
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Cardiovasc. Med. , 02 September 2020
Sec. General Cardiovascular Medicine
Volume 7 - 2020 | https://doi.org/10.3389/fcvm.2020.00129
This article is part of the Research Topic What do we know about COVID-19 implications for cardiovascular disease? View all 109 articles
The Coronavirus Disease 2019 (COVID-19) pandemic, being caused by an easily and rapidly spreading novel betacoronavirus, has created a state of emergency for people, the scientific community, healthcare systems and states, while the global financial consequences are still unfolding. Cardiovascular complications have been reported for COVID-19-infected patients and are associated with a worse prognosis. ECG and biomarkers may raise suspicion of cardiac involvement. However, transthoracic echocardiography is a fast and reliable bedside method to establish the diagnosis of cardiac complications, including acute coronary syndromes, pericarditis, myocarditis, and pulmonary embolism. Early detection of cardiac dysfunction by speckle tracking echocardiography during off-line analysis may be used to identify a high-risk population for development of heart failure in the acute setting. Precautionary measures are mandatory for operators and equipment to avoid viral dispersion. No specific treatment is yet available for severe acute respiratory syndrome coronavirus 2 (SARS-CoV 2), and a variety of antiviral, immune-modifying, and antioxidant agents are therefore under intense investigation. Echocardiography, including assessment of myocardial deformation, may provide a useful tool to monitor the effects of the various treatment regimens on cardiac function both acutely and in the midterm.
A novel enveloped, single-stranded, positive-sense RNA betacoronavirus belonging to the family of coronaviruses has been identified as the causative agent of the novel viral pneumonia that started in the city of Wuhan, Hubei Province, China, on December 12, 2019, (1) and has turned into a global health emergency. The Coronavirus Disease 2019 pandemic counts, as of June 4, 2020, over 6.5 million confirmed cases in 188 countries and regions in the world and 384.815 fatalities (2), rapidly doubling the number of deaths within a month. The consequences of the pandemic in terms of its effects on the world population and global economy are still unfolding. The disease varies considerably from an asymptomatic or mild form without pneumonia to mild forms of pneumonia to severe pneumonia with lung consolidation that can lead to respiratory failure, sepsis, and multiorgan failure (3). Compared to the previous two coronaviruses that cause severe disease in humans, SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome), SARS exhibits environmental stability (4) and MERS requires close and prolong contact for contamination (5), while the current coronavirus shows easy and high transmissibility, partly related to the high viral load early in the course of the disease (6).
Cardiovascular complications are relatively common, occurring in up to 25% of COVID-19 patients (7, 8) (Table 1). Myocardial injury is associated with a 37% in-hospital mortality even in patients without prior cardiovascular disease (9, 15). Cases of acute myocarditis have been reported presenting either as fulminant myocarditis or with symptoms mimicking an acute coronary syndrome (ACS) (16, 17). Pathology evidence of myocardial infiltration by a limited number of monocytes, lymphocytes, and/or neutrophils (18), and rarely associated epicarditis (19) may be suggestive of either activation of the systemic immune response or myocardial inflammatory infiltration due to viral-induced myocyte lysis. Patients presenting with ST elevation myocardial infarction (STEMI) either as an initial manifestation of the disease or during the course of hospitalization for COVID-19 disease (20) were treated with primary percutaneous intervention (PCI). Interestingly, 39% of patients had no evidence of obstructive coronary artery disease on coronary angiography, a finding that questions thrombolysis as a therapeutic alternative to timely coronary angiography and possibly primary PCI. Myocardial injury (11, 14) may also be attributed to myocardial supply/demand mismatch precipitated by hypoxemia, hypotension, tachycardia, and an uncontrolled inflammatory response, leading to cytokine release syndrome (10).
Pulmonary embolism (12, 13, 21, 22) occurs frequently occurring in up to a quarter of all COVID-19 patients despite prophylactic antithrombotic treatment. The activation of the coagulation cascade by inflammatory cytokines, direct endothelial injury of lung microcirculation, antiplatelet activation, and suppression of the fibrinolytic system are all involved synergistically in the mechanism of venous thrombosis (23). Sudden hemodynamic compromise, the need for increased oxygen supplementation in discordance with radiological disease severity or elevations in D-dimers, especially >1 g/l, should prompt further diagnostic work-up with Computed Tomography Pulmonary Angiography (CTPA) to confirm pulmonary embolism.
