- 1Cancer Care Program, Nova Scotia Health, Halifax, NS, Canada
- 2Physical Activity and Cancer Lab, Dalhousie University and Nova Scotia Health, Halifax, NS, Canada
- 3School of Health and Human Performance, Division of Kinesiology, Faculty of Health, Dalhousie University, Halifax, NS, Canada
- 4John W. Lindsay YMCA, Health Management, Halifax, NS, Canada
- 5Faculty of Kinesiology, University of Calgary, Calgary, AB, Canada
- 6Department of Oncology, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
- 7Department of Psychosocial Resources, Tom Baker Cancer Centre, Alberta Health Services, Calgary, AB, Canada
- 8Faculty of Health, Dalhousie University, Halifax, NS, Canada
- 9Faculty of Rehabilitation, University of Alberta, Edmonton, AB, Canada
- 10Division of Medical Oncology, Department of Medicine, Dalhousie University, Nova Scotia Health, Halifax, NS, Canada
- 11Beatrice Hunter Cancer Research Institute, Halifax, NS, Canada
Introduction: Strong evidence supports the recommendation that individuals living with or beyond cancer (LWBC) should be physically active and engage in physical exercise to enhance health and improve cancer-related outcomes. Many individuals LWBC are not achieving these benefits, partly due to a lack of resources. To address this, Activating Cancer Communities through an Exercise Strategy for Survivors (ACCESS) was developed to provide exercise programming and investigate exercise strategies and barriers for those LWBC.
Methods: Using an effectiveness-implementation design, adults LWBC joined ACCESS by healthcare provider or self-referral. A clinical exercise physiologist triaged participants to either a hospital-based site or one of two community-based sites to complete a 12-week, 24-session multimodal individualized exercise program. Physical fitness and multiple patient-reported outcomes were measured pre- and post-intervention.
Results: Between January 2018 and March 2020, there were 332 referrals. Of these, 122 participants consented and completed the study. Completing ACCESS was associated with improvements in physical fitness and participant-reported outcomes, including general wellbeing, fatigue, negative emotional states, sleep quality, and exercise self-efficacy. The program was well-received by participants and was deemed feasible and acceptable from an implementation perspective.
Discussion: The ACCESS program demonstrably improved several health outcomes for individuals LWBC. Implementation outcomes have and continue to guide ongoing efforts to improve accessibility to ACCESS and work with the regional health authority and cancer care program to support the adoption of exercise into standard oncology care.
Clinical trial registration: clinicaltrials.gov, identifier [NCT03599843].
1 Introduction
The field of exercise oncology has grown rapidly in the past few decades (1–4), with over 2,500 clinical trials providing evidence of the safety, feasibility and benefits of exercise for those living with or beyond cancer (LWBC) (5). The benefits of exercise include reduced disease and treatment-related side effects (e.g., anxiety, depression, muscle loss/weakness, cancer-related pain, fatigue), as well as a reduced risk of disease recurrence and cancer mortality (5–8). Drawing on this research, several international evidence-based guidelines for improving health outcomes for individuals LWBC have been published and continue to evolve as additional evidence becomes available (5, 9, 10). To achieve these benefits however, individuals must be able to adopt and maintain a regular exercise program. However, even with the development of evidence-based guidelines, exercise has not yet been widely implemented as a standard of care in the oncology setting. While there are many contributing factors, a lack of resources, exercise expertise, and awareness of benefits are notable barriers (11–13). Consequently, a large majority of individuals LWBC do not meet exercise guidelines, and promoting and supporting habitual exercise behavior remains an ongoing challenge (14, 15).
Building on the work of the Alberta Cancer Exercise (ACE) model (16), we created Activating Cancer Communities through an Exercise Strategy for Survivors (ACCESS). ACCESS is a hybrid effectiveness-implementation study designed to pilot the implementation of exercise programming in cancer care and to examine the effectiveness of individualized exercise programming to improve health outcomes in persons LWBC in Nova Scotia, Canada. Given the relative lack of cancer-specific exercise programming in our region (13), ACCESS will improve access to programming through (a) the development of a network of clinical and community partnerships, (b) provision of cancer-specific exercise education and training to qualified exercise professionals (QEP; www.thrivehealthservices.com), (c) building clinician and self-referral pathways, and (d) participant screening and triage by a Clinical Exercise Physiologist (CEP) to appropriate exercise programming. Implementing ACCESS in “real-world,” practice-based conditions provides a pragmatic model of a standard of care (17–19). This type of practical approach to research settings is critical when investigating the implementation of exercise programming in cancer care (20, 21).
The primary purpose of ACCESS is to achieve the following in a real-world setting: (1) examine implementation outcomes (e.g., clinician and self-referral to hospital and/or community-based cancer exercise program, recruitment/accrual, participant retention and program adherence, safety); and (2) explore benefits of program participation in hospital and community-based programming (i.e., participant satisfaction, fitness, quality of life). This interim report is based on the data from participants that completed ACCESS prior to the COVID-19 pandemic, which forced a temporary pause of programming.
2 Materials and methods
2.1 Study design and procedures
ACCESS is a type 2 effectiveness-implementation study that models a standard of care in an oncology setting (22). The hybrid design allows for the testing and evaluation of both the effectiveness of the intervention as well as insight into implementation strategies in real-world settings. The study was approved by the Nova Scotia Health Research Ethics Board and is registered at ClinicalTrials.gov (NCT03599843). All participants included in this report partook in the study between the study's first recruitment in January 2018 until March 2020, when the program temporarily shut down due to the COVID-19 pandemic. Following a virtual restart in August 2020, ACCESS continues to be active in a hybrid (online or in-person) format as of January 2024. Consenting participants underwent baseline and post-intervention testing including mental and physical health-related measures (described below). Additional questionnaires regarding program evaluation and implementation were completed following the exercise intervention. The Consort guidelines were referenced when preparing this manuscript (23).
Following informed consent, participants were screened by the study's coordinator and CEP using the Physical Activity Readiness Questionnaire (PAR-Q+) (24) and by self-reporting their relevant medical history (e.g., cancer diagnosis and treatment status, co-morbid disease, medication use, etc.) to confirm readiness to participate in an exercise program. If participants were not able to recall diagnostic and treatment details, the ACCESS CEP sought explicit consent to review/abstract relevant medical history to design a safe and effective exercise program. If safety issues were identified, the coordinator and/or CEP consulted the participant's oncologist or primary healthcare provider for additional evaluation and/or recommendation to participate in a supervised exercise program.
2.2 Recruitment
Participants were directly referred to ACCESS either by healthcare provider referral (e.g., oncologists, oncology nurses, primary care physicians, etc.) or self-referral. Following clinical or self-referral and confirmation of participant interest, prospective participants were contacted by phone to discuss the study in greater detail and explore study participation and/or other physical activity options (e.g., education) that were most suited to the individuals' needs, abilities, and activity goals.
2.3 Eligibility criteria
Eligible participants for ACCESS had to: (1) have a diagnosis of cancer or a history of cancer; (2) be ≥18 years old; (3) be able to participate in low/mild levels of physical activity at a minimum [assessed via PAR-Q+ (24) with consideration for cancer specific concerns]; (4) be pre-treatment, receiving active treatment, or have received cancer treatment within the past 5 years or have late occurring/ongoing side-effects as a result of the cancer diagnosis (e.g., fatigue); (5) be able and willing to attend a twice weekly exercise program at our central hub (high and low-to-moderate risk groups) under the supervision of a CEP, or at one of our community partner exercise facilities (low-to-moderate risk group only) under the supervision of a QEP; and (6) be able to provide informed written consent in English. For the purpose of this study, the CEP identified participants as either “high risk” or “low-to-moderate risk.” “High risk” was defined as those who: (1) had a previous cardiac event (e.g., myocardial infarction, stroke) or have unstable cardiovascular disease; (2) were currently receiving a known cardiotoxic agent (e.g., currently receiving an anthracycline-based therapy); and (3) those with known bone metastases or advanced stage disease. High risk participants were always supervised by a CEP and required physician/oncologist clearance. Anyone not classified as “high risk” was deemed “low-to-moderate risk.” All community-based programming was supported by the ACCESS CEP and served those participants cleared to exercise under a “Cancer and Exercise” trained QEP (see www.thrivehealthservices.com), but without medical needs that would require direct CEP supervision.
