
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bird Sci., 30 July 2024
Sec. Bird Ecology and Behavior
Volume 3 - 2024 | https://doi.org/10.3389/fbirs.2024.1440063
This article is part of the Research TopicBioenergetic and Behavioral Effects of Rapid Anthropogenic Change and Eco-evolutionary ImplicationsView all 3 articles
 Melanie G. Kimball*
Melanie G. Kimball* Danna F. Masri
Danna F. Masri Eve B. Gautreaux
Eve B. Gautreaux Keegan R. Stansberry
Keegan R. Stansberry Tosha R. Kelly
Tosha R. Kelly Christine R. Lattin
Christine R. LattinSome individuals respond to new objects, foods, or environments with wariness (neophobia), whereas others are willing to approach and explore. Because novel stimuli can represent both dangers and resources, group-living species may show adaptive plasticity in neophobia in response to social cues. To better understand how conspecific calls can influence neophobia in a highly gregarious species, we exposed individual house sparrows (Passer domesticus) to either conspecific alarm calls (n = 12), conspecific contact calls (n = 12), or no playback (n = 12) and measured latency to feed in the presence of novel objects. We also measured novelty responses with no sound the week before and after the sound treatment week for all individuals. Relative to no playback and contact calls, we predicted that conspecific alarm calls would increase neophobia behavior during the acoustic trial and that these effects would persist the week after exposure. Instead, we found that individuals in the contact call and no playback groups became less neophobic as weeks progressed, while the alarm call group showed no attenuation of neophobia. There was a significant interaction between week and treatment, where neophobia responses over the three weeks were significantly different for individuals exposed to alarm calls compared to the contact and no playback groups combined. These results suggest that house sparrows learn social information about potentially threatening stimuli from conspecific alarm calls; here, that novel objects may be dangerous.
Neophobia is fearful or aversive behavior towards novelty, and it has been described in a wide variety of taxa (Dardenne et al., 2013; Cohen et al., 2015; Joyce et al., 2016; Mazza et al., 2021). With the rise of urbanization (Marzluff, 2002), animals are encountering more novel stimuli in human-altered landscapes, including novel objects, foods, scents, and spaces. Neophobia has broad ecological relevance because it influences whether an individual can exploit novel resources (Greenberg and Mettke-Hofmann, 2001). Conversely, when new things are dangerous (e.g., novel predators like domestic cats, novel environments like roads), neophobia can help animals survive (Crane and Ferrari, 2017; Feyten et al., 2019). Therefore, neophobia is a behavior that ultimately affects both individual survival and population persistence (Candler and Bernal, 2015; Damas-Moreira et al., 2019; Magory Cohen et al., 2020). Neophobia can also show plasticity based on different environmental cues (Brown et al., 2013; Mitchell et al., 2016).
One type of environmental cue that can affect neophobia is communication from conspecifics. The effect of alarm cues on neophobia has been well-studied in aquatic vertebrates, with alarm or disturbance scents from conspecifics as stimuli (Brown et al., 2020; Rivera-Hernández et al., 2022). In juvenile rainbow trout (Oncorhynchus mykiss), exposure to conspecific alarm cues during development decreased spatial and object neophobia (Poisson et al., 2017). In contrast, acute and embryonic exposure to alarm cues increased neophobia in other species of fish and amphibians (Brown et al., 2013; Mitchell et al., 2016; Rivera-Hernández et al., 2022). We would expect conspecific cues to be most salient for social species that live in groups as adults and frequently communicate information about potential resources and threats to other group members. Group-living species benefit from group vigilance (Olson et al., 2015) and use behavioral cues, like alarm calls (Griesser, 2009), to communicate with conspecifics. For example, different alarm call types elicited context-specific anti-predator behavior in noisy miners (Manorina melanocephala) (Farrow et al., 2017) and Siberian jays (Perisoreus infaustus) (Griesser, 2008), and conspecific playback influenced perception of group size during foraging in pied babblers (Turdoides bicolor) (Radford and Ridley, 2007). Because neophobia can be adaptive or non-adaptive, depending on whether the novel stimulus in question represents a danger or a resource, group-living species may show adaptive plasticity in neophobia in response to social cues, for example learning that a novel food is not dangerous after witnessing conspecifics approach and feed. This, in turn, could affect the ability of social species to exploit habitats full of novelty, such as urban and suburban environments.
House sparrows (Passer domesticus) are small gregarious songbirds (Anderson, 2007) that often forage in groups (Barnard and Sibly, 1981; Katsnelson et al., 2008). House sparrows show repeatable variation in neophobia responses (Ensminger and Westneat, 2012; Kelly et al., 2020; Kimball et al., 2022), with individuals either consistently displaying neophobic or non-neophobic behavior, which makes them an excellent model species to investigate the effects of social cues on neophobia behavior. House sparrows show evidence of social learning when they are able to watch conspecifics during neophobia trials (Tuliozi et al., 2018; Kelly et al., 2020). Additionally, house sparrows use a wide array of conspecific vocal cues. Low frequency alarm calls are used in response to threats (Reyer et al., 1998; Kopisch et al., 2005; Klvaňová et al., 2011), and higher frequency contact calls and chirrups can be used to recruit other sparrows to foraging flocks (Elgar, 1986), to indicate submissiveness, or to attract mates during the breeding season (Nivison, 1978; Fitzwater, 1994). To our knowledge, there are no studies on the impact of different types of conspecific calls on neophobia.
Here, we investigated the effects of conspecific alarm and contact calls on object neophobia responses. We assessed time to feed in the presence of a novel object from the start of the trial immediately after researchers replaced food dishes and left the room (latency to feed), which is a commonly used measure of neophobia (Greenberg, 1990; Webster and Lefebvre, 2001; Kelly et al., 2022; Kimball et al., 2022). If novelty were presented in the absence of a positive stimulus (here, the normal food dish) then neophilia (an interest in or preference for novelty) would be measured, not neophobia (Mettke-Hofmann et al., 2002; Kimball and Lattin, 2023a). During novel object trials, we predicted that conspecific alarm call playback would increase neophobia, and conspecific contact call playback and no playback would induce no change in neophobia. Neophobia was measured for one week before sound treatments, one week with sound treatments, and one more week after sound treatments; we predicted any changes in behavior due to sound treatments would persist in the week following exposure.
