
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 19 February 2015
Sec. Cardiovascular Imaging
Volume 2 - 2015 | https://doi.org/10.3389/fcvm.2015.00005
 Andrew G. Sherrah1,2
Andrew G. Sherrah1,2 Stuart M. Grieve1,3,4,5
Stuart M. Grieve1,3,4,5 Richmond W. Jeremy1,2
Richmond W. Jeremy1,2 Paul G. Bannon1,2
Paul G. Bannon1,2 Michael P. Vallely1,2,6
Michael P. Vallely1,2,6 Rajesh Puranik1,7*
Rajesh Puranik1,7*The acute event of thoracic aortic dissection carries with it high mortality and morbidity. Despite optimal initial surgical or medical management strategies, the risk of further complications in the long-term, including aneurysmal dilatation and false lumen (FL) expansion, are not insignificant. Adequate follow-up of such conditions requires dedicated imaging where relevant prognostic indicators are accurately assessed. We perform a systematic review of the literature and report the current evidence for the use of magnetic resonance imaging (MRI) in assessment of chronic aortic dissection. We then make a comparison with traditional imaging modalities including computed tomography and echocardiography. We discuss new ways in which MRI may extend existing aortic assessment, including identification of blood-flow dynamics within the TL and FL using phase-contrast imaging.
Aortic dissection is a catastrophic complication of aortic wall disease associated with high mortality and morbidity. The underlying process of the aortic wall disruption is most commonly secondary to atherosclerotic disease (especially with older age) or a known connective tissue disease [such as Marfan syndrome (MFS); thoracic aortic aneurysm and dissection syndrome (TAAD); or bicuspid aortic valve (BAV)]. Using data from the International Registry of Acute Aortic Dissection (IRAD), aortic dissection is more common in men, with mean age of 63 years and an incidence of up to 0.8% at autopsy (1–4). According to the Stanford classification, type A aortic dissections involve the proximal/ascending aorta (and may extend distally) while type B aortic dissections involve the descending thoracic aorta without any proximal extension (5, 6). The aims of surgical intervention in type A dissection are the prevention of aortic rupture, severe aortic valve regurgitation, coronary artery dissection, and cardiac tamponade (2). Compared with type B dissection, type A confers a higher risk of neurological complications, including stroke. For type B, surgical or endovascular intervention is typically reserved only for those with clinical compromise or who are inadequately managed with medical therapy alone (2, 3).
It is well recognized that persisting or chronic aortic dissection (>2 weeks after initial intimal injury) is a risk factor for further aortic dilatation and dissection extension (7–9). In these cases, intervention may be required to prevent progressive aortic dilatation (1). Re-operation rates in type A dissection for areas of aneurysmal dilatation or persisting dissection range from 10 to 20% in the first 10 years following initial surgery (10).
A key issue regarding the management of chronic aortic dissections is a progressive increase in the size of the false lumen (FL). The reported incidence of partial or complete distal aortic FL patency in type A dissection patients is significant, ranging between 31 and 89% (9). For type B dissection patients who have been medically managed, a persisting FL in the chronic phase correlates with an increased risk of aortic enlargement (11). Hence, regardless of the initial management in aortic dissection, careful follow-up with appropriate imaging is mandated (12). Several international guidelines recommend the close follow-up of these patients; however multiple imaging modalities are currently used without a clear consensus of the gold standard (2–5). This “gap” in the evidence is specifically noted in the current European Society of Cardiology guidelines on aortic disease management (4).
The most commonly used modalities are computed tomography (CT) and echocardiography, however magnetic resonance imaging (MRI) has recently emerged as a comprehensive, non-ionizing imaging tool well suited to serial measurements in this group of patients (Figure 1) (13). In the acute setting of aortic dissection, the most suitable imaging modality has been extensively examined, given the multiple critical clinical factors that affect time to diagnosis and appropriate management (2, 14, 15). Here, the utility of imaging modalities is limited by availability, time to acquire diagnostic imaging, cost, degree of invasiveness, the need for intravenous contrast, radiation exposure, and ease of intra-operative access. The availability of computed tomography angiography (CTA), together with the accurate visualization of the aortic root using trans-esophageal echocardiography (TEE), form the standard work-up in the majority of centers and offers a good combination of whole aortic coverage, characterization of dissection severity, and timely imaging (6). In the chronic setting, options for follow-up imaging are less constrained by the need for rapid image acquisition.
