
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 28 July 2016
Sec. General Cardiovascular Medicine
Volume 3 - 2016 | https://doi.org/10.3389/fcvm.2016.00024
 Waqas Qureshi1*
Waqas Qureshi1* Zeeshan Ali2
Zeeshan Ali2 Waseem Amjad3
Waseem Amjad3 Zaid Alirhayim4
Zaid Alirhayim4 Hina Farooq5
Hina Farooq5 Shayan Qadir6
Shayan Qadir6 Fatima Khalid7
Fatima Khalid7 Mouaz H. Al-Mallah8
Mouaz H. Al-Mallah8
Cancer patients are at major risk of developing venous thromboembolism (VTE), resulting in increased morbidity and economic burden. While a number of theories try to explain its pathophysiology, its risk stratification can be broadly done in cancer-related, treatment–related, and patient-related factors. Studies report the prophylactic use of thrombolytic agents to be safe and effective in decreasing VTE-related mortality/morbidity especially in postoperative cancer patients. Recent data also suggest the prophylactic use of low molecular weight Heparins (LMWHs) and Warfarin to be effective in reducing VTEs related to long-term central venous catheter use. In a double-blind, multicenter trial, a new ultra-LMWH Semuloparin has shown to be efficacious in preventing chemotherapy-associated VTE’s along with other drugs, such as Certoparin and Nadoparin. LMWHs are reported to be very useful in preventing recurrent VTEs in advanced cancers and should be preferred over full dose Warfarin. However, their long-term safety beyond 6 months has not been established yet. Furthermore, this paper discusses the safety and efficacy of different drugs used in the treatment and prevention of recurrent VTEs, including Bemiparin, Semuloparin, oral direct thrombin inhibitors, parenteral and direct oral factor Xa inhibitors.
◦ Risk factors for venous thromboembolism can be grouped into three broad categories: cancer-related, patient-related, and treatment-related factors.
◦ Prophylactic use of anticoagulants is safe and efficacious in preventing VTE.
◦ LMWHs prove to be a good treatment option for VTE in advanced cancers, being simpler and more efficacious in preventing recurrence.
Cancer continues to pose a costly and growing international threat toward modern day society. Among its many direct and indirect complications is its role as a major risk factor for venous thromboembolism (VTE), discovered in a fifth of all cancer patients and as many as half on postmortem examination (1, 2). Such VTE events include, but are not limited to, central venous catheter (CVC)-related thrombosis and pulmonary embolism (PE) (3, 4). It was Professor Armand Trosseau who first described the association between cancer and thrombosis in 1865, almost 150 years ago, yet its exact pathophysiology remains poorly understood. Cancer-associated VTE bears several clinical and economic implications, including increased hospitalization rates, the need for anticoagulation (and its associated bleeding complications), in addition to the risk for recurrent VTE and the potential for delays in cancer therapy (5). This article presents an overview of VTE risk assessment in cancer patients, current treatment guidelines and the role of newer anticoagulants in the treatment of cancer-related VTE.
To the practicing clinician, cancer remains the most significant acquired risk factor for the development of VTE, with an annual incidence of 1 in 200, ultimately affecting at least 15% of this population (6). VTE in patients with underlying malignancy as opposed to those without cancer can be particularly more serious given their increased likelihood of VTE recurrence, risk of major bleeding complications from anticoagulants, and their reduced survival from such events. Prandoni et al. for instance, reported that patients with cancer and VTE were approximately four times more likely to develop recurrent thromboembolic complications and twice as likely to develop major bleeding while on anticoagulation when compared to patients without underlying malignancy (7). In a retrospective analysis, Khorana et al. found that inhospital mortality was two- to fivefold higher among neutropenic cancer patients hospitalized with thromboembolism as compared to those without thromboembolism. Chew and colleagues analyzed the effect of VTE on survival between cancer patients and found that a diagnosis of thromboembolism was associated with reduced survival rates during the first year, regardless of the type of cancer studied [hazard ratio (HR) 1.6–4.2, P > 0.1] (8). In addition to its human cost, VTE in cancer patients confers additional economic burden. Of cancer patients who develop deep vein thrombosis (DVT), the mean cost of hospitalization in 2002 was US $20,065 (9) compared to an average between $7712 and $10,804 for a similar episode in the general population (10).
While the exact mechanism of VTE in cancer patients is unclear, several theories seem to bear credence. It is recognized, for example, that tissue factor (TF) (which initiates the coagulation cascade) is itself expressed in a variety of malignancies and released into the circulation, suggesting its potential role in cancer-related VTE (8). One study was able to demonstrate a consistent relationship between cell surface expression of TF and prothrombotic potential across a range of sites, including breast, colorectal, and pancreatic tumor cell lines (11). Other observations have lent themselves to other theories. Falanga and Gordon (12), for example, described a cysteine protease that directly activates factor X in the absence of factor VII while Denko and Giaccia had proposed that tumor cell hypoxia stimulates production of procoagulant and angiogenic factors (13). Other theories based on animal models have raised the possibility of oncogene activation to explain the manifestations of Trousseau’s syndrome (14). Yet others describe release of mucins and their interaction with L- and P-selectins particularly in patients with mucinous adenocarcinomas (15, 16). It would not be unreasonable, therefore, to assume that some of these pathways operate and overlap in ways that ultimately predispose the cancer patient to thromboembolic events.
It is difficult to directly compare the rates of cancer-related VTE among patients as the studies vary in their study periods, the methods employed in detecting and reporting VTE, the patient populations, and their follow-up periods. Additionally, with the temporal rise in the incidence of VTE, newer studies seem to report higher rates than those that are less recent (17, 18). Nevertheless, there is broad agreement in the literature with regards to most risk factors for cancer-related VTE. These can be broadly divided into three categories: cancer-related factors, treatment-related factors, and patient-related factors.
The site of the primary tumor has been established as a risk factor for VTE in a number of studies. Specific incidence rates vary based on the clinical setting, but some of the highest rates have been described in patients with primary brain tumors (19, 20), pancreatic (21, 22), stomach (23), uterine (24, 25), and lung carcinomas (26, 27). More recent studies suggest high incidence rates of VTE in association with hematologic malignancies as well. In a large population-based case-control study, hematologic malignancies were in fact found to confer the highest risk of venous thrombosis, followed by lung and gastrointestinal cancers (28). Even among cancer patients with the same primary site, VTE rates seem to vary markedly based on grade and histology. Blom et al., for instance, showed that lung cancer patients with adenocarcinoma had a greater incidence of venous thrombosis as compared to those with squamous cell carcinoma (29). Indeed the stage of cancer is also important, with more advanced stages of cancers conferring ever increasing risk (18, 30). It appears that this risk is highest in the period immediately following cancer diagnosis. In a large case-control study, it was reported that the risk of VTE was highest in the first 3 months following the diagnosis of cancer [adjusted odds ratio (OR), 53.5; 95% confidence interval (CI), 8.6–334.3], subsiding gradually over a 15-year period to levels observed in the general population (28).