Adult house sparrows (n = 16 males, n = 20 females) were captured with mist nets at several bird feeder locations in East Baton Rouge Parish, Louisiana, United States from February through April 2022, and February through April 2023. Females caught during the breeding season were not included if they had an active brood patch. Having a cage mate has been shown to affect neophobia in house sparrows (Kelly et al., 2020), therefore sparrows were singly housed in a vivarium at Louisiana State University. However, house sparrows are social and therefore were not acoustically isolated from other individuals in their treatment group. Cages were 56 cm length x 45 cm width x 33 cm height with solid cage dividers separating adjacent cages on the left and/or right. Sparrows had unlimited access to mixed seeds, grit, a vitamin-rich food supplement (Mazuri small songbird diet), and water. Each cage contained multiple perches. The three different experimental groups for this study (alarm call playback, contact call playback, and no playback) were housed in two different rooms, with the alarm call group and contact call groups being tested at the same time in separate rooms (n = 12 per room; n = 24 total) in the summer of 2022, and the no playback group being tested in the same room as the alarm call group in the summer of 2023 (n = 12). Therefore, all birds were only exposed to eleven other individuals for the duration of the experiment, and the auditory environment should have been relatively homogenous among individuals. Sparrows could hear but not see the eleven other birds in the same room with them during trials, and they could not hear individuals or playback from the other colony room. To acclimate to captivity, sparrows were maintained at natural day length (13L:11D) for three weeks prior to behavior trials. Animals were collected under a Louisiana State Scientific Collecting Permit and approved methods were used for capture, transport and husbandry per Ornithological Council’s Guidelines to the to the Use of Wild Birds in Research (Fair et al., 2023). All experimental procedures were approved by the Louisiana State University Institutional Animal Care and Use Committee under protocol 56-2022.
We conducted three consecutive weeks of neophobia trials, with each week consisting of five trials over five days (Monday – Friday), and each sparrow randomly receiving three of nine possible objects and two days of control (no object) trials each week in a random order (Figure 1). The purpose of control trials was twofold. First, control trials helped ensure that birds that did not approach food dishes and feed during novel object trials were showing neophobia and not an aversion to the entire testing procedure (in which case, they would also fail to approach during control trials; typically, these subjects are excluded). Secondly, control trials allowed us to examine if the effects of sound treatments on feed latencies were specific to the object trials. Trials took place in the two colony rooms. The first week of behavior was a control week with no sound treatment, the second week involved 15 min of playback exposure at the beginning of trials (n = 12 alarm, n = 12 contact) or no sound (n = 12 no playback), and the third week was another control week with no sound treatment. The contact call playback and no playback groups were two kinds of controls for this experiment, with the contact call group mimicking the vocalizations that normally occur in the treatment rooms and the no playback group controlling for the effect of playing sound. However, because sparrows were not acoustically isolated it is likely that both control groups were exposed to contact calls from neighboring sparrows during trials, and therefore these groups were combined for analyses. It is also likely that sparrows in all three groups produced some alarm calls when animal husbandry staff entered the rooms for ~30 min daily to replace food and water; there were never more than two staff in the room at a time. Sound trials were treated as an acute stimulus and were only played for the first 15 min of the 1 h trial to be able to assess the effects of sound trials during and immediately post-stimulus, which did not differ. Each playback session consisted of a unique 15 min playlist of several different house sparrow contact calls or several different alarm calls that were edited using Raven Lite (Cornell Lab of Ornithology, 2023). Sound files were from the collections of the Macaulay Library (Cornell Lab of Ornithology, Ithaca, NY) and the Borror Laboratory of Bioacoustics (The Ohio State University, Columbus, OH). The order of sound files in playlists were randomly assigned. Average sound file length for alarm playlists was 49.1 ± 5.0 s, and 90.2 ± 12.8 s for contact playlists, and sparrows heard 18 total sound files for alarm playlists and 10 total sound files for contact playlists. On average, sparrows heard 94 ± 52 alarm calls, and 143 ± 44 contact calls in 1 min of playback. The average time interval between calls during both alarm and contact call playback was 1.4 ± 0.8 s. Volume was standardized to 60 dBA from cage racks to the speaker using a sound level pressure meter.
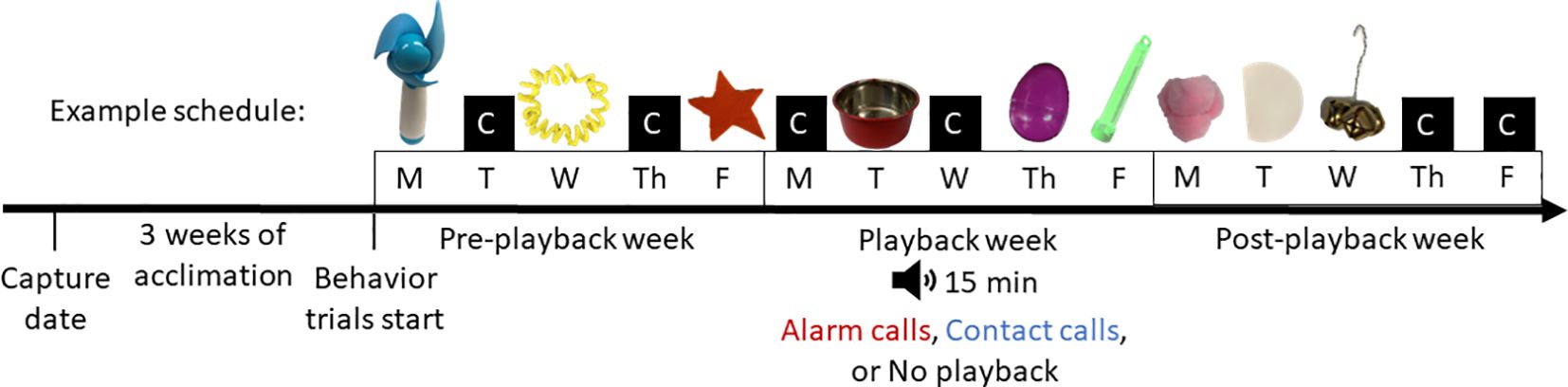
Figure 1 Example object and treatment order timeline for one individual house sparrow for the three weeks of the experiment. Novel objects were placed in, around, or near the food dish during object trials. Control trials involved replacing the regular food dish. The order of control/object trials and object presentation order was randomly determined, but all sparrows eventually saw all objects. Trials lasted 1 h. Control trials are represented by a black box with a “C”.