2.4 Intervention
The intervention consisted solely of a multimodal exercise program which included a combination of aerobic, resistance, balance, and flexibility exercises delivered in a circuit-type class setting or group personal training format, twice weekly, for 12-weeks. If participants could not attend a session, accommodations were made so they would complete the missed session at a later date with the aim that each participant would complete 24 sessions total. Exercise programs were tailored to each participants' unique needs, goals, interests, and abilities. Participants self-identified their fatigue and energy before each session, and their rating of perceived exertion immediately following each session, on a 10-point scale, where higher values mean higher fatigue, energy, and perceived exercise intensity. Each training session lasted between 45–60 min and included time for both a warm-up and cool-down. Participants were also instructed to inform the CEP and/or QEP if they felt that any exercise was beyond or below their comfort level or ability, so that the exercises could be modified to better suit their needs.
2.5 Cancer specific education and training
All fitness professionals (CEPs and QEPs) completed the “Cancer and Exercise: Training for Fitness Professionals” course offered through Thrive Health Services. To create a supply of QEPs, all cancer-specific exercise training was subsidized using grant funds. This online training program provides fitness professionals with the skills and knowledge to work with individuals LWBC to ensure their ongoing safety and success in an exercise setting.
2.6 Outcomes to support implementation
The RE-AIM (Reach Effectiveness-Adoption Implementation Maintenance) framework was referenced when designing the evaluation of effectiveness and implementation outcomes (25, 26). Embedded within RE-AIM is a cost-effectiveness analysis pertaining to individual and institution costs associated with program participation and delivery. Reach was assessed using data on referral frequency, accrual rate (number of participants who consented to participate divided by the number of eligible participants), reasons for exclusions/refusals to participate, and characteristics of participants who consented/declined participation. Effectiveness was assessed using fitness and patient reported outcomes as described above. Program retention and costs associated with program delivery were recorded. Adoption was assessed by recording rates of clinician and self-referral. Implementation assessment included investigating barriers/facilitators to delivery for participants using a 0%−100% scoring chart across multiple domains. Thirteen questions related to exercise preference were provided alongside nine questions identifying barriers to exercise, including key barriers like travel and motivation. Participants were provided with these questions at the start of the exercise program. Program fidelity was assessed by examining how many participants adhered to the protocol's weekly frequency and total exercise sessions. Maintenance will be assessed in future reports by considering factors that promote delivery of the intervention over the long term at the site and individual level. These implementation outcomes fit into a broader taxonomy outlined by Proctor and colleagues, which are discussed in detail below (27).
2.7 Effectiveness outcome measures
2.7.1 Physical measurements
Participants' height, weight, waist and hip circumference, resting heart rate, resting blood pressure, 6-min walk test (6MWT), hand grip strength, 30-second chair stand, single legged stance, sit-and-reach, and shoulder flexibility were assessed based on protocols from the Canadian Society of Exercise Physiology's Physical Activity Training for Health Protocol (CSEP-PATH) (28) and the Senior Fitness Test (29). Improvements in physical fitness measures were assessed to determine effectiveness. Non-modifiable characteristics and smoking/alcohol history were recorded for descriptive purposes.
2.7.2 Participant-reported outcomes
Participant-reported outcome measures included quality of life [Functional Assessment of Cancer Therapy–General (FACT-G)] (30), fatigue [Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F)] (31), physical activity behavior (Godin Leisure Time Exercise Questionnaire, GLTEQ) (32–34), sedentary time (International Sedentary Assessment Tool, ISAT) (35), sleep quality (Pittsburgh Sleep Quality Index, PSQI) (36), negative emotional states [Depression Anxiety Stress Scales-42 (DASS-42)] (37), and a post-intervention participant satisfaction survey. General health was self-reported on a 1–5 scale, where higher values indicate worse health. The participant satisfaction survey asked participants to rate the program across different dimensions in two domains: program review (i.e., a critique of the program's administration) and instructional review (i.e., a critique of the program's instruction), both on a 5-point scale of agreeableness. Participants also completed a 13-item survey designed to assess self-efficacy related to exercising, exercise adherence, and physical function on a 10-point scale ranging from 0%−100%.
2.8 Medical record follow-up
An exploratory post hoc analysis of emergency department visits, inpatient stays, and survival was performed between participants that finished the program vs. those that withdrew. This was performed to better understand what differences in medical system usage may be present between participants that completed the ACCESS program vs. those that practically forewent it for a variety of reasons, providing a surrogate control group. Of note, only 13% of participants withdrew from the program as a result of poor health (Figure 1), suggesting that any differences observed are only partially due to changes in health status.
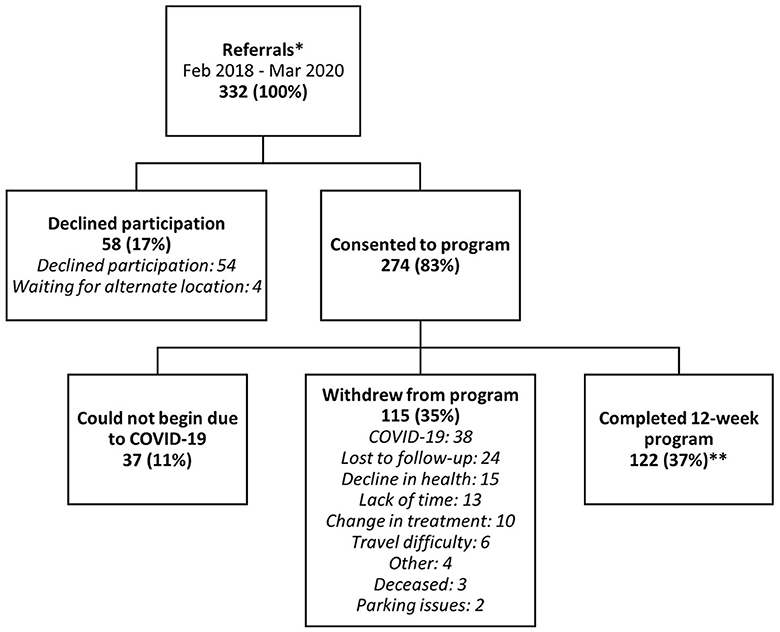
Figure 1. Participant flow and total number of participants for each stage of ACCESS, with percentage retained from the original referral number (332) in brackets. *See Supplementary Figure 1 for a breakdown of referral source, program location, and ethnicity of participants. **Completion was 61% while omitting COVID-19-related withdrawals.
2.9 Statistical approach
2.9.1 Health outcomes
All health-related outcomes were analyzed using only data from participants that completed the 12-week exercise program. Continuous outcomes with two serial measurements were analyzed using a paired t-test. Data were dichotomized when guidance was available (e.g., so-called “normal” or “disturbed” sleepers for the PSQI) and analyzed using a two-factor (time × baseline level) analysis of variance (ANOVA) followed by Šídák's multiple comparisons post hoc test.
2.9.2 Record review
For hospital visits and stays, a Mann-Whitney U-test was performed. For survival, contingency tables were created for those that did or did not complete the program crossed with whether or not survival was present at the end of follow-up and analyzed using a χ2 test.
2.9.3 General
Data are presented as mean ± standard deviation unless otherwise stated and were analyzed using GraphPad Prism version 9.5.1 for Windows, GraphPad Software, Boston, Massachusetts USA, www.graphpad.com. Statistical significance was considered at or below the error rate of α = 0.05. Currency was converted to USD from CAD on 15 March 2024 unless otherwise specified (exchange rate: $1 USD to $1.35 CAD).
3 Results
3.1 Recruitment
A total of 332 persons LWBC were referred to the ACCESS program between January 2018 and March 2020 (Figure 1). Referrals peaked approximately 1-year intro recruitment, and declined gradually until the trial was paused due to COVID-19 (Supplementary Figure 1). Fifty-seven percent of the referrals came from oncologists, 11% from oncology nurses, whereas 23% were self-referrals (Supplementary Figure 2). The remaining referrals were from other health care providers (e.g., non-oncology physician or other non-oncology healthcare providers).
