- 1Department of Biology, University of Saskatchewan, Saskatoon, SK, Canada
- 2Department of Ecosystem Science and Management, University of Northern British Columbia, Prince George, BC, Canada
- 3Science and Technology Branch, Environment and Climate Change Canada, Saskatoon, SK, Canada
Drivers of global change are creating strongly contrasting early life conditions for developing offspring, which may have carry-over effects on lifetime fitness. We tested for “silver-spoon” effects of natal conditions (environmental conditions and maternal quality) and individual quality (pre-fledging) on the lifetime reproductive success (LRS) of aerial insectivorous adult tree swallows (Tachycineta bicolor) hatched in distinct populations with contrasting environments in Saskatchewan and British Columbia, Canada. In both populations, LRS of adults was influenced by environmental conditions they experienced as developing nestlings, but silver-spoon effects were context-dependent, indicating population-specific responses to the local environment. Higher natal temperature in Saskatchewan had positive silver-spoon effects on the LRS of adult swallows, but the opposite was observed in British Columbia, likely because the highest temperatures local recruits experienced as nestlings occurred during heat extremes. In Saskatchewan, where wetter conditions reflect higher wetland abundance and food supply, we detected a negative effect of good natal wetland conditions on adult LRS, contrary to our hypothesis. However, since current breeding wetland conditions are a strong driver of adult fitness, and adults experiencing high natal wetland abundance generally bred when wetland abundance was lower, we suspect any potential benefits of natal wetland abundance on LRS were overridden by wetland conditions during breeding. As hypothesized, wetter natal conditions in British Columbia, which reflect an unfavorable environment for developing nestlings, had negative silver-spoon effects on the LRS of adults. No maternal or pre-fledging quality effects were detected at either site. Therefore, LRS of individuals within distinct populations is influenced, at least in part, by carry-over effects of the natal environment that vary locally. Consequently, natal environmental conditions that affect fitness, with putative population-level consequences, may underly spatially-varying population trends of regionally distinct populations within a species’ range.
Introduction
Global changes in land use and climate are currently having negative influences on numerous taxa (Spooner et al., 2018; Northrup et al., 2019; Rosenberg et al., 2019; Halupka et al., 2023), with effects predicted to increase over the next several decades (Visconti et al., 2016; Newbold, 2018; Powers and Jetz, 2019). As animals are exposed to novel environmental conditions caused by the loss of habitat and climate change, it is important to understand the long-term fitness consequences of varying conditions, including those experienced during early life. Studies across diverse taxa have shown that early life conditions, including environmental quality and parental effects (reviewed in Lindström, 1999), can have persistent, or carry-over, effects into adulthood affecting the fitness prospects of entire cohorts of individuals born or hatched in the same year (Ancona and Drummond, 2013; Herfindal et al., 2015; Payo-Payo et al., 2023). Therefore, a better understanding of how varying early life conditions may influence fitness is important for the conservation of numerous taxa, including migratory birds, as it may provide deeper insights into mechanisms underlying spatially-varying population trends of species across their range.
“Good” or “poor” early life conditions that are positively or negatively associated with adult fitness, respectively, are called “silver-spoon effects” (Grafen, 1988). For example, offspring born or hatched in years with “good” environmental conditions, such as favorable weather, high-quality habitats, or plentiful prey availability, are generally higher quality, i.e., heavier or in better body condition (Hamel et al., 2009; Ancona and Drummond, 2013) and may perform better at later life stages regardless of the adult conditions experienced (Reid et al., 2003; van de Pol et al., 2006; Brown et al., 2022; Poli et al., 2022). However, even in years of “good” environmental conditions, it is possible that offspring may experience poor-quality or degraded environmental conditions if food resources or local weather vary temporally or deteriorate seasonally during the breeding season at temperate latitudes (Harriman et al., 2017; Shipley et al., 2020). While studies have demonstrated evidence in support of silver-spoon effects (van de Pol et al., 2006; Spagopoulou et al., 2020; Poli et al., 2022), fitness outcomes of early life conditions may also depend on the environment individuals experience as adults (e.g., environmental matching hypothesis; Monaghan, 2008).