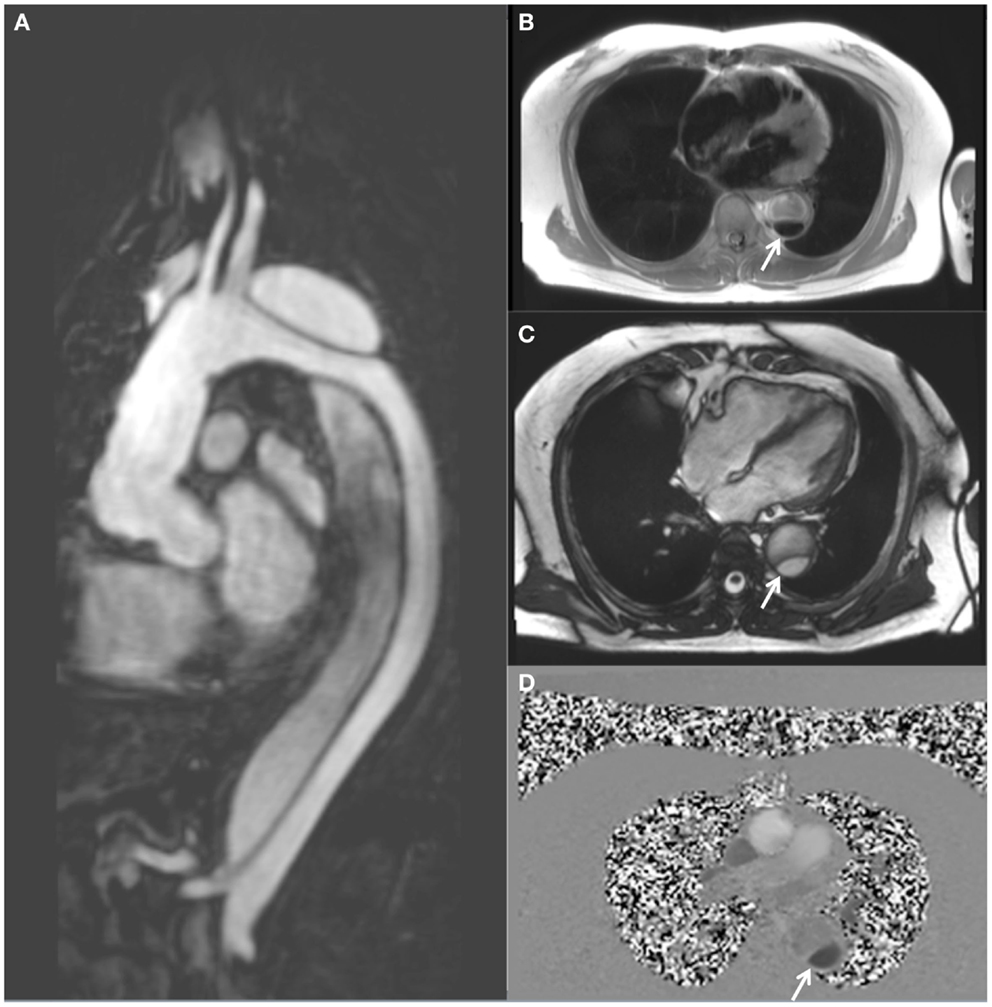
Figure 1. MRI follow-up of a 63-year-old male with chronic descending thoracic aortic dissection. The patient had undergone surgical replacement of the ascending aorta for type A aortic dissection 4 years earlier. (A) Sagittal gadolinium-contrast-enhanced MRA (magnetic resonance angiography) view; (B) axial black blood view of the proximal descending thoracic aorta; (C) axial true FISP (steady state-free precession) cine view; and (D) axial phase-contrast view, showing flow patterns in the true and false lumens of the descending aorta. The true lumen is indicated by the white arrow (Courtesy: Cardiovascular Magnetic Resonance, Sydney, Australia).
This review aims to compare the clinical utility of alternative imaging modalities for the follow-up of chronic aortic dissection by means of systematic review of the current literature, with emphasis upon the advantages and disadvantages of MRI in this setting, and discuss the future role of MRI in assessment of chronic aortic dissection.
We performed a systematic review of the current literature using pre-existing guidelines (16), to ascertain whether in patients with chronic aortic dissection, there is benefit from MRI-based follow-up when compared to other imaging modalities. The Cochrane Central Register of Controlled Trials, MEDLINE, and EMBASE were searched using the terms [(chronic dissection) and (aort*) and (MRI OR magnetic resonance imag*)]. Exclusion criteria were existing topic reviews, studies without a direct comparison of MRI and another imaging modality, and studies not in English. Case reports and conference abstracts were included due to a predicted paucity of available prospective trials.
Twelve studies were included for this review from the literature selection process (summarized in Figure 2). No prospective studies were identified where patients with chronic dissection were randomized to follow-up with MRI or follow-up with another imaging modality. The included studies compared MRI and at least one other imaging modality, namely CT (17–21), TEE (22–26), traditional aortography (21, 27), or intravascular ultrasound (IVUS) (26, 28), in the follow-up of individual patients with heterogeneity in both MRI techniques utilized and reported radiological findings (Table 1). Additionally, the majority of included studies (10 of 12) were published in the year 2000 and earlier, when MRI technology was vastly different to today. Pertinent aspects of the included studies, related to the era of MRI use, are discussed below.
In addition to adequate assessment of FL patency, degree of thrombosis, and aortic diameter, MRI is capable of quantifying potentially prognostic hemodynamic parameters such as complex flow patterns, localized wall shear stress, valvular function, and pulse wave velocity. MRI technology is rapidly advancing, and recent improvements in gradient technology, radiofrequency coils, parallel imaging, pulse sequence design, and post-processing have greatly improved image quality. Despite the relatively rudimentary nature of early (from greater than 10 years ago) MRI studies utilizing low-field strengths (0.5–1.0 T) to assess chronic aortic dissection, results are favorable. Compared with CTA, the assessment of FL partial thrombosis was shown to be comparable (20, 30). Using spin-echo (SE) “black blood” techniques, the near absence of signal from blood flowing at a normal velocity affords a natural contrast that highlights flowing versus static blood in the aorta, in comparison to CTA which requires exogenous contrast to accurately define fluid compartments (20). Clinically useful assessment of FL patency was also demonstrated in early studies using phase-contrast (PC) gradient-echo (GRE) sequences to differentiate thrombus from slow flow (31).
While these early studies highlighted the potential of MRI in imaging aortic dissection, current MRI scanners possess far superior technology and the ability to assess additional dynamic aspects of blood-flow and the aortic wall environment. This key potential advantage that MRI affords over other imaging modalities offers the prospect of developing new prognostic indicators that move beyond the simple measurement of vessel dimensions alone (32).
