Introduction
The time has come for cardiovascular disease (CVD) prevention to play a more prominent role in cardio-oncology (Figure 1A). Cardio-oncology is an emerging subspecialty within internal medicine, and particularly cardiology, which involves the prevention and management of cardiovascular injury from cancer therapies (4–6). A small section of the field is dedicated to diagnosing and managing primary or secondary tumors to the heart (7). The majority of the field focuses on cardiotoxicity from radiotherapy, chemotherapy, and immunotherapy. While anthracyclines are the most commonly studied drugs and are frequently associated with cardiomyopathy, a myriad of novel cardiotoxic chemotherapeutic and immunotherapeutic drugs are continuously being developed in Oncology, with diverse cardiovascular (CV) effects (Figure 1B). Radiation to the chest can result in accelerated atherosclerosis, pericardial disease, valve disease, conduction abnormalities, and cardiomyopathies (8–10). Traditional chemotherapies, endocrine therapies, and targeted or immunotherapies can also associate with these cardiovascular toxicities in addition to myocarditis (3, 11–18), with valve disease possibly being no exception (19–22).
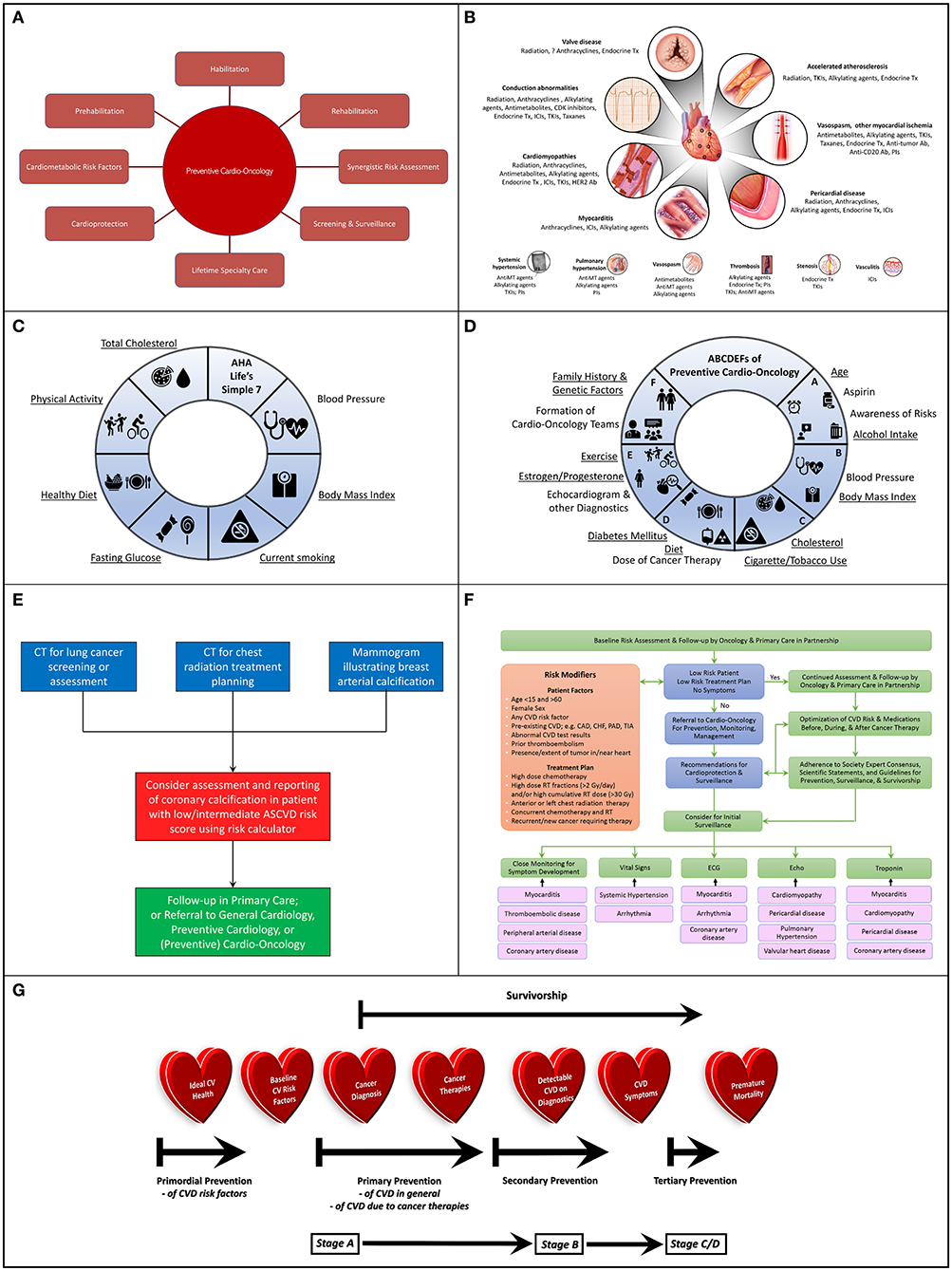
Figure 1. (A) Potential Scope of Preventive Cardio-Oncology: Preventive Cardio-Oncology can potentially consist of prehabilitation, habilitation, and rehabilitation, with synergistic risk assessment in cardiology and oncology screening tests, as well as optimization of cardiometabolic risk factors (pre-existing, or consequential from cancer/therapies), in addition to adequate cardioprotection in the setting of cancer therapy, with appropriate screening and surveillance guided by lifetime specialty care. (B) Cardiovascular Toxic Effects of Cancer Therapies: A wide spectrum of cancer therapeutics can injure or aggravate a variety of components of the cardiac (top) and vascular (bottom) system, knowledge of which can assist with vigilant monitoring, prevention if possible, and appropriate diagnosis if present. (C) American Heart Association (AHA) Life's Simple 7: Seven different domains can guide conceptualization and realization of Ideal Cardiovascular Health, recognizing specific factors (underlined) that underlie risk of development of both CVD and cancer. (D) ABCDEFs of Preventive Cardio-Oncology: A proposed ABCDEF approach to prevention of CVD and cancer in the general population and in those with a prior history of cancer addresses all seven factors in the AHA Life's Simple 7, as well as other characteristics that can contribute to CVD, cancer (new or recurrent), or both (underlined), with expansion of a previously published ABCDE algorithm (1, 2) to include Family History and Genetic Factors and Formation of Cardio-Oncology Teams to prevent or mitigate the impact of these multiple hits and risk factors relevant to the development of CV toxicities from cancer therapies. (E) Algorithm for Synergistic Screening in Preventive Cardio-Oncology: Patients presenting for screening or assessment for cancer could also be considered for simultaneous or subsequent CV screening and reporting, particularly if the initial imaging test is of the chest, with radiology teams scheduling or reading chest x-rays or chest CTs contacting patients' ordering clinician to consider CV assessment and reporting in tandem, or with ordering clinician teams (primary care, oncology, cardiology, cardio-oncology, preventive cardio-oncology) also requesting assessment and reporting of CV findings when ordering chest x-rays or CTs for patients at low/intermediate risk for CVD, especially for patients with any previous cancers who received radiation therapy or chemotherapy with drugs that associate with coronary artery atherosclerosis or thrombosis. (F) Algorithm for Risk Assessment and Follow-up: Initial assessment of patients in oncology can occur with the oncologist in partnership with primary care, with those patients at low risk continuing to be followed predominantly in oncology and primary care, while other patients can be referred to (preventive) cardio-oncology for recommendations on additional screening and preventive measures prior to, during, and after cancer therapies, recognizing that close monitoring for symptoms, abnormal vitals, or findings on lab testing, ECG, or Echo can help guide the need for more advanced testing related to potential cardiotoxicities; various specific cardiotoxicities may benefit most from particular initial monitoring methods (black arrows), e.g., myocarditis from ICIs may first manifest with symptoms with or without changes in the ECG or troponin (which can further be assessed with cardiac MRI), while VEGFI toxicity most commonly manifests as hypertension; high risk determinants are adapted from (3). (G) Preventive Cardio-Oncology Paradigm Shift: Efforts at prevention in Cardio-Oncology may need to be shifted earlier than traditionally implemented or currently considered; in typical Preventive Cardiology, primordial prevention targets the general population prior to development of risk factors for CVD, such as hypertension or diabetes; primordial prevention in Preventive Cardio-Oncology, if truly merging Preventive Cardiology and Cardio-Oncology, could benefit from maintaining this place of primordial prevention on the prevention spectrum; as such, indeed, primordial prevention would remain in the general population before cancer is diagnosed; at the time of cancer diagnosis, individuals in the general population would then become cancer survivors, with a focus on primary prevention for those with CV risk factors, then secondary prevention following cancer therapy, especially once structural heart disease is visible on imaging and manifested with signs or symptoms; with advanced CVD, tertiary prevention would be in effect, in hopes to optimize quality while delaying premature mortality. CAD, coronary artery disease; CHF, congestive heart failure; CV, cardiovascular; CVD, cardiovascular disease; Echo, echocardiogram; ECG, electrocardiogram; Gy, Gray; ICIs, immune checkpoint inhibitors; PAD, peripheral artery disease; RT, radiation therapy; TIA, transient ischemic attack; VEGFI, vascular endothelial growth factor inhibitor (a tyrosine kinase inhibitor).
Various chemotherapies and targeted or immunologic therapies can also lead to peripheral vascular toxicities, such as systemic hypertension, pulmonary hypertension, thrombosis, stenosis, vasospasm, or vasculitis (3, 11–18) (Figure 1B). Systemic hypertension can further increase the risk of cardiovascular toxicity, as do all baseline CV risk factors (e.g., diabetes) and known CVD itself (11, 17, 23). Systemic hypertension occurs in more than one fourth of all individuals treated with vascular endothelial growth factor inhibitors (small molecule tyrosine kinase inhibitors), while all patients experience some increase in their blood pressure (24). These drugs also associate with cardiomyopathy, vasculopathy, coagulopathy, and nephropathy largely due to systemic endothelial dysfunction, reduced nitric oxide production, and destruction of the glomerular filtration barrier resulting from alteration of the balance between angiogenic and antiangiogenic, or vasoconstrictor and vasodilator, factors (24–26).
With all of this in mind, we should no longer direct the bulk of our efforts at intervention toward management of cardiovascular toxicities that have already occurred. It is time for us to swing the pendulum further in the direction of prevention of cardiovascular toxicities. Prevention can take different forms and can be pursued in different ways, with definition, scope, awareness, partnership, screening, surveillance, and intervention. Further, primordial (or health promotion), primary, secondary, and tertiary prevention may be defined somewhat differently in the traditional field of Preventive Cardiology (27, 28), than in a more recent proposal for consideration of prevention in cardio-oncology (20). It would be useful to reconcile these two slightly disparate paradigms, and attempt to continue to envision the potential scope of preventive efforts in cardio-oncology. Thus, as we advocate for moving from a traditionally reactive model to a more proactive model for prevention of cardiovascular toxicities in response to cancer therapies, we are entering the era of Preventive Cardio-Oncology. This concept is further introduced here, with a suggested near-future scope for the field (Figure 1A).