Parental effects, such as the quality of parents themselves and/or decisions about when in a season to breed, may influence the early life conditions experienced by offspring during development (Lindström, 1999; Rödel et al., 2009; Bouwhuis et al., 2010). For instance, parents in good body condition, because they can invest more care or resources, often produce offspring that are heavier or in better condition with greater fitness prospects, regardless of environmental quality (i.e., silver-spoon condition-transfer effects; Bonduriansky and Crean, 2018). Likewise, across taxa, offspring produced earlier in the season are often heavier (Plard et al., 2015), more likely to recruit to the breeding population (Blums et al., 2002), and sometimes have higher fitness as adults (Rödel et al., 2009; Saino et al., 2012). This could be due to having higher quality parents (i.e., older, more experienced, in better condition) or better access to food resources during development (reviewed in Verhulst and Nilsson, 2008). Therefore, in seasonally deteriorating environments there could be interactions between parental quality and environmental conditions. For example, even in years with “good” conditions, offspring may still have lower fitness prospects if they are raised by poor-quality parents and/or later in the season. Alternatively, in “good” conditions, offspring may have high fitness prospects even when raised by parents that are poor quality and/or later in the season, as such parental effects may be more pronounced or only detected in poor-quality environments (Engqvist and Reinhold, 2016).
Early life conditions can have both short- and long-term consequences for fitness-related traits of offspring (Lindström, 1999). Short-term effects of early life conditions on offspring have been well characterized, and shown to affect offspring quality (e.g., pre-fledging body mass or condition) and survival, or probability of recruitment to the breeding population (Ancona et al., 2018; Halliwell et al., 2023). However, short-term effects of early life conditions on offspring are not always detected on fitness components over the long term, i.e., after local recruitment to the breeding population (Nevoux et al., 2010; Cam and Aubry, 2011; Mumme et al., 2015). Nevertheless, evidence of early life conditions having carry-over effects on adult survival after recruitment (Brown et al., 2022; Poli et al., 2022) or lifetime reproductive success (van de Pol et al., 2006; Rödel et al., 2009; Plard et al., 2015) have been demonstrated. For example, red-billed choughs (Pyrrhocorax pyrrhocorax) hatched in good environments were more likely to recruit to the breeding population and produced more recruits themselves as adults (Reid et al., 2003). Regardless, the context in which early life conditions carry-over to affect fitness and how they may vary across ecological conditions have not been fully evaluated.
In an aerial insectivore, the tree swallow (Tachycineta bicolor), we have previously explored the short-term fitness consequences of variation in early life (natal) conditions at two distinct breeding populations in Canada (Saskatchewan [SK] and British Columbia [BC]) that experience strongly contrasting local environments (Figure 1; Table 1). In SK, located in the Canadian prairies, temperatures during the breeding season are generally warmer and there are few days with temperatures below 18.5°C (Berzins et al., 2020), i.e., “cold snaps” (the critical temperature for insect flight; Winkler et al., 2013). This contrasts the BC site, located in the interior of the province, where temperatures are cooler and cold snaps are more frequent (Griebel and Dawson, 2019). However, warmer temperatures during development at both sites, e.g., ambient temperature (SK) (Berzins et al., 2021) or experimentally heated nest boxes (BC) (Dawson et al., 2005), enhance the pre-fledging body mass of nestlings, and therefore reflect “good” environmental conditions. Wetter natal conditions at the semi-arid SK site are indicative of a “good” environment reflecting a high abundance of ponds (i.e., wetland basins containing water; Weegman et al., 2017; Clark et al., 2018). Abundant ponds during development have positive effects on the quality (i.e., pre-fledging body mass) and apparent first-year survival of offspring (Harriman, 2014; Berzins et al., 2021), presumably because of greater insect food availability or quality. In contrast, wetter natal conditions at the BC site are indicative of a “poor” environment, strongly associated with severe weather, such as cold snaps and precipitation, which negatively affect fledging survival (Berzins et al., 2020). Overall, the unfavorably cooler and wetter natal conditions likely underly the reported lower fledging success, and lighter nestling body mass prior to fledging, at the BC site compared to SK (Harriman et al., 2017; Weegman et al., 2017).
