- 1Department of Behavior, Social, and Health Education Sciences, Rollins School of Public Health, Atlanta, GA, United States
- 2Good Samaritan Health Center, Atlanta, GA, United States
- 3Emory Primary Care, Emory Healthcare, Atlanta, GA, United States
- 4Division of General Internal Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, GA, United States
- 5Department of Family and Preventive Medicine, Emory University School of Medicine, Atlanta, GA, United States
Introduction: While there is strong evidence supporting family cancer history screening as a tool for risk-stratified cancer screening, challenges in implementation remain. Many efforts tend to focus solely on the high-risk pathway neglecting the entire patient population. This study aims to capture primary care providers' perspectives on implementing genetic-informed, risk-stratified mammography screening guidelines.
Methods: Semistructured interviews were conducted involving 14 providers and 5 practice leaders across 2 Georgia healthcare systems between November 2020 and May 2021. Interviews assessed the barriers and facilitators at patient, provider, and system levels using the Consolidated Framework for Implementation Research. Thematic analysis was conducted using MAXQDA, and Fishbone analysis was applied to summarize the results.
Results: Barriers and facilitators differed between high- and low-risk pathways. For high-risk pathways, barriers included limited provider knowledge and unclear referral protocols, while facilitators included established relationships between providers and genetic professionals and effective electronic health record systems. For low-risk pathways, barriers centered on provider acceptance, guideline inconsistency, and risk communication challenges.
Conclusion: Effective implementation of risk-stratified breast cancer screening requires tailored strategies to address pathway-specific barriers. Integrating ongoing education, clinical decision support, and workflow alignment may enhance program adoption.
Introduction
The U.S. Preventive Services Task Force (USPSTF) endorses family history-based screening as a frontline public health strategy to risk-stratify populations for tailored cancer prevention services (also known as precision public health) (1). With hereditary breast and ovarian cancer (HBOC), brief screening tools have been validated for identifying the 5%−10% of women who should be referred for genetic counseling and testing. Those with BRCA mutations can receive tailored life-saving prevention and treatment options (1). However, using these family history screenings will result in 85%−90% of women finding out they are not at risk for HBOC. These women, in turn, meet the criteria for initiating mammogram screening at age 40 and continuing biennial screenings thereafter. Strong evidence now supports risk-stratified screening regimens as the veritable “win–win,” affording early cancer detection and reducing patient burden and health care costs (2).
Controversy persists regarding the appropriate age to begin mammography screening and the best screening interval for women with an average risk for breast cancer (3). Specifically, the USPSTF, the American Cancer Society (ACS), and the American College of Radiology (ACR) each have different screening guidelines (4). Although mammography is widely acknowledged to be a critically important tool for breast cancer screening, its use can have adverse effects, including the possibility of false-positive results, which can cause anxiety and psychological stress and expose women to unnecessary treatment, pain, and side effects (5, 6). In addition, racial disparities in screening mammography use are evident in Black and Hispanic populations (7, 8). For these women, the pursuit of unwarranted mammography presents substantial logistical challenges and increased demand for limited resources. While we must ensure access to mammography screening, risk-stratified recommendations would mitigate an inappropriate demand for limited resources.
There are various challenges in implementing risk-stratified screening guidelines. Mammography screening practices operate within complex health system structures, including provider and patient behaviors. Our pilot work showed that patients struggle to distinguish between inherited vs. sporadic breast cancer risk (9). Additionally, providers fear that deviating from a single community-standard care pathway for screening would increase the risk of medical malpractice claims (10). Although electronic health record (EHR) prompts can help bridge care gaps (such as those related to screenings and immunizations), the logic behind them may be unclear or based on outdated recommendations. These factors can interact [for e.g., populations with low trust in medical systems may view that varied screening intervals are not based on risk but rather on providers refusing to offer necessary care (11)]. Successfully adopting risk-based guidelines requires prospectively identifying barriers to a seamless workflow integration and strategies for increasing patient and provider buy-in (12).
The overarching goal of this study is to characterize provider perceptions of facilitators and barriers to implementing genetic-informed risk-stratified mammography screening in primary care practices in Georgia. The specific aims are to (1) explore health care providers' awareness and perceptions of the genetic-informed risk-stratified mammography screening guidelines, perceived barriers, and facilitators to its implementation in primary care practice and (2) identify implementation strategies to address barriers that providers raise that are most amenable to interventions.