Object trials followed methods detailed in Kimball et al. (2022). Briefly, sparrows underwent an overnight fast to standardize motivation to feed in trials. The morning of trials, researchers entered the room 30 min after lights on, started cameras (and sound treatments, during playback weeks), replaced food dishes, and left the room. Controls were the normal food dish with no novel object, and treatments involved novel objects placed in, on, around, or directly over the food dish. The objects used were: a purple plastic egg placed in the food dish, a white cover over part of the dish, a red-painted dish, yellow coiled pipe cleaners around the edge of the dish, an orange felt star taped to the front of the dish, a green glowstick taped across the top of the dish, gold metallic jingle bells hung over the food dish, three pink puffs glued into a pyramid and placed in the food dish, and a blue foam fan turned on and placed directly below the food dish. Object and control trial order was randomly determined for each sparrow, but all sparrows eventually saw all nine objects. One individual saw the glowstick twice due to an experimenter error, so the second glowstick exposure was excluded from analyses; to ensure this bird saw all objects, we cut a control trial, so this bird had five instead of six control trials over the course of the experiment. Novel objects were selected to share few common features (e.g., red color) that might target ecologically-relevant perceptual bias (Greggor et al., 2015), and have all been shown to significantly increase house sparrows’ average latency to feed (Kelly et al., 2020; Kimball et al., 2022; Kimball and Lattin, 2023b). To determine latency to approach and feed during trials (defined as the duration between the start of trials and the time sparrows first approached or fed from their food dish, respectively), 1 h of behavior was recorded using pole-mounted cameras (ZOSI Z18.5.T.2) connected to a DVR (ANNKE Model DM310). Video recordings were stopped at the end of the hour, and novel objects were removed from food dishes. Because sparrows do not eat in the lab when lights are out (Lattin et al., 2021), behavior trials only represent an additional 2 h of fasting at maximum for birds that do not feed during neophobia trials. Videos were scored for time to first approach (defined as the first time a sparrow was close enough to the food dish to feed) and feed from the food dish; however, these measures were significantly correlated (r = 0.97, p < 0.0001), therefore we used feed latency times for all analyses. One sparrow was removed from analysis because it did not approach during control trials, indicating an aversion to the entire testing procedure rather than neophobia specifically. Therefore, the final sample size for the combined contact call and no playback group was n = 23.
We used R Studio v 4.3.0 for all behavior analyses (R Core Team, 2024). To investigate differences in latency to feed in the presence of a novel object between contact call playback, alarm call playback, and no playback groups, we used Cox proportional hazard models via the “coxme” function in the coxme package (Therneau, 2020). We did not have an a priori reason to expect an effect of sex on neophobia responses (Ensminger and Westneat, 2012; Kimball et al., 2022), therefore sex was not included as a covariate in models. The first model assessed whether neophobia responses on object days differed from control days, and included object type as a fixed effect, individual ID as a random effect, feed status (fed vs. did not feed) as a censoring variable, and time to feed as the dependent variable for data that only included feed latencies from the first control week. After assessing this effect, control days were removed from subsequent neophobia analyses, because we were interested in comparing responses during novel object trials. The second model assessed whether our two control groups (no playback vs contact calls) differed in latency to feed over time and included a treatment*week interaction and trial number as fixed effects, individual ID as a random effect, feed status (fed vs. did not feed) as a censoring variable, and time to feed as the dependent variable. The data set did not include data from the alarm group. Because latency to feed over time did not differ between the no playback and contact call groups (week x treatment; β = -0.17, HR(95% CI) = 1.18(1.86 – 0.75), z = 0.72, p = 0.47), and because sparrows were not acoustically isolated during trials and likely heard conspecific contact calls during trials, these groups were combined for subsequent analyses.
To evaluate whether alarm calls affected neophobia responses relative to the control groups, we created a Cox proportional hazard model using a treatment*week interaction and trial number as fixed effects, individual ID as a random effect feed status (fed vs. did not feed) as a censoring variable, and time to feed as the dependent variable. We also assessed whether there was an effect of week on neophobia responses using two additional Cox proportional hazard models (one for alarm and one for the combined control groups) with week and trial number as fixed effects, individual ID as a random effect, and time to feed and feed status as the dependent variables. Week was treated as a continuous variable for all models because we were interested in the change in neophobia responses over time. The trial number variable re-started every week (values were only 1-5) and was included as a covariate in models to take into account the order of trials within weeks. We repeated these three Cox proportional hazard models using control trial data, to determine whether the effects of treatment and time were specific to novel object trials (and therefore neophobia) or whether effects were habituation to the testing procedure itself.
We assessed whether the data met the proportional hazards assumptions (In and Lee, 2019; Kuitunen et al., 2021), by correlating scaled Schoenfeld residuals with transformed time using the “cox.zph” function in the coxme package (Therneau, 2020), which were then visualized using the “plot” function. All data fit the assumption of proportional hazard models (all X2 < 1.53, all p > 0.22), except for the combined contact call and no playback dataset used for the second model described above (X2 = 4.39, all p = 0.04). For data that failed to meet the assumptions, we used the “coxphw” function in the coxphw package (Dunkler et al., 2018) to create weighted Cox regression models (non-proportional hazards). We report effect sizes as hazard ratios (HR), with an HR greater than 1 indicating that individuals fed faster during trials (e.g., the effect of treatment x week in Table 1b means that individuals in the combined control group fed faster during novel object trials over the three weeks compared to individuals in the alarm group). 95% confidence intervals are given for HR estimates. We created Kaplan-Meier survival curves of sparrow feed latencies with the “survfit” command in the survival package (Therneau, 2021) and visualized them using the “ggsurvplot” command in the survminer package (Kassambara et al., 2021), as done previously (Kelly et al., 2022; Kimball et al., 2022; Kimball and Lattin, 2023b).