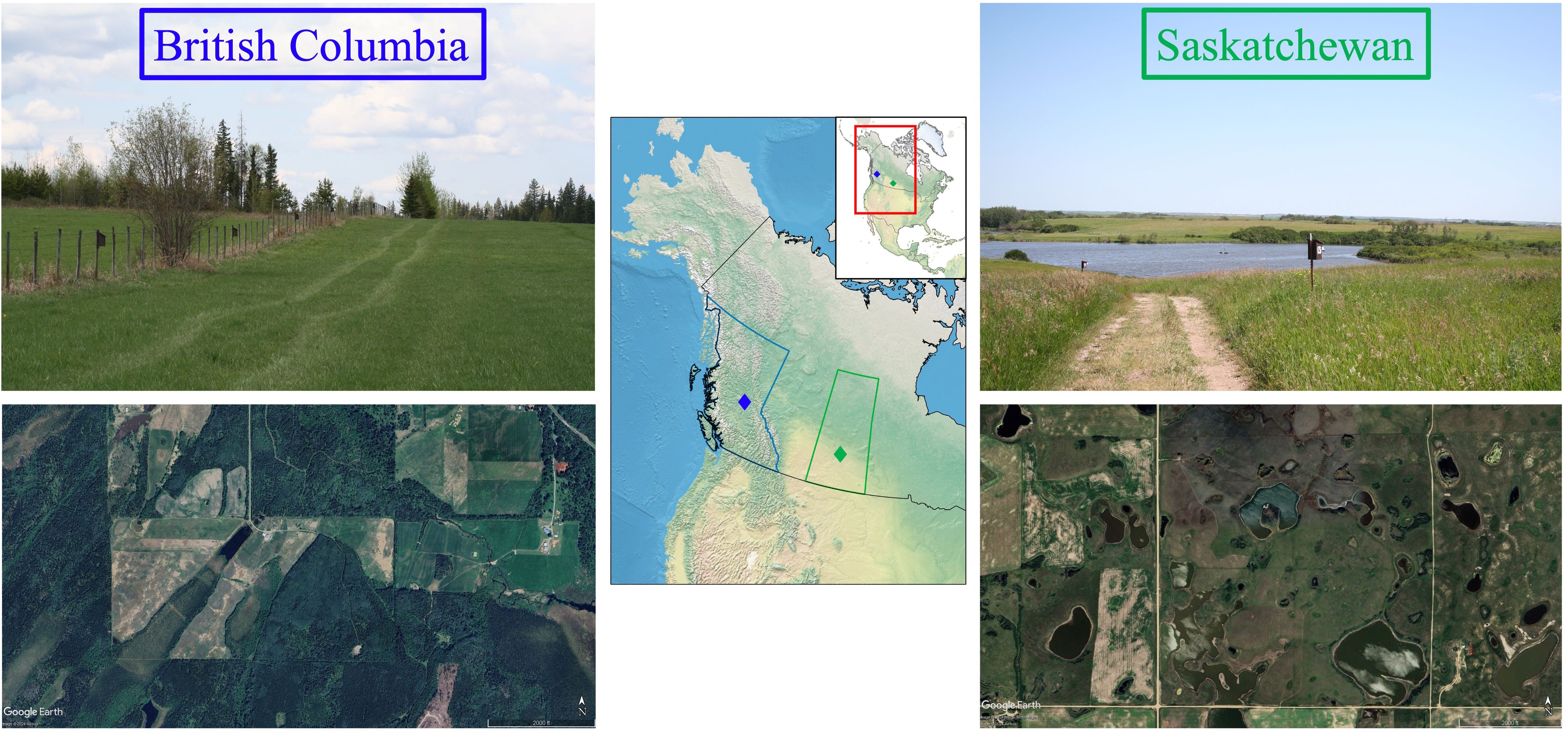
Figure 1 Map of North America showing tree swallow (Tachycineta bicolor) study sites located in distinct biomes (temperate [evergreen] forest versus temperate prairies) >1200 km apart. The site in British Columbia (left panel; blue) is in the interior of the province, and contains few small permanent wetlands, with stands of deciduous and coniferous trees that are intermixed among open hay fields. The site in Saskatchewan (right panel; green) is in the semi-arid prairies, and contains >100 wetland basins with ponds of varying size and permanency, as well as numerous small groves of trembling aspen (Populus tremuloides), cropland, and native and tame grassland. The map of North America was created using map data from Nature Earth (naturalearthdata.com) in QGIS v3.2.6 (QGIS.org, 2024). Photos (top) courtesy of Lisha Berzins, and satellite imagery (bottom) was sourced from Google Earth Pro version 7.3.6.9796, accessed April 2024 [British Columbia. (June 7, 2023). 53°N, 122°W. Eye alt 11682 ft. Terrain layer. Airbus, 2024; Saskatchewan. (June 27, 2023). 52°N, 106°W, Eye alt 11635 ft. Terrain layer. Airbus, Maxar Technologies 2024].
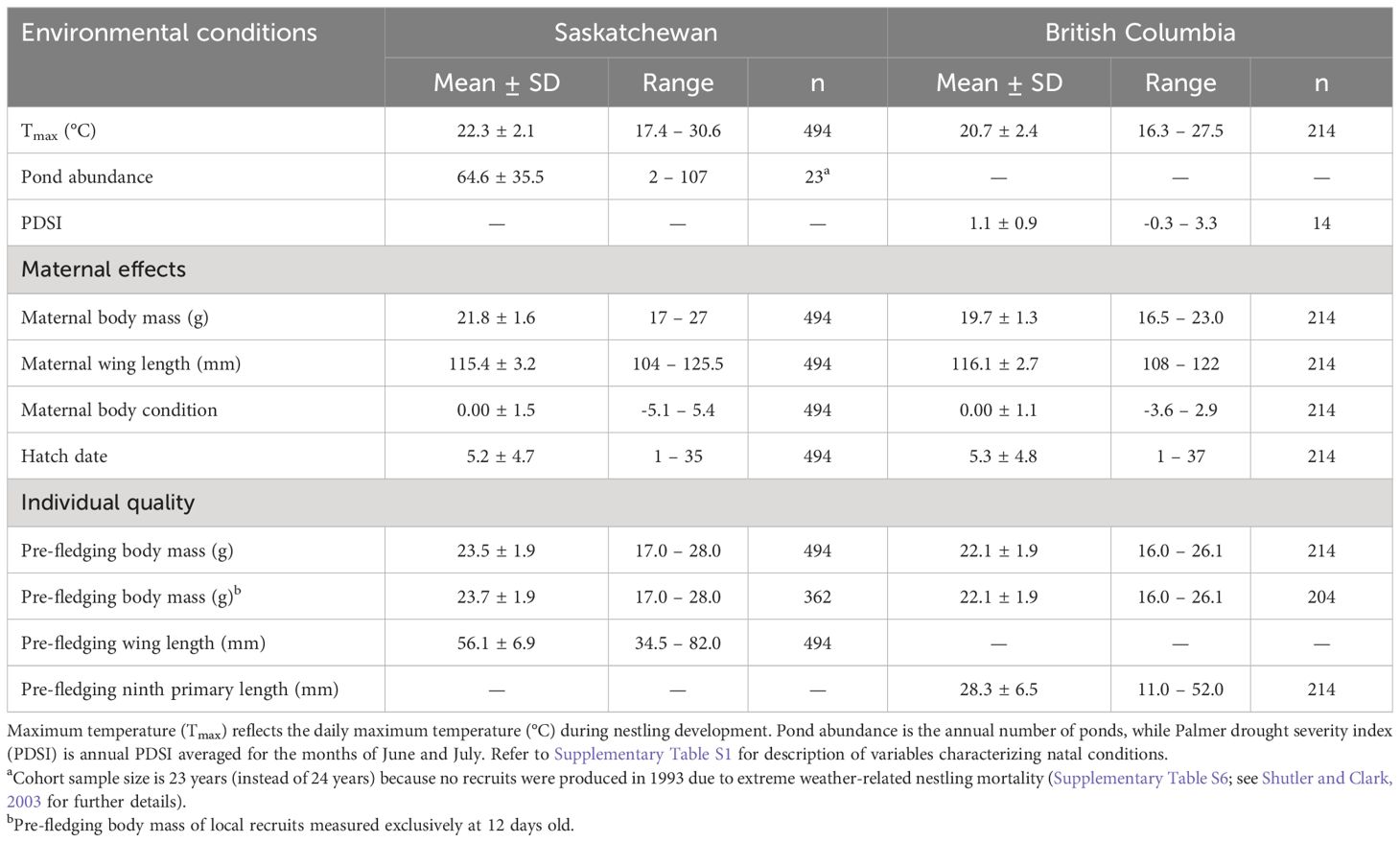
Table 1 Mean ± standard deviation (SD) of variables characterizing natal conditions experienced by locally recruited tree swallows (Tachycineta bicolor) as nestlings hatched in Saskatchewan (1991–2014) and British Columbia (2001–2014), Canada.
Maternal effects, such as timing of egg laying within a season, can also have short-term benefits for nestlings, with those hatching from nests initiated earlier both in SK and BC being more likely to recruit locally (Shutler et al., 2006; Harriman, 2014). The quality of the female