Echocardiography is a first-line imaging method to diagnose overt and subtle myocardial dysfunction (14). Localized wall motion abnormalities may be suggestive of a culprit coronary artery lesion leading to an ACS, whereas a diffuse pattern of abnormal segmental longitudinal myocardial strain by echocardiography may support the diagnosis of myocarditis over this of an acute coronary syndrome. Signs indicative of acute pulmonary embolism (PE) should be sought. Right ventricular dilatation from a PLAX view or a basal RV/LV ratio > 1 in a four chamber view can be easily measured as well as pulmonary artery diameter from a short axis view. RV dysfunction can be assessed both qualitatively and with the integration of simple and fast measurements of TDI and TAPSE. Right ventricular systolic hypokinesis is associated with worse 30–day prognosis (24). The presence of a Mc-ConnelI sign increases the sensitivity for the diagnosis though specificity on its own is only 33% (25). Echocardiographic evidence of RV pressure overload expressed by systolic and diastolic septal flattening can be noticed (26). Estimation of pulmonary artery systolic pressure from tricuspid systolic gradient and a usually increased inferior vena cava diameter combined with a short acceleration time and midsystolic notch in the PW Doppler of the RVOT(‘60/60; sign) (26) further indicates increased PA pressure and proximal thromboemboli (27). In clinical practice, hemodynamic instability should lead to a cardiac Point of care Ultrasound (POCUS) (28, 29) to determine the presence of left ventricular dysfunction and/or right ventricular dilatation or a large pericardial effusion (Figure 1). Handheld echo devices are the most suitable for this indication as they are portable, more easily disinfected compared to traditional ultrasound machines, while images can be stored and transferred to a PC. On the other hand, in an hemodynamically stable patient with COVID-19 infection, a high clinical suspicion of cardiac involvement, supported by one abnormal diagnostic parameter acquired on admission-that is ECG, CXR, hs Troponin I, NT-proBNP, D-dimers, FDP's—should lead to consideration of a transthoracic echocardiogram (TTE). Solely one abnormal laboratory test cannot be the only criterion for a TTE in a stable COVID-19 infection patient, since their positive predictive value for a specific disease may be low, especially in patients with concomitant chronic diseases and thus lead to a TTE patients' without cardiac involvement. For example, D-dimers may be elevated in various diseases where activation of coagulation and fibrinolysis is present such as cancer patients with COVID 19 (30) or chronic kidney disease (31). For NT-pro BNP values, a level greater that 300 pg/ml (32) should be considered as abnormal. Although, when taking age into consideration, a higher NTproBNP level >1,800 pg/ml should be used to suspect acute heart failure in patients older than >75 years, (33, 34), anything lower than those NT pro-BNP values, at a cut-off level of 940 pg/ml, has been related to adverse outcomes in critically ill patients admitted to ICU (35). Elevated hs cardiac Troponin I is even more specific to myocardial injury than CTnT (36) and may be attributed to multiple and overlapping mechanisms. Cardiac Troponin I can be measured on admission and during hospitalization of COVID- 19 patients in conjunction to NT-pro BNP and thus guide the need for TTE.