Cancer Survivorship and Cardiometabolic Risk
As cancer therapies become more effective over time, the prevalence of cancer survivorship continues to rise. For 2019, almost 2 million new cancer diagnoses and just over 600,000 new cancer deaths are estimated; as cancer incidence continues to increase for most cancers, cancer mortality continues to decrease, leading to millions of cancer survivors each year (29). CVD remains a leading cause of death in cancer survivors (second only to cancer recurrence) (4, 20, 30, 31), and shares some risk factors with various cancers. For example, CVD and breast cancer have several risk factors in common, such as unhealthy Western diet, red or processed meat, physical inactivity or sedentary lifestyle, smoking, early menarche, and hormone replacement therapy (20) (Figures 1C,D, underlined). Yet, some factors affect risk differently for each disease. Premenopausal obesity and early menopause can increase CVD, while decreasing the risk of breast cancer; light to moderate intake of alcohol can attenuate cardiovascular risk, while increasing breast cancer risk (20). Given this reality, individuals diagnosed with breast cancer may already have baseline risk factors for CVD, as is also the case for individuals diagnosed with a variety of other cancer types. Overlapping risk factors for developing CVD or cancer (or both) (12, 16, 20, 32, 33) are underlined in Figures 1C,D.
Baseline cardiometabolic risk in individuals diagnosed with cancer can also conceivably increase even before beginning cancer therapies, because of cardiometabolic derangements or systemic inflammation due to the presence of the cancer itself (34, 35). Metabolic derangements and barriers to cardiometabolic health can also occur due to cancer therapies (4, 36–38). For example, chemotherapy can affect the vasculature and lead to initial diagnosis of, or aggravation of pre-existing, hypertension (12, 39). Radiation to the abdomen, brain, or whole body can affect the endocrine system resulting in abnormalities with hypothalamus-pituitary, thyroid, or pancreatic function (36, 40, 41), as well as hypertension, hyperlipidemia, and elevated waist circumference and levels of free fatty acids (40). Surgery to the brain can potentially also affect endocrine function, but appears to have very limited effect on cardiovascular risk, if any at all (42). A greater effect is seen when radiation is combined with surgery, and even greater so when chemotherapy also is added (42). In addition to direct physiological contributions to cardiovascular risk, cancer therapies can also have indirect effects. Fatigue, musculoskeletal discomfort, decrease in quality of life, or early menopause related to cancer therapies can limit individuals' ability to continue to pursue ideal cardiovascular health during therapy and can contribute to obesity and the metabolic syndrome (34, 36, 41). Thus, the CV impact of therapies for cancer (and potentially the presence of cancer itself) can superimpose on pre-existing CVD risk factors to worsen CV outcomes, which is described as the “multiple hit” hypothesis (23).
Given the co-existence of risk for cancer and CVD based on baseline factors, it has been suggested that joint screening for cancer and CVD should perhaps be pursued (32). This could include assessing for coronary artery calcification on chest CT scans initially ordered to screen for or evaluate known lung cancer or for radiation treatment planning in appropriate individuals, or following up on breast arterial calcification noted during mammography while screening for breast cancer (32, 43–47) (Figure 1E). When ordering chest x-rays or CTs for patients at low/intermediate risk for CVD, especially for patients with any previous cancers who received radiation therapy or chemotherapy with drugs that associate with coronary artery atherosclerosis or thrombosis, ordering clinician teams (primary care, oncology, cardiology, cardio-oncology, preventive cardio-oncology) could also request assessment and reporting of CV findings. Additionally, for patients with appointments for chest x-rays or chest CTs, radiology team schedulers and clinicians could contact patients' ordering clinicians to consider concurrent or subsequent CV assessment and reporting. A clinical trial (the BRAGATSTON Study) is currently in progress to provide low-cost automated quantification of coronary artery calcification on radiation planning chest CTs for CV risk prediction1. Similar studies could be pursued for chest CTs screening for or assessing cancers in the chest. Indeed, cardiovascular risk assessment in individuals diagnosed with cancer can uncover cardiometabolic risk factors out of proportion to the general population. CVD is more prevalent in individuals with cancer, compared to age-matched controls (48), and patients with cancer who develop CVD experience worse outcomes (31). Cardiovascular risk assessment should therefore be considered in tandem with screening for or managing various cancers.
Risk scores can play a role. In addition to the CVD risk scores used in the general population, such as the American Heart Association (AHA) and American College of Cardiology (ACC) pooled cohort atherosclerotic cardiovascular disease risk equations, there are also scores being developed for assessing CVD risk in individuals with a history of adult or childhood cancer (49, 50). Such risk scores may become invaluable in preventive cardio-oncology.
Risk Mitigation
To mitigate cardiovascular risk in individuals with cancer, typical cardiovascular risk management should be pursued as in the general population with pharmacotherapy and lifestyle modification as appropriate (17, 51, 52), while recognizing that a history of cancer therapy increases overall cardiovascular risk and should inform the approach to lifestyle intervention (23) (proposed ABCDEF approach, Figure 1D).
Pharmacologic Therapies and Oncologic Treatment Maneuvers in Prevention of Cardiomyopathy
Additional cardioprotective measures in the setting of cancer therapies should also be pursued as indicated (17). These can possibly include pharmacotherapy such as dexrazoxane, beta blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, statins, and other medications (53–55). Dose limitation or reduction for chemoradiation, as well as drug formulation and length of infusion for pharmacological cancer therapies can also be addressed (17, 55). Contemporary radiotherapy methods can also be adopted, with proton beam therapy, deep inspiration breath hold, prone imaging, and CT treatment planning, as well as dose limitation as appropriate (8–10, 55). Lifetime appropriate screening and surveillance would also be key (3, 4, 55). Meta-analyses and systematic reviews have suggested cardioprotective effects of beta blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, statins, dexrazoxane, and continuous infusion of a limited dose of liposomal doxorubicin, particularly for individuals with breast cancer receiving chemotherapy with anthracyclines, trastuzumab, or both (56–59). A single-center study determined deep inspiration breath hold as an effective method to reduce CV toxicity in patients with breast cancer (60). A multi-center randomized clinical trial is underway to assess benefits of proton vs. photon beam radiation therapy for individuals with non-metastatic breast cancer (61). Accordingly, cardioprotective pharmacotherapeutics and oncologic treatment maneuvers are currently most often used for patients with breast cancer who will undergo treatment with anthracyclines, trastuzumab, or both, especially those deemed to be high risk, e.g., based on chemotherapy dose, concurrent radiation, or pre-existing CVD, or those who have developed cardiotoxicity (17, 55) (Figure 1F). While further data needs to be obtained to strengthen recommendations for pharmacologic cardioprotection, studies so far support the likelihood of clinical utility in these patients.
Pharmacologic Therapies in Optimization of Hypertension
For patients treated with vascular endothelial growth factors, antihypertensives usually provide sufficient control of blood pressure to allow for continuation of cancer therapy (14, 18). Most commonly, ACE inhibitors, ARBs, calcium channel blockers, and diuretics are used (18, 26, 62). ACE inhibitors have been thought to be preferred for concurrently impacting proteinuria, as well as release of endothelial nitric oxide and expression of plasminogen activator inhibitor-1 (63, 64). In fact, ACE inhibitors also associated with significant improvement in overall survival of patients with metastatic renal cell carcinoma (65). Of note, two recent retrospective studies were pursued using robust databases, with differences in study design, patient baseline characteristics, cancer type, and timing of use of antihypertensive therapy (26, 62). One study showed a greater extent of blood pressure control with ACE inhibitors and ARBS, compared to other antihypertensive drug classes (including calcium channel blockers and diuretics) (62). Conversely, the other recent study of patients treated with VEGF inhibitors identified blood pressure reductions most effectively associated with the use of calcium-channel blockers and potassium-sparing diuretics, compared to ACE inhibitors, ARBs, and other antihypertensive classes (26). Indeed, results from a preclinical study indicated a preference for nifedipine over captopril to achieve optimal control of severe blood pressure increases of 35–50 mm Hg (66). Thus, conclusions regarding inhibition of the renin-angiotensin-aldosterone system as the preferred method for managing hypertension in these patients have been divergent (67). Therefore, no clear broad recommendation can currently confidently be made for use of one antihypertensive class of medications over the other in all patients (64, 67). Care of the patient should continue to be individualized, and collaborations for patient care and further research in this area should persist (67). Nevertheless, a singular consensus remains: non-dihydropyridine calcium channel blockers specifically should be avoided, as these drugs inhibit the cytochrome P450 3A4 enzyme which metabolizes VEGFIs; use of non-dihydropyridine calcium channel blockers such as diltiazem or verapamil could lead to high levels of VEGFIs, potentially even further aggravating VEGFI-induced hypertension (18, 67, 68).
Nutrition Counseling
Nutritional counseling could be personalized and re-assessed before, during, and after cancer therapy. Currently, dietitians have the option of training in Oncology as a nutrition subspecialty, which is particularly helpful for patients with cancer who develop malnutrition [estimated at 40% of all cancer patients, or >50% in patients with gastrointestinal or head and neck cancers (69, 70)]; dietitians can also become certified in Obesity and Weight Management2. Perhaps dieticians or nutritionists could be trained in preventive cardio-oncology and opt for certification in both Oncology Nutrition and Obesity And Weight Management, to merge philosophies from oncology and preventive cardiology in the context of individual patient nutrition needs. Indeed, studies in nutrition oncology encourage individualization of patients' nutritional care (71, 72), which may be a good fit as a component of Preventive Cardio-Oncology. A multicenter study indicated that a majority of clinicians in Oncology assessed patients for obesity and pursued weight management counseling during and after patients' cancer treatment, but did not refer patients for continued lifestyle intervention and support to optimize or maintain a healthy weight (73). Of note, a recent document providing recommendations and guidelines for obesity management in cancer highlights obesity as a risk factor for cancer development, recurrence, or outcomes, and cautions clinicians to be aware that obesity can sometimes mask acute/subacute malnutrition in individuals with cancer (74). Given the intricacies of obesity and nutrition for cancer patients and survivors, the American Society of Clinical Oncology has established a multifaceted initiative focused on education, awareness, research, policy, and advocacy for obesity and weight management in cancer patients and survivors (75). Perhaps partnerships among Oncology and Cardiology societies, including the American Society for Preventive Cardiology would be key in the advancement of Preventive Cardio-Oncology (75).