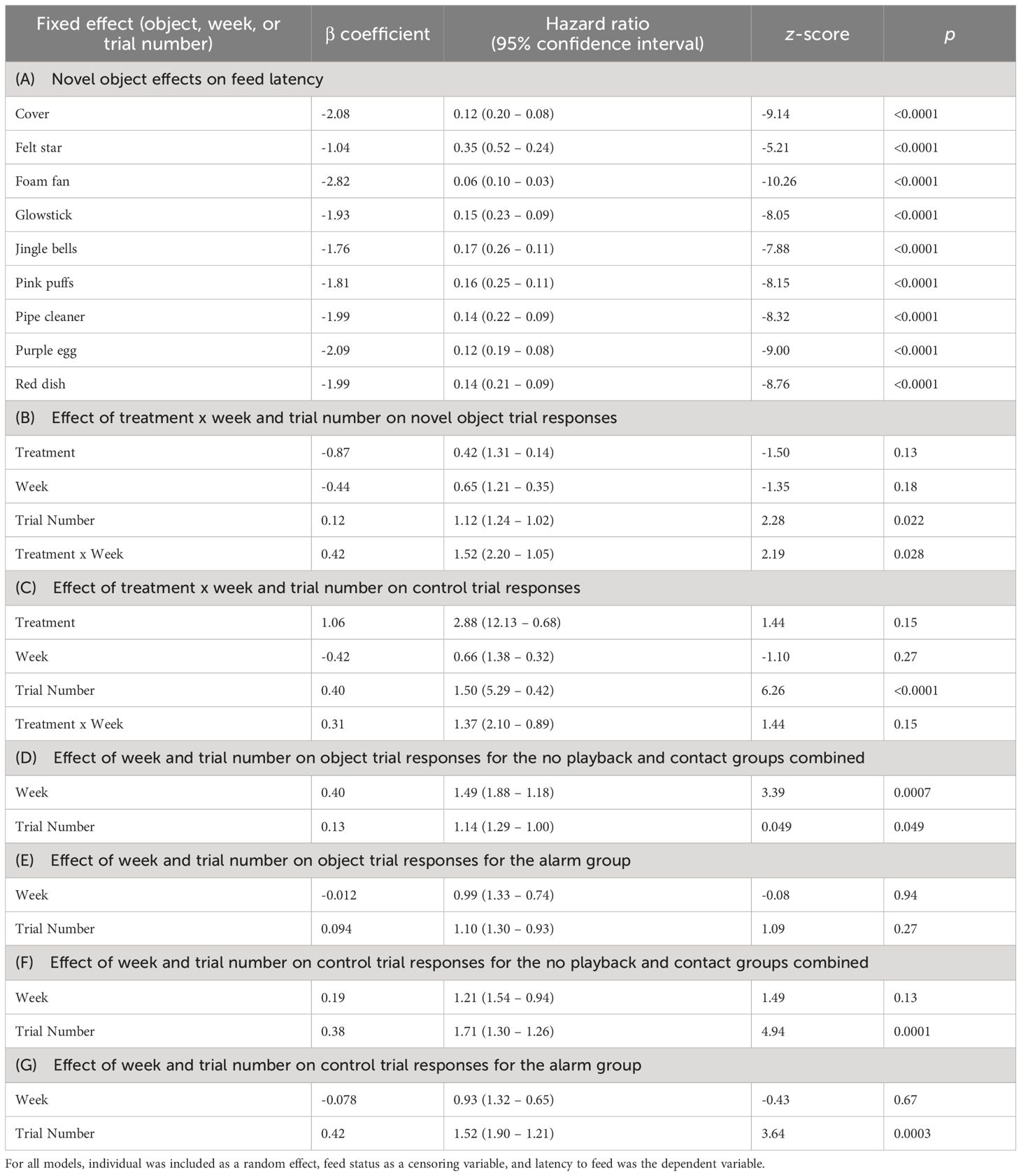
Table 1 Results of Cox proportional hazard models of house sparrow feeding probability for (A) each object type contrasted with control trials, the effect of treatment x week on responses during (B) object and (C) control trials, the effect of week and trial number on responses during object trials for the (D) combined control groups (n = 23) and (E) the alarm call group (n = 12), and the effect of week and trial number on responses during control trials for the (F) combined control groups and (G) the alarm call group.
All latency to feed times in the presence of a novel object were significantly different from control responses (Table 1A, all z < -2.39, all p < 0.017). We found a significant treatment x week interaction, where individuals in the alarm call group had significantly longer latency to feed responses to novel objects over the three weeks compared to the combined no playback and contact call group (Table 1B, z = 2.19, p = 0.028, Figure 2). Additionally, there was a significant effect of week on feed latency for the combined contact call and no playback groups (Table 1D, z = 3.39, p = 0.0007), indicating an attenuation of neophobia behavior over the three weeks of the study (Figure 2A). However, there was no significant effect of week for the alarm call group (Table 1E, z = -0.08, p = 0.94), indicating no attenuation of latency to feed in the presence of novel objects over time (Figure 2B). There was no significant treatment x week interaction for control trials (Table 1C, z = 1.44, p = 0.15), and no significant effect of week for control trials for the combined control group (Table 1F, z = 1.49, p = 0.13) and the alarm group (Table 1G, z = -0.43, p = 0.67), therefore effects were specific to novel object trials.