Methods
Study design
Between November 2020 and May 2021, semistructured phone interviews were conducted involving 14 providers and 5 leaders recruited from Emory Healthcare primary care clinics and Phoebe Health Care. The structured interview questions were based on the Consolidated Framework for Implementation Research (CFIR) to assess barriers and facilitators at multiple levels (13). In this study, we define the “high-risk screening pathway” according to the USPSTF guidelines, which recommend that “primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women who had a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing” (1). The “low-genetic risk screening pathway” refers to the discussion of biennial mammography screening for average-risk women aged 50–74 years, which was supported by the 2016 USPSTF guidelines (14) and several international mammography screening guidelines (3). Average-risk women were defined as asymptomatic women who do not have preexisting breast cancer or a previously diagnosed high-risk breast lesion and who are not at a high risk for breast cancer because of a known underlying genetic mutation (such as a BRCA1 or BRCA2 gene mutation or other familial breast cancer syndrome) or a history of chest radiation at a young age. The institutional review board of Emory University approved this study (IRB00113501).
Recruitment
We enlisted key stakeholders, including primary care providers and organizational leadership staff, who were involved in the breast cancer risk assessment and screening. We targeted two primary care settings to represent health care organizations with different insurance structures that serve rural and urban catchment areas and diverse patient populations. Primary care clinics of Emory Healthcare are part of a large academic medical center, with a mix of multiple payers. Phoebe Putney is the major healthcare system in southwestern Georgia that serves a relatively large rural population covered by Medicaid. Gaining insights from these two different primary care settings is aimed at characterizing a comprehensive array of provider and system barriers and facilitators to inform intervention strategies with the potential scalability for implementation in diverse primary care practices across Georgia.
Recruitment strategies included (1) email outreach, (2) snowball sampling, and (3) recruitment at training sessions and events such as Grand Rounds and the monthly Emory Primary Care Forum. If the providers were willing to participate in the Zoom interview, they were sent a consent form via email. At the beginning of the interview, the study team confirmed the participant's eligibility and reviewed the information included in the informed consent. The study team explained the purpose of the study and stated that participation was completely voluntary and that non-completion or withdrawal would not affect their employment status or academic standing at their institution. Participants were given the opportunity to ask questions, and if they agreed, verbal consent was obtained.
Data collection
The interview questions elicited descriptions of each participant's perceived role and experience with genetic-informed risk-stratified mammogram screening. Supplementary Table S1 shows how the constructs adapted from the CFIR are used to understand the implementation of the genetic-informed risk-stratified mammography screening guidelines. Participants were instructed to comment on a list of barriers and facilitators based on the CFIR, giving special attention to understanding which factors might be unique to their clinical setting and which are more universal, and therefore generalizable, to other healthcare systems. In addition, the interviewer (YG) asked participants to provide insights into approaches to address the identified barriers and facilitate the implementation process in primary care practice, with a particular focus on how readily the strategies can or cannot be integrated into the routine workflow.
Data analysis
Interview data were audio-recorded, transcribed, and imported to MAXQDA for analysis. We used structured methods, such as codebook development, double coding, and data interpretation/presentation. Each transcript was independently coded by two coders. Discrepancies between coders were discussed and resolved through consensus meetings to ensure reliability and consistency in the coding process. We conducted standard content analysis and thematic analysis (15) to identify distinct concepts and categories related to each interview question, such as why to accept or not the genetic-informed risk-stratified screening, barriers and facilitators to implementation, and recommended strategies for addressing barriers that are most amenable to an intervention to promote implementing guidelines in primary care practices. Extracted barriers and facilitators to screening guideline recommendations were grouped into three themes: patient-, provider-, and health care system–level factors.
Results
A total of 19 health professionals participated in semistructured qualitative interviews. Of these, 14 were primary care providers, and 5 were practice leaders (i.e., chiefs and practice directors). After de-identifying the qualitative data, interviews revealed that most participants were employed by Emory Healthcare (n = 9, 47.4%).
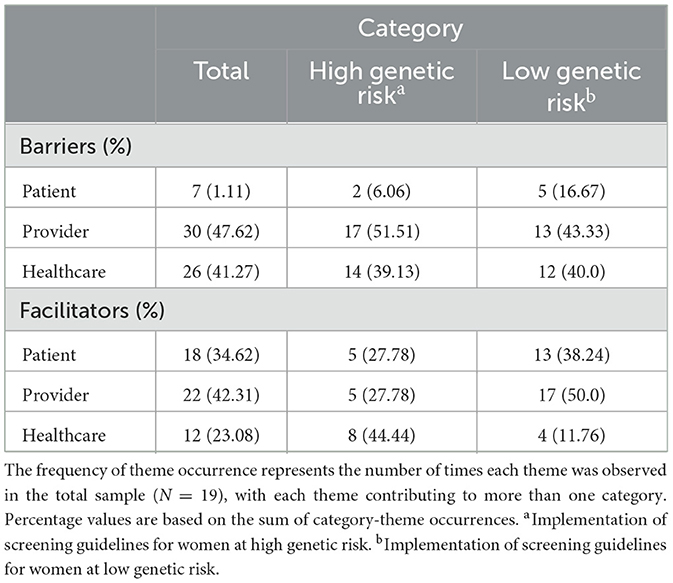
Table 1 illustrates the frequency of theme occurrence conveyed through the interview process. The most frequently reported barriers operated at the provider (n = 30, 47.6%) and healthcare system (n = 26, 41.3%) levels, regardless of risk-stratified mammography screening guidelines. Conversely, the provider (n = 22, 42.3%) and patient (n = 18, 34.6%) levels were most frequently cited as facilitators among both risk-stratified screening regimens.