Cardio-Oncology or Cardiovascular Oncology Prehabilitation, Habilitation, Rehabilitation
Incorporation of various methodologies in typical cardiac rehabilitation could also have utility for secondary prevention (76), and possibly also primary prevention, of cardiovascular toxicity from a variety of cancer therapies. Some have suggested a multidimensional system consisting of cardiovascular oncology prehabilitation (30, 77, 78), habilitation (30, 79, 80), and rehabilitation (30, 78, 81), to optimize cardiovascular health before, during, and after cancer therapies, respectively. Prehabilitation focuses on optimizing baseline cardiovascular health and cardiopulmonary fitness prior to initiation of cancer therapies (30, 77, 78). Habilitation refers to ongoing optimization of cardiovascular health and cardiopulmonary fitness while patients are undergoing cancer therapies (30, 79, 80). Rehabilitation refers to efforts to recover cardiopulmonary function after completion of cancer therapies (30, 78, 81).
Potential Challenges in Cardio-Oncology Prehabilitation, Habilitation, Rehabilitation
There is often little time from diagnosis of cancer to initiation of chemotherapy, which can limit the time window for pursuing cardio-oncology prehabilitation in the cardiology community. Nevertheless in the oncology community, timely calls to action for developing high-quality cancer rehabilitation programs propose prospective models that actually begin upon cancer diagnosis and continue throughout cancer therapy then for a lifetime (82, 83), which could also be termed cancer prehabilitation (before therapy), habilitation (during therapy), and rehabilitation (after therapy). These multidisciplinary programs aim to optimize physical and psychosocial health and function. The models include establishment of baseline functioning prior to cancer therapies. This can include tests such as the 6-min walk or Get-Up-And-Go, or formal fitness assessment as in the general population prior to exercise training (84). This can help assure both patients and clinicians of the safety of pursuing exercise training (84). Fitness testing as part of baseline assessment can also identify potential physical vulnerabilities that may limit or modify exercise regimens (84). Pre-existing conditions that may associate with high risk for cardiovascular and other toxicities can be identified and addressed; exercise level, mobility, strength, and gait, among other parameters, are objectively assessed. Ongoing surveillance subsequently occurs during and after therapy, with referrals made for exercise, nutrition, and weight management. With further collaboration between the cardiology and oncology communities, efforts could be combined to establish and pool resources and protocols for comprehensive multidimensional cardio-oncology programs pursued before (prehabilitation), during (habilitation), or after (rehabilitation) cancer therapy.
Cardio-oncology habilitation can be challenging for some patients, due to physical or psychosocial effects of cancer therapy. Studies have shown reduction in physical activity levels in cancer patients following diagnosis and during therapy (85–87). In fact, patients have traditionally often been advised to limit their physical activity during therapy, particularly for those who are older or experience fatigue or side effects of cancer therapy (82). The most frequent symptoms in cancer patients include fatigue and peripheral neuropathy. Of note, aerobic, resistance, and strength training have been shown to improve fatigue; strength and balance training also improve fatigue (88).
It should be recognized that patients often maintain lower physical activity levels even after completion of cancer therapies, and generally do not return to their pretreatment levels of physical activity (85–87). In spite of a myriad of guidelines and recommendations from various societies regarding the safety and efficacy of exercise (75, 89, 90)3, ~70% of cancer survivors do not meet recommendations for physical activity in cancer survivors (91, 92). It should be noted too that adverse effects of cancer therapies extend to the entire cardiovascular and skeletal muscle axis (76). As a result, cardiorespiratory fitness (CRF) declines are in part due to impairment of components of the cardiovascular and respiratory muscle system. Thus, survivors may have impairments in mobility and function beyond the general population (93). For example, CRF in survivors can be 30% lower than in age-matched healthy yet sedentary control individuals (94), and as stated often does not recover following cancer therapy (94–96). However, physical function and cardiorespiratory fitness can both improve with structured exercise regimens in cancer rehabilitation (85–87, 97). Additionally and importantly, post-treatment exercise reduces the risk of mortality (98–102).
In one established program, oncology rehabilitation is modeled based on cardiac rehabilitation and is available to all cancer survivors, with no restrictions on length of time since diagnosis4 (103). Evaluation by a physical therapist clears patients for safe and effective exercise therapy. Physical activity is tailored to each patient's capabilities and limitations. The initial focus is on brisk walking and resistance training. More than 500 individuals have gone through the program, with significant improvements in peak oxygen consumption and the 6-min walk test, as well as upper and lower extremity strength. Patients start slowly and are encouraged to aim for similar recommended physical activity levels as the general population5 (89, 104). The system is similar to recommendations in the AHA statement on Cardio-Oncology rehabilitation, which also includes a suggestion for “opt-out” referrals to maximize utilization (76).
Survivors find it quite helpful to have supervised exercise in the context of social support, encouragement, motivation, and accountability from other patients or from oncologic/cardiac rehabilitation staff. The convenience of having cardiac rehabilitation facilities close to and affiliated with the medical institution that hosts the cancer center may help cancer patients and survivors embrace cardio-oncology rehabilitation programs in the post-adjuvant period (i.e., after completion of cancer therapies). This should also apply to cardio-oncology prehabilitation (before cancer therapies; neoadjuvant?) and habilitation (while undergoing cancer therapies; adjuvant) programs. Studies show that cardiovascular fitness is preserved or improved if exercise training is initiated prior to completion of cancer therapy, rather than waiting to recover cardiopulmonary function after completion of cancer therapy (105, 106). This provides support for the benefit of habilitation even over rehabilitation. If programs initiate exercise training before the first dose of cancer therapy, this points toward the potential for prehabilitation.
In the general population, outpatient cardiac rehabilitation has low utilization rates, although there is potential to decrease morbidity and mortality by ~25% (107). Women in particular face a myriad of barriers in cardiac rehabilitation (108, 109), and large numbers of women are not referred at all (108, 109). High intensity interval training (HIIT) may be among practical solutions to meet women's needs in cardiac rehabilitation (108). HIIT provides more effective and efficient regimens than typical exercise programs, at all stages of prevention (104). HIIT has been shown to improve cardiovascular health components such as fitness (110, 111), body fat percentage (111, 112), waist circumference (112), insulin resistance (111), blood glucose levels (113), blood pressure (113), and lipids (111, 112) in the general population, as well as those with a history of cardiac disease (114, 115) and in patients with a history of cancer (116). Thus, HIIT should play role in Cardio-Oncology prehabilitation, habilitation, and rehabilitation. However, HIIT will not overcome all barriers. Besides time limitations, accessibility, education level, multiple comorbidities, language, social support, and family responsibilities also play a role. Automated referrals or “opt-out” methods may improve referral and participation of women, as may enrollment assistance, incentives, and home-based exercise regimens (109). The remaining barriers of health literacy, multiple comorbidities, language, family responsibilities, and side effects from cancer therapy will also need to be addressed.
Outpatient cardiac rehabilitation utilization in the general population is also particularly limited in rural communities, as well as in those with limited education, lower socioeconomic status, or older age (107). Research is therefore needed to continue to assess the feasibility and effectiveness of these proposed cardio-oncology prehabilitation, habilitation, and rehabilitation programs in academic and community centers or in patients' homes preferably with virtual exercise trainers or accountability groups. Innovative approaches with telemedicine, mobile health, and remote monitoring are being evaluated as potentially cost-effective means of delivery of these structured programs.6
Cardiopulmonary Stress Testing in Prehabilitation, Habilitation, Rehabilitation
It would also be ideal for cardiopulmonary stress testing to be incorporated to assess cardiopulmonary functional status before, during, or after cancer therapies. This could potentially be helpful for preventive counseling and subsequent management for any abnormalities in cardiopulmonary function, given that various cancer therapies can injure the heart and lungs and lead to reduced cardiopulmonary function and fitness (94, 95, 117–119), which associates with increased risk of long-term cardiovascular toxicities, cardiopulmonary symptoms, and mortality (120, 121).
Rehabilitation Too Late?
Currently, the AHA has issued a Scientific Statement endorsing cardio-oncologic rehabilitation, including cardiopulmonary stress testing after patients have completed cancer therapy, particularly for those who received high doses of cardiotoxic chemotherapy or radiation and who have known cardiopulmonary symptoms, CVD, or untreated CVD risk factors (76). The statement is supported by various studies suggesting the benefit of structured exercise therapy for improving cardiovascular health and cardiopulmonary or fitness for cancer survivors (30, 81, 97, 122). In turn, cardiopulmonary fitness associates with lower levels of short-term and long-term cardiovascular and other toxicities, as well as a lower risk of mortality in individuals with cancer (94, 120, 121). Studies also indicate benefits of exercise prior to initiation of or during cancer therapy (30, 116, 123). The statement also encourages optimization of patient evaluation, nutrition and dietary counseling, obesity management, blood pressure and lipids management, diabetes, tobacco cessation, and management of psychosocial contributing factors (76). Additionally, the AHA statement encourages continued research to help determine best practices for implementation of cardio-oncology rehabilitation and determine of clinical indication and feasibility to support reimbursement.
Perhaps once rehabilitation becomes more established, we can then also strategize and implement cardio-oncology prehabilitation and habilitation. Yet, will it be too late to wait? Should we primarily focus our efforts on rehabilitation after cancer therapies, or should we turn our attention to habilitation during cancer therapies while patients are undergoing cycles of chemotherapy and radiation before or after surgery? In fact, should we start sooner? Should we advocate for initiating cardio-oncology prehabilitation before patients undergo chemotherapy, radiation, or surgery? In the overall surgical world, exercise or nutritional prehabilitation (initiated before surgery) seems to improve outcomes and lower healthcare costs compared to waiting for rehabilitation (initiated after surgery) alone (124–137). Clinical trials are underway to further define the prehabilitation period (before surgical therapy) as optimal for fitness intervention (138–140). Data for cardio-oncology prehabiltiation is also promising. Most recently, a study suggested that long-term survivors of breast cancer who had higher levels of physical activity (and presumably fitness) prior to their cancer diagnosis demonstrated a graded reduction in subsequent CV outcomes (e.g., coronary artery disease, heart failure, peripheral artery disease, cardiovascular death), compared to their counterparts with lower levels of physical activity pre-diagnosis (123). Does this also provide indirect support for stronger consideration of primordial and primary prevention in cardio-oncology (Figure 1G)? Primordial prevention focuses on preventing development of CVD risk factors such as hypertension, hyperlipidemia, or obesity. Primary prevention focuses on managing existing CVD risk factors such as hypertension, hyperlipidemia, obesity, or diabetes, in efforts to prevent development of CVD itself. If through primordial and primary prevention in the general population we can optimize ideal cardiovascular health (e.g., based on the AHA Life's Simple 7) before cancer diagnoses occur, then perhaps when individuals from among the general population are diagnosed with cancer and become cancer survivors, those with greater CV health may already be ahead of the curve. The AHA Life's Simple 7 campaign encourages optimization of seven modifiable cardiovascular risk factors, specifically diet, physical activity, weight, smoking, hypertension, hyperlipidemia, and diabetes (141, 142) (Figure 1C).