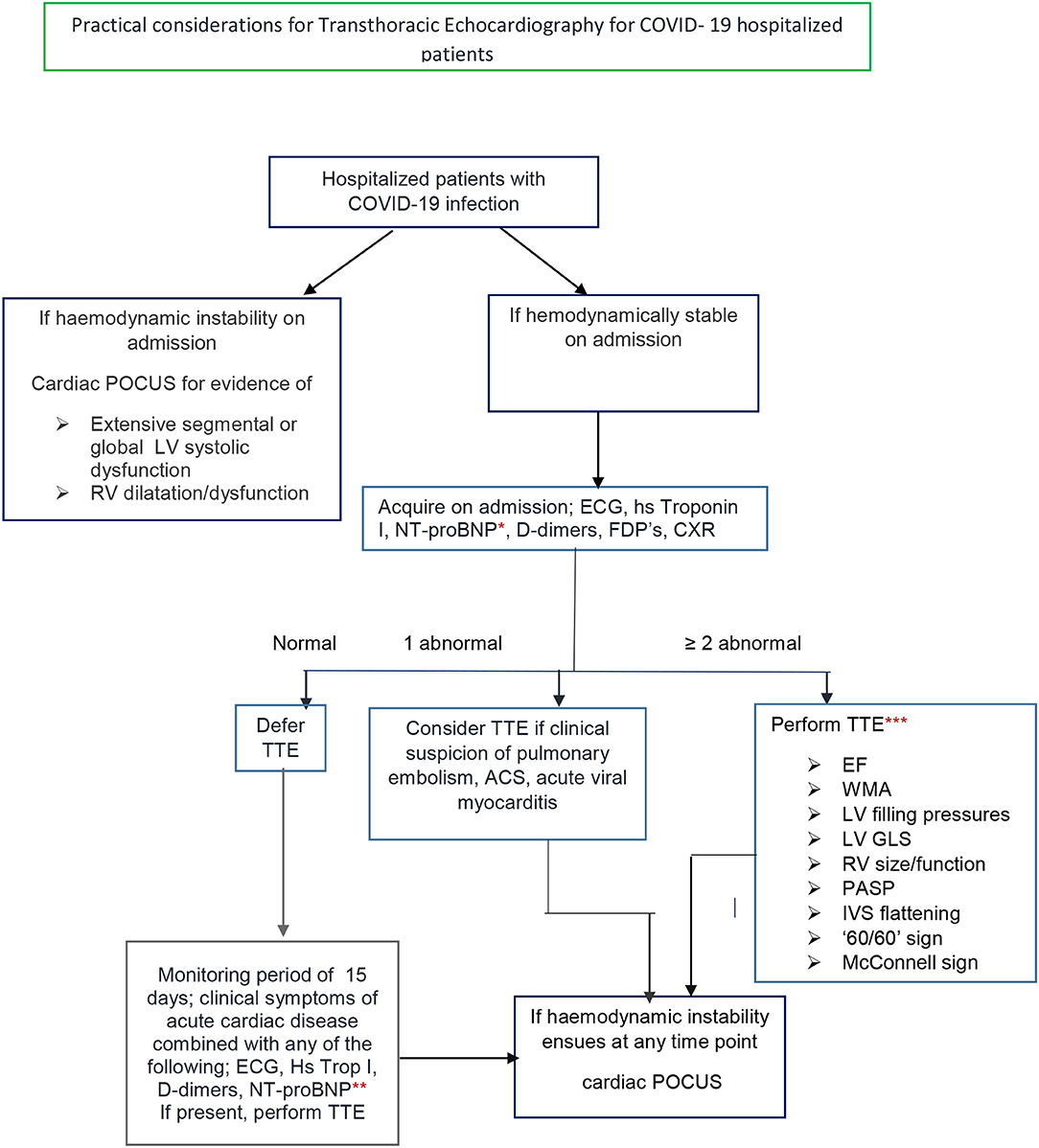
Figure 1. Transthoracic echocardiography for hospitalized COVID 19 disease patients. *NT-pro BNP level on admission ≥300 pg/ml (9). **NT-pro BNP level during hospitalization ≥940 pg/ml (16, 17). ***One experienced sonographer/cardiologist. Use PPE. Disinfect equipment in and out of the ward. EF, ejection fraction; WMA, wall motion abnormality; LV, left ventricle; RV, right ventricle, IVS, interventricular septum; GLS, global longitudinal strain by offline analysis; Cardiac POCUS, cardiac point of care cardiac ultrasound.
On the contrary, when two of the initial diagnostic parameters are abnormal, a complete TTE should be performed to diagnose possible cardiac involvement and ventricular dysfunction. Even the combination of two elevated laboratory biomarkers alone, should lead to a complete TTE, as hsTrop T and NT-proBNP levels were linearly correlated and considerably increased in non-survivor COVID-19 patients (15). As cardiac complications may occur within 15 days of admission monitoring of NT-proBNP, troponins and D-dimers in combination with the patients' clinical status are recommended throughout this period (37).
Notable considerations exist when estimating left and right ventricular systolic function, one being the presence of tachycardia, related to numerous factors such as fever, hypoxemia, cytokine production, and systemic inflammation. Ventricular systolic function is negatively affected in the presence of tachycardia due to the force–frequency relationship. Diastolic function estimations parameters are also affected by tachycardia, since fusion of transmitral E and A waves makes estimation of their ratio and the DT time inaccurate (38). In that case, a TR maximum velocity jet for estimation of PA systolic pressure may be an indicator of LV filling pressures. An impaired global longitudinal strain of the LV or RV may also indicate the initiation of myocardial damage particularly in patients with elevated troponins.
Echocardiography exams should be performed by experienced practitioners to ensure quick acquisition of high quality images (24) and thus minimize possible viral exposure.
TEE carries a high risk of spreading aerosolized viral material within an exam environment. It should be avoided during the pandemic (39). It should be only carried out when there is an absolute indication (e.g., bacterial endocarditis), and the results are expected to modify patient's management. In that case, the exam should be carefully designed by the patient's medical team (40).
