- 1Division of Cardiology, Department of Medical Sciences, University of Turin, “Città della Salute e della Scienza” Hospital, Turin, Italy
- 2Department of General and Interventional Cardiology, Helios Klinikum Erfurt, Erfurt, Germany
- 3Department of Cardiology, Campus Benjamin Franklin, Charite' Medical University Berlin, Berlin, Germany
- 4Department of Cardiology, Kantonsspital Luzern, Lucerne, Switzerland
- 5Division of Cardiology, Fondazione Cardiocentro Ticino, Lugano, Switzerland
- 6Department of Internal Medicine, Hirslanden Klinik St. Anna, Lucerne, Switzerland
- 7Azienda Sanitaria Locale Torino 4, Ospedale di Ciriè, Ciriè, Italy
- 8Department of Biomedical Sciences, University of Italian Switzerland, Lugano, Switzerland
In the last decades, transcatheter aortic valve replacement (TAVR) revolutionized the treatment of symptomatic severe aortic stenosis. The efficacy and safety of TAVR were first proven in inoperable and high-risk patients. Then, subsequent randomized clinical trials showed non-inferiority of TAVR as compared to surgical aortic valve replacement also in intermediate- and low-risk populations. As TAVR was progressively studied and clinically used in lower-risk patients, issues were raised questioning its opportunity in a younger population with a longer life-expectancy. As long-term follow-up data mainly derive from old studies with early generation devices on high or intermediate surgical risk patients, results can hardly be extended to most of currently treated patients who often show a low surgical risk and are treated with newer generation prostheses. Thus, in this low-risk younger population, decision making is difficult due to the lack of supporting data. The aim of the present review is to revise current literature regarding TAVR in younger patients.
Introduction
Aortic stenosis (AS) is the most common valvular heart disease in the adult population and its prevalence progressively increases according to population aging (1). Medical therapy yields poor outcomes in severe symptomatic AS treatment and surgical aortic valve replacement (SAVR) has long been considered the standard of care in suitable symptomatic patients with severe AS (2). The development of transcatheter aortic valve replacement (TAVR) provided a new strategy for their treatment that rapidly became a valid alternative approach in a progressively larger proportion of patients. From the interventional perspective, the procedure of TAVR has significantly evolved over the years. From the very first pioneering implant by Cribier, performed through a trans-venous, trans-septal, retrograde approach (3), nowadays procedural steps have been clearly defined. The percutaneous transfemoral approach performed under local anesthesia with conscious sedation is the most widely adopted in current practice, due to its easiness, low invasiveness and better short- and long-term outcomes (4, 5).
Aim of the present review is to revise the history of TAVR by focusing on its adoption in “young” patients and address current open issues regarding TAVR in this group.
The Evolution of TAVR
From Young to Old and Back to Young Patients
The first-in-man TAVR procedure was performed on a middle-aged patient. Back in 2002, Professor A. Cribier implanted a prototype balloon expandable valve on a 57-year-old man with severe calcific AS who was judged inoperable due to hemodynamic instability and significant associated comorbidities (3). The technical success of this procedure laid the foundation for subsequent studies investigating TAVR in inoperable and high-risk surgical candidates so as to offer a therapeutic option for such critical patients. When comparing the development of TAVR to the evolution of percutaneous coronary interventions it is clear that their development moved in opposite directions. While coronary interventions were first performed in extremely selected low-risk patients moving to higher-risk challenging cases (6), TAVR first focused on older, high/extreme surgical risk patients, and gradually approached younger and lower-risk subjects.
In this context, indication to TAVR was judged according to surgical risk scores such as the Society of Thoracic Surgeons (STS) score or the EuroScore (ES), both estimating the risk of 30-days mortality after open surgery.
According to the STS score, three categories of patients were identified, namely at high, intermediate or low risk (> 8%, 4–8%, or <4%, respectively), with only slight variations of the cut-off values among the studies or the less precise EuroScore II (7). Nevertheless, due to the limited numbers of criteria considered as variables in conventional surgical risk scores and their derivation from surgical series, the STS score and the EuroScore II have been proven to perform poorly in patients undergoing TAVR, thus highlighting the utmost role of appropriate clinical judgment in the choice between surgical vs. interventional approaches (8).