Paradigm Shift
In Preventive Cardiology, the prevention spectrum includes primordial, primary, secondary (and often tertiary) components. For Cardio-Oncology, a prevention timeline has been suggested in a recent AHA Scientific Statement on the intersection of CVD and breast cancer (20), specifically for the development of cardiovascular toxicity from cancer therapies, regardless of the presence of baseline cardiovascular health and risk factors. In this timeline, primordial prevention of cardiotoxicity would begin upon cancer diagnosis before cancer therapies are administered, since administration of cancer therapies is the key risk factor for development of cardiotoxicity from cancer therapies. Primary prevention would then begin after receiving cancer therapies as the main risk factor for cardiotoxicity. Secondary prevention would begin upon diagnosis of structural heart disease, such as decline in left ventricular systolic function, thought to be due to administration of cancer therapy. Treatment of subsequently developed CVD could then be considered tertiary prevention in this model.
In pursuit of preventive cardio-oncology, we will need to determine how to align the typical paradigm in preventive cardiology with the timeline proposed in the recent scientific statement. Perhaps the two paradigms could be reconciled as follows (Figure 1G). Ideal cardiovascular health based largely on the AHA Life's Simple 7 would be the initial baseline in the general population, from among which cancer survivors will ultimately be diagnosed. This ideal baseline would include optimal parameters for diet, physical activity, obesity, smoking, hypertension, hyperlipidemia, and diabetes, as well as nontraditional cardiovascular risk factors. Primordial prevention of CVD in the general population would limit or avoid development of CVD risk factors (e.g., hypertension). If baseline CVD risk factors develop (e.g., hypertension or hyperlipidemia), then primary prevention would ensue in two different and complementary categories. The first category would be typical primary prevention of CVD in the general population, optimizing management of existing traditional and nontraditional cardiovascular risk factors. The second category would add cardioprotective measures if indicated for those who have been diagnosed with cancer and are deemed to be high risk for developing cardiotoxicity (Figures 1F,G). Such measures may include utilization of beta blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, statins, dexrazoxane, continuous infusion of a limited dose of liposomal doxorubicin, and potentially proton vs. photon beam therapy, and so on.
Once cancer therapies are administered, subclinical cardiovascular injury undetected by standard diagnostic testing may be presumed, since studies have shown microscopic evidence of cardiovascular injury (preclinical) and development of macroscopic CVD (clinical) with even low doses of cancer therapy (143–150). Thus, one could argue that secondary prevention of cardiovascular injury could start following administration of any dose of cancer therapies. However, undetected subclinical injury could be considered Stage A akin to the corresponding ACC stage of heart failure (143), consistent with the presence of risk factors for CVD without overt evidence of macroscopic CVD detected on standard diagnostic tests (151, 152). Once detection of CVD on diagnostic testing occurs, then this would be classified as Stage B, if no symptoms have yet developed, with continuation of secondary prevention. Traditionally, change in left ventricular ejection fraction has been most commonly used to identify cardiotoxicity, heralding Stage B/C CVD; more recently, studies are underway (including an international multicenter prospective randomized controlled trial) to assess the efficacy of global longitudinal strain as a more sensitive and earlier echocardiographic marker of cardiovascular injury in Stage B (153, 154). Once cardiovascular symptoms develop, then Stage C CVD would be diagnosed. Tertiary prevention would be initiated at this point, to avoid complications or further events, or progression of CVD to Stage D, or eventually premature mortality (Figure 1G).
Partnerships for Monitoring, Surveillance, and Intervention in Prevention
It is interesting to consider who should be responsible for CV screening, monitoring, and treatment in Preventive Cardio-Oncology, in the short-term and long-term, before, during, and after cancer therapies. There are several excellent algorithms in literature to guide the flow of patient care in Cardio-Oncology (3, 11, 14, 17, 18, 155–158). Most of these algorithms focus on the relationships among the cardiologist, oncologist, hematologist, and patient, or on steps to follow regarding surveillance for or management of left ventricular dysfunction or other cardiovascular toxicities, or monitoring recommendations for patients being treated with particular cancer therapies. Very few if any of the algorithms explicitly incorporate partnership with patients' primary care providers, and if so, where in the algorithm their input would be present. It goes without saying that patients' primary care providers are understood to be part of their care at all stages. Nevertheless, it may be helpful to clearly include primary care expertise in Preventive Cardio-Oncology algorithms. In Figure 1F, for example, baseline screening and follow-up for all patients in Cardio-Oncology traditionally begins in Oncology (or Hematology), and this should be in partnership with our colleagues in Primary Care. Involving Primary Care clinicians from early on may help streamline long-term follow-up of cancer patients and survivors. A study revealed the prevalent interest and practice of Primary Care clinicians in optimal long-term care of cancer survivors, juxtaposed with their concerns about limited information received regarding patients' detailed treatment plan and survivorship care recommendations (159). Primary Care clinicians also appear to generally desire more guidance, communication, and training to best manage shared care and delegation of responsibility for complications that arise for cancer survivors (159).
Perhaps in Preventive Cardio-Oncology, we can establish distinct methods and training to help guide assessment and follow-up of patients in the context of their (Preventive) Cardio-Oncology team (in F portion of ABCDEF, Figure 1D). At baseline, asymptomatic patients with low risk medical history and low risk treatment plans could continue to be followed closely in Oncology and Primary Care, with a goal of optimizing CVD risk and medications before, during, and after cancer therapy, guided by Cardiology if needed (Figure 1F). In fact, if at any point before, during, or after cancer therapy, patients are symptomatic or are not deemed to be at low risk based on their medical history or treatment plan, these patients should be evaluated to determine the best roles for prevention and management at that moment in their Cardio-Oncology care. A role for Preventive Cardio-Oncology would include providing recommendations for cardioprotection, screening, and surveillance, especially prior to initiation of cancer therapy. All care decisions for patients should be guided by individualization of care, with adherence to any available society expert consensus or scientific statements, or guidelines for prevention, surveillance, and survivorship, as well as management (12, 16, 18, 20, 55, 76).
Minimal surveillance prior to most if not all cancer therapies should include close monitoring for development of symptoms, as well as vital signs and ECG, and often echocardiogram, and troponin (Figure 1F). On subsequent testing, these modalities could most often capture initial signs of cardiovascular toxicity. Various forms of early toxicity may be more likely to show up on some modalities than others (black arrows, Figure 1F). For example, most groups recommend ECG and troponin for monitoring of early toxicity from immune checkpoint inhibitors, of course along with observation for symptoms and assessment of vitals; with escalation to cardiac MRI if suspicion for myocarditis persists, or endomyocardial biopsy, if indicated, in conjunction with Cardio-Oncology consultation and management (13, 160–163). Weekly monitoring of troponin may be pursued for the first 6 weeks, given that aggressive and often fulminant myocarditis typically occurs at a median of 30 days from initiation of therapy (13, 164). However, it should be recognized that troponin levels, echocardiography, or electrocardiography can be normal in patients presenting with myocarditis (162); a high index of suspicion is paramount for any non-specific symptoms or signs noted in patients treated with immunotherapy. As another example, tyrosine kinase inhibitors such as vascular endothelial growth factor inhibitors most commonly aggravate hypertension; accordingly, monitoring and close management of vital signs is of utmost importance in patients being treated with these drugs (165, 166). Weekly monitoring of blood pressure (goal <140/90 mm Hg) for the first 4–6 weeks is recommended, and subsequently every 2–3 weeks until completion of therapy, as systemic hypertension as a form of CV toxicity usually manifests within 4 weeks (14, 18, 26, 62, 67, 167, 168). For patients receiving anthracyclines, an echocardiogram (or cardiac MRI or MUGA if echocardiography is not available or technically feasible) is usually pursued prior to initiation of therapy and within 6–12 months of completion of therapy particularly in high-risk patients, or sooner if concerning CV signs or symptoms arise for any patient during therapy (17, 55). For treatment with trastuzumab, an echocardiogram is commonly pursued at baseline and at 3, 6, and 9 months and often indefinitely while on therapy, although the timing of surveillance remains debatable7 (55). Recognizing the need for differential monitoring of patients based on their treatment regimen is critical to excellent team-based care in (Preventive) Cardio-Oncology, particularly since some drugs are more cardiotoxic than others (Figure 1B).
Following therapy, surveillance and screening in Preventive Cardio-Oncology can be guided by algorithms based on individual patients' treatment plans. All patients could pursue an ABCDEF approach (Figure 1D), which expands on the established ABCDE approach (1, 2), to optimize CV health. For asymptomatic patients with normal cardiac function following therapy with anthracyclines or trastuzumab, while no strict recommendations are available for frequency and duration of echocardiographic or biomarker surveillance, management of CV risk factors should be pursued lifelong (55). Those treated with vasotoxic drugs with persistent cardiovascular risk after therapy (e.g., cisplatin, nilotinib) could consider imaging formal screening tests for peripheral artery disease every 1–2 years and non-invasive stress testing every 5 years (3). Non-invasive stress testing could also be considered every 5 years, along with a concomitant transthoracic echocardiogram, for those who received radiation therapy (3, 158). Most recently, a study illustrated a strong correlation between coronary artery radiation exposure and subsequent segmental coronary artery calcification scores (169). New coronary artery calcification was noted in some patients with breast cancer, lung cancer, lymphoma, or myeloma at a median of 32 months after radiation therapy. Recommendations could be developed for using coronary artery calcification for surveillance in cancer survivors starting 2–3 years after radiation therapy, for those individuals who would otherwise be at low/intermediate CVD risk. The current ACC/AHA prevention guidelines suggest consideration of statin therapy for any CAC > 0 (51). Perhaps this recommendation could also be considered for cancer survivors, who are at an even greater risk for developing CVD than the general population. Perhaps in the future prophylactic statin initiation may be considered for those with higher levels of mean heart dose or coronary artery radiation exposure, even in the absence of (1) known CAC score or (2) CAC score > 0. In the current prevention guidelines, a CAC score of 0 does not preclude statin therapy, if individuals have a personal history of diabetes or cigarette smoking, or a family history of premature coronary heart disease (51). Preventive Cardio-Oncology recommendations could include a history of treatment with radiation therapy or vasotoxic drugs with persistent risk (e.g., cisplatin, nilotinib) in this list of risk modifiers.
Discussion
We can no longer wait to intensify our focus on prevention in cardio-oncology, as the number of survivors of childhood and adult cancers approaches 17 million in 2019 and is projected at ~22 million by 20308. The presence of baseline cardiovascular risk factors raises CVD risk after cancer therapies, but at a higher rate than in the general population due to cardiotoxic effects of cancer therapies. Risk mitigation strategies in the general population are also applied in cardio-oncology, while recognizing the higher risk. Cardioprotection specific to cardio-oncology (e.g., dexrazoxane) is also potentially used, along with appropriate screening and surveillance. A paradigm shift may be beneficial, to initiate preventive efforts sooner than currently considered.
