- 1Arrhythmia Center, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Cardiology, Shanghai Institute of Cardiovascular Diseases, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Department of Cardiology, Beijing Hospital, Beijing, China
- 4Department of Cardiology, First Affiliated Hospital of Harbin Medical University, Harbin, China
- 5Department of Cardiology, Nanjing Drum Tower Hospital, Nanjing, China
Objective: To clarify the impact of sex on physical activity (PA) levels among patients with implantable cardioverter-defibrillators/cardiac resynchronization therapy defibrillators (ICD/CRT-D) and its association with cardiac death and all-cause mortality.
Methods: Overall, data of 820 patients with ICD/CRT-D from the SUMMIT registry were retrospectively analyzed. Baseline PA from 30 to 60 days after device implantation was measured using Biotronik accelerometer sensors. The primary and secondary endpoints were cardiac death and all-cause mortality, respectively.
Results: Baseline PA levels were significantly higher in male patients than in female patients (11.40 ± 5.83% vs. 9.93 ± 5.49%, P = 0.001). Males had higher predictive PA cut-off values for cardiac death (11.16 vs. 7.15%) and all-cause mortality (11.33 vs. 7.17%). During the median follow-up time of 75.7 ± 29.1 months, patients with baseline PA<cut-off values had higher cumulative incidence of cardiac death and all-cause mortality in both males and females. At a PA level between the cut-off values of males and females, males had a higher risk of cardiac death (hazard ratio = 4.952; 95%CI = 1.055-23.245, P = 0.043) and all-cause mortality (hazard ratio = 2.432; 95%CI = 1.095-5.402, P = 0.029).
Conclusions: Males had higher predictive PA cut-off values for cardiac death and all-cause mortality in patients with ICD/CRT-D. Sex should be considered as an important contributing factor when deciding for PA targets.
Introduction
Implantable cardioverter-defibrillators (ICD) and cardiac resynchronization therapy defibrillators (CRT-D) have become the standard modalities for the prevention of sudden cardiac death and management of some patients with heart failure (HF) (1, 2). It is important to identify patients at high risk for cardiac death and all-cause mortality after ICD/CRT-D implantation to improve their clinical outcomes (3–6).
Physical activity (PA) is a quantitative index reflecting individual functional status.
A number of studies have indicated that PA is significantly associated with clinical prognosis in the healthy population and in patients with structural heart disease (7–9).
PA data can be collected constantly and automatically using accelerometer sensors (10–12). Previous studies evaluating PA, when used alone or when integrated with other diagnostic procedures in patients with ICD/CRT-D, suggested an inverse relationship with HF regarding hospitalization and survival, after adjusting for other clinical factors (6, 13–17). PA level in males is different from that in females in various diseases (18–20). Despite the important role of PA in the prognostication of outcomes after ICD/CRT-D implantation, little is known about the differences between sex in PA levels among those patients.
The present study aimed to clarify the impact of sex on PA levels among patients with ICD/CRT-D and their predictive value for cardiac death and all-cause mortality risk.
Methods
Study Population and Study Design
We retrospectively analyzed patients who underwent ICD or CRT-D implantation between June 2010 and June 2014 from the SUMMIT registry study. The present study complied with the Declaration of Helsinki, and ethics committees from all participating institutions approved this study. All patients provided written informed consent before enrollment. Inclusion criteria: (1) patients with indications for ICD/CRT-D (Biotronik SE & Co. KG; Berlin, Germany) implantation according to Class I therapy practice guidelines; (2) patients with ICD/CRT-D devices equipped to process daily home monitoring (HM) transmissions; (3) patients aged over 18 years; (4) patients who survived more than three months after ICD/CRT-D implantation. Exclusion criteria: (1) unable to follow up as required or had missing HM data; (2) with diagnosed malignant tumor or life expectancy <1 year; (3) scheduled heart transplant. Eight hundred and forty-five patients from a total of 1,008 patients had PA data recorded. Nineteen patients with incomplete data, 3 patients who died within three months after implantation, 3 patients lost to follow-up were excluded, and 820 patients were eventually enrolled.