The Evolution of TAVR: A Path Through Randomized Clinical Trials and Registries
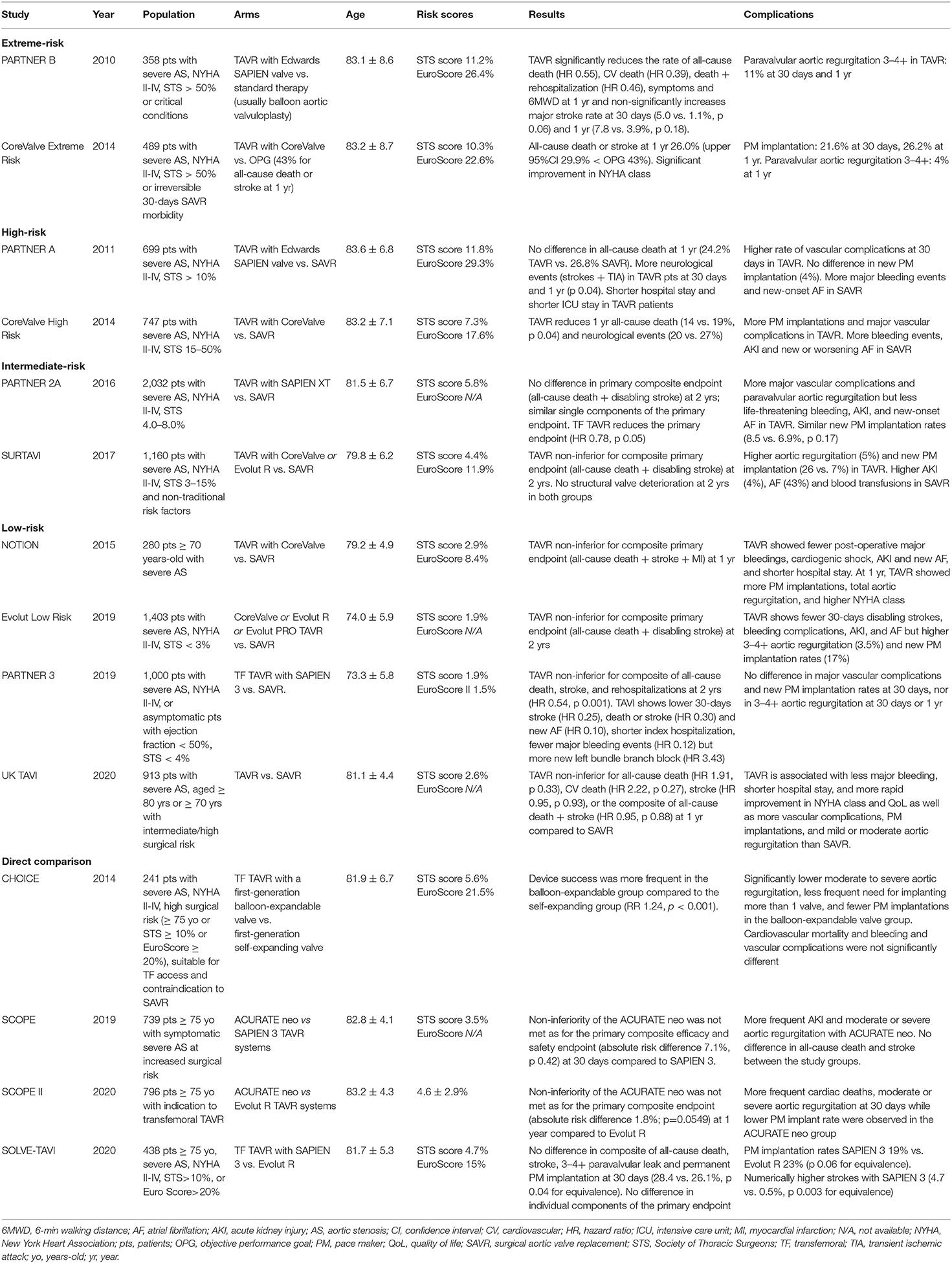
The first randomized controlled trial on TAVR, the PARTNER 1B, was published in 2010 and compared TAVR with a balloon-expandable bovine pericardial Edwards SAPIEN Valve against medical therapy on extreme-risk patients (mean age 83.1 ± 8.6 years, mean STS score 11.2 ± 5.8). The trial reported a significantly lower rate of death at 1 year in the TAVR group compared to the medical treatment group, thus opening this therapeutic possibility (9). The evidence that a percutaneous treatment in non-surgical candidates was superior to standard medical therapy laid the ground for the subsequent PARTNER 1A trial, demonstrating the non-inferiority of TAVR vs. SAVR in high-risk patients (10). In parallel, the efficacy of the self-expandable Medtronic CoreValve bioprosthesis was demonstrated in non-operable as well as high-risk patients with the CoreValve Extreme risk study and the CoreValve High risk trial, respectively (11, 12).
In the following years, trials comparing, TAVR vs. SAVR on intermediate-risk patients demonstrated the non-inferiority of TAVR vs. SAVR with both the second generation balloon-expandable SAPIEN XT valve in the PARTNER 2A trial and the self-expandable CoreValve valve in the SURTAVI trial (13, 14). Recently, TAVR was evaluated in low-risk patients, with the PARTNER 3 trial showing the superiority of the balloon-expandable SAPIEN 3 valve compared to SAVR and the Evolut Low Risk Trial demonstrating non-inferiority against SAVR. (12, 15) Characteristics of the landmark TAVR trials are reported in Table 1.
The consistent reduction of the estimated surgical risk throughout the years in trials' populations paralleled the reduction in the mean age (with an inverse increase in expected survival) of patients enrolled in the different trials (from mean age 83.1 ± 8.6 years in the PARTNER IB to mean age 74.0 ± 5.9 years in the Evolut Low Risk Trial), as age represents one of the main determinants of surgical risk. This trend clearly urges to consider several aspects of TAVR, such as procedural safety, long term efficacy, freedom from reinterventions, and patient's expectations, all of which have tremendous impact when evaluating different therapeutic options in young patients with severe AS.