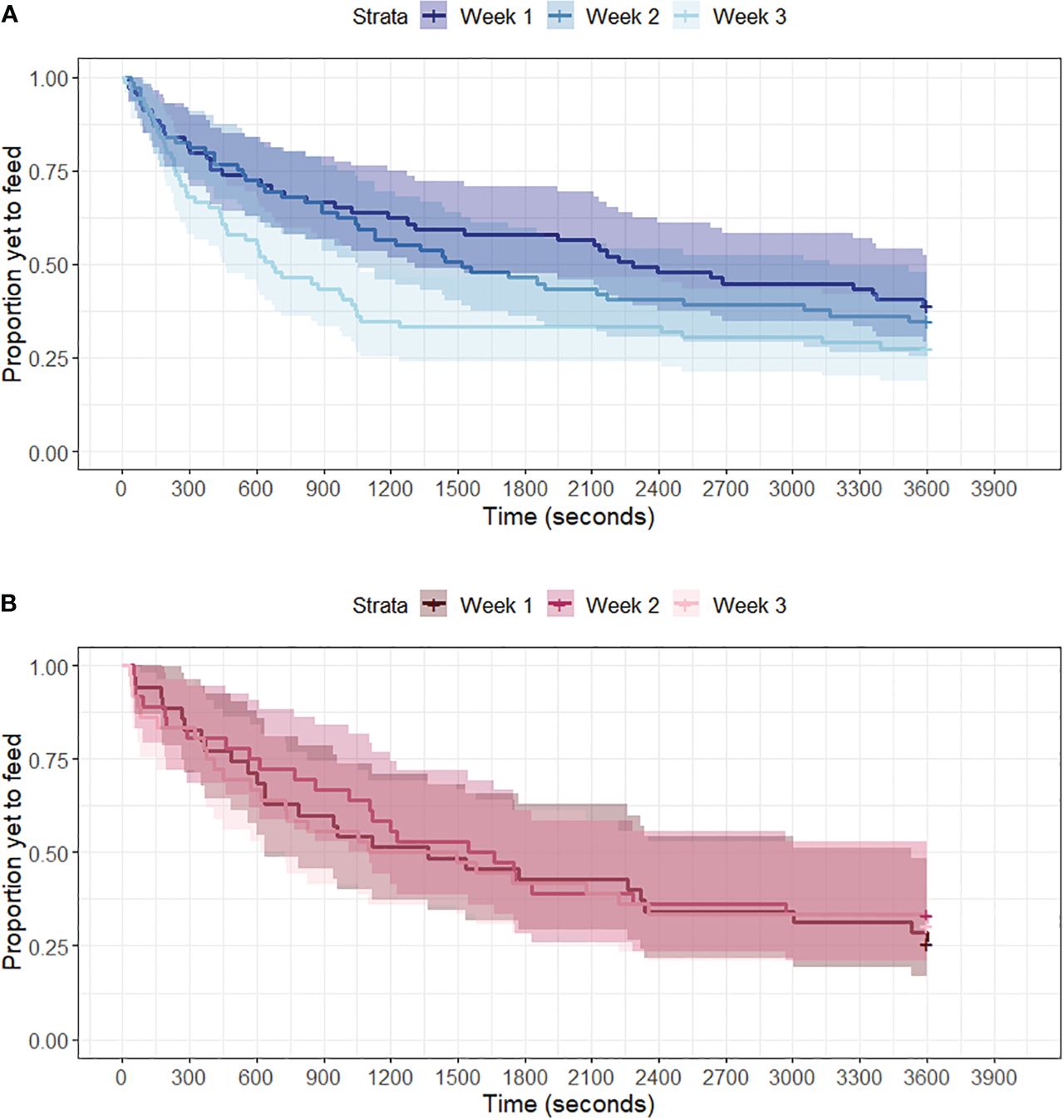
Figure 2 Kaplan-Meier survival curves of average house sparrow feeding likelihood in the presence of nine different novel objects over three weeks of testing. Playback treatments were played during week 2 only. Latency to feed in presence of a novel object significantly decreased over three weeks for individuals exposed to (A) no playback and contact calls (blue, n = 23), but stayed consistent for individuals exposed to (B) alarm calls (red; n = 12). Shading shows 95% confidence intervals.
The goal of this research was to determine the effect of conspecific calls on neophobia behavior in a gregarious songbird. We predicted that sparrows exposed to alarm calls would increase neophobia (measured as the latency to feed in the presence of a novel object), and sparrows exposed to contact call playback and no playback would show no change in neophobia, and that these patterns would persist post-exposure. Instead, we found that sparrows exposed to contact calls and no playback decreased their latency to feed in the presence of novel objects as weeks progressed, whereas sparrows exposed to alarm calls showed no change in their responses to novel objects over time. These results suggest that alarm calls prevented the attenuation of neophobia seen in the other two groups, which provides evidence for social learning. Because the novel objects were not actually dangerous, this mismatched social information (danger cues paired with non-dangerous novel objects) resulted in neophobic sparrows in the alarm call group responding non-adaptively to novel objects and prevented them from accessing their food. Previous work has shown that neophobia in social birds (including house sparrows) can be influenced by visual information from conspecifics (Stöwe and Kotrschal, 2007; King et al., 2015; Kelly et al., 2020), like watching cage mates approach and feed in the presence of a novel object. Our project demonstrates that even conspecific vocalizations can affect neophobia and suggests that sparrows may also learn information about potentially threatening stimuli from some conspecific call types.
Social learning has often been studied in a visual context, where individual personality traits are measured in both group and solo trials (e.g. Coleman and Mellgren, 1994). However, social information can also be transferred in the absence of visual social cues. In social birds such as Corvids and Parids, vocal alarm signals are often used to transmit knowledge of danger (Templeton et al., 2005; Cornell et al., 2012; Lee et al., 2019; Keen et al., 2020). Here we found that house sparrows exposed to alarm calls did not decrease neophobia over the three weeks of novel object trials, which suggests that sparrows may have learned to associate alarm calls with novel objects. Overall, this implies that social species may make decisions about novelty not just using information from visual social cues (e.g., witnessing a conspecific approach a novel object) but also from auditory social cues, and that the presence or absence of alarm calls may influence whether an individual approaches a new potential food source or nesting site, for example. This study is the first to highlight the importance of considering how the auditory environment can affect individual behavior during neophobia trials and more broadly during novelty encounters in the wild. It would be interesting to test how auditory social cues affect novelty responses in a group setting compared to an individual setting, where individuals are in mixed flocks and visually isolated from each other respectively.
