left ventricular ejected fraction (LVEF) +1 (if the sCr level was > 2 mg/dL) (11), and AGEF = age/LVEF (%) +1 (if the estimated glomerular filtration rate (eGFR) was < 60 mL/min/1.73 m2) (3). In addition, the eGFR was calculated using the 4-variable modification of diet in renal disease equation (12). The CACS score was calculated on a scale of 0–4 at admission, based on four clinical parameters (1 point for each): age ≥ 75 years, Killip class > 1, systolic blood pressure < 100 mmHg, and heart rate > 100 beats/min) (4). For CHADS2 score, 2 points were assigned for history of stroke or transient ischemic attack and 1 point for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus (5). The GRACE score, which included eight variables (i.e., age, heart rate, systolic blood pressure, creatinine, Killip's classification, cardiac arrest at admission, increased cardiac markers and ST-segment deviation), was calculated according to the computational method (Supplementary Table 1) (6). Mehran score, which included the following eight variables: hypotension, congestive heart failure, chronic kidney disease, age > 75 years, anemia, diabetes, contrast medium volume, and use of intra-aortic balloon pump, was defined according to the specifications of Mehran et al. (13). In order to compare the differences among risk scores, patients were assigned into categories according to tertiles from the present data. Patients in tertiles I, II, and III of each risk score were considered as low-risk, moderate-risk, and high-risk populations, respectively.
Study Endpoints
The primary endpoint was infection during hospitalization, which was diagnosed based on signs, symptoms and laboratory tests compatible with infection. In addition, infection was categorized as pulmonary infections, urinary infections or others (i.e., primary bacteremia, abdominal sepsis, and unidentified primary infection site), according to the clinical records during hospitalization. Appropriate antibiotics were administered once infection was confirmed (14). The hospital infection control committee validated the antibiotics and approved their initiation for each infection case according to the hospital's infection prevention and control regime. The secondary endpoints were in-hospital MACE, which was defined as a composite endpoint of all-cause death, recurrent myocardial infarction, target vessel revascularization, stroke, and renal replacement therapy during hospitalization.
Statistical Analysis
Chi-squared test or Fisher's exact test was used, as appropriate, to compare the categorical variables, which were expressed as percentages. Comparisons between normally distributed continuous variables, which were expressed as mean ± SD, were performed using two-sample t-tests. Non-normally distributed continuous variables, presented as median and interquartile range, were analyzed using Wilcoxon rank sum tests. Area under the receiver-operating characteristic curves (AUC) was constructed to assess the discrimination of risk scores for infection and MACE, which was expressed by c-statistic. An AUC < 0.60 was considered to reflect poor discrimination, 0.60–0.75 good discrimination, and > 0.75 best discrimination (15). AUCs were compared using the non-parametric approach of DeLong et al. (16). An objective assessment of calibration was analyzed using the Hosmer-Lemeshow goodness-of-fit test (17); calibration slope (plotting the observed proportions vs. the predicted probabilities), with 1 indicated perfect calibration. In addition, decision curve analysis was introduced to evaluate the utility of the risk scores; the risk scores with a higher net benefit across the range of thresholds indicated better clinical usefulness (18). All tests were two tailed, and a P < 0.05 was considered statistically significant. All data analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Clinical Characteristics
The study flow is shown in Figure 1. A total of 2,260 consecutive patients with STEMI (62.32 ± 12.36 years; 81.3% males) were finally included. All patients' baseline clinical characteristics and the mean of all risk scores are presented in Table 1. Briefly, 51.3% patients had hypertension, 28.4% had diabetes, and 30.5% had prior myocardial infraction. Mean eGFR, LVEF, and contrast volume were 80.87 ± 29.53 mL/min/1.73 m2, 51.60 ± 11.77% and 112.56 ± 45.12 mL, respectively. The overall incidence of infection during hospitalization was 16.0%, the incidence of pulmonary infections and urinary infections was 70.6 and 15.0%, respectively. The rate of mechanical ventilation was 6.2% during hospitalization, and the rate of in-hospital MACE was 7.6% (Table 1).