parent, i.e., body condition, age class (second-year [SY] or after-second-year [ASY]), or breeding site experience (previously banded or unbanded when captured), also appear to benefit nestlings in the short-term, although the patterns appear site- and/or possibly year-dependent. Specifically, Harriman et al. (2017) reported that in SK, females in better body condition produced nestlings with better body condition prior to fledging (see also Griebel et al., 2019), but this was not observed at the BC site, although other studies in BC have shown that the age class of females can influence nestling body mass (Bitton and Dawson, 2017). Maternal breeding site experience was also found to be a predictor of apparent first-year survival of offspring in SK, but not at the BC site (Harriman, 2014). Finally, Lombardo et al. (2020) reported that the influence of maternal quality traits on the recruitment potential of nestling tree swallows in Michigan, USA, varied annually but were not found to be influential when the data were pooled over a 12-year time series.
In the current study, we tested whether natal conditions, i.e., environmental conditions, maternal effects, or pre-fledging quality, influenced the lifetime reproductive success (LRS) of locally recruited adult tree swallows. Local recruits are defined as nestlings hatched at our study sites that returned to breed in subsequent years. Given that nestlings which permanently emigrated from our study sites were rarely detected, we hereafter use “apparent LRS” (LRSa; see Berzins et al., 2020). Based on the short-term benefits of “good” natal conditions in these study systems, we hypothesized (Supplementary Table S1) that local recruits experiencing warmer temperatures during