In the combined contact call and no playback group, we saw a decrease in neophobia over the three weeks, indicating that in the absence of social information conveying danger, individuals began to acclimate to novel object trials. Specifically, sparrows on average fed 38 min faster as weeks progressed. Habituation to neophobia trials has been reported previously in house sparrows (Ensminger and Westneat, 2012; Moldoff and Westneat, 2017). This type of learning has been called generalization, defined as the process of grouping novel stimuli into pre-existing cognitive categories and responding in a similar way (Ghirlanda and Enquist, 2003). A recent study showed house sparrows reduced neophobia towards novel objects that differed in color and shape but had the same texture, thus showing generalization (McLaughlin and Westneat, 2023). Here, we show evidence of generalization about novel objects with few shared characteristics including both physical appearance and location; this suggests that what might matter for generalization is not necessarily the specific shared features of the objects themselves, but rather, a similar context, in this case, a rule such as “something weird near the food dish.”
Overall, we found that house sparrows exposed to playback of conspecific contact calls and no playback decreased neophobia over three weeks of trials, whereas house sparrows exposed to conspecific alarm calls did not. This is evidence for social learning, where sparrows may be associating alarm calls with novel objects, thus preventing generalization. Although neophobia is repeatable (Dingemanse et al., 2002; Kimball et al., 2022), it also shows plasticity in response to learning and social context (Kelly et al., 2020; St. Lawrence et al., 2021), which can have adaptive benefits. The ability to learn from conspecifics may help individuals navigate dangerous situations (Griffin, 2004; Mertes et al., 2022) and make decisions about novel resources (Moretti et al., 2015; Greggor et al., 2016). Therefore, social learning capacity may ultimately affect survival, and has broad conservation consequences (Brakes et al., 2019).
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
The animal study was approved by Louisiana State University Institutional Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
MK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. DM: Data curation, Writing – review & editing. EG: Data curation, Writing – review & editing. KS: Data curation, Writing – review & editing. TK: Data curation, Formal analysis, Writing – review & editing. CL: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The publication of this work was made possible by NSF grant IOS-2237423 awarded to CL.
Tosha R. Kelly is a Merck Awardee of the Life Sciences Research Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbirs.2024.1440063/full#supplementary-material
Anderson T. (2007). Biology of the ubiquitous house sparrow: from genes to populations, biology of the ubiquitous house sparrow: from genes to populations. New York: Oxford University Press. doi: 10.1093/acprof:oso/9780195304114.001.0001
Barnard C. J., Sibly R. M. (1981). Producers and scroungers: A general model and its application to captive flocks of house sparrows. Anim. Behav. 29, 543–550. doi: 10.1016/S0003-3472(81)80117-0
Brakes P., Dall S. R. X., Aplin L. M., Bearhop S., Carroll E. L., Ciucci P., et al. (2019). Animal cultures matter for conservation. Science 80-, ). doi: 10.1126/science.aaw3557
Brown G. E., Demers E. E. M., Goldman J. A., Singh A., Chivers D. P., Ferrari M. C. O. (2020). Unpredictable risk enhances induced neophobia in northern red-bellied dace. Anim. Behav. 168, 121–127. doi: 10.1016/j.anbehav.2020.08.012
Brown G. E., Ferrari M. C. O., Elvidge C. K., Ramnarine I., Chivers D. P. (2013). Phenotypically plastic neophobia: A response to variable predation risk. Proc. R. Soc B Biol. Sci. 280, 20122712. doi: 10.1098/rspb.2012.2712
Candler S., Bernal X. E. (2015). Differences in neophobia between cane toads from introduced and native populations. Behav. Ecol. 26, 97–104. doi: 10.1093/beheco/aru162
Cohen S., Benjamini Y., Golani I. (2015). Coping with space neophobia in drosophila melanogaster: the asymmetric dynamics of crossing a doorway to the untrodden. PloS One 10, 1–14. doi: 10.1371/journal.pone.0140207
Coleman S. L., Mellgren R. L. (1994). Neophobia when feeding alone or in flocks in zebra finches. Anim. Behav. 48, 903–907. doi: 10.1006/anbe.1994.1315
Cornell H. N., Marzluff J. M., Pecoraro S. (2012). Social learning spreads knowledge about dangerous humans among American crows. Proc. R. Soc B Biol. Sci. 279, 499–508. doi: 10.1098/rspb.2011.0957
Cornell Lab of Ornithology (2023). Raven lite: interactive sound analysis software (Version 2.0.4), K.L.Y.C. for C.B. at the. Ithaca, NY: Cornell Lab Ornithol.
Crane A. L., Ferrari M. C. O. (2017). Patterns of predator neophobia: A meta-analytic review. Proc. R. Soc B Biol. Sci. 284, 20170583. doi: 10.1098/rspb.2017.0583
Damas-Moreira I., Riley J. L., Harris D. J., Whiting M. J. (2019). Can behaviour explain invasion success? A comparison between sympatric invasive and native lizards. Anim. Behav. 151, 195–202. doi: 10.1016/j.anbehav.2019.03.008
Dardenne S., Ducatez S., Cote J., Poncin P., Stevens V. M. (2013). Neophobia and social tolerance are related to breeding group size in a semi-colonial bird. Behav. Ecol. Sociobiol. 67, 1317–1327. doi: 10.1007/s00265-013-1560-3
Dingemanse N. J., Both C., Drent P. J., Van Oers K., Van Noordwijk A. J. (2002). Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 64, 929–938. doi: 10.1006/anbe.2002.2006
Dunkler D., Ploner M., Schemper M., Heinze G. (2018). Weighted cox regression using the R package coxphw. J. Stat. Software 84. doi: 10.18637/jss.v084.i02
Elgar M. A. (1986). House sparrows establish foraging flocks by giving chirrup calls if the resources are divisible. Anim. Behav. 34, 169–174. doi: 10.1016/0003-3472(86)90020-5
Ensminger A. L., Westneat D. F. (2012). Individual and sex differences in habituation and neophobia in house sparrows ( Passer domesticus ). Ethology 118, 1085–1095. doi: 10.1111/eth.12009
Fair J., Paul E., Jones J., Bies L. (2023). “Guidelines to the use of wild birds in research,” in Ornithological council (Washington, D.C.: The Ornithological Council).