Baseline Clinical Characteristics
Information regarding baseline clinical characteristics was collected from patient medical records before ICD/CRT-D implantation, which included age, sex, and body mass index (BMI), New York Heart Association (NYHA) class, ischemic cardiomyopathy (ICM) history, old myocardial infarction and valvular disease history, comorbidities [hypertension, diabetes, atrial fibrillation (AF), stroke and pre-implantation syncope], echocardiographic characteristics [left ventricular ejection fraction (LVEF), and left ventricular end-diastolic diameter (LVEDD)], and medication history [beta-blockers, amiodarone, diuretics, statins, digoxin, aldosterone antagonist, and angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB)].
PA Measurement
PA in ICD/CRT-D was measured through an integrated circuit accelerometer embedded in the pulse generator. The accelerometer detects both the frequency and amplitude of patient motion and translates this into a proportional electrical signal updated each minute. Automatic PA measurements using Biotronik accelerometer sensors were cumulatively collected and recorded as the time when the device delivered higher than the devices' basic rates. The PA resolution was 2 s, and the data were converted into % per 24 h. The Biotronik remote monitoring system can automatically transmit data stored in implantable devices to the Biotronik service center every day. Considering clinical procedural recovery, the baseline PA was defined as the average PA measured from 30 to 60 days after device implantation, as recommended by previous studies (14–16).
Follow-Up and Study Endpoints
Patients were immediately informed in case of interruption (failure or disruption) in data transmission. Routine follow-up clinic visits and phone calls were conducted by designated clinical coordinators. The primary endpoint of this study was cardiac death. The secondary endpoint was all-cause mortality. In case of patient death, the date and cause of death was confirmed with their families based on the death certificate.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviations (SD), and were compared between the male group and female group using Student's t-test. Categorical variables are expressed as numbers and percentages and were compared between the two groups using Pearson's χ2 test. Receiver operating characteristic (ROC) curves were plotted to identify a PA cut-off value in the male group and female group, which was used to predict cardiac death and all-cause mortality. Kaplan–Meier survival curves (Log-rank tests) evaluated survival time from the date of ICD or CRT-D implantation to the date of cardiac death and all-cause mortality between subgroups of PA<cutoff AND PA ≥cut-off in males and females, respectively. Univariate and multivariate Cox regression analyses were used to evaluate the relationship among the baseline characteristics, which included the PA level and the study endpoints. Hazard ratios and 95% confidence intervals (CIs) were calculated for the endpoints. Based on the two PA cut-offs for the male and female groups, the participants were categorized into three subgroups. The endpoints were compared between males and females when PA was between the cut-off values of males and females. All statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) and Graph Pad Prism Software (version 5.0; GraphPad Software, La Jolla, CA, USA). Two-sided statistical significance was defined as P value < 0.05 for all analyses.
Results
Baseline Characteristics
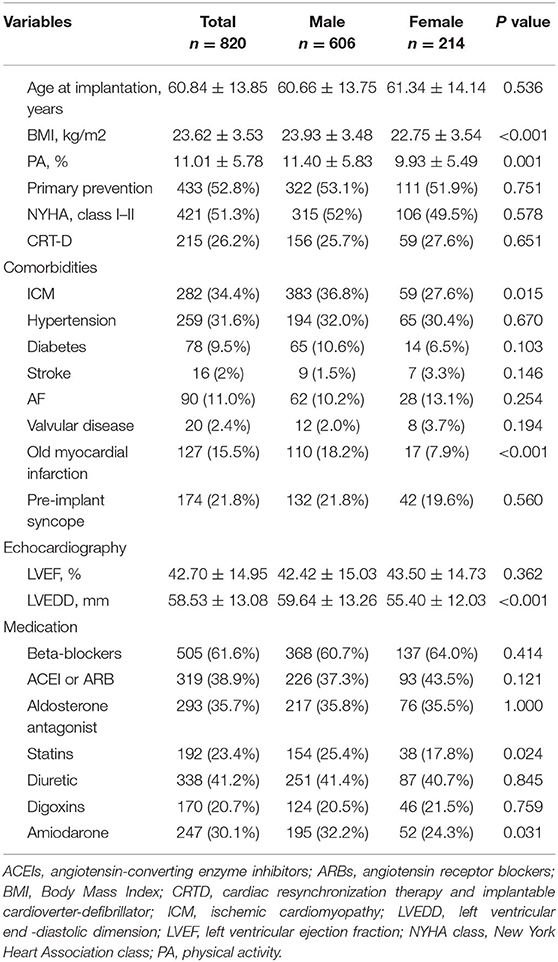
Baseline demographic and clinical characteristics are shown in Table 1. Overall, 820 patients, of which 73.9% were males (n = 606), were enrolled in this study. Their mean age was 60.84 ± 13.85 years. The mean LVEF and LVEDD were 42.70 ± 14.95% and 58.53 ± 13.08 mm, respectively, with 26.2% of patients receiving CRT-D devices. The mean baseline PA level was significantly higher in the male group than in the female group (11.40 ± 5.83% vs. 9.93 ± 5.49%, P = 0.001). Compared to patients in the female group, those in the male group had higher BMI (P < 0.001) and LVEDD (P < 0.001), more concomitant with ICM (P = 0.015) and received statin (P = 0.024) and amiodarone therapy (P = 0.031).