Barriers and facilitators to high-risk screening pathway
Regarding the reported barriers and facilitators for women at high genetic risk (Figure 1), the most noted barriers among interviewees were time constraints (n = 10, 52.6%) and logistics (i.e., referral support; n = 9, 47.3%). In comparison, the most common facilitator was feasibility (i.e., user-friendly EHRs and referral streaming; n = 8, 42.1%).
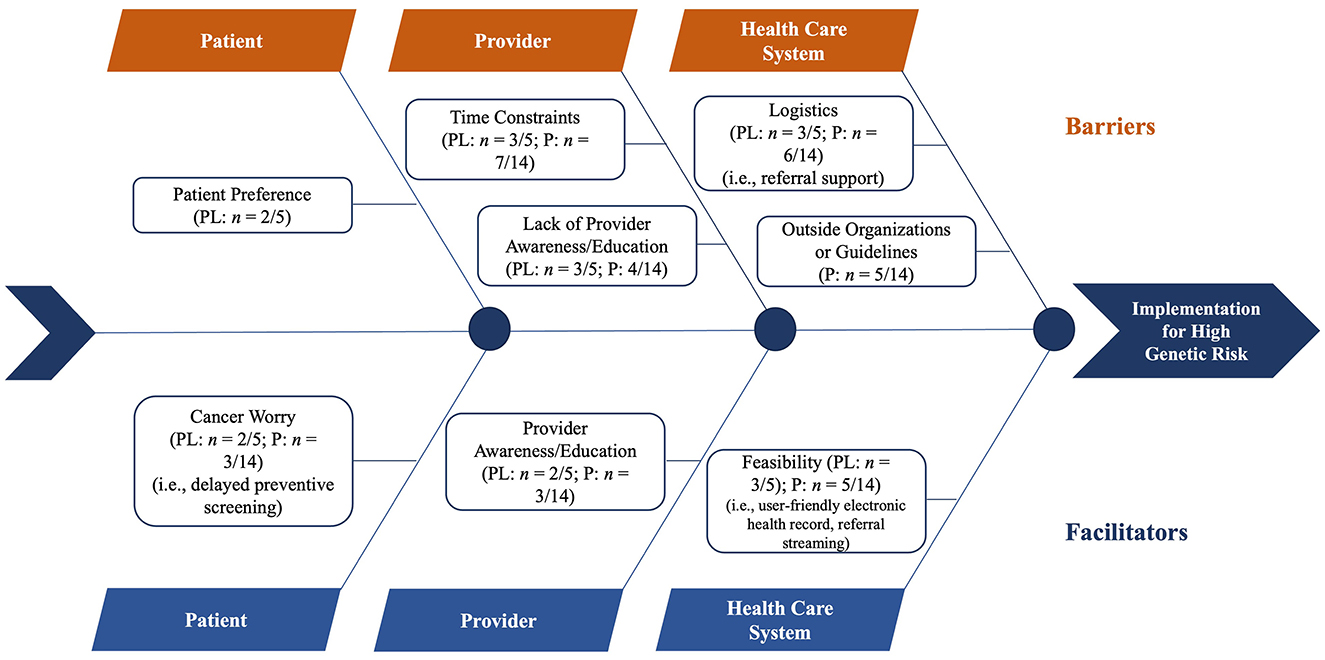
Figure 1. Meta-fishbone diagram of facilitators and barriers to implementing screening guidelines for women at high genetic risk as perceived by health professionals during in-depth interviews. PL, practice leaders; P, providers.