Of those referred, 274 people consented to the program (83% accrual). Due to the proximity to the COVID-19 pandemic, 37 participants were unable to begin the study and were thus censored from completion analyses given the extraordinary nature of the pandemic. A total of 122 completed the program at the location most appropriate and convenient for them. Most participants (n = 89, 73%) completed ACCESS at the hospital site, while 27% (n = 33) were split between the two community centers. Notably, 38 enrolled participants had to withdraw from the program due to the perceived risk of COVID-19. Thus, the program retained 51%, or 122 of 237 of participants (i.e., attrition was 49%). However, this was still contaminated with the 38 people that began the program but were forced to withdraw due to COVID-19. Applying the rates of attrition and completion calculated without these 38 provides an estimated final attrition of 39% and a completion rate of 61%. The main reasons for withdrawal are in Figure 1.
3.2 Participants
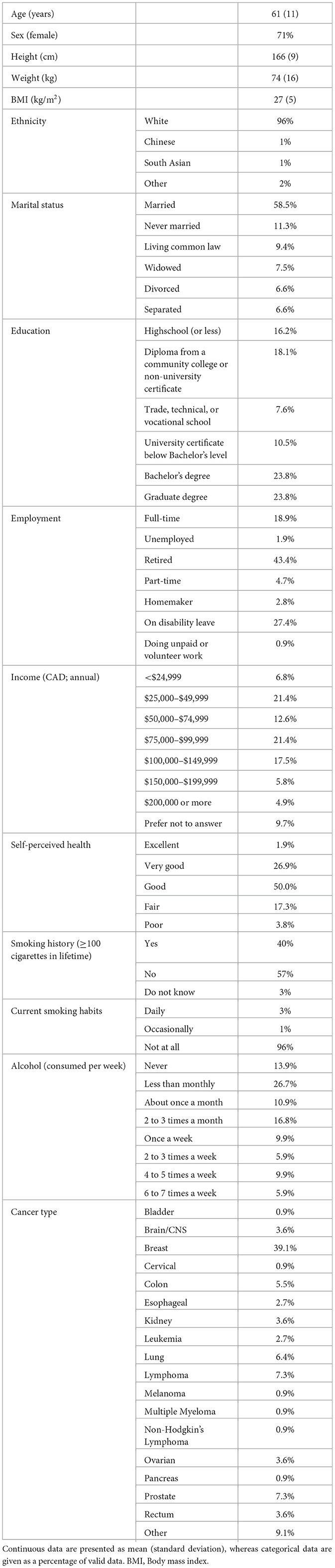
The baseline characteristics for participants that completed the program are listed in Table 1. Participant age ranged from 29–85 years and had a median self-assessed health of “Good” (Supplementary Figure 3). The most common cancer types for participants that completed ACCESS were breast (39%), followed by prostate (7%), lymphoma (7%), and lung (6%).
3.3 Program fidelity
The protocol of ACCESS was to invite all participants to complete two exercise sessions per week for 12-weeks to accrue 24 sessions total. At this frequency it should theoretically take 84 days. However, this was difficult to maintain for most participants. The average length of time to complete the program was 121-days, averaging 1.4 sessions per week across participants. Eight participants completed the 24-sessions either in 12-weeks or within seven days of the 12-week mark, while five participants completed all 24-sessions of ACCESS in fewer than 12-weeks. The range of time-to-completion was 6–38 weeks. The proportion of participants that finished the program by completing all 24 sessions was 92%, with 8% completing 20-sessions or fewer. Average attendance to the program was 22.8 sessions (95%).
3.4 Outcomes to support implementation at the individual level
3.4.1 Costs for participants
Participants were asked to share information about costs associated with attending the program related to purchasing clothes, parking costs, and travel costs (Table 2). Eighteen participants provided data on all three, with the average cost of $224 USD with a standard deviation of $135. The range was $5 to $471 USD.
3.4.2 Exercise preferences and facilitators
Participants shared their facilitators (13 questions) and perceived barriers (nine questions) for exercise and using an arbitrary cut-off of a median response above 75%, the following attributes were found to be important for participants: having a trained instructor; being able to stay involved in a regular program; doing exercise that makes them feel good; exercising outdoors; receiving feedback; having challenging exercises; and exercising to improve health (Supplementary Figure 4).
3.4.3 Barriers to exercise
Barriers were assessed using the same format as facilitators and are reported in Supplementary Figure 5. Using an arbitrary cutoff of a median response above 25% (i.e., below the uppermost 75%), barriers were wanting more information about recommended exercises; feeling too tired to exercise; and it being too difficult to begin exercising.
3.5 Outcomes to support implementation at the program level
Between April 2018 and March 2020, a total of $36,631.14 USD was spent to support the entire ACCESS program, or $18,315.57 USD per 12-month period (Supplementary Table 1). The per capita cost for the 122 participants that completed the program was $300.25 USD. When including consenting participants that withdrew (excluding the 37 that could not begin due to the pandemic), the per capita cost was $155 USD for 237 participants. Participants were reimbursed for parking costs, which totaled $3,239 USD. Programming fees for participants that attended satellite sites totaled $2,839 USD ($86 per participant), which was paid to the community center to subsidize costs for participants on a per-person basis. It is important to note that these costs do not include the purchasing of exercise equipment or administrative tools, like computers, as these were re-purposed from existing resources.
3.5.1 Program staffing
The program was sustained by a combined part-time research coordinator and CEP position with an annual salary of $15,042 USD ($30,084 between April 2018 and March 2020). QEPs at community centers were paid by their centers. In addition, students from a local kinesiology program (approximately six to eight, which varied by semester) supported the program and were supervised by the CEP as part of an experiential learning opportunity.
3.5.2 Equipment maintenance
A total of $469 USD was spent on maintaining equipment between April 2018 and March 2020. These funds were spent on water station refilling and exercise machine maintenance.
3.6 Physical measures, fitness, and activity levels
Weight and body mass index (kg/m2) were unchanged following the program (p = 0.211 and 0.556, respectively). Waist circumference decreased on average by 2 cm (p < 0.001). Left and right shoulder flexibility remained unchanged (p = 0.552 and 0.569, respectively), and there were no changes in single-leg balance with eyes open or closed for left or right sides (p > 0.37 for all). However, participants that completed the intervention had improved personal fitness and activity levels following the exercise intervention (Figure 2). Mean 6 MWT distance was 55 m greater following ACCESS (p < 0.001), chair stands increased by an average of 4.4 stands/30 seconds (p < 0.001), mean sit and reach scores increased by 4 cm (p < 0.001), and combined grip strength scores increased by 1.8 kg (p = 0.016). Average levels of physical activity (GLTEQ's leisure score index) increased by 11.6 points (p < 0.001), while average sedentary hours per week measured by the ISAT dropped by 3.22 h (p = 0.016).