Clinical Outcomes and Baseline PA Level as a Predictor
During a mean follow-up time of 75.7 ± 29.1 months (range from 3.1 to 116.6 months), 90 cardiac (11.0%) and 181 all-cause mortality events (22.1%) occurred.
In the ROC analysis of baseline PA levels as a predictor for cardiac death (Figure 1), the cut-off value was 11.16% in the male group (area under the curve (AUC) = 0.666, P < 0.001, Figure 1A) and 7.15% in the female group (AUC = 0.695, P = 0.006, Figure 1B), respectively. The cut-of value of baseline PA levels as a predictor for all-cause mortality was 11.33% in the male group (AUC = 0.702, P < 0.001, Figure 1C), and 7.17% in the female group (AUC = 0.737, P < 0.001), respectively (Figure 1D).
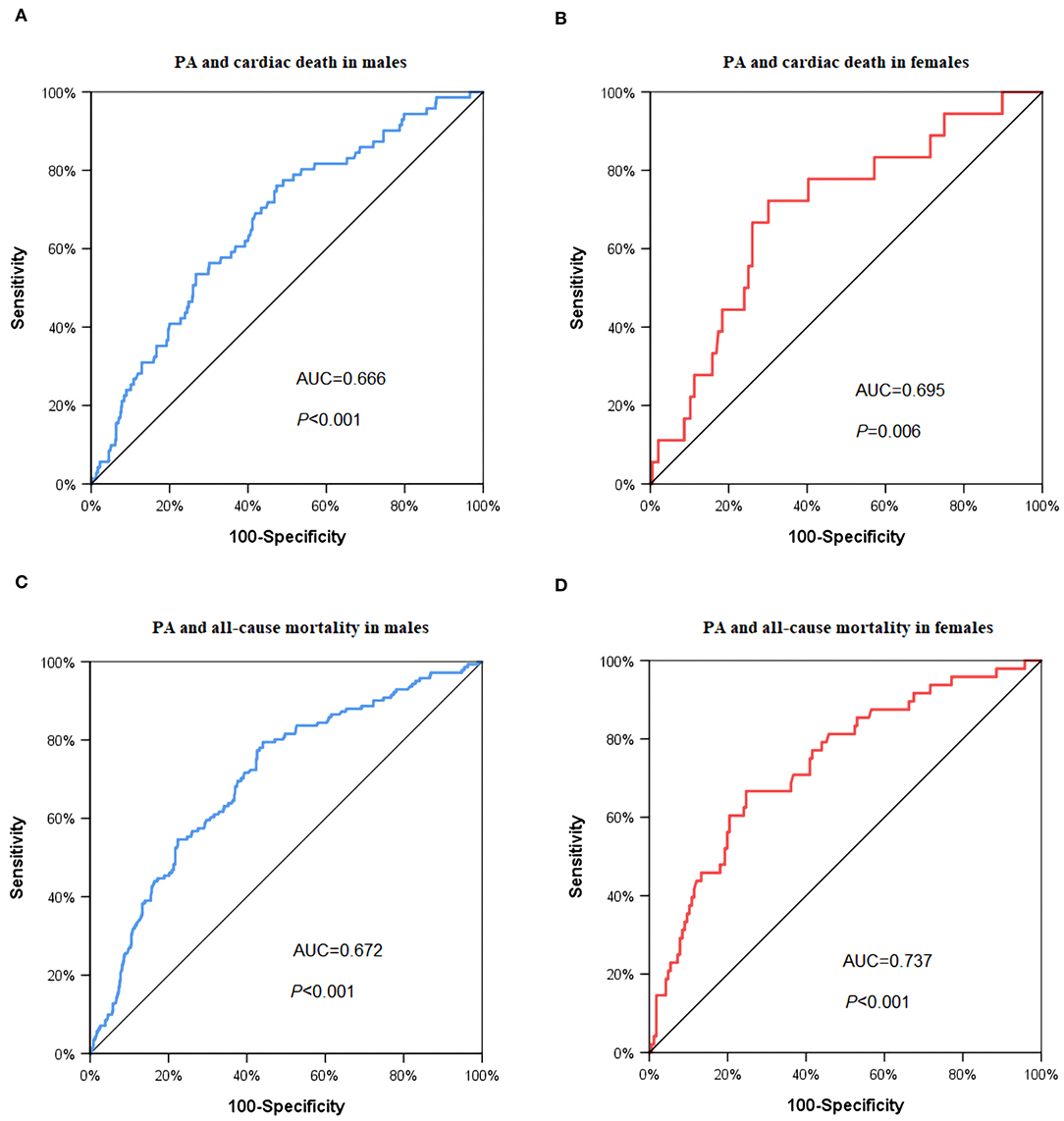
Figure 1. ROC curve to predict the cut-off values of cardiac death and all-cause mortality. (A) Cardiac death in male group: AUC = 0.666, P < 0.001, PA cut off value = 11.16%; (B) cardiac death in female group: AUC = 0.695, P = 0.006, PA cut-off value = 7.15%; (C) all-cause mortality in male group: AUC = 0.702, P < 0.001, PA cut off value = 11.33%; (D) all-cause mortality in female group: AUC = 0.737, P < 0.001, PA cut off value = 7.17%. PA: physical activity.