Patient-level barriers and facilitators
The sole barrier that emerged at the patient level was patient preference (n = 2, 10.5%). Such resistance to risk-based screening results in diagnostic delays. Based on a practice leader's prior experience, they shared:
Not every patient necessarily wants genetic screening for a few different reasons – “Do I potentially want to be pigeon-holed into this is what's wrong and now I know, and I have to do something, and I may not be able to get life insurance or certain types of insurance? So, there was definitely some things that I had to think about being at a young age and kind of what my future looks, I ended up wanting to know if I did or I didn't because I wanted to know whatever I have, I want to take care of it.” (2)
Health professionals reported cancer worry (n = 5, 26.3%) as a facilitator. The patient's family history and degree of “cancer worry” were related to identifying cancer worry as a facilitator for considering increasing genetic counseling referrals for women who had a positive result on the risk assessment tool. Describing factors that would encourage patients to consider screening recommendations, one primary care provider stated:
“Especially a lot of people may – as I mentioned – have a family history so they want to make sure that they are doing everything they should be and make sure that they are doing what is best for their health. (11)”
Provider-level barriers and facilitators
Barriers identified at the provider level encompass a lack of provider awareness or education and time constraints. Health professionals more often cited time constraints (n = 10, 52.6%) rather than a lack of provider awareness or education (n = 7, 36.8%) as a barrier to referring women for genetic counseling if they had a positive result on the risk assessment tool. Providers cited a need for additional time, mainly to fully capture all of a patient's medical history. If a patient presented with multiple complaints during an office visit, one provider stated:
Our uptake in that [high-risk screening] procedure is relatively speaking, too low. It should be higher for the types of patients that we take care of. It just seems that those tasks that involve deeper, thoughtful time-consuming discussions may not take place as quickly as, “This is something that's recommended for you. You should get it, I'm going to order it.” (8)
Given the time needed for a preventive care visit, providers suggested scheduling an additional office visit with the sole focus on high-risk screening. Recognizing the importance, a provider stated:
Time would always be helpful and certainly perhaps maybe this visit – this topic [high-risk screening] could certainly be a whole visit in and of itself, very frankly, and especially if somebody is high risk, I would want to sit down and make sure I take the time to have a proper conversation with that patient instead of just a shorter version of what I may do for a recommending routine for breast cancer surveillance. (1)
In addition to time and workload pressures in primary care, providers expressed a lack of their peers' awareness or education as a barrier to successfully implementing high-risk screening guidelines. Describing what would happen if there were a higher volume of genetic counseling referrals, one provider shared:
I think the main [barrier] is awareness of the tools and specifically when to refer someone. (13)
When referencing a lack of provider awareness, a primary care provider stated:
But truthfully, I do not know the best way to risk stratify these patients in terms of their low, medium, high risk. I would have a general understanding of, “Okay, if this patient did have a family history of breast cancer” it would raise my suspicion as more of a higher category. But then afterwards, I will say I'm not very knowledgeable on the recommended risk stratifying protocol afterwards. (1)
Participants perceived provider awareness or education (n = 5, 26.3%) as a facilitator for implementing the high-risk screening pathway. Participants mentioned that provider awareness and education promote the uptake of risk-based screening. A provider stated:
So, two ways. One is I listen to a podcast. And so, I mean this is covered in several podcasts, but the JAMA podcast, the Journal of the American Medical Association, they interviewed – they do this for each of the USPSTF guidelines. … And then the second way is through continuing medical education. (18)
Healthcare system-level barriers and facilitators
Health professionals perceived logistics (n = 9, 47.4%) and outside organizations or guidelines (n = 5, 26.3%) as barriers to implementing the high-risk screening pathway. The lack of EHR support or disruption to workflow was related to identifying logistics as a barrier when considering increasing genetic counseling referrals for women who had a positive result on the risk assessment tool. When discussing family history analyses and EHR system integration, a practice leader stated:
[I]t used to be – when we did paper records, we actually drew pedigrees and boxes and relations and stuff like that color in squares and circles and make notes and things like that. And now that we're working on a computer system, I haven't seen the ability to easily include those types of family pedigrees with relevant information. (8)
Furthermore, participants indicated a lack of institutional support in genetic-informed risk-stratified mammography referral. When discussing the decision to refer a patient to genetic counseling, a primary care provider said:
I want to refer, but who do I refer them to? And, then you've got to stop, and you've got to dig, and if you're a practice that's working with a skeletal staff, who has the time to stop and figure all that stuff out? (15)
Similarly, another primary care provider stated:
I think the main one is awareness of the tools and specifically when to refer someone. It requires me to step away from what I'm doing, go look at the screening tool, do the screening - how to do the referral. And I'll be honest, those aren't things that I have incorporated into my practice. (13)
Health professionals less frequently reported outside organizations or guidelines as a barrier when considering increasing referral of women with a positive result on the risk assessment tool for genetic counseling. The reputations of existing genetic counseling professional organizations or inadequate insurance coverage for services were related to identifying outside organizations or guidelines as a barrier.
The sole barrier that emerged at the healthcare system level was feasibility (n = 8, 42.1%). The capacity to build referral partnerships or ease of access to genetic counseling was related to identifying feasibility as a barrier.
Barriers and facilitators to low-risk screening pathway
Figure 2 illustrates the meta-fishbone diagram of reported barriers and facilitators for implementing screening guidelines for women with low genetic risk. Across practice leaders and primary care providers, the most common barriers were provider acceptance (n = 10, 52.6%) and logistics (i.e., EHR; n = 9, 47.6%). Conversely, the most common facilitators were provider awareness or education (n = 9, 47.3%) and cost (n = 7, 36.6%).

Figure 2. Meta-fishbone diagram of facilitators and barriers for implementing screening guidelines for women with low genetic risk as perceived by health professionals during in-depth interviews. PL, practice leaders; P, providers.