As cancer patients too often know unfortunately, the remedy can sometimes be more toxic than the malady itself. Chemotherapy is associated with a two- to sixfold increase in the risk of VTE compared to the general population and in patients starting new chemotherapy regimens, accounts for 9% of deaths (3, 31). These trends seem to be increasing over time, perhaps owing to the development of additional chemotherapeutic options. In hospitalized patients receiving chemotherapy, rates of VTE rose from 3.9 to 5.7% from 1995 to 2003, an increase of 47% (32). Some chemotherapy agents appear to confer greater risk than others. Patients with multiple myeloma receiving Thalidomide in combination with dexamethasone, for example, have DVT rates as high as 28% in some instances (33, 34). Additional predictors for Thalidomide associated VTE include its combined use with Doxorubicin (OR = 4.3), newly diagnosed disease (OR = 2.5), and Chromosome 11 abnormalities (OR = 1.8) (35). Another commonly used agent, Lenalidomide, has significant survival benefits in myeloma patients while also being associated with rates of VTE as high as 75% (36). Another agent, Bevacizumab (an anti-angiogenic in use for a variety of cancers) has been associated with increased risk of both arterial (37) as well as venous (38) events. Strategies to mitigate VTE events in such patients continue to be investigated, though intermittently dosed chemotherapy regimens appear to lessen such risks when compared to continuous treatment (39).
Even common and seemingly innocuous practices, such as the administration of erythropoiesis-stimulating agents (ESAs) to treat anemia, can be harmful in the cancer patient. In a systematic review of 57 trials on cancer patients, thromboembolic events were observed in 229 of 3,728 patients treated with Epoetin or Darbepoetin and in 118 of 3,041 untreated controls (RR = 1.7; 95% CI, 1.4–2.1) (40). In hospitalized cancer patients, it is often necessary to transfuse blood and platelet products both of which are associated with an increased risk of thromboembolic events as well as mortality (41).
While surgery and a prolonged postoperative period are well-known risk factors for VTE, recent data suggest these may not be as significant as other factors, possibly owing to increased thromboprophylaxis rates among the surgical patient population (31, 42–44). Nevertheless, cancer patients undergoing surgery are still at risk for VTE events, particularly in those >60 years of age (OR = 2.6, 95% CI, 1.2–5.7), with prior episodes of VTE (OR = 6, 95% CI, 2.1–16.8), advanced stages of cancer (OR = 2.7, 95% CI, 1.4–5.2), anesthesia lasting more than 2 h (OR = 4.5, 95% CI, 1.1 to 19), and bed rest exceeding 3 days (45).
Central venous catheters are widely used in patients with cancer for the administration of chemotherapy. Verso et al. reported that the incidence of symptomatic catheter-related DVT in adults ranges from 0.3 to 28% while that of catheter-related DVT screened by venography ranges from 27 to 66% (46). The specific chemotherapy agent administered through the catheter can also influence the risk of DVT (47). Other treatment-related risk factors for VTE that have been described in the literature include hospitalization (48, 49) and radiation (31, 50).
The overall risk of VTE is often affected by a multitude of patient-related factors, including a history of prior thrombotic events, comorbid conditions, genetic factors, immobility, age, sex, and race (Table 1). Prior thrombotic episodes significantly increase future thrombotic risk in a wide range of cancer patients including those with prostate cancer, myeloma, and cancer patients undergoing surgery to name a few (OR = 6.0, 95% CI, 2.1–16.8) (51, 52). Concurrent thrombotic events, either venous or arterial are also thought to increase the risk for VTE (32). In patients with hepatocellular carcinoma, for instance, it is noted that the incidence of systemic VTE is higher in patients with concurrent portal vein thrombosis when compared to those without it (11.5 vs. 4.4%, P = 0.04) (53). It is suggested that locally occurring thrombotic events may propagate pathways that result in systemic hemostatic activation. In addition to obtaining a history of prior VTE events, one must elucidate details of a patient’s comorbid conditions as these are invariably apt to influence thrombotic risk. Khorana et al. for instance, showed that infections (OR = 1.77), renal disease (OR = 1.53), arterial thromboembolism (OR = 1.45), pulmonary disease (OR = 1.37), and anemia (OR = 1.35) (32) are strongly associated with VTE risk in cancer patients. Not surprisingly then, in a study on patients with ovarian cancers, Rodriguez et al. observed that the risk of VTE continued to increase with the number of such comorbid conditions; (HR = 2.1 with one comorbidity, HR = 2.6 with two comorbidities and HR = 3.9 with ≥ three comorbidities) (44).
Finally, one must obtain a detailed family history when assessing a patient’s thrombotic risk. This is important as a number of predisposing genetic factors, such as Factor V Leiden and Prothrombin gene mutations are known to confer an increased risk of VTE in cancer patients when compared to those without the mutations (63, 67). Even when the above-mentioned factors have been evaluated, a comprehensive VTE risk assessment in the cancer patient is incomplete until weighed in the context of their functional status. In a prospective study on patients with non-small cell lung carcinoma receiving chemotherapy, VTE developed in 31% patients with poor performance status as compared to 15% with better performance status (26).
In order to reduce the disease burden of VTE, it is important to identify cancer patients at high risk for VTE and who may, therefore, benefit from thromboprophylaxis. Conversely, identifying patients at low risk for VTE may allow us to determine those patients in whom prophylactic anticoagulation can be foregone in order to mitigate the risk of iatrogenic bleeding. Though both intuitive and appealing, the utility of such an approach has had conflicting results (68–70). Recently, a validated risk model for use in the ambulatory setting was published to identify cancer patients whom are at high risk for VTE (Table 2) (62). Five predictive variables were identified in the development cohort, before initiation of chemotherapy. Rates of VTE in the development cohort were 0.8% in the low-risk category (score = 0), 1.8% in the intermediate risk category (score = 1–2), and 7.1% in the high risk category (score = 3 or above), while rates in the validation cohorts were 0.3, 2, and 6.7%, respectively (over a median period of 2.5 months, C statistics = 0.7 for both cohorts).
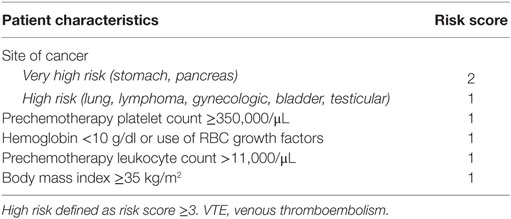
Table 2. Predictive model for chemotherapy-associated VTE (62).
The model has a negative predictive value of 98.5% at the cutoff point for high risk (score ≥ 3). This score is being used to define cancer patients at high risk for VTE in a study on thromboprophylaxis.1 Additionally, this model is being assessed for its use in clinical practice as well.2
In surgical oncology patients without thromboprophylaxis, the incidence of lower extremity DVT, as shown by venography, ranges from 40 to 80% while that of proximal DVT varies between 10 and 20%. The risk of fatal postoperative PE associated with cancer surgery is about four times higher in comparison to non-oncologic surgery (71). Strong risk factors associated with VTE in this setting have been described above (treatment-related risk factors). One commonly used prophylactic regimen consists of a pre-operative dose of subcutaneously administered Heparin, followed by scheduled dosing 12–24 h postoperatively. Typically, unfractionated Heparin (UFH) is given two to three times a day and low molecular weight Heparin (LMWH) is injected once daily. International guidelines direct the use of anti-thrombotic prophylaxis for at least 7–10 postoperative days in patients with cancer surgery (5, 72–74).