Safety is of utmost importance for the personnel involved in echocardiography of suspected or confirmed COVID-19 patients. Frequent and meticulous handwashing is mandatory. Personal Protective Equipment (PPE) should be used depending on the risk level. Face masks, headcovers, eye shields, gloves, gowns, and shoe covers should be used when examining high risk patients. Detailed description for the PPE is provided by WHO, ASE and EACVI (24, 34, 41). Institutions provide their own detailed protocols in line with international societies' guidance and local experience.
Equipment used for Echocardiography studies should be thoroughly disinfected at the end of the exam, in the examination room and again at the hallway (8). Dedicated machines for scanning suspected or confirmed patients may be preferable at this time. Manufacturer's guidance for proper disinfection of the different types of machines should be followed as well as instructions given by certain disinfectant producers.
A number of different pharmacological agents (42)—antivirals, investigational antivirals, and immune-system-mediating agents—are currently under investigation for COVID-19 treatment in 1,833 clinical trials enrolled at Clinical Trials.gov as of May 30, 2020, under the search terms COVID-19 and SARS-CoV-2.
Chloroquine and hydroxychloroquine are used as antimalarial chemotherapeutic agents. They are also used in the treatment of different autoimmune diseases due to their multitargeted mechanism of action. They inhibit release of inflammatory cytokines by mononuclear cells (43) and interfere with Toll-like receptor signaling pathways and cyclic GMP-AMP (cGamP) synthase (cGaS) activity. In COVID-19 infection, it has been shown in vitro that chloroquine can inhibit viral binding to ACE2 receptor (44). They are both contraindicated in G6PD deficiency. QT prolongation and possible TdP may occur, especially in patients with hypokalemia, hypomagnesemia, hypocalcemia, or on concomitant use of QT prolonging drugs. Serious cardiac side effects occur especially in high cumulative doses after long term treatment, though low cumulative doses (45) may also result in heart failure. Conduction disorders were the main side effect reported in a systematic review (38), affecting 85% of patients. Other non-specific adverse cardiac events include ventricular hypertrophy (22%), hypokinesia (9.4%), heart failure (26.8%), pulmonary arterial hypertension (3.9%), and valvular dysfunction (7.1%), which can be readily ruled in by echocardiography (37). Both agents increase the bioavailability of metoprolol via inhibition of CYP2D6-catalyzed pathways (46). Frequent ECG is recommended, while TTE may reveal early myocardial dysfunction leading to possible treatment discontinuation. A number of ongoing clinical trials (47, 48) examine the therapeutic benefit hydroxychloroquine in COVID-19-infected patients as well as its role in chemoprophylaxis for exposed healthcare workers (49).
Recombinant human angiotensin converting enzyme 2 (ACE−2) has experimental (50) and clinical data (51) on the attenuation of acute lung injury by lessening angiotensin II levels and possibly IL-6.
Convalescent plasma treatment may be promising in terms of viral load and even mortality (52).
Corticosteroids have conflicting evidence for their effect on SARS CO-V 2 infection as they may delay viral clearance from blood and respiratory tract based on data from previous coronaviruses outbreaks (53). On the contrary, a small retrospective clinical trial of early, low-dose, short-term administration of methylprednisolone was associated with improved outcomes in patients with COVID 19 pneumonia (54), revealing the need for further clinical studies.
Remdesevir, a nucleotide analog inhibiting viral RNA polymerases, has an Emergency Use Authorization (EUA) from FDA for suspected or confirmed COVID 19 adult and children patients with severe disease since May 2020 (55). It has been shown to inhibit SARS and MERS in an in vitro model of human epithelial airway cells (56) and is also under clinical investigation (57–60). Preliminary results in limited number of patients point to further clinical studies (61).
The combination lopinavir/ritonavir is used in treating HIV-1 infection—they are both aspartase protease inhibitors, and ritonavir increases its plasma half-life. This drug combination has been reported to reduce viral load in clinical case reports (62), although the first clinical trial did not show statistically significant benefit (63). HAART (Highly Active Antiretroviral Treatment), especially protease inhibitors, have been associated with endothelial dysfunction and subclinical atherosclerosis (64–66). HAART may promote metabolic factors such as hyperlipidemia and induce atherosclerotic lesion formation through a CD-36 dependent accumulation of cholesterol in macrophages (30, 67, 68). These mechanisms, combined with the possible myocardial injury associated with the infection itself (9), may contribute to vascular and myocardial dysfunction. Therefore, for patients that have recovered from SARS-CoV-2 infection under protease inhibitor treatment, vascular, and ventricular function should be assessed by TTE at the end of the treatment and possibly at a 3- to 6-month intervals. The combination can also promote QT and PR interval prolongation as well as second and third degree AV block (11). Moreover, lopinavir/ritonavir are CYP3A4 inhibitors. They therefore cannot be used concomitantly with chloroquine (69), while antiplatelet and anticoagulant drugs may need dose adjustment or monitoring (59). Combination therapy of lopinavir /ritonavir, ribavirin and interferon b-1b was superior to lopinavir/ritonavir in a phase 2 clinical trial in terms of symptom alleviation, viral shedding, and hospital stay (70).