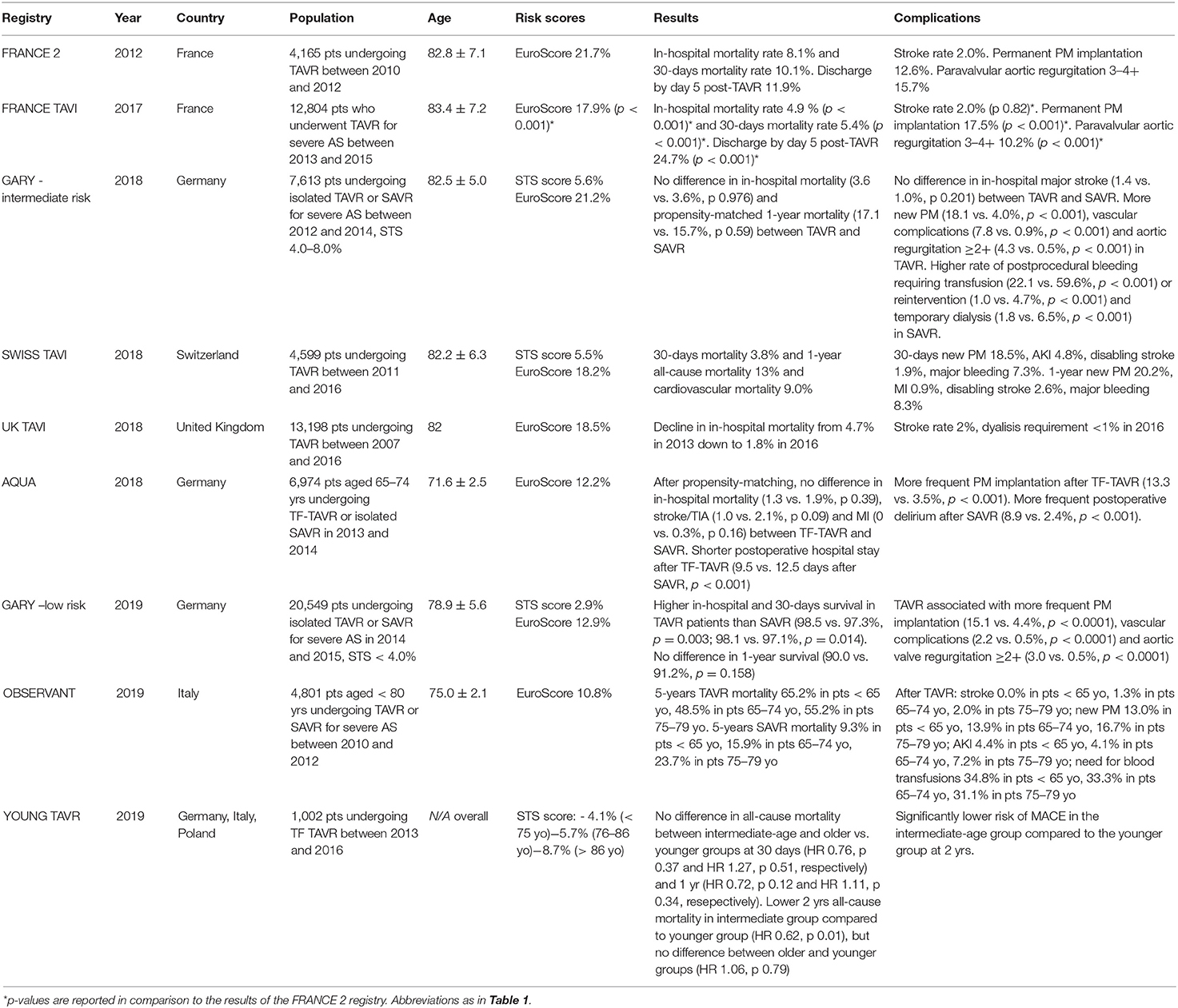
The outspread performance of TAVR procedures boosted by the promising trials' results fueled the birth of national registries, providing encouraging TAVR data on wider populations in real-life scenarios (16–21). The progressive trend in lowering of the surgical risk of the TAVR patients included in randomized trials was also evident in real life all-comers registries (18, 20). When only low-risk patients were considered, the mean logistic EuroScore decreased down to 10.8% (21). Overall, the mean age of patients enrolled in TAVR registries remained stable at about 80–82 years across all reports, but more attention has been recently given to younger patients, with latest data focusing specifically on this peculiar population (22). Basic features of TAVR registries are reported in Table 2.
While only few studies specifically focused on younger patients, current randomized trials enrolled patients according their high or intermediate surgical risk, thus intrinsically selecting elderly patients. This is evident when considering that mean age only decreased from 83 years in the PARTNER 1B trial to 73 years in the PARTNER 3 trial despite a concomitant drop in STS score from 11.2% down to 1.9% (9, 15).
Beside age and surgical risk, several clinical parameters, such as myocardial fibrosis, renal dysfunction, diabetes mellitus and pulmonary hypertension, have been identified as predictors short term all-cause mortality (23–25).
While only, preliminary evidence is currently available supporting the treatment of younger patients, patients requests for a percutaneous treatments in particular in younger subjects are becoming a matter of daily negotiation. The initial data deriving from the AQUA registry, addressing symptomatic patients younger than 75 years-old with an average intermediate surgical risk (EuroScore 12.2%), reported similar in-hospital outcomes for TAVR and SAVR with the exception of a more frequent need for new pacemaker implantation and a less frequent incidence of postoperative dialysis and delirium in TAVR (22).
Beside the progressive extension of TAVR to lower risk, younger populations, current research is focused on head to head comparisons between different transcatheter devices in order to ascertain the best fit between patient and current commercially available prostheses. Table 1 reports details on currently available direct comparisons between different devices (26–29). The results of recent trials support the safe implantation of newer generation valves, which have the advantage of better adaptation to the individual valve anatomy (26).