Kaplan–Meier Survival Curves
As shown in Figure 2, in the Kaplan-Meier survival analysis, patients with baseline PA levels lower than cut-off values had higher cumulative incidence of cardiac death in both males (Log rank P < 0.001, Figure 2A) and females (Log rank P < 0.001, Figure 2B). The results were similar regarding all-cause mortality. With baseline PA levels lower than cut-off values, males (Log rank P < 0.001, Figure 2C) and females (Log rank P < 0.001, Figure 2D) both had higher cumulative incidence of all-cause mortality.
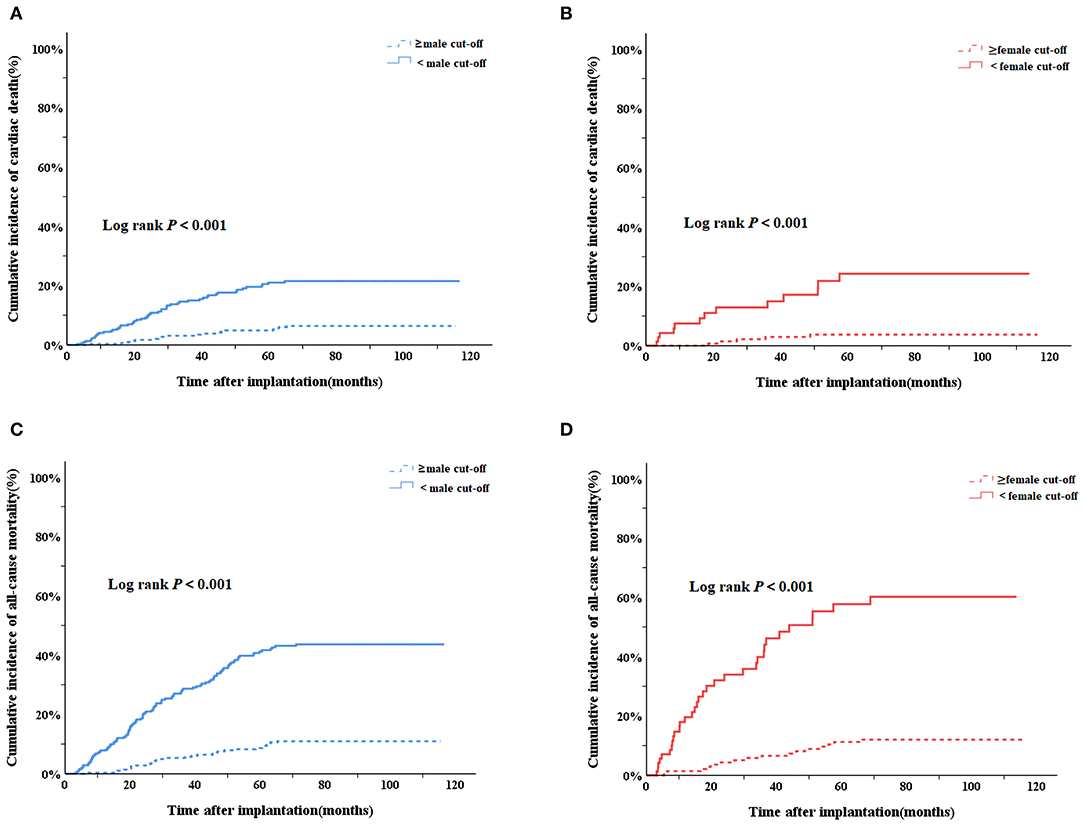
Figure 2. Kaplan–Meier survival curves to evaluate the cumulative incidence of cardiac death and all-cause mortality between subgroups of PA<cutoff AND PA ≥cut-off in males and females, respectively. (A) Cardiac death in male group; (B) cardiac death in female group; (C) all-cause mortality in male group; (D) all-cause mortality in female group. PA, physical activity.