Enoxaparin and Cancer (ENOXACAN) II was the first study to demonstrate the benefits of extended thromboprophylaxis with enoxaparin in reducing postoperative VTE among cancer patients undergoing surgery (75). These benefits have been demonstrated across a wide range of patient profiles among oncologic patients, including those undergoing abdominal, pelvic, and thoracic surgery. Rasmussen et al. showed that prolonged prophylaxis with LMWH significantly reduces the risk of VTE compared with prophylaxis given for 7–10 days, without an increase in bleeding complications (76). The overall incidence of VTE was 14.3% (95% CI, 11.2–17.8%) in the control group as compared to 6.1% (95% CI, 4.0–8.7%) in the patients receiving out-of-hospital LMWH (76). The ESMO and AIOM guidelines (73, 74) recommend extended prophylaxis for all patients undergoing elective cancer surgery while the ASCO panel (5) recommends extended prophylaxis for up to 4 weeks in patients undergoing major abdominal or pelvic cancer surgery in the presence of strong risk factors (please see above).
If pharmacologic prophylaxis is contraindicated, mechanical prophylaxis, such as continued use of intermittent pneumatic compression devices with or without compression stockings, should be employed. If compression stockings are used, it is important to ensure that they are of the appropriate size in order to be effective in preventing DVT (77).
The incidence of asymptomatic CVC-related DVT has been reported to be about 20% while that of overt DVT of the upper extremities ranges between 2 and 4% (71). Thromboprophylaxis for CVC-related thrombosis is controversial. Both LMWH and Warfarin have been found to be safe and effective in these patients (68, 70, 78, 79). International guidelines, however, do not recommend routine prophylaxis for this indication (5, 73, 74).
In one of the earliest studies examining this topic, Levine et al. reported that low-dose Warfarin is safe and effective for VTE prevention in stage IV breast cancer patients receiving chemotherapy, the relative risk reduction vs. placebo being 85% (80). In the more recent PROTECHT study, Nadroparin use was found to be associated with a statistically significant 50% relative risk reduction in thromboembolic events among cancer patients in the ambulatory setting receiving chemotherapy for advanced cancers of the breast, lung, gastrointestinal tract, head/neck region, ovary, and pancreas. Fifteen (2.0%) of 769 patients treated with Nadroparin and 15 (3.9%) of 381 patients treated with placebo had a thromboembolic event (single-sided P = 0.02) (81).
Thromboprophylaxis with Certoparin; a LMWH, primarily active against factor Xa, was evaluated in the TOPIC-1 and TOPIC-2 studies that examined patients with advanced breast cancer (TOPIC-1) and stage III/IV non-small cell lung cancer (TOPIC-2), respectively (69). VTE occurrence was not different between the treatment groups in TOPIC-1 (4% treated with Certoparin vs. 4% receiving placebo, OR = 1.02, 95% CI, 0.3–3.48) and TOPIC-2 [which showed a non-significant trend toward efficacy in lung cancer group (4.5 vs. 8.3%, OR = 0.52, 95% CI, 0.23–1.12)].
More recently, Semuloparin, a novel ultra LMWH, was tested for its safety and efficacy in preventing VTE among patients with advanced solid cancers receiving chemotherapy (82). In a double-blind, multicenter trial, patients were randomly assigned to receive subcutaneous Semuloparin 20 mg once daily, or placebo. The primary efficacy outcome was the composite of any symptomatic DVT, any non-fatal PE, and VTE-related death. VTE occurred in 20 of 1,608 patients (1.2%) receiving Semuloparin, compared with 55 of 1,604 patients (3.4%) on placebo (HR: 0.36; 95% CI, 0.21–0.60). Major bleeding rates of patients receiving Semuloparin and placebo rates were similar, occurring in 1.2 and 1.1%, respectively (HR: 1.05; 95% CI, 0.55–1.99).
International guidelines do not recommend routine prophylaxis of cancer patients in the ambulatory setting who receive anti-neoplastic agents (5, 72–74). In this setting, thromboprophylaxis is only recommended for patients with Multiple Myeloma on Thalidomide or Lenalidomide-based combination chemotherapy. Table 3 summarizes the commonly used anticoagulant regimens for VTE prophylaxis.
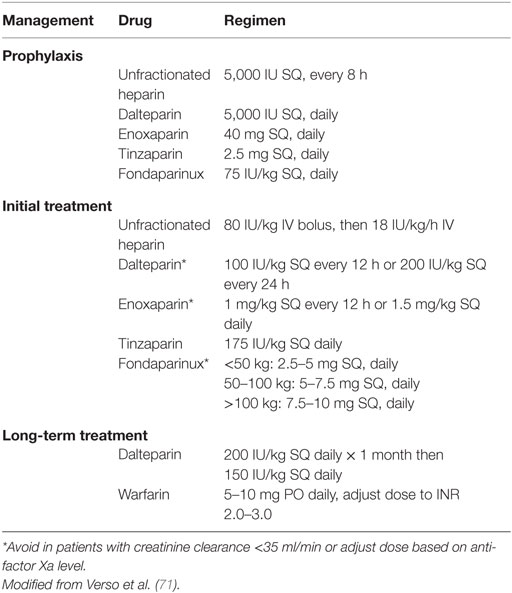
Table 3. Recommended anticoagulant regimens for venous thromboembolism prophylaxis and treatment in patients with cancer.
Patients with acute leukemia and hematopoietic stem cell transplant (HSCT) recipients present a special challenge in this regard as both groups are at great risk for both thrombotic and bleeding events. The incidence of VTE in patients with acute leukemia, for example, ranges from 1.7 to 12% (83). Thrombocytopenia experienced by many of these patients as a result of chemotherapy places them at great risk for bleeding complications. In one retrospective study, of 1,514 HSCT transplant recipients, 4.6% patients developed symptomatic VTE within 180 days of HSCT (84), while 3.6% of patients had had a fatal bleeding episode. Randomized clinical trials are required in order to devise the best regimen for VTE prophylaxis in this high-risk patient population.