One in three men and women will develop cancer over the course of their lifetime9, and many of these individuals will become long-term cancer survivors. A greater emphasis on encouraging and facilitating healthy behaviors, screening and surveillance, and other methods of cardioprotection will be needed for cancer survivors, as well as novel methods of identifying those at greatest CVD risk (170). Cardiopulmonary function testing and cardio-oncology prehabilitation, habilitation, and rehabilitation will likely help promote efforts at achieving and maintaining ideal cardiovascular health characterized by optimization of Life's Simple 7, along with non-modifiable and nontraditional risk factors. These will need to be applied for survivors of adult and childhood cancers, with improved care transition when children and adolescents become adults. Lifetime access to specialty care will need to continually be established, as particularly desired by childhood cancer survivors (171, 172). Disparities in the medically underserved and in ethnic minorities will also need to be addressed (170), particularly as efforts in precision cardio-oncology and innovation in cardio-oncology unfold.
Research should continue in all of these arenas, to help inform best practices for prevention in cardio-oncology. Several clinical trials are underway to assess CV risk factors and profiles in individuals with a variety of cancer types being treated with a myriad of chemotherapies and endocrine therapies at various stages following diagnosis10. These studies are utilizing traditional or innovative (e.g., Clinical Informatics using the electronic health records) methods to assess cardiovascular risk profiles in the short-term or long-term for patients with breast, endometrial, prostate, testicular, or colorectal carcinoma, or Hodgkin's or Non-Hodgkin's lymphoma, or other cancers treated with endocrine therapies or other forms of cancer therapy. Dozens of clinical trials are also ongoing to assess potential benefits and cost-effectiveness of preventive efforts with pharmacotherapeutics (e.g., statins, ACE inhibitors, beta blockers) before, during, or after cancer therapies, and nutrition and exercise initiated before, during, or after cancer therapies (for cardio-oncology prehabilitation, habilitation, or rehabilitation)11. Pharmacologic cardioprotection will likely continue to be the most feasible intervention to institute, for high risk patients prior to initiation of cancer therapies. Prehabilitation with nutrition and exercise for those diagnosed with cancer may have a limited timeframe for initiation. Nevertheless, it may be prudent to counsel those diagnosed with cancer about nutrition and exercise benefits even prior to starting canscer therapies. Valuable efforts in prehabilitation may indeed also be to partner with Preventive Cardiologists and Primary Care clinicians to encourage health promotion and synergistic surveillance and screening in the general population before cancer diagnoses are made. Perhaps with a somewhat thorough approach to preventive cardio-oncology, we may be able to prevent some injury, protect more hearts, and facilitate cancer remission or cure, with a goal of greater quality and quantity of life.
Author Contributions
S-AB conceived of designed, wrote, revised, and approved the submitted manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^http://www.clinicaltrials.gov
2. ^https://www.cdrnet.org/certifications/board-certification-as-a-specialist-in-oncology-nutrition
3. ^https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf
4. ^https://professional.heart.org/professional/ScienceNews/UCM_503836_Exercise-focused-Rehabilitation-for-Cancer-Survivors-Creating-a-CORE-Component.jsp
5. ^https://professional.heart.org/professional/ScienceNews/UCM_503836_Exercise-focused-Rehabilitation-for-Cancer-Survivors-Creating-a-CORE-Component.jsp
6. ^https://professional.heart.org/professional/ScienceNews/UCM_503836_Exercise-focused-Rehabilitation-for-Cancer-Survivors-Creating-a-CORE-Component.jsp (82).
7. ^National Comprehensive Cancer Network, NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 3.2014. https://jnccn.org/abstract/journals/jnccn/12/4/article-p542.xml
8. ^National Cancer Institute Office of Cancer Survivorship. Statistics: National Cancer Institute; 2019 [Available from: https://cancercontrol.cancer.gov/ocs/statistics/index.html]
9. ^The American Cancer Society. Lifetime Risk of Developing or Dying From Cancer: American Cancer Society; 2018. Available from: https://www.cancer.org/cancer/cancer-basics/lifetime-probability-of-developing-or-dying-from-cancer.html
10. ^Examples of trials at http://www.clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT01080170, https://clinicaltrials.gov/ct2/show/NCT00885612, https://clinicaltrials.gov/ct2/show/NCT00705094, https://clinicaltrials.gov/ct2/show/NCT00688532, https://clinicaltrials.gov/ct2/show/NCT03935282
11. ^Examples of trials at http://www.clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT02842658, https://clinicaltrials.gov/ct2/show/NCT02969577, https://clinicaltrials.gov/ct2/show/NCT03964142, https://clinicaltrials.gov/ct2/show/NCT02454777, https://clinicaltrials.gov/ct2/show/NCT03131024, https://clinicaltrials.gov/ct2/show/NCT03277898, https://clinicaltrials.gov/ct2/show/NCT03334071, https://clinicaltrials.gov/ct2/show/NCT03748550, https://clinicaltrials.gov/ct2/show/NCT02471053, https://clinicaltrials.gov/ct2/show/NCT03748550, https://clinicaltrials.gov/ct2/show/NCT03975491
References
1. Montazeri K, Unitt C, Foody JM, Harris JR, Partridge AH, Moslehi J. ABCDE steps to prevent heart disease in breast cancer survivors. Circulation. (2014) 130:e157–9. doi: 10.1161/CIRCULATIONAHA.114.008820
2. Bhatia N, Santos M, Jones LW, Beckman JA, Penson DF, Morgans AK, et al. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. Circulation. (2016) 133:537–41. doi: 10.1161/CIRCULATIONAHA.115.012519
3. Iliescu CA, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, et al. SCAI Expert consensus statement: Evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of India, and sociedad Latino Americana de Cardiologia intervencionista). Catheter Cardiovasc Interv. (2016) 87:E202–23. doi: 10.1002/ccd.26375
4. Chow EJ, Leger KJ, Bhatt NS, Mulrooney DA, Ross CJ, Aggarwal S, et al. Paediatric cardio-oncology: epidemiology, screening, prevention, and treatment. Cardiovasc Res. (2019) 115:922–34. doi: 10.1093/cvr/cvz031
5. Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. (2014) 3:e000665. doi: 10.1161/JAHA.113.000665
6. Bellinger AM, Arteaga CL, Force T, Humphreys BD, Demetri GD, Druker BJ, et al. Cardio-oncology: how new targeted cancer therapies and precision medicine can inform cardiovascular discovery. Circulation. (2015) 132:2248–58. doi: 10.1161/CIRCULATIONAHA.115.010484
7. Maleszewski JJ, Bois MC, Bois JP, Young PM, Stulak JM, Klarich KW. Neoplasia and the heart: pathological review of effects with clinical and radiological correlation. J Am Coll Cardiol. (2018) 72:202–27. doi: 10.1016/j.jacc.2018.05.026
8. Desai MY, Jellis CL, Kotecha R, Johnston DR, Griffin BP. Radiation-associated cardiac disease: a practical approach to diagnosis and management. JACC Cardiovasc Imaging. (2018) 11:1132–49. doi: 10.1016/j.jcmg.2018.04.028
9. Menezes KM, Wang H, Hada M, Saganti PB. Radiation matters of the heart: a mini review. Front Cardiovasc Med. (2018) 5:83. doi: 10.3389/fcvm.2018.00083
10. Sylvester CB, Abe JI, Patel ZS, Grande-Allen KJ. Radiation-induced cardiovascular disease: mechanisms and importance of linear energy transfer. Front Cardiovasc Med. (2018) 5:5. doi: 10.3389/fcvm.2018.00005
11. Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc. (2014) 89:1287–306. doi: 10.1016/j.mayocp.2014.05.013
12. Campia U, Moslehi JJ, Amiri-Kordestani L, Barac A, Beckman JA, Chism DD, et al. Cardio-oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Association. Circulation. (2019) 139:e579–602. doi: 10.1161/CIR.0000000000000641
13. Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. (2019) 115:854–68. doi: 10.1093/cvr/cvz026
14. Cameron AC, Touyz RM, Lang NN. Vascular complications of cancer chemotherapy. Can J Cardiol. (2016) 32:852–62. doi: 10.1016/j.cjca.2015.12.023
15. Ederhy S, Cautela J, Ancedy Y, Escudier M, Thuny F, Cohen A. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc Imaging. (2018) 11:1187–90. doi: 10.1016/j.jcmg.2017.11.036
16. Blaes AH, Thavendiranathan P, Moslehi J. Cardiac toxicities in the era of precision medicine: underlying risk factors, targeted therapies, and cardiac biomarkers. Am Soc Clin Oncol Educ Book. (2018) 38:764–74. doi: 10.1200/EDBK_208509
17. Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol. (2017) 70:2536–51. doi: 10.1016/j.jacc.2017.09.1096
18. Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J Am Coll Cardiol. (2017) 70:2552–65. doi: 10.1016/j.jacc.2017.09.1095
19. Haque R, Shi J, Schottinger JE, Chung J, Avila C, Amundsen B, et al. Cardiovascular disease after aromatase inhibitor use. JAMA Oncol. (2016) 2:1590–7. doi: 10.1001/jamaoncol.2016.0429
20. Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. (2018) 137:e30–66. doi: 10.1161/CIR.0000000000000556
21. Crawford MH. Chemotherapy-induced valvular heart disease. JACC Cardiovasc Imaging. (2016) 9:240–2. doi: 10.1016/j.jcmg.2015.07.019
22. Murbraech K, Wethal T, Smeland KB, Holte H, Loge JH, Holte E, et al. Valvular dysfunction in lymphoma survivors treated with autologous stem cell transplantation: a national cross-sectional study. JACC Cardiovasc Imaging. (2016) 9:230–9. doi: 10.1016/j.jcmg.2015.06.028
23. Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. (2007) 50:1435–41. doi: 10.1016/j.jacc.2007.06.037
24. Pandey AK, Singhi EK, Arroyo JP, Ikizler TA, Gould ER, Brown J, et al. Mechanisms of VEGF (Vascular Endothelial Growth Factor) inhibitor-associated hypertension and vascular disease. Hypertension. (2018) 71:e1–8. doi: 10.1161/HYPERTENSIONAHA.117.10271
25. Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Oncol. (2018) 2:13. doi: 10.1038/s41698-018-0056-z
26. Waliany S, Sainani KL, Park LS, Zhang CA, Srinivas S, Witteles RM. Increase in blood pressure associated with tyrosine kinase inhibitors targeting vascular endothelial growth factor. JACC CardioOncol. (2019) 1:24–36. doi: 10.1016/j.jaccao.2019.08.012
28. Mensah GA, Dietz WH, Harris VB, Henson R, Labarthe DR, Vinicor F, et al. Prevention and control of coronary heart disease and stroke–nomenclature for prevention approaches in public health: a statement for public health practice from the Centers for disease control and prevention. Am J Prev Med. (2005) 29:152–7. doi: 10.1016/j.amepre.2005.07.035
29. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
30. Squires RW, Shultz AM, Herrmann J. Exercise training and cardiovascular health in cancer patients. Curr Oncol Rep. (2018) 20:27. doi: 10.1007/s11912-018-0681-2
31. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. (2011) 13:R64. doi: 10.1186/bcr2901
32. Handy CE, Quispe R, Pinto X, Blaha MJ, Blumenthal RS, Michos ED, et al. Synergistic opportunities in the interplay between cancer screening and cardiovascular disease risk assessment. Circulation. (2018) 138:727–34. doi: 10.1161/CIRCULATIONAHA.118.035516
33. Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. (2016) 375:1457–67. doi: 10.1056/NEJMra1100265
34. Gilliland TM, Villafane-Ferriol N, Shah KP, Shah RM, Tran Cao HS, Massarweh NN, et al. Nutritional and metabolic derangements in pancreatic cancer and pancreatic resection. Nutrients. (2017) 9:E243. doi: 10.3390/nu9030243
35. Pavo N, Raderer M, Hülsmann M, Neuhold S, Adlbrecht C, Strunk G, et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. (2015) 101:1874–80. doi: 10.1136/heartjnl-2015-307848
36. Tonorezos ES, Jones LW. Energy balance and metabolism after cancer treatment. Semin Oncol. (2013) 40:745–56. doi: 10.1053/j.seminoncol.2013.09.011
37. Madssen TS, Thune I, Flote VG, Lundgren S, Bertheussen GF, Frydenberg H, et al. Metabolite and lipoprotein responses and prediction of weight gain during breast cancer treatment. Br J Cancer. (2018) 119:1144–54. doi: 10.1038/s41416-018-0211-x
38. Nottage KA, Ness KK, Li C, Srivastava D, Robison LL, Hudson MM. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia - from the St. jude lifetime cohort. Br J Haematol. (2014) 165:364–74. doi: 10.1111/bjh.12754
40. van Waas M, Neggers SJ, Raat H, van Rij CM, Pieters R, van den Heuvel-Eibrink MM. Abdominal radiotherapy: a major determinant of metabolic syndrome in nephroblastoma and neuroblastoma survivors. PLoS ONE. (2012) 7:e52237. doi: 10.1371/journal.pone.0052237
41. Gibson TM, Ehrhardt MJ, Ness KK. Obesity and metabolic syndrome among adult survivors of childhood leukemia. Curr Treat Options Oncol. (2016) 17:17. doi: 10.1007/s11864-016-0393-5
42. Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: childhood cancer survivor study. Cancer. (2003) 97:663–73. doi: 10.1002/cncr.11095
43. Bui QM, Daniels LB. A review of the role of breast arterial calcification for cardiovascular risk stratification in women. Circulation. (2019) 139:1094–101. doi: 10.1161/CIRCULATIONAHA.118.038092
44. Hendriks EJE, Beulens JWJ, Mali WPTM, Beijerinck D, van der Graaf Y, de Jong PA, et al. Breast arterial calcifications and their association with incident cardiovascular disease and diabetes: the Prospect-EPIC cohort. J Am Coll Cardiol. (2015) 65:859–60. doi: 10.1016/j.jacc.2014.12.015
45. Hendriks EJ, de Jong PA, van der Graaf Y, Mali WP, van der Schouw YT, Beulens JW. Breast arterial calcifications: a systematic review and meta-analysis of their determinants and their association with cardiovascular events. Atherosclerosis. (2015) 239:11–20. doi: 10.1016/j.atherosclerosis.2014.12.035
46. Margolies L, Salvatore M, Hecht HS, Kotkin S, Yip R, Baber U, et al. Digital mammography and screening for coronary artery disease. JACC Cardiovasc Imaging. (2016) 9:350–60. doi: 10.1016/j.jcmg.2015.10.022
47. Cuddy S, Payne DL, Murphy DJ, Dunne RM, Bueno R, Blankstein R, et al. Incidental coronary artery calcification in cancer imaging. JACC CardioOncol. (2019) 1:135–7. doi: 10.1016/j.jaccao.2019.08.005
48. Al-Kindi SG, Oliveira GH. Prevalence of preexisting cardiovascular disease in patients with different types of cancer: the unmet need for onco-cardiology. Mayo Clin Proc. (2016) 91:81–3. doi: 10.1016/j.mayocp.2015.09.009
49. Chen Y, Chow EJ, Oeffinger KC, Border WL, Leisenring WM, Meacham LR, et al. Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst. (2019). doi: 10.1093/jnci/djz108. [Epub ahead of print].
50. Abdel-Qadir H, Thavendiranathan P, Austin PC, Lee DS, Amir E, Tu JV, et al. Development and validation of a multivariable prediction model for major adverse cardiovascular events after early stage breast cancer: a population-based cohort study. Eur Heart J. (2019). doi: 10.1093/eurheartj/ehz460. [Epub ahead of print].
51. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2019) 140:e596–646. doi: 10.1161/CIR.0000000000000678
52. Bittner VA. The new 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation. (2019) 140:e596–646. doi: 10.1161/CIRCULATIONAHA.119.040625
53. Asselin BL, Devidas M, Chen L, Franco VI, Pullen J, Borowitz MJ, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-hodgkin lymphoma: a report of the children's oncology group randomized trial pediatric oncology group 9404. J Clin Oncol. (2016) 34:854–62. doi: 10.1200/JCO.2015.60.8851
54. Ganatra S, Nohria A, Shah S, et al. Upfront dexrazoxane for the reduction of anthracycline-induced cardiotoxicity in adults with preexisting cardiomyopathy and cancer: a consecutive case series. Cardio-Oncology. (2019) 5:1. doi: 10.1186/s40959-019-0036-7
55. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2017) 35:893–911. doi: 10.1200/JCO.2016.70.5400
56. Vaduganathan M, Hirji SA, Qamar A, et al. Efficacy of Neurohormonal Therapies in Preventing Cardiotoxicity in Patients With Cancer Undergoing Chemotherapy. JACC CardioOncol. (2019) 1:54–65. doi: 10.1016/j.jaccao.2019.08.006
57. Macedo AVS, Hajjar LA, Lyon AR, Nascimento BR, Putzu A, Rossi L, et al. Efficacy of dexrazoxane in preventing anthracycline cardiotoxicity in breast cancer. JACC CardioOncol. (2019) 1:68–79. doi: 10.1016/j.jaccao.2019.08.003
58. Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer. (2013) 49:2900–9. doi: 10.1016/j.ejca.2013.04.030
59. van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. (2010) CD005006. doi: 10.1002/14651858.CD005006.pub3
60. Zagar TM, Kaidar-Person O, Tang X, Jones EE, Matney J, Das SK, et al. Utility of deep inspiration breath hold for left-sided breast radiation therapy in preventing early cardiac perfusion defects: a prospective study. Int J Radiat Oncol Biol Phys. (2017) 97:903–9. doi: 10.1016/j.ijrobp.2016.12.017
61. Bekelman JE, Lu H, Pugh S, Baker K, Berg CD, de Gonzalez AB, et al. Pragmatic randomised clinical trial of proton vs. photon therapy for patients with non-metastatic breast cancer: the Radiotherapy Comparative Effectiveness (RadComp) Consortium trial protocol. BMJ Open. (2019) 9:e025556. doi: 10.1136/bmjopen-2018-025556
62. Bottinor WJ, Shuey MM, Manouchehri A, Farber-Eger EH, Xu M, Nair D, et al. Renin-angiotensin-aldosterone system modulates blood pressure response during vascular endothelial growth factor receptor inhibition. JACC CardioOncol. (2019) 1:14–23. doi: 10.1016/j.jaccao.2019.07.002
63. Dincer M, Altundag K. Angiotensin-converting enzyme inhibitors for bevacizumab-induced hypertension. Ann Pharmacother. (2006) 40:2278–9. doi: 10.1345/aph.1H244
64. Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. (2010) 102:596–604. doi: 10.1093/jnci/djq091
65. Izzedine H, Derosa L, Le Teuff G, Albiges L, Escudier B. Hypertension and angiotensin system inhibitors: impact on outcome in sunitinib-treated patients for metastatic renal cell carcinoma. Ann Oncol. (2015) 26:1128–33. doi: 10.1093/annonc/mdv147
66. Curwen JO, Musgrove HL, Kendrew J, Richmond GH, Ogilvie DJ, Wedge SR. Inhibition of vascular endothelial growth factor-a signaling induces hypertension: examining the effect of cediranib (recentin; AZD2171) treatment on blood pressure in rat and the use of concomitant antihypertensive therapy. Clin Cancer Res. (2008) 14:3124–31. doi: 10.1158/1078-0432.CCR-07-4783
67. Touyz RM, Lang NN. Hypertension and antiangiogenesis. Janus Face of VEGF Inhibitors. (2019) 1:37–40. doi: 10.1016/j.jaccao.2019.08.010
68. Humphreys BD, Atkins MB. Rapid development of hypertension by sorafenib: toxicity or target? Clin Cancer Res. (2009) 15:5947–9. doi: 10.1158/1078-0432.CCR-09-1717
69. Gangadharan A, Choi SE, Hassan A, Ayoub NM, Durante G, Balwani S, et al. Protein calorie malnutrition, nutritional intervention and personalized cancer care. Oncotarget. (2017) 8:24009–30. doi: 10.18632/oncotarget.15103
70. Righini CA, Timi N, Junet P, Bertolo A, Reyt E, Atallah I. Assessment of nutritional status at the time of diagnosis in patients treated for head and neck cancer. Eur Ann Otorhinolaryngol Head Neck Dis. (2013) 130:8–14. doi: 10.1016/j.anorl.2012.10.001
71. Hartmuller VW, Desmond SM. Professional and patient perspectives on nutritional needs of patients with cancer. Oncol Nurs Forum. (2004) 31:989–96. doi: 10.1188/04.ONF.989-996
72. Capra S, Ferguson M, Ried K. Cancer: impact of nutrition intervention outcome–nutrition issues for patients. Nutrition. (2001) 17:769–72. doi: 10.1016/S0899-9007(01)00632-3
73. Ligibel JA, Jones LW, Brewster AM, Clinton SK, Korde LA, Oeffinger KC, et al. Oncologists' attitudes and practice of addressing diet, physical activity, and weight management with patients with cancer: findings of an ASCO survey of the oncology workforce. J Oncol Pract. (2019) 15:e520–8. doi: 10.1200/JOP.19.00124