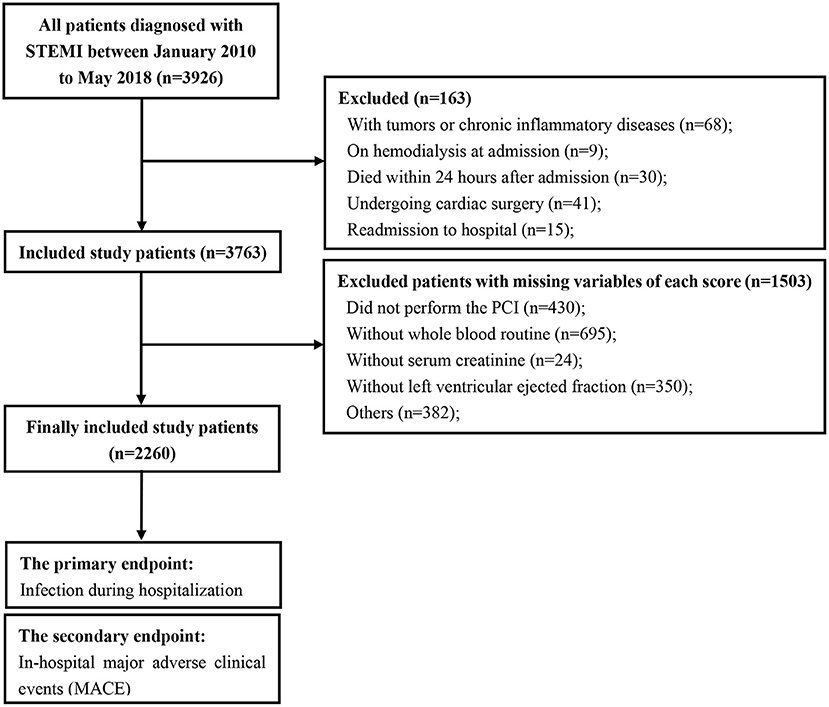
Figure 1. Study flow of participants. STEMI, ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention.
All six risk scores showed abnormal distribution (P < 0.01) in Supplementary Figure 1. Clinical outcomes according to tertiles (low-, moderate-, and high-risk groups) of all the six risk scores are shown in Figure 2. A significant positive gradient of risk with respect to infection and MACE was observed as the risk scores increased.
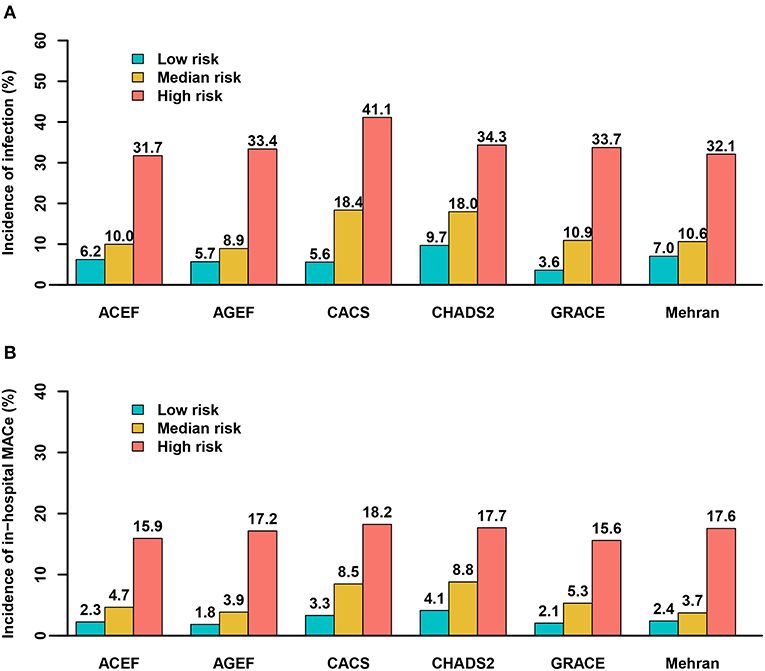
Figure 2. Predictive ability of the risk scores for infection and major adverse clinical events by tertiles. (A) infection; (B) MACE. ACEF (≤1.05, 1.05–1.41, >1.41); AGEF (≤1.07, 1.07–1.58, >1.58); CACS (0, 0–1, >1); CHADS2 (≤1, 1–2, >2); GRACE (≤144, 144–172, >172); Mehran (≤6, 6–10, >10).