Farrow L. F., Doohan S. J., McDonald P. G. (2017). Alarm calls of a cooperative bird are referential and elicit context-specific antipredator behavior. Behav. Ecol. 28, 724–731. doi: 10.1093/beheco/arx020
Feyten L. E. A., Demers E. E. E. M., Ramnarine I. W., Chivers D. P., Ferrari M. C. O., Brown G. E. (2019). Who’s where? Ecological uncertainty shapes neophobic predator avoidance in Trinidadian guppies. Behav. Ecol. Sociobiol. 73, 70 doi: 10.1007/s00265-019-2687-7
Ghirlanda S., Enquist M. (2003). A century of generalization. Anim. Behav. 66, 15–36. doi: 10.1006/anbe.2003.2174
Greenberg R. (1990). Feeding neophobia and ecological plasticity: a test of the hypothesis with captive sparrows. Anim. Behav. 39, 375–379. doi: 10.1016/S0003-3472(05)80884-X
Greenberg R., Mettke-Hofmann C. (2001). “Ecological aspects of neophobia and neophilia in birds,” in Current ornithology (Boston, MA: Springer US), 119–178. doi: 10.1007/978-1-4615-1211-0_3
Greggor A. L., McIvor G. E., Clayton N. S., Thornton A. (2016). Contagious risk taking: Social information and context influence wild jackdaws’ responses to novelty and risk. Sci. Rep. 6, 1–7. doi: 10.1038/srep27764
Greggor A. L., Thornton A., Clayton N. S. (2015). Neophobia is not only avoidance: improving neophobia tests by combining cognition and ecology. Curr. Opin. Behav. Sci. 6, 82–89. doi: 10.1016/j.cobeha.2015.10.007
Griesser M. (2008). Referential calls signal predator behavior in a group-living bird species. Curr. Biol. 18, 69–73. doi: 10.1016/j.cub.2007.11.069
Griesser M. (2009). Mobbing calls signal predator category in a kin group-living bird species. Proc. R. Soc B Biol. Sci. 276, 2887–2892. doi: 10.1098/rspb.2009.0551
Griffin A. S. (2004). Social learning about predators: A review and prospectus. Learn. Behav. 34, 124–130. doi: 10.3758/BF03193188
In J., Lee D. K. (2019). Survival analysis: Part II – applied clinical data analysis. Korean J. Anesthesiol. 72, 441–457. doi: 10.4097/kja.19183
Joyce B. J., Demers E. E. M., Chivers D. P., Ferrari M. C. O., Brown G. E. (2016). Risk-induced neophobia is constrained by ontogeny in juvenile convict cichlids. Anim. Behav. 114, 37–43. doi: 10.1016/j.anbehav.2016.01.007
Kassambara A., Kosinski M., Biecek P., Scheipl F. (2021). survminer: drawing survival curves using “ggplot2. R package version 0.4.9. R Packag. version 0.4.7.
Katsnelson E., Motro U., Feldman M. W., Lotem A. (2008). Early experience affects producer-scrounger foraging tendencies in the house sparrow. Anim. Behav. 75, 1465–1472. doi: 10.1016/j.anbehav.2007.09.020
Keen S. C., Cole E. F., Sheehan M. J., Sheldon B. C. (2020). Social learning of acoustic anti-predator cues occurs between wild bird species. Proc. R. Soc B Biol. Sci. 287, 20192513. doi: 10.1098/rspb.2019.2513
Kelly T. R., Kimball M. G., Stansberry K. R., Lattin C. R. (2020). No, you go first: phenotype and social context affect house sparrow neophobia. Biol. Lett. 16, 20200286. doi: 10.1098/rsbl.2020.0286
Kelly T. R., Lynch K. I., Couvillion K. E., Gallagher J. N., Stansberry K. R., Kimball M. G., et al. (2022). A transient reduction in circulating corticosterone reduces object neophobia in male house sparrows. Horm. Behav. 137, 105094. doi: 10.1016/j.yhbeh.2021.105094
Kimball M. G., Gautreaux E. B., Couvillion K. E., Kelly T. R., Stansberry K. R., Lattin. C. R. (2022). Novel objects alter immediate early gene expression globally for ZENK and regionally for c-Fos in neophobic and non-neophobic house sparrows. Behav. Brain Res. 428, 113863. doi: 10.1016/j.bbr.2022.113863
Kimball M. G., Lattin C. R. (2023a). The “Seven deadly sins“ of neophobia experimental design. Integr. Comp. Biol., 1–17. doi: 10.1093/icb/icad127
Kimball M. G., Lattin C. R. (2023b). Exploration of A novel environment is not correlated with object neophobia in wild-caught house sparrows (Passer domesticus). Behav. Processes 210, 104913. doi: 10.1016/j.beproc.2023.104913
King A. J., Williams L. J., Mettke-Hofmann C. (2015). The effects of social conformity on Gouldian finch personality. Anim. Behav. 99, 25–31. doi: 10.1016/j.anbehav.2014.10.016
Klvaňová A., Hořáková D., Exnerová A. (2011). Nest defence intensity in house sparrows passer domesticus in relation to parental quality and brood value. Acta Ornithol. 46, 47–54. doi: 10.3161/000164511X589910
Kopisch A. D., Schwagmeyer P. L., Mock D. W. (2005). Individual consistency in parental effort across multiple stages of care in the house sparrow, Passer domesticus. Ethology 111, 1062–1070. doi: 10.1111/j.1439-0310.2005.01137.x
Kuitunen I., Ponkilainen V. T., Uimonen M. M., Eskelinen A., Reito A. (2021). Testing the proportional hazards assumption in cox regression and dealing with possible non-proportionality in total joint arthroplasty research: methodological perspectives and review. BMC Musculoskelet. Disord. 22, 1–7. doi: 10.1186/s12891-021-04379-2
Lattin C. R., Kelly T. R., Kelly M. W., Johnson K. M. (2021). Constitutive gene expression differs in three brain regions important for cognition in neophobic and non-neophobic house sparrows (Passer domesticus). bioRxiv. 17, e0267180. doi: 10.1101/2021.07.06.451290