Venous thromboembolism treatment in cancer patients is no different from that of other medical patients. While diagnostic evaluation is underway, anticoagulation should be started in all patients in whom VTE is a serious consideration. Options for acute management include adjusted-dose UFH, fixed dose LMWHs or Fondaparinux. Options for chronic anticoagulation have been summarized in Table 3. If an oral Vitamin K antagonist (VKA) is chosen, initial overlap with a parenteral agent (e.g., UFH, etc.) is required for at least 5–7 days until the international normalized ratio (INR) is between 2 and 3 for at least 24 h. Studies have shown that in patients with advanced cancers, LMWHs significantly reduce the incidence of recurrent VTE (by as much as 50% in some studies) when compared to oral VKA, without any difference in bleeding complications (85–88). LMWHs also have the advantage of simplifying initial treatment, thus, making it feasible to manage VTE in the outpatient setting as well. According to international guidelines, LMWHs are preferable to full dose Warfarin for initial treatment of cancer-related VTE, particularly in the first 3–6 months (5, 72–74). In these patients, it is recommended that subsequent anticoagulant therapy with oral VKAs or LMWHs should be continued indefinitely or until the cancer has resolved. The safety and efficacy of LMWHs in cancer patients beyond a treatment duration of 6 months is unknown but currently under investigation.3
In an observational study, Kovacs et al. noted that upper extremity DVTs secondary to central catheters in cancer patients respond well to anticoagulation without removal of the catheter (89). Therefore, it is preferable in this setting to treat upper extremity DVT without removal of the CVC. Guidelines further recommend that anticoagulation should be continued for as long as the catheter is in place and for at least 3 months after its removal (90).
The significance of diagnosing and treating calf vein DVT has been questioned in the past (91, 92). In an observational study by Galanaud et al., no difference in the proportion of recurrent VTE between patients with distal DVT or isolated proximal DVT was detected (93). In a randomized study on patients with calf vein thrombosis, Lagerstedt et al. observed that progressive thromboembolism developed in 29% of the patients in the absence of 3 months of oral anticoagulation therapy (94). The NCCN guidelines recommend a minimum of 3 months of therapy until larger studies supporting a shorter treatment duration become available (95). Given the inherent risk of major and rarely fatal bleeding (96, 97), the NCCN guidelines recommend that thrombolytic therapy should be restricted to life- or limb-threatening thrombotic events (95).
Vena cava filter insertion is commonly performed for recurrent PE, extension of DVT while on anticoagulation, and in instances where anticoagulation is contraindicated. Their use, however, is not associated with any mortality benefit based on results from observational studies and large randomized controlled trials (9, 98–100). Additionally, there are serious safety concerns for complications, such as filter thrombus, embolization, fatal PE as well as fracture, and migration of retrievable filters (9, 101, 102). The NCCN guidelines, therefore, recommend that Vena Cava filters should only be used in the setting of acute VTE where a patient cannot receive anticoagulation.
A large randomized clinical trial evaluating thrombolytic therapy for sub-massive PE failed to show any mortality benefit (103). Additionally, there were concerns for bleeding complications with this form of therapy. The NCCN guidelines, therefore, recommend that use of systemic thrombolytics be reserved for massive PE. Recommendations for superficial venous thrombosis have been summarized in Table 4.
Recurrent VTE despite adequate anticoagulation is not uncommon among cancer patients and an empiric approach has been proposed for such instances (104, 105). In all cases of symptomatic recurrent VTE, it is important to ensure drug compliance. Heparin-induced thrombocytopenia (HIT) must also be excluded. Patients on treatment with oral VKA should be switched to LMWH and those being managed with LMWH should have an increase in their dose by 25% (or increased to weight adjusted doses if receiving lower doses) (106, 107). In patients who do not respond, another dose escalation should be considered and an anti Xa level may be used to estimate the next dose escalation (108). Anatomic risk factors that may account for recurrent thrombosis (e.g., target vessel compression by tumor, May–Thurner syndrome, Thoracic outlet syndrome) should be excluded. Vena cava filter insertion may also be considered though this intervention has no impact on patient survival as described above.
Bemiparin is a LMWH with anti-factor Xa/anti-factor IIa activity that has been studied for VTE prophylaxis in cancer patients undergoing abdominal or pelvic surgery (109). In the CANBESURE study, 703 cancer patients undergoing surgery were randomized to receive 3,500 IU of Bemiparin subcutaneously daily for 8 days. They were then randomized to receive Bemiparin or placebo for an additional 20 days. Major VTE occurred in 0.8% of patients in the Bemiparin group compared with 4.6% in the placebo group (relative risk reduction 82.4%; 95% CI, 21.5–96.1%; P = 0.010). The study authors concluded that 4 weeks of Bemiparin use (when compared to 1 week of Bemiparin) significantly reduced the rate of major VTE without an associated increase in bleeding risk in cancer patients undergoing surgery.
Semuloparin is a subcutaneous ultra LMWH that acts as a factor Xa inhibitor with residual factor IIa activity (110). The TREK study evaluated the dose–response profile of Semuloparin in patients undergoing total knee replacement (110). A significant dose–response correlation was observed across five tested doses of Semuloparin and the incidence of VTE ranged from 5.3% (at a dose of 60 mg/day) to 44.1% (at a dose of 10 mg/day). A similar dose–response effect was observed for incidents of major bleeding (P = 0.0231).
It was concluded that a dose between 20 and 40 mg/day provides an adequate benefit-to-risk profile. Agnelli et al. evaluated Semuloparin for thromboprophylaxis in cancer patients receiving chemotherapy (82).
Fondaparinux is a synthetic pentasaccharide that acts by binding to antithrombin and increasing its inhibitory effect against factor Xa by a factor of ~300. Turpie et al., in a meta-analysis of four multicenter, randomized, double-blind trials, evaluated the safety and efficacy of Fondaparinux in comparison with Enoxaparin (111). The incidence of VTE was reduced by half from 13.7% with LMWH to 6.8% with Fondaparinux. The US Food and Drug Administration has approved Fondaparinux as a substitute for Heparin or LMWH in the initial treatment of VTE. Additional studies are needed to evaluate the safety and efficacy of this agent in patients with malignancy.
Rivaroxaban (109, 112–115) and Apixaban (116–118) are two promising oral inhibitors of factor Xa that have been predominantly studied for prevention of VTE in patients undergoing orthopedic surgery.
In one study, extended duration prophylaxis with Rivaroxaban (10 mg once daily for 35 days) was compared with shorter duration prophylaxis with Enoxaparin (40 mg once daily for 10 days) in acutely ill medical patients, including patients with cancer (MAGELLAN trial) (119). The primary efficacy outcomes were symptomatic VTE, VTE-related death, or asymptomatic proximal DVT detected by routine compressive ultrasonography. Rivaroxaban was associated with a reduction in the risk of venous thrombosis compared to 10 days of treatment with Enoxaparin. Bleeding rates were significantly increased with this new factor Xa inhibitor.
In an interim analysis of a phase II trial, Levine et al. noted that Apixaban was well tolerated by patients with metastatic cancer. The incidence of major bleeding and thrombosis were very low among 125 patients (two patients receiving Apixaban at a dose of 20 mg and one patient in the placebo group developed major bleeding). Thrombosis was reported among three cases in the placebo group (120).