Discrimination for Infection and In-hospital MACE
The prognostic accuracy of the six risk scores for infection and in-hospital MACE are presented in Table 2 and Supplementary Figure 2. Each score showed a clear discrimination for infection, with AUCs ranging from 0.746 to 0.791, except for CHADS2 risk score [AUC, 0.682; 95% confidence interval [CI], 0.652–0.712] (Table 2 and Supplementary Figure 2). Additionally, the prognostic accuracy of all risk scores was assessed according to the subtypes of infection. CHADS2 score had a relatively poor predictive value for pulmonary infection (AUC, 0.675; 95% CI, 0.639–0.710). CHADS2 and Mehran risk score showed poor predictive value for urinary infection (AUC, 0.653 for CHADS2; AUC, 0.697 for Mehran, respectively), and other risk scores displayed good predictive power for both pulmonary infection and urinary infection (AUC > 0.70).
Furthermore, each score had a best discrimination for in-hospital MACE, with AUCs ranging from 0.700 to 0.786, except for CHADS2 risk score, with AUC of 0.696 (Table 2 and Supplementary Figure 2).
Predictive Values of Risk Scores for Subgroup Analysis
Subgroup analysis was performed according to the WBC count (≥10 × 109/L or <10 × 109/L), gender and hypertension. The results showed similar discrimination of these 6 risk scores for infections in these subgroups with the primary analysis (Supplementary Tables 2–4). However, the discriminative ability of these 6 risk scores for infection was relative lower for female than those for male (Supplementary Table 3).
Calibration of Risk Scores for Infection and In-hospital MACE
Figure 3 and Supplementary Figure 3 illustrated the calibration plots of the six risk scores for infection and MACE during hospitalization. All risk scores exhibited best calibration for in-hospital infection (calibration slope nearly at 1), while CACS risk score displayed good calibration (calibration slope, 0.77; 95%CI 0.18–1.35) (Figure 3). Moreover, for in-hospital MACE, the best calibration was found in all risk scores (calibration slope nearly at 1) (Supplementary Figure 3).