Lee V. E., Régli N., McIvor G. E., Thornton A. (2019). Social learning about dangerous people by wild jackdaws. R. Soc Open Sci. 6, 191031. doi: 10.1098/rsos.191031
Magory Cohen T., Kumar R. S., Nair M., Hauber M. E., Dor R. (2020). Innovation and decreased neophobia drive invasion success in a widespread avian invader. Anim. Behav. 163, 61–72. doi: 10.1016/j.anbehav.2020.02.012
Marzluff J. M. (2002). Avian ecology and conservation in an urbanizing world. Auk 119, 889–892. doi: 10.2307/4090001
Mazza V., Czyperreck I., Eccard J. A., Dammhahn M. (2021). Cross-context responses to novelty in rural and urban small mammals. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.661971
McLaughlin A. L., Westneat D. F. (2023). House sparrows exhibit individual differences in generalization when confronted with different novel stimuli. Ethology. 129, 369–379. doi: 10.1111/eth.13374
Mertes K., Ressijac C. A., Moraes R. N., Hughey L. F., Alegre L. H. P., Horning M., et al. (2022). Assessing neophobia and exploration while accounting for social context: an example application in scimitar-horned oryx. Mamm. Biol. 102, 1357–1371. doi: 10.1007/s42991-022-00271-1
Mettke-Hofmann C., Winkler H., Leisler B. (2002). The significance of ecological factors for exploration and neophobia in parrots. Ethology 108, 249–272. doi: 10.1046/j.1439-0310.2002.00773.x
Mitchell M. D., Chivers D. P., Brown G. E., Ferrari M. C. O. (2016). Living on the edge: How does environmental risk affect the behavioural and cognitive ecology of prey? Anim. Behav. 115, 185–192. doi: 10.1016/j.anbehav.2016.03.018
Moldoff D. E., Westneat D. F. (2017). Foraging sparrows exhibit individual differences but not a syndrome when responding to multiple kinds of novelty. Behav. Ecol. 28, 732–743. doi: 10.1093/beheco/arx014
Moretti L., Hentrup M., Kotrschal K., Range F. (2015). The influence of relationships on neophobia and exploration in wolves and dogs. Anim. Behav. 107, 159–173. doi: 10.1016/j.anbehav.2015.06.008
Nivison J. J. (1978). The vocal behavior and displays of the House Sparrow, Passer domesticus L., in the United States 1–293.
Olson R. S., Haley P. B., Dyer F. C., Adami C. (2015). Exploring the evolution of a trade-off between vigilance and foraging in group-living organisms. R. Soc Open Sci. 2, 150135. doi: 10.1098/rsos.150135
Poisson A., Valotaire C., Borel F., Bertin A., Darmaillacq A. S., Dickel L., et al. (2017). Embryonic exposure to a conspecific alarm cue triggers behavioural plasticity in juvenile rainbow trout. Anim. Behav. 133, 35–45. doi: 10.1016/j.anbehav.2017.09.013
Radford A. N., Ridley A. R. (2007). Individuals in foraging groups may use vocal cues when assessing their need for anti-predator vigilance. Biol. Lett. 3, 249–252. doi: 10.1098/rsbl.2007.0110
R Core Team (2024). R: A language and environment for statistical computing (Vienna, Austria: R Found. Stat. Comput).
Reyer H. U., Fischer W., Steck P., Nabulon T., Kessler P. (1998). Sex-specific nest defense in house sparrows (Passer domesticus) varies with badge size of males. Behav. Ecol. Sociobiol. 42, 93–99. doi: 10.1007/s002650050416
Rivera-Hernández I. A. E., Crane A. L., Pollock M. S., Ferrari M. C. O. (2022). Disturbance cues function as a background risk cue but not as an associative learning cue in tadpoles. Anim. Cogn. 25, 881–889. doi: 10.1007/s10071-022-01599-4
St. Lawrence S., Rojas-Ferrer I., Morand-Ferron J. (2021). Does the presence of a conspecific increase or decrease fear? Neophobia habituation zebra finches. Ethology 127, 1033–1041. doi: 10.1111/eth.13224
Stöwe M., Kotrschal K. (2007). Behavioural phenotypes may determine whether social context facilitates or delays novel object exploration in ravens (Corvus corax). J. Ornithol. 148, 179–184. doi: 10.1007/s10336-007-0145-1
Templeton C. N., Greene E., Davis K. (2005). Allometry of alarm calls: black-capped chickadees encode information about predator size. Science. 308, 1934–1937. doi: 10.1126/science.1108841
Therneau T. M. (2021). survival: A package for survival analysis in R. Available online at: https://CRAN.R-project.org/package=survival.
Tuliozi B., Fracasso G., Hoi H., Griggio M. (2018). House sparrows’ (Passer domesticus) behaviour in a novel environment is modulated by social context and familiarity in a sex-specific manner. Front. Zool. 15. doi: 10.1186/s12983-018-0267-8
Keywords: vocal behavior, social learning, social cues, songbird, behavioral plasticity
Citation: Kimball MG, Masri DF, Gautreaux EB, Stansberry KR, Kelly TR and Lattin CR (2024) Conspecific alarm calls prevent the attenuation of neophobia behavior in wild-caught house sparrows (Passer domesticus). Front. Bird Sci. 3:1440063. doi: 10.3389/fbirs.2024.1440063
Received: 28 May 2024; Accepted: 16 July 2024;
Published: 30 July 2024.
Edited by:
Jacquelyn K. Grace, Texas A and M University, United StatesReviewed by:
Herbert Hoi, University of Veterinary Medicine Vienna, AustriaCopyright © 2024 Kimball, Masri, Gautreaux, Stansberry, Kelly and Lattin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melanie G. Kimball, bWtpbWJhNkBsc3UuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.