development (SK/BC) or hatched in years with abundant ponds (SK) or relatively drier conditions (i.e., lower Palmer drought severity index (PDSI); BC) would have higher LRSa (environmental conditions), consistent with the silver-spoon hypothesis. Furthermore, we hypothesized that LRSa of local recruits would be higher if reared by mothers in better body condition or that bred earlier in the breeding season (maternal effects), and that these effects on LRSa may depend on environmental conditions in the natal year (maternal effect*environmental condition interactions). For instance, LRSa of local recruits may be (1) lower if reared by mothers in poor body condition or later in the season even when environmental conditions are good, i.e., warmer temperatures, or higher pond abundance (SK) or relatively drier PDSI (BC); or (2) higher if reared by mothers in better body condition or breeding earlier in the season when temperatures are cooler, or pond abundance is lower (SK) or PDSI is relatively wet (BC). Lastly, while natal environmental conditions may have direct effects on long-term fitness (van de Pol et al., 2006; Spagopoulou et al., 2020), some studies have shown that natal environmental conditions can affect fitness indirectly via their effects on juvenile or yearling body mass which persist into adulthood (Plard et al., 2015; Brown et al., 2022). We hypothesized that local recruits fledging with heavier body mass, and therefore being higher quality individuals, would achieve greater LRSa as adults. Again, positive effects of pre-fledging body mass may be more pronounced if local recruits were hatched earlier within the season or reared by mothers in better body condition (pre-fledging body mass*maternal effects interactions).