Patient-level barriers and facilitators
Health professionals perceived patient concern (n = 5, 26.3%) as the sole barrier to implementing the low-risk screening pathway at the patient level. Health professionals suggested that women are more likely to undergo screening should somebody they know receive an abnormal mammogram. A primary care provider stated: “Because a friend was tested or was found to have at an earlier age than 40, and they just want to get ahead of it. And I don't have any problem with it, yeah” (18). To avoid undue worry caused by delaying screening, a practice leader stated: “I think it's become too of like everybody knows somebody who has had breast cancer. So, you see a friend, a colleague, a family member, go through it and it sparks your interest” (10).
For those women who routinely screen for breast cancer, a primary care provider said:
“I think many women are uncomfortable waiting until 50 … they've done it every year, they've been told for years and years and years to do a breast self-exam every month, to get a mammogram every year. When they come in – and I'm often the first person to tell them, you don't need a mammogram until 50. (13)”
Under the patient level, facilitators that emerged include concerns about screening risks, patient preference, and cost of frequent screening. Health professionals frequently perceived the cost associated with frequent screening (n = 7, 36.8%) as a facilitator to considering delaying or reducing mammography screening before the age of 50 years. Describing the risks of the mammogram procedure and its associated out-of-pocket costs, one primary care provider stated:
If you're not having issues, it's really an unnecessary test and an additional cost to you. We talk about the risks and harms of the procedure, that it may not detect all breast cancers. It also could detect benign lumps that then we have to do further workup and there are extra costs and procedures involved to make sure that it's benign. (14)
Health professionals less frequently reported factors surrounding patient preference (n = 2, 10.5%) as a facilitator to considering delaying or reducing mammography screening before age 50. One primary care provider said: “[T]he most important thing is probably patient preference for whether they want to engage in the service early or frequently” (19). Health professionals also alluded to the shared decision-making related to screening mammography. A primary care provider described this phenomenon:
[I]f patients tell me, “I do not want to get a mammogram,” I can't force them. So, it is definitely patient preference, and it's ultimately an informed decision between the patient and the provider, and the patient has to make the final decision on whether or not they're going to get it done. (14)
Provider-level barriers and facilitators
Barriers at the provider level include provider acceptance and malpractice concerns. Health professionals frequently perceived provider acceptance (n = 10, 52.6%) as a barrier to adopting the low-risk screening guidelines. The provider's comfort level with delaying or reducing mammography screening before the age of 50 years was related to identifying provider acceptance as a barrier to the USPSTF 2016 guideline implementation. A primary care provider mentioned:
“When recommendations change to longer and less, it's sort of hard for us to get used to. Like when pap smears went from every year to every 3 years, you know? And there's still doctors that do them every year now. So, I think that moving from 40 to 50 would take us a while to feel comfortable with probably. (9)”
When asked about their major concerns regarding delaying screening, the provider stated, “Just missing something in that 10 years, you know?” (9).
Additionally, health professionals reported malpractice concerns (n = 3, 15.8%) as a barrier to implementation. The provider's awareness of the medical liability associated with failing to order mammography screening was related to identifying malpractice concerns when discussing barriers to delaying or reducing mammography screening before age 50. A primary care provider said:
“I think everyone, like providers, are pretty aware that that's one of the high liability. Missing breast cancer is pretty high liability” (12). Similarly, in reference to the 2016 USPSTF's standard of care for breast cancer, another primary care provider said, “I think providers are concerned probably about not only missing patients that've been there, I think honestly always worried about malpractice and they don't want to be blamed if they didn't order a test” (11).
Facilitators that materialized under the provider level include provider awareness or education and effective provider communication. Health professionals frequently perceived provider awareness or education (n = 9, 47.4%) as a facilitator of adopting low-risk guidelines. A primary care provider mentioned:
“[W]e stick to the habits that we've learned. So, for clinicians who are training now, if they're strongly taught 50, probably that will naturally start to delay because they just won't recommend it anymore. And, then, people like me, who have been trained a long time, we have to reeducate. (4)”
Continuing education opportunities provide an avenue for providers to stay up to date with the latest recommendations.
Furthermore, health professionals indicated the importance of effective provider communication (n = 8, 42.1%). The provider's ability to clearly communicate the benefits and harms of screening enhances adherence to risk-stratified screening regimens. One primary care provider said: “I think the most important factor is discussing with the patients their age, medical history, family history, and then having an educated conversation with them about the risks and benefits of preventive care, whether it is breast cancer screening, colon cancer screening, prostate cancer screening, among others. Furthermore, in most situations, when we have an educated discussion with them, they are happy to comply with the guidelines in the majority of cases” (5).