A typical MRI protocol for imaging of the aorta will include GRE and black blood-weighted images for anatomic definition (Figure 1B). The addition of steady state-free precession cine imaging (true FISP) affords a high blood/tissue contrast without the need for intravenous contrast administration (Figure 1C). Gadolinium-contrast-enhanced magnetic resonance angiography (MRA) (Figure 1A) provides high resolution three-dimensional (3D) data, similar to CT. However uncommon, gadolinium toxicity can nonetheless occur when such intravenous contrast is used, especially in those with pre-existing renal impairment (33).
The ability to assess and quantify blood-flow movement within the aorta is gained when PC imaging is utilized (Figure 1D). The ability to more reliably assess FL thrombosis (compared with CT) is an advantage of MRI in this context as FL thrombosis has been shown to be associated with reduced aortic expansion rates (7, 10, 34). Amano et al. reported their series of 16 chronic thoracic aortic dissection patients who underwent MRI at 1.5 T utilizing cardiac-gating, respiratory compensation, and fat suppression acquisition techniques together with 3D PC imaging of flow patterns (9, 35). They demonstrated that time-resolved 3D MRI may be used to assess the presence of blood-flow within the FL which has previously been shown to be prognostically significant (35, 36). In a group of 70 patients undergoing MRI (1.5 T) following surgically repaired type A aortic dissection, Almeida et al. demonstrated that the initial dimension of the descending thoracic aorta and the non-invasive pulse pressure to be independent predictors of late progression to aneurysm (37). Computational 3D models of descending thoracic aortic dissection have previously demonstrated a positive correlation between wall shear stress and disease progression (38).
An important application of MRI is in the identification of a number of important post-operative conditions. For example, the use of contrast-enhanced 3D MRA can distinguish aortic pseudoaneurysm from periprosthetic hematoma via identified areas of high signal intensity that are indicative of peri-graft flow (39). Furthermore, contrast-enhanced “breath-hold” MRA has been shown to be superior to “black blood” MRI for the assessment of intimal flaps and in assessing aortic branch vessel involvement (40). This is attributed to both the higher spatial resolution and the delineation of a hypointense intimal flap surrounded by contrast-enhanced bright blood (40). This benefit becomes particularly useful when classifying the dissection according to intimal tear location (41). A major advantage of MRI is the existence of multiple processing techniques that all provide specific and unique information. This permits a “problem-based” approach, where a number of different MRI sequences are used to characterize the features of the dissected aorta (17–21, 40).
Blood-flow quantification using PC or velocity mapping can demonstrate bidirectional flow within a FL; such turbulent flow may induce aortic wall shear stress which may be associated with elevated risk of aneurysmal dilatation or tear (22–26, 42). Pressure-dependent movement of a dissection membrane and consequent branch obstruction also indicates that morphologic assessment alone may be insufficient (21, 27, 42). Additionally, MRI affords the ability to accurately assess blood-flow at low velocities and hence differentiate this from FL thrombosis (17).
Echocardiography is generally tolerated well by patients and does not require use of contrast or ionizing radiation. It is also widely available and may be performed at the bedside. It can assess FL flow, FL thrombosis, and FL communication with the true lumen (TL); all significant prognostic markers (26, 28, 34, 43). Assessment of flow using echocardiography is optimal owing to high temporal resolution and is able to quantitate maximum flow velocity. However, the quantitative assessment of flow is limited due to the angular dependence of Doppler measurements, which makes this a highly operator-dependent modality. The recent addition of a 3D component to TEE has helped better quantify entry tear site and size (44). Early reports comparing it with MRI in the setting of chronic aortic dissection showed both modalities to be useful in follow-up, even when limited by inferior technology compared with more modern techniques (24, 25). In several studies examining patients following surgery for acute type A dissection, no difference between MRI and TEE was shown regarding the assessment of persistence and extent of aortic dissection (23). FL flow was better assessed with TEE in some cases, however, slow FL flow was unreliably detected by MRI sequences at this stage of technology development (23). A major limitation of echocardiography is the difficulty in viewing all sections of the thoracic aorta (23–25). MRI, as a cross-sectional modality, gives excellent coverage of the aorta throughout its course. Furthermore, MRI has shown lower inter-observer variability (compared with echocardiography) for aortic diameter measurement, a critical prognostic indicator (45).
In a study in 2000 by Di Cesare et al., 29 patients who had undergone surgery for type A dissection all underwent follow-up imaging with TEE, conventional MRI, and contrast-enhanced 3D breath-hold MRA (22). Imaging follow-up time ranged from 1 to 110 months post-operatively. A high correlation co-efficient was observed for diameter of the descending aorta in all three imaging types, however, TEE showed greater inter-observer variability of measurements made at the distal surgical anastomosis (22). Contrast-enhanced MRA was the most reliable in detection of FL flow; MRI was considered the modality of choice for the follow-up of surgically treated patients with persisting distal aortic dissection (22).
Although TEE can provide useful prognostic information in the acute setting, current recommendations suggest MRI or CT to be more useful for long-term follow-up (5, 43). Given its comparatively low cost, favorable temporal resolution, and bedside utility, previous recommendations have included TEE as a first line follow-up imaging modality in chronic dissection (23), however, this may not be entirely appropriate when measurement of absolute aortic dimensions are of such high importance.