Edoxaban, another oral direct factor Xa inhibitor has been recommended as an alternative agent to treat VTE in cancer patients by the latest Chest guidelines (Grade 2C) (121). In an indirect comparison with apixaban, rivaroxaban, and dabigatran; edoxaban did not show statistically significant difference in risk of recurrent VTE or all – cause mortality (122). In addition, for the composite end – point of major or clinically relevant non-major bleeding, the relative risk for apixaban vs. edoxaban was 1.50 (95% CI, 1.17–1.92, P = 0.001), was 1.15 (95% CI, 0.95–1.39, P = 0.16) for rivaroxaban vs. edoxaban and 1.31 (95% CI, 1.02–1.68, P = 0.04) for edoxaban vs. dabigatran. However, this analysis did not look at the cancer patients. Therefore, edoxaban appears a safer alternative to Warfarin except patients with creatinine clearance >95 ml/min in whom it had shown to increase the risk of ischemic stroke. It does carry the benefit of once a day dosing as compared to its contemporary agents.
Ximelagatran was the first oral direct thrombin inhibitor studied in clinical trials but its development was discontinued because of potentially severe hepatotoxicity. Dabigatran etexilate is the most developmentally advanced oral direct thrombin inhibitor. In randomized, double-blinded trials, it has been shown to be non-inferior to LMWH in reducing the risk of major VTE in patients undergoing orthopedic surgery.
Thromboembolism in cancer patients is associated with significant consequences, including its association with morbidity, mortality, the need for long-term anticoagulation, and its consumption of healthcare resources. Recent studies have enabled us to better understand its clinical risk factors. New oral anticoagulants present an attractive treatment option because of their ease of (oral) administration and their lack of need for laboratory monitoring. Some of their limitations include:
(a) Published data on the safety and efficacy of NOACs in cancer-associated VTE is lacking. In clinical trials evaluating NOACs in VTE treatment, a very small percentage of the study population randomized to receive a NOAC had a diagnosis of cancer. Therefore, it remains unknown whether the safety and efficacy outcomes observed in these trials of largely non-cancer patients also apply to cancer patients.
(b) Recent trials comparing Fondaparinux or Rivaroxaban with LMWH have shown that specific factor Xa inhibition might be less efficacious than LMWH inhibition in cancer patients (123, 124).
(c) The oral administration route may not be practical in patients experiencing nausea, vomiting, and diarrhea as side effects of chemotherapy.
(d) No antidote is available to reverse the anticoagulant effects of these agents.
(e) The lack of assays to measure their anticoagulant effect makes it difficult to manage patients presenting with recurrent thrombosis or bleeding.
(f) Drug–drug interactions with anti-neoplastic agents may lead to clinically important changes in drug levels (105). The ISTH guidance statement, therefore, recommends against the use of NOACs for the treatment of cancer-associated thrombosis (108). It is imperative that the efficacy and safety of these agents be investigated in randomized controlled trials, specifically, in cancer patients with VTE.
The authors contributed to the idea, drafting, and main editing of the manuscript.
All of the authors report no conflict of interest and have no biases based on industry, employment, consultancies, stock ownership, honoraria, paid expert testimony, or patent applications/registrations.
This work is supported by National Institute of Health Ruth L. Kirschstein NRSA Institutional Training Grant 5T32HL076132-10 received by Waqas Qureshi MD.
1. Gao S, Escalante C. Venous thromboembolism and malignancy. Expert Rev Anticancer Ther (2004) 4(2):303–20. doi:10.1586/14737140.4.2.303
2. Khorana AA. Cancer and thrombosis: implications of published guidelines for clinical practice. Ann Oncol (2009) 20(10):1619–30. doi:10.1093/annonc/mdp068
3. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med (2000) 160(6):809–15. doi:10.1001/archinte.160.6.809
4. Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med (2007) 167(14):1471–5. doi:10.1001/archinte.167.14.1471
5. Lyman GH, Khorana AA, Falanga A, Clarke-Pearson D, Flowers C, Jahanzeb M, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol (2007) 25(34):5490–505. doi:10.1200/JCO.2007.14.1283
6. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation (2003) 107(23 Suppl 1):I17–21. doi:10.1161/01.CIR.0000078466.72504.AC
7. Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood (2002) 100(10):3484–8. doi:10.1182/blood-2002-01-0108
8. Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost (2007) 5(3):520–7. doi:10.1111/j.1538-7836.2007.02369.x
9. Elting LS, Escalante CP, Cooksley C, Avritscher EB, Kurtin D, Hamblin L, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med (2004) 164(15):1653–61. doi:10.1001/archinte.164.15.1653
10. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy (2009) 29(8):943–53. doi:10.1592/phco.29.8.943
11. Welsh J, Smith JD, Yates KR, Greenman J, Maraveyas A, Madden LA. Tissue factor expression determines tumour cell coagulation kinetics. Int J Lab Hematol (2012) 34(4):396–402. doi:10.1111/j.1751-553X.2012.01409.x
12. Falanga A, Gordon SG. Isolation and characterization of cancer procoagulant: a cysteine proteinase from malignant tissue. Biochemistry (1985) 24(20):5558–67. doi:10.1021/bi00341a041
13. Denko NC, Giaccia AJ. Tumor hypoxia, the physiological link between Trousseau’s syndrome (carcinoma-induced coagulopathy) and metastasis. Cancer Res (2001) 61(3):795–8.
14. Boccaccio C, Sabatino G, Medico E, Girolami F, Follenzi A, Reato G, et al. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature (2005) 434(7031):396–400. doi:10.1038/nature03357
15. Varki NM, Varki A. Heparin inhibition of selectin-mediated interactions during the hematogenous phase of carcinoma metastasis: rationale for clinical studies in humans. Semin Thromb Hemost (2002) 28(1):53–66. doi:10.1055/s-2002-20564
16. Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest (2003) 112(6):853–62. doi:10.1172/JCI200318882
17. Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med (2006) 119(1):60–8. doi:10.1016/j.amjmed.2005.06.058
18. Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med (2006) 166(4):458–64. doi:10.1001/archinte.166.4.458
19. Brandes AA, Scelzi E, Salmistraro G, Ermani M, Carollo C, Berti F, et al. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur J Cancer (1997) 33(10):1592–6. doi:10.1016/S0959-8049(97)00167-6
20. Semrad TJ, O’Donnell R, Wun T, Chew H, Harvey D, Zhou H, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg (2007) 106(4):601–8. doi:10.3171/jns.2007.106.4.601
21. Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res (2007) 13(10):2870–5. doi:10.1158/1078-0432.CCR-06-2351
22. Mandalà M, Reni M, Cascinu S, Barni S, Floriani I, Cereda S, et al. Venous thromboembolism predicts poor prognosis in irresectable pancreatic cancer patients. Ann Oncol (2007) 18(10):1660–5. doi:10.1093/annonc/mdm284
23. Tetzlaff ED, Correa AM, Baker J, Ensor J, Ajani JA. The impact on survival of thromboembolic phenomena occurring before and during protocol chemotherapy in patients with advanced gastroesophageal adenocarcinoma. Cancer (2007) 109(10):1989–95. doi:10.1002/cncr.22626
24. Franchi M, Ghezzi F, Riva C, Miglierina M, Buttarelli M, Bolis P. Postoperative complications after pelvic lymphadenectomy for the surgical staging of endometrial cancer. J Surg Oncol (2001) 78(4):232–7. doi:10.1002/jso.1158
25. Satoh T, Matsumoto K, Uno K, Sakurai M, Okada S, Onuki M, et al. Silent venous thromboembolism before treatment in endometrial cancer and the risk factors. Br J Cancer (2008) 99(7):1034–9. doi:10.1038/sj.bjc.6604658
26. Numico G, Garrone O, Dongiovanni V, Silvestris N, Colantonio I, Di Costanzo G, et al. Prospective evaluation of major vascular events in patients with nonsmall cell lung carcinoma treated with cisplatin and gemcitabine. Cancer (2005) 103(5):994–9. doi:10.1002/cncr.20893
27. Tagalakis V, Levi D, Agulnik JS, Cohen V, Kasymjanova G, Small D. High risk of deep vein thrombosis in patients with non-small cell lung cancer: a cohort study of 493 patients. J Thorac Oncol (2007) 2(8):729–34. doi:10.1097/JTO.0b013e31811ea275
28. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA (2005) 293(6):715–22. doi:10.1001/jama.293.6.715
29. Blom JW, Osanto S, Rosendaal FR. The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost (2004) 2(10):1760–5. doi:10.1111/j.1538-7836.2004.00928.x
30. Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost (2002) 87(4):575–9.