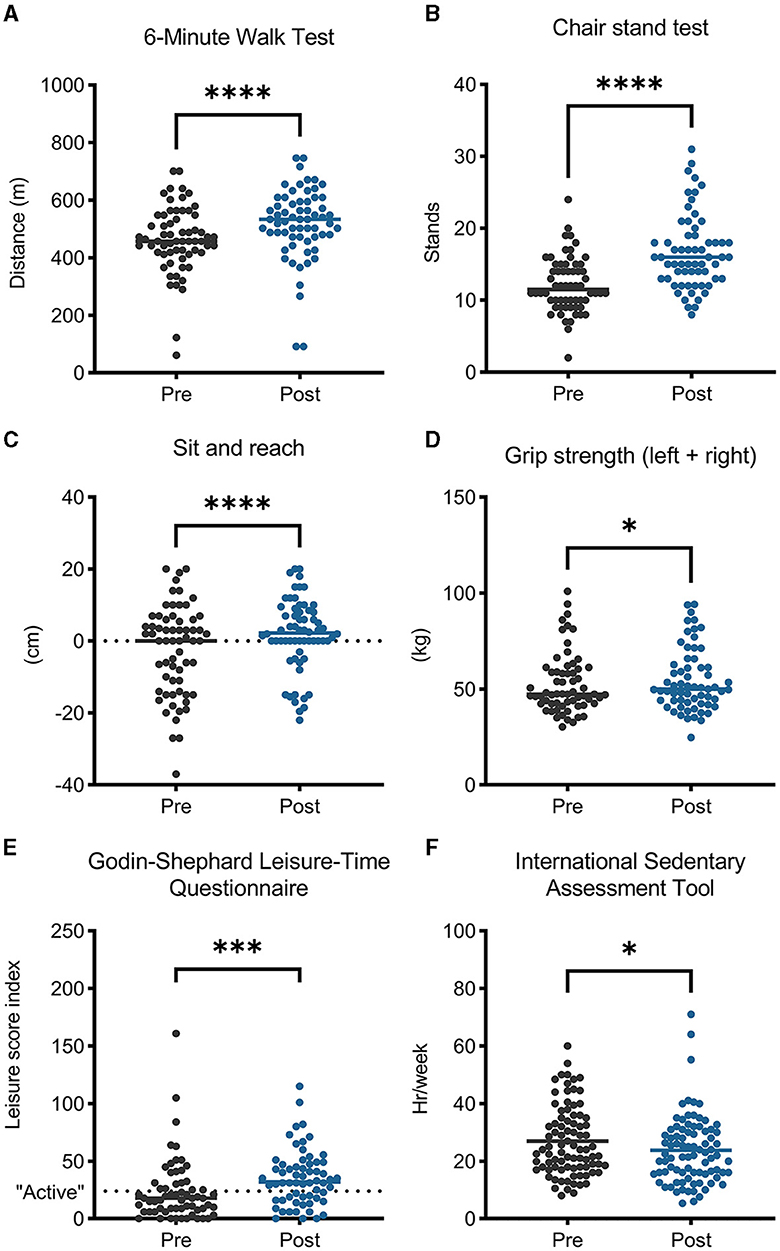
Figure 2. Physical fitness, activity levels, and sedentary behavior improved after participating in the ACCESS program. *p < 0.05; ***p < 0.001; ****p < 0.0001.
3.7 Patient-reported outcomes
3.7.1 General wellbeing and fatigue
General wellbeing mean scores, measured using the FACT-G, increased (improved) by 4.3 points (p < 0.001; Figure 3). An analysis of the four subdomains revealed that mean scores improved for the physical (p = 0.002), emotional (p = 0.001), and functional subdomains (p < 0.001), while the social wellbeing subdomain did not change (p = 0.889). Cancer-related fatigue measured using the FACIT-F improved following ACCESS, with mean scores increasing by 4.2 points (p < 0.001).
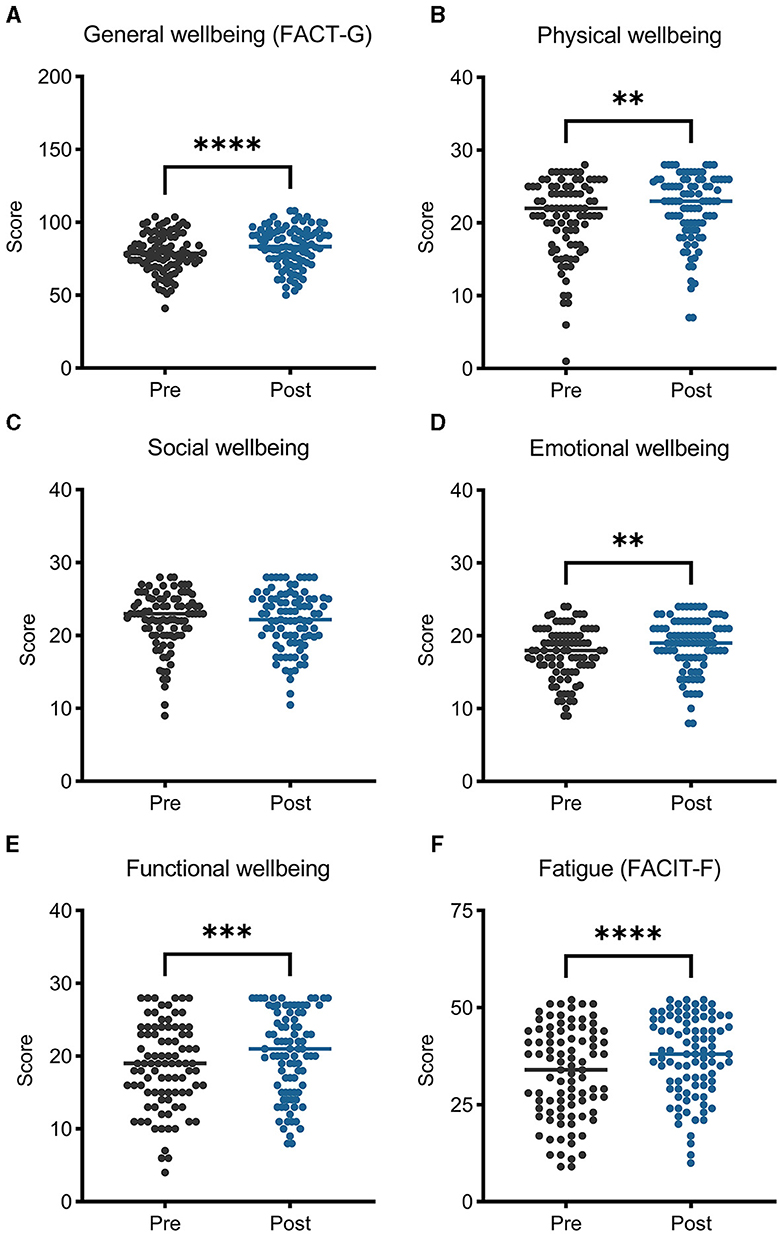
Figure 3. General wellbeing (A–E) improved after participating in ACCESS, measured by the Functional Assessment of Cancer Therapy–General (FACT-G), broken down into its physical, social, emotional, and functional subscales. Panel (F) depicts fatigue levels, which improved and were assessed with the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) fatigue subscale. Higher scores equal better wellbeing/less fatigue. **p < 0.01; ***p < 0.001; ****p < 0.0001.
3.7.2 Negative emotional states
Combined average levels of depression, anxiety, and stress did not change significantly (p = 0.101; Supplementary Figure 6), although there were significant changes in the depression and anxiety subscales (p = 0.037 and 0.029, respectively). A two-factor (time × severity) analysis of variance revealed that individuals experienced significant improvement if they entered the ACCESS program with higher than normal (37) levels of depression, anxiety, or stress (p < 0.01 for all interactions). Post hoc tests revealed significant improvements in depression (p < 0.0001), anxiety (p < 0.0001), and stress (p = 0.013) for those that began with higher-than-normal levels, while those within the normal ranges tended to stay there (Figure 4).
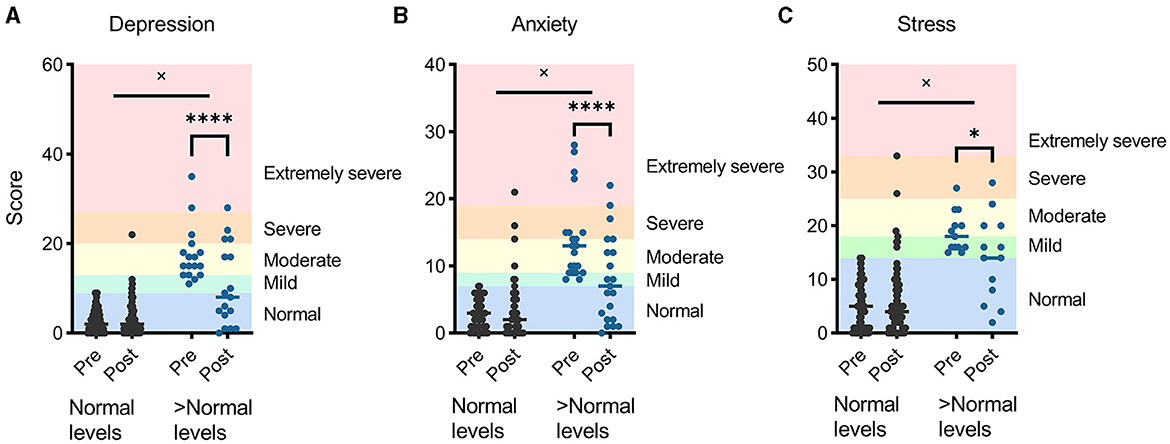
Figure 4. Negative emotional states of depression (A), anxiety (B), and stress (C), measured using the Depression, Anxiety, and Stress Subscales-42, improved for participants that began ACCESS with higher than “normal” levels. *p < 0.05; ****p < 0.0001; × denotes a significant interaction between baseline levels and time.