Cox Regression Analysis in Males and Females
As shown in Table 2, uni-variate and multivariate cox regression analysis were performed in males and females, respectively. In the males group, the uni-variate Cox regression model showed that PA levels <11.16% were associated with an increased risk of cardiac death (HR = 3.460; 95%CI = 2.029–5.902, P < 0.001) and PA levels <11.33% were associated with an increased all-cause mortality (HR = 4.065; 95%CI = 2.716–6.084, P < 0.001). Both PA measurements (<11.16% and <11.33%) remained as independent predictors of cardiac death (HR = 3.071, 95%CI = 1.741–5.417, P < 0.001) and all-cause mortality (HR = 3.445, 95%CI = 2.246–5.283, P < 0.001), when adjusted using a multivariate model (adjusted for age, NYHA class, BMI, primary indication, CRT-D, hypertension, diabetes, AF, stroke, ICM, LVEF, LVEDD, old myocardial infarction, beta-blockers, aldosterone antagonist, ACEI or ARB, diuretics, amiodarone and statins).
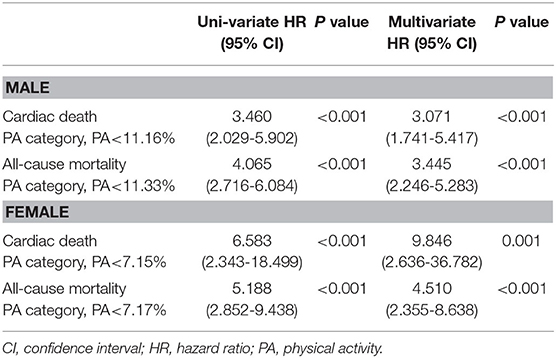
Table 2. Predictors of cardiac death and all-cause mortality according to PA in male and female patients.
In the female group, PA levels <7.15% were associated with an increased risk of cardiac death (HR = 6.583; 95%CI = 2.343–18.499, P < 0.001) and PA levels <7.17% were associated with an increased all-cause mortality (HR = 5.188; 95%CI = 2.852– 9.438, P < 0.001) in a uni-variate Cox regression model. PA levels <7.15% and <7.17% remained as independent predictors of cardiac death (HR = 9.846, 95%CI = 2.636–36.782, P = 0.001) and all-cause mortality (HR = 4.510, 95%CI = 2.355–8.638, P < 0.001), when adjusted in a multivariate model (adjusted for age, NYHA class, BMI, primary indication, CRT-D, hypertension, diabetes, AF, stroke, ICM, LVEF, LVEDD, old myocardial infarction, beta-blockers, aldosterone antagonist, ACEI or ARB, diuretics, amiodarone and statins).
Sex Differences in the Subgroup of PA Levels Between the Cut-Off Values of Males and Females
As shown in Table 3, in the subgroup of PA levels between the cut-off values of males and females, males had a higher risk of cardiac death (hazard ratio = 4.952; 95%CI = 1.055-23.245, P = 0.043) and all-cause mortality (hazard ratio = 2.432; 95% CI = 1.095−5.402, P = 0.029) after adjusting for confounders (age, PA, NYHA class, BMI, primary indication, CRT-D, hypertension, diabetes, AF, stroke, ICM, LVEF, LVEDD, old myocardial infarction, betablockers, aldosterone antagonist, ACEI or ARB, diuretics, amiodarone and statins).