Computed tomography angiography has essentially replaced conventional angiography in the assessment of aortic disease secondary to the reduced associated morbidity, lack of invasiveness, and lower cost associated with this modality. CTA is additionally readily available in the vast majority of clinical centers, and has a high reproducibility and low intra- and inter-operator variability (46). Its drawbacks include the need for iodinated contrast and the exposure to radiation. For patients greater than 60 years of age with normal renal function, the potential negative effects of such radiation exposure may be negligible when compared with the risks of their aortic disease (2), however the increasing recognition of multiple types of genetically defined aortopathies means that for younger patients requiring long-term monitoring, radiation dose may be an important consideration. New advances in reconstruction algorithms, prospective gating, and detector efficiency have permitted large reductions in radiation dose. Although not specific to chronic aortic dissection, this has been demonstrated in several recent reports, where “low radiation” CT assessment of aortic coarctation (47), endoleak (48), and the ascending aorta (49) has been achieved.
The time to complete a CTA examination is relatively short (4–20 s) and the use of electrocardiographic (ECG) gating techniques allows reliable artifact-free imaging of the aortic root and coronary arteries when necessary (5, 50, 51). CTA can show superior visualization of vessel calcification (compared with echocardiography or MRI where it is often observed as artifact or “signal drop-out”) and extremely precise aortic lumen diameter measurements. In the context of endovascular aortic stent-grafts and some mechanical heart valves, CT is deemed as the imaging modality of choice (4). MRI may become feasible in this subset of patients, however, inadequate visualization of stent struts and incompatibility with stainless steel implants remains at present (52). The presence of stainless steel wires used for operative sternal closure additionally inhibits image acquisition due to artifact.
Despite improvements in modern multi-detector CT technology, limited temporal resolution can prevent reliable resolution of rapidly moving structures such as an intimal flap or native valve leaflets (53). Ganten et al. have shown the potential for ECG-gated CTA imaging in chronic aortic dissection patients, particularly regarding determination of vessel distensibility (51). Thirty-two patients with conservatively treated type B dissection showed a reduction in aortic distensibility as measured by CTA (versus healthy age-matched controls), which may be a predisposing factor for dissection or a part of the vascular remodeling process following dissection (51). Such information may prove prognostically useful regarding the progression of aneurysmal disease. The feasibility of other novel CTA measures may also have potential as prognostic markers in chronic aortic dissection, for example, aortic displacement (a potential contributor to vessel wall shear stress) during the cardiac cycle (54), or four-dimensional (4D) CTA to assess aortic pulsatility (50).
As the traditional gold standard (2), aortography has been superseded by the less invasive approaches. Its high specificity and sensitivity are countered by risk of further iatrogenic aortic dissection and need for intravenous iodinated contrast (27, 55). Its use in the chronic dissection setting is difficult to justify given other available modalities. IVUS has similarly been reported to have both high sensitivity and specificity, however, is similarly compromised by its invasiveness (2, 29). The use of positron emission tomography (PET) has also been reported; in the acute setting areas of elevated metabolic activity at freshly disrupted segments of aortic wall show increased uptake of radionuclide tracer (12). In asymptomatic patients with chronic aortic dissection, however, no noticeable uptake is detected, hindering its use as a prognostic indicator in this setting (12). Furthermore, PET has limited spatial resolution, where precise delineation of fine structural detail is not always possible.
The integration of computational fluid dynamics (CFD) as an adjunctive assessor of prognosis in chronic aortic dissection has been explored in several studies (56–58). Such approaches utilize computer-based algorithms involving Newtonian fluid flow and complement MRI to provide specific hemodynamic parameters. A case study from Karmonik et al. of a 45-year-old male with chronic aortic dissection where dual phase MRA and two-dimensional (2D) PC results were compared with CFD studies demonstrated that such simulation could quantify changes in both total pressure and wall shear stress during follow-up using patient-derived data (56). Karmonik’s group have additionally compared such changes with that which occurs in the healthy aorta, albeit with a small case series (n = 2) (58). When using MRI at 1.5 T and CFD, both the ascending aorta and TL diameter increased by a factor of 1.36 times in the chronic dissection patient compared with the patient with a healthy aorta. Abnormal wall shear stress values (considerably lower in the healthy aorta) were attributed to aneurysmal dilatation, rather than simply an increase in FL pressure or increase in the intra-arterial pressure gradient (58). As is noted in these studies, however, CFD simulations describe fixed mechanical forces and effects and do not take into account the multiple biological factors that exist in a native vessel.
Hemodynamic quantification appears to be the major advantage of MRI over other imaging modalities in chronic aortic dissection. Encoding of all three spatial directions of a volumetric data set utilizing 3D velocity-encoded cine MRI relative to the cardiac cycle, is commonly referred to as 4D flow MRI (59). The velocity and direction of aortic blood-flow can be represented as a “streamline” image (Figure 3). A reported MRI at 1.5 T of a single patient with chronic dissection from Müller-Eschner et al. with the use of velocity-colored “streamlines” of blood-flow showed acceleration of flow entering the FL through the primary entry tear (59). This evident vortical flow in the FL may be a further contributing factor to progressive expansion of the FL and aneurysmal dilatation. Maj et al. have similarly published hemodynamic information acquired using time-resolved, contrast-enhanced MRA (60). They show that with a sufficient blood velocity difference between the FL and TL, it is possible to achieve separate contrast enhancement of the dissected luminal channels (60).