3.7.3 Sleep quality
A statistically significant mean decrease (improvement) of 0.5 points was observed across all participants (Figure 5). A subsequent two-factor ANOVA (time × baseline level) using a cut-off of 5 to indicate disturbed sleep was performed (38). A post hoc analysis revealed that disturbed sleepers improved their sleep scores following the ACCESS program by an average of 1.2 points (p = 0.0002), while those that scored below the cutoff of 5 at baseline did not see a significant change.
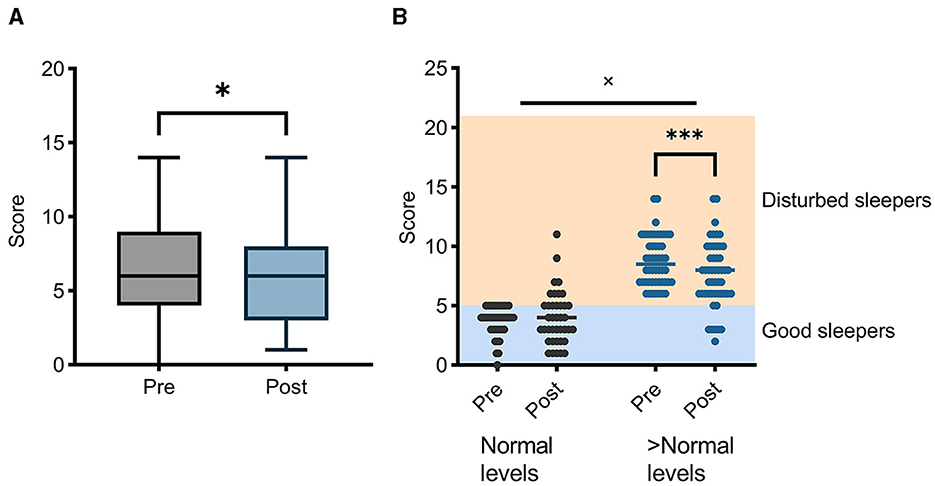
Figure 5. Pittsburgh Sleep Quality Index (PSQI) levels improved following participation in ACCESS (A). This effect was driven by individuals with poorer sleep quality (B). *p < 0.05; ***p < 0.001; × denotes a significant interaction between baseline levels and time.
3.7.4 Self-efficacy
Confidence in being able to exercise while feeling tired, lacking discipline, nauseated, or disinterested, or lacking time was improved (p < 0.05 for all; Supplementary Figure 7). On the other hand, confidence in exercising when is it not a priority, in poor weather, when it is not enjoyable, or without social support remained unchanged. Self-rated confidence to walk briskly, jog, climb stairs, and/or exercise at a higher intensity also increased (p < 0.05 for all).
3.7.5 Intra-session states
Ratings of fatigue and energy remained similar across each session, although ratings of exertion were significantly higher in session two vs. session one and continued to rise until approximately session four (Supplementary Figure 8).
3.8 Self-rated program satisfaction
Participants reported that the program was enjoyable, well-organized, sufficiently challenging, and generally effective at increasing strength, and physical function (Supplementary Figure 9). Further, session length, the training location, timing, and modalities were well-received. On the other hand, ratings of parking convenience revealed that many participants found parking difficult. Participants rated levels of preparedness, knowledge, and interpersonal skills of the instructors favorably (Supplementary Figure 10). Overall, participants rated levels of satisfaction with the ACCESS program very high overall, with all respondents stating they would recommend the program to others LWBC (Supplementary Figure 11).
3.9 Missing data
Missing data was present across several measures and is presented in Supplementary Table 2.
3.10 Medical record follow-up
To explore healthcare usage and overall survival between participants that completed the program and participants that withdrew for any variety of reasons (Figure 1), healthcare records for 162 participants that enrolled in the program and either completed (n = 96) or withdrew (n = 66) were reviewed for hospital admissions and mortality since their program start date. The mean follow-up time was 4.6 ± 0.5 years. A non-parametric analysis suggested no significant difference in the frequency of emergency room visits (p = 0.191), or inpatient stays (p = 0.420). Participants that withdrew had a higher frequency of mortality during the follow-up period (p < 0.001). In the group that completed ACCESS, 92% were alive at follow-up, vs. 72% for those that withdrew. Values for each measure are visualized in Supplementary Figure 12. No large differences in cancer type frequency were present between groups, although participants affected by kidney or lung cancer had ≥5% differences in proportions within the withdrawal group vs. the completion group (Supplementary Table 3). However, total numbers in all cancer types other than breast were low (<10). Participants that volitionally withdrew from ACCESS had worse self-reported health and baseline wellbeing (FACT-G) scores, lower levels of leisure activity, and higher (worse) DASS-42 scores, but were similar in age, weight, BMI, physical fitness measures, fatigue scores, and sleep quality scores in comparison to those that completed the program (Supplementary Table 4). The history of chemotherapy, radiotherapy, or surgery did not differ between those who completed the program or withdrew (p = 0.763, 0.667, and 0.273 respectively).
4 Discussion
The evaluation of exercise interventions in a cancer care setting is crucial to inform evidence-based recommendations for program creation that can facilitate systemic change in cancer care. This effectiveness-implementation trial demonstrated that a 12-week individualized multimodal exercise program for individuals LWBC improved physical fitness, increased activity levels, improved general wellbeing, reduced fatigue, reduced negative emotional states when starting levels are high, and improved sleep quality. The long-term goal of this work is to support efforts to embed exercise programming as a standard of care through evaluation of effectiveness and implementation success. Programs like ACCESS provide much needed exercise oncology services that align with national guidelines on exercise and physical activity recommendations (5, 39). The challenge now is to demonstrate effectiveness and functionality during real-world conditions and meet the call for healthcare systems to integrate exercise oncology as a standard of care (21, 40). ACCESS achieves this by providing an individualized exercise program for people affected by cancer using highly inclusive inclusion/exclusion criteria and engaging with local health authorities and community partners to support implementation.
4.1 Implementation
The merits of ACCESS regarding implementation can be assessed through a critical analysis using existing frameworks for judging relevant outcomes for implementation science, like those defined by Proctor and colleagues (27). In the order of presentation in the mentioned article and availability of data (27), acceptability is the perception that ACCESS was well-received by participants and can be evaluated based on participant feedback about the program. Program satisfaction, enjoyability, and organization were all scored exclusively positively by participants, which likely explains why 100% of respondents would recommend ACCESS to other LWBC. Despite most participants agreeing with statements about the program's effectiveness, quality, and administration, the statement “Parking was convenient” was highly variable, highlighting the need for flexibility in access to care. Virtual programming has been shown to effective for those LWBC (41), and we have already created a secure videoconferencing stream for current ACCESS participants, as was necessitated by the COVID-19 pandemic.