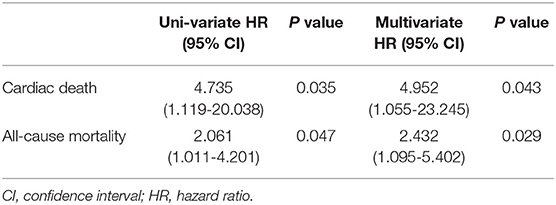
Table 3. Sex differences in the subgroup of PA levels between the cut-off values of males and females.
Discussion
Main Findings
Our main findings were as follows: (1) patients with baseline PA levels lower than cut-off values had higher cumulative incidence of cardiac death and all-cause mortality in both males and females; (2) male patients have higher baseline and predictive PA cut-off values than female patients; and (3) the difference between males and females was observed when the PA level was between the cut-off values of males and females.
Relationship Between PA and Outcomes
A series of studies evaluated the relationship between daily PA levels and outcomes in ICD/CRT-D patients, including hospitalization due to HF, cardiac death, and all-cause mortality (6, 13–17). Previous studies discovered that an integrated diagnostic algorithm derived from device-measured dynamic parameters, including PA, could identify CRT-D patients with a higher risk of hospitalization due to HF within the subsequent months (6, 13). Conraads et al. demonstrated that the PA during the early 30-day period post device implantation as an independent significant predictor of hospitalization due to HF and all-cause mortality even after correcting for HF severity (14). Palmisano et al. provided more evidence about the relationship between PA and hospitalization due to HF, and Kramer et al. discovered that regardless of baseline and time-varying PA levels, a low PA level was associated with a higher risk of all-cause mortality (15, 17). Zhao et al. demonstrated that a PA cut-off value of 7.84% can predict the risk of cardiac death and all-cause mortality in patients with ICD/CRT-D implantation (16). In the present study, significant clinical factors such as age, NYHA class, LVEF, CRT-D, comorbidities and medications were taken into consideration in the multivariate Cox regression analysis, and more evidence was provided to support the inverse association between daily PA and cardiac death and all-cause mortality. Additionally, PA was recorded with home monitoring and the association was accessed in males and females, respectively.
Sex Differences in PA and Its Effects on Endpoints Risk
Previous studies have examined sex differences in PA levels after a cardiac event (18–20). The present study showed conclusive evidence that PA levels were higher in males than in females among patients with ICD/CRT-D. In addition, the PA cut-off value predicting negative outcomes was higher in male patients than in female patients. When the PA level was between the cut-off values of PA in males and females, male patients had a 5-fold increased risk for cardiac death and 2.5-fold increased risk for all-cause mortality, compared with female patients. Therefore, female sex may be a protective factor in patients with insufficient PA. Our results were consistent with those of previous studies suggesting that females may have a lower risk of exertion-related sudden cardiac death (21–23). Although specific underlying mechanisms have not been elucidated, possible explanations may be due to the important role of the autonomic nervous system in cardiac death. Estrogen, a predominant hormone in females, modulates the autonomic nervous system and increases vagal tone (24). In a study conducted by Zhao et al. a high PA level was an indicator of efficient autonomic function because of a significant correlation between PA and heart rate variability (16). Thus, both high PA levels and female sex contributed to a better prognosis in patients with ICD/CRT-D by modulating autonomic function.
Clinical Implications
Male patients were shown to present with more severe disease, have more comorbidities, and receive more medications than females. Male patients were also shown to engage in more PA after ICD/CRT-D implantation than female patients. Female patients were shown to have greater benefits from increased PA. Sex differences were shown to impact PA levels, cardiac death, and all-cause mortality of patients who received ICD/CRT-D. Thus, interventions to improve the outcomes in patients with ICD/CRT-D must consider sex as an important contributing factor.