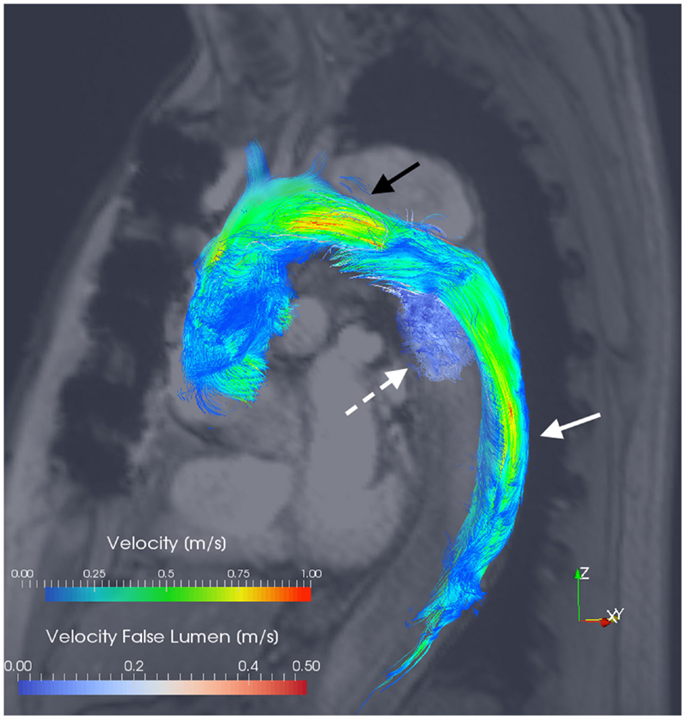
Figure 3. 3D velocity-encoded (4D) view with velocity streamlines of the thoracic aorta of the patient presented in Figure 1. Blood-flow vectors passing through the true lumen (solid white arrow) and the false lumen (dotted white arrow) have been isolated; the reconstructed outline of the entire thoracic aorta is shown. Notably, flow acceleration is observed within the true lumen at the aortic arch (black arrow). Courtesy by Dr. F. Callaghan, Sydney Translational Imaging Laboratory, The Charles Perkins Centre, and The University of Sydney, Sydney, Australia.
Clough et al. have shown excellent accuracy of 4D PC MRI in velocity assessment (when compared with the MRI gold standard of 2D PC MRI) (17). In their series of 12 patients, the utilization of a 4D PC sequence demonstrated a correlation between the rate of rotation of helical blood-flow and the rate of aortic expansion in the FL (17). Francois et al. have similarly demonstrated the feasibility of integration of 4D flow techniques using MRI at 3 T, where the entire thoracic aorta can be imaged in a single acquisition without significant additional scan time (32). Their computation of blood-flow streamlines has allowed the observation of such helicity and vortical blood-flow as well as significantly more retrograde flow in the FL compared with the TL (32). Such approaches raise the potential to derive secondary biomarkers or wall shear stress forces from acquired 4D fields as clinical prognosticators (32, 61). The significance of such observations upon aortic disease progression, risk of future acute events, and ultimately patient mortality and morbidity is yet to be determined.
In the setting of chronic aortic dissection, the ideal imaging modality for use in follow-up has high sensitivity and specificity, is non-invasive, and can accurately identify not only aortic dimensions but also progressive changes in relative flow between true and FLs. MRI can achieve these objectives, as well as the assessment of significant prognostic indicators such as FL thrombosis. CT and echocardiography remain the two most widely used alternatives, given their easy access, rapid acquisition, and non-invasiveness. There is a paucity in the current literature comparing these imaging modalities in the context of chronic aortic disease. In patients requiring multiple studies, the risks of repeated contrast/radiation exposure (especially in the young) and inter-observer variability in assessed aortic dimensions remain a concern. New techniques in MRI include more accurate hemodynamic assessment and the use of 4D imaging to demonstrate blood-flow and potential wall shear stress. However, the relevance of such techniques to long-term prognosis warrants further prospective investigation. Given its advantages, and when available, MRI is suggested as a suitable imaging modality in the follow-up of chronic aortic dissection.
This manuscript was presented in poster format at the 2014 Australian and New Zealand Society of Cardiac and Thoracic Surgeons Annual Scientific Meeting (9–12 November, Gold Coast, Australia).
Cardiovascular Magnetic Resonance Sydney receives sponsorship funding from Siemens Ltd. (Australia). There was no involvement from Siemens in the development or preparation of this manuscript.
The authors wish to acknowledge Dr. Fraser Callaghan, Ph.D. of The Sydney Translational Imaging Laboratory; and the Radiology Department of Royal Prince Alfred Hospital (Sydney, Australia) for the image acquisition, processing of MRI data, and provision of the image used in Figure 3. We also wish to thank Cardiovascular Magnetic Resonance Sydney (www.cmrs.org.au) for the image acquisition and provision of images used in Figure 1.
2D, two-dimensional; 3D, three-dimensional; 4D, four-dimensional; BAV, bicuspid aortic valve; CFD, computational fluid dynamics; CT, computed tomography; CTA, computed tomography angiography; ECG, electrocardiography; FL, false lumen; GRE, gradient-echo; IRAD, international registry of acute aortic dissection; IVUS, intravascular ultrasound; MFS, Marfan syndrome; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; PC, phase-contrast; PET, positron emission tomography; SE, spin-echo; T, Tesla; TAAD, thoracic aortic aneurysm and dissection syndrome; TL, true lumen; TEE, trans-esophageal echocardiography.
1. Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the international registry of acute aortic dissection (IRAD). Eur J Vasc Endovasc Surg (2009) 37(2):149–59. doi:10.1016/j.ejvs.2008.11.032
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, et al. Diagnosis and management of aortic dissection: recommendations of the task force on aortic dissection, European society of cardiology. Eur Heart J (2001) 22(18):1642–81. doi:10.1053/euhj.2001.2782
3. Fattori R, Cao P, De Rango P, Czerny M, Evangelista A, Nienaber C, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol (2013) 61(16):1661–78. doi:10.1016/j.jacc.2012.11.072
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J (2014) 35(41):2873–926. doi:10.1093/eurheartj/ehu281
5. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. Circulation (2010) 121(13):1544–79. doi:10.1161/CIR.0b013e3181d47d48
6. Braverman AC. Acute aortic dissection: clinician update. Circulation (2010) 122(2):184–8. doi:10.1161/CIRCULATIONAHA.110.958975
7. Jonker FHW, Trimarchi S, Rampoldi V, Patel HJ, O’Gara P, Peterson MD, et al. Aortic expansion after acute type B aortic dissection. Ann Thorac Surg (2012) 94(4):1223–9. doi:10.1016/j.athoracsur.2012.05.040
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Elefteriades JA, Lovoulos CJ, Coady MA, Tellides G, Kopf GS, Rizzo JA. Management of descending aortic dissection. Ann Thorac Surg (1999) 67:2002–5. doi:10.1016/S0003-4975(99)00428-2
9. Kirsch M, Legras A, Bruzzi M, Louis N. Fate of the distal aorta after surgical repair of acute DeBakey type I aortic dissection: a review. Arch Cardiovasc Dis (2011) 104(2):125–30. doi:10.1016/j.acvd.2010.11.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Albrecht F, Eckstein F, Matt P. Is close radiographic and clinical control after repair of acute type A aortic dissection really necessary for improved long-term survival? Interact Cardiovasc Thorac Surg (2010) 11(5):620–5. doi:10.1510/icvts.2010.239764
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Blount KJ, Hagspiel KD. Aortic diameter, true lumen, and false lumen growth rates in chronic type B aortic dissection. AJR Am J Roentgenol (2009) 192(5):W222–9. doi:10.2214/AJR.07.3986
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Reeps C, Pelisek J, Bundschuh RA, Gurdan M, Zimmermann A, Ockert S, et al. Imaging of acute and chronic aortic dissection by 18F-FDG PET/CT. J Nucl Med (2010) 51(5):686–91. doi:10.2967/jnumed.109.072298
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Sherrah AG, Vallely MP, Grieve SM, Jeremy RW, Hendel PN, Puranik R. Clinical utility of magnetic resonance imaging in the follow-up of chronic aortic type B dissection. Heart Lung Circ (2014) 23(7):e157–9. doi:10.1016/j.hlc.2014.02.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Moore AG, Eagle KA, Bruckman D, Moon BS, Malouf JF, Fattori R, et al. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: international registry of acute aortic dissection (IRAD). Am J Cardiol (2002) 89:1235–8. doi:10.1016/S0002-9149(02)02316-0
15. Shiga T, Wajima Z, Apfel CC, Inoue T, Ohe Y. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med (2006) 166:1350–6. doi:10.1001/archinte.166.13.1350
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Cook DA, West CP. Conducting systematic reviews in medical education: a stepwise approach. Med Educ (2012) 46(10):943–52. doi:10.1111/j.1365-2923.2012.04328.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Clough RE, Waltham M, Giese D, Taylor PR, Schaeffter T. A new imaging method for assessment of aortic dissection using four-dimensional phase contrast magnetic resonance imaging. J Vasc Surg (2012) 55(4):914–23. doi:10.1016/j.jvs.2011.11.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Bijnens BH, Falaschi B, Pineda V, Cuellar H, Laynez A, Lopez-Lima I, et al. The ratio of the false and true lumen area is more predictive than the diameter for further dilatation of the aorta in chronic dissection. Eur Heart J (2010) 31(Suppl 1):311–2. doi:10.1093/eurheartj/ehq288
19. Rofsky NM, Weinreb JC, Grossi EA, Galloway AC, Libes RB, Colvin SB, et al. Aortic aneurysm and dissection: normal MR imaging and CT findings after surgical repair with the continuous-suture graft-inclusion technique. Radiology (1993) 186:195–201. doi:10.1148/radiology.186.1.8416564
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Grenier P, Pernes JM, Desbleds M-T, DeBrux J-L. Magnetic resonance imaging of aneurysms and chronic dissections of the thoracic aorta. Ann Vasc Surg (1987) 1:534–41. doi:10.1016/S0890-5096(06)61436-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Pernes JM, Grenier P, Desbleds M-T, de Brux JL. MR evaluation of chronic aortic dissection. J Comput Assist Tomogr (1987) 11(6):975–81. doi:10.1097/00004728-198711000-00009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Di Cesare E, Giordano AV, Cerone G, De Remigis F, D’Eusanio G, Masciocchi C. Comparative evaluation of TEE, conventional MRI and contrast-enhanced 3D breath-hold MRA in the post-operative follow-up of dissecting aneurysms. Int J Card Imaging (2000) 16:135–47. doi:10.1023/A:1006404824873
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Cecconi M, La Canna G, Manfrin M, Coletti L, Lanza R, De Pinto F, et al. Postoperative follow-up in type A aortic dissection: comparison of transesophageal echocardiography and magnetic resonance imaging. Cardiovasc Imaging (1997) 9:119–22.
24. Masani ND, Banning AP, Jones RA, Ruttley MST, Fraser AG. Follow-up of chronic thoracic aortic dissection: comparison of transesophageal echocardiography and magnetic resonance imaging. Am Heart J (1996) 131:1156–63. doi:10.1016/S0002-8703(96)90091-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Deutsch HJ, Sechtem U, Meyer H, Theiseen P, Schicha H, Erdmann E. Chronic aortic dissection: comparison of MR imaging and transesophageal echocardiography. Radiology (1994) 192:645–50. doi:10.1148/radiology.192.3.8058928
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Williams DM, Joshi A, Dake MD, Deeb GM, Miller DC, Abrams GD. Aortic cobwebs: an anatomic marker identifying the false lumen in aortic dissection – Imaging and pathologic correlation. Radiology (1994) 190(1):167–74. doi:10.1148/radiology.190.1.8259399
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Maspes F, Gandini R, Pocek M, Mazzoleni C, Fiaschetti V, Ascoli Marchetti A, et al. Breath-hold gadolinium-enhanced three-dimensional MR angiography: personal experience in the thoracoabdominal district. Radiol Med (1999) 98(4):275–82.