31. Blom JW, Vanderschoot JP, Oostindiër MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost (2006) 4(3):529–35. doi:10.1111/j.1538-7836.2006.01804.x
32. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer (2007) 110(10):2339–46. doi:10.1002/cncr.23062
33. Cavo M, Zamagni E, Tosi P, Cellini C, Cangini D, Tacchetti P, et al. First-line therapy with thalidomide and dexamethasone in preparation for autologous stem cell transplantation for multiple myeloma. Haematologica (2004) 89(7):826–31.
34. Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR, Eastern Cooperative Oncology Group. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol (2006) 24(3):431–6. doi:10.1200/JCO.2005.03.0221
35. Zangari M, Barlogie B, Thertulien R, Jacobson J, Eddleman P, Fink L, et al. Thalidomide and deep vein thrombosis in multiple myeloma: risk factors and effect on survival. Clin Lymphoma (2003) 4(1):32–5. doi:10.3816/CLM.2003.n.011
36. Zonder JA, Barlogie B, Durie BG, McCoy J, Crowley J, Hussein MA. Thrombotic complications in patients with newly diagnosed multiple myeloma treated with lenalidomide and dexamethasone: benefit of aspirin prophylaxis. Blood (2006) 108(1):403. doi:10.1182/blood-2006-01-0154
37. Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst (2007) 99(16):1232–9. doi:10.1093/jnci/djm086
38. Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA (2008) 300(19):2277–85. doi:10.1001/jama.2008.656
39. Mandalà M, Barni S, Floriani I, Isa L, Fornarini G, Marangolo M, et al. Incidence and clinical implications of venous thromboembolism in advanced colorectal cancer patients: the ‘GISCAD-alternating schedule’ study findings. Eur J Cancer (2009) 45(1):65–73. doi:10.1016/j.ejca.2008.09.005
40. Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst (2006) 98(10):708–14. doi:10.1093/jnci/djj189
41. Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med (2008) 168(21):2377–81. doi:10.1001/archinte.168.21.2377
42. Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Kakkar AK, Deslandes B, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet (2008) 371(9610):387–94. doi:10.1016/S0140-6736(08)60202-0
43. Alcalay A, Wun T, Khatri V, Chew HK, Harvey D, Zhou H, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol (2006) 24(7):1112–8. doi:10.1200/JCO.2005.04.2150
44. Rodriguez AO, Wun T, Chew H, Zhou H, Harvey D, White RH. Venous thromboembolism in ovarian cancer. Gynecol Oncol (2007) 105(3):784–90. doi:10.1016/j.ygyno.2007.02.024
45. Agnelli G, Bolis G, Capussotti L, Scarpa RM, Tonelli F, Bonizzoni E, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg (2006) 243(1):89–95. doi:10.1097/01.sla.0000193959.44677.48
46. Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol (2003) 21(19):3665–75. doi:10.1200/JCO.2003.08.008
47. Linenberger ML. Catheter-related thrombosis: risks, diagnosis, and management. J Natl Compr Canc Netw (2006) 4(9):889–901.
48. Kröger K, Weiland D, Ose C, Neumann N, Weiss S, Hirsch C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol (2006) 17(2):297–303. doi:10.1093/annonc/mdj068
49. Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol (2006) 24(3):484–90. doi:10.1200/JCO.2005.03.8877
50. Lin J, Wakefield TW, Henke PK. Risk factors associated with venous thromboembolic events in patients with malignancy. Blood Coagul Fibrinolysis (2006) 17(4):265–70. doi:10.1097/01.mbc.0000224845.27378.c3
51. Secin FP, Jiborn T, Bjartell AS, Fournier G, Salomon L, Abbou CC, et al. Multi-institutional study of symptomatic deep venous thrombosis and pulmonary embolism in prostate cancer patients undergoing laparoscopic or robot-assisted laparoscopic radical prostatectomy. Eur Urol (2008) 53(1):134–45. doi:10.1016/j.eururo.2007.05.028
52. Srkalovic G, Cameron MG, Rybicki L, Deitcher SR, Kattke-Marchant K, Hussein MA. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer (2004) 101(3):558–66. doi:10.1002/cncr.20405
53. Connolly GC, Chen R, Hyrien O, Mantry P, Bozorgzadeh A, Abt P, et al. Incidence, risk factors and consequences of portal vein and systemic thromboses in hepatocellular carcinoma. Thromb Res (2008) 122(3):299–306. doi:10.1016/j.thromres.2007.10.009
54. Komrokji RS, Uppal NP, Khorana AA, Lyman GH, Kaplan KL, Fisher RI, et al. Venous thromboembolism in patients with diffuse large B-cell lymphoma. Leuk Lymphoma (2006) 47(6):1029–33. doi:10.1080/10428190600560991
55. Czaykowski PM, Moore MJ, Tannock IF. High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J Urol (1998) 160(6 Pt 1):2021–4. doi:10.1097/00005392-199812010-00022
56. Chew HK, Davies AM, Wun T, Harvey D, Zhou H, White RH. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost (2008) 6(4):601–8. doi:10.1111/j.1538-7836.2008.02908.x
57. Pritchard KI, Paterson AH, Paul NA, Zee B, Fine S, Pater J. Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group. J Clin Oncol (1996) 14(10):2731–7.