Limitations
The present study was a retrospective study. Of the 820 patients recruited, only 214 female patients (26.1%), compared to the 606 (73.9%) male patients, were included, which may lead to selection bias. In addition, the present study included a mixture of patients receiving ICDs or CRT-Ds for primary or secondary prevention. Moreover, despite of adjustment for multiple covariates, some important basal biochemical and clinical characteristics, like the levels of glucose and lipid profile, smoke or alcohol intake, were not included. And the specific underlying mechanisms explaining the impact of sex on PA levels and their association with outcomes were not elucidated, although we attempted to provide possible explanations. Thus, more prospective studies with larger samples are needed to further validate our findings.
Conclusions
Baseline PA levels are significantly higher in male patients than in female patients with ICD/CRT-D. Male patients have higher predictive PA cut-off values for cardiac death and all-cause mortality than female patients. The present study suggests that sex should be considered as an important contributing factor when deciding for PA targets in patients with ICD/CRT-D.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by ethics committee of Fuwai Hospital (the chief institute) and all other participating organizations (Zhongshan Hospital Fudan University et al.). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XL and SZhang contributed to the conception or design of the work. XL, ZLi, and SZhao contributed to the acquisition, analysis, and interpretation of data for the work. XL, XX, and XS drafted the manuscript. KC, WH, YS, JY, ZLia, WX, and SZhang critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
This study was supported by the National Natural Science Foundation of China (81470466).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
PA, physical activity; SCD, sudden cardiac death; CIED, cardiovascular implantable electronic devices; HM, home monitoring; ICD, implantable cardioverter-defibrillator; CRTD, cardiac resynchronization therapy defibrillator; AF, atrial fibrillation; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; LVEDD, left ventricular end diastolic dimension; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; HR, hazard ratio; CI, confidence interval.
References
1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis treatment of acute chronic heart failure: The Task Force for the diagnosis treatment of acute chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
2. Gopinathannair R, Cornwell WK, Dukes JW, Ellis CR, Hickey KT, Joglar JA, et al. Device therapy and arrhythmia management in left ventricular assist device recipients: a Scientific statement from the american heart association. Circulation. (2019) 139:e967–e89. doi: 10.1161/CIR.0000000000000673
3. Chen S, Ling Z, Kiuchi MG, Yin Y, Krucoff MW. The efficacy and safety of cardiac resynchronization therapy combined with implantable cardioverter defibrillator for heart failure: a meta-analysis of 5674 patients. Europace. (2013) 15:992–1001. doi: 10.1093/europace/eus419
4. Huang Y, Wu W, Cao Y, Qu N. All cause mortality of cardiac resynchronization therapy with implantable cardioverter defibrillator: a meta-analysis of randomized controlled trials. Int J Cardiol. (2010) 145:413–7. doi: 10.1016/j.ijcard.2010.05.016
5. Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation. (2010) 122:2359–67. doi: 10.1161/CIRCULATIONAHA.110.960633
6. Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar S, Porter CB, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to access and review trending information and evaluate correlation to symptoms in patients with heart failure) study. J Am Coll Cardiol. (2010) 55:1803–10. doi: 10.1016/j.jacc.2009.11.089
7. Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. (2017) 390:2643–54. doi: 10.1016/s0140-6736(17)31634-3
8. Mok A, Khaw KT, Luben R, Wareham N, Brage S. Physical activity trajectories and mortality: population based cohort study. BMJ. (2019) 365:l2323. doi: 10.1136/bmj.l2323
9. Jeong SW, Kim SH, Kang SH, Kim HJ, Yoon CH, Youn TJ, et al. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur Heart J. (2019) 40:3547–55. doi: 10.1093/eurheartj/ehz564
