- 1Department of Internal Medicine II, Medical University of Vienna, Vienna, Austria
- 2Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria
- 3Core Facilities, Medical University of Vienna, Vienna, Austria
- 4Ludwig Boltzmann Institute for Cardiovascular Research, Vienna, Austria
- 5Department of Blood Group Serology and Transfusion Medicine, Medical University of Vienna, Vienna, Austria
- 6Department of Internal Medicine I, Cardiology and Intensive Care Medicine, Landesklinikum Mistelbach-Gänserndorf, Mistelbach, Austria
Background: Peripheral artery disease (PAD) patients undergoing infrainguinal angioplasty with stenting suffer high rates of target lesion restenosis and ischemic events. Blood-based prognostic markers in these patients are currently limited. The IL-33/ST2-system is involved in atherothrombosis. Soluble ST2 has been proposed as a biomarker in patients with cardiovascular disease.
Aim: To investigate the association of sST2 with platelet activation and monocyte tissue factor (TF) in 316 patients undergoing elective angioplasty and stenting for cardiovascular disease, and its predictive value for ischemic outcomes following infrainguinal angioplasty with stent implantation in 104 PAD patients within this cohort.
Methods and Results: Circulating levels of sST2, platelet surface P-selectin, monocyte TF expression as well as soluble P-selectin were determined in 316 consecutive patients on dual antiplatelet therapy following angioplasty and stenting. sST2 was independently associated with soluble P-selectin (B = 6.4, 95% CI 2.0–10.7, p = 0.004) and TF expression (B = 0.56, 95% CI 0.02–1.1, p = 0.041) but not with platelet surface P-selectin (B = 0.1, 95% CI −0.1–0.3, p = 0.307) after adjustment for age, sex, clinical risk factors and inflammatory parameters. During the follow-up of 24 months, the primary endpoint occurred in 41 of 104 PAD patients (39.4%). However, circulating levels of sST2 did not predict the primary endpoint in PAD patients (HR 1.1, 95% CI 0.76–1.71, p = 0.527).
Conclusion: sST2 is associated with soluble P-selectin and monocyte TF expression in atherosclerosis but not with ischemic outcomes following infrainguinal angioplasty with stent implantation for PAD.
Introduction
Peripheral artery disease (PAD) remains a great therapeutic challenge with angioplasty and stent implantation being an effective therapeutic intervention in patients with high-grade stenosis (1, 2). However, target vessel restenosis and ischemic events are frequent and severe complications after infrainguinal endovascular interventions. Blood-based biomarkers allowing risk stratification of patients with PAD following angioplasty and stenting are limited (3, 4).
The main components of the interleukin (IL)-33/suppression of tumorigenesis-2 (ST2) system include the ligand IL-33 as well as transmembrane ST2 [ST2L or IL-1 Receptor Like 1 (IL1RL1)] and soluble ST2 (sST2) representing two receptor isoforms (5). sST2 acts as a decoy receptor through binding free IL-33, which prevents cytokine signaling via ST2L (6).
Association of IL-33 and sST2 with the pathogenesis of atherosclerosis and thrombosis has been demonstrated in several previous studies (7–10). In monocytes, the IL-33/ST2 system induces tissue factor (TF) expression and the release of prothrombotic microvesicles (9). Increased levels of IL-33 are associated with an increased risk of in-stent restenosis and sST2 was associated with disease severity and outcome in patients with coronary artery disease (11, 12). Very high IL-33 levels predicted mortality in STEMI patients (11). Both IL-33 and sST2 are elevated in patients with carotid artery stenosis, and correlated with the vulnerability of atherosclerotic plaques (13). The biomarker potential of sST2 was proven in patients with acute myocardial infarction (AMI) (11, 14, 15), heart failure (HF) (16, 17), and critically ill patients (18). In detail, higher sST2 levels were associated with poor outcomes. In contrast, circulating sST2 levels did not predict future cardiovascular events in patients with carotid artery stenosis over a follow-up of 3 years (19). Therefore, the predictive value of circulating sST2 for ischemic outcomes seems to be dependent among others on the localization and manifestation of atherosclerosis.
Data on sST2 in PAD are limited. A previous study demonstrated higher levels of sST2 in patients with PAD compared with healthy controls (20). Another study investigated the influence of IL1RL1 single nucleotide polymorphisms on sST2 levels in PAD patients and found lower sST2 levels in rs950880 AA homozygotes (21). The authors subsequently demonstrated that the combination of a high sST2 level and rs950880 AA homozygosity was a strong predictor of all-cause death, but not for secondary endpoints defined as cardiovascular death, AMI, hospitalization for HF, stroke, and amputation in PAD patients (21). Therefore, the predictive value of circulating sST2 for target vessel restenosis in PAD is still unclear. Moreover, data linking sST2 with platelet activation in atherosclerosis are scarce. We therefore sought to investigate the association of sST2 with platelet activation and monocyte tissue factor (TF) expression in 316 patients undergoing elective angioplasty and stenting for cardiovascular disease, and its ability to predict ischemic outcomes following infrainguinal angioplasty with stent implantation in 104 PAD patients within this cohort.
Methods
Study Population
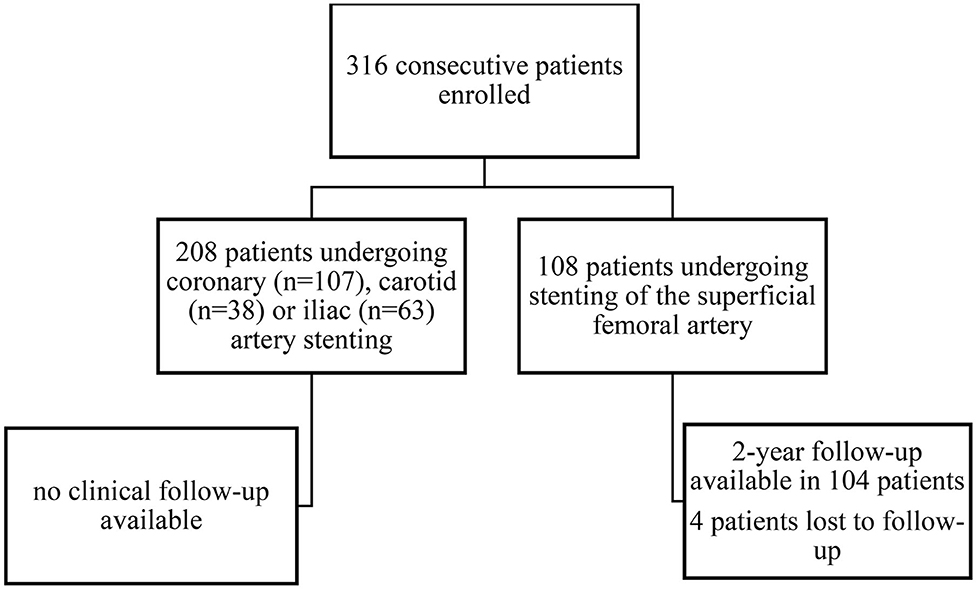
In this prospective cohort study, 316 consecutive patients undergoing successful angioplasty with endovascular stent implantation were enrolled at the Department of Internal Medicine II at the Medical University of Vienna. Study design, patient enrollment and follow-up are depicted in Figure 1. All patients received 100 mg of aspirin and 75 mg of clopidogrel per day. Patients undergoing peripheral or carotid angioplasty and stenting received DAPT for 3 months followed by aspirin monotherapy. Patients undergoing coronary angioplasty and stenting received DAPT for 6 months, followed by aspirin monotherapy. In 104 patients undergoing infrainguinal angioplasty and stenting clinical follow-up was assessed 1 and 2 years after the endovascular intervention.
Exclusion criteria were a known aspirin or clopidogrel intolerance (allergic reactions, gastrointestinal bleeding), a therapy with vitamin K antagonists (warfarin, phenprocoumon, acenocoumarol) or direct oral anticoagulants (dabigatran, rivaroxaban, apixaban, edoxaban), a treatment with ticlopidine, dipyridamole or non-steroidal anti-inflammatory drugs, a family or personal history of bleeding disorders, malignant paraproteinemias, myeloproliferative disorders or heparin-induced thrombocytopenia, severe hepatic failure, acute and chronic inflammatory diseases, known qualitative defects in thrombocyte function, a major surgical procedure within 1 week before enrolment, a platelet count <100,000 or >450,000/L and a hematocrit <30% as previously described (22, 23).
The study protocol was approved by the Ethics Committee of the Medical University of Vienna in accordance with the Declaration of Helsinki and written informed consent was obtained from all study participants.
Blood Sampling
Blood was drawn for flow cytometry 1 day after the percutaneous intervention as previously described (24). Flow cytometry was performed by a single operator, blinded to clinical follow-up and sST2 measurements.
Quantification of sST2
Circulating sST2 was assessed using human ST2/IL-1 R4 DuoSet® ELISA Kit (R&D Systems), as previously described (11, 17, 18, 25).
Soluble P-Selectin (sP-Selectin)
sP-selectin was quantified in duplicates using 100 μL platelet-poor plasma diluted 20-fold in sample diluent using Human sP-selectin/CD62P ELISA reagent set (R&D Systems), as previously described (26, 27).
Platelet Surface Expression of P-Selectin
The expression of P-selectin was determined in citrate-anticoagulated blood, as previously described (28, 29). In brief, whole blood was diluted in phosphate-buffered saline to obtain 20 × 103 platelets/μL in 20μL, and incubated with the platelet specific monoclonal antibody anti-CD42b (clone HIP1, allophycocyanin labelled; Becton Dickinson (BD), San Jose, CA, USA). Samples were then incubated with an antibody against P-selectin (anti-CD62p-phycoerythrin, clone CLB-Thromb6; Immunotech, Beckman Coulter, Fullerton, CA, USA). After 15min of incubation in the dark, the reaction was stopped by adding 500 μL PBS and samples were acquired immediately on a FACS Calibur flow cytometer (BD) with excitation by an argon laser at 488 nm and a red diode laser at 635 nm at a rate of 200–600 events per second. Isotype-matched control antibodies were used in separate vials for the determination of non-specific binding. At acquisition, the platelet population was identified by its characteristics in the forward scatter versus side scatter plot. A total of 10,000 events were acquired within this gate. This population was further identified by platelets stained with the platelet-specific monoclonal antibody anti-CD42b versus side scatter. Binding of the antibody against P-selectin was determined in a histogram for P-selectin. The MFI based on all events was used for statistical calculations. The gated events were analyzed using the CellQuest Pro software (BD). Standard BD Calibrite beads were used for daily calibration of the cytometer.
Monocyte TF
Monocyte TF was measured as previously described (30), using fluorochrome-conjugated monoclonal antibodies: APC-labeled monoclonal antibody for the constitutive platelet marker CD42b (glycoprotein Ib of von Willebrand factor receptor complex), a FITC-labeled monoclonal antibody for human TF (BD, Franklin Lakes, NJ, USA), a PE-Cy5–labeled monoclonal antibody for monocyte CD14 (endotoxin receptor), and corresponding isotype controls. All antibodies were purchased from BD, except the anti-TF monoclonal antibody which came from American Diagnostica (Stamford, CT, USA). In brief, 100 μL of citrate-anti-coagulated whole blood was stained with saturating concentrations of the above mentioned fluorochrome-conjugated monoclonal antibodies. After 10 min of pre-incubation with antibodies in the dark at room temperature, samples were fixed and erythrolysed with Optilyse B (Instrumentation Laboratories, Bedford, MA, USA). Flow cytometry was performed on a FACSCalibur BD. Acquisition was stopped when 5,000 CD14+ events were acquired. Monocytes were identified by gating CD14+ events, and all additional analyses were performed on this population. The negative and positive delineators were set by gating ~2% background staining on the isotype control fluorescence. TF expression was calculated as percentage of monocytes staining positive for TF (TF+-monocytes).
Clinical Endpoints
Clinical follow-up was assessed at regular visits of the study participants to the outpatient department of the Division of Vascular Medicine at the Medical University of Vienna and via telephone calls, respectively. The primary endpoint was defined as the composite of the first occurrence of any of the following events: non-fatal AMI, non-fatal stroke or transient ischemic attack (TIA), cardiovascular death, and sonographically confirmed >80% target vessel restenosis or reocclusion within 2 years after peripheral angioplasty and stenting.
Statistical Analysis
Categorical variables are summarized as counts and percentages and are compared by the χ2-test or the Fisher's exact test as appropriate. Continuous variables are expressed as median and interquartile range (IQR) and compared by the t-test or the Mann-Whitney U-test in case of non-normal distribution. Univariate and multivariate linear regression models were fit to evaluate the associations of sST2 with platelet surface P-selectin, sP-selectin, and monocyte TF expression. The multivariate linear regression model was adjusted for age, sex, active smoking, type 2 diabetes, hypertension, hyperlipidemia and high sensitivity C-reactive protein (hs-CRP). Forest plots were plotted using Beta values and 95% CI from linear regression models. The univariate Cox proportional hazard regression model was fit to assess whether sST2 could significantly predict the dichotomous clinical outcome (without/with adverse ischemic events). Hazard ratios (HR) are given as HR per one increase of standard deviation (HR per 1-SD). Kaplan-Meier failure plots were constructed in groups according to sST2 expression above or below the median value to compare time-dependent discriminative power of circulating sST2. Two-sided p-values of 0.05 indicated statistical significance. SPSS 22.0 (IBM Corporation, Armonk, NY, USA), GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) and STATA version 12 (StataCorp LLC, College Station, TX, USA) were used for all statistical analyses.
Results
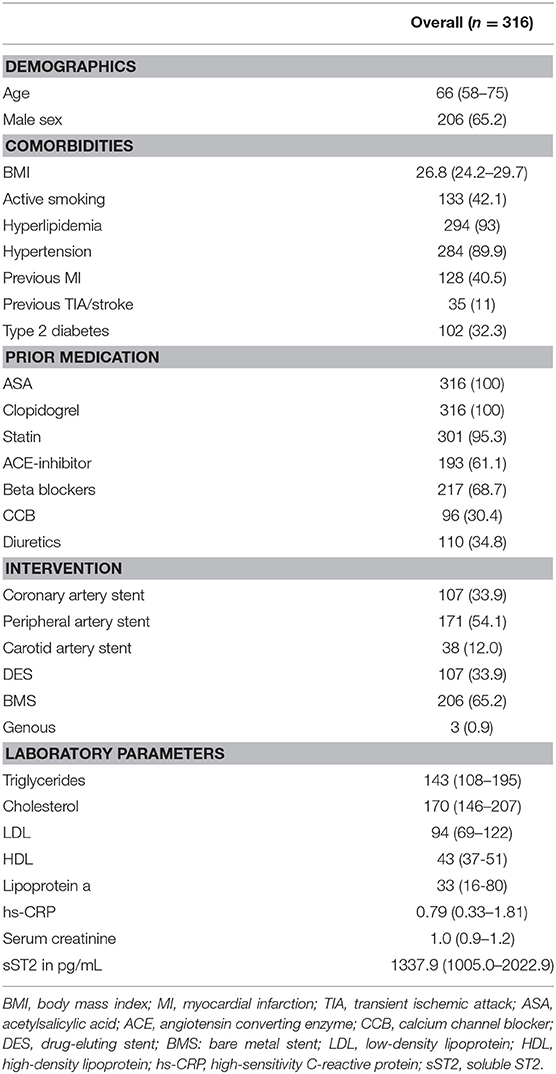
Clinical characteristics of the overall patient population are given in Table 1. Median age was 66 years (IQR 58–75), and 65.2% of patients were male. Most common comorbidities were hyperlipidemia (93%) and hypertension (90%), followed by active smoking (42%), previous MI (40.5%) and type 2 diabetes (32%).
In the overall patient cohort (n = 316), sST2 was associated with monocyte TF expression (B = 0.55, 95% CI 0.02–1.1, p = 0.042, Figure 2A). Furthermore, sST2 was significantly linked to sP-selectin expression (B = 5.4, 95% CI 1.1–9.7, p = 0.014, Figure 2B) but not to platelet surface P-selectin (B = 0.1, 95% CI −0.1–0.3, p = 0.307, Figure 2C). Figure 2D depicts respective univariate linear regression coefficient Beta with 95% confidence interval. In the multivariate regression model, sST2 remained significantly associated with sP-selectin (B = 6.4, 95% CI 2.0–10.7, p = 0.004, Figure 2E) and monocyte TF expression (B = 0.56, 95% CI 0.02–1.1, p = 0.041, Figure 2E) after adjustment for age, sex, clinical risk factors and inflammatory parameters.
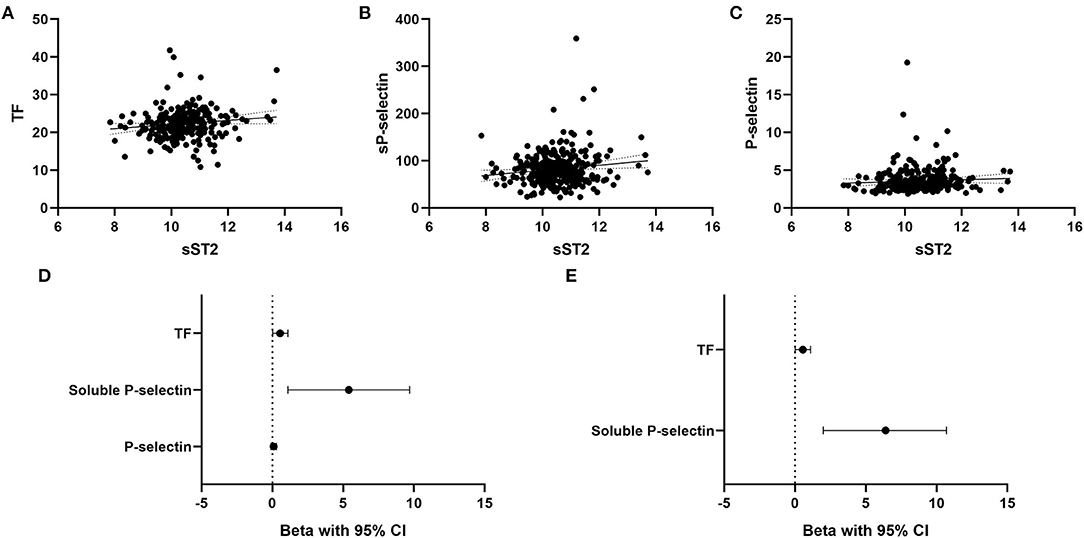
Figure 2. Association of circulating sST2 with monocyte TF, soluble P-selectin, and platelet surface P-selectin. Univariate linear regression analysis for association of sST2 with monocyte TF (A), soluble P-selectin (B), and platelet surface P-selectin (C) was performed as described in the methods section. Forest plots depict the univariate (D) and multivariate (E) regression coefficients Beta with 95% confidence interval. The multivariate regression model was adjusted for age, sex, arterial hypertension, hyperlipidemia, active smoking, type 2 diabetes and high-sensitivity CRP. A p < 0.05 was considered statistically significant.
Two year follow-up was assessed in 104 patients (32.9% of the overall cohort) undergoing elective infrainguinal angioplasty and stenting for PAD. During this time, the primary endpoint occurred in 41 patients (39.4%). Clinical risk factors were similarly distributed between patients without and with the primary endpoint (Table 2). Interestingly, patients suffering the primary endpoint had lower baseline cholesterol and triglyceride levels as compared to patients without the primary endpoint during follow-up. Both groups had similarly high circulating levels of sST2 (1,214 pg/mL, IQR 884–1,775 vs. 1,165 pg/mL, IQR 893–1,838, p = 0.976, Table 2). Circulating levels of sST2 did not predict the primary endpoint in Cox regression analysis (HR 1.1, 95% CI 0.76–1.71, p = 0.527). Kaplan-Meier survival curves showed no difference in survival for patients with high and low levels of sST2 (log-rank p = 0.785, Figure 3).
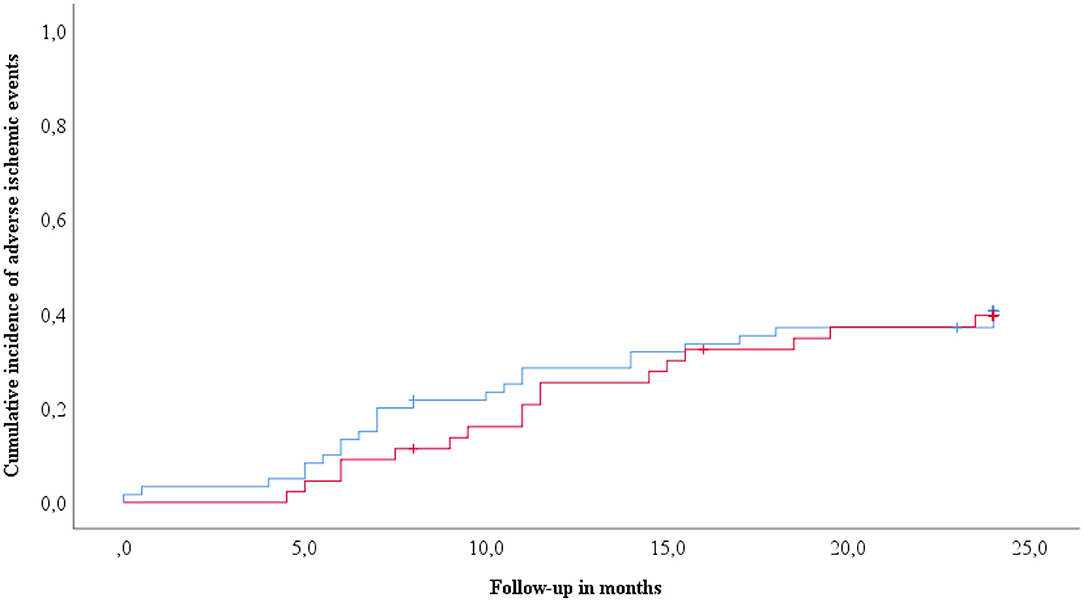
Figure 3. Cumulative incidence of adverse ischemic events according to circulating sST2. Kaplan-Meyer analyses for the cumulative incidence of adverse ischemic events (primary endpoint) following infrainguinal angioplasty and stenting stratified according to circulating sST2 levels. sST2 above the median is indicated by the red line. The blue line indicates sST2 below median.
Discussion
In the present study we investigated the association of sST2 with platelet activation and monocyte TF expression after elective angioplasty and stenting for cardiovascular disease, and its prognostic value following infrainguinal angioplasty with stent implantation in PAD. We could show here for the first time that sST2 is independently associated with increased levels of sP-selectin and enhanced monocyte TF expression. However, the association of sST2 with a prothrombotic milieu did not translate into a higher risk of adverse ischemic events in patients undergoing infrainguinal angioplasty and stenting for PAD.
Conflicting results have been reported on the role of the IL-33/ST2 system in different inflammatory conditions including atherosclerosis (7–10, 31, 32). While our group previously demonstrated pro-inflammatory and pro-angiogenic effects of IL-33 in human vascular endothelial cells (7, 10) as well as pro-thrombotic effects of IL-33 in human monocytes and endothelial cells (8, 9), another study reported that the injection of IL-33 in atherosclerosis-prone apolipoprotein E (ApoE) knockout (ApoE−/−) mice reduced the plaque area via induction of IL-5 and antioxidized low-density lipoprotein (ox-LDL) antibodies (33). However, the knockout of ST2 or IL-33 showed no beneficial effect on atherosclerosis development in ApoE−/− mice (34).
Different blood cells including monocytes, eosinophils, basophils, mast cells, B-cells, innate lymphoid cells type 2, different subtypes of T-cells, dendritic cells, and M2 polarized macrophages express ST2L (31). Platelets on the other hand, do not express ST2L on their surface to our best knowledge (unpublished correspondence). In the present study, we found a strong association of circulating sST2 with monocyte TF expression and sP-selectin but not with platelet surface P-selectin. Platelets shed P-selectin from their surface, which is then detectable in the circulation in its soluble form. Therefore, sP-selectin may be a more stable marker reflecting continuously ongoing platelet activation, while platelet surface P-selectin may represent platelet activation at the time of blood sampling (26). It was previously shown that P-selectin expression on activated platelets as well as sP-selectin are major determinants of leukocyte-platelet interaction and can trigger monocyte TF expression (35, 36). Furthermore, sP-selectin has been repeatedly associated with thromboembolic events (37, 38). We could show previously that IL-33 stimulates increased TF expression in monocytes and the release of prothrombotic extracellular vesicles via ST2L (9). Thus, one could hypothesize that the observed associations of sST2 with increased monocyte TF expression and sP-selectin might reflect complex platelet-monocyte interactions, with increased monocyte/platelet interactions and monocyte TF expression, contributing to a prothrombotic milieu in patients with cardiovascular disease (9).
The association of increased sST2 with adverse outcomes was demonstrated in different cardiovascular pathologies. sST2 concentrations were correlated with short-term as well as long-term mortality in patients with AMI (11, 14). In the large cohort from the Ludwigshafen risk and cardiovascular health study, sST2 predicted all-cause mortality also in patients with stable coronary artery disease (CAD) during a follow-up of 9.8 years (39). Moreover, sST2 is an established biomarker for mortality in patients with acute or chronic HF (16). sST2 independently predicted all-cause mortality in non-ischemic, dilated cardiomyopathy during a follow-up of 7 years, but it was inferior to growth differentiation factor-15 (GDF-15) for the prediction of fatal arrhythmic events in this patient population (17). In children and adolescent with HF sST2 performed poorly in contrast to midregional (MR) pro-atrial natriuretic peptide (proANP) that could accurately detect HF with diagnostic performance comparable with NT-proBNP. However, NT-proBNP, sST2, MR-proANP, and GDF-15 were able to distinguish between pediatric HF patients with preserved and poor functional status (40).
In spite of the observed association of sST2 with increased sP-selectin and monocyte TF expression, the present study showed that sST2 is not capable to identify PAD patients at higher risk for poor outcomes following angioplasty and stenting. Our findings are in line with a previous study by Lin et al., who demonstrated that in patients with PAD, increased sST2 levels were an independent predictor of all-cause mortality, but did not predict other endpoints including cardiovascular death, MI, stroke, and amputation (21). Both groups of patients, i.e., with and without event during the follow-up had similarly elevated sST2 levels, reflecting high cardiovascular risk and prothrombotic environment characteristic for PAD patients. The vast majority of patients with ischemic outcomes in the present study suffered target vessel restenosis (88%) during follow-up, and only 4.8% of patients experienced an AMI. Thus, sST2 is not associated with target vessel restenosis in PAD. Due to the low number of other ischemic events, we cannot draw conclusions on cardiovascular mortality based on the present study in this group of patients.
The present study had several limitations. Blood samples were acquired 24 h after the intervention and all patients were still on dual antiplatelet therapy at the time of blood sampling. Dual antiplatelet therapy can inhibit platelet P-selectin expression, possibly confounding the association of sST2 with platelet activation (41). Thus, we cannot provide data on the variability of sST2 formation, platelet activation and monocyte TF expression over time. Choosing this time point we sought to investigate whether or not a single post-procedural measurement early after intervention may be used for risk stratification. Another limitation was the small sample size in the follow-up cohort. Due to its small sample size, the results of our study should be confirmed in a larger cohort of patients with PAD. We have only used CD14 antibodies and therefore were not able to identify non-classical monocytes. However, based on previous studies, classical and intermediate monocytes are responsible for the most TF expression and prothrombotic potential of monocytes (9). Finally, currently available ELISA cannot distinguish between free and complexed sST2 (42).
In conclusion, circulating levels of sST2 are associated with sP-selectin and monocyte TF expression in atherosclerosis but not with ischemic outcomes following infrainguinal angioplasty with stent implantation for PAD.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethikkommission der Medizinischen Universität Wien, Borschkegasse 8b/6, 1090 Vienna, Austria. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SS, SD, and TG: conceptualization. CK, SP, BE, and TG: data curation. SS and TG: formal analysis. TG: funding acquisition and project administration. SP and TG: investigation. SS and SP: methodology. JW and CH: resources. SS, SD, and TG: writing—original draft. CK, JW, CH, BE, and SP: writing—review and editing. All authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ApoE, apolipoprotein; ox-LDL, antioxidized low-density lipoprotein; HF, heart failure; HR, hazard ratio; hs-CRP, high sensitivity CRP; IL-33, interleukin 33; IQR, interquartile range; MFI, mean fluorescence intensity; MI, myocardial infarction; PAD, peripheral artery disease; SD, standard deviation; sP-selectin, soluble P-selectin; sST2, soluble suppression of tumorigenesis 2; TF, tissue factor; TIA, transient ischemic attack.
References
1. Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. (2018) 39:763–816. doi: 10.1093/eurheartj/ehx095
2. Hiramoto JS, Teraa M, de Borst GJ, Conte MS. Interventions for lower extremity peripheral artery disease. Nat Rev Cardiol. (2018) 15:332–50. doi: 10.1038/s41569-018-0005-0
3. Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cifkova R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. (2015) 241:507–32. doi: 10.1016/j.atherosclerosis.2015.05.007
4. Signorelli SS, Vanella L, Abraham NG, Scuto S, Marino E, Rocic P. Pathophysiology of chronic peripheral ischemia: new perspectives. Ther Adv Chronic Dis. (2020) 11:2040622319894466. doi: 10.1177/2040622319894466
5. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. (2005) 23:479–90. doi: 10.1016/j.immuni.2005.09.015
6. Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. (2007) 117:1538–49. doi: 10.1172/JCI30634
7. Demyanets S, Konya V, Kastl SP, Kaun C, Rauscher S, Niessner A, et al. Interleukin-33 induces expression of adhesion molecules and inflammatory activation in human endothelial cells and in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. (2011) 31:2080–9. doi: 10.1161/ATVBAHA.111.231431
8. Stojkovic S, Kaun C, Basilio J, Rauscher S, Hell L, Krychtiuk KA, et al. Tissue factor is induced by interleukin-33 in human endothelial cells: a new link between coagulation and inflammation. Sci Rep. (2016) 6:25171. doi: 10.1038/srep25171
9. Stojkovic S, Thulin A, Hell L, Thaler B, Rauscher S, Baumgartner J, et al. IL-33 stimulates the release of procoagulant microvesicles from human monocytes and differentially increases tissue factor in human monocyte subsets. Thromb Haemost. (2017) 117:1379–90. doi: 10.1160/TH16-10-0784
10. Stojkovic S, Kaun C, Heinz M, Krychtiuk KA, Rauscher S, Lemberger CE, et al. Interleukin-33 induces urokinase in human endothelial cells–possible impact on angiogenesis. J Thromb Haemost. (2014) 12:948–57. doi: 10.1111/jth.12581
11. Demyanets S, Speidl WS, Tentzeris I, Jarai R, Katsaros KM, Farhan S, et al. Soluble ST2 and interleukin-33 levels in coronary artery disease: relation to disease activity and adverse outcome. PLoS ONE. (2014) 9:e95055. doi: 10.1371/journal.pone.0095055
12. Demyanets S, Tentzeris I, Jarai R, Katsaros KM, Farhan S, Wonnerth A, et al. An increase of interleukin-33 serum levels after coronary stent implantation is associated with coronary in-stent restenosis. Cytokine. (2014) 67:65–70. doi: 10.1016/j.cyto.2014.02.014
13. Stankovic M, Ljujic B, Babic S, Maravic-Stojkovic V, Mitrovic S, Arsenijevic N, et al. IL-33/IL-33R in various types of carotid artery atherosclerotic lesions. Cytokine. (2019) 120:242–50. doi: 10.1016/j.cyto.2019.05.010
14. Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. (2004) 109:2186–90. doi: 10.1161/01.CIR.0000127958.21003.5A
15. Dhillon OS, Narayan HK, Quinn PA, Squire IB, Davies JE, Ng LL. Interleukin 33 and ST2 in non-ST-elevation myocardial infarction: comparison with Global Registry of Acute Coronary Events Risk Scoring and NT-proBNP. Am Heart J. (2011) 161:1163–70. doi: 10.1016/j.ahj.2011.03.025
16. Aimo A, Januzzi JL Jr, Bayes-Genis A, Vergaro G, Sciarrone P, Passino C, et al. Clinical and prognostic significance of sST2 in heart failure: JACC review topic of the week. J Am Coll Cardiol. (2019) 74:2193–203. doi: 10.1016/j.jacc.2019.08.1039
17. Stojkovic S, Kaider A, Koller L, Brekalo M, Wojta J, Diedrich A, et al. GDF-15 is a better complimentary marker for risk stratification of arrhythmic death in non-ischaemic, dilated cardiomyopathy than soluble ST2. J Cell Mol Med. (2018) 22:2422–9. doi: 10.1111/jcmm.13540
18. Krychtiuk KA, Stojkovic S, Lenz M, Brekalo M, Huber K, Wojta J, et al. Predictive value of low interleukin-33 in critically ill patients. Cytokine. (2018) 103:109–13. doi: 10.1016/j.cyto.2017.09.017
19. Willems S, Quax PH, de Borst GJ, de Vries JP, Moll FL, de Kleijn DP, et al. Soluble ST2 levels are not associated with secondary cardiovascular events and vulnerable plaque phenotype in patients with carotid artery stenosis. Atherosclerosis. (2013) 231:48–53. doi: 10.1016/j.atherosclerosis.2013.08.024
20. Jirak P, Mirna M, Wernly B, Paar V, Thieme M, Betge S, et al. Analysis of novel cardiovascular biomarkers in patients with peripheral artery disease. Minerva Med. (2018) 109:443–50. doi: 10.23736/S0026-4806.18.05628-8
21. Lin JF, Wu S, Juang JJ, Chiang FT, Hsu LA, Teng MS, et al. IL1RL1 single nucleotide polymorphism predicts sST2 level and mortality in coronary and peripheral artery disease. Atherosclerosis. (2017) 257:71–7. doi: 10.1016/j.atherosclerosis.2016.12.020
22. Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. In vivo and protease-activated receptor-1-mediated platelet activation but not response to antiplatelet therapy predict two-year outcomes after peripheral angioplasty with stent implantation. Thromb Haemost. (2014) 111:474–82. doi: 10.1160/TH13-07-0558
23. Gremmel T, Wadowski PP, Mueller M, Kopp CW, Koppensteiner R, Panzer S. Serum Cholinesterase Levels Are Associated With 2-Year Ischemic Outcomes After Angioplasty and Stenting for Peripheral Artery Disease. J Endovasc Ther. (2016) 23:738–43. doi: 10.1177/1526602816655521
24. Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Comparison of methods to evaluate clopidogrel-mediated platelet inhibition after percutaneous intervention with stent implantation. Thromb Haemost. (2009) 101:333–9. doi: 10.1160/TH08-09-0577
25. Demyanets S, Kaun C, Pentz R, Krychtiuk KA, Rauscher S, Pfaffenberger S, et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J Mol Cell Cardiol. (2013) 60:16–26. doi: 10.1016/j.yjmcc.2013.03.020
26. Gremmel T, Koppensteiner R, Kaider A, Eichelberger B, Mannhalter C, Panzer S. Impact of variables of the P-selectin - P-selectin glycoprotein ligand-1 axis on leukocyte-platelet interactions in cardiovascular disease. Thromb Haemost. (2015) 113:806–12. doi: 10.1160/TH14-08-0690
27. Gremmel T, Ay C, Riedl J, Kopp CW, Eichelberger B, Koppensteiner R, et al. Platelet-specific markers are associated with monocyte-platelet aggregate formation and thrombin generation potential in advanced atherosclerosis. Thromb Haemost. (2016) 115:615–21. doi: 10.1160/th15-07-0598
28. Gremmel T, Xhelili E, Steiner S, Koppensteiner R, Kopp CW, Panzer S. Response to antiplatelet therapy and platelet reactivity to thrombin receptor activating peptide-6 in cardiovascular interventions: differences between peripheral and coronary angioplasty. Atherosclerosis. (2014) 232:119–24. doi: 10.1016/j.atherosclerosis.2013.10.027
29. Gremmel T, Koppensteiner R, Panzer S. Comparison of aggregometry with flow cytometry for the assessment of agonists -induced platelet reactivity in patients on dual antiplatelet therapy. PLoS ONE. (2015) 10:e0129666. doi: 10.1371/journal.pone.0129666
30. Moertl D, Berger R, Hammer A, Hutuleac R, Koppensteiner R, Kopp CW, et al. Dose-dependent decrease of platelet activation and tissue factor by omega-3 polyunsaturated fatty acids in patients with advanced chronic heart failure. Thromb Haemost. (2011) 106:457–65. doi: 10.1160/TH11-03-0169
31. Altara R, Ghali R, Mallat Z, Cataliotti A, Booz GW, Zouein FA. Conflicting vascular and metabolic impact of the IL-33/sST2 axis. Cardiovasc Res. (2018) 114:1578–94. doi: 10.1093/cvr/cvy166
32. Aimo A, Migliorini P, Vergaro G, Franzini M, Passino C, Maisel A, et al. The IL-33/ST2 pathway, inflammation and atherosclerosis: trigger and target? Int J Cardiol. (2018) 267:188–92. doi: 10.1016/j.ijcard.2018.05.056
33. Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, et al. IL-33 reduces the development of atherosclerosis. J Exp Med. (2008) 205:339–46. doi: 10.1084/jem.20071868
34. Martin P, Palmer G, Rodriguez E, Woldt E, Mean I, James RW, et al. Atherosclerosis severity is not affected by a deficiency in IL-33/ST2 signaling. Immun Inflamm Dis. (2015) 3:239–46. doi: 10.1002/iid3.62
35. Ivanov, II, Apta BHR, Bonna AM, Harper MT. Platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes. Sci Rep. (2019) 9:13397. doi: 10.1038/s41598-019-49635-7
36. Ushiyama S, Laue TM, Moore KL, Erickson HP, McEver RP. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J Biol Chem. (1993) 268:15229–37.
37. Ay C, Jungbauer LV, Sailer T, Tengler T, Koder S, Kaider A, et al. High concentrations of soluble P-selectin are associated with risk of venous thromboembolism and the P-selectin Thr715 variant. Clin Chem. (2007) 53:1235–43. doi: 10.1373/clinchem.2006.085068
38. Blann AD, Draper Z. Platelet activation as a marker of heart attack. Clin Chim Acta. (2011) 412:841–2. doi: 10.1016/j.cca.2011.02.022
39. Dieplinger B, Egger M, Haltmayer M, Kleber ME, Scharnagl H, Silbernagel G, et al. Increased soluble ST2 predicts long-term mortality in patients with stable coronary artery disease: results from the Ludwigshafen risk and cardiovascular health study. Clin Chem. (2014) 60:530–40. doi: 10.1373/clinchem.2013.209858
40. Hauser JA, Demyanets S, Rusai K, Goritschan C, Weber M, Panesar D, et al. Diagnostic performance and reference values of novel biomarkers of paediatric heart failure. Heart. (2016) 102:1633–9. doi: 10.1136/heartjnl-2016-309460
41. Moshfegh K, Redondo M, Julmy F, Wuillemin WA, Gebauer MU, Haeberli A, et al. Antiplatelet effects of clopidogrel compared with aspirin after myocardial infarction: enhanced inhibitory effects of combination therapy. J Am Coll Cardiol. (2000) 36:699–705. doi: 10.1016/S0735-1097(00)00817-2
Keywords: peripheral artery disease, sST2, ischemic outcomes, platelet reactivity, tissue factor
Citation: Stojkovic S, Demyanets S, Kopp CW, Hengstenberg C, Wojta J, Eichelberger B, Panzer S and Gremmel T (2020) Association of Soluble Suppression of Tumorigenesis 2 (sST2) With Platelet Activation, Monocyte Tissue Factor and Ischemic Outcomes Following Angioplasty and Stenting. Front. Cardiovasc. Med. 7:605669. doi: 10.3389/fcvm.2020.605669
Received: 12 September 2020; Accepted: 26 November 2020;
Published: 22 December 2020.
Edited by:
Paul H. A. Quax, Leiden University, NetherlandsReviewed by:
Margreet R. De Vries, Leiden University Medical Center, NetherlandsHetty De Boer, Leiden University Medical Center, Netherlands
Copyright © 2020 Stojkovic, Demyanets, Kopp, Hengstenberg, Wojta, Eichelberger, Panzer and Gremmel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Gremmel, dGhvbWFzLmdyZW1tZWxAbWVkdW5pd2llbi5hYy5hdA==
 Stefan Stojkovic
Stefan Stojkovic Svitlana Demyanets
Svitlana Demyanets Christoph W. Kopp
Christoph W. Kopp Christian Hengstenberg
Christian Hengstenberg Johann Wojta
Johann Wojta Beate Eichelberger5
Beate Eichelberger5 Thomas Gremmel
Thomas Gremmel