It Is Never too Late to Be Young: TAVR in “Young” Patients
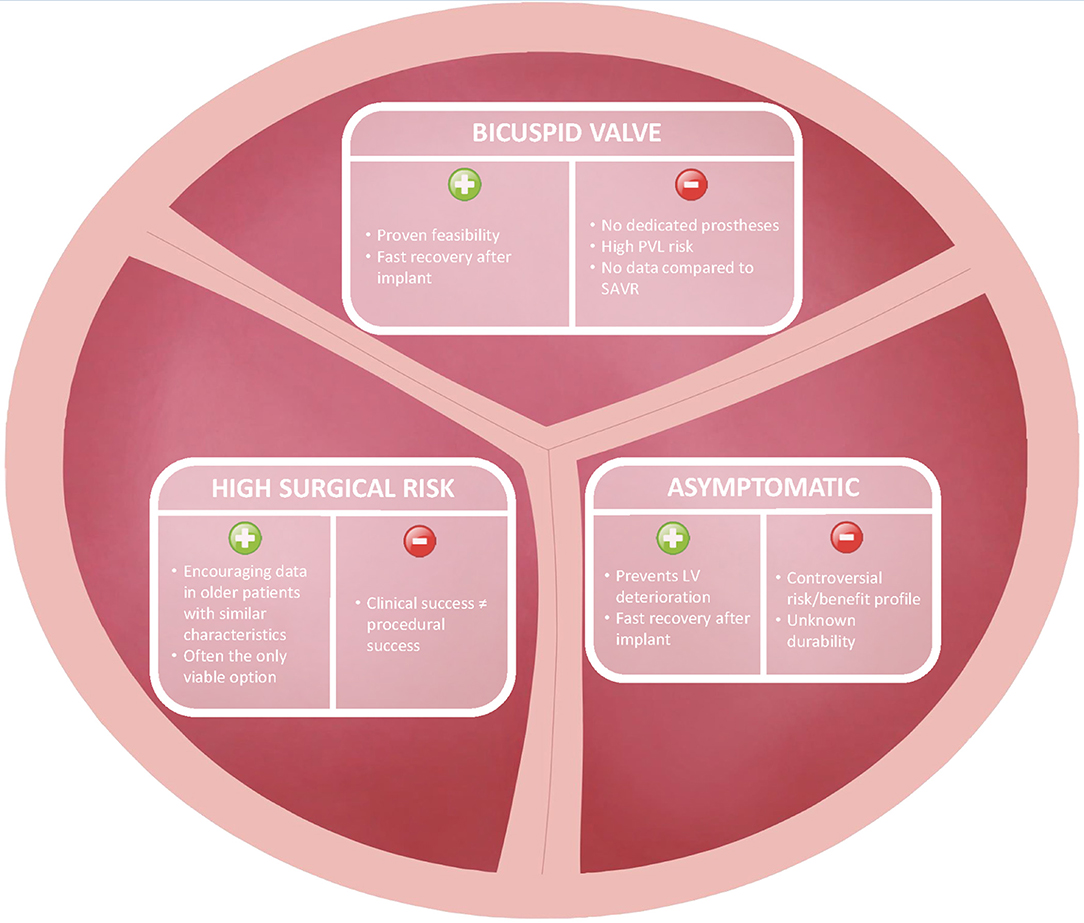
Apart from rare congenital cardiovascular diseases, severe AS is commonly diagnosed in middle-aged and elderly patients. By “young” AS patients we define individuals between 60 and 75 years of age, who have been only marginally included in current TAVR trials but might personally prefer or deserve percutaneous approach according to peculiar clinical characteristics. In our practice, we encountered three different groups of young patients in whom TAVR might be considered as an alternative to surgery, namely asymptomatic active patients with degenerative AS on a tricuspid valve, severe AS patients on a bicuspid aortic valve and severely comorbid “young” patients with severe AS unsuitable for the traditional surgical approach (i.e., the “Cribier's patient”) (Figure 1).
Asymptomatic Patients
As new severe AS therapy opportunities were put forward and guidelines indications were updated, doubts and controversies were raised regarding the treatment of asymptomatic patients (1). While no data are yet available to support the treatment of asymptomatic AS patients with TAVR, some evidence is becoming available for SAVR. The recent RECOVERY trial compared early SAVR vs. conservative management in asymptomatic patients with severe AS (mean age 63.4 ± 10.7 years) and highlighted a significantly lower incidence of operative mortality or cardiovascular death in the early intervention group (30). The mean age of patients included in this trial clearly highlights that severe AS is not unique to elderly patients. Likewise, propensity-matched data from CURRENT AS registry stress that early aortic valve replacement (98% SAVR, mean age 71.6 ± 8.7 years) provides better 5-years survival and fewer heart failure hospitalizations compared to conservative strategies (31). Following the RECOVERY trial and the CURRENT AS registry, a meta-analysis encompassing a total of 29 observational studies with 4,075 patients showed high rates of all-cause death, cardiac death and progression to aortic valve intervention in untreated patients and demonstrated that early AS intervention was associated with a significant reduction in long-term mortality (HR 0.38; 95%CI 0.25–0.58) compared to a conservative strategy (32). These studies mostly comprised SAVR-treated patients in the intervention group. If similar results were confirmed with a percutaneous approach, it would pave the road to a paradigm shift from a symptomatic approach to “prophylactic” treatment of severe AS in the absence of symptoms.
It is common knowledge that a certain number of asymptomatic severe AS patients are currently treated with TAVR despite the lack of strong supporting evidence derived from dedicated studies. The paucity of limiting symptoms and their relative younger age usually allow these patients to be still professionally or physically active. In addition, asymptomatic subjects might have reduced perception of the valvular disease, thus preferring or accepting only a percutaneous solution able to shorten their absence from professional and personal duties as compared to conventional surgery. Clearly, when referring asymptomatic patients to TAVR procedural safety and long-term efficacy must be warranted in order not to dissipate its clinical benefit.
Research is moving faster to address unresolved issues regarding young AS patients. From a physiological and structural point of view, myocardial fibrosis represents a key progressive phase in left ventricular hypertrophic response to severe AS and its detection at late gadolinium enhancement cardiac magnetic resonance was proved to be an independent predictor of all-cause mortality in patients with AS (25). The EVoLVeD trial (NCT03094143) is currently enrolling asymptomatic severe AS patients older than 18 years old, with mid-wall late gadolinium enhancement as an early marker of left ventricle decompensation, to assess if early SAVR would improve the composite outcome of all-cause mortality and AS-related hospitalizations (33). Other interesting insights are expected from the EARLY-TAVR trial (NCT03042104), which started enrollment in 2017 and is randomizing asymptomatic patients aged 65 years or older to undergo either TAVR with the SAPIEN 3 bioprosthesis or clinical surveillance. Asymptomatic patients are likely bound to represent the next frontier for TAVR in the upcoming years.