Healthcare system–level barriers and facilitators
Barriers identified at the health care system–level included logistics and inconsistent medical institution guidelines. Health professionals perceived logistics (n = 8, 42.1%) more frequently than inconsistent medical institution guidelines (n = 4, 21.1%) as a barrier to guideline implementation. Navigating the EHR system was related to identifying logistics when discussing barriers delaying or reducing mammography screening before age 50. During a discussion about healthcare system–level decision-making related to screening mammography, a practice leader shared:
It's harder to do when we have to go into the chart to figure out whether something has been done and shared decision-making can be done extremely well, or it can be done in a way that is very cursory. And so, that's one of those difficult problems. An example would be that advanced care planning, which is now reimbursed for providers in primary care to receive funding for the work that they do there.
Similarly, another primary care provider indicated that the EHR presents difficulties in decision support with automated patient reminders for routine screening. When asked if they could override the EHR system reminder to screen for breast cancer, one primary care provider stated:
Yeah, so you can override it, and usually in my case if I'm saying we're going to not get it this year just because of low risk, I mean, I usually document in the note too. I may write it out. But I just usually document as to, “We discussed the pros and cons of getting a mammogram at 40 and due to her low risk, patient” – and I usually will put, “Patient prefers to wait after a discussion of pros and cons.” But yeah, there is a way to get that recommendation off the list if you're not going to do it. (5)
Health professionals less frequently reported factors surrounding inconsistent medical institution guidelines as a barrier to considering delaying or reducing mammography screening before age 50. One primary care provider said:
So, if each institution has its own guidelines, it's very hard for us – or each organization has its own guidelines – so it's very difficult for an institution to adopt a firm guideline. “This is the age we're gonna start, this is the age we're gonna stop.” I think overall – as we mentioned – it's really best for each patient to really have that conversation with her provider regarding this test and then determine a plan that's best for her. (1)
The facilitators that emerged at the health care system level include logistics and consistent guidelines or recommendations. Health professionals mentioned logistics (n = 2, 10.5%) as an implementation facilitator for the low-risk screening pathway. Improvements in the EHR system were related to identifying logistics as a facilitator of guideline implementation. Health professionals also perceived consistent guidelines or recommendations (n = 2, 10.5%) as a facilitator for adopting low-risk guidelines. When asked about their thoughts on delaying or reducing mammography screening before age 50, one practice leader responded:
[I]f we're going down the line of saying that everyone is gonna go through genetic testing, and we can certainly stratify that point if you are low risk or high risk, then I think it might be more palatable to a physician to say, “Okay, I'm going to follow the United States Preventative Task Force Guidelines and starting at 50. And this is why you can start at 50, because we've tested you and you were at low risk.” (7)
Discussion
The emphasis on genetic-informed risk-stratified breast cancer screening in primary care is the logical step in implementing precision medicine. However, current efforts to promote screening uptake have primarily targeted those at the highest risk of carrying a BRCA1/2 mutation. McBride et al. (16) suggest that precision public health means carefully addressing the needs of high- and low-risk individuals, emphasizing that genomic-informed screening should be individualized for those with “negative” results as well as those at high risk. Our study showed that barriers and facilitators differ significantly between the high- and low-risk pathways, highlighting the need for tailored strategies to ensure successfully implementing a program for all.
For the high-risk pathway, primary care providers and practice leaders reported that knowledge barriers and a lack of clarity on referral pathways impeded using genetic counseling resources effectively. Facilitators of accurate, appropriate referrals for the high-risk pathway included a strong knowledge of genetics and established connections with genetic professionals, which is shown in other studies (17). These interrelationships demonstrate that provider-level and system-level resources influence referral decisions within the high-risk pathway.
EHR accessibility and streamlined referral processes emerged as critical facilitators in the high-risk pathway. These findings align with previous studies that identify EHR systems (e.g., integrating a risk assessment algorithm or platform into the EHR) as essential for supporting genomic-informed decision-making in primary care (18, 19). However, quality improvements to the EHR often require healthcare system involvement and coordinated implementation efforts. Proper training in EHR functionality may enhance adopting risk-stratified screening guidelines for high-risk cases (20).
In contrast, the low-risk screening pathway presents distinct challenges. Most women who undergo family history screening did not have BRCA1/2 mutations (21, 22), yet their risk of breast cancer is not zero. This context highlights the importance of clear communication, as implicit assumptions about negative results may lead patients to overlook ongoing risks. Conversely, women who overestimate their risk may distrust negative results and seek frequent mammograms, increasing their exposure to false positives (23). Few studies have applied theory-based communication approaches (e.g., dual-processing models and operant learning theory) to address barriers in low-risk pathways, and their effects remain limited (24). Further research is necessary to develop targeted communication strategies that effectively convey risk information and promote acceptance.
Institutional inconsistency in screening guidelines further complicates screening low-risk pathways (4). Our study found that varied guideline adoption among medical institutions contributes to barriers at the provider and system levels, particularly in screening practices for women younger than 50. Research has shown that inconsistent guidelines shape provider decision-making, potentially misaligning with USPSTF recommendations (4). To mitigate these issues, medical boards should rigorously evaluate national guidelines while institutions establish clear policies and supportive workflows.