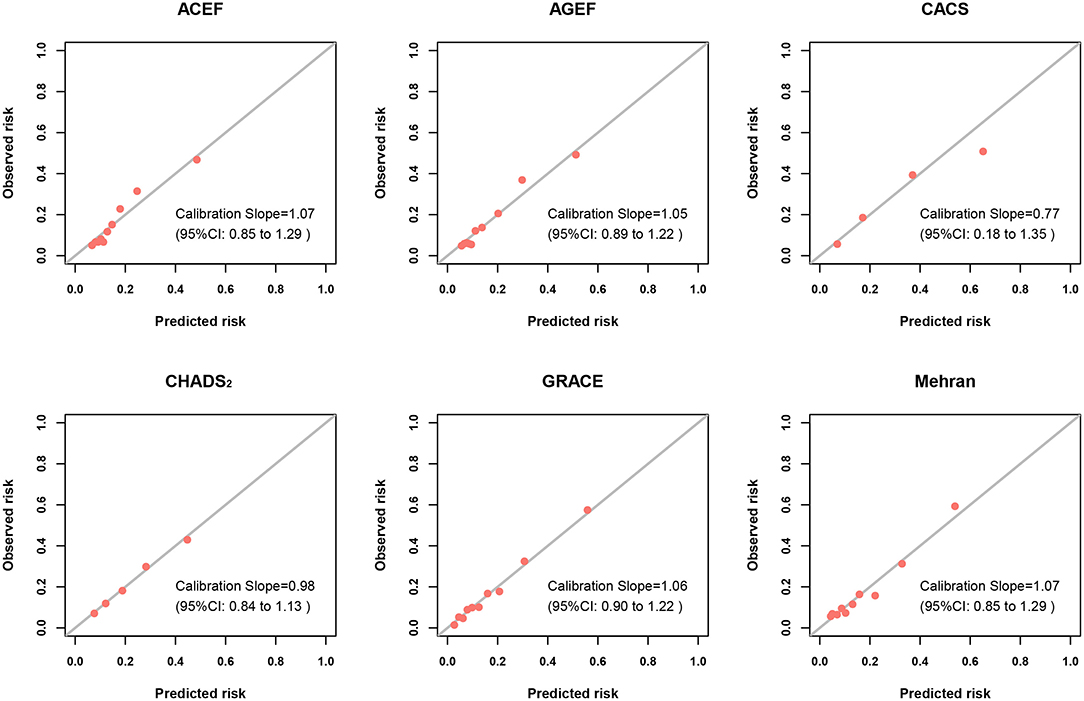
Figure 3. Calibration plots of risk scores for infection. Calibration plots showing the predicted probability vs. observed incidence of infection of risk scores. The diagonal line represents the perfect calibration (curve with a slope of 1 and an intercept of 0).
Utility of Risk Scores
When used to predict infection during hospitalization in patients with STEMI undergoing PCI, ACEF, AGEF, CACS, GRACE, and Mehran scores showed best discrimination (AUC ≥ 0.75) and good calibration abilities. And CHADS2 risk score had a relatively good calibration but low discrimination for infection. With regard to the prediction for in-hospital MACE, all risk scores showed best calibration abilities (calibration slope nearly at 1), and all risk scores except CHADS2 risk scores showed good discrimination (AUC ≥ 0.70).
In addition, the decision curves of the risk scores for infection and in-hospital MACE are shown in Figure 4 and Supplementary Figure 4, respectively. Except CHADS2 risk score, the other risk scores had a good standardized net benefit for infection; GRACE risk score had the greatest standardized net benefit. The risk scores, except CHADS2 and CACS risk score, exhibited a good standardized net benefit for in-hospital MACE.
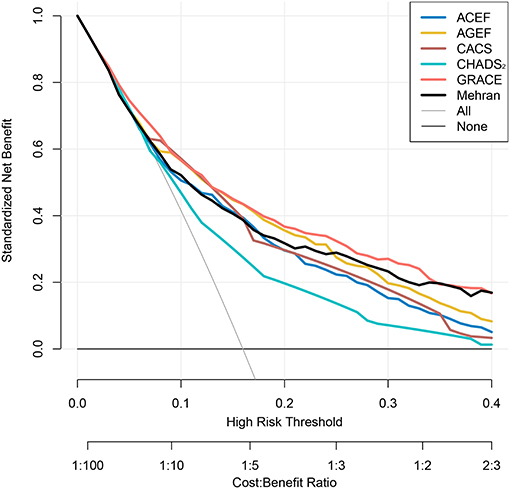
Figure 4. Decision curves of risk scores for infection. Net benefit of using models to predict infection development are shown. The GRACE risk score (Red) demonstrated the greatest standardized net benefit while CHADS2 risk score showed the lowest (Green).