Monoclonal antibodies, such as tocilizumab (71), sarilumab (72), and bevasizumab (73), are under investigation to control the cytokine surge associated with the severe form of COVID-19 infection manifested as acute respiratory distress syndrome and multiorgan failure. IL-6 inhibition with biological agents such as tocilizumab and sarilumab may show a beneficial effect in controlling the excessive cytokine production (74) and evolution to alveoli consolidation. As has been recently shown in mechanically ventilated patients with COVID-19 infection (75), excessive IL-6 production is associated with lymphopenia and immunoparesis as assessed by low expression of the humanleukocyte antigen (HLA)-DR on CD14-monocytes, and this effect is reversed by tocilizumab. Additionally, IL-1b production is major factor contributing to the macrophage activation syndrome (Haemophagocytic lymphohistiocytosis syndrome) which characterizes significant number of the critically ill COVID-19 infected patients. Another possible mechanism for monoclonal antibodies beneficial effect could be mediated by preserving endothelial glycocalyx integrity. Damage of endothelial glycocalyx increases vascular permeability to circulating blood cell inflammatory markers and proteins (76) and may thus mediate lung injury and initiate SARS in COVID-19 as has been previously shown in septic patients. Anti-inflammatory treatment may exert beneficial effect on endothelial glycocalyx and thus may offer protection from evolution to alveoli exudation (77). Moreover, anti-inflammatory treatment with tocilizumab but also anakinra—an IL 1 receptor antagonist—exhibit beneficial effects on vascular function and myocardial function (78), as has been shown in patients with rheumatoid arthritis.
Additional protective mechanisms for IL-6 and IL-1 inhibitors may be related to regulation of ROS production, which hampers cellular functions, such as with the proteasome, leading to impaired endogenous protein degradation and mitochondrial dysfunction, augmenting the damage promoted by the direct interaction of SARS-COV proteins with the proteasome (79). ROS may activate the STAT/IL-6 axis (80) and promote IL-8 expression in pulmonary epithelial cells stimulated with lipid-associated membrane proteins from Mycoplasma pneumonia (81), triggering cytokine release and immune cell infiltration in the lung cells. Agents with inherent antioxidant properties such as N-acetylcysteine (NAC) and vitamin C may also be shown to be effective. The beneficial anti-inflammatory effect of monoclonal antibody treatment on myocardial function in COVID-19 infected patients may by easily monitored by an improvement in global longitudinal strain (GLS) toward normal values (81), as has been previously shown in patients with rheumatoid arthritis and uncontrolled inflammation (78, 79). The lowest expected normal values for GLS are −16.7% in men and −17.8% in women, according to a recently proposed consensus document, and these are similar to the values reported after remission of the acute inflammatory exacerbations by biological agents in patients with rheumatoid arthritis (78, 79).
The COVID-19 pandemic, still unfolding around the world, has created a significant worldwide human, scientific, financial, and psychological burden, requiring innovative and cooperative strategies to combat the pandemic and its unprecedented consequences. TTE is required to guide clinical management of patients with abnormal ECG and/or biomarkers, as it may diagnose early cardiac involvement in the acute setting. Antiviral, anti-inflammatory, and antioxidant treatment agents as well as hyperimmune plasma are being investigated in a multitude of clinical trials. Echocardiography provides a valid method to monitor myocardial effect of potential treatments for COVID-19 during hospitalization and in the mid-term follow up.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
II had the original idea of the manuscript. JP reviewed the biomarkers and treatment section. A-RV wrote the initial version of the manuscript including figure. All authors offered comments on the manuscript's sections.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
2. Johns Hopkins Coronavirus Resource Center. Available online at: https://coronavirus.jhu.edu
3. He F, Deng Y, Li W. Coronavirus disease 2019 (COVID-19): what we know? J Med Virol. (2020) 92:719–25. doi: 10.1002/jmv.25766
4. Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. (2005) 194:1–6. doi: 10.1007/s00430-004-0219-0
5. Mackay IM, Arden KE. MERS coronavirus: diagnostics, epidemiology and transmission. Virol J. (2015) 12:222. doi: 10.1186/s12985-015-0439-5
6. To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. (2020) 20:565–74. doi: 10.1016/S1473-3099(20)30196-1
7. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
8. Yang X, Yu Y, Xu J, Shu H, Jia'an X, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-COV 2 pneumonia in Wuhan, China: a single –centered, retrospective observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
9. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. doi: 10.1001/jamacardio.2020.0950