10. Hawkins MS, Storti KL, Richardson CR, King WC, Strath SJ, Holleman RG, et al. Objectively measured physical activity of USA adults by sex, age, and racial/ethnic groups: a cross-sectional study. Int J Behav Nutr Phys Act. (2009) 6:31. doi: 10.1186/1479-5868-6-31
11. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. (2002) 346:793–801. doi: 10.1056/NEJMoa011858
12. Vegh EM, Kandala J, Orencole M, Upadhyay GA, Sharma A, Miller A, et al. Device-measured physical activity versus six-minute walk test as a predictor of reverse remodeling and outcome after cardiac resynchronization therapy for heart failure. Am J Cardiol. (2014) 113:1523–8. doi: 10.1016/j.amjcard.2014.01.430
13. Cowie MR, Sarkar S, Koehler J, Whellan DJ, Crossley GH, Tang WH, et al. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur Heart J. (2013) 34:2472–80. doi: 10.1093/eurheartj/eht083
14. Conraads VM, Spruit MA, Braunschweig F, Cowie MR, Tavazzi L, Borggrefe M, et al. Physical activity measured with implanted devices predicts patient outcome in chronic heart failure. Circ Heart Fail. (2014) 7:279–87. doi: 10.1161/CIRCHEARTFAILURE.113.000883
15. Kramer DB, Mitchell SL, Monteiro J, Jones PW, Normand SL, Hayes DL, et al. Patient activity and survival following implantable cardioverter-defibrillator implantation: the aLTITUDE activity study. J Am Heart Assoc. (2015) 4:5. doi: 10.1161/JAHA.115.001775
16. Zhao S, Chen K, Su Y, Hua W, Chen S, Liang Z, et al. Association between patient activity and long-term cardiac death in patients with implantable cardioverter-defibrillators and cardiac resynchronization therapy defibrillators. Eur J Prev Cardiol. (2017) 24:760–7. doi: 10.1177/2047487316688982
17. Palmisano P, Guerra F, Ammendola E, Ziacchi M, Luigi Pisano EC, Dell'Era G, et al. Physical activity measured by implanted devices predicts atrial arrhythmias and patient outcome: results of iMPLANTED (Italian multicentre observational registry on patients with implantable devices remotely monitored). J Am Heart Assoc. (2018) 7:5. doi: 10.1161/JAHA.117.008146
18. Blair SN, Kohl HW 3rd, Paffenbarger RS Jr., Clark DG, Cooper KH, et al. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. (1989) 262:2395–401. doi: 10.1001/jama.262.17.2395
19. Minges KE, Strait KM, Owen N, Dunstan DW, Camhi SM, Lichtman J, et al. Gender differences in physical activity following acute myocardial infarction in adults: a prospective, observational study. Eur J Prev Cardiol. (2017) 24:192–203. doi: 10.1177/2047487316679905
20. Bennett DA, Du H, Clarke R, Guo Y, Yang L, Bian Z, et al. Association of physical activity with risk of major cardiovascular diseases in chinese men and women. JAMA Cardiol. (2017) 2:1349–58. doi: 10.1001/jamacardio.2017.4069
21. Whang W, Manson JE, Hu FB, Chae CU, Rexrode KM, Willett WC, et al. Physical exertion, exercise, and sudden cardiac death in women. JAMA. (2006) 295:1399–403. doi: 10.1001/jama.295.12.1399
22. Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. (1998) 97:2110–6. doi: 10.1161/01.cir.97.21.2110
23. Siscovick DS. Exercise and its role in sudden cardiac death. Cardiol Clin. (1997) 15:467–72. doi: 10.1016/s0733-8651(05)70352-0
24. Linde C, Bongiorni MG, Birgersdotter-Green U, Curtis AB, Deisenhofer I, Furokawa T, et al. Sex differences in cardiac arrhythmia: a consensus document of the European heart rhythm association, endorsed by the heart rhythm society and Asia Pacific heart rhythm society. Europace. (2018) 20:1565. doi: 10.1093/europace/euy067
Keywords: physical activity, sex differences, cardiac death, all-cause mortality, implantable cadioverter defibrillators
Citation: Li X, Xue X, Sun X, Zhao S, Chen K, Hua W, Su Y, Yang J, Liang Z, Xu W, Li Z and Zhang S (2020) Sex Differences in Physical Activity and Its Association With Cardiac Death and All-Cause Mortality in Patients With Implantable Cardioverter-Defibrillators. Front. Cardiovasc. Med. 7:588622. doi: 10.3389/fcvm.2020.588622
Received: 29 July 2020; Accepted: 19 November 2020;
Published: 14 December 2020.
Edited by:
Saskia C. A. De Jager, Utrecht University, NetherlandsReviewed by:
Monika Gladka, Hubrecht Institute (KNAW), NetherlandsMora Murri, University of Málaga, Spain
Copyright © 2020 Li, Xue, Sun, Zhao, Chen, Hua, Su, Yang, Liang, Xu, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Zhang, emhhbmdzaHVmd0AxNjMuY29t
†These authors have contributed equally to this work
 Xiaoyao Li
Xiaoyao Li Xiaodi Xue1†
Xiaodi Xue1† Shu Zhang
Shu Zhang