Strengths and limitations
This study has several limitations, including the relatively small sample size of practice leaders and representatives from two southeastern healthcare systems and the subjective nature of the health professionals' responses, which reflect individual perceptions and knowledge. In addition, the findings are based solely on the perspectives of health professionals; future studies should investigate patients' perceived barriers and facilitators to risk-stratified breast cancer screening. However, these findings have implications for various healthcare settings, as we intentionally included two primary care sites with varied insurance structures, serving both rural and urban populations with a diverse patient base. Future research should examine whether these findings apply to other regions or healthcare systems with different policies, infrastructures, and resources. Our data collection and analysis, guided by fishbone diagrams and the comprehensive CFIR, enabled structured visualization of results at each level. Future research could enhance data analysis by integrating qualitative methods with natural language processing techniques, providing quantitative insights into theme importance and enabling cross-group comparisons (e.g., institutions and demographics) to identify subtle variations in perspectives (25, 26). A unique strength is the timing of data collection, completed before the recent changes to the USPSTF guidelines, allowing us to capture insights that can guide strategies for adapting to evolving and sometimes conflicting guideline recommendations in other healthcare settings, including the de-implementation of outdated practices.
Conclusion
Overall, our findings show that ongoing medical education for primary care providers and accessible clinical decision-making support for screening referrals serve as implementation facilitators for risk-stratified recommendations. By identifying the unique barriers and facilitators for high- and low-risk screening pathways, primary care clinics are better positioned to design and pilot targeted interventions that promote uptake and integration into clinical practice. Future risk-stratified screening programs should consider these insights, addressing the specific needs of high- and low-risk pathways simultaneously to optimize program effectiveness and sustainability.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Emory University. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because that the research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside of the research context.
Author contributions
YG: Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation. HB: Visualization, Formal analysis, Writing – original draft. CE: Validation, Visualization, Writing – review & editing, Conceptualization, Supervision. MC: Investigation, Resources, Validation, Visualization, Writing – review & editing. SA: Investigation, Resources, Validation, Visualization, Writing – review & editing. TJ: Conceptualization, Investigation, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Winship Invest$ Grant from the Winship Cancer Institute of Emory University (2020). The research reported in this publication was supported in part by the Intervention Development, Dissemination and Implementation Shared Resource of Winship Cancer Institute of Emory University and the National Institutes of Health/National Cancer Institute under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
The research reported in this publication was supported in part by the Intervention Development, Dissemination and Implementation Shared Resource of Winship Cancer Institute of Emory University and the National Institutes of Health/National Cancer Institute under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. US Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US preventive services task force recommendation statement. JAMA. (2019) 322:652–65. doi: 10.1001/jama.2019.10987
2. Owens DK, Whitlock EP, Henderson J, Pignone MP, Krist AH, Bibbins-Domingo K, et al. Use of decision models in the development of evidence-based clinical preventive services recommendations: methods of the US Preventive Services Task Force. Ann Intern Med. (2016) 165:501–8. doi: 10.7326/M15-2531
3. Ren W, Chen M, Qiao Y, Zhao F. Global guidelines for breast cancer screening: a systematic review. Breast. (2022) 64:85–99. doi: 10.1016/j.breast.2022.04.003
4. Onega T, Haas JS, Bitton A, Brackett C, Weiss J, Goodrich M, et al. Alignment of breast cancer screening guidelines, accountability metrics, and practice patterns. Am J Manag Care. (2017) 23:35–40.