28. Yamada T, Tada S, Harada J. Aortic dissection diagnosis without intimal rupture: diagnosis with MR imaging and CT. Radiology (1988) 168:347–52. doi:10.1148/radiology.168.2.3393653
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Yamada E, Matsumura M, Kyo S, Omoto R. Usefulness of a prototype intravascular ultrasound imaging in evaluation of aortic dissection and comparison with angiographic study, transesophageal echocardiography, computed tomography, and magnetic resonance imaging. Am J Cardiol (1995) 75:161–5. doi:10.1016/S0002-9149(00)80067-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Gaubert J-Y, Moulin G, Mesana T, Chagnaud C, Caus T, Delannoy L, et al. Type A dissection of the thoracic aorta: use of MR imaging for long-term follow-up. Radiology (1995) 196:363–9. doi:10.1148/radiology.196.2.7617845
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Di Cesare E, Costanzi A, Fedele F, Di Renzi P, D’Eusanio G, Lupattelli L, et al. MRI postoperative monitoring in patients surgically treated for aortic dissection. Magn Reson Imaging (1996) 14(10):1149–56. doi:10.1016/S0730-725X(96)00221-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Francois CJ, Markl M, Schiebler ML, Niespodzany E, Landgraf BR, Schlensak C, et al. Four-dimensional, flow-sensitive magnetic resonance imaging of blood flow patterns in thoracic aortic dissections. J Thorac Cardiovasc Surg (2013) 145(5):1359–66. doi:10.1016/j.jtcvs.2012.07.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Do C, Barnes JL, Tan C, Wagner B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol (2014) 307(7):F844–55. doi:10.1152/ajprenal.00379.2014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Sueyoshi E. Growth rate of aortic diameter in patients with type B aortic dissection during the chronic phase. Circulation (2004) 110(11 Suppl 1):II–256–II–261. doi:10.1161/01.CIR.0000138386.48852.b6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Amano Y, Sekine T, Suzuki Y, Tanaka K, Takagi R, Kumita S. Time-resolved three-dimensional magnetic resonance velocity mapping of chronic thoracic aortic dissection: a preliminary investigation. Magn Reson Med Sci (2011) 10(2):93–9. doi:10.2463/mrms.10.93
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Tsai TT, Evangelista A, Nienaber CA, Myrmel T, Meinhardt G, Cooper JV, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med (2007) 357:349–59. doi:10.1056/NEJMoa063232
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Almeida AG, Nobre AL, Pereira RA, Costa-Pereira A, Tavares C, Cravino J, et al. Impact of aortic dimensions and pulse pressure on late aneurysm formation in operated type A aortic dissection. A magnetic resonance imaging study. Int J Card Imaging (2008) 24(6):633–40. doi:10.1007/s10554-008-9296-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Rudenick PA, Bordone M, Bijnens BH, Soudah E, Oñate E, Garcia-Dorado D, et al. Influence of tear configuration on false and true lumen haemodynamics in type B aortic dissection. Conf Proc IEEE Eng Med Biol Soc (2010) 2010:2509–12. doi:10.1109/IEMBS.2010.5626689
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Garcia A, Ferreiros J, Santamaria M, Bustos A, Abades JL, Santamaria N. MR angiographic evaluation of complications in surgically treated type A aortic dissection. Radiology (2006) 26(4):981–92. doi:10.1148/rg.264055082
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Kunz RP, Oberholzer K, Kuroczynski W, Horstick G, Krummenauer F, Thelen M, et al. Assessment of chronic aortic dissection: contribution of different ECG-gated breath-hold MRI techniques. AJR Am J Roentgenol (2004) 182:1319–26. doi:10.2214/ajr.182.5.1821319
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Dake MD, Thompson M, van Sambeek M, Vermassen F, Morales JP, Investigators TD. DISSECT: a new mnemonic-based approach to the categorization of aortic dissection. Eur J Vasc Endovasc Surg (2013) 46(2):175–90. doi:10.1016/j.ejvs.2013.04.029
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Strotzer M, Aebert H, Lenhart M, Nitz W, Wild T, Manke C, et al. Morphology and hemodynamics in dissection of the descending aorta. Acta Radiol (2000) 41(6):594–600. doi:10.1080/028418500127345965
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Evangelista A, Flachskampf FA, Erbel R, Antonini-Canterin F, Vlachopoulos C, Rocchi G, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr (2010) 11(8):645–58. doi:10.1093/ejechocard/jeq056
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
44. Evangelista A, Aguilar R, Cuellar H, Thomas M, Laynez A, Rodriguez-Palomares J, et al. Usefulness of real-time three-dimensional transoesophageal echocardiography in the assessment of chronic aortic dissection. Eur J Echocardiogr (2011) 12(4):272–7. doi:10.1093/ejechocard/jeq191
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Bhatla P, Nielsen JC. Cardiovascular magnetic resonance as an alternate method for serial evaluation of proximal aorta: comparison with echocardiography. Echocardiography (2013) 30(6):713–8. doi:10.1111/echo.12105
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
46. Sharma UK, Gulati MS, Mukhopadhyay S. Aortic aneurysm and dissection: evaluation with spiral CT angiography. JNMA J Nepal Med Assoc (2005) 44:8–12.