Materials and methods
Study species
Tree swallows are aerial insectivores, which are birds characterized by their reliance on flying insects. As a guild, aerial insectivores have experienced steep population declines in North America since the 1970’s (Rosenberg et al., 2019). Across their breeding distribution in northern North America, tree swallows produce a single clutch per season that generally contains ~4–7 eggs (Winkler et al., 2020). The lifespan of tree swallows is ~ 2.7 years on average (Winkler et al., 2020), and ~50 to 60% of individuals breed only once (Lombardo and Thorpe, 2010; Berzins et al., 2020). Tree swallows are migratory birds that overwinter in the southern United States, Mexico, and the Caribbean (Knight et al., 2018). Apparent survival estimates indicate that adult survival is ~50% (Weegman et al., 2017; Clark et al., 2018), while juvenile survival is ~5–6% at the BC and SK sites (Weegman et al., 2017).
Study areas
Tree swallows were studied at two sites in Canada: Saskatchewan (SK) from 1991–2017, and British Columbia (BC) from 2001–2017. The SK site is located in the Canadian prairies, 40 km east of Saskatoon (52°N, 106°W) at the St. Denis National Wildlife Area. This site has >100 wetland basins containing ponds of varying permanency (Berzins et al., 2021), numerous small groves of trembling aspen (Populus tremuloides), cropland, and native and tame grassland (Figure 1). The BC site is in the interior sub-boreal forest and consists of three sub-sites within the vicinity of Prince George (53°N, 123°W). This area is characterized by a few small permanent wetlands, stands of deciduous and coniferous trees that are intermixed among hay fields (Figure 1).
At both sites, nest boxes are mounted ~1.5 m above the ground on metal or wooden posts in lines or grids and spaced ~30 m apart (see Shutler and Clark, 2003; Harriman et al., 2017). Nest boxes used in this study were constructed of plywood (10 mm thickness) similar to the “Long Point” nest box design (Hussell, 2003), i.e., ~38 mm diameter entrance hole centered ~155 mm above the floor of the box, and an interior floor measuring ~140 x 140 mm (Griebel et al., 2020). The number of nest boxes available varied annually at sites, ranging from 75 to >200 (SK) and 196 to >300 (BC) (Harriman, 2014). Nest box occupancy rates are nearly 100% annually at the SK site, but range from ~30 to 60% at the BC site (Shutler et al., 2012).
General field methods
Study sites were monitored from May to August to record data on annual reproductive success and individual quality. At both sites, tree swallows begin egg-laying in May, eggs hatch in June, and the nestling rearing period extends from June into July. Starting in May, nest boxes were visited every 1–2 days to record nest building, dates of clutch initiation and completion, and clutch size. Clutches were presumed complete, and incubation had begun, when the number of eggs was unchanged for three consecutive days and eggs were warm. Approximately 12 days after clutch completion, we monitored nests daily to determine the hatching date (the day the first egg within a clutch hatched = nestling age of day 0; Dawson, 2008). Eggs within a clutch hatch asynchronously, with the hatching of the first- and last-laid eggs usually occurring within a 24 hr period (Bitton et al., 2006), although some nests can take up to three days for all eggs to hatch (Winkler et al., 2020).