Discussion
To our knowledge, this study is the first to validate and compare the prognostic values of six risk scores for infection and in-hospital MACE in patients with STEMI undergoing PCI. The major findings are as follows: (1) the risk scores, except CHADS2 risk score, showed good discrimination and calibration for infection, and the GRACE risk score was slightly superior to the other risk scores; (2) all six risk scores showed good discrimination and calibration for in-hospital MACE; and (3) among the six risk scores, the CACS risk score was preferentially recommended for clinical use as its clinical variables were simpler and more practical.
Infection, which is an important complication of patients with STEMI following PCI, is associated with significantly worse clinical outcomes (1). A recent prospective study reported that in-hospital mortality was twice higher in patients with MI concurrent of infection than those patients without infection (19). In our study, 361 patients (16.0%) had infection, which was slightly higher than the incidences in the previous studies (3.9 and 10%) (14, 19). Several studies have reported that infection is a common problem for intensive care unit (ICU) patients, and the risk of infection increases with the duration of ICU stay (20); thus, the higher rate of infection in our study may be associated with prolonged ICU stay (6 days vs. 1 day) (21). In addition, about 30% patients in Killip class ≥ II, suggesting a complicated situation, that can prolong hospital stay and the patients can be more prone to infections. Hence, identifying patients with STEMI with a high risk of infection is crucial. However, data on infection risk assessment in such patients are scarce.
Scoring systems currently used for clinical event risk stratification in patients with STEMI are based on multivariable models that integrate elements from medical history, admission electrocardiogram, biochemical evidence of myocyte necrosis, and renal function (2–7). Previous reports have proposed criteria for predicting infection in cardiovascular diseases, and mostly, in cardiac surgery (22, 23). Before the ACEF, AGEF, and CACS risk scores were firstly validated in our recent studies, a prediction score for infection in patients with STEMI undergoing PCI had not been reported (8, 9). Although none of the six risk scores were developed specifically for predicting infection, this study found that they could predict infection well and thus their use was expanded. The variables included in these risk scores were associated with the risk of infection. Thus, the good predictive value for infection was somehow expected. Previous studies demonstrated that renal function and hemodynamic status, including heart rate, and blood pressure, are associated with the development of infection (24–26). In addition, the complex and vicious interaction between heart failure and infection has been reported in many studies (27, 28). An increase in pulmonary pressure, that is induced by the decline in LVEF, results in pulmonary edema and accumulation of pneumonia related pathogens, such as Streptococcus pneumoniae, which has a deleterious effect and could lead to respiratory infection (29).
The GRACE risk score, which is a useful scoring model to predict short-term mortality in acute MI, has a high diagnostic accuracy for the prediction of 6-month post-discharge death in patients with acute coronary syndrome treated with primary PCI (6). Among the six risk scores, the GRACE risk score provided the greatest discrimination for infection risk prediction. However, the GRACE risk score requires evaluation of eight variables, and should be calculated using a computer, thereby making its use in the clinical setting challenging; a simpler risk score is required for widespread adoption. Moreover, compared to the GRACE and Mehran risk scores, the ACEF, AGEF, and CACS risk scores, which include three to four variables, are less complicated and also had best discrimination and good calibration for infection. Furthermore, the decision curves showed that these three risk scores had a good standardized net benefit for infection. Nevertheless, the ACEF and AGEF risk scores include the heart function evaluated by echocardiography and renal function measured by serum creatinine, whereas, the CACS risk score included accessible clinical variables only in clinical practice, which are simple to remember, and calculate, and thus could be time-saving. The CHADS2 score is a useful prognostic tool for predicting cardiovascular or cerebrovascular events in patients with acute coronary syndrome (30). In our study, the CHADS2 score showed good calibration but a relatively low discrimination, and bad standardized net benefit for infection, which in turn is of limited clinical use. A potential explanation of such low discrimination is that unlike hypotension, hypertension in the CHADS2 risk score was not proven to be associated with the risk of infection (26), thereby reducing the predictive value of CHADS2 risk score to some extent.