Bicuspid Aortic Valve
Severe AS on a bicuspid aortic valve (BAV) shows unique features such as presentation at an earlier age and technical issues related to peculiar anatomical characteristics. The first landmark trial on TAVR addressing BAV patients was published by Mylotte in 2014 (mean age 78.0 ± 8.9 years), demonstrating the feasibility of a percutaneous approach with promising outcomes on the short- and intermediate-term, albeit with a higher incidence of post-implantation aortic regurgitation mainly due to a more complex anatomy compared to tricuspid valves (34). Notably, although no differences between patients treated with TAVR with either a bicuspid or tricuspid anatomy were evident at 30 day (35), higher bailout TAVR-in-TAVR and lower device success rates were reported in BAV patients; indeed, careful patient selection, and anatomical assessment are paramount to warrant procedural safety and long-term efficacy (36).
Based on the STS/American College of Cardiology TVT Registry, including 81,822 consecutive patients with AS undergoing TAVR, Makkar et al. compared 2,691 BAV patients to a matched cohort of tricuspid aortic valve patients (median age 74 years, mean STS score 5%) (37). Similar 30-day (2.6 vs. 2.5%) and 1-year mortality (10.5 vs. 12.0%) rates and incidence of moderate/severe paravalvular leak at 30 days and 1 year were reported, alongside an increased 30-day risk for stroke in patients with BAV (2.5 vs. 1.6%) (37).
The recently published BEAT registry compared 242 BAV patients treated with Sapien 3 vs. 111 patients treated with Evolut R/PRO (mean age 77.8 years, mean STS score 4.4%) and confirmed the good procedural results with both platforms but with a higher rate of moderate-sever paravalvular aortic regurgitation at 1 year for the Evolut R/PRO group and a more frequent occurrence of annular rupture with balloon-expandable valves (38). As a result, the presence of a BAV, mainly due to the associated ellipticity of the aortic annulus, has a definite impact of the prosthesis' choice, as showed by a recent survey, with current evidences supporting the adoption of a self-expandable device (39).
Young Patients at High Surgical Risk
TAVR use is increasing rapidly among young adult patients in recent years (40). Although TAVR may be an appealing option for young adults with severe AS in general due to faster recovery, avoidance of a chest scar, no need for general anesthesia, in certain subgroups of young but frail patients TAVR might de facto be the only feasible option due to their high surgical risk (38). To investigate the risk profile and baseline clinical characteristics of young patients undergoing percutaneous or surgical treatments for severe symptomatic AS, the OBSERVANT study analyzed data on 4,801 patients younger than 80 years undergoing isolated TAVR or SAVR (21). The study reported a remarkable difference in the clinical characteristics of patients undergoing TAVR compared to those undergoing SAVR, with the logistic EuroScore being significantly higher in TAVR patients as compared to SAVR among all age subgroups (7.90 vs. 2.40% in patients < 65 years, 10.57 vs. 4.91% in those aged 65–74 years and 11.19 vs. 7.52% in patients aged 75–79 years, respectively). Moreover, TAVR patients younger than 65 years showed the highest short and long term mortality as compared to older patients, mainly due to higher baseline surgical risk and frailty rather than procedural complications (21).
The YOUNG TAVR multicenter registry described characteristics and mortality after TAVR in different age groups (41). Patients aged 75 years or less had significantly higher rates of chronic obstructive pulmonary disease, diabetes and coronary artery disease, lower ejection fraction. and slightly higher left ventricular end-diastolic diameter compared to intermediate (76–86 years) and older (more than 86 years) age groups, despite having a significantly lower estimated STS 30-days mortality score (4.11 vs. 5.65 vs. 8.65% in younger, intermediate and older age groups, respectively; p < 0.001). Compared with younger patients, intermediate-age and older patients showed no difference in 30-day and 1-year all-cause mortality, but the intermediate age group showed lower all-cause mortality at 2 years (HR, 0.62; p = 0.01). Thus, according to clinical experience and YOUNG TAVR registry findings, “young” patients currently treated with TAVR are a unique subgroup with peculiar comorbidities impacting on mid-term outcomes. Their surgical risk, however, remains only partially captured by surgical risk scores, as age still plays a pivotal role in the STS risk assessment (41).
These data outline that not all young patients carry a low surgical risk but this group might hide patients that are not suitable for any other option, as in the case of the first patients treated by Professor Cribier. Moreover, the mortality risk assessment by means of the STS tool might be inadequate in this young patients' cohort. As a result, careful patient selection and clinical assessment for TAVR treatment must be warranted in this population to identify higher-risk individuals and guarantee a patient-tailored treatment.
Unanswered Questions: Valve Durability, Conduction Disturbances, and Coronary Access
The overall encouraging and reassuring results of TAVR trials in lower risk, younger patients must be faced against the risk of procedural complications and unanswered questions coming with the TAVR procedures.