10. Guzik T, Mohiddin S, DiMarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. (2020) 116:1666–87. doi: 10.1093/cvr/cvaa106
11. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. (2020) 368:m1091. doi: 10.1136/bmj.m1091
12. Bompard F, Monnier H, Saab I, Tordjman M, Abdoul H, Fournier L, et al. Pulmonary embolism in patients with Covid-19 Pneumonia. Eur Respir J. (2020) 56:2001365. doi: 10.1183/13993003.01365-2020
13. Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller M, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. (2020) 18:1995–2002. doi: 10.20944/preprints202004.0345.v1
14. Wei JF, Huang FY, Xiong TY, Liu Q, Chen H, Wang H, et al. Acute myocardial injury is common in patients with Covid-19 and impairs their prognosis. Heart. (2020) 106:1154–9. doi: 10.1136/heartjnl-2020-317007
15. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5:1–8. doi: 10.1001/jamacardio.2020.1017
16. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. (2020) 45:230–2. doi: 10.1007/s00059-020-04909-z
17. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 COVID-19. JAMA Cardiol. (2020) 5:1–6. doi: 10.1001/jamacardio.2020.1096
18. National Health Commission of the People's Republic of China. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment. 7th ed. Available online at: http://kjfy.meetingchina.org/msite/news/show/cn/3337.html 2020
19. Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, Märkl B, et al. Post mortem examinations of patients with COVID-19. JAMA. (2020) 323:2518–20. doi: 10.1001/jama.2020.8907
20. Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. (2020) 141:2113–6. doi: 10.1161/CIRCULATIONAHA.120.047525
21. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. (2020) 191:9–14. doi: 10.1016/j.thromres.2020.04.024
22. Inciardi R, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. (2020) 41:1821–9. doi: 10.1093/eurheartj/ehaa388
23. Iba T, Levy J, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. (2020). doi: 10.1097/CCM.0000000000004458
24. Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med. (2005) 165:1777–81. doi: 10.1001/archinte.165.15.1777
25. Casazza F, Bongarzoni A, Capozi A, Agostoni O. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur J Echocardiogr. (2005) 6:11–4. doi: 10.1016/j.euje.2004.06.002
26. Konstantinides S, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. (2020) 41:543–603. doi: 10.1093/eurheartj/ehz405
27. Torbicki A, Kurzyna M, Ciurzynski M, Pruszczyk P, Pacho R, Kuch-Wocial A, et al. Proximal pulmonary emboli modify right ventricular ejection pattern. Eur Respir J. (1999) 13:616–21. doi: 10.1183/09031936.99.13361699
28. Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. J Am Soc Echocardiogr. (2020) 33:648–53. doi: 10.1016/j.echo.2020.04.001
29. Johri AM, Galen B, Kirkpatrick JN, Lanspa M, Mulvagh S, Thamman R. ASE statement on point-of-care ultrasound (POCUS) during the 2019 novel coronavirus pandemic. J Am Soc Echocardiogr. (2020) 33:670–3. doi: 10.1016/j.echo.2020.04.017
30. Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital system. Cancer Discov. (2020) 10:935–41. doi: 10.1158/2159-8290.CD-20-0516
31. Sharain K, Hoppensteadt D, Bansal V, Singh A, Fareed J. Progressive increase of inflammatory biomarkers in chronic kidney disease and end-stage renal disease. Clin Appl Thromb Hemost. (2013) 19:303–8. doi: 10.1177/1076029612454935
32. Ponikowski P, Voors AA, Anker S, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
33. Fu S, Ping P, Zhu Q, Ye P, Luo L. Brain natriuretic peptide and its biochemical, analytical, and clinical issues in heart failure: a narrative review. Front Physiol. (2018) 9:692. doi: 10.3389/fphys.2018.00692
34. Ikonomidis I, Nikolaou M, Dimopoulou I, Paraskevaidis I, Lekakis J, Mavrou I, et al. Association of left ventricular diastolic dysfunction with elevated NT-pro-BNP in general intensive care unit patients with preserved ejection fraction: a complementary role of tissue Doppler imaging parameters and NT-pro-BNP levels for adverse outcome. Shock. (2010) 33:141–8. doi: 10.1097/SHK.0b013e3181ad31f8
35. Kotanidou A, Karsaliakos P, Tzanella M, Mavrou I, Kopterides P, Papadomichelakis E, et al. Prognostic importance of increased plasma amino-terminal pro-brain natriuretic peptide levels in a large noncardiac, general intensive care unit population. Shock. (2009) 31:342–7. doi: 10.1097/SHK.0b013e31818635b6
36. Thygesen K, Alpert J, Jaffe A, Chaitman BR, Bax JJ, Morrow DA, et al. Universal definition of myocardial infarction 2018. Eur Heart J. (2019) 40:237–69. doi: 10.1093/eurheartj/ehy462
37. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
38. Nagueh S, Smiseth OA, Appleton C, Byrd BF III, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2016) 29:277–314. doi: 10.1016/j.echo.2016.01.011
39. Skulstad H, Cosyns B, Popescu BA, Galderisi M, Salvo GD, Donal E, et al. COVID-19 pandemic and cardiac imaging:EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. (2020) 21:592–8. doi: 10.1093/ehjci/jeaa072
41. World Health Organization. CORONAVIRUS DISEASE (COVID-19) Outbreak: Rights, Roles and Responsibilites of Health Workers Including Key Considerations for Occupational Safety and Health.