58. Osman K, Comenzo R, Rajkumar SV. Deep venous thrombosis and thalidomide therapy for multiple myeloma. N Engl J Med (2001) 344(25):1951–2. doi:10.1056/NEJM200106213442516
59. Knight R, DeLap RJ, Zeldis JB. Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med (2006) 354(19):2079–80. doi:10.1056/NEJMc053530
60. Kuenen BC, Levi M, Meijers JC, van Hinsbergh VW, Berkhof J, Kakkar AK, et al. Potential role of platelets in endothelial damage observed during treatment with cisplatin, gemcitabine, and the angiogenesis inhibitor SU5416. J Clin Oncol (2003) 21(11):2192–8. doi:10.1200/JCO.2003.08.046
61. Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer (2005) 104(12):2822–9. doi:10.1002/cncr.21496
62. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood (2008) 111(10):4902–7. doi:10.1182/blood-2007-10-116327
63. Kennedy M, Andreescu AC, Greenblatt MS, Jiang H, Thomas CA, Chassereau L, et al. Factor V Leiden, prothrombin 20210A and the risk of venous thrombosis among cancer patients. Br J Haematol (2005) 128(3):386–8. doi:10.1111/j.1365-2141.2004.05327.x
64. Simanek R, Vormittag R, Ay C, Alguel G, Dunkler D, Schwarzinger I, et al. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost (2010) 8(1):114–20. doi:10.1111/j.1538-7836.2009.03680.x
65. Tefferi A, Gangat N, Wolanskyj A. The interaction between leukocytosis and other risk factors for thrombosis in essential thrombocythemia. Blood (2007) 109(9):4105. doi:10.1182/blood-2007-01-066985
66. Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost (2008) 6(11):1983–5. doi:10.1111/j.1538-7836.2008.03156.x
67. Eroglu A, Kurtman C, Ulu A, Cam R, Akar N. Factor V Leiden and PT G20210A mutations in cancer patients with and without venous thrombosis. J Thromb Haemost (2005) 3(6):1323–4. doi:10.1111/j.1538-7836.2005.01346.x
68. Couban S, Goodyear M, Burnell M, Dolan S, Wasi P, Barnes D, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol (2005) 23(18):4063–9. doi:10.1200/JCO.2005.10.192
69. Haas SK, Freund M, Heigener D, Heilmann L, Kemkes-Matthes B, von Tempelhoff GF, et al. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost (2012) 18(2):159–65. doi:10.1177/1076029611433769
70. Verso M, Agnelli G, Bertoglio S, Di Somma FC, Paoletti F, Ageno W, et al. Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double-blind, placebo-controlled, randomized study in cancer patients. J Clin Oncol (2005) 23(18):4057–62. doi:10.1200/JCO.2005.06.084
71. Verso M, Agnelli G. New and old anticoagulants in cancer. Thromb Res (2012) 129(Suppl 1):S101–5. doi:10.1016/S0049-3848(12)70027-0
72. Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest (2008) 133(6 Suppl):381s–453s. doi:10.1378/chest.08-0656
73. Mandalà M, Falanga A, Piccioli A, Prandoni P, Pogliani EM, Labianca R, et al. Venous thromboembolism and cancer: guidelines of the Italian Association of Medical Oncology (AIOM). Crit Rev Oncol Hematol (2006) 59(3):194–204. doi:10.1016/j.critrevonc.2006.05.001
74. Mandala M, Falanga A, Roila F. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guidelines for the management. Ann Oncol (2010) 21(Suppl 5):v274–6. doi:10.1093/annonc/mdq199
75. Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nilsson PE, Le Moigne-Amrani A, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med (2002) 346(13):975–80. doi:10.1056/NEJMoa012385
76. Rasmussen MS, Jorgensen LN, Wille-Jorgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev (2009) (1):Cd004318. doi:10.1002/14651858.CD004318.pub2
77. CLOTS Trials Collaboration, Dennis M, Sandercock PA, Reid J, Graham C, Murray G, et al. Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet (2009) 373(9679):1958–65. doi:10.1016/S0140-6736(09)60941-7
78. Farge D, Durant C, Villiers S, Long A, Mahr A, Marty M, et al. Lessons from French National Guidelines on the treatment of venous thrombosis and central venous catheter thrombosis in cancer patients. Thromb Res (2010) 125(Suppl 2):S108–16. doi:10.1016/S0049-3848(10)70027-X
79. Karthaus M, Kretzschmar A, Kröning H, Biakhov M, Irwin D, Marschner N, et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: final results of a double-blind, placebo-controlled phase III trial. Ann Oncol (2006) 17(2):289–96. doi:10.1093/annonc/mdj059
80. Levine M, Hirsh J, Gent M, Arnold A, Warr D, Falanga A, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet (1994) 343(8902):886–9. doi:10.1016/S0140-6736(94)90008-6
81. Agnelli G, Gussoni G, Bianchini C, Verso M, Mandalà M, Cavanna L, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol (2009) 10(10):943–9. doi:10.1016/S1470-2045(09)70232-3
82. Agnelli G, George DJ, Kakkar AK, Fisher W, Lassen MR, Mismetti P, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med (2012) 366(7):601–9. doi:10.1056/NEJMoa1108898
83. Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol (2009) 27(29):4848–57. doi:10.1200/JCO.2009.22.8197
84. Gerber DE, Segal JB, Levy MY, Kane J, Jones RJ, Streiff MB. The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention. Blood (2008) 112(3):504–10. doi:10.1182/blood-2007-10-117051
85. Deitcher SR, Kessler CM, Merli G, Rigas JR, Lyons RM, Fareed J, et al. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin Appl Thromb Hemost (2006) 12(4):389–96. doi:10.1177/1076029606293692
86. Hull RD, Pineo GF, Brant RF, Mah AF, Burke N, Dear R, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med (2006) 119(12):1062–72. doi:10.1016/j.amjmed.2006.02.022
87. Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med (2003) 349(2):146–53. doi:10.1056/NEJMoa025313
88. Meyer G, Marjanovic Z, Valcke J, Lorcerie B, Gruel Y, Solal-Celigny P, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med (2002) 162(15):1729–35. doi:10.1001/archinte.162.15.1729
89. Kovacs MJ, Kahn SR, Rodger M, Anderson DR, Andreou R, Mangel JE, et al. A pilot study of central venous catheter survival in cancer patients using low-molecular-weight heparin (dalteparin) and warfarin without catheter removal for the treatment of upper extremity deep vein thrombosis (The Catheter Study). J Thromb Haemost (2007) 5(8):1650–3. doi:10.1111/j.1538-7836.2007.02613.x
90. Wagman LD, Baird MF, Bennett CL, Bockenstedt PL, Cataland SR, Fanikos J, et al. Venous thromboembolic disease. Clinical practice guidelines in oncology. J Natl Compr Canc Netw (2006) 4(9):838–69.
91. Righini M. Is it worth diagnosing and treating distal deep vein thrombosis? No. J Thromb Haemost (2007) 5(Suppl 1):55–9. doi:10.1111/j.1538-7836.2007.02468.x
92. Schellong SM, Distal DVT. Worth diagnosing? Yes. J Thromb Haemost (2007) 5(Suppl 1):51–4. doi:10.1111/j.1538-7836.2007.02490.x
93. Galanaud JP, Sevestre-Pietri MA, Bosson JL, Laroche JP, Righini M, Brisot D, et al. Comparative study on risk factors and early outcome of symptomatic distal versus proximal deep vein thrombosis: results from the OPTIMEV study. Thromb Haemost (2009) 102(3):493–500. doi:10.1160/TH09-01-0053
94. Lagerstedt CI, Olsson CG, Fagher BO, Oqvist BW, Albrechtsson U. Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis. Lancet (1985) 2(8454):515–8. doi:10.1016/S0140-6736(85)90459-3
95. Streiff MB. The National Comprehensive Cancer Center Network (NCCN) guidelines on the management of venous thromboembolism in cancer patients. Thromb Res (2010) 125(Suppl 2):S128–33. doi:10.1016/S0049-3848(10)70030-X
96. Enden T, Kløw NE, Sandvik L, Slagsvold CE, Ghanima W, Hafsahl G, et al. Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost (2009) 7(8):1268–75. doi:10.1111/j.1538-7836.2009.03464.x
97. Vedantham S. Deep venous thrombosis: the opportunity at hand. AJR Am J Roentgenol (2009) 193(4):922–7. doi:10.2214/AJR.09.3214
98. Barginear MF, Gralla RJ, Bradley TP, Ali SS, Shapira I, Greben C, et al. Investigating the benefit of adding a vena cava filter to anticoagulation with fondaparinux sodium in patients with cancer and venous thromboembolism in a prospective randomized clinical trial. Support Care Cancer (2012) 20(11):2865–72. doi:10.1007/s00520-012-1413-z
99. Decousus H, Leizorovicz A, Parent F, Page Y, Tardy B, Girard P, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med (1998) 338(7):409–15. doi:10.1056/NEJM199802123380701
100. White RH, Zhou H, Kim J, Romano PS. A population-based study of the effectiveness of inferior vena cava filter use among patients with venous thromboembolism. Arch Intern Med (2000) 160(13):2033–41. doi:10.1001/archinte.160.13.2033
101. Getzen TM, Rectenwald JE. Inferior vena cava filters in the cancer patient: current use and indications. J Natl Compr Canc Netw (2006) 4(9):881–8.
102. Nicholson W, Nicholson WJ, Tolerico P, Taylor B, Solomon S, Schryver T, et al. Prevalence of fracture and fragment embolization of Bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. Arch Intern Med (2010) 170(20):1827–31. doi:10.1001/archinternmed.2010.316
103. Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W, Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med (2002) 347(15):1143–50. doi:10.1056/NEJMoa021274
104. Lee AY. Thrombosis in cancer: an update on prevention, treatment, and survival benefits of anticoagulants. Hematology Am Soc Hematol Educ Program (2010) 2010:144–9. doi:10.1182/asheducation-2010.1.144
105. Lee AY. Treatment of established thrombotic events in patients with cancer. Thromb Res (2012) 129(Suppl 1):S146–53. doi:10.1016/S0049-3848(12)70035-X
106. Brose KM, Lee AY. Cancer-associated thrombosis: prevention and treatment. Curr Oncol (2008) 15(Suppl 1):S58–67. doi:10.3747/co.2008.177
107. Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost (2009) 7(5):760–5. doi:10.1111/j.1538-7836.2009.03326.x
108. Carrier M, Khorana AA, Zwicker J, Noble S, Lee AY, Subcommittee on Haemostasis and Malignancy for the SSC of the ISTH. Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH. J Thromb Haemost (2013) 11(9):1760–5. doi:10.1111/jth.12338
109. Kakkar VV, Balibrea JL, Martínez-González J, Prandoni P, CANBESURE Study Group. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost (2010) 8(6):1223–9. doi:10.1111/j.1538-7836.2010.03892.x
110. Lassen MR, Dahl OE, Mismetti P, Destrée D, Turpie AG. AVE5026, a new hemisynthetic ultra-low-molecular-weight heparin for the prevention of venous thromboembolism in patients after total knee replacement surgery – TREK: a dose-ranging study. J Thromb Haemost (2009) 7(4):566–72. doi:10.1111/j.1538-7836.2009.03301.x
111. Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Arch Intern Med (2002) 162(16):1833–40. doi:10.1001/archinte.162.16.1833
112. Eriksson BI, Kakkar AK, Turpie AG, Gent M, Bandel TJ, Homering M, et al. Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J Bone Joint Surg Br (2009) 91(5):636–44. doi:10.1302/0301-620X.91B5.21691
113. Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med (2008) 358(26):2776–86. doi:10.1056/NEJMoa076016
114. Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet (2009) 373(9676):1673–80. doi:10.1016/S0140-6736(09)60734-0
115. Buller HR, Lensing AW, Prins MH, Agnelli G, Cohen A, Gallus AS, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-Ranging Study. Blood (2008) 112(6):2242–7. doi:10.1182/blood-2008-05-160143
116. Lassen MR, Davidson BL, Gallus A, Pineo G, Ansell J, Deitchman D. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost (2007) 5(12):2368–75. doi:10.1111/j.1538-7836.2007.02764.x
117. Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet (2010) 375(9717):807–15. doi:10.1016/S0140-6736(09)62125-5
118. Botticelli Investigators, Writing Committe, Buller H, Deitchman D, Prins M, Segers A. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. J Thromb Haemost (2008) 6(8):1313–8. doi:10.1111/j.1538-7836.2008.03054.x
119. Cohen A, et al. Rivaroxaban vs. enoxaparin for the prevention of venous thromboembolism in acutely ill medical patients: Magellan subgroup analyses. J Thromb Haemostasis (2011) 9:21.
120. Levine MN, Gu C, Liebman HA, Escalante CP, Solymoss S, Deitchman D, et al. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost (2012) 10(5):807–14. doi:10.1111/j.1538-7836.2012.04693.x
121. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest (2016) 149(2):315–52. doi:10.1016/j.chest.2015.11.026
122. Mantha S, Ansell J. Indirect comparison of dabigatran, rivaroxaban, apixaban and edoxaban for the treatment of acute venous thromboembolism. J Thromb Thrombolysis (2015) 39(2):155–65. doi:10.1007/s11239-014-1102-5
123. Cohen AT, Spiro TE, Spyropoulos AC, MAGELLAN Steering Committee. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med (2013) 368(20):1945–6. doi:10.1056/NEJMc1303641
Keywords: venous thromboembolism, risk stratification, newer oral anticoagulants, thromboprophylaxis, low molecular weight heparin, apixaban, rivaroxaban
Citation: Qureshi W, Ali Z, Amjad W, Alirhayim Z, Farooq H, Qadir S, Khalid F and Al-Mallah MH (2016) Venous Thromboembolism in Cancer: An Update of Treatment and Prevention in the Era of Newer Anticoagulants. Front. Cardiovasc. Med. 3:24. doi: 10.3389/fcvm.2016.00024
Received: 15 May 2016; Accepted: 06 July 2016;
Published: 28 July 2016
Edited by:
Guillaume Christian Mahé, CHU de Rennes, FranceReviewed by:
Nazareno Paolocci, Johns Hopkins University, USACopyright: © 2016 Qureshi, Ali, Amjad, Alirhayim, Farooq, Qadir, Khalid and Al-Mallah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Waqas Qureshi, d3F1cmVzaGlAd2FrZWhlYWx0aC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.