Although the six risk scores could predict clinical outcomes in patients with STEMI, differences in their prediction of in-hospital MACE remained unclear. Our study is the first to investigate the difference in predicting MACE among the risk scores. Of the six risk scores, ACEF, AGEF, GRACE, and Mehran risk scores showed best discrimination, CACS risk score and CHADS2 risk score showed good discrimination, and all risk scores showed best calibration. Nevertheless, considering its discrimination, calibration, and clinical simplicity, the CACS risk score may be a good tool for predicting MACE.
Notably, recent study has demonstrated that gender played an active role in the incidence and outcomes of major infectious diseases, such as community-acquired pneumonia (31). However, another investigation reported an opposite result that gender did not appear to play a role in acquisition of an intensive care unit-acquired infection in critically ill patients (32). In current study, the discriminative ability of these 6 risk scores for infection was relative lower for female than those for male, which suggested that gender difference might alter the prognostic capacity to some extent. Furthermore, Ishigami et al. (33) revealed that patients with severe hypertension on admission appeared to be at increased risk of stroke-associated pneumonia in elderly subjects with acute ischemic stroke. The result indicated that hypertension might contribute to the infection development. However, we demonstrated that all six risk scores displayed similar fair discriminative capacity for infection in patients with or without hypertension. Thus, further investigations might be required to examine the effects of hypertension on the prediction of these risk scores.
Our study also had several limitations. First, this study was performed at a single center; thus, the results should be interpreted prudently. Second, the predictive values observed were exclusive to patients with STEMI; hence, caution should be taken in applying the findings to other patients. Lastly, we only included six risk scores to predict infection because we believe that these risk scores are commonly used and those not included are excessively complicated. Future research may consider examining the predictive values of other risk scores in different populations.
In summary, for patients with STEMI undergoing PCI, ACEF, AGEF, CACS, GRACE, and Mehran risk score have good discrimination and calibration for predicting infection and in-hospital MACE during hospitalization. Of the six risk scores investigated in this study, the CACS risk score is preferentially recommended for clinical use as its variables are simpler and practical.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics and Research Committee of Guangdong Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
NT, PH, and YL: study design. LW, WC, LZ, HF, and YD: data collection. CD, YL, and LW: data analysis and interpretation. YL, LW, and WC: manuscript preparation. All authors: critical revision of manuscript.
Funding
This study was supported by the Shuangqing talent program of Guangdong Provincial People's Hospital (Grant No. KJ012019095), Science and Technology Planning Project of Guangzhou City (Grant No. 201906010089), The first clinical research training course seed fund of Guangdong Provincial People's Hospital (No. 2018lcpx04), and National Key R&D Program of China (2016YFC1301100). The funders had no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors appreciate the patients who participated in this trial and their relatives, the clinical and research teams, and the nursing teams in our hospital for their work on the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2020.621002/full#supplementary-material
References