42. Klerkin J, Fried JA, Raikhelkar JK, Sayer G, Griffin JM, Masoumi A, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. (2020) 141:1648–55. doi: 10.1161/CIRCULATIONAHA.120.046941
43. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. (2020) 16:155–66. doi: 10.1038/s41584-020-0372-x
44. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. (2020) 30:269–71. doi: 10.1038/s41422-020-0282-0
45. Chatre L, Roubille F, Vernhet H, Jorgensen C, Pers YM. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. (2018) 41:919–31. doi: 10.1007/s40264-018-0689-4
46. Somer M, Kallio J, Pesonen U, Pyykkö K, Huupponen R, Scheinin M, et al. Influence of hydroxychloroquine on the bioavailability of oral metoprolol. Br J Clin Pharmacol. (2000) 49:549–54. doi: 10.1046/j.1365-2125.2000.00197.x
47. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19:results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. (2020) 56:105949. doi: 10.1016/j.ijantimicag.2020.105949
48. ClinicalTrials.gov. Hydroxychloroquine for the Treatment of Patients With Mild to Moderate COVID-19 to Prevent Progression to Severe Infection or Death. Identifier: NCT04323631.
49. Clinical trials.gov. Chemoprophylaxis of SARS-CoV-2 Infection (COVID-19) in Exposed Healthcare Workers (COVIDAXIS). Identifier: NCT04328285. Available online at: https://clinicaltrials.gov/ct2/show/NCT04328285
50. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. (2005) 436:112–6. doi: 10.1038/nature03712
51. Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. (2017) 21:234. doi: 10.1186/s13054-017-1823-x
52. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. (2020) 130:2757–65. doi: 10.1172/JCI138745
53. Russel CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. (2020) 395:473–5. doi: 10.1016/S0140-6736(20)30317-2
54. Wang Y, Jiang W, He Q, Wang B, Zhou P, Dong N, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 Pneumonia. Signal Transduct Target Ther. (2020) 5:57. doi: 10.1038/s41392-020-0158-2
56. Ko WC, Rolain JM, Lee NY, Chen PL, Huang CT, Lee PI, et al. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. (2020) 55:105933. doi: 10.1016/j.ijantimicag.2020.105933
57. ClinicalTrials.gov. Severe 2019-nCoV Remdesivir RCT. Identifier: NCT04257656 (2020). Available online at: https://clinicaltrials.gov/ct2/show/NCT04257656
58. ClinicalTrials.gov. Mild/Moderate 2019-nCoV Remdesivir RCT. NCT04252664 (2020). Available online at: https://clinicaltrials.gov/ct2/show/NCT04252664
59. ClinicalTrials.gov. Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Severe Coronavirus Disease (COVID-19). Identifier: NCT04292899 (2020). Available online at: https://clinicaltrials.gov/ct2/show/NCT04292899
60. ClinicalTrials.gov. Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment. Identifier: NCT04292730 (2020). Available online at: https://clinicaltrials.gov/ct2/show/NCT04292730
61. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of Remdesevir for patients with Severe Covid−19. N Engl J Med. (2020) 382:2327–36. doi: 10.1056/NEJMc2015312
62. Lim J, Jeon S, Shin H-Y, Kim MJ, Seong YM, Lee WJ, et al. Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 Pneumonia monitored by quantitative RT-PCI. J Korean Med Sci. (2020) 35:e79. doi: 10.3346/jkms.2020.35.e79
63. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. (2020) 382:1787–99. doi: 10.1056/NEJMc2008043
64. Palios J, Ikonomidis I, Lekakis J, Tsiodras S, Poulakou G, Antoniadou A, et al. Microcirculatory vascular dysfunction in HIV-1 infected patients receiving highly active antiretroviral therapy. Microcirculation. (2010) 17:303–10. doi: 10.1111/j.1549-8719.2010.00023.x
65. Lekakis J, Tsiodras S, Ikonomidis I, Palios J, Poulakou G, Rallidis L, et al. HIV-positive patients treated with protease inhibitors have vascular changes resembling those observed in atherosclerotic cardiovascular disease. Clin Sci. (2008) 115:189–96. doi: 10.1042/CS20070353