Cost is a key implementation outcome, which was addressed at the individual level and administrative level for ACCESS. As an aggregate it was found that participants spent on average $224 USD to participate in ACCESS, which is comparable to $350 USD reported by a similar trial, although the latter was an estimate by a community partner (42). Other programs cited personal costs in the range of an estimated $59 to $1,273 USD, which vary by institution, insurance coverage, and what was purchased (43–46). Of these programs, all but one reported that their program would cost more than $416.50 USD per person, and this program also incorporated nutritional counseling and was heavily subsidized by participant health insurance (44). This suggests that the cost for ACCESS for participants was similar to other programs, but the evidence supporting this claim is not extensive. From the program's perspective, it was estimated that the total cost of the program for 2-years was approximately $37,000 USD, or $18,000 per year, inclusive of staffing, satellite site subsidies, parking subsidies, and equipment maintenance. This is lower than a similar, multimodal exercise program designed to support patients during and after treatment that reported an annual running cost of $41,766 USD with four part-time employees and multiple student volunteers (44). This difference may be due to a greater involvement of student volunteers with ACCESS, who helped substantially and lowered operational costs. In our experience, ACCESS would benefit from further administrative and training support, which has motivated our group to seek additional funds to support current endeavors and would raise the current administrative cost estimate. Greater administrative support will allow us to reach more people LWBC and ensure quality data.
From the perspective of feasibility, which is the extent to which a new program can be carried out in a given setting, it was deemed that ACCESS is feasible based on accrual and attrition rates. In our experience there were several clinical champions that contributed to referring to ACCESS, including oncologists, oncology nurses, and other physicians and healthcare providers. Approximately 80% of referrals came from healthcare providers despite self-referral from advertisements being available, highlighting the importance of clinician referrals. The accrual rate for our study was 83%, which is slightly higher than similar trials, which ranged from 23%−72% (43, 44, 47, 48). Attrition during ACCESS was estimated to be 39%, which aligns with seven similar studies identified in a systematic review that reported an average attrition of 38.4% with a range of 22%−56% (21). These studies investigated exercise interventions offered alongside cancer care inclusive of survivorship. The average attendance of ACCESS was 95%, which is high when compared to 16 comparable studies that reported an average of 63.7% and a range of 30%−83% (21). This likely relates to our flexibility of scheduling sessions and allowing participants to complete 24-sessions on their own terms, but a qualitative analysis of ACCESS participants would be required to determine this. One possible reason for the high attendance is a reduction in the program's fidelity, which is how closely an intervention follows the original protocol (27). We allowed a high level of flexibility to the program to accommodate participants' schedules and as such, the proposed twice-weekly exercise schedule was not strictly followed. In fact, only 11% of participants completed ACCESS within or before the end of the 13th week. This highlights an issue regarding the feasibility of achieving the desired frequency of two sessions per week with available staff, and points to the need for more flexible programming or increased CEP staff members. Further, the impact of scheduling flexibility on the effectiveness of the program is difficult to judge, although frequency is a key factor in exercise prescription, and reduced frequency would likely detrimentally impact health gains from exercise, given that volume is not matched. Future research investigating what impact this flexibility has on health gains would be enlightening.
4.2 Effectiveness
This study included a robust assessment of effectiveness using five functional assessments, measures of physical activity and sedentary behavior, fatigue, wellbeing, negative emotional states, sleep health, and self-efficacy related to exercise. The improvements in aerobic fitness following ACCESS were significant and surpassed the estimated minimal clinically important difference of 30.5 m for adults with pathology or 32 m for postoperative recovery (49, 50). Further, improvements in lower-limb strength-endurance (i.e., 30-second chair stands) were similar to another 12-week multimodal exercise programs aimed at older adults with cancer and is indicative of increased physical function (51). The average increase in physical activity levels and reduction in sedentary time is also noteworthy, given the high relative prevalence of physical inactivity for those LWBC (52, 53). These outcomes, alongside increases to self-efficacy to exercise, support evidence for the message that individuals LWBC should avoid inactivity (5, 54).
Both fitness and physical activity levels are linked with health-related quality of life, which is an important concern for those LWBC, who tend to have diminished levels both across treatment and into survivorship (55). Indeed, scores on the physical wellbeing domain in the FACT-G improved following ACCESS, underpinning an important improvement in physical health. Such an improvement is particularly meaningful in this population, where physical impairments play a large role in health-related quality of life impairments (56, 57). Mean score improvements of the combined four subdomains of the FACT-G were close to achieving a minimal clinically important difference of 5-6 points (58). Fatigue scores measured using FACIT-F surpassed a minimal clinically significant difference of 3–4 points (59). Interestingly, this was not captured in the more granular 0–10 daily fatigue scale used to assess fatigue before each exercise session. The daily fatigue scale may thus serve best as a clinical indicator to the CEP/QEP to direct the intensity of the exercise session.
An interesting finding from this trial was the improvements to depression, anxiety, and stress subscales of the DASS-42 for those experiencing negative affective outcomes. The DASS-42 or a similar measure could be useful to include within a screening tool to facilitate recruitment of those that would derive the most benefit from programs like ACCESS. Negative emotional states are a concern for those LWBC, making this an important consideration (60, 61). Likewise, although there was a statistically significant change in sleep quality overall, we found that disturbed sleepers were driving this change. In a relatively smaller trial, a minimum clinically important difference using the PSQI was 1.3, which was 0.1 higher than the change detected in this study for disturbed sleepers (62). Overall, the improvements across mental and physical domains of health speaks to the plurality of benefits available from exercise programs for persons LWBC.
4.3 Considerations
The results of this trial should be interpreted with some caution. One key consideration is that missing data was present and substantial across multiple outcomes, limiting validity. Further, more work is needed in additional implementation outcomes, as highlighted by Proctor and colleagues (27). Adoption is an implementation outcome used to denote the intention or action to try a new evidence-based practice. The spike in referrals approximately 1-year into recruiting followed by a steady decline suggests that efforts to maintain adoption would be fruitful. Adoption is connected to appropriateness, which is the perceived fit of ACCESS in this setting by the provider. In this case, the appropriateness of this intervention should likely be assessed at the level of program administrators, hospital administrators, and regional health system leaders. As discussed by Proctor and colleagues, appropriateness is separated from acceptability given its focus on the provider and how congruent the proposed intervention is with the provider's perceived scope and ability, which was not identified in the present study (27). A future analysis of the appropriateness of ACCESS within its specific healthcare setting would likely aid further implementation efforts. Further, understanding penetration (i.e., how many providers referred compared to the total number of providers) would be valuable. Penetration appears understudied in exercise oncology effectiveness-implementation research, so increasing efforts to investigate this could prove fruitful (21). In combination, these topics will be further explored in future reports on ACCESS by investigating the “maintenance” outcomes from the RE-AIM framework (63). A future investigation of such processes would better inform us on the room and routes for growth at the health system level. Lastly, it was our experience that missing data can occur, especially at centers without adequate supervision by research-trained staff. Future trials using community partners would benefit from support by research trained personnel to ensure adequate data collection. Further, reducing the burden of research on participants (e.g., lowering the number of questionnaires, tests, etc.) is an important next step when transitioning solely to implementation and program evaluation.
An exploratory medical record review of participants that consented to the program that either withdrew or completed the program revealed that individuals that withdrew were more likely to pass away during the follow-up period. While it is tempting to infer the difference is due to a sustained or increased level of fitness or physical activity, which predicts better survivorship in cancer survivors (64), it is almost certainly multideterminant. Participants that withdrew had worse average scores of self-reported health, measures of wellbeing, and leisure activity, and at least 15 participants withdrew due to a decline in health. Although not recorded, it is possible these participants were candidates for palliative care, who would still benefit from an exercise program (65). Further, 21 participants withdrew due to accessibility issues like lack of time or travel. When taken in consideration with the low percentage of participants that completed the program on schedule, this suggests that there are populations of individuals LWBC that we may be missing, such as those with poorer health and/or lower accessibility, and that efforts should be made to ensure programs are accessible to them. In response to these considerations, ACCESS now operates using a hybrid model, allowing participants to join via secure video conferencing. We are striving to increase accessibility and acknowledge that our sample has not adequately reached some communities that are present in our region, namely Black and Indigenous people.