74. Greenlee H, Santiago-Torres M, McMillen KK, Ueland K, Haase AM. Helping patients eat better during and beyond cancer treatment: continued nutrition management throughout care to address diet, malnutrition, and obesity in cancer. Cancer J. (2019) 25:320–8. doi: 10.1097/PPO.0000000000000405
75. Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. (2014) 32:3568–74. doi: 10.1200/JCO.2014.58.4680
76. Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. (2019) 139:e997-e1012. doi: 10.1161/CIR.0000000000000679
77. Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. (2013) 92:715–27. doi: 10.1097/PHM.0b013e31829b4afe
78. Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. (2014) 25:1293–311. doi: 10.1093/annonc/mdu012
79. Bennett C. Exercise during adjuvant therapy improves cardiac function. Oncol Times. (2019) 41:31. doi: 10.1097/01.COT.0000553543.22371.44
80. Young-McCaughan S, Mays MZ, Arzola SM, Yoder LH, Dramiga SA, Leclerc KM, et al. Research and commentary: change in exercise tolerance, activity and sleep patterns, and quality of life in patients with cancer participating in a structured exercise program. Oncol Nurs Forum. (2003) 30:441–54; discussion−54. doi: 10.1188/03.ONF.441-454
81. Lee K, Tripathy D, Demark-Wahnefried W, Courneya KS, Sami N, Bernstein L, et al. Effect of aerobic and resistance exercise intervention on cardiovascular disease risk in women with early-stage breast cancer: a randomized clinical trial. JAMA Oncol. (2019) 5:710–4. doi: 10.1001/jamaoncol.2019.0038
82. Alfano CM, Cheville AL, Mustian K. Developing high-quality cancer rehabilitation programs: a timely need. Am Soc Clin Oncol Educ Book. (2016) 35:241–9. doi: 10.14694/EDBK_156164
83. Stout NL, Binkley JM, Schmitz KH, Andrews K, Hayes SC, Campbell KL, et al. A prospective surveillance model for rehabilitation for women with breast cancer. Cancer. (2012) 118:2191–200. doi: 10.1002/cncr.27476
84. Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T, et al. Exercise for people with cancer: a systematic review. Curr Oncol. (2017) 24:e290–315. doi: 10.3747/co.24.3519
85. Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. (2003) 97:1746–57. doi: 10.1002/cncr.11227
86. Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. (1997) 3:215–26. doi: 10.1089/acm.1997.3.215
87. Mustian KM, Griggs JJ, Morrow GR, McTiernan A, Roscoe JA, Bole CW, et al. Exercise and side effects among 749 patients during and after treatment for cancer: a University of Rochester Cancer Center Community Clinical Oncology Program Study. Support Care Cancer. (2006) 14:732–41. doi: 10.1007/s00520-005-0912-6
88. Ferioli M, Zauli G, Martelli AM, Vitale M, McCubrey JA, Ultimo S, et al. Impact of physical exercise in cancer survivors during and after antineoplastic treatments. Oncotarget. (2018) 9:14005–34. doi: 10.18632/oncotarget.24456
89. Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. (2010) 42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112
90. Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. (2012) 62:30–67. doi: 10.3322/caac.20140
91. Thraen-Borowski KM, Gennuso KP, Cadmus-Bertram L. Accelerometer-derived physical activity and sedentary time by cancer type in the United States. PLoS ONE. (2017) 12:e0182554. doi: 10.1371/journal.pone.0182554
92. Irwin ML. Physical activity interventions for cancer survivors. Br J Sports Med. (2009) 43:32–8. doi: 10.1136/bjsm.2008.053843
93. Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst. (2006) 98:521–9. doi: 10.1093/jnci/djj130
94. Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. (2012) 30:2530–7. doi: 10.1200/JCO.2011.39.9014
95. Peel AB, Barlow CE, Leonard D, DeFina LF, Jones LW, Lakoski SG. Cardiorespiratory fitness in survivors of cervical, endometrial, and ovarian cancers: the Cooper Center Longitudinal Study. Gynecol Oncol. (2015) 138:394–7. doi: 10.1016/j.ygyno.2015.05.027
96. Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc. (2014) 3:e000432. doi: 10.1161/JAHA.113.000432
97. Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. (2018) 36:2297–305. doi: 10.1200/JCO.2017.77.5809
98. Dennett AM, Peiris CL, Shields N, Prendergast LA, Taylor NF. Moderate-intensity exercise reduces fatigue and improves mobility in cancer survivors: a systematic review and meta-regression. J Physiother. (2016) 62:68–82. doi: 10.1016/j.jphys.2016.02.012
99. Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. (2005) 293:2479–86. doi: 10.1001/jama.293.20.2479
100. Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. (2008) 26:3958–64. doi: 10.1200/JCO.2007.15.9822
101. Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer. (2013) 133:1905–13. doi: 10.1002/ijc.28208
102. Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. (2011) 29:726–32. doi: 10.1200/JCO.2010.31.5226
103. Dittus KL, Lakoski SG, Savage PD, Kokinda N, Toth M, Stevens D, et al. Exercise-based oncology rehabilitation: leveraging the cardiac rehabilitation model. J Cardiopulm Rehabil Prev. (2015) 35:130–9. doi: 10.1097/HCR.0000000000000091
104. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for Americans. JAMA. (2018) 320:2020–8. doi: 10.1001/jama.2018.14854
105. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. (2016) 9:CD005001. doi: 10.1002/14651858.CD005001.pub3
106. Van Moll CC, Schep G, Vreugdenhil A, Savelberg HH, Husson O. The effect of training during treatment with chemotherapy on muscle strength and endurance capacity: a systematic review. Acta Oncol. (2016) 55:539–46. doi: 10.3109/0284186X.2015.1127414
107. Arena R, Williams M, Forman DE, Cahalin LP, Coke L, Myers J, et al. Increasing referral and participation rates to outpatient cardiac rehabilitation: the valuable role of healthcare professionals in the inpatient and home health settings: a science advisory from the American Heart Association. Circulation. (2012) 125:1321–9. doi: 10.1161/CIR.0b013e318246b1e5
108. Way KL, Reed JL. Meeting the needs of women in cardiac rehabilitation. Circulation. (2019) 139:1247–8. doi: 10.1161/CIRCULATIONAHA.118.037754
109. Supervía M, Medina-Inojosa JR, Yeung C, Lopez-Jimenez F, Squires RW, Pérez-Terzic CM, et al. Cardiac rehabilitation for women: a systematic review of barriers and solutions. Mayo Clin Proc. (2017) 92:565–77. doi: 10.1016/j.mayocp.2017.01.002
110. Su L, Fu J, Sun S, Zhao G, Cheng W, Dou C, et al. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: a meta-analysis. PLoS ONE. (2019) 14:e0210644. doi: 10.1371/journal.pone.0210644
111. Fisher G, Brown AW, Bohan Brown MM, Alcorn A, Noles C, Winwood L, et al. High intensity interval- vs moderate intensity- training for improving cardiometabolic health in overweight or obese males: a randomized controlled trial. PLoS ONE. (2015) 10:e0138853. doi: 10.1371/journal.pone.0138853
112. Stavrinou PS, Bogdanis GC, Giannaki CD, Terzis G, Hadjicharalambous M. High-intensity interval training frequency: cardiometabolic effects and quality of life. Int J Sports Med. (2018) 39:210–7. doi: 10.1055/s-0043-125074
113. Batacan RB, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med. (2017) 51:494–503. doi: 10.1136/bjsports-2015-095841
114. Dun Y, Thomas RJ, Smith JR, Medina-Inojosa JR, Squires RW, Bonikowske AR, et al. High-intensity interval training improves metabolic syndrome and body composition in outpatient cardiac rehabilitation patients with myocardial infarction. Cardiovasc Diabetol. (2019) 18:104. doi: 10.1186/s12933-019-0907-0
115. Wewege MA, Ahn D, Yu J, Liou K, Keech A. High-intensity interval training for patients with cardiovascular disease-is it safe? A systematic review. J Am Heart Assoc. (2018) 7:e009305. doi: 10.1161/JAHA.118.009305
116. Mugele H, Freitag N, Wilhelmi J, Yang Y, Cheng S, Bloch W, et al. High-intensity interval training in the therapy and aftercare of cancer patients: a systematic review with meta-analysis. J Cancer Surviv. (2019) 13:205–23. doi: 10.1007/s11764-019-00743-3
117. Klassen O, Schmidt ME, Scharhag-Rosenberger F, Sorkin M, Ulrich CM, Schneeweiss A, et al. Cardiorespiratory fitness in breast cancer patients undergoing adjuvant therapy. Acta Oncol. (2014) 53:1356–65. doi: 10.3109/0284186X.2014.899435
118. Brubaker P, Jensen A, Jordan J, Lamar Z, Mihalko S, Haykowsky M, et al. Exercise capacity is reduced in cancer survivors previously treated with anthracycline-based chemotherapy despite a preserved cardiac output response. JACC Cardiovasc Imaging. (2019) 12:2267–9. doi: 10.1016/j.jcmg.2019.05.016
119. Miller AM, Lopez-Mitnik G, Somarriba G, Lipsitz SR, Hinkle AS, Constine LS, et al. Exercise capacity in long-term survivors of pediatric cancer: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Pediatr Blood Cancer. (2013) 60:663–8. doi: 10.1002/pbc.24410
120. Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. (2009) 27:4605–12. doi: 10.1200/JCO.2008.20.0634
121. Lakoski SG, Willis BL, Barlow CE, Leonard D, Gao A, Radford NB, et al. Midlife cardiorespiratory fitness, incident cancer, and survival after cancer in men: the Cooper Center Longitudinal Study. JAMA Oncol. (2015) 1:231–7. doi: 10.1001/jamaoncol.2015.0226
122. Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation. (2018) 137:1176–91. doi: 10.1161/CIRCULATIONAHA.117.024671
123. Okwuosa TM, Ray RM, Palomo A, Foraker RE, Johnson L, Paskett ED, et al. Pre-diagnosis exercise and cardiovascular events in primary breast cancer. Women's Health Initiative. (2019) 1:41–50. doi: 10.1016/j.jaccao.2019.08.014
124. Wynter-Blyth V, Moorthy K. Prehabilitation: preparing patients for surgery. BMJ. (2017) 358:j3702. doi: 10.1136/bmj.j3702
125. Shaughness G, Howard R, Englesbe M. Patient-centered surgical prehabilitation. Am J Surg. (2018) 216:636–8. doi: 10.1016/j.amjsurg.2017.04.005
126. Killewich LA. Strategies to minimize postoperative deconditioning in elderly surgical patients. J Am Coll Surg. (2006) 203:735–45. doi: 10.1016/j.jamcollsurg.2006.07.012
127. Boereboom CL, Williams JP, Leighton P, Lund JN, Group EPiCCDS. Forming a consensus opinion on exercise prehabilitation in elderly colorectal cancer patients: a Delphi study. Tech Coloproctol. (2015) 19:347–54. doi: 10.1007/s10151-015-1317-2
128. Halloway S, Buchholz SW, Wilbur J, Schoeny ME. Prehabilitation interventions for older adults: an integrative review. West J Nurs Res. (2015) 37:103–23. doi: 10.1177/0193945914551006
129. Jack S, West M, Grocott MP. Perioperative exercise training in elderly subjects. Best Pract Res Clin Anaesthesiol. (2011) 25:461–72. doi: 10.1016/j.bpa.2011.07.003
130. Topp R, Ditmyer M, King K, Doherty K, Hornyak J. The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clin Issues. (2002) 13:263–76. doi: 10.1097/00044067-200205000-00011
131. Kim DJ, Mayo NE, Carli F, Montgomery DL, Zavorsky GS. Responsive measures to prehabilitation in patients undergoing bowel resection surgery. Tohoku J Exp Med. (2009) 217:109–15. doi: 10.1620/tjem.217.109
132. Waite I, Deshpande R, Baghai M, Massey T, Wendler O, Greenwood S. Home-based preoperative rehabilitation (prehab) to improve physical function and reduce hospital length of stay for frail patients undergoing coronary artery bypass graft and valve surgery. J Cardiothorac Surg. (2017) 12:91. doi: 10.1186/s13019-017-0655-8
133. Trépanier M, Minnella EM, Paradis T, Awasthi R, Kaneva P, Schwartzman K, et al. Improved disease-free survival after prehabilitation for colorectal cancer surgery. Ann Surg. (2019) 270:493–501. doi: 10.1097/SLA.0000000000003465
134. Santa Mina D, Hilton WJ, Matthew AG, Awasthi R, Bousquet-Dion G, Alibhai SMH, et al. Prehabilitation for radical prostatectomy: a multicentre randomized controlled trial. Surg Oncol. (2018) 27:289–98. doi: 10.1016/j.suronc.2018.05.010
135. Howard R, Yin YS, McCandless L, Wang S, Englesbe M, Machado-Aranda D. Taking control of your surgery: impact of a prehabilitation program on major abdominal surgery. J Am Coll Surg. (2019) 228:72–80. doi: 10.1016/j.jamcollsurg.2018.09.018
136. Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos-Reed N, et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta-analysis. Gastroenterology. (2018) 155:391–410.e4. doi: 10.1053/j.gastro.2018.05.012
137. Carli F, Silver JK, Feldman LS, McKee A, Gilman S, Gillis C, et al. Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject matter experts. Phys Med Rehabil Clin N Am. (2017) 28:49–64. doi: 10.1016/j.pmr.2016.09.002
138. van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. (2019) 19:98. doi: 10.1186/s12885-018-5232-6
139. Allen S, Brown V, Prabhu P, Scott M, Rockall T, Preston S, et al. A randomised controlled trial to assess whether prehabilitation improves fitness in patients undergoing neoadjuvant treatment prior to oesophagogastric cancer surgery: study protocol. BMJ Open. (2018) 8:e023190. doi: 10.1136/bmjopen-2018-023190
140. Le Roy B, Pereira B, Bouteloup C, Costes F, Richard R, Selvy M, et al. Effect of prehabilitation in gastro-oesophageal adenocarcinoma: study protocol of a multicentric, randomised, control trial-the PREHAB study. BMJ Open. (2016) 6:e012876. doi: 10.1136/bmjopen-2016-012876
141. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. (2010) 121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703
142. Sanchez E. Life's simple 7: vital but not easy. J Am Heart Assoc. (2018) 7:e009324. doi: 10.1161/JAHA.118.009324
143. Jones DN, Jordan JH, Meléndez GC, Lamar Z, Thomas A, Kitzman DW, et al. Frequency of transition from stage A to stage B heart failure after initiating potentially cardiotoxic chemotherapy. JACC Heart Fail. (2018) 6:1023–32. doi: 10.1016/j.jchf.2018.08.005
144. Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. (2010) 28:1276–81. doi: 10.1200/JCO.2009.26.5751
145. Carver JR, Szalda D, Ky B. Asymptomatic cardiac toxicity in long-term cancer survivors: defining the population and recommendations for surveillance. Semin Oncol. (2013) 40:229–38. doi: 10.1053/j.seminoncol.2013.01.005
146. Vandecruys E, Mondelaers V, De Wolf D, Benoit Y, Suys B. Late cardiotoxicity after low dose of anthracycline therapy for acute lymphoblastic leukemia in childhood. J Cancer Surviv. (2012) 6:95–101. doi: 10.1007/s11764-011-0186-6
147. Amigoni M, Giannattasio C, Fraschini D, Galbiati M, Capra AC, Madotto F, et al. Low anthracyclines doses-induced cardiotoxicity in acute lymphoblastic leukemia long-term female survivors. Pediatr Blood Cancer. (2010) 55:1343–7. doi: 10.1002/pbc.22637
148. Lightfoot JC, D'Agostino RB, Hamilton CA, Jordan J, Torti FM, Kock ND, et al. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. (2010) 3:550–8. doi: 10.1161/CIRCIMAGING.109.918540
149. Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol. (2010) 7:564–75. doi: 10.1038/nrcardio.2010.121
150. Cascales A, Pastor-Quirante F, Sánchez-Vega B, Luengo-Gil G, Corral J, Ortuño-Pacheco G, et al. Association of anthracycline-related cardiac histological lesions with NADPH oxidase functional polymorphisms. Oncologist. (2013) 18:446–53. doi: 10.1634/theoncologist.2012-0239
151. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. (2013) 62:e147–239. doi: 10.1161/CIR.0b013e31829e8776
152. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. (2017) 136:e137–61. doi: 10.1161/CIR.0000000000000509
153. Cubbon RM, Lyon AR. Cardio-oncology: concepts and practice. Indian Heart J. (2016) 68(Suppl 1):S77–85. doi: 10.1016/j.ihj.2016.01.022
154. Negishi T, Thavendiranathan P, Negishi K, Marwick TH, Investigators S. Rationale and design of the strain surveillance of chemotherapy for improving cardiovascular outcomes: the SUCCOUR trial. JACC Cardiovasc Imaging. (2018) 11:1098–105. doi: 10.1016/j.jcmg.2018.03.019
155. Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. (2010) 102:14–25. doi: 10.1093/jnci/djp440
156. Barac A, Murtagh G, Carver JR, Chen MH, Freeman AM, Herrmann J, et al. Cardiovascular health of patients with cancer and cancer survivors: a roadmap to the next level. J Am Coll Cardiol. (2015) 65:2739–46. doi: 10.1016/j.jacc.2015.04.059
157. Koutsoukis A, Ntalianis A, Repasos E, Kastritis E, Dimopoulos MA, Paraskevaidis I. Cardio-oncology: a focus on cardiotoxicity. Eur Cardiol. (2018) 13:64–9. doi: 10.15420/ecr.2017:17:2
158. Desai MY, Windecker S, Lancellotti P, Bax JJ, Griffin BP, Cahlon O, et al. Prevention, diagnosis, and management of radiation-associated cardiac disease: JACC scientific expert panel. J Am Coll Cardiol. (2019) 74:905–27. doi: 10.1016/j.jacc.2019.07.006
159. McDonough AL, Rabin J, Horick N, Lei Y, Chinn G, Campbell EG, et al. Practice, preferences, and practical tips from primary care physicians to improve the care of cancer survivors. J Oncol Pract. (2019) 15:e600–6. doi: 10.1200/JOP.18.00740
160. Itzhaki Ben Zadok O, Ben-Avraham B, Nohria A, Orvin K, Nassar M, Iakobishvili Z, et al. Immune-checkpoint inhibitor-induced fulminant myocarditis and cardiogenic shock. JACC CardioOncol. (2019) 1:141–4. doi: 10.1016/j.jaccao.2019.07.004
161. Ganatra S, Neilan TG. Immune checkpoint inhibitor-associated myocarditis. Oncologist. (2018) 23:879–86. doi: 10.1634/theoncologist.2018-0130
162. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. (2018) 71:1755–64. doi: 10.1016/j.jacc.2018.02.037
163. Neilan TG, Rothenberg ML, Amiri-Kordestani L, Sullivan RJ, Steingart RM, Gregory W, et al. Myocarditis associated with immune checkpoint inhibitors: an expert consensus on data gaps and a call to action. Oncologist. (2018) 23:874–8. doi: 10.1634/theoncologist.2018-0157
164. Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. (2018) 19:1579–89. doi: 10.1016/S1470-2045(18)30608-9
165. Maurea N, Spallarossa P, Cadeddu C, Madonna R, Mele D, Monte I, et al. A recommended practical approach to the management of target therapy and angiogenesis inhibitors cardiotoxicity: an opinion paper of the working group on drug cardiotoxicity and cardioprotection, Italian Society of Cardiology. J Cardiovasc Med. (2016) 17(Suppl 1):e93–104. doi: 10.2459/JCM.0000000000000383
166. Dobbin SJH, Cameron AC, Petrie MC, Jones RJ, Touyz RM, Lang NN. Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors. Heart. (2018) 104:1995–2002. doi: 10.1136/heartjnl-2018-313726
167. Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. (2007) 370:2011–9. doi: 10.1016/S0140-6736(07)61865-0
168. Pal S, Gong J, Mhatre SK, Lin SW, Surinach A, Ogale S, et al. Real-world treatment patterns and adverse events in metastatic renal cell carcinoma from a large US claims database. BMC Cancer. (2019) 19:548. doi: 10.1186/s12885-019-5716-z
169. Milgrom SA, Varghese B, Gladish GW, Choi AD, Dong W, Patel ZS, et al. Coronary artery dose-volume parameters predict risk of calcification after radiation therapy. J Cardiovasc Imaging. (2019) 27:268–79. doi: 10.4250/jcvi.2019.27.e38
170. Centers for Disease Control and Prevention (CDC). Cancer survivors–United States. MMWR Morb Mortal Wkly Rep. (2011) 60:269–72. Available online at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6009a1.htm
171. Absolom K, Eiser C, Michel G, Walters SJ, Hancock BW, Coleman RE, et al. Follow-up care for cancer survivors: views of the younger adult. Br J Cancer. (2009) 101:561–7. doi: 10.1038/sj.bjc.6605213
Keywords: cardio-oncology, prevention, preventive cardiology, cancer, cancer survivorship, cardiac rehabilitation, cardiometabolic, risk factors
Citation: Brown S-A (2020) Preventive Cardio-Oncology: The Time Has Come. Front. Cardiovasc. Med. 6:187. doi: 10.3389/fcvm.2019.00187
Received: 17 September 2019; Accepted: 10 December 2019;
Published: 10 January 2020.
Edited by:
Rhian M. Touyz, University of Glasgow, United KingdomReviewed by:
Gavin Oudit, University of Alberta, CanadaAriane Vieira Scarlatelli Macedo, Santa Casa of Sao Paulo, Brazil
Ninian Nicholas Lang, University of Glasgow, United Kingdom
Ian Paterson, University of Alberta, Canada
Copyright © 2020 Brown. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sherry-Ann Brown, YnJvd24uc2hlcnJ5YW5uQG1heW8uZWR1
 Sherry-Ann Brown
Sherry-Ann Brown