TAVR durability and efficacy in younger patients with fewer comorbidities and longer life-expectancy have been questioned (42). TAVR long-term follow-up data mainly derive from studies performing TAVR with early generation devices on high or intermediate surgical risk patients and results are hardly extendable to current low-risk patients with newer valve prostheses. For instance, the NOTION trial randomized low surgical risk patients (mean age 79.4 years) with severe AS to TAVR or SAVR in a 1:1 fashion and described similar rates of all-cause mortality at 6 years (42.5 vs. 37.7%, respectively, P = 0.58) with higher rates of structural valve deterioration for SAVR than TAVR (24.0 vs. 4.8%, respectively; P < 0.001) (43). Nevertheless, all TAVR patients received a CoreValve bioprosthesis and results may hardly be generalizable to balloon-expandable or newer-generation self-expanding valves. Long-term outcomes of the PARTNER 1 trial demonstrated similar all-cause mortality at 5-years follow-up for TAVR and SAVR (67.8 vs. 62.4%; P = 0.76) with higher rates of moderate or severe aortic regurgitation in the TAVR group compared to SAVR (14 vs. 1%; P < 0.0001), which was associated with increased 5-years risk of all-cause death in the TAVR group (44). A recent meta-analysis on structural durability of TAVR with both balloon-expandable and self-expanding valves vs. SAVR showed high rates of paravalvular regurgitation, moderate or severe aortic regurgitation, and reintervention at 5-years follow-up in the TAVR group compared to SAVR, thus highlighting that valve deterioration is still one of the open issues of percutaneous devices (45). Again, data were derived from trials using earlier generation TAVR valves in higher surgical risk (46, 47). Bioprosthetic valve failure and severe structural valve deterioration rates have been estimated to be around 4.6 and 1.3% at long-term follow-up, respectively (48).
The clinical impact of conduction disturbances after TAVR has been largely debated as well. High grade atrio-ventricular block and new-onset left bundle branch block are the most frequent complications following TAVR (49). Apart from the mechanical interaction between the prosthesis and the conduction system, there is evidence supporting the association between severe aortic stenosis itself, causing calcium deposition on the conduction system and the development of left ventricular dysfunction, and conduction disturbances.
The 2020 SOLVE-TAVI trial comparing newer generation self-expanding vs. balloon-expandable valves in TAVR procedures showed a near-significant trend toward higher permanent pacemaker implantation rate at 30 days in the self-expanding group and compared to the balloon-expanding group (23.0 vs. 19.2%, P = 0.06) (26). A meta-analysis of 30 studies reported that new-onset persistent left bundle branch block and permanent pacemaker implantation after TAVR are associated with an increased risk of death and heart failure hospitalization at 1 year, while periprocedural new-onset left bundle branch block was also associated with higher cardiac mortality and pacemaker implantation rates within the year following the procedure (50). As morbidity and mortality associated with these conditions are not trivial, careful patient evaluation and selection for TAVR procedures, in particular in the younger patients subgroups, are paramount (51).
TAVR assessment in younger patients must also take into account that their longer life expectancy might require future percutaneous procedures, either coronary or valvular or both. This undoubtedly impacts on valve selection, as prostheses allowing rapid and effective access to the coronary arteries or eventual valve-in-valve TAVR shall be preferred (39). Due to the peculiar design of most prostheses, current self-expandable devices might hamper the access to coronary ostia and thus limit the possibility of future percutaneous coronary interventions, which comes as a relevant prognostic drawback in the setting of acute coronary syndromes.
Conclusions
Adoption of a percutaneous approach for the treatment of severe AS in progressively younger patients is one of the latest trends in contemporary interventional cardiology. As detailed in our review, different subgroups of patients with their peculiar clinical characteristics are currently encountered in clinical practice. In particular, the paradigm shift from a symptomatic to a “prophylactic” treatment of severe AS represents one of the major challenges of modern cardiology, radically changing the decision-making process in the treatment of valvular heart diseases. While opening newer therapeutic possibilities and potentially amplifying the prognostic approach of surgical and percutaneous treatments, this paradigm shift will challenge operators to achieve absolute procedural safety and ascertained long-term efficacy.
As severe AS is a complex pathology requiring comprehensive clinical and instrumental multidisciplinary evaluation and to date, no single algorithm can provide a patient-tailored approach, the Heart Team plays an essential role in deciding the optimal treatment strategy, especially when treating young patients with scarce data guiding the decision-making process.
Author Contributions
PPB, FA, BA, FC, and LB conceived the study and carried out the first draft of the manuscript and its major revisions. GC, GT, GS, GP, and GMDF revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro heart survey on valvular heart disease. Eur Heart J. (2003) 24:1231–43. doi: 10.1016/s0195-668x(03)00201-x
2. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91. doi: 10.1016/j.rec.2017.12.013
3. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. (2002) 106:3006–8. doi: 10.1161/01.cir.0000047200.36165.b8
4. Conrotto F, D'Ascenzo F, Giordana F, Colaci C, Scacciatella P, Biondi-Zoccai G, et al. Impact of access on TAVI procedural and midterm follow-up: a meta-analysis of 13 studies and 10,468 patients. J Interv Cardiol. (2014) 27:500–8. doi: 10.1111/joic.12141
5. Biasco L, Ferrari E, Pedrazzini G, Faletra F, Moccetti T, Petracca F, et al. Access sites for TAVI: patient selection criteria, technical aspects, and outcomes. Front Cardiovasc Med. (2018) 5:88. doi: 10.3389/fcvm.2018.00088
6. Canfield J, Totary-Jain H. 40 Years of percutaneous coronary intervention: history and future directions. J Pers Med. (2018) 8:33. doi: 10.3390/jpm8040033
7. Shroyer AL, Coombs LP, Peterson ED, Eiken MC, DeLong ER, Chen A, et al. The society of thoracic surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg. (2003) 75:1856–64. doi: 10.1016/s0003-4975(03)00179-6
8. D'Ascenzo F, Ballocca F, Moretti C, Barbanti M, Gasparetto V, Mennuni M, et al. Inaccuracy of available surgical risk scores to predict outcomes after transcatheter aortic valve replacement. J Cardiovasc Med. (2013) 14:894–98. doi: 10.2459/JCM.0b013e3283638e26
9. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. (2010) 363:1597–607. doi: 10.1056/NEJMoa1008232
10. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. (2011) 364:2187–98. doi: 10.1056/NEJMoa1103510
11. Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS, Heimansohn D, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. (2014) 63:1972–81. doi: 10.1016/j.jacc.2014.02.556
12. Adams DH, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. (2014) 370:1790–98. doi: 10.1056/NEJMoa1816885
13. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2016) 374:1609–20. doi: 10.1056/NEJMoa1514616
14. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2017) 376:1321–31. doi: 10.1056/NEJMoa1700456
15. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380:1695–705. doi: 10.1056/NEJMoa1814052
16. Werner N, Zahn R, Beckmann A, Bauer T, Bleiziffer S, Hamm CW, et al. Patients at intermediate surgical risk undergoing isolated interventional or surgical aortic valve implantation for severe symptomatic aortic valve stenosis. Circulation. (2018) 138:2611–23. doi: 10.1161/CIRCULATIONAHA.117.033048
17. Bekeredjian R, Szabo G, Balaban Ü, Bleiziffer S, Bauer T, Ensmingeret S, et al. Patients at low surgical risk as defined by the society of thoracic surgeons score undergoing isolated interventional or surgical aortic valve implantation: in-hospital data and 1-year results from the german aortic valve registry (GARY). Eur Heart J. (2019) 40:1323–30. doi: 10.1093/eurheartj/ehy699
18. Ludman PF. UK TAVI registry. Heart. (2019) 105(Suppl. 2):s2–5. doi: 10.1136/heartjnl-2018-313510
19. Ferrari E, Stortecky S, Heg D, Muller O, Nietlispach F, Tueller D, et al. The hospital results and 1-year outcomes of transcatheter aortic valve-in-valve procedures and transcatheter aortic valve implantations in the native valves: the results from the Swiss-TAVI registry. Eur J Cardiothorac Surg. (2019) 56:55–63. doi: 10.1093/ejcts/ezy471
20. Auffret V, Lefevre T, Van Belle E, Eltchaninoff H, Iung B, Koning R, et al. Temporal trends in transcatheter aortic valve replacement in France: FRANCE 2 to 1 FRANCE TAVI. J Am Coll Cardiol. (2017) 70:42–55. doi: 10.1016/j.jacc.2017.04.053
21. Tarantini G, Nai Fovino L, D'Errigo P, Rosato S, Barbanti M, Tamburino C, et al. Factors influencing the choice between transcatheter and surgical treatment of severe aortic stenosis in patients younger than 80 years: results from the OBSERVANT study. Catheter Cardiovasc Interv. (2020) 95:E186–95. doi: 10.1002/ccd.28447
22. Eggebrecht H, Bestehorn K, Rassaf T, Bestehorn M, Voigtländer T, Fleck E, et al. In-hospital outcomes after transcatheter or surgical aortic valve replacement in younger patients less than 75 years old: a propensity-matched comparison. EuroIntervention. (2018) 14:50–7. doi: 10.4244/EIJ-D-17-01051
23. D'Ascenzo F, Conrotto F, Salizzoni S, Rossi ML, Nijhoff F, Gasparetto V, et al. Incidence, predictors, and impact on prognosis of systolic pulmonary artery pressure and its improvement after transcatheter aortic valve implantation: a multicenter registry. J Invasive Cardiol. (2015) 27:114–9.
24. Conrotto F, Salizzoni S, Andreis A, D'Ascenzo F, D'Onofrio A, Agrifoglio M, et al. Transcatheter aortic valve implantation in patients with advanced chronic kidney disease. Am J Cardiol. (2017) 119:1438–42. doi: 10.1016/j.amjcard.2017.01.042
25. Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. (2011) 58:1271–9. doi: 10.1016/j.jacc.2011.03.064
26. Thiele H, Kurz T, Feistritzer HJ, Stachel G, Hartung P, Eitel I, et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: the randomized SOLVE-TAVI trial. Eur Heart J. (2020) 41:1890–9. doi: 10.1093/eurheartj/ehaa036
27. Abdel-Wahab M, Mehilli J, Frerker C, Neumann FJ, Kurz T, Tölg R, et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. (2014) 311:1503–14. doi: 10.1001/jama.2014.3316
28. Lanz J, Kim WK, Walther T, Burgdorf C, Möllmann H, Linke A. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: a randomised non-inferiority trial. Lancet. (2019) 394:1619–28. doi: 10.1016/S0140-6736(19)32220-2
29. Tamburino C, Bleiziffer S, Thiele H, Scholtz S, Hildick-Smith D, Cunnington M, et al. Comparison of self-expanding bioprostheses for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: the SCOPE 2 randomized clinical trial. Circulation. (2020) doi: 10.1161/CIRCULATIONAHA.120.051547. [Epub ahead of print].