5 Conclusion
The ACCESS program is piloting an innovative model of care for our region that functions as a clinic-to-community exercise oncology program. An analysis of outcomes from participants that completed the program prior to March of 2020 suggests that ACCESS can improve multiple aspects of physical fitness and patient-reported outcomes including quality of life, wellbeing, fatigue, and sleep. Results from this analysis have spurred further development of this program internally and through collaboration with local community centers, the regional health authority and cancer care program to enhance implementation through scale and accessibility of programs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Nova Scotia Health Research Ethics Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SH: Formal analysis, Writing – original draft, Writing – review & editing. CC: Project administration, Writing – original draft, Writing – review & editing. TC: Project administration, Writing – original draft, Writing – review & editing. SC-R: Conceptualization, Writing – original draft, Writing – review & editing. SK: Project administration, Writing – original draft, Writing – review & editing. JL: Project administration, Writing – original draft, Writing – review & editing. MM: Conceptualization, Writing – original draft, Writing – review & editing. MK: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. SG: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. We would like to acknowledge the generous support of the following funders that make this work possible: the Queen Elizabeth II Foundation; Beatrice Hunter Cancer Research Institute; and Ultramar, in partnership with the Quebec Breast Cancer Foundation and the New Brunswick Health Research Foundation under the Health Research Value Demonstration Initiative Grant (2018-HRSI1401).
Acknowledgments
We would like to express our sincere thanks to our colleague Dr. Chris Blanchard and the Department of Medicine at Dalhousie University for providing access to a shared research lab and exercise space. This work would not have been possible without their generous support. The authors would also like to acknowledge the generous assistance of Deborah Wright, Alyssa Dickinson, Olivia Mercer, Caroline Straub, Olaide-Afolabi Laoye, several oncology care providers at Nova Scotia Health, kinesiology students at Dalhousie that assisted with program delivery, and to the participants that took part in ACCESS. SH was supported by a Health System Impact Fellowship funded by the Canadian Institutes of Health Research and is a trainee with Beatrice Hunter Cancer Research Institute.
Conflict of interest
CC was employed by John W. Lindsay YMCA.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The reviewer DM declared a past co-authorship with the author SC-R to the handling editor.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcacs.2024.1389084/full#supplementary-material
References
1. Winningham ML, MacVicar MG. The effect of aerobic exercise on patient reports of nausea. Oncol Nurs Forum. (1988) 15:447–50.
2. MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients' functional capacity. Nurs Res. (1989) 38:348–51. doi: 10.1097/00006199-198911000-00007
3. Winningham ML, MacVicar MG, Bondoc M, Anderson JI, Minton JP. Effect of aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncol Nurs Forum. (1989) 16:683–9.
4. Winningham ML. Walking program for people with cancer. Getting started Cancer Nurs. (1991) 14:270–6. doi: 10.1097/00002820-199114050-00007
5. Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. (2019) 51:2375–90. doi: 10.1249/MSS.0000000000002116
6. Garcia DO, Thomson CA. Physical activity and cancer survivorship. Nutr Clin Pract. (2014) 29:768–79. doi: 10.1177/0884533614551969
7. Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev. (2017) 39:71–92. doi: 10.1093/epirev/mxx007
8. Misiag W, Piszczyk A, Szymańska-Chabowska A, Chabowski M. Physical activity and cancer care-a review. Cancers. (2022) 14:4154. doi: 10.3390/cancers14174154
9. Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. (2010) 42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112
10. Cormie P, Atkinson M, Bucci L, Cust A, Eakin E, Hayes S, et al. Clinical Oncology Society of Australia position statement on exercise in cancer care. Med J Aust. (2018) 209:184–7. doi: 10.5694/mja18.00199
11. Santa Mina D, Alibhai SM, Matthew AG, Guglietti CL, Steele J, Trachtenberg J, et al. Exercise in clinical cancer care: a call to action and program development description. Curr Oncol. (2012) 19:e136–144. doi: 10.3747/co.19.912
12. Keogh JW, Olsen A, Climstein M, Sargeant S, Jones L. Benefits and barriers of cancer practitioners discussing physical activity with their cancer patients. J Cancer Educ. (2017) 32:11–5. doi: 10.1007/s13187-015-0893-1
13. Shea KM, Urquhart R, Keats MR. Physical activity and cancer care in the Atlantic Canadian provinces: an examination of provider beliefs, practices, resources, barriers, and enablers. J Cancer Educ. (2020) 35:946–53. doi: 10.1007/s13187-019-01546-x
14. Sweegers MG, Boyle T, Vallance JK, Chinapaw MJ, Brug J, Aaronson NK, et al. Which cancer survivors are at risk for a physically inactive and sedentary lifestyle? Results from pooled accelerometer data of 1447 cancer survivors. Int J Behav Nutr Phys Act. (2019) 16:66. doi: 10.1186/s12966-019-0820-7
15. National Cancer Institute. Cancer survivors and physical activity. (2023). Available online at: https://progressreport.cancer.gov/after/physical_activity (accessed October 19, 2023).
16. McNeely ML, Sellar C, Williamson T, Shea-Budgell M, Joy AA, Lau HY, et al. Community-based exercise for health promotion and secondary cancer prevention in Canada: protocol for a hybrid effectiveness-implementation study. BMJ Open. (2019) 9:e029975. doi: 10.1136/bmjopen-2019-029975
18. Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv Ther. (2018) 35:1763–74. doi: 10.1007/s12325-018-0805-y
19. Brownson RC, Fielding JE, Green LW. Building capacity for evidence-based public health: reconciling the pulls of practice and the push of research. Annu Rev Public Health. (2018) 39:27–53. doi: 10.1146/annurev-publhealth-040617-014746
20. Santa Mina D, Au D, Brunet J, Jones J, Tomlinson G, Taback N, et al. Effects of the community-based Wellspring Cancer Exercise Program on functional and psychosocial outcomes in cancer survivors. Curr Oncol Tor Ont. (2017) 24:284–94. doi: 10.3747/co.23.3585
21. Czosnek L, Richards J, Zopf E, Cormie P, Rosenbaum S, Rankin NM, et al. Exercise interventions for people diagnosed with cancer: a systematic review of implementation outcomes. BMC Cancer. (2021) 21:643. doi: 10.1186/s12885-021-08196-7
22. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. (2012) 50:217–26. doi: 10.1097/MLR.0b013e3182408812
23. Butcher NJ, Monsour A, Mew EJ, Chan AW, Moher D, Mayo-Wilson E, et al. Guidelines for reporting outcomes in trial reports: the CONSORT-outcomes 2022 extension. JAMA. (2022) 328:2252–64. doi: 10.1001/jama.2022.21022
24. Warburton DER, Jamnik VK, Bredin SSD, Gledhill N. The physical activity readiness questionnaire for everyone (PAR-Q+) and electronic physical activity readiness medical examination (ePARmed-X+). Health Fit J Can. (2011) 4:3–17.
25. Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health. (2007) 28:413–33. doi: 10.1146/annurev.publhealth.28.021406.144145
26. White SM, McAuley E, Estabrooks PA, Courneya KS. Translating physical activity interventions for breast cancer survivors into practice: an evaluation of randomized controlled trials. Ann Behav Med Publ Soc Behav Med. (2009) 37:10–9. doi: 10.1007/s12160-009-9084-9
27. Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. (2011) 38:65–76. doi: 10.1007/s10488-010-0319-7
28. Members CSEP. Canadian Society for Exercise Physiology-Physical Activity Training for Health (CSEP-PATH). Ottawa, Ontario: Canadian Society for Exercise Physiology. (2013).
29. Langhammer B, Stanghelle JK. The senior fitness test. J Physiother. (2015) 61:163. doi: 10.1016/j.jphys.2015.04.001
30. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. (1993) 11:570–9. doi: 10.1200/JCO.1993.11.3.570
31. Cella D, Lai JS, Stone A. Self-reported fatigue: one dimension or more? Lessons from the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) questionnaire. Support Care Cancer. (2011) 19:1441–50. doi: 10.1007/s00520-010-0971-1
32. Godin G, Shephard RJA. simple method to assess exercise behavior in the community. Can J Appl Sport Sci. (1985) 10:141–6.
33. Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health Rev. (1986) 77:359–62.