Adult tree swallows were captured in nest boxes and their sex was determined based on the presence of a cloacal protuberance (male) or brood patch (female) (Pyle, 1997). Adults were banded with an aluminum band if newly captured or band numbers were recorded if captured previously, weighed with a spring scale to the nearest 0.5 g and wing length was measured with a ruler to the nearest 0.5 mm. Nestlings were weighed, measured, and banded as described above for adults, with a few exceptions due to site-specific field protocols. First, nestlings were weighed with a spring scale to the nearest 0.25 g in SK and 0.125 g in BC. Second, wing length was measured in SK, whereas the ninth primary length was measured in BC. Since wing length is more or less determined by ninth primary length (the outermost feather) both provide similar estimates of structure size. Most nestlings (73% SK, 95% BC) were measured on day 12, but in some cases, they were measured on a different day (i.e., day 11 or a day from 13 to 16 days old). Starting on day 22, we checked nest boxes daily until all nestlings fledged, and recorded band number(s) of those that did not fledge due to mortality.
Apparent lifetime reproductive success
The number of local recruits hatched at our study sites in plywood nest boxes between 1991–2014 (SK) and 2001–2014 (BC) that returned to breed in subsequent years was 678 and 278, respectively. LRSa was estimated for local recruits until the 2017 breeding season which allowed for up to two breeding seasons to recapture recruiting offspring of birds hatched in 2014. The majority of recruits, 81% (SK) and 96% (BC), are captured breeding in nest boxes 1–2 years after hatching (Supplementary Figure S1), and previous capture-mark-recapture analyses at our study sites indicate that recapture rates of adult swallows are high (Weegman et al., 2017; Clark et al., 2018). LRSa reflects whether a local recruit produced no recruits (0) or produced at least one recruit detected as a breeding adult (1), and is expressed as a binary outcome since a low percentage (30% [SK] and 19% [BC]) of individuals produced more than one recruit in their lifetime (Berzins et al., 2020; Supplementary Table S2). That the majority of tree swallows produce no recruits in their lifetime (Berzins et al., 2020) is a pattern reported across taxa (e.g., tufted duck, Aythya fuligula; Blums and Clark, 2004; elephant seals, Mirounga angustirostris; Le Boeuf et al., 2019). Our measure of LRSa is based on all offspring local recruits raised that recruited, regardless of whether they were biologically related due to egg or nestling swaps between nests (e.g., Shutler et al., 2006; Harriman et al., 2017), which occurred in approximately 15% (SK) and 12% (BC) of nests, or for males specifically, because rates of extra-pair paternity are high (e.g., >70% at the BC site; Berzins and Dawson, 2020).
Quantifying natal conditions
Definitions of variables characterizing natal conditions (environmental conditions and maternal effects) and pre-fledging quality, and the predicted relationships to LRSa of local recruits, are provided in Supplementary Table S1. Means and standard deviations (SD) for variables describing the natal conditions are provided in Table 1.
Environmental conditions
Based on our current knowledge of tree swallows at the study sites (Berzins et al., 2020, 2021; see also Cox et al., 2019), we selected two variables to characterize the natal environment: maximum temperature (Tmax) and moisture conditions represented by pond abundance (SK) or Palmer drought severity index (PDSI; BC). Scatter plots showing variation between annual pond abundance or PDSI and seasonal Tmax are provided in Supplementary Figure S2.
