- Shanghai Chest Hospital, Shanghai Jiaotong University, Shanghai, China
Objectives: We attempted to develop more precisely quantified risk models for predicting cardiogenic stroke risk in non-valvular atrial fibrillation (NVAF) patients.
Methods: We conducted a case-control study, using data from hospitalized patients with AF who underwent transesophageal echocardiography at Shanghai Chest Hospital. A total of 233 high cardiogenic stroke risk patients with left atrial appendage thrombus (LAT) or left atrial spontaneous echo contrast (LA-SEC) and 233 controls matched for age, sex, AF type.
Results: AF history, LA diameter enlargement, larger left ventricular end diastolic diameter, lower ejection fraction, greater serum uric acid (SUA), and brain natriuretic peptide (BNP) levels showed association with high stroke risk. The multivariate logistic regression analysis revealed that AF duration, left atrial diameter (LAd), left ventricular ejection fraction (LVEF), SUA, and BNP were independent risk factors of the LAT/LA-SEC. We used LAd, LVEF, SUA, and BNP to construct a combined predictive model for high stroke risk in NVAF patients (the area under ROC curve: 0.784; sensitivity 66.1%; specificity 76.8%; 95% CI 0.744–0.825, P < 0.001).
Conclusion: Comprehensive evaluation of LAd, LVEF, SUA, and BNP may help stratify the cardiogenic stroke risk among non-valvular AF patients, guiding anticoagulation therapy.
Introduction
Cardiogenic stroke is defined as the ischemic stroke caused by the shedding of a cardiogenic embolus and embolism corresponding to the cerebral artery. According to reports, it accounts for 14% of all ischemic strokes (1, 2). Atrial fibrillation (with or without other cardiovascular diseases) related stroke accounts for more than 79% of all cardiogenic stroke, which is the most important risk factor of cardiogenic stroke (3, 4). Compared with non-AF related stroke, AF related stroke has more severe symptoms, higher disability rate, higher mortality rate, and is easy to relapse; the mortality rate is twice as high as non-AF related stroke; the medical cost is 1.5 times as high as non-AF related stroke (5). Left atrial appendage thrombus (LAT) and left atrial spontaneous echo contrast (LA-SEC) caused by atrial fibrillation are high risk factors of cardiogenic stroke. The majority of AF is non-valvular AF. At present, esophageal ultrasound is still the gold standard for monitoring thrombus in left atrial appendage. Although there is evidence that standardized anticoagulation therapy can significantly improve the prognosis of patients with high risk of thromboembolic events, in fact, most patients with atrial fibrillation do not use anticoagulation therapy. Strategies for identifying patients at risk for thromboembolism are commonly based on the basis of the CHA2DS2 -VASc score (6), however study found biomarkers could further refine stroke risk differentiation among patients initially classified as low risk (7). Clinically, we also found patients with low CHA2DS2 -VASc score still have a risk of thromboembolic events, more valuable forecast indicators of biomarkers in patients with AF seems to be necessary. Therefore, we aimed to develop a more precisely quantified risk models for predicting cardiogenic stroke risk in non-valvular atrial fibrillation (NVAF) patients.
Methods
Study Population
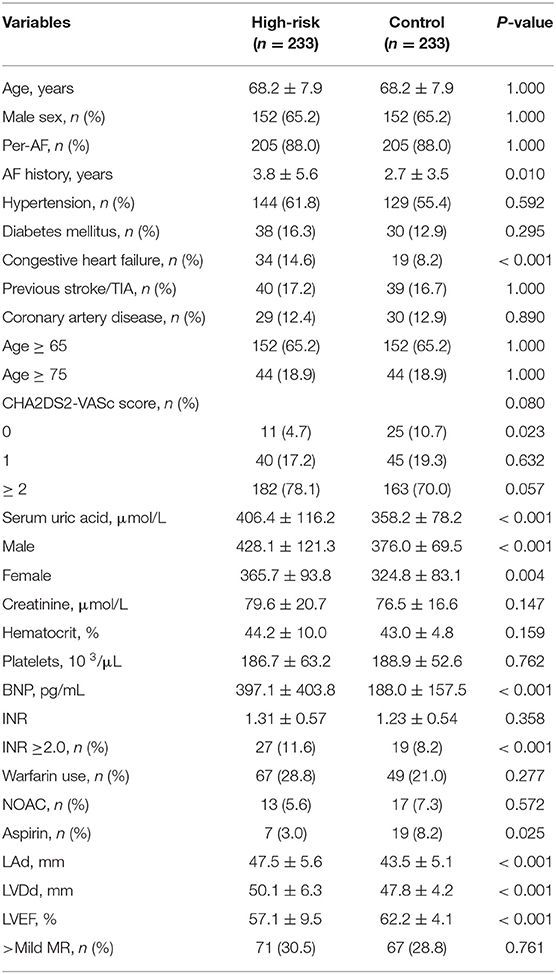
The study population comprised 233 high cardiogenic stroke risk patients with LAT or LA-SEC and 233 controls matched for age, sex, AF type, between January 2017 and July 2019. AF was confirmed by a 12-lead surface electrocardiogram and Holter. Paroxysmal AF and non-paroxysmal AF were defined according to the published guideline. Stroke risk was then evaluated according to the Congestive Heart Failure, Hypertension, Age>75 Years, Diabetes Mellitus, Stroke, Vascular Disease, Age 65–74 Years, Sex Category (CHA2DS2 -VASc) score. All patients underwent echocardiography and TEE before catheter ablation, with written informed consent was obtained. LA thrombus was diagnosed by a well-circumscribed echogenic mass contrasted with the adjacent myocardium. LA-SEC was diagnosed by the presence of dynamic smog-like echoes in the left atrial cavity and left atrial appendage. The left atrial diameter (LAD) and left ventricular ejection fraction (LVEF) were measured by transthoracic two-dimensional echocardiography.
Data on the clinical baseline characteristics of all patients were collected from electronic medical records and analyzed. Patients were categorized into a thrombosis group and a normal group according to the TEE results. The Ethics Study Committee at Shanghai Chest Hospital approved the study protocols and agreed that informed consent was not necessary because of the observational nature of the study.
Statistical Analysis
All analyses were performed with SPSS software version 25.0 (IBM Inc., NY, USA). All continuous data are presented as the mean ± SD deviation and were compared using Student t-test. Categorical variables were compared using Pearson's chi-square test or Fisher exact test whenever needed. The receiver operating characteristic (ROC) curve was constructed by plotting sensitivity vs. specificity used to discriminate the power of parameters in identifying the risk of stroke (LA/LAA thrombus, LAS-EC). Multivariable and univariable logistic regression was used to identify the risks of LAT or LA-SEC. All probability values were 2-sided and a P < 0.05 was considered statistically significant.
Results
Baseline Characteristics of the High Stroke Risk Group and Control Group
From January 1, 2017 to December 31, 2018, a total of 3,522 patients underwent TEE at the Shanghai Chest Hospital. After applying the exclusion criteria, 55 (1.56%) patients with non-valvular AF were LAT and 178 (5.05%) were LA-SEC. A case-control study was performed on 233 patients with LAT or LA-SEC and 233 age, sex, and AF-type matched control patients selected from a list of subjects who had undergone TEE. The baseline characteristics of patients in the high risk and control groups are summarized in Table 1.
As shown in Table 1, the patients in high risk group had greater proportion of congestive heart failure, larger LA diameter, larger left ventricular end diastolic diameter, lower ejection fraction, greater SUA, and BNP than control group. The mean AF history (3.8 ± 5.6 vs. 2.7 ± 3.5 years, P = 0.01) was markedly longer in patients with LAT/LA-SEC. There were no statistically significant differences in hypertension, diabetes mellitus, previous stroke/TIA, coronary artery disease, CHA2DS2-VASc Score, and INR, more than moderate mitral regurgitation.
Factors Predict High Stroke Risk and ROC Curve Analysis
Compared with the normal group, the high risk group had longer AF history, higher serum uric acid and BNP levels, LA enlargement, LVD enlargement, lower LVEF in high risk patients than in control group. All the above differences were statistically significant (P < 0.05). However, the CHA2DS2-VASc were similar between the two groups.
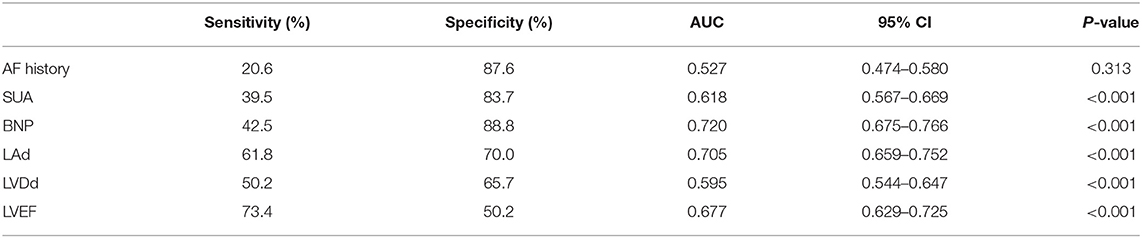
ROC curve analysis was conducted to evaluate the diagnostic value of statistically significant parameters in high risk patients (Table 2). The best cut-off value of SUA was ≥429.5 μmol/L (AUC 0.618, sensitivity 39.5%, specificity 83.7%, 95%CI 0.567–0.669, P < 0.001). The best cut-off value of BNP was ≥334.5 pg/mL (AUC 0.720, sensitivity 42.5%, specificity 88.8%, 95%CI 0.675–0.766, P < 0.001). The best cut-off value of LAd was ≥45.5 mm (AUC 0.705, sensitivity 61.8%, specificity 70.0%, 95%CI 0.659–0.752, P < 0.001). The best cut-off value of LVEF was ≤51.5% (AUC 0.677, sensitivity 73.4%, specificity 50.2%, 95%CI 0.629–0.725, P < 0.001).
Multivariable Analysis for LAT or LA-SEC
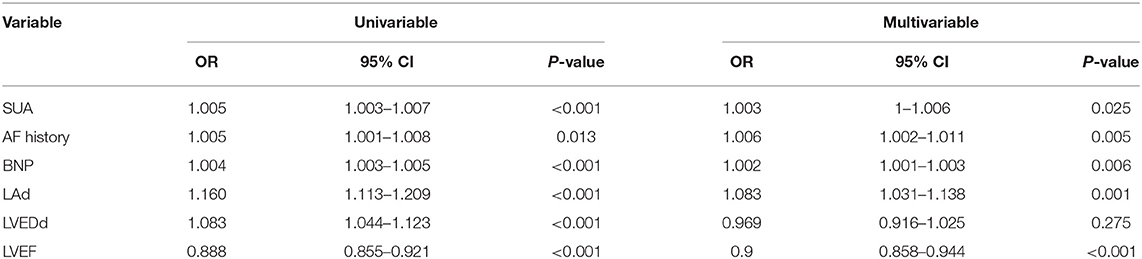
Multiple candidate clinical predictors and echocardiography measurements was performed to identify the independent predictors for LAT/LA-SEC. Our results demonstrated that AF duration, LAd, LVEF, SUA, and BNP were significantly correlated with the presence of LAT/LA-SEC. Univariable and multivariable analysis showed that these parameters were found to be significantly predictive of high stroke risk in NVAF patients (Table 3).
Combined Predictive Model
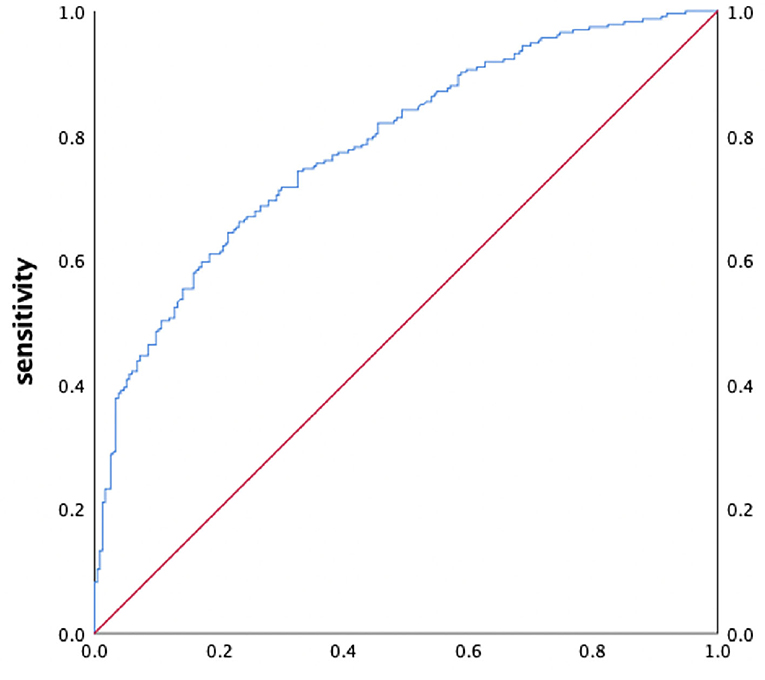
We used SUC, BNP, LAd, and LVEF as independent variables for further multivariate logistic regression. The results show that the combined predictive mode had an excellent discriminatory capacity in predicting high stroke risk (AUC 0.784; sensitivity 66.1%; specificity 76.8%; 95% CI 0.744–0.825, P < 0.001, Figure 1).
Subgroup Analyses
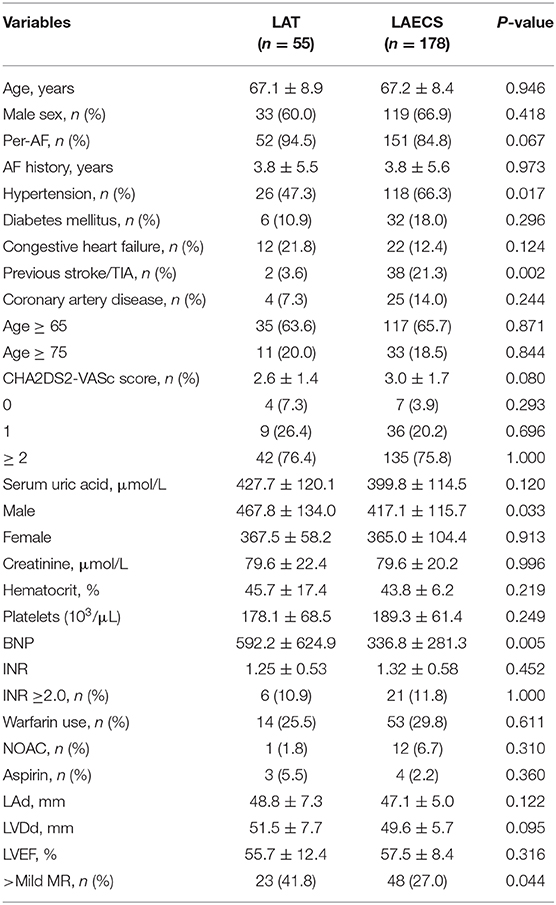
Stratified analyses were performed to assess the predicted value of parameters in LAT and LA-SEC group. As shown in Table 4, the patients in LAT group had greater proportion of hypertension than LA-SEC group. There were no statistically significant differences in age, sex, AF type, AF history, diabetes mellitus, previous stroke/TIA, coronary artery disease, CHA2DS2-VASc Score, congestive heart failure, LAd, LVEDd, LVEF, creatinine, hematocrit, platelets, and use of anticoagulant or aspirin.
The SUA levels in LAT group were no statistically significant differences greater than in LA-SEC group. However, the mean male SUA level (467.8 ± 134.0 vs. 417.1 ± 115.7 μmol/L, P = 0.033) was significantly higher in patients with LAT than LA-SEC (Table 4).
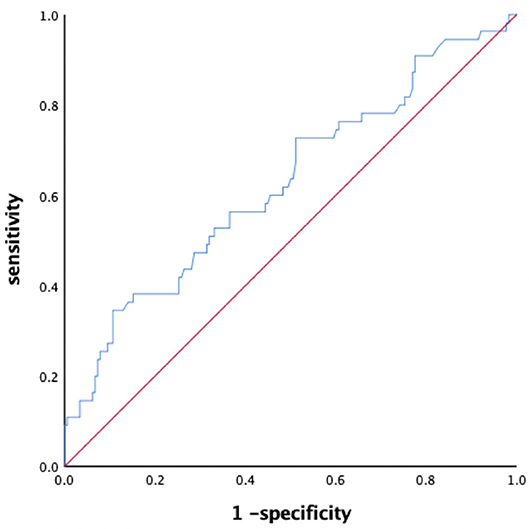
The BNP level in LAT group were significantly greater than in LA-SEC group (592.2 ± 624.9 vs. 336.8 ± 281.3 pg/mL, P = 0.005). The corresponding AUC for BNP predicting LAT was 0.627 (95% CI: 0.539–0.715) and the best cut-off point for BNP predicting LAT was 627 pg/mL, the sensitivity and specificity were 34.5 and 89.3%, respectively (Figure 2).
Discussion
Main Findings
In this case-control study, we demonstrated a significant positive association between SUA, BNP, LAd, LVEF and high stroke risk in non-valvular atrial fibrillation patients. The main findings were as follows: (1) Patients in the LAT/LA-SEC group had significantly higher SUA, BNP levels, LAd and lower LVEF than the control group; (2) Increased SUA and BNP, LA enlargement, LVEF reduction were independent risk factors and combining these four factors above is stronger than using any one single factor for predicting high stroke risk in non-valvular AF patients; (3) BNP levels in LAT group were significantly higher than LA-SEC group, which can be a modest predictor of higher stroke risk in AF patients with LA-SEC.
SUA is the final product of purine metabolism catalyzed by xanthine oxidase, which plays an important role in the formation of free radical superoxide anion and oxidative stress, consequently resulting in calcium overload and decreasing sodium channels and aggravating cellular damage (8–10). These pathological processes promote electrical remodeling and structural remodeling of the left atrium, leading to an increase of its size and contribute to the occurrence and development of AF (11–15). High SUA level is an independent risk factor for stroke and cardiovascular death (16–19). Studies have shown that hyperuricemia is an important risk factor for stroke and may improve the clinical risk stratification of patients with atrial fibrillation (20). Although it is still unable to explain the mechanism of hyperuricemia and stroke. However, we found that patients with LAT and LASEC had higher SUA levels, which means hyperuricemia is associated with a high risk of cardiac stroke in patients with non-valvular atrial fibrillation. This may provide clues for screening high-risk groups and strengthening anticoagulation therapy.
BNP is a sensitive indicator reflecting the increase of cardiac pressure and volume load, and its level is related to the functional load of cardiac pump. When atrial fibrillation occurs, the left atrium cannot contract effectively, the damage of left ventricular diastolic function and the increase of left ventricular filling pressure can lead to left atrial blood stasis, presenting as SEC, and increase the risk of LAA thrombosis (21, 22). Studies have shown that BNP can predict the risk of atrial fibrillation (23), thromboembolism (22, 24–27), and general cardiovascular risk stratification in NVAF patients (28–30). Recent studies have suggested that BNP is not only a predictor of AF, but also an early predictor of cerebral embolism in patients with AF (31). Our study has demonstrated that BNP is associated with LAT and LA-SEC, and BNP levels in LAT patients are higher than those in SEC patients. BNP can predict the risk of cardiogenic stroke independently of CHADS 2 and CHA2DS 2-vasc scores, and a higher BNP value means a higher risk of stroke.
In our analysis, decreased LVEF was revealed to be a powerful and independent predictor of LAT/LA-SEC formation in AF patients, which means high cardiogenic stroke risk. Previous studies have suggested that incidence of LAT depending on LVEF, and severe LV systolic dysfunction (confirmed by echocardiography) was a strong predictor of stroke (32, 33).
Studies found that LA enlargement is association with LA-SEC and embolic events (34–36). Left atrial enlargement may lead to thrombotic stroke by promoting endothelial damage, atrial blood stasis, and thrombosis (37). Atrial cardiomyopathy caused by fibrosis of the left atrium can lead to atrial fibrillation over time. LA enlargement is the manifestation of the severity of atrial cardiomyopathy, and co-exist with AF (38).
Despite that CHA2DS2-VASc score is mostly used to predict the stroke risk in atrial fibrillation (39). However, we found that there was no relationship between the score and LAT/LA-SEC formation. In this study, we found that there was an independent correlation between SUA, BNP, LAd, LVEF and LAT/LA-SEC in AF patients, and subgroup analysis found that the BNP level in LAT group was higher than that in LA-SEC group, with significant statistical difference, which may provide clues for high BNP to increase the risk of cardiogenic stroke and risk in non-valvular AF patients.
Clinical Implication
The main strength of our study was the generalization of the different features of the real-world non-valvular atrial fibrillation population in a matched cohort. Our present study found that a comprehensive evaluation of left atrial diameter, left ventricular ejection fraction, serum uric acid, and BNP may help stratify the cardiogenic stroke risk among non-valvular AF patients, which may help clinicians in the decision-guiding anticoagulation therapy.
Limitations
The present study had several limitations. The number of patients is relatively insufficient to determine the actual prediction value of these parameters for LA-SEC. In addition, most of the study population met the criteria for catheter ablation of AF, so selection bias may limit the current statistical analysis, and the population in this study may not reflect all patients with non-valvular AF. Considering that this study is a retrospective study, further prospective clinical trials are necessary to verify the predictive value of these parameters on the risk of cardiogenic stroke caused by atrial fibrillation and the guiding significance of anticoagulation decision-making. The pathophysiological mechanism of LAT and LA-SEC has not been well-explored. The mechanism of combined predictive model for cardiogenic stroke risk in AF needs further study.
Conclusion
This study found that the combined predictive model has a moderate predictive value for cardiogenic stroke risk among non-valvular AF patients, which will help us strengthen the screening of high-risk populations and strengthen anticoagulation therapy.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This research was supported by Natural Science Foundation of China Grants (Grant No: 81670305) and (Grant No: 81770324), National Key Research and Development Project (Grant No: 2018YFC1312503), and Shanghai Sailing Program (Grant No: 20YF1444300).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Murtagh B, Smalling RW. Cardioembolic stroke. Curr Atheroscler Rep. (2006) 8:310–16. doi: 10.1007/s11883-006-0009-9
2. Di Tullio MR, Homma S. Mechanisms of cardioembolic stroke. Curr Cardiol Rep. (2002) 4:141–8. doi: 10.1007/s11886-002-0027-3
3. Khoo CW, Lip GY. Clinical outcomes of acute stroke patients with atrial fibrillation. Expert Rev Cardiovasc Ther. (2009) 7:371–4. doi: 10.1586/erc.09.11
4. Pujadas Capmany R, Arboix A, Casañas-Muñoz R, Anguera-Ferrando N. Specific cardiac disorders in 402 consecutive patients with ischaemic cardioembolic stroke. Int J Cardiol. (2004) 95:129–34. doi: 10.1016/j.ijcard.2003.02.007
5. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012): the joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. (2012) 42:S1–44. doi: 10.1093/ejcts/ezs455
6. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2020) 29:ehaa612. doi: 10.1093/eurheartj/ehaa612
7. Rivera-Caravaca JM, Roldán V, Esteve-Pastor MA, Valdés M, Vicente V, Lip GYH, et al. Long-term stroke risk prediction in patients with atrial fibrillation: comparison of the ABC-Stroke and CHA2DS2-VASc scores. J Am Heart Assoc. (2017) 6:e006490. doi: 10.1161/JAHA.117.006490
8. Maharani N, Kuwabara M, Hisatome I. Hyperuricemia and atrial fibrillation. Int Heart J. (2016) 57:395–9. doi: 10.1536/ihj.16-192
9. Korantzopoulos P, Letsas KP, Liu T. Xanthine oxidase and uric acid in atrial fibrillation. Front Physiol. (2012) 3:150. doi: 10.3389/fphys.2012.00150
10. Tsai CT, Lai LP, Kuo KT, Hwang JJ, Hsieh CS, Hsu KL, et al. Angiotensin II activates signal transducer and activators of transcription 3 via Rac1 in atrial myocytes and fibroblasts: implication for the therapeutic effect of statin in atrial structural remodeling. Circulation. (2008) 117:344–55. doi: 10.1161/CIRCULATIONAHA.107.695346
11. Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Impact of gout on the risk of atrial fibrillation. Rheumatology. (2016) 55:721–8. doi: 10.1093/rheumatology/kev418
12. Tamariz L, Hernandez F, Bush A, Palacio A, Hare JM. Association between serum uric acid and atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. (2014) 11:1102–8. doi: 10.1016/j.hrthm.2014.04.003
13. Chen Y, Xia Y, Han X, Yang Y, Yin X, Qiu J, et al. Association between serum uric acid and atrial fibrillation: a cross-sectional community-based study in China. BMJ Open. (2017) 7:e019037. doi: 10.1136/bmjopen-2017-019037
14. Koza Y. Uric acid elevation in atrial fibrillation: is it simply an epiphenomenon or not?. Int J Cardiol. (2014) 174:869. doi: 10.1016/j.ijcard.2014.04.215
15. Nyrnes A, Toft I, Njølstad I, Mathiesen EB, Wilsgaard T, Hansen JB, et al. Uric acid is associated with future atrial fibrillation: an 11-year follow-up of 6308 men and women–the Tromso Study. Europace. (2014) 16:320–6. doi: 10.1093/europace/eut260
16. Wu AH, Gladden JD, Ahmed M, Ahmed A, Filippatos G. Relation of serum uric acid to cardiovascular disease. Int J Cardiol. (2016) 213:4–7. doi: 10.1016/j.ijcard.2015.08.110
17. Zhang W, Iso H, Murakami Y, Miura K, Nagai M, Sugiyama D, et al. Serum uric acid and mortality form cardiovascular disease: EPOCH-JAPAN study. J Atheroscler Thromb. (2016) 23:692–703. doi: 10.5551/jat.31591
18. Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. national health and nutrition examination survey. JAMA. (2000) 283:2404–10. doi: 10.1001/jama.283.18.2404
19. Brombo G, Bonetti F, Volpato S, Morieri ML, Napoli E, Bandinelli S, et al. Uric acid within the “normal” range predict 9-year cardiovascular mortality in older individuals. the InCHIANTI study. Nutr Metab Cardiovasc Dis. (2019) 29:1061–7. doi: 10.1016/j.numecd.2019.06.018
20. Chao TF, Hung CL, Chen SJ, Wang KL, Chen TJ, Lin YJ, et al. The association between hyperuricemia, left atrial size and new-onset atrial fibrillation. Int J Cardiol. (2013) 168:4027–32. doi: 10.1016/j.ijcard.2013.06.067
21. Doukky R, Khandelwal A, Garcia-Sayan E, Gage H. External validation of a novel transthoracic echocardiographic tool in predicting left atrial appendage thrombus formation in patients with nonvalvular atrial fibrillation. Eur Heart J Cardiovasc Imaging. (2013) 14:876–81. doi: 10.1093/ehjci/jes313
22. Doukky R, Garcia-Sayan E, Patel M, Pant R, Wassouf M, Shah S, et al. Impact of diastolic function parameters on the risk for left atrial appendage thrombus in patients with nonvalvular atrial fibrillation: a prospective study. J Am Soc Echocardiogr. (2016) 29:545–53. doi: 10.1016/j.echo.2016.01.014
23. Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. (2010) 121:200–7. doi: 10.1161/CIRCULATIONAHA.109.882241
24. Shimizu H, Murakami Y, Inoue S, Ohta Y, Nakamura K, Katoh H, et al. High plasma brain natriuretic polypeptide level as a marker of risk for thromboembolism in patients with nonvalvular atrial fibrillation. Stroke. (2002) 33:1005–10. doi: 10.1161/hs0402.105657
25. Pant R, Patel M, Garcia-Sayan E, Wassouf M, D'Silva O, Kehoe RF, et al. Impact of B-type natriuretic peptide level on the risk of left atrial appendage thrombus in patients with nonvalvular atrial fibrillation: a prospective study. Cardiovasc Ultrasound. (2016) 14:4. doi: 10.1186/s12947-016-0047-6
26. Harada M, Tabako S, Fujii Y, Takarada Y, Hayashi K, Ohara H, et al. Correlation between plasma brain natriuretic peptide levels and left atrial appendage flow velocity in patients with non-valvular atrial fibrillation and normal left ventricular systolic function. J Echocardiogr. (2018) 16:72–80. doi: 10.1007/s12574-017-0362-4
27. Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, et al. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health study. Circulation. (2009) 120:1768–74. doi: 10.1161/CIRCULATIONAHA.109.873265
28. Hayashi K, Tsuda T, Nomura A, Fujino N, Nohara A, Sakata K, et al. Impact of B-Type natriuretic peptide level on risk stratification of thromboembolism and death in patients with nonvalvular atrial fibrillation - the hokuriku-plus AF registry. Circ J. (2018) 82:1271–8. doi: 10.1253/circj.CJ-17-1085
29. Wasser K, Weber-Krüger M, Gröschel S, Uphaus T, Liman J, Hamann GF, et al. Brain natriuretic peptide and discovery of atrial fibrillation after stroke: a subanalysis of the find-AFRANDOMISED Trial. Stroke. (2020) 51:395–401. doi: 10.1161/STROKEAHA.119.026496
30. Okada Y, Shibazaki K, Kimura K, Matsumoto N, Iguchi Y, Aoki J, et al. Brain natriuretic peptide is a marker associated with thrombus in stroke patients with atrial fibrillation. J Neurol Sci. (2011) 301:86–9. doi: 10.1016/j.jns.2010.10.017
31. Zecca B, Mandelli C, Maino A, Casiraghi C, Bolla G, Consonni D, et al. A bioclinical pattern for the early diagnosis of cardioembolic stroke. Emerg Med Int. (2014) 2014:242171. doi: 10.1155/2014/242171
32. Khan MN, Usmani A, Noor S, Elayi S, Ching CK, Di Biase L, et al. Low incidence of left atrial or left atrial appendage thrombus in patients with paroxysmal atrial fibrillation and normal EF who present for pulmonary vein antrum isolation procedure. J Cardiovasc Electrophysiol. (2008) 19:356–8. doi: 10.1111/j.1540-8167.2007.01070.x
33. Uziebło-Zyczkowska B, Krzesiński P, Jurek A, Kapłon-Cieślicka A, Gorczyca I, Budnik M, et al. Left ventricular ejection fraction is associated with the risk of thrombus in the left atrial appendage in patients with atrial fibrillation. Cardiovasc Ther. (2020) 2020:3501749. doi: 10.1155/2020/3501749
34. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. the Framingham Heart study. Circulation. (1995) 92:835–41. doi: 10.1161/01.CIR.92.4.835
35. Frenkel D, D'Amato SA, Al-Kazaz M, Markowitz SM, Liu CF, Thomas G, et al. Prevalence of left atrial thrombus detection by transesophageal echocardiography: a comparison of continuous non-vitamin k antagonist oral anticoagulant versus warfarin therapy in patients undergoing catheter ablation for atrial fibrillation. JACC Clin Electrophysiol. (2016) 2:295–303. doi: 10.1016/j.jacep.2016.01.004
36. Calvo N, Mont L, Vidal B, Nadal M, Montserrat S, Andreu D, et al. Usefulness of transoesophageal echocardiography before circumferential pulmonary vein ablation in patients with atrial fibrillation: is it really mandatory? Europace. (2011) 13:486–91. doi: 10.1093/europace/euq456
37. Yaghi S, Moon YP, Mora-McLaughlin C, Willey JZ, Cheung K, Di Tullio MR, et al. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke study. Stroke. (2015) 46:1488–93. doi: 10.1161/STROKEAHA.115.008711
38. Jordan K, Yaghi S, Poppas A, Chang AD, Mac Grory B, Cutting S, et al. Left atrial volume index is associated with cardioembolic stroke and atrial fibrillation detection after embolic stroke of undetermined source. Stroke. (2019) 50:1997–2001. doi: 10.1161/STROKEAHA.119.025384
39. Huang J, Wu SL, Xue YM, Fei HW, Lin QW, Ren SQ, et al. Association of CHADS2 and CHA2DS2-VASc scores with left atrial thrombus with nonvalvular atrial fibrillation: a single center based retrospective study in a cohort of (2695). Chinese subjects. Biomed Res Int. (2017) 2017:6839589. doi: 10.1155/2017/6839589
Keywords: atrial fibrillation, cardiogenic stroke, left atrial appendage thrombus, LA-SEC, risk model
Citation: Song Z, Xu K, Hu X, Jiang W, Wu S, Qin M and Liu X (2020) A Study of Cardiogenic Stroke Risk in Non-valvular Atrial Fibrillation Patients. Front. Cardiovasc. Med. 7:604795. doi: 10.3389/fcvm.2020.604795
Received: 10 September 2020; Accepted: 12 October 2020;
Published: 05 November 2020.
Edited by:
Tong Liu, Tianjin Medical University, ChinaReviewed by:
Osmar Antonio Centurion, National University of Asunción, ParaguayMartin Ibarrola, Independent Researcher, Bella Vista, Argentina
Copyright © 2020 Song, Xu, Hu, Jiang, Wu, Qin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Liu, eGtkcmxpdXh1JiN4MDAwNDA7MTI2LmNvbQ==; Mu Qin, cWlubXUtMTAwMSYjeDAwMDQwO2xpdmUuY24=
†These authors have contributed equally to this work
 Ziliang Song
Ziliang Song Kai Xu†
Kai Xu†