66. Lekakis J, Ikonomidis I, Palios J, Tsiodras S, Karatzis E, Poulakou G, et al. Association of highly active antiretroviral therapy with increased arterial stiffness in patients infected with human immunodeficiency virus. Am J Hypertens. (2009) 22:828–34. doi: 10.1038/ajh.2009.90
67. Tsiodras S, Mantzoros C, Hammer S, Samore S. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med. (2000) 160:2050–6. doi: 10.1001/archinte.160.13.2050
68. Dressman J, Kincer J, Matveev SV, Guo L, Greenberg RN, Guerin T, et al. HIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophages. J Clin Invest. (2003) 111:389–39. doi: 10.1172/JCI200316261
69. Naksuk N, Lazar S, Peeraphatdit T, Bee T. Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol. Eur Heart J Acute Cardiovasc Care. (2020) 9:215–21. doi: 10.1177/2048872620922784
70. Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. (2020) 395:1695–704. doi: 10.1016/S0140-6736(20)31042-4
71. Clinical Trials.gov. Tocilizumab in COVID−19 Pneumonia (TOCIVID-19) NCT04317092. Available online at: https://clinicaltrials.gov/ct2/show/NCT04317092
72. ClinicalTrials.gov. Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID−19 NCT04315298. (2020). Available online at: https://clinicaltrials.gov/ct2/show/NCT04315298
73. ClinicalTrials.gov. Bevacizumab in Severe or Critically Severe Patients With COVID−19 Pneumonia-RCT (BEST-RCT) NCT04305106. (2020). Available online at: https://clinicaltrials.gov/ct2/show/NCT04305106
74. Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. (2020) 43:203–8. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013
75. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. (2020) 27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009
76. Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol. (2015) 80:389–402. doi: 10.1111/bcp.12629
77. Ikonomidis I, Pavlidis G, Katsibri P, Andreadou I, Triantafyllidi E, Tsoumani M, et al. Effects of interleukin 6 inhibitor tocilizumab on endothelial glycocalyx, vascular and myocardial function compared to prednisolone. Eur Heart J. (2019) 40:ehz745.0837. doi: 10.1093/eurheartj/ehz745.0837
78. Ikonomidis I, Pavlidis G, Katsimbri P, Andreadou I, Triantafyllidi H, Tsoumani M, et al. Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clin Res Cardiol. (2019) 108:1093–101. doi: 10.1007/s00392-019-01443-9
79. Wang Q, Li C, Zhang Q, Wang T, Li J, Guan W, et al. Interactions of SARS Coronavirus Nucleocapsid Protein with the host cell proteasome subunit p42. Virol J. (2010) 7:99. doi: 10.1186/1743-422X-7-99
80. Choi SY, Lim JW, Shimizu T, Kuwano K, Kim JM, Kim H, et al. Reactive oxygen species mediate Jak2/Stat3 activation and IL-8 expression in pulmonary epithelial cells stimulated with lipid-associated membrane proteins from Mycoplasma pneumoniae. Inflamm Res. (2012) 61:493–501. doi: 10.1007/s00011-012-0437-7
Keywords: coronavirus, transthoracic echo, Coronavirus disease 2019, SARS-CoV 2, global longitudinal strain, antiviral treatment, anti-inflammatory treatment
Citation: Vrettou A-R, Parissis J and Ikonomidis I (2020) The Dual Role of Echocardiography in the Diagnosis of Acute Cardiac Complications and Treatment Monitoring for Coronavirus Disease 2019 (COVID-19). Front. Cardiovasc. Med. 7:129. doi: 10.3389/fcvm.2020.00129
Received: 21 April 2020; Accepted: 25 June 2020;
Published: 02 September 2020.
Edited by:
Masanori Aikawa, Harvard Medical School, United StatesReviewed by:
Rosa Sicari, Institute of Clinical Physiology, Italian National Research Council, ItalyCopyright © 2020 Vrettou, Parissis and Ikonomidis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ignatios Ikonomidis, aWdub2lrQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.