1. Truffa AA, Granger CB, White KR, Newby LK, Mehta RH, Hochman JS, et al. Serious infection after acute myocardial infarction: incidence, clinical features, and outcomes. JACC Cardiovasc Interv. (2012) 5:769–76. doi: 10.1016/j.jcin.2012.03.018
2. Stähli BE, Wischnewsky MB, Jakob P, Klingenberg R, Obeid S, Heg D, et al. Predictive value of the age, creatinine, and ejection fraction (ACEF) score in patients with acute coronary syndromes. Int J Cardiol. (2018) 270:7–13. doi: 10.1016/j.ijcard.2018.05.134
3. Andò G, Morabito G, de Gregorio C, Trio O, Saporito F, Oreto G. Age, glomerular filtration rate, ejection fraction, and the AGEF score predict contrast-induced nephropathy in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Catheter Cardiovasc Interv. (2013) 82:878–85. doi: 10.1002/ccd.25023
4. Huynh T, Kouz S, Yan AT, Yan A, Danchin N, O'Loughlin J, et al. Canada acute coronary syndrome risk score: a new risk score for early prognostication in acute coronary syndromes. Am Heart J. (2013) 166:58–63. doi: 10.1016/j.ahj.2013.03.023
5. Poçi D, Hartford M, Karlsson T, Herlitz J, Edvardsson N, Caidahl K. Role of the CHADS2 score in acute coronary syndromes: risk of subsequent death or stroke in patients with and without atrial fibrillation. Chest. (2012) 141:1431–40. doi: 10.1378/chest.11–0435
6. Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. (2004) 291:2727–33. doi: 10.1001/jama.291.22.2727
7. Sgura FA, Bertelli L, Monopoli D, Leuzzi C, Guerri E, Spartà I, et al. Mehran contrast-induced nephropathy risk score predicts short- and long-term clinical outcomes in patients with ST-elevation-myocardial infarction. Circ Cardiovasc Interv. (2010) 3:491–8. doi: 10.1161/CIRCINTERVENTIONS.110.955310
8. Wang L, Huang G, Peng Q, Duan C, Dai Y, Shao S, et al. Risk predictive ability of ACEF score for infection in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. Eur J Prev Cardiol. (2020) 27:220–2. doi: 10.1177/2047487319873142
9. Liu Y, Dai Y, Chen J, Huang C, Duan C, Shao S, et al. Predictive value of the Canada Acute Coronary Syndrome risk score for post-acute myocardial infarction infection. Eur J Intern Med. (2020) 71:57–61. doi: 10.1016/j.ejim.2019.10.012
10. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
11. Ranucci M, Castelvecchio S, Menicanti L, Frigiola A, Pelissero G. Risk of assessing mortality risk in elective cardiac operations: age, creatinine, ejection fraction, and the law of parsimony. Circulation. (2009) 119:3053–61. doi: 10.1161/CIRCULATIONAHA.108.842393
12. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. (2006) 17:2937–44. doi: 10.1681/ASN.2006040368
13. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. (2004) 44:1393–9. doi: 10.1016/S0735–1097(04)01445–7
14. Piccaro de Oliveira P, Gonzales V, Lopes RD, Schmidt MM, Garofallo S, Santos RP, et al. Serious infections among unselected patients with ST-elevation myocardial infarction treated with contemporary primary percutaneous coronary intervention. Am Heart J. (2016) 181:52–9. doi: 10.1016/j.ahj.2016.08.005
15. Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: users' guides to the medical literature. JAMA. (2017) 318:1377–84. doi: 10.1001/jama.2017.12126
16. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45. doi: 10.2307/2531595
17. Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness- of-fit tests for the logistic regression model. Stat Med. (1997) 16:965–80. doi: 10.1002/(SICI)1097–0258(19970515)16:9<965::AID-SIM509>3.0.CO;2-O
18. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. (2006) 26:565–74. doi: 10.1177/0272989X06295361
19. Putot A, Chague F, Manckoundia P, Cottin Y, Zeller M. Post-infectious myocardial infarction: new insights for improved screening. J Clin Med. (2019) 8:E827. doi: 10.3390/jcm8060827
20. Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. (2009) 302:2323–9. doi: 10.1001/jama.2009.1754