47. Xu J, Zhao H, Wang X, Bai Y, Liu L, Liu Y, et al. Accuracy, image quality, and radiation dose of prospectively ECG-triggered high-pitch dual-source CT angiography in infants and children with complex coarctation of the aorta. Acad Radiol (2014) 21(10):1248–54. doi:10.1016/j.acra.2014.04.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
48. Koike Y, Ishida K, Hase S, Kobayashi Y, Nishimura J, Yamasaki M, et al. Dynamic volumetric CT angiography for the detection and classification of endoleaks: application of cine imaging using a 320-row CT scanner with 16-cm detectors. J Vasc Interv Radiol (2014) 25(8):1172–80. doi:10.1016/j.jvir.2014.03.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Liu Y, Xu J, Li J, Ren J, Liu H, Xu J, et al. The ascending aortic image quality and the whole aortic radiation dose of high-pitch dual-source CT angiography. J Cardiothorac Surg (2013) 8(228):1–5. doi:10.1186/1749-8090-8-1
50. Weber TF, Ganten MK, Boeckler D, Geisbuesch P, Kauczor HU, Tengg-Kobligk von H. Heartbeat-related displacement of the thoracic aorta in patients with chronic aortic dissection type B: quantification by dynamic CTA. Eur J Radiol (2009) 72(3):483–8. doi:10.1016/j.ejrad.2008.07.045
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
51. Ganten MK, Weber TF, Tengg-Kobligk von H, Boeckler D, Stiller W, Geisbuesch P, et al. Motion characterization of aortic wall and intimal flap by ECG-gated CT in patients with chronic B-dissection. Eur J Radiol (2009) 72(1):146–53. doi:10.1016/j.ejrad.2008.06.024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
52. Grabenwoger M, Alfonso F, Bachet J, Bonser R, Czerny M, Eggebrecht H, et al. Thoracic endovascular aortic repair (TEVAR) for the treatment of aortic diseases: a position statement from the EACTS and the ESC, in collaboration with the EAPCI. Eur Heart J (2012) 33(13):1558–63. doi:10.1093/eurheartj/ehs074
53. Silverman JM, Raissi S, Tyszka JM, Trento A, Herfkens RJ. Phase-contrast cine MR angiography detection of thoracic aortic dissection. Int J Card Imaging (2000) 16:461–70. doi:10.1023/A:1010781305922
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
54. Weber TF, Ganten MK, Boeckler D, Geisbuesch P, Kopp-Schneider A, Kauczor HU, et al. Assessment of thoracic aortic conformational changes by four-dimensional computed tomography angiography in patients with chronic aortic dissection type B. Eur Radiol (2008) 19(1):245–53. doi:10.1007/s00330-008-1103-x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
55. Razavi M. Acute dissection of the aorta: options for diagnostic imaging. Cleve Clin J Med (1995) 62(6):360–5. doi:10.3949/ccjm.62.6.360
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
56. Karmonik C, Partovi S, Müller-Eschner M, Bismuth J, Davies MG, Shah DJ, et al. Longitudinal computational fluid dynamics study of aneurysmal dilatation in a chronic DeBakey type III aortic dissection. J Vasc Surg (2012) 56(1):260–3. doi:10.1016/j.jvs.2012.02.064
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Karmonik C, Partovi S, Davies MG, Bismuth J, Shah DJ, Bilecen D, et al. Integration of the computational fluid dynamics technique with MRI in aortic dissections. Magn Reson Med (2012) 69(5):1438–42. doi:10.1002/mrm.24376
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
58. Karmonik C, Müller-Eschner M, Partovi S, Geisbusch P, Ganten MK, Bismuth J, et al. Computational fluid dynamics investigation of chronic aortic dissection hemodynamics versus normal aorta. Vasc Endovascular Surg (2013) 47(8):625–31. doi:10.1177/1538574413503561
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
59. Müller-Eschner M, Ren J, Partovi S, Unterhinninghofen R, Böckler D, Ley S, et al. Tridirectional phase-contrast magnetic resonance velocity mapping depicts severe hemodynamic alterations in a patient with aortic dissection type Stanford B. J Vasc Surg (2011) 54(2):559–62. doi:10.1016/j.jvs.2011.02.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
60. Maj E, Cieszanowski A, Rowinski O, Wojtaszek M, Szostek M, Tworus R. Time-resolved contrast-enhanced MR angiography: value of hemodynamic information in the assessment of vascular diseases. Pol J Radiol (2010) 75(1):52–60.
61. Meierhofer C, Schneider EP, Lyko C, Hutter A, Martinoff S, Markl M, et al. Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: a prospective study. Eur Heart J Cardiovasc Imaging (2013) 14(8):797–804. doi:10.1093/ehjci/jes273
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: chronic aortic dissection, aortic type B dissection, aortic type A dissection, magnetic resonance imaging, follow-up
Citation: Sherrah AG, Grieve SM, Jeremy RW, Bannon PG, Vallely MP and Puranik R (2015) MRI in chronic aortic dissection: a systematic review and future directions. Front. Cardiovasc. Med. 2:5. doi: 10.3389/fcvm.2015.00005
Received: 26 September 2014; Paper pending published: 21 October 2014;
Accepted: 05 February 2015; Published online: 19 February 2015.
Edited by:
Sebastian Kelle, German Heart Institute Berlin, GermanyReviewed by:
Peter Bernhardt, University of Ulm, GermanyCopyright: © 2015 Sherrah, Grieve, Jeremy, Bannon, Vallely and Puranik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajesh Puranik, RPAH Medical Centre, Suite 401, Newtown, NSW 2042, Australia e-mail:cmFqLnB1cmFuaWtAY21ycy5vcmcuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.