30. Kang DH, Park SW. Early surgery or conservative care for asymptomatic aortic stenosis. N Engl J Med. (2020) 382:111–9. doi: 10.1056/NEJMc2016167
31. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. (2015) 66:2827–38. doi: 10.1016/j.jacc.2015.10.001
32. Gahl B, Çelik M, Head SJ, Vanoverschelde JL, Pibarot P, Reardon MJ, et al. Natural history of asymptomatic severe aortic stenosis and the association of early intervention with outcomes: a systematic review and meta-analysis. JAMA Cardiol. (2020) 5:1–11. doi: 10.1001/jamacardio.2020.2497
33. Bing R, Everett RJ, Tuck C, Semple S, Lewis S, Harkess R, et al. Rationale and design of the randomized, controlled early valve replacement guided by biomarkers of left ventricular decompensation in asymptomatic patients with severe aortic stenosis (EVOLVED) trial. Am Heart J. (2019) 212:91–100. doi: 10.1016/j.ahj.2019.02.018
34. Mylotte D, Lefevre T, Søndergaard L, Watanabe Y, Modine T, Dvir D, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol. (2014) 64:2330–9. doi: 10.1016/j.jacc.2014.09.039
35. De Biase C, Mastrokostopoulos A, Philippart R, Desroche LM, Blanco S, Rehal K, et al. Aortic valve anatomy and outcomes after transcatheter aortic valve implantation in bicuspid aortic valves. Int J Cardiol. (2018) 266:56–60. doi: 10.1016/j.ijcard.2018.01.018
36. Bax JJ, Delgado V, Hahn RT, Leipsic J, Min JK, Grayburn P, et al. Transcatheter aortic valve replacement: role of multimodality imaging in common and complex clinical scenarios. JACC Cardiovasc Imaging. (2020) 13:124–39. doi: 10.1016/j.jcmg.2018.10.037
37. Makkar RR, Yoon SH, Leon MB, Chakravarty T, Rinaldi M, Shah PB, et al. Association between transcatheter aortic valve replacement for bicuspid vs tricuspid aortic stenosis and mortality or stroke. JAMA. (2019) 321:2193. doi: 10.1001/jama.2019.7108
38. Mangieri A, Tchetchè D, Kim WK, Pagnesi M, Sinning JM, Landes U, et al. balloon versus self-expandable valve for the treatment of bicuspid aortic valve stenosis: insights from the beat international collaborative registry. Circ Cardiovasc Interv. (2020) 13:e008714. doi: 10.1161/CIRCINTERVENTIONS.119.008714
39. Biasco L, Cerrato E, Tersalvi G, Pedrazzini G, Wilkins B, Faletra F, et al. WorldwIde survey on clinical and anatomical factors driving the choice of transcatheter aortic valve prostheses. Front Cardiovasc Med. (2020) 7:38. doi: 10.3389/fcvm.2020.00038
40. Nelson J, Maul T, Wearden P, Najm HK, Baloglu O, Johnston D, et al. Aortic valve replacement in young and middle-aged adults: current and potential roles of TAVR. Ann Thorac Surg. (2020) S0003-4975(20)31292-3. doi: 10.1016/j.athoracsur.2020.05.180. [Epub ahead of print].
41. Navarese EP, Andreotti F, Kołodziejczak M, Wanha W, Lauten A, Veulemans V, et al. Age-related 2-year mortality after transcatheter aortic valve replacement: the YOUNG TAVR registry. Mayo Clin Proc. (2019) 94:1457–66. doi: 10.1016/j.mayocp.2019.01.008
42. Doshi R. Extended benefits of TAVR in young patients with low-intermediate risk score: proceed with care. EuroIntervention. (2018) 14:e485. doi: 10.4244/EIJ-D-18-00236L
43. Søndergaard L, Ihlemann N, Capodanno D, Jørgensen TH, Nissen H, Kjeldsen BJ, et al. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol. (2019) 73:546–53. doi: 10.1016/j.jacc.2018.10.083
44. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. (2015) 385:2477–84. doi: 10.1016/S0140-6736(15)60308-7
45. Ler A, Ying YJ, Sazzad F, Choong AMTL, Kofidis T. Structural durability of early-generation transcatheter aortic valve replacement valves compared with surgical aortic valve replacement valves in heart valve surgery: a systematic review and meta-analysis. J Cardiothorac Surg. (2020) 15:127. doi: 10.1186/s13019-020-01170-7
46. Yater WM, Cornell VH. Heart block due to calcareous lesions of the bundle of his: review and report of a case with detailed histopathologic study. Ann Intern Med. (1935) 8:777–89.
47. MacMillan RM, Demorizi NM, Gessman LJ, Maranhao V. Correlates of prolonged HV conduction in aortic stenosis. Am Heart J. (1985) 110:56–60. doi: 10.1016/0002-8703(85)90514-9
48. Capodanno D, Søndergaard L, Tamburino C. Durability of transcatheter bioprosthetic aortic valves: the story so far. EuroIntervention. (2019) 15:846–9. doi: 10.4244/EIJV15I10A160
49. Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. (2017) 136:1049–69. doi: 10.1161/CIRCULATIONAHA.117.028352
50. Faroux L, Chen S, Muntané-Carol G, Regueiro A, Philippon F, Sondergaard L, et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta-analysis. Eur Heart J. (2020) 41:2771–81. doi: 10.1093/eurheartj/ehz924
Keywords: transaortic valve replacement, TAVR, severe aortic stenosis, valvular heart disease, young patients, low risk
Citation: Bocchino PP, Angelini F, Alushi B, Conrotto F, Cioffi GM, Tersalvi G, Senatore G, Pedrazzini G, De Ferrari GM and Biasco L (2020) Transcatheter Aortic Valve Replacement in Young Low-Risk Patients With Severe Aortic Stenosis: A Review. Front. Cardiovasc. Med. 7:608158. doi: 10.3389/fcvm.2020.608158
Received: 19 September 2020; Accepted: 25 November 2020;
Published: 14 December 2020.
Edited by:
Crochan John O'Sullivan, Triemli Hospital, SwitzerlandReviewed by:
Moritz Seiffert, University Heart and Vascular Center Hamburg (UHZ), GermanyDavid Chistian Reineke, Clinic for Cardiovascular Surgery, Inselspital, Switzerland
Copyright © 2020 Bocchino, Angelini, Alushi, Conrotto, Cioffi, Tersalvi, Senatore, Pedrazzini, De Ferrari and Biasco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luigi Biasco, bHVpZ2kuYmlhc2NvQGdtYWlsLmNvbQ==
 Pier Paolo Bocchino
Pier Paolo Bocchino Filippo Angelini
Filippo Angelini Brunilda Alushi
Brunilda Alushi Federico Conrotto1
Federico Conrotto1 Gregorio Tersalvi
Gregorio Tersalvi Gaetano Maria De Ferrari
Gaetano Maria De Ferrari Luigi Biasco
Luigi Biasco