34. Amireault S, Godin G, Lacombe J, Sabiston CM. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol. (2015) 15:60. doi: 10.1186/s12874-015-0045-7
35. Prince SA, LeBlanc AG, Colley RC, Saunders TJ. Measurement of sedentary behaviour in population health surveys: a review and recommendations. PeerJ. (2017) 5:e4130. doi: 10.7717/peerj.4130
36. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
37. Lovibond S, Lovibond P. Manual for the Depression Anxiety Stress Scales. 2nd ed. Sydney: Psychology Foundation. (1995). doi: 10.1037/t01004-000
38. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A, et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. (2016) 25:52–73. doi: 10.1016/j.smrv.2015.01.009
39. Ligibel JA, Bohlke K, May AM, Clinton SK, Demark-Wahnefried W, Gilchrist SC, et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J Clin Oncol. (2022) 40:2491–507. doi: 10.1200/JCO.22.00687
40. Czosnek L, Rankin NM, Cormie P, Murnane A, Turner J, Richards J, et al. “Now is the time for institutions to be investing in growing exercise programs as part of standard of care”: a multiple case study examining the implementation of exercise oncology interventions. Support Care Cancer. (2023) 31:422. doi: 10.1007/s00520-023-07844-x
41. Wonders KY, Gnau K, Schmitz KH. Measuring the feasibility and effectiveness of an individualized exercise program delivered virtually to cancer survivors. Curr Sports Med Rep. (2021) 20:271–6. doi: 10.1249/JSR.0000000000000846
42. Rogers LQ, Goncalves L, Martin MY, Pisu M, Smith TL, Hessong D, et al. Beyond efficacy: a qualitative organizational perspective on key implementation science constructs important to physical activity intervention translation to rural community cancer care sites. J Cancer Surviv Res Pract. (2019) 13:537–46. doi: 10.1007/s11764-019-00773-x
43. Beidas RS, Paciotti B, Barg F, Branas AR, Brown JC, Glanz K, et al. A hybrid effectiveness-implementation trial of an evidence-based exercise intervention for breast cancer survivors. J Natl Cancer Inst Monogr. (2014) 2014:338–45. doi: 10.1093/jncimonographs/lgu033
44. Kirkham AA, Van Patten CL, Gelmon KA, McKenzie DC, Bonsignore A, Bland KA, et al. Effectiveness of oncologist-referred exercise and healthy eating programming as a part of supportive adjuvant care for early breast cancer. Oncologist. (2018) 23:105–15. doi: 10.1634/theoncologist.2017-0141
45. Marker RJ, Cox-Martin E, Jankowski CM, Purcell WT, Peters JC. Evaluation of the effects of a clinically implemented exercise program on physical fitness, fatigue, and depression in cancer survivors. Support Care Cancer. (2018) 26:1861–9. doi: 10.1007/s00520-017-4019-7
46. Culos-Reed SN, Dew M, Shank J, Langelier DM, McDonough M. Qualitative evaluation of a community-based physical activity and yoga program for men living with prostate cancer: survivor perspectives. Glob Adv Health Med. (2019) 8:2164956119837487. doi: 10.1177/2164956119837487
47. Irwin ML, Cartmel B, Harrigan M, Li F, Sanft T, Shockro L, et al. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer. (2017) 123:1249–58. doi: 10.1002/cncr.30456
48. Santa Mina D, Au D, Auger LE, Alibhai SMH, Matthew AG, Sabiston CM, et al. Development, implementation, and effects of a cancer center's exercise-oncology program. Cancer. (2019) 125:3437–47. doi: 10.1002/cncr.32297
49. Antonescu I, Scott S, Tran TT, Mayo NE, Feldman LS. Measuring postoperative recovery: what are clinically meaningful differences? Surgery. (2014) 156:319–27. doi: 10.1016/j.surg.2014.03.005
50. Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. (2017) 23:377–81. doi: 10.1111/jep.12629
51. Mikkelsen MK, Lund CM, Vinther A, Tolver A, Johansen JS, Chen I, et al. Effects of a 12-week multimodal exercise intervention among older patients with advanced cancer: results from a randomized controlled trial. Oncologist. (2022) 27:67–78. doi: 10.1002/onco.13970
52. Courneya KS, Katzmarzyk PT, Bacon E. Physical activity and obesity in Canadian cancer survivors: population-based estimates from the 2005 Canadian Community Health Survey. Cancer. (2008) 112:2475–82. doi: 10.1002/cncr.23455
53. Neil SE, Gotay CC, Campbell KL. Physical activity levels of cancer survivors in Canada: findings from the Canadian community health survey. J Cancer Surviv Res Pract. (2014) 8:143–9. doi: 10.1007/s11764-013-0322-6
54. Jones TL, Edbrooke L, Rawstorn JC, Hayes SC, Maddison R, Denehy L, et al. Self-efficacy, motivation, and habits: psychological correlates of exercise among women with breast cancer. Support Care Cancer. (2023) 31:584. doi: 10.1007/s00520-023-08040-7
55. Firkins J, Hansen L, Driessnack M, Dieckmann N. Quality of life in “chronic” cancer survivors: a meta-analysis. J Cancer Surviv Res Pract. (2020) 14:504–17. doi: 10.1007/s11764-020-00869-9
56. Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. (2013) 63:295–317. doi: 10.3322/caac.21186
57. Martín-Cuesta J, Calatayud J, Casaña J, Smith L, Pardhan S, López-Sánchez GF, et al. Association of difficulties in daily physical activities and handgrip strength with cancer diagnoses in 65,980 European older adults. Aging Clin Exp Res. (2023) 35:2971–8. doi: 10.1007/s40520-023-02577-7
58. Eton DT, Cella D, Yost KJ, Yount SE, Peterman AH, Neuberg DS, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. (2004) 57:898–910. doi: 10.1016/j.jclinepi.2004.01.012
59. Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J, et al. Validation of the functional assessment of chronic illness therapy fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. (2005) 32:811–9.
60. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin North Am. (2017) 101:1099–113. doi: 10.1016/j.mcna.2017.06.005
61. Proctor CJ, Reiman AJ, Best LA. Cancer, now what? A cross-sectional study examining physical symptoms, subjective well-being, and psychological flexibility. Health Psychol Behav Med. (2023) 11:2266220. doi: 10.1080/21642850.2023.2266220
62. Longo UG, Berton A, De Salvatore S, Piergentili I, Casciani E, Faldetta A, et al. Minimal clinically important difference and patient acceptable symptom state for the pittsburgh sleep quality index in patients who underwent rotator cuff tear repair. Int J Environ Res Public Health. (2021) 18:8666. doi: 10.3390/ijerph18168666
63. Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. (2019) 7:64. doi: 10.3389/fpubh.2019.00064
64. Klepin HD, Geiger AM, Tooze JA, Newman AB, Colbert LH, Bauer DC, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc. (2010) 58:76–82. doi: 10.1111/j.1532-5415.2009.02620.x
Keywords: cancer, living with and beyond cancer, oncology, exercise, physical activity, implementation, intervention, re-aim
Citation: Heinze SS, Chiekwe CJ, Christensen T, Culos-Reed SN, Kendall SJ, Langley JE, McNeely ML, Keats MR and Grandy SA (2024) Activating cancer communities through an exercise strategy for survivors: an effectiveness-implementation trial. Front. Cancer Control Soc. 2:1389084. doi: 10.3389/fcacs.2024.1389084
Received: 20 February 2024; Accepted: 27 March 2024;
Published: 10 April 2024.
Edited by:
Carolina Ximena Sandler, Western Sydney University, AustraliaReviewed by:
Jelena Roganovic, Clinical Hospital Centre Rijeka, CroatiaDavid Mizrahi, The University of Sydney, Australia
Copyright © 2024 Heinze, Chiekwe, Christensen, Culos-Reed, Kendall, Langley, McNeely, Keats and Grandy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scott A. Grandy, c2NvdHQuZ3JhbmR5QGRhbC5jYQ==; Melanie R. Keats, bWVsYW5pZS5rZWF0c0BkYWwuY2E=
†These authors have contributed equally to this work and share last authorship
 Stefan S. Heinze
Stefan S. Heinze C. Joy Chiekwe
C. Joy Chiekwe Thomas Christensen2
Thomas Christensen2 S. Nicole Culos-Reed
S. Nicole Culos-Reed Jodi E. Langley
Jodi E. Langley Melanie R. Keats
Melanie R. Keats Scott A. Grandy
Scott A. Grandy