Maximum temperature (Tmax) is a nest-level variable reflecting the average daily maximum temperature (°C) local recruits experienced as nestlings aged 2 to 12 days old (Berzins et al., 2021), and ranged from 17.4 to 30.6°C (SK) and 16.3 to 27.5°C (BC). Tmax data were retrieved from weather stations operated by Environment and Climate Change Canada at the airports in Saskatoon (~42 km from the SK site) and Prince George (~15 to 24 km from the BC sub-sites). Nestlings fledged at heavier masses in SK when daily Tmax was warmer (Berzins et al., 2021) and in BC when nest boxes were experimentally heated (Dawson et al., 2005) during growth. We therefore predicted that local recruits reared when temperatures were higher, reflecting good natal conditions, would have higher LRSa.
Pond abundance is a cohort-level variable reflecting the number of wetland basins flooded with water at the SK site (Berzins et al., 2020). The number of ponds was recorded annually in the month of May preceding the hatching of local recruits (Clark et al., 2018). Pond numbers recorded in May and July in sub-set of years were positively correlated (Rotella et al., 2003), which suggests that our measure of pond abundance should provide a good estimate of moisture conditions during nestling rearing (Berzins et al., 2021). In this region, climate extremes drive annual fluctuations in the number of ponds during wet-dry cycles (Johnson et al., 2004), and pond numbers at the SK site annually ranged from 2 to 107 (Berzins et al., 2021). We considered pond abundance as a categorical variable, where low and high categories represent the extremes, by dividing years into three groups: <41 ponds representing “low” pond abundance, 52 to 70 ponds as “intermediate” pond abundance, and >80 ponds as “high” pond abundance (Supplementary Figure S3). These categories divide the distribution of pond numbers into roughly thirds, and also take advantage of natural breaks in pond numbers between 41 to 52 and 70 to 80. Our previous work has shown that pond abundance in the natal year is a strong predictor of nestling pre-fledging body mass and recruitment potential (Berzins et al., 2021). Therefore, we predicted that local recruits hatched in years of intermediate to high pond abundances would have greater LRSa than those hatched in years of low pond numbers, if the benefits of good pond conditions in the natal year carry-over into adulthood.
The BC site has few permanent wetlands, in contrast to SK, so we instead used PDSI as a cohort-level variable characterizing local moisture conditions (Harriman, 2014; Berzins et al., 2020). Values of PDSI reflect the average for the months of June to July (Berzins et al., 2020), which overlap with the nestling rearing period. In general, negative and positive PDSI values represent drier and wetter conditions, respectively, but while PDSI values ranged from -0.26 to 3.33, there was only a single year with a negative PDSI value at the BC site (Supplementary Figure S4). To represent PDSI variability, we divided values into categories such that <0.5 represented relatively “dry” conditions, 0.5 to 1.5 “intermediate” conditions, and >1.5 relatively “wet” conditions. Data for PDSI were obtained from Agriculture and Agri-Food Canada (station #1096453). Wetter PDSI conditions are positively correlated with precipitation during the nestling period (Spearman correlation: rs = 0.71, p < 0.001, n = 14 years), and these inclement weather conditions presumably reduce the activity and availability of insect prey (Cox et al., 2019), resulting in a “poor” natal environment. Therefore, we predicted that local recruits hatched in relatively wetter years would have lower LRSa if negative effects of being raised in wetter conditions carry-over into adulthood.
Maternal effects
Early life rearing conditions experienced by local recruits as nestlings may vary as a result of maternal quality or decisions about when to initiate breeding. Two variables were selected to characterize variation in natal conditions due to maternal quality effects: maternal body condition and hatching date within a season.
Maternal body condition is a nest-level variable reflecting body mass corrected for structural size and stage of the breeding season. Body condition was calculated as the residual from linear models that regressed the body mass of local recruits’ mothers (response variable) against their wing length (structural size) and the number of days prior to or after hatching of nestlings (O’Brien and Dawson, 2013), which ranged from before hatch to 16-days old (Supplementary Table S3). Because the body mass of local recruits’ mothers was higher at the SK site (Table 1), we used linear models to calculate maternal body condition for each site separately. Maternal body condition was used as a variable to characterize maternal effects in this system because it reflects differences in the age class and/or breeding experience of female tree swallows (Harