- 1Primary Care Unit, Department of Public Health and Primary Care, University of Cambridge, Cambridge, United Kingdom
- 2Department of Psychology, University of Cambridge, Cambridge, United Kingdom
- 3School of Clinical Medicine, University of Cambridge, Cambridge, United Kingdom
- 4Wolfson Institute of Population Health, Queen Mary University of London, London, United Kingdom
- 5School of Cancer and Pharmaceutical Sciences, King's College London, London, United Kingdom
Introduction: New technologies and innovations are emerging that enable stratification of individuals based on their risk of cancer and enable screening or diagnostic investigations to be targeted to those at greatest need. This study aimed to explore, in depth, attitudes of the UK public toward this concept; specifically, anticipated acceptability and uptake, including barriers and enablers toward uptake.
Methods: A survey was completed independently by a representative population sample and alongside a researcher in think aloud interviews. Participants considered three of six exemplars of innovations that enable risk assessment: polygenic risk scores, geodemographic segmentation, continuous biomarker monitoring, minimally invasive tests, artificial intelligence analysis of medical records, and wearable devices. Questions about likelihood of taking up the risk assessment, acceptability of risk-stratified healthcare, and comfort about risk results being used within healthcare generally were set in asymptomatic then symptomatic scenarios. Descriptive statistics and multivariable logistic regression were used to explore differences between the exemplars and contexts and the impact of individual characteristics. Interviews were analyzed using codebook thematic analysis guided by the Theoretical Framework of Acceptability. Free-text comments were also analyzed thematically.
Results: 999 participants completed the survey independently and 21 participants completed interviews. Most were extremely or somewhat likely to take up risk assessments, ranging from 62.0% for geodemographic segmentation to 85.2% for minimally invasive tests in the asymptomatic scenario, and from 64.2% for geodemographic segmentation to 94.0% for minimally invasive tests in the symptomatic scenario. Acceptability of using the exemplars within risk-stratified screening or referral pathways followed a similar pattern, as did comfort with the results being used widely. Qualitative analyses showed that the innovations and risk-based approach were viewed as proactive and logical. Tests requiring low burden were preferred, although most participants did not consider the burden of any of the innovations to be too high, particularly in the symptomatic context.
Conclusions: Risk-based innovations for cancer early detection are intuitive. Study participants would be likely to engage and support their use for risk stratification, particularly for decisions about symptom investigations. These findings justify and promote ongoing research to develop these technologies and highlight features that increase public acceptability.
1 Introduction
There is a growing interest in the utilization of new technologies in cancer research, motivated both by the “push” of healthcare needs but also the “pull” of technological advances. The burden of cancer is projected to continue to increase and healthcare resources are continually under pressure (1, 2). Early detection and prevention of cancer is, therefore, a policy priority as well as pertaining to individual benefits (3, 4). Through risk stratification, screening tests and tests to investigate symptoms that are possibly indicative of cancer can be allocated according to risk, with screening at an increased intensity or urgent and more invasive tests offered to those at higher risk of cancer, and screening at a reduced intensity or more routine tests offered to those at lower than population-level risk of cancer. This can benefit individuals through earlier diagnosis, improved decision making, and avoidance of interventions that are unlikely to be necessary. Clinicians may also benefit through improved decision making, and service providers through increased efficiency in resource use, decreased costs and improved service delivery (5).
New technologies and innovations have the potential to significantly impact healthcare, including in the field of early cancer detection and diagnosis (6, 7). Such developments include tests and sensors for (novel) cancer biomarkers, smartwatches, robotics, and new materials for wearable sensors (6–15). For instance, nanomaterial-enabled optical biosensors have the potential to detect cancer biomarkers at the low levels present in early-stage disease (14). Furthermore, the UK government recently outlined how wearable devices could contribute to transforming how people monitor their health (16). Many of these technologies utilize artificial intelligence (AI) (such as machine learning, deep learning or large language modeling) to analyse and summarize the data they collect (6, 17, 18). Amid the host of technological and governance issues that must be addressed, it is hoped that such technologies can improve individual healthcare and reduce inequality in access and outcomes (7). In this study, we consider the use of such innovations to calculate individual cancer risk and, in turn, inform risk stratification.
In addition to evidence of cost effectiveness, efficacy and acceptability to healthcare providers, innovations that enable risk assessment must meet the needs of and be acceptable to members of the public. This is needed in order to maintain positive population perspectives toward health services and ensure engagement with innovations. A growing body of research evidences high public acceptability toward risk-stratified cancer screening, although concerns about reduced screening for those with lower cancer risk persist (19, 20). The studies included in these reviews typically focus on phenotypic and/or genetic risk prediction methods. Public attitudes toward unfamiliar and novel technologies are unknown, and studies evaluating specific innovations are yet to be conducted.
Consequently, the overall aim of this study was to explore the receptiveness of members of the public to the concept of using risk-based innovations to inform risk stratification in asymptomatic and symptomatic contexts using a set of exemplars. Specifically, we aimed (1) to quantify public attitudes toward innovations for risk assessment and describe how these are influenced by individual level characteristics and beliefs about risk of cancer in the survey; (2) to understand participants' reasons for their likelihood of taking up the offer of each risk-based innovation based on the free text survey data; and (3) to understand public attitudes to risk-based innovations in depth at an individual level and identify key barriers and enablers toward uptake in think aloud interviews.
2 Methods
2.1 Study design
This paper reports the findings of a survey that was completed independently online by a population-based sample and in the context of an interview by a separate sample that completed the same survey whilst thinking out loud. This multi-methods approach was used to gain understanding of representative public attitudes plus in-depth insights toward these perspectives (21, 22). Ethical approval was obtained from the University of Cambridge Psychology Research Ethics Committee (PRE.2023.064). Data were collected in August/September 2023.
2.2 Survey design
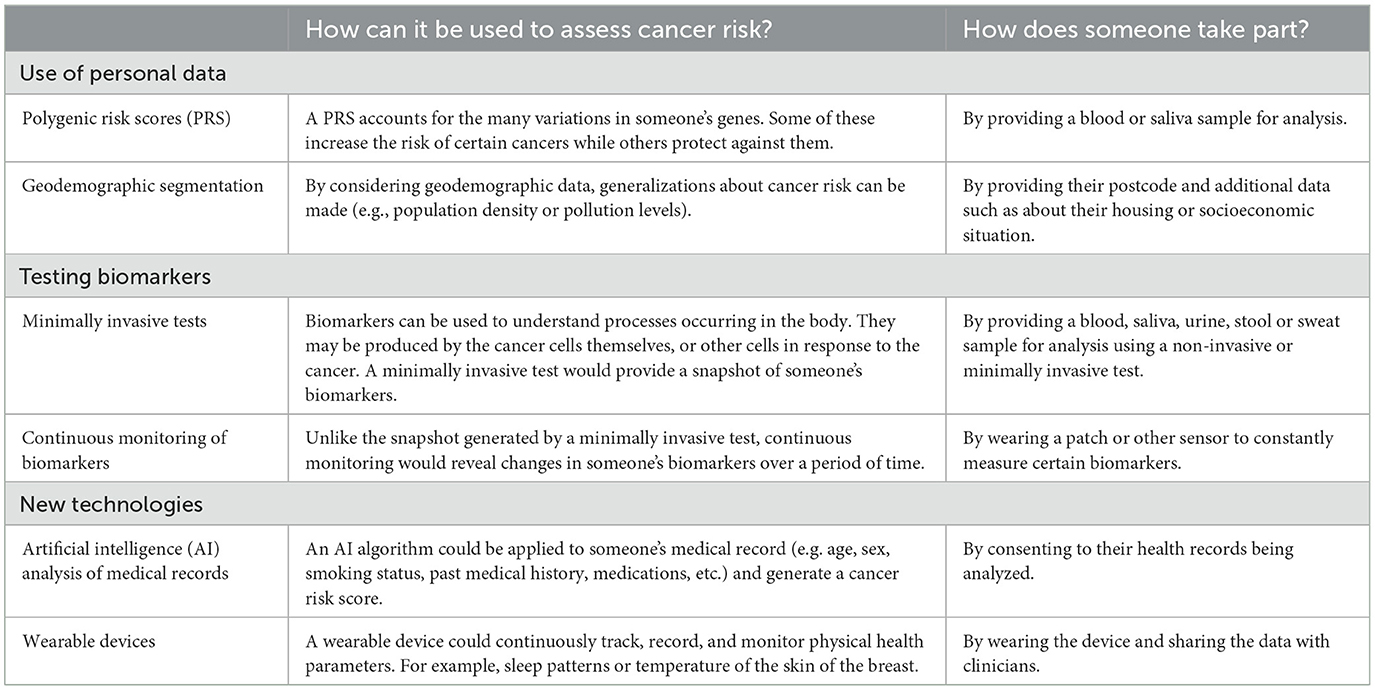
In the main survey, participants answered a set of questions on one exemplar of risk-based innovations from each of three categories (use of personal data, testing biomarkers, and new technologies) (Table 1). The six possible exemplars were polygenic risk scores (PRS), geodemographic segmentation, minimally invasive tests, continuous monitoring of biomarkers, artificial intelligence (AI) analysis of medical records, and wearable devices. The exemplars had been identified and prioritized using a survey with 20 experts or academics in the field to reflect a diversity of emerging technologies that are relevant to screening, early detection and diagnosis in multiple cancer types, as described elsewhere (Dennison et al., unpublished).1 One exemplar from each category was used and the three exemplars were displayed in a random order. A similar number of responses were collected on each exemplar.
For each exemplar, participants were provided with a short video clip that described the innovation, how it could be used to assess cancer risk and what providing the data/taking part would involve. They had the option to read the transcript if they did not want to watch the video, and all participants were provided with the same information in a written text descriptor (summarized in Table 1).
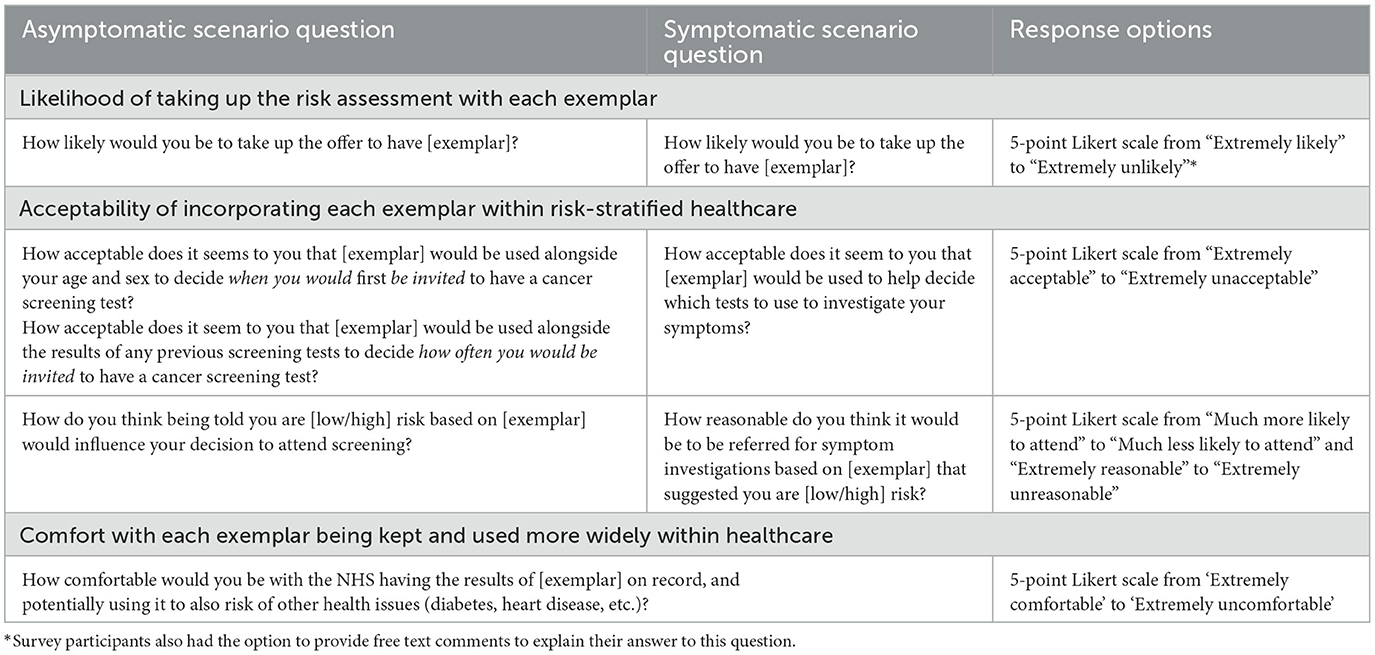
The questions were presented in the form of scenarios. First, participants were asked to imagine that they felt fine (that they had no symptoms of cancer) and were invited to take part in a risk assessment using the innovation and that the risk result could inform cancer screening. Second, they were asked to imagine that they had a vague symptom that could have various causes (that they had lost weight without trying) and were invited to take part in a risk assessment using the innovation so that the general practitioner (GP) could use the risk result to help decide which tests to use to investigate the symptom. As shown in Table 2, outcomes included the likelihood of taking up the risk assessment, the acceptability of risk-stratified healthcare and, lastly, how comfortable they would be with the innovation being used within healthcare more generally. Five-point Likert response scales were used throughout. Survey participants were also given the option to explain their reasons for being likely or unlikely to take up the offer of each risk assessment by providing a free text response to the question “In a few words, why?”.
Before these questions, participants were given a short description of the context for the study. At the end of the survey, participants provided demographic information and completed measures about lifestyle and screening history, thoughts and beliefs about cancer, and attitudes toward online privacy. A copy of the survey is available via the repository (see Data availability statement).
2.3 Participants and recruitment
For the online survey, a sample of ~1,000 participants was recruited through an online participant recruitment platform. A sample of 958 participants would enable us to estimate 66% likelihood of uptake of risk-based innovations with a 95% confidence level and 3% error margin. Participants were resident in the UK and representative of the UK population in terms of age, sex, ethnicity, and socioeconomic status. The first 600 participants were recruited by age, sex and ethnicity using the recruitment platform's representative sample feature. The sample was then enriched with 400 participants of lower socioeconomic status (self-reported socioeconomic decile 1–3 based on the response to the question “Where would you put yourself on the socioeconomic ladder?” previously given on the recruitment platform) using the recruitment platform's pre-screeners to match the demographics of the UK population.
For the interview study, a separate sample of 21 adult participants were recruited using purposeful sampling by a market research company. Based on our experience of conducting qualitative research on similar topics, this sample size was anticipated to meet the concept of information power (considering the study aim, sample specificity, use of theory, dialogue quality, and analysis method) (23). Participants with a variety of ages, socioeconomic backgrounds, ethnicities and both sexes were sought. We also interviewed two participants with a previous cancer diagnosis and three interviews used an interpreter with participants who would not have taken part in English; these participants were identified from South Asian communities with which the recruitment company had existing connections. People with medical expertise were not approached to take part.
All participants were compensated for their time at the rate recommended by the recruitment agencies. Survey participants read the study information and gave informed written consent at the start of the survey; consent was obtained prior to the interviews by the recruitment agency.
2.4 Data collection
The survey was hosted by Qualtrics and survey study participants completed it independently. The interviews took place using the screensharing function on Zoom video conferencing, which was also used to video-record them. One or two researchers conducted each interview. After ensuring they understood the purpose of and procedure for the interview, the researchers encouraged the participants to work through the survey whilst verbalizing their thoughts on the information provided, reasons for their answers to the questions, etc. English-language interviews lasted for up to 1 h, and non-English-language interviews lasted for 1.5 h. If necessitated by time restrictions, participants were able to complete two exemplars within the survey rather than three.
2.5 Analysis
2.5.1 Quantitative analysis
All quantitative analyses were performed using Stata 15. Descriptive statistics were used to summarize the characteristics of the participants and their responses to each of the three key outcomes for each of the six exemplars and in both the asymptomatic and symptomatic contexts, plus the impact of a low- or high-risk result on perspectives toward risk stratification. For analysis of the acceptability of incorporating each exemplar within risk-stratified screening, one variable was created based on the mean responses to the questions about screening frequency and starting age. Differences between asymptomatic and symptomatic scenarios were assessed using the Wilcoxon signed-rank non-parametric test to identify the significance of asymptomatic/symptomatic context on views within the sample.
Multivariable logistic regression was used to explore differences between the six exemplars within asymptomatic and symptomatic contexts and the impact of binary individual level characteristics: age (older vs. younger than 40 years), sex (female vs. male), smoking status (ever smoker vs. never smoker), ethnicity (ethnic minority vs. white), education level (university level vs. below university level), socioeconomic status decile (higher vs. lower socioeconomic status i.e. deciles 4–10 vs. 1–3), prior history of cancer (yes vs. no), technology use (at least one of using an app, tracking device or wearable daily vs. no use or use of only an electronic device daily), and perceived risk of cancer (likely vs. neither likely nor unlikely or unlikely). In all these analyses the outcomes were dichotomised by combining ‘extremely' with ‘somewhat'. The order in which exemplars were presented and an interaction between the exemplar and the order were also included.
Differences between the six exemplars are presented as predicted probabilities ±95% confidence interval using the -margins- command. The impact of individual characteristics on likelihood of uptake are presented as odds ratios ±95% confidence interval.
2.5.2 Qualitative free-text comment analysis
The free-text comments were analyzed thematically following a previously used approach (24) using Microsoft Excel spreadsheets. The comments were analyzed in 12 groups according to the dichotomy described above, and for each of the six exemplars of innovations. Comments given in asymptomatic and symptomatic scenarios were combined.
First, any comments that were generic to cancer, screening, symptoms, and/or risk assessments (that is, not specific to the innovation) or were inconsistent with the participant's previous answer (such as if they answered “very unlikely” to take part but provided a free text answer suggesting they would take up the test) were excluded.
For each group, at least two researchers familiarized themselves with a subset of the comments (20% of each group) and suggested a list of potential codes. The researchers met to agree initial code lists then piloted these on a subset of the comments (20%). After comparing and reviewing the coding decisions, they developed consolidated code lists for each group, repeating the codes across groups where possible. The remaining free text comments were then coded into the consolidated lists. Each comment was coded by two researchers and any discrepancies were discussed to reach an agreement. If a comment contained multiple meanings, it was assigned to as many codes as appropriate to cover the content.
Individual codes were then mapped into overarching themes based on the Theoretical Framework of Acceptability (TFA) (25). Four of the TFA domains were relevant—affective attitude, burden, ethicality and perceived effectiveness. A fifth domain, anticipated benefits and costs, combined the opportunity costs and anticipated benefits domains. No comments related to intervention coherence or self-efficacy. Summaries (definitions) of each code were developed and the proportion of included comments relating to each TFA domain within each exemplar was presented.
2.5.3 Qualitative interview analysis
Recordings of the interviews were transcribed verbatim. For the interviews completed in multiple languages, only the parts in English were transcribed (i.e., the participant's words that were spoken through the interpreter). Data analysis was conducted using codebook thematic analysis (26), using NVivo 12 software. An initial deductive coding frame was developed based on the six exemplars of risk-based innovations and the structure of the survey, and further codes were added inductively based on participants' responses. An initial sample of the transcripts was coded by two researchers to develop and refine this coding frame. The remaining transcripts were coded by one researcher. After summarizing and reviewing each code, we defined themes according to key and repeating concepts. These were guided by the TFA where applicable (25).
3 Results
3.1 Participant characteristics and survey responses
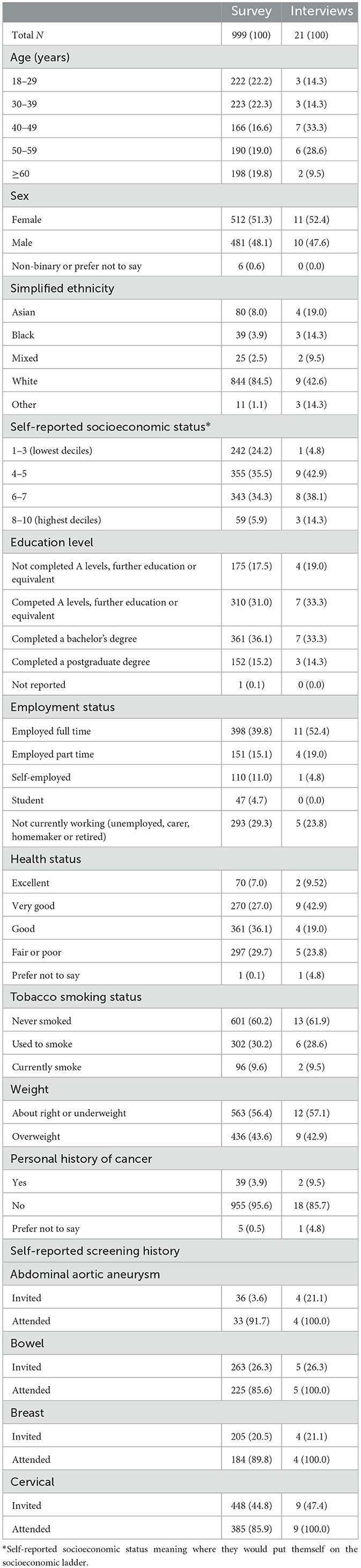
The survey was live for ~80 h. In that time, 1,052 individuals registered with the recruitment platform accessed the survey, and 999 completed the survey and their responses were included (41 individuals withdrew, 11 individuals timed-out, and one was excluded because they completed the survey exceptionally quickly in 3 min). The 999 participants took a median 18 min 45 s to complete the survey (IQR 14 min 57 s to 25 min 10 s). A total of 21 interviews were also conducted: 18 were completed in English, two in Bengali, and one in Indian Punjabi.
Participant demographics are shown in Table 3. Reflecting the sampling strategy, survey participants were largely representative of the UK population with respect to age, sex, ethnicity, and self-reported socioeconomic status. There was a greater proportion of interview participants aged 40–59 years, and nine participants reported White ethnicity and the remaining 12 participants were of Asian, Black or mixed ethnicity, or another ethnicity. Only one interview participant reported socioeconomic status 1–3, although education level and employment distributions were similar across the samples. All interview participants attended screening that they had been invited to, while attendance ranged from 85.6 to 91.7% of survey respondents, depending on the screening programme. Thoughts and beliefs about cancer and screening were also comparable between samples (Supplementary Table 1), but interview participants had more familiarity with health apps and more positive attitudes toward technology and use of data (Supplementary Table 2).
All but one participant provided at least one free-text comment to explain their likelihood of taking up the risk assessment in both asymptomatic and symptomatic scenarios. Across the six risk-based innovations, a total of 4,616 comments were given to explain why someone was likely to take up the risk assessment (positive comments) and 1,342 comments were given to explain why someone was unlikely to take up the risk assessment (negative comments). 3,536 comments were omitted because they were inconsistent with the stated likelihood of taking up the risk assessment (3.7% comments), were too vague to classify their meaning (47.1% comments), or were not specific to risk assessment using the innovation in question (49.2% comments). This included comments that the participant would do whatever their GP recommended to benefit their health, stating the aim of early diagnosis, or that they did not want any risk assessment.
In the remainder of the Results section, we report the findings of the survey in relation to the three main outcomes: (Section 3.2) likelihood of taking up the risk assessment (plus explanations of reasons for likelihood based on free text comments, considering each innovation in turn), (Section 3.3) acceptability of risk-stratified healthcare, and (Section 3.4) comfort with the risk assessment being kept on record. Results of the think aloud interviews relating to barriers and enablers of risk-based innovations are then described (Section 3.5).
3.2 Likelihood of taking up the risk assessment
The majority of survey participants responded that they would be extremely or somewhat likely to take up the offer of risk assessment with each of the exemplars both in asymptomatic and symptomatic scenarios, as summarized in Figure 1A (with unadjusted results shown in Supplementary Table 3A). The percentage of participants likely to take up risk assessment in the asymptomatic scenario ranged from 62.0% for geodemographic segmentation to 85.2% for minimally invasive tests, and from 64.2% for geodemographic segmentation to 94.0% for minimally invasive tests in the symptomatic scenario. However, more than one in five participants would be unlikely to take up geodemographic segmentation, AI or wearable devices in the asymptomatic scenario or geodemographic segmentation in the symptomatic scenario. Across all exemplars, participants indicated greater likelihood of taking up the offer of risk assessment in the symptomatic scenario than in the asymptomatic scenario (p ≤ 0.001 across the five-point Likert scale).
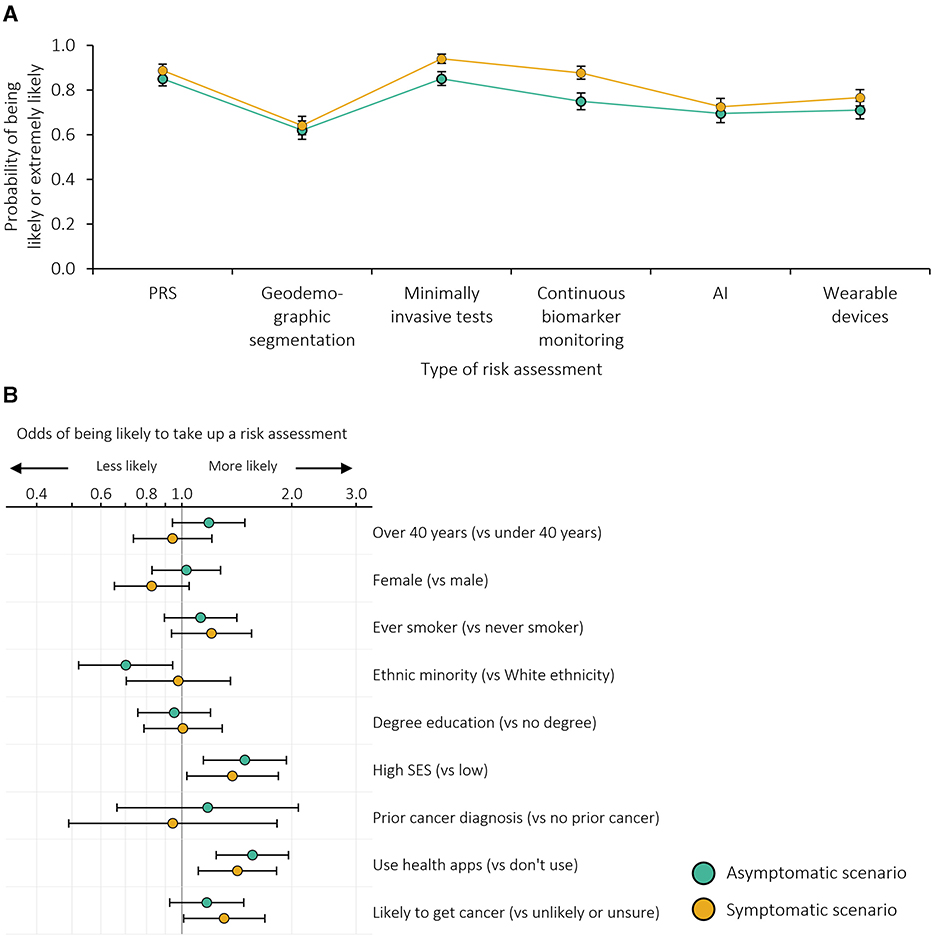
Figure 1. Likelihood of taking up the risk assessment: (A) probability of being likely to take up each risk assessment adjusted for individual demographics and the order in which the exemplars were presented; (B) odds ratios for being more or less likely to be likely to take up any risk assessment. n = 999 survey participants. Error bars represent 95% confidence intervals. SES, socioeconomic status. Based on dichotomised outcomes (i.e. likely or extremely likely to take up the risk assessment vs. neither likely nor unlikely, somewhat unlikely or extremely unlikely).
The order in which participants viewed different risk assessments impacted how likely they were to take it up (Supplementary Figure 1A). Of note, geodemographic segmentation tended to be more likely to be taken up when it was considered first, rather than when it was the second or third exemplar seen.
The impact of participant characteristics on likelihood of uptake across all exemplars is shown in Figure 1B. Participants from ethnic minority groups were less likely to take up risk assessment in the asymptomatic scenario (p = 0.019). Those with higher socioeconomic status and greater technology use were more likely to take up risk assessment in both the asymptomatic (p = 0.003 and p < 0.001, respectively) and symptomatic scenarios (p = 0.030 and p = 0.005, respectively). The impact of personal characteristics differed somewhat by exemplar (Supplementary Table 4A); for example, in the symptomatic scenario, the association with familiarity with health apps was only statistically significant when applied to wearable devices (p < 0.001).
3.2.1 Reasons for the likelihood of taking up the risk assessment across different exemplars
An explanation of how each TFA domain contributed to participants' reasoning, with illustrative quotations, and the proportion of participants citing each reason are presented in Supplementary Table 5, Figure 2, respectively. Notably, several of the concepts applied both positively and negatively. For example, some participants were likely to take up PRS, minimally invasive tests and continuous monitoring of biomarkers because they considered them trustworthy and reliable whereas other participants would not want to take them up because they considered them not trustworthy or reliable enough. Similarly, some participants would take up wearable devices and continuous monitoring of biomarkers because they required low effort (describing them as easy, non-invasive, and convenient) whereas other participants thought they were intrusive and required too much effort (describing them as burdensome and invasive).
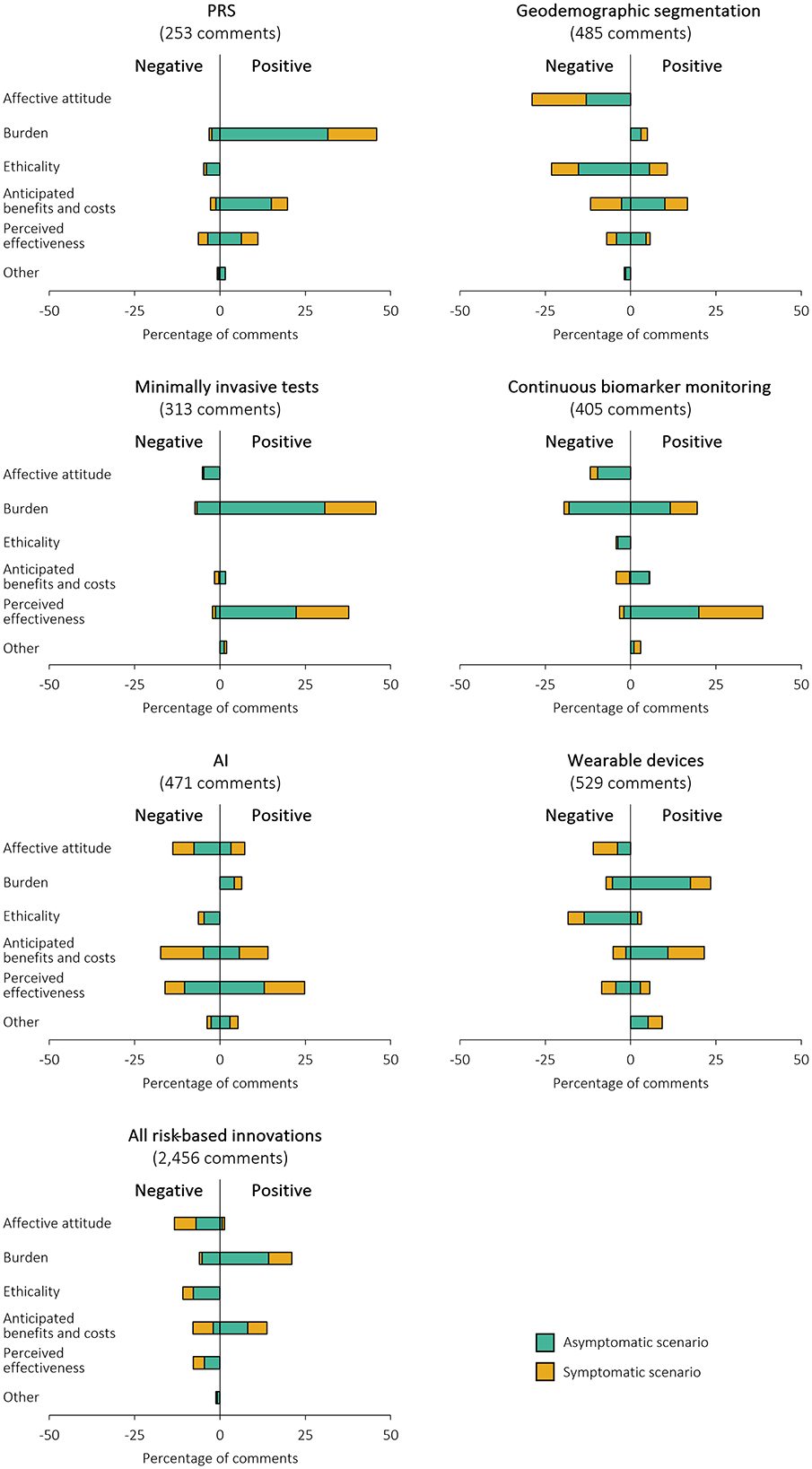
Figure 2. Percentage of positive and negative free text comments included in each domain of the TFA to describe survey participants' reasons for the likelihood of taking up the risk assessment. Percentage of comments add up to more than 100% as some comments contained multiple meanings. Positive comments indicate reasons for being likely to take up the risk assessment; negative comments indicate reasons for being unlikely to take up the risk assessment.
3.2.1.1 PRS
The most common reason for taking up PRS was the low burden and simplicity of providing a saliva or blood sample, referred to in 46% of the included comments (n = 116). Conversely, the perceived effectiveness (mistrust of PRS algorithms, methodology or technology), ethicality (concerns about the storage and sharing of genetic data) and anticipated costs (potential to be penalized for “good genes” with fewer screening or investigative tests) were all suggested reasons for not taking part.
3.2.1.2 Geodemographic segmentation
Several different benefits were anticipated with regards to geodemographic segmentation: personal interest or reassurance, to aid their GP in understanding their health, and contributing to research and helping others. This domain amounted to 17% of the included, non-generic comments (n = 81). Other participants said that they were simply willing to share geodemographic information (11% comments [n = 52]) but over twice as many participants saw geodemographic information as irrelevant or too impersonal to be useful for cancer risk prediction (29% comments [n = 140]).
3.2.1.3 Minimally invasive tests
Reasons for taking up minimally invasive tests tended to either be associated with the low burden or perceived effectiveness (particularly that biomarkers were reliable and accurate therefore leading to early detection of cancer), meaning that they were considered to have little disadvantage. However, half of the negative comments included that the burden of completing a minimally invasive test was still too great in terms of time or invasiveness (7% comments [n = 23]), particularly for screening.
3.2.1.4 Continuous biomarker monitoring
Like minimally invasive tests, burden and perceived effectiveness were the most frequently noted reasons for being willing to take up continuous monitoring of biomarkers, being included in 20% (n = 79) and 39% comments (n = 157), respectively. However, the same number of participants felt that the burden was too high in terms of discomfort, intrusiveness and mental health impact (20% comments [n = 79]), and others generally disliked the concept (12% comments classified as affective attitude [n = 48]).
3.2.1.5 AI analysis of medical records
The most frequently cited reason for being willing for AI to assess cancer risk was its perceived effectiveness, mentioned in 25% of the included comments (n = 117). These comments focused on the potential of AI to analyse large quantities of data, making it powerful at identifying trends and without the inevitable error, bias and time limitations of humans. Conversely, many did not trust it (either in general or due to the newness of AI; affective attitude), and did not want or think AI would be accurate enough to take on a role that they considered to belong to GPs (anticipated costs and perceived effectiveness). These domains each covered a third of the negative comments (n = 65 to 85).
3.2.1.6 Wearable devices
There were more positive comments on the burden of wearable devices than negative, with participants indicating that it would be easy since they already had a device or anticipated it to be a low effort risk assessment. 18% of the included comments (n = 97) were negatively related to ethicality: specifically, participants were concerned about data security, surveillance, and the potential to attribute blame for a high cancer risk to the individual.
3.3 Acceptability of risk-stratified screening or referral
The majority of survey participants also responded that use of the exemplars within risk-stratified screening or referral pathways would be extremely or somewhat acceptable (Figure 3A; with unadjusted results shown in Supplementary Table 3B). Notably, however, over 13.5% of participants considered the use of geodemographic segmentation, AI or wearable devices unacceptable in both asymptomatic and screening contexts, and 10% considered the use of geodemographic segmentation extremely unacceptable in the symptomatic context. Acceptability was higher in the symptomatic context than in the asymptomatic context (p ≤ 0.001 across the five-point Likert scale) for all exemplars except minimally invasive tests where no difference was seen (p = 0.224). In adjusted analyses (Supplementary Figure 1B), the pattern of acceptability was similar to that of the uptake of risk assessment for each exemplar, with geodemographic segmentation less acceptable, and PRS, minimally invasive tests and continuous biomarker monitoring more acceptable, in both the asymptomatic and symptomatic contexts.
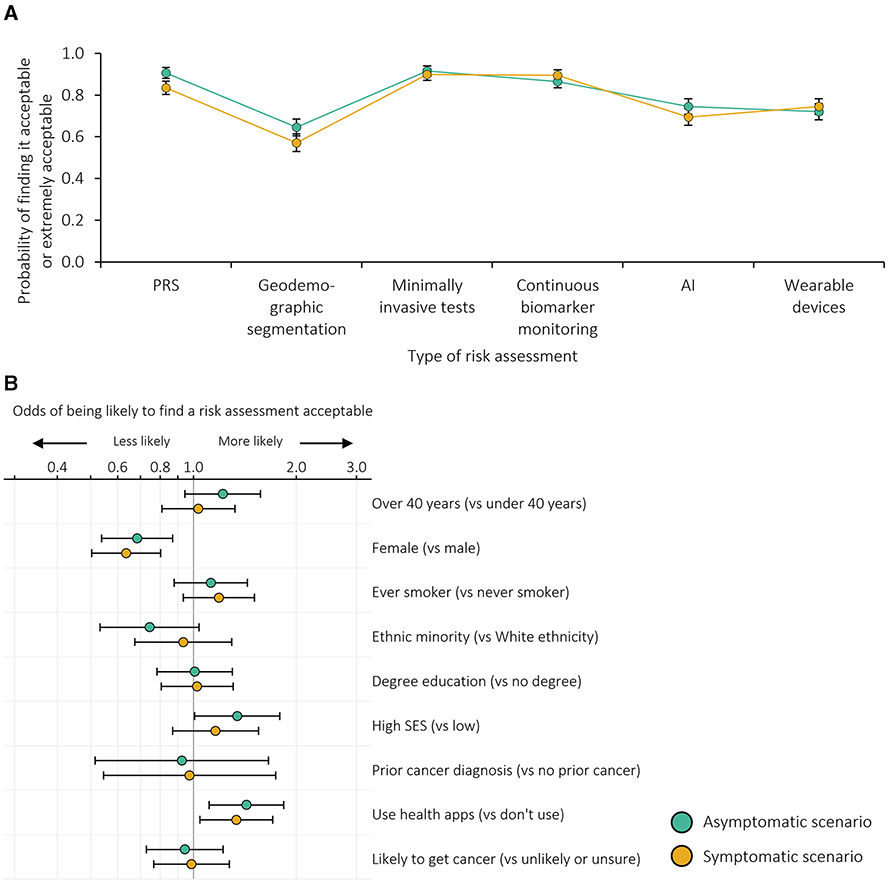
Figure 3. Acceptability of risk-stratified screening or referral: (A) probability of finding risk-stratified screening or referral acceptable adjusted for individual demographics and the order in which the exemplars were presented; (B) odds ratios for being more or less likely to find risk-stratified screening or referral acceptable. n = 999 survey participants. Error bars represent 95% confidence intervals. SES, socioeconomic status. Based on dichotomised outcomes (i.e., likely or extremely acceptable vs. neither acceptable nor unacceptable, somewhat unacceptable or extremely unacceptable).
In both contexts, female participants were less likely to consider use of any of the exemplars acceptable (p = 0.002 in the asymptomatic context and p < 0.001 in the symptomatic context) and those with greater technology use were more likely to take up risk assessment (p = 0.005 in the asymptomatic context and p = 0.021 in the symptomatic context; Figure 3B, Supplementary Table 4B). Participants with higher socioeconomic status were more likely to consider risk assessment acceptable in the asymptomatic context than those with lower socioeconomic status (p = 0.044).
Supplementary Figure 2 shows the anticipated impact of a risk estimate, based on each exemplar, on the likelihood of attending screening. For most participants (62.3 to 68.0%), a low risk estimate would not change the likelihood of attending screening whereas most (69.1 to 82.1%) considered they would be more likely to attend screening if they received a high risk estimate. Supplementary Figure 2 also shows the reasonableness of referral for different priority tests based on each risk estimate. Across both risk levels and all exemplars, the most frequent response was that this was reasonable. For each exemplar, it was more frequently considered reasonable in the high risk scenario compared to the low risk scenario (79.1 to 95.0% vs. 48.6 to 73.8%; p < 0.001). Participants considered that using geodemographic segmentation to inform referral was least reasonable compared to the other innovations.
3.4 Comfort with results from risk assessments being kept on record
Comfort with the results from risk assessments being kept on record and used within the NHS to assess risk of other health conditions was generally high (Figure 4A, Supplementary Table 3C, Supplementary Figure 1C). Over 83.0% of respondents were comfortable with the NHS holding data on PRS, minimally invasive tests and continuous biomarker monitoring. Between 66.5% and 74.5% were comfortable with data from geodemographic segmentation, AI and wearable devices being held by the NHS.
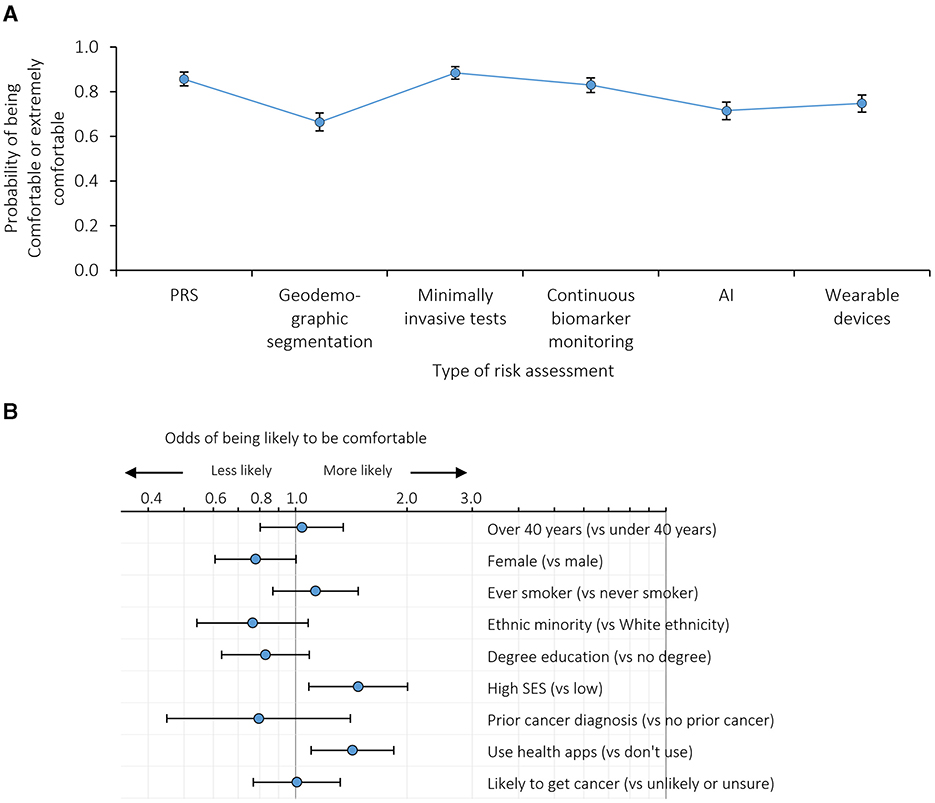
Figure 4. Comfort with results from risk assessments being kept on record: (A) probability of being comfortable with the results from different risk assessments being kept on record adjusted for individual demographics and the order in which the exemplars were presented; (B) odds ratios for being more or less likely to be comfortable with results from risk assessments being kept on record. n = 999 survey participants. Error bars represent 95% confidence intervals. SES, socioeconomic status. Based on dichotomised outcomes (i.e. likely or extremely comfortable with the NHS having the results from different risk assessments on record vs. neither comfortable nor uncomfortable, somewhat uncomfortable or extremely uncomfortable).
Participants with higher socioeconomic status and technology use were more likely to be comfortable across all the exemplars than participants with low socioeconomic status or lower technology use (p = 0.013 and p = 0.007, respectively; Figure 4B, Supplementary Table 4C).
3.5 General barriers and enablers to risk-based innovations
3.5.1 How participants felt about risk-based innovations (affective attitude)
Overall, using innovations to assess cancer risk prior to a healthcare decision was viewed as a “proactive” approach that made “absolute sense”, with individuals generally welcoming their use. Also, the participants were positive toward each of the specific exemplars presented. However, concerns were expressed that the ease of some of the methods may lead some people to undertake them without considering the potential consequences or implications. This particularly related to minimally invasive tests, but also applied to other exemplars (Table 4; Quote 1).
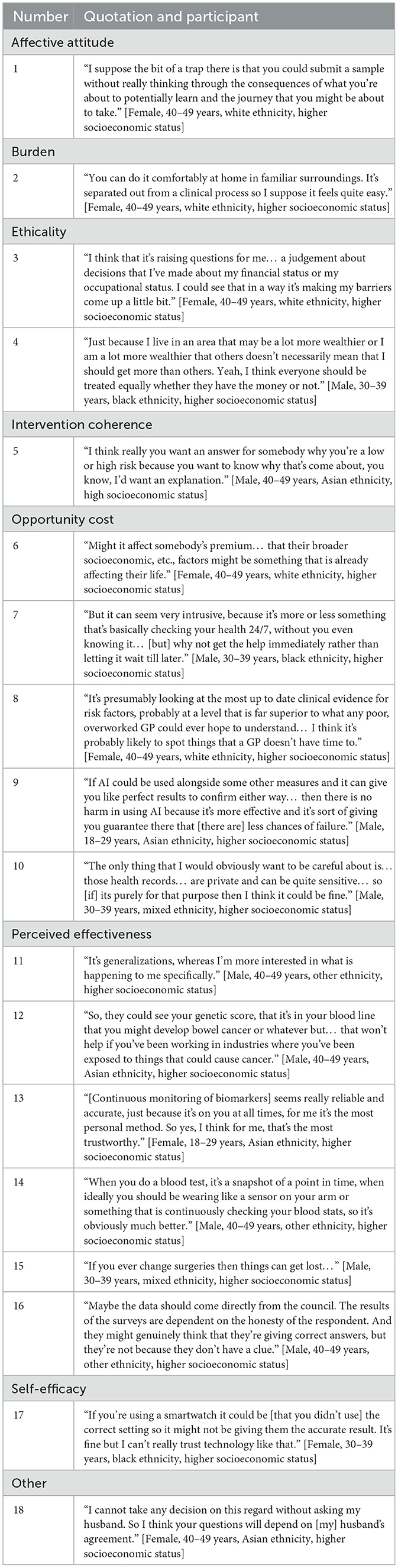
Table 4. Think-aloud interview participant quotations by Theoretical Framework of Acceptability (TFA) domain.
3.5.2 Perceived level of effort required to participate (burden)
The perceived level of effort required to participate was a key consideration for participants. A key benefit of AI and geodemographic segmentation was that data retrieval does not require high levels of involvement by individuals. Wearing a smart device or sensor to continuously monitor biomarkers, or providing a sample for a PRS were also viewed as low effort because they only require one appointment or setting up a device that then works autonomously. The perceived burden of minimally invasive tests was dependent on two factors: the type of test required and the test location. Urine and stool samples made some participants feel uncomfortable so they preferred saliva and blood tests. Tests that could be carried out at home were preferred to those taking place in a clinical setting. They were considered to be “less stigmatizing” and a “very natural step” (Table 4; Quote 2).
3.5.3 The extent to which risk-based innovations fit with an individual's value system (ethicality)
This domain tended not to be mentioned within the interviews, with the exception of concerns about the impact of geodemographic segmentation on people of lower socioeconomic status. In this context, firstly, participants were uncomfortable about the potential of causing individuals from this group to feel judged: that geodemographic segmentation may make people feel “guarded” and inadvertently cause negative views toward the decisions that people had made such as lifestyle choices, occupation, etc. Secondly, prior to correction by the interviewer, participants were concerned that geodemographic segmentation may disadvantage the poorest in society because they may not have the opportunity to move to areas that have fewer health risks. This idea was viewed as unethical and went against participants' value systems. Overall, members of the public placed importance on fair cancer healthcare, particularly for people who may already face disadvantages (Table 4; Quotes 3 and 4).
3.5.4 The extent to which participants understand the risk-based innovation and how it works (intervention coherence)
Overall, understanding was high across all risk assessment methods. Participants had prior knowledge of some of the exemplars (such as wearable devices and minimally invasive tests). Other methods were newly introduced during the interviews but were considered to be easily understood (such as continuous monitoring of biomarker levels and PRS).
In terms of coherence of the risk estimate, participants did not want to receive a risk categorization that could not be explained and expected to receive, at a minimum, a general explanation as to why they received their risk score. A lack of explanation would make them “untrust[ing]” of the NHS, particularly if they had a high risk of cancer. In particular, they would feel more positive toward the use of AI if a report could explain and justify their result (Table 4; Quote 5). This principle also applied more generally: by understanding the components of their risk score, they would treat it in a more serious manner, which could motivate people to engage in behavior change to lower their cancer risk or “make important decisions about their health” in conversation with healthcare professionals.
Furthermore, communication strategies were viewed as important in terms of the acceptability of cancer risk assessments to the public. Participants suggested that any changes to current practice should be communicated through public campaigns, letters formally stamped with the NHS logo, and conversations with GPs as the point of contact. More specifically, conflicting opinions were expressed regarding the communication of the results from individuals' risk assessments. Numeric scores were viewed as precise but had the potential to be “morbid” and it could lead to comparison amongst friends and family. Presenting the risk score categorically (high, medium or low risk) was equally conflicting as although it may be easier for the public to understand, individuals on the lower or higher ends of these scales could get either a false sense of security or anxiety.
3.5.5 Benefits, profits or values that must be given up to engage with the risk-based innovations (opportunity cost)
A variety of opportunity costs were associated with different exemplars of risk assessments. For example, participants considered the implications for health insurance associated with geodemographic segmentation. Specifically, they worried if they were found to be at higher risk of cancer based on where they lived, it could cause companies to raise insurance premiums that may, in turn, exacerbate inequalities (Table 4; Quote 6). Additionally, it felt inevitable that continuous monitoring would be accompanied by an invasion of their privacy. This was particularly true for wearable or mobile technologies that would show lifestyle factors. However, they felt that the benefits outweighed invasion of privacy (Table 4; Quote 7).
Notably, participants considered the impact of AI on their relationship with healthcare professionals (the “human vs. technology” trade-off) with mixed emotions. Positively, some participants felt that AI would be more accurate than a GP. It could also be more efficient, which, in turn, may lead to positive financial implications and reduced workload and time pressure. In contrast, other participants expressed a strong preference for maintaining the traditional GP-patient relationship, valuing the interactions and conversations with healthcare professionals. They felt that the AI algorithm might generate a less accurate risk assessment because GPs could notice additional, external factors that an algorithm may miss. Participants with concerns about AI therefore did not want their healthcare to be informed solely by the output of the algorithm. They felt that AI should be used as a side-by-side or secondary check to ensure other risk assessments did not miss anything and to confirm the risk categorization (Table 4; Quotes 8 and 9).
Irrespective of exemplar, participants spontaneously mentioned concerns for data access and storage. Health data is sensitive information, therefore, they were accepting of it being accessed as long as it was for a risk assessment, as intended, and that it was safeguarded appropriately to prevent misuse (Table 4; Quote 10). Participants generally trusted the NHS with their medical data, and felt it was logical to use it to assess risk of other health conditions, not just cancer. They also expressed an interest in having access to their own personal data.
3.5.6 The extent to which risk-based innovations are perceived as likely to achieve their purpose (perceived effectiveness)
Participants were quick to share their perspectives on the effectiveness of the exemplars. Perceived effectiveness was impacted by the type and frequency of data collection, in addition to anticipation of errors and inaccuracies to be introduced within data-based innovations.
Participants tended to place a greater value on the collection of biological data than lifestyle or environmental data. It was seen to be “more sophisticated” and objective because it examines individualized internal processes and therefore also incorporates the effects of lifestyle and environmental factors. Nevertheless, both were valued: geodemographic segmentation alone could be too generalized or generic (Table 4; Quote 11) yet PRS would not take important environmental and lifestyle factors into consideration; for example, an individual may have a low genetic risk of developing cancer but live in a polluted environment or lead an unhealthy lifestyle, which could place them at higher risk (Table 4; Quote 12). Furthermore, the potential of biological data extended beyond cancer prediction, such as alerting individuals to biological indicators of other diseases, as well as benefiting society by providing further information on disease processes.
When considering the frequency of data collection, continuous methods were favored over one-off snapshots. Continuous data collection was viewed as comprehensive, reliable and accurate as it provides real time data (Table 4; Quote 13). Although single data collection events required less effort, upon reflection, participants felt that they had more potential for inaccuracies as the results reflect a person's health at just one moment in time (Table 4; Quote 14). They were also concerned that additional, non-fixed factors may impact the results. For example, the quality of the test conducted, potential for contamination, or additional biological and lifestyle related factors that change regularly (sleep, stress, diet etc). On the other hand, concerns about the accuracy and trustworthiness of wearable devices were heightened by the need to remove it and disrupt constant wear (e.g., to charge it or to wash).
For AI, participants were concerned that medical records might not be up to date. This applied to individuals not attending their GP regularly or only attending if they became symptomatic, and in cases where health events had not been reported and therefore would be unknown to the algorithm (Table 4; Quote 15). Participants proposed that a standardized check of health records should be conducted prior to running AI to ensure that there is a consistent baseline level of information and that no biases are present within the algorithm. They also worried that individuals may not report geodemographic segmentation data correctly leading to errors in risk categorization (Table 4; Quote 16).
3.5.7 Confidence that they can perform the behavior required to participate in the risk-based innovation (self-efficacy)
Self-efficacy was not considered for AI, continuous monitoring of biomarkers, geodemographic segmentation or PRS. These risk assessments tend not to require an individual to perform a behavior independently for data collection.
Conversely, participants showed a lack of confidence that they or others could complete the tests that required more active engagement. As described for the burden of completing certain minimally invasive tests, some were unsure about providing urine or stool samples at home. Moreover, they worried that a lack of self-efficacy would impact the accuracy of risk assessments based on data collected by wearable devices, which could lead to an inaccurate risk category (Table 4; Quote 17). These technologies might be difficult for individuals who are not “tech savvy”. Participants felt that they may not be able to correctly operate the technologies or understand the outputs, which could lead to them feeling “overwhelmed” and “agitated”.
3.5.8 Individual personality and context (other)
Lastly, throughout the interviews it became apparent that the level of receptiveness to risk assessment methods varied according to individual tendencies, irrespective of the exemplar risk assessment method they reviewed. Some participants demonstrated an avoidant coping style toward health and therefore were not enthusiastic to take up a risk assessment as they “would rather not know”. In contrast, some participants proactively “want[ed] to keep on top of their health” and therefore were enthusiastic about taking up the innovations and finding out the results. Others explained that such decisions should be taken in conjunction with other family members (Table 4; Quote 18).
4 Discussion
This research highlights the receptiveness of members of the UK public to the concept of using novel innovations to estimate cancer risk and inform cancer screening or, in particular, referral to investigate symptoms. Across the six exemplars, 69 to 94% of the sample reported they were likely to take up a risk assessment and 69 to 91% considered it acceptable to inform risk stratification (excluding geodemographic segmentation, as discussed below). Interview participants were fundamentally positive about risk-based innovations, particularly when they found the concepts logical and intuitive. Together with the learnings about optimizing acceptability, these findings suggest that engagement with novel risk assessments is likely to be high if offered to the public prior to screening or if they presented with symptoms in primary care.
4.1 Discussion of findings
4.1.1 Overall summary
We observed moderate or high overall intended uptake and acceptability of the use of risk-based innovations to stratify care in both asymptomatic and symptomatic contexts; this is consistent with previous studies that have shown public willingness to participate in cancer risk assessments (19, 27, 28). Analyses of interview and free text data highlighted underlying positive attitudes toward such approaches: they are considered logical, intuitive and likely to be effective. Of note, though, acceptability of risk stratification tended to be slightly lower than likelihood of risk assessment uptake. This suggests that participants were more eager to learn of their cancer risk than for it to be used to inform screening or investigations for symptoms (more so in the symptomatic context). Although we were not able to explore why this might be in the qualitative analyses, it is known that the public anticipate gaining knowledge of their personal risk of cancer to be empowering and motivational for behavior change (19, 29, 30), as well as benefitting health by enabling those at high risk to access increased monitoring and/or prophylactic intervention (31–34). Risk assessment in the symptomatic context may additionally be considered a form of investigation and more participants considered the effort required to complete a risk assessment worthwhile when symptomatic.
Differences in views according to individual participant characteristics are important to consider for implementation of risk-based innovations, alongside individual differences that are not easy to gauge such as avoidant or proactive tendencies. Across the three main outcomes, participants were statistically more likely to respond positively if they reported use of health apps. This was particularly true for wearable devices. Other associations with participant characteristics varied by outcome and context. For example, female participants were less likely to find risk-stratified healthcare acceptable, but there were no differences compared to males for anticipated uptake. Conversely, participants of minority ethnicity and low socioeconomic status were less likely to say they would take up a risk assessment for screening but did not find risk stratification significantly less acceptable than their comparators. This is consistent with socioeconomic and ethnic inequalities in uptake of screening itself (35–37). Similar patterns have been found for returning a fecal immunochemical test kit in symptomatic patients (38), help-seeking for possible cancer symptoms (39), and in a study assessing uptake of breast cancer risk assessments based on phenotypic and genetic risk and mammographic density (40). It is therefore necessary to ensure that those already less likely to engage in screening and present with symptoms are not discouraged further through the prospect of a risk assessment.
4.1.2 Minimally invasive tests
Almost all participants were likely to take up the offer of minimally invasive tests, find risk stratification based on the results acceptable and be comfortable with the results being kept on record (a minimum 85.0% across all outcomes), making them the most popular risk assessment method examined. Qualitative findings suggest that this was driven by understanding that such tests would require low effort, particularly where they could be completed in comfort at home rather than needing an appointment, and were perceived as effective, reliable and accurate due to their biological nature. This aligns with some of the reasons recent focus group participants gave for their enthusiasm for multi-cancer early detection blood tests: their familiarity with and the relatively non-invasive and standardized nature of blood tests (41). Likewise, discrete choice experiments have reported minimally invasive tests (e.g., stool tests, breath analysis and blood tests) to be preferred over more invasive tests for population cancer screening, providing that sensitivity is sufficiently high (42, 43). As such, while our study is the first to ask participants to consider such tests for cancer risk assessments rather than screening itself, the findings are consistent with views on their use in other contexts.
4.1.3 Continuous biomarker monitoring
The second innovation based on biomarkers—continuous monitoring of biomarkers—was regarded similarly positively to minimally invasive tests, albeit with perceptions of a greater burden yet higher potential effectiveness. This may explain why 12.8% more participants were likely to take up the offer in the symptomatic rather than asymptomatic scenario. There is a lack of information about perceptions of continuous biomarker monitoring in the context of cancer healthcare. Observational research is needed to understand to what extent experiences in other contexts such as continuous glucose monitoring in people with diabetes, where empowerment and reassurance are experienced alongside burdens due to device complexity and the non-stop nature (44, 45), would be experienced if it were used for cancer risk assessments. Nevertheless, our findings suggest that the public would be open to this idea.
4.1.4 Wearable devices
The use of wearables in healthcare is a rapidly growing area of interest to both individuals and policy makers, both in those with cancer diagnoses and healthy or asymptomatic individuals (16). There is a paucity of data on acceptability in certain areas relating to cancer; for example, while there is promise in the feasibility, efficacy and acceptability of wearable devices to promote physical activity in cancer survivors, public perspectives toward portable, non-invasive technologies for early breast cancer detection remain unknown as research has focused on technological developments so far (13, 46). In this study we found that participants were moderately receptive to the use of wearable devices compared to the other exemplars included. They described positive aspects including the acceptable burden because data are collected automatically once the device is set up, which may only take one appointment as for some of the other innovations, and/or they may already own a wearable device (e.g., a smartwatch). This aligns with the observed association between receptiveness and familiarity with health apps. Participants also expressed a range of potential added benefits from motivating behavior change to altruistically contributing to medical knowledge. Ethicality due to the focus on lifestyle factors was the greatest concern; specifically, the potential to attribute blame for a high cancer risk on lifestyle choices as well as privacy concerns over continuous surveillance. This issue is of paramount importance to policymakers and researchers too (47).
4.1.5 PRS
Participants were receptive to PRS with 84.7% and 88.1% participants likely to take up this test in asymptomatic and symptomatic contexts, respectively, with more certainty in uptake in the symptomatic context. This is comparable to findings on the acceptability of PRS for risk assessment in hypothetical contexts (48–51), as well as 84 to 96% uptake observed in research settings (52). Our study reveals that the low burden of PRS was the key driver in this context: participants were encouraged by the requirement to only provide one sample at home or at a single appointment. Furthermore, only a few participants reported concerns about PRS, although these are important to consider as they have been observed in other settings. Some raised issues of ethicality or data protection (52). Others considered a potential cost in which access to other tests may be delayed or withdrawn whilst waiting for the PRS result or upon receiving a low genetic risk of cancer. Although fear of delays could be mitigated by appropriate policies, the concern about being “penalized for good genes” echoes public objections about risk stratification for those with lower cancer risk and again highlights the value of screening within society (19). Lastly, other participants expressed mistrust of the accuracy of the algorithms, methodology or technology.
4.1.6 AI analysis of medical records
Receptiveness to AI analysis of medical records fell in the midpoint between the other exemplars. The qualitative work suggests there were mixed views accounting for the different perceived impacts of AI on relationships with healthcare professionals: some considered AI favorably, suggesting that it could outperform or help their overworked GP, whereas others were concerned that it was more fallible, would negatively affect this valued relationship or would have unintended impacts such as deskilling GPs. This echoes public perspectives toward AI in a multitude of clinical contexts, albeit not specifically for analyses of medical records, where authors of a systematic review report that “the acceptability of AI generally hinged on its use as a support, rather than a replacement, of healthcare providers” (53). It was also important to our participants that they could understand how their risk classification came about, which put some people off the prospect of AI. A recent review suggested that inability to understand an AI algorithm reduced patient autonomy in decision making and led to greater psychological harms caused by misdiagnoses (54). Nevertheless, amongst our participants, the minimal burden meant there was little to lose. Mixed perspectives as reported here were also observed in a recent survey of 600 members of the US public: 52.0% were comfortable with AI being used to predict future medical conditions compared to, for example, 84.2% who were comfortable with its use in administrative tasks such as scheduling appointments (55). Together, these findings suggest that using AI to estimate risk of cancer based on general practice medical records has the potential to be acceptable to the public provided that suitable safeguards are in place.
4.1.7 Geodemographic segmentation
Finally, we observed that geodemographic segmentation was the least preferred option with only approximately two-thirds of participants considering that they would be likely to take it up and fewer finding it acceptable to inform healthcare, especially in the symptomatic context. Interestingly, this was the example in which the order effect was greatest: if participants saw it first, they were more receptive to it. This suggests geodemographic segmentation was considered as a somewhat good idea, but not as good as the other exemplars. As the least preferred option, implementation efforts may be best focussed on other innovations while further research is conducted into the utility of geodemographic segmentation and public perspectives toward it.
Reasons for likely uptake included the low burden. Although the impact of environmental factors on cancer risk resonated with many [as in other research where pollution and radiation were perceived to have some of the highest impacts on cancer risk (56)], this was not an obvious part of demographic segmentation to others. The interviews revealed misunderstanding in which participants associated personal finances with socioeconomics. They therefore assumed that personal finances would inform the cancer risk assessment and healthcare, which raised issues of ethicality and negative affective attitudes. This misunderstanding could be corrected by the interviewer, but not in the survey that was completed independently. As such, the population-level acceptability of geodemographic segmentation should be interpreted with this in mind. It also highlights two important implications: it is necessary for policies to be coherent and fair for society in order to be acceptable. For example, the message that screening and referral informed by geodemography may help to reduce inequalities could be received positively, subject to evaluation (57).
4.2 Strengths and limitations
A major strength of this research is the use of multiple research methods, giving a comprehensive overview of public receptiveness to risk-based innovations. The qualitative free text analysis and interviews helped us to interpret the quantitative data collected in the population survey. The think aloud interviews provided insight into how people might personally feel if they were offered specific risk assessments. Furthermore, our analyses were informed by the TFA, meaning we considered recognized domains of acceptability.
Throughout the research, we used a set of six exemplars of risk-based innovations. Choice of exemplars was informed by expert consensus to ensure that the findings were relevant and up to date within this rapidly advancing field. Specific examples likely made the complex concepts easier for participants to grasp and helped engagement despite the theoretical nature of the questions we were asking. However, the nature of the survey context meant that descriptions needed to be brief and a degree of vagueness was unavoidable in order to avoid overwhelming the participants. We observed misunderstanding related to geodemographic segmentation in particular, but it is likely that other misunderstandings remain unknown. We were unable to provide participants with specific details such as risk assessment accuracy statistics or cost of each type of innovation, which they would expect if innovations were implemented in practice. Moreover, participants' preconceptions about the technologies will have influenced their perspectives; in particular, many people are familiar with AI, although less so in the clinical context (53), therefore may have unrealistic concerns or even hopes that impact their receptiveness. However, AI was only named overtly in one exemplar despite the fact that it would likely contribute to interpreting other data such as from wearable devices. We also grouped innovations: wearable devices included both smartwatches and sensors worn on other parts of the body; minimally invasive tests included blood, saliva and other samples, which participants may have had nuanced perspectives toward. A recent study showed blood-based tests were preferred over stool tests, yet such comparisons were not possible in our study as a result of this grouping (43). Furthermore, we observed an order effect in the survey meaning that the participants had been influenced by the other innovations they had seen. Providing more details on the innovation and/or only asking each participant to consider one innovation could have mitigated this. Nevertheless, we sought to identify overall receptiveness and acceptability rather than focusing entirely on the chosen exemplars themselves, in order for our findings to be relevant to all potential innovations.
We recruited participants with a wide range of demographic characteristics from across the UK, including three participants in the think aloud interviews who did not have sufficient competency or confidence to complete the interviews in English. This means that the findings are more likely to be applicable across the population. However, the participants may not be representative of the UK population according to characteristics that we were not able to measure or recruit according to, such as lived experience, and all were registered with survey or qualitative recruitment agencies. Importantly, people who showed an interest in research on this topic when approached to take part may also have different perspectives from people who were not interested.
4.3 Implications
As well as demonstrating overall positive views toward risk-based innovations, we identified the characteristics of innovations that enable risk assessment that matter most to the public and that should be taken into account by developers (summarized in Figure 5). The effort to complete a test was important: although this does not mean that risk assessments with high burden will not be taken up, a low burden was often highlighted as an appealing factor, particularly in the asymptomatic context. Burden should therefore be minimized through test design or interventions to facilitate participation. The burden should also be proportional to the potential gains, meaning that physically or personally invasive tests may only be offered to symptomatic individuals. Based on the findings of these studies, from the perspective of public acceptability, minimally invasive tests and PRS could be implemented immediately.
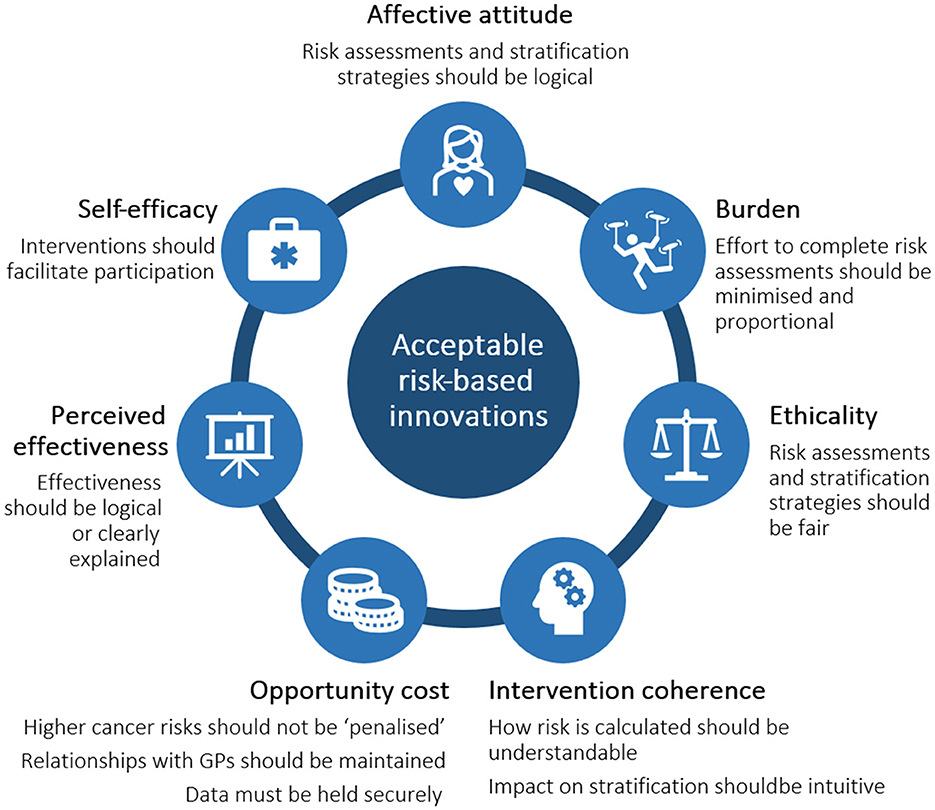
Figure 5. Summary of requirements for risk-based innovations to be acceptable to the public, based on the Theoretical Framework of Acceptability.
Additionally, risk-based innovations and the stratified healthcare that follow a risk assessment should be logical and coherent. Risk calculations should be intuitive and explainable; for example, that someone's risk classification has arisen due to the presence of certain genetic variations, biomarkers, environmental risk factors, etc. It is also important that fairness, ethicality and data protection are seen to be maintained, alongside innovation-specific needs such as the interaction between healthcare providers and AI.
Specific strategies to communicate, promote, and adapt future innovations to the population and/or specific subgroups will be required. The differences in receptiveness observed between the examples of potential innovations used in these studies further highlights the importance of engaging the public early in the development of individual innovations in the future.
5 Conclusion
Our findings show that members of the UK public are receptive to the concept of using novel innovations to estimate risk of cancer and inform stratification. Research effort and investment to develop these technologies should, therefore, continue and also take into account that greater receptiveness was seen in the context of referral decisions to investigate symptoms than in the asymptomatic context for screening. We identified a diverse range of unifying priorities across innovations, such as burden and coherence, that will guide the development, policymaking and communication of technologies. Patients and the public should be involved throughout this process to ensure changes to practice are acceptable.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: University of Cambridge repository: https://doi.org/10.17863/CAM.112960.
Ethics statement
The studies involving humans were approved by University of Cambridge Psychology Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. RC: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. JT: Investigation, Methodology, Writing – original draft, Writing – review & editing. AS: Formal analysis, Writing – original draft, Writing – review & editing. MS: Formal analysis, Writing – original draft, Writing – review & editing. PP: Formal analysis, Writing – original draft, Writing – review & editing. LT: Formal analysis, Writing – original draft, Writing – review & editing. JW: Formal analysis, Supervision, Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Investigation, Methodology, Project administration. JU-S: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by a Cancer Research UK commissioned research award (reference PICATR-2022/100003). Juliet A. Usher-Smith, Advanced Fellow, NIHR300861, was funded by the National Institute for Health and Care Research for this research project. Rebecca A. Dennison was also funded on this NIHR fellowship.
Acknowledgments
We would like to thank our patient and public involvement representatives, Mary Adeson, Phil Alsop, Philip Dondi and Ruth Katz, for their invaluable contributions throughout this project. We also thank our participants for giving their time and opinions within the survey and interviews. We would additionally like to thank the following for supporting the identification and prioritization of examples of risk-based innovations: Dr. Lyndsy Ambler (Senior Strategic Evidence Manager, Cancer Research UK, UK), Prof. Antonis Antoniou (Professor of Cancer Risk Prediction, University of Cambridge, UK), Prof. Mireille Broeders (Professor of Personalized Cancer Screening, Radboud University Medical Center, the Netherlands), Dr. Alice Brookes (Strategic Evidence Manager, Cancer Research UK, UK), Prof. Anna Chiarelli (Senior Scientist, Cancer Care Ontario, Canada), Mr. Joseph Day (Senior Business Development Executive, Cancer Research UK, UK), Prof. Evelien Dekker (Professor of Gastrointestinal Oncology, University of Amsterdam, the Netherlands), Prof Stephen Duffy (Professor of Cancer Screening, Queen Mary University of London, UK), Prof. Rebecca Fitzgerald (Professor of Cancer Prevention, University of Cambridge, UK), Prof. David French (Professor of Health Psychology, University of Manchester, UK), Prof. Fiona Gilbert (Professor of Radiology, University of Cambridge, UK), Prof. Vincent Gnanapragasam (Professor of Urology, University of Cambridge, UK), Samantha Harrison (Head of Strategic Evidence, Cancer Research UK, UK), Dr. Sarah Hindmarch (Research Associate, Manchester Center for Health Psychology, University of Manchester, UK), Prof. Anne Mackie (Director of Screening, Department of Health and Social Care, UK), Prof. Ranjit Manchanda (Professor of Gynecological Oncology, Queen Mary University of London, UK), Dr. Sowmiya Moorthie (Senior Policy Analyst, PHG Foundation, UK), Prof. Richard Neal (Professor of Primary Care, University of Exeter, UK), Prof. Peter Sasieni (Academic Director of King's Clinical Trials Unit and Professor of Cancer Prevention, King's College London, UK), Prof. Samuel Smith (Professor of Behavioral Oncology, University of Leeds, UK), and Hope Walters (Strategic Evidence Manager, Cancer Research UK, UK). Stephen Sharp (MRC Epidemiology Unit, University of Cambridge, UK) gave advice on the quantitative analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR, NHS, or the UK Department of Health and Social Care.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcacs.2025.1522609/full#supplementary-material
Footnotes
1. ^Dennison R, Cline R, Tung J, John S, Moorthie S, Waller J, et al. Societal views on using risk-based innovations to inform cancer screening and referral policies: findings from three community juries. Health Expect. 25:1789–806.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Page B, Irving D, Amalberti R, Vincent C. Health services under pressure: a scoping review and development of a taxonomy of adaptive strategies. BMJ Qual Saf. (2023) 2023:016686. doi: 10.1136/bmjqs-2023-016686
3. Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. JNCI. (2011) 103:117–28. doi: 10.1093/jnci/djq495
4. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Gastroenterol Rev. (2019) 14:89–103. doi: 10.5114/pg.2018.81072
5. Hull MA, Rees CJ, Sharp L, Koo S. A risk-stratified approach to colorectal cancer prevention and diagnosis. Nat Rev Gastroenterol Hepatol. (2020) 17:773–80. doi: 10.1038/s41575-020-00368-3
6. Fitzgerald RC, Antoniou AC, Fruk L, Rosenfeld N. The future of early cancer detection. Nat Med. (2022) 28:666–77. doi: 10.1038/s41591-022-01746-x
7. Thacharodi A, Singh P, Meenatchi R, Tawfeeq Ahmed ZH, Kumar RRS, V N, et al. Revolutionizing healthcare and medicine: The impact of modern technologies for a healthier future—a comprehensive review. Health Care Science. (2024). doi: 10.1002/hcs2.115
8. Mahmoudi T, de la Guardia M, Baradaran B. Lateral flow assays towards point-of-care cancer detection: A review of current progress and future trends. TrAC - Trend Analyt Chemist. (2020) 125:115842. doi: 10.1016/j.trac.2020.115842
9. Kwong GA, Ghosh S, Gamboa L, Patriotis C, Srivastava S, Bhatia SN. Synthetic biomarkers: a twenty-first century path to early cancer detection. Nat Rev Cancer. (2021) 21:655–68. doi: 10.1038/s41568-021-00389-3
10. Ray PP, Dash D, De D, A. systematic review of wearable systems for cancer detection: current state and challenges. J Med Syst. (2017) 41:180. doi: 10.1007/s10916-017-0828-y
11. Kim J, Campbell AS, de Ávila BE-F, Wang J. Wearable biosensors for healthcare monitoring. Nat Biotechnol. (2019) 37:389–406. doi: 10.1038/s41587-019-0045-y
12. Triantafyllidis A, Kondylakis H, Katehakis D, Kouroubali A, Alexiadis A, Segkouli S, et al. Smartwatch interventions in healthcare: a systematic review of the literature. Int J Med Inform. (2024) 190:105560. doi: 10.1016/j.ijmedinf.2024.105560
13. Aboagye SO, Hunt JA, Ball G, Wei Y. Portable noninvasive technologies for early breast cancer detection: a systematic review. Comput Biol Med. (2024) 182:109219. doi: 10.1016/j.compbiomed.2024.109219
14. Ahmed Taha B, Kadhim AC, Addie AJ, Haider AJ, Azzahrani AS, Raizada P, et al. Advancing cancer diagnostics through multifaceted optical biosensors supported by nanomaterials and artificial intelligence: a panoramic outlook. Microchem J. (2024) 205:111307. doi: 10.1016/j.microc.2024.111307
15. Chaudhary V, Taha BA, Lucky, Rustagi S, Khosla A, Papakonstantinou P, et al. Nose-on-chip nanobiosensors for early detection of lung cancer breath biomarkers. ACS Sens. (2024) 9:4469–94. doi: 10.1021/acssensors.4c01524
16. UK Department of Health and Social Care Press Release. Government Issues Rallying Cry to the Nation to Help Fix NHS. Available at: https://www.gov.uk/government/news/government-issues-rallying-cry-to-the-nation-to-help-fix-nhs (accessed November 04, 2024).
17. Kann BH, Hosny A, Aerts HJWL. Artificial intelligence for clinical oncology. Cancer Cell. (2021) 39:916–27. doi: 10.1016/j.ccell.2021.04.002
18. Cabral BP, Braga LAM, Syed-Abdul S, Mota FB. Future of artificial intelligence applications in cancer care: a global cross-sectional survey of researchers. Current Oncology. (2023) 30:3432–46. doi: 10.3390/curroncol30030260
19. Taylor LC, Hutchinson A, Law K, Shah V, Usher-Smith JA, Dennison RA. Acceptability of risk stratification within population-based cancer screening from the perspective of the general public: a mixed-methods systematic review. Health Expect. (2023) 26:989–1008. doi: 10.1111/hex.13739
20. Dunlop KLA, Singh N, Robbins HA, Zahed H, Johansson M, Rankin NM, et al. Implementation considerations for risk-tailored cancer screening in the population: a scoping review. Prev Med (Baltim). (2024) 181:107897. doi: 10.1016/j.ypmed.2024.107897
21. Fox MC, Ericsson KA, Best R. Do procedures for verbal reporting of thinking have to be reactive? A meta-analysis and recommendations for best reporting methods. Psychol Bull. (2011) 137:316–44. doi: 10.1037/a0021663
22. Durning SJ, Artino AR, Beckman TJ, Graner J, van der Vleuten C, Holmboe E, et al. Does the think-aloud protocol reflect thinking? Exploring functional neuroimaging differences with thinking (answering multiple choice questions) vs. thinking aloud. Med Teach. (2013) 35:720–6. doi: 10.3109/0142159X.2013.801938
23. Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies. Qual Health Res. (2016) 26:1753–60. doi: 10.1177/1049732315617444
24. Freer-Smith C, Harvey-Kelly L, Mills K, Harrison H, Rossi SH, Griffin SJ, et al. Reasons for intending to accept or decline kidney cancer screening: thematic analysis of free text from an online survey. BMJ Open. (2021) 11:e044961. doi: 10.1136/bmjopen-2020-044961
25. Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: An overview of reviews and development of a theoretical framework. BMC Health Serv Res. (2017) 17:1–13. doi: 10.1186/s12913-017-2031-8
26. Braun V, Clarke V. Conceptual and design thinking for thematic analysis. Qualit Psychol. (2022) 9:3–26. doi: 10.1037/qup0000196
27. Banks J, Hollinghurst S, Bigwood L, Peters TJ, Walter FM, Hamilton W. Preferences for cancer investigation: a vignette-based study of primary-care attendees. Lancet Oncol. (2014) 15:232–40. doi: 10.1016/S1470-2045(13)70588-6
28. Venning B, Bergin R, Pearce A, Lee A, Emery JD. Factors affecting patient decisions to undergo testing for cancer symptoms: an exploratory qualitative study in Australian general practice. BJGP Open. (2023) 7:BJGPO.2022.0168. doi: 10.3399/BJGPO.2022.0168
29. Woof VG, Ruane H, French DP, Ulph F, Qureshi N, Khan N, et al. The introduction of risk stratified screening into the NHS Breast Screening Programme: views from British-Pakistani women. BMC Cancer. (2020) 20:452. doi: 10.1186/s12885-020-06959-2
30. Meisel SF Side L, Fraser L, Gessler S, Wardle J, Lanceley A. Population-based, risk-stratified genetic testing for ovarian cancer risk: a focus group study. Public Health Genomics. (2013) 16:184–91. doi: 10.1159/000352028
31. Dunlop K, Rankin NM, Smit AK, Salgado Z, Newson AJ, Keogh L, et al. Acceptability of risk-stratified population screening across cancer types: qualitative interviews with the Australian public. Health Expect. (2021) 24:1326–36. doi: 10.1111/hex.13267
32. Kelley-Jones C, Scott S, Waller J, UK. women's views of the concepts of personalised breast cancer risk assessment and risk-stratified breast screening: a qualitative interview study. Cancers. (2021) 13:5813. doi: 10.3390/cancers13225813
33. Rahman B, Meisel SF, Fraser L, Side L, Gessler S, Wardle J, et al. Population-based genetic risk prediction and stratification for ovarian cancer: views from women at high risk. Fam Cancer. (2015) 14:135–44. doi: 10.1007/s10689-014-9769-5
34. Rainey L, van der Waal D, Broeders MJM. Dutch women's intended participation in a risk-based breast cancer screening and prevention programme: a survey study identifying preferences, facilitators and barriers. BMC Cancer. (2020) 20:965. doi: 10.1186/s12885-020-07464-2
35. Hirst Y, Stoffel S, Baio G, McGregor L, von Wagner C. Uptake of the English Bowel (Colorectal) Cancer Screening Programme: an update 5 years after the full roll-out. Eur J Cancer. (2018) 103:267–73. doi: 10.1016/j.ejca.2018.07.135
36. Douglas E, Waller J, Duffy SW, Wardle J. Socioeconomic inequalities in breast and cervical screening coverage in England: are we closing the gap? J Med Screen. (2016) 23:98–103. doi: 10.1177/0969141315600192
37. Young B, Robb KA. Understanding patient factors to increase uptake of cancer screening: a review. Future Oncology. (2021) 17:3757–75. doi: 10.2217/fon-2020-1078
38. Bailey JA, Morton AJ, Jones J, Chapman CJ, Oliver S, Morling JR, et al. Sociodemographic variations in the uptake of faecal immunochemical tests in primary care: a retrospective study. Br J Gen Pract. (2023) 73:e843–9. doi: 10.3399/BJGP.2023.0033
39. Whitaker KL, Scott SE, Wardle J. Applying symptom appraisal models to understand sociodemographic differences in responses to possible cancer symptoms: a research agenda. Br J Cancer. (2015) 112:S27–34. doi: 10.1038/bjc.2015.39
40. Evans DGR, Warwick J, Astley SM, Stavrinos P, Sahin S, Ingham S, et al. Assessing individual breast cancer risk within the UK National Health Service Breast Screening Program: A new paradigm for cancer prevention. Cancer Prevent Rese. (2012) 5:943–51. doi: 10.1158/1940-6207.CAPR-11-0458
41. Schmeising-Barnes N, Waller J, Marlow LAV. Attitudes to multi-cancer early detection (MCED) blood tests for population-based screening: a qualitative study in Great Britain. Soc Sci Med. (2024) 347:116762. doi: 10.1016/j.socscimed.2024.116762
42. Peters Y, Siersema PD. Public preferences and predicted uptake for esophageal cancer screening strategies: a labeled discrete choice experiment. Clin Transl Gastroenterol. (2020) 11:e00260. doi: 10.14309/ctg.0000000000000260
43. Ong C, Cook AR, Tan K-K, Wang Y. Advancing colorectal cancer detection with blood-based tests: qualitative study and discrete choice experiment to elicit population preferences. JMIR Public Health Surveill. (2024) 10:e53200. doi: 10.2196/53200
44. Natale P, Chen S, Chow CK, Cheung NW, Martinez-Martin D, Caillaud C, et al. Patient experiences of continuous glucose monitoring and sensor-augmented insulin pump therapy for diabetes: a systematic review of qualitative studies. J Diabetes. (2023) 15:1048–69. doi: 10.1111/1753-0407.13454
45. Messer LH, Johnson R, Driscoll KA, Jones J. Best friend or spy: a qualitative meta-synthesis on the impact of continuous glucose monitoring on life with Type 1 diabetes. Diabetic Med. (2018) 35:409–18. doi: 10.1111/dme.13568
46. Coughlin SS, Caplan LS, Stone R. Use of consumer wearable devices to promote physical activity among breast, prostate, and colorectal cancer survivors: a review of health intervention studies. J Cancer Survivorship. (2020) 14:386–92. doi: 10.1007/s11764-020-00855-1
47. Klonoff DC, Price WN. The need for a privacy standard for medical devices that transmit protected health information used in the precision medicine initiative for diabetes and other diseases. J Diabetes Sci Technol. (2017) 11:220–3. doi: 10.1177/1932296816680006
48. Dennison RA, Taylor LC, Morris S, Boscott RA, Harrison H, Moorthie SA, et al. Public preferences for determining eligibility for screening in risk-stratified cancer screening programs: a discrete choice experiment. Medical Decision Making. (2023) 43:374–86. doi: 10.1177/0272989X231155790
49. Dennison RA, Thomas CV, Morris S, Usher-Smith JA, A. discrete choice experiment to understand public preferences and priorities for risk-stratified bowel cancer screening programmes in the UK. Prev Med (Baltim). (2023) 177:107786. doi: 10.1016/j.ypmed.2023.107786
50. Dennison RA, Clune RJ, Morris S, Thomas C, Usher-Smith JA. Understanding the preferences and considerations of the public towards risk-stratified screening for colorectal cancer: insights from think-aloud interviews based on a discrete choice experiment. Health Expectat. (2024) 27:14153. doi: 10.1111/hex.14153
51. Usher-Smith JA, Harvey-Kelly LLW, Rossi SH, Harrison H, Griffin SJ, Stewart GD. Acceptability and potential impact on uptake of using different risk stratification approaches to determine eligibility for screening: a population-based survey. Health Expect. (2021) 24:341–51. doi: 10.1111/hex.13175
52. Dannhauser FC, Taylor LC, Tung JSL, Usher-Smith JA. The acceptability and clinical impact of using polygenic scores for risk-estimation of common cancers in primary care: a systematic review. J Community Genet. (2024) 15:217–34. doi: 10.1007/s12687-024-00709-8
53. Young AT, Amara D, Bhattacharya A, Wei ML. Patient and general public attitudes towards clinical artificial intelligence: a mixed methods systematic review. Lancet Digit Health. (2021) 3:e599–611. doi: 10.1016/S2589-7500(21)00132-1
54. Xu H, Shuttleworth KMJ. Medical artificial intelligence and the black box problem: a view based on the ethical principle of “do no harm.” Intellig Med. (2023) 4:52–7. doi: 10.1016/j.imed.2023.08.001
55. Witkowski K, Okhai R, Neely SR. Public perceptions of artificial intelligence in healthcare: ethical concerns and opportunities for patient-centered care. BMC Med Ethics. (2024) 25:74. doi: 10.1186/s12910-024-01066-4
56. Petrova D, Borrás JM, Pollán M, Bayo Lozano E, Vicente D, Jiménez Moleón JJ, et al. Public perceptions of the role of lifestyle factors in cancer development: results from the Spanish Onco-barometer 2020. Int J Environ Res Public Health. (2021) 18:10472. doi: 10.3390/ijerph181910472
Keywords: cancer screening, health policy, personalized medicine, risk factors, artificial intelligence, genetic risk
Citation: Dennison RA, Clune RJ, Tung JSL, Schumacher AA, Solovyeva M, Pandey P, Taylor LC, Waller J and Usher-Smith JA (2025) The public are receptive to risk-based innovations: a multi-methods exploration of anticipated acceptability and uptake of novel technologies for cancer early detection in symptomatic and asymptomatic scenarios. Front. Cancer Control Soc. 3:1522609. doi: 10.3389/fcacs.2025.1522609
Received: 04 November 2024; Accepted: 20 January 2025;
Published: 12 February 2025.
Edited by:
Vishal Chaudhary, University of Delhi, IndiaReviewed by:
Bakr Taha, Universiti Kebangsaan Malaysia, MalaysiaMagnus Dustler, Lund University, Sweden
Sonu Sonu, Shoolini University, India
Copyright © 2025 Dennison, Clune, Tung, Schumacher, Solovyeva, Pandey, Taylor, Waller and Usher-Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca A. Dennison, cmw0MjNAbWVkc2NobC5jYW0uYWMudWs=
†These authors share senior authorship
 Rebecca A. Dennison
Rebecca A. Dennison Reanna J. Clune1,2
Reanna J. Clune1,2 Pranjal Pandey
Pranjal Pandey Lily C. Taylor
Lily C. Taylor