5. Bond M, Pavey T, Welch K, Cooper C, Garside R, Dean S, et al. Systematic review of the psychological consequences of false-positive screening mammograms. Health Technol Assess. (2013) 17:1–170. doi: 10.3310/hta17130
6. Tosteson AN, Fryback DG, Hammond CS, Hanna LG, Grove MR, Brown M, et al. Consequences of false-positive screening mammograms. JAMA Intern Med. (2014) 174:954–61. doi: 10.1001/jamainternmed.2014.981
7. Ahmed AT, Welch BT, Brinjikji W, Farah WH, Henrichsen TL, Murad MH, et al. Racial disparities in screening mammography in the United States: a systematic review and meta-analysis. J Am Coll Radiol. (2017) 14:157–65.e9. doi: 10.1016/j.jacr.2016.07.034
8. Ganguly AP, Baker KK, Redman MW, McClintock AH, Yung RL. Racial disparities in the screening mammography continuum within a heterogeneous health care system. Cancer. (2023) 129:3171–81. doi: 10.1002/cncr.34632
9. Guan Y, Condit CM, Escoffery C, Bellcross CA, McBride CM. Do women who receive a negative BRCA1/2 risk result understand the implications for breast cancer risk? Public Health Genom. (2019) 22:102–9. doi: 10.1159/000503129
10. Norton WE, Chambers DA. Unpacking the complexities of de-implementing inappropriate health interventions. Implement Sci. (2020) 15:2. doi: 10.1186/s13012-019-0960-9
11. Guo Y, Cheng TC, Yun Lee H. Factors associated with adherence to preventive breast cancer screenings among middle-aged African American Women. Soc Work Public Health. (2019) 34:646–56. doi: 10.1080/19371918.2019.1649226
12. Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S, et al. Tailored interventions to address determinants of practice. Cochrane Database Syst Rev. (2015) 2015:CD005470. doi: 10.1002/14651858.CD005470.pub3
13. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. (2009) 4:50. doi: 10.1186/1748-5908-4-50
14. Siu AL, Force UPST. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. (2016) 164:279–96. doi: 10.7326/M15-2886
15. Huberman M, Miles MB. The Qualitative Researcher's Companion. New York: Sage. (2002). doi: 10.4135/9781412986274
16. McBride CM, Guan Y, Hay JL. Regarding the yin and yang of precision cancer- screening and treatment: are we creating a neglected majority? Int. J Environ Res Public Health. (2019) 16:4168. doi: 10.3390/ijerph16214168
17. Seibel E, Gunn G, Ali N, Jordan E, Kenneson A. Primary care providers' use of genetic services in the southeast United States: barriers, facilitators, and strategies. J Prim Care Community Health. (2022) 13:21501319221134752. doi: 10.1177/21501319221134752
18. Wurtmann EJ, Baldinger S, Olet S, Daley A, Swenson KK. An electronic health record tool increases genetic counseling referral of individuals at hereditary cancer risk: an intervention study. Public Health Genom. (2022) 27:1–7. doi: 10.1159/000525447
19. Del Fiol G, Kohlmann W, Bradshaw RL, Weir CR, Flynn M, Hess R, et al. Standards-based clinical decision support platform to manage patients who meet guideline-based criteria for genetic evaluation of familial cancer. JCO Clin Cancer Inform. (2020) 4:1–9. doi: 10.1200/CCI.19.00120
20. Musa S, Dergaa I, Al Shekh Yasin R, Singh R. The impact of training on electronic health records related knowledge, practical competencies, and staff satisfaction: a pre-post intervention study among wellness center providers in a primary health-care facility. J Multidiscip Healthc. (2023) 16:1551–63. doi: 10.2147/JMDH.S414200
21. Brannon Traxler L, Martin ML, Kerber AS, Bellcross CA, Crane BE, Green V, et al. Implementing a screening tool for identifying patients at risk for hereditary breast and ovarian cancer: a statewide initiative. Ann Surg Oncol. (2014) 21:3342–7. doi: 10.1245/s10434-014-3921-1
22. Joseph G, Kaplan C, Luce J, Lee R, Stewart S, Guerra C, et al. Efficient identification and referral of low-income women at high risk for hereditary breast cancer: a practice-based approach. Public Health Genom. (2012) 15:172–80. doi: 10.1159/000336419
23. Guan Y, Nehl E, Pencea I, Condit CM, Escoffery C, Bellcross CA, et al. Willingness to decrease mammogram frequency among women at low risk for hereditary breast cancer. Sci Rep. (2019) 9:9599. doi: 10.1038/s41598-019-45967-6
24. Guan Y, Haardörfer R, McBride CM, Escoffery C, Lipscomb J. Testing theory-based messages to encourage women at average risk for breast cancer to consider biennial mammography screening. Ann Behav Med. (2023) 57:696–707. doi: 10.1093/abm/kaad018
25. Abram MD, Mancini KT, Parker RD. Methods to integrate natural language processing into qualitative research. Int J Qual Methods. (2020) 19:608. doi: 10.1177/1609406920984608
Keywords: cancer screening, family history and cancer, mammogram, public health genomics, CFIR
Citation: Guan Y, Barge H, Escoffery C, Cellai M, Alfonso S and Johnson II TM (2025) Examining barriers and facilitators to implementing evidence-based genetic risk-stratified breast cancer screening in primary care. Front. Cancer Control Soc. 3:1521486. doi: 10.3389/fcacs.2025.1521486
Received: 01 November 2024; Accepted: 10 March 2025;
Published: 02 April 2025.
Edited by:
Edīns Miklaševičs, Riga Stradiņš University, LatviaReviewed by:
Luis Mariano Esteban, University of Zaragoza, SpainYufan Guan, Virginia Commonwealth University, United States
Copyright © 2025 Guan, Barge, Escoffery, Cellai, Alfonso and Johnson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Guan, eXVlLmd1YW5AZW1vcnkuZWR1
 Yue Guan
Yue Guan Haley Barge
Haley Barge Cam Escoffery
Cam Escoffery Michele Cellai3,4
Michele Cellai3,4