21. Shavadia JS, Chen AY, Fanaroff AC, de Lemos JA, Kontos MC, Wang TY. Intensive care utilization in stable patients with ST-segment elevation myocardial infarction treated with rapid reperfusion. JACC Cardiovasc Interv. (2019) 12:709–17. doi: 10.1016/j.jcin.2019.01.230
22. Kansy A, Jacobs JP, Pastuszko A, Mirkowicz-Małek M, Manowska M, Jezierska E, et al. Major infection after pediatric cardiac surgery: external validation of risk estimation model. Ann Thorac Surg. (2012) 94:2091–5. doi: 10.1016/j.athoracsur.2012.07.079
23. Sarmiento E, Navarro J, Fernandez-Yañez J, Palomo J, Muñoz P, Carbone J. Evaluation of an immunological score to assess the risk of severe infection in heart recipients. Transpl Infect Dis. (2014) 16:802–12. doi: 10.1111/tid.12284
24. Schwarz A, Linnenweber-Held S, Heim A, Framke T, Haller H, Schmitt C. Viral origin, clinical course, and renal outcomes in patients with BK virus infection after living-donor renal transplantation. Transplantation. (2016) 100:844–53. doi: 10.1097/TP.0000000000001066
25. Ahmad S, Tejuja A, Newman KD, Zarychanski R, Seely AJ. Clinical review: a review and analysis of heart rate variability and the diagnosis and prognosis of infection. Crit Care. (2009) 13:232. doi: 10.1186/cc8132
26. Yilmaz HO, Babazade R, Leung S, Zimmerman NM, Makarova N, Saasouh W, et al. Postoperative hypotension and surgical site infections after colorectal surgery: a retrospective cohort study. Anesth Analg. (2018) 127:1129–36. doi: 10.1213/ANE.0000000000003666
27. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Risk of heart failure after community acquired pneumonia: prospective controlled study with 10 years of follow-up. BMJ. (2017) 356:j413. doi: 10.1136/bmj.j413
28. Remick J, Georgiopoulou V, Marti C, Ofotokun I, Kalogeropoulos A, Lewis W, et al. Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulation. (2014) 129:1781–9. doi: 10.1161/CIRCULATIONAHA.113.004574
29. Al-Kindi SG, ElAmm C, Ginwalla M, Mehanna E, Zacharias M, Benatti R, et al. Heart failure in patients with human immunodeficiency virus infection: epidemiology and management disparities. Int J Cardiol. (2016) 218:43–6. doi: 10.1016/j.ijcard.2016.05.027
30. Kang IS, Pyun WB, Shin GJ. Predictive value of CHADS2 score for cardiovascular events in patients with acute coronary syndrome and documented coronary artery disease. Korean J Intern Med. (2016) 31:73–81. doi: 10.3904/kjim.2016.31.1.73
31. Barbagelata E, Cillóniz C, Dominedò C, Torres A, Nicolini A, Solidoro P. Gender differences in community-acquired pneumonia. Minerva Med. (2020) 111:153–65. doi: 10.23736/S0026–4806.20.06448–4
32. Guidry CA, Swenson BR, Davies SW, Dossett LA, Popovsky KA, Bonatti H, et al. Sex- and diagnosis-dependent differences in mortality and admission cytokine levels among patients admitted for intensive care. Crit Care Med. (2014) 42:1110–20. doi: 10.1097/CCM.0000000000000139
Keywords: risk score, infection, ST-segment elevation myocardial infarction, percutaneous coronary intervention, major adverse clinical events
Citation: Liu Y, Wang L, Chen W, Zeng L, Fan H, Duan C, Dai Y, Chen J, Xue L, He P and Tan N (2021) Validation and Comparison of Six Risk Scores for Infection in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 7:621002. doi: 10.3389/fcvm.2020.621002
Received: 24 October 2020; Accepted: 24 December 2020;
Published: 22 January 2021.
Edited by:
Chen Liu, The First Affiliated Hospital of Sun Yat-Sen University, ChinaReviewed by:
Jianqing She, The First Affiliated Hospital of Xi'an Jiaotong University, ChinaLilei Yu, Renmin Hospital of Wuhan University, China
Copyright © 2021 Liu, Wang, Chen, Zeng, Fan, Duan, Dai, Chen, Xue, He and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Tan, Z2R0YW5uaW5nQDEyNi5jb20=; Pengcheng He, Z2RocGMxMDBAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Yuanhui Liu
Yuanhui Liu Litao Wang
Litao Wang Wei Chen4†
Wei Chen4† Hualin Fan
Hualin Fan Chongyang Duan
Chongyang Duan Jiyan Chen
Jiyan Chen Pengcheng He
Pengcheng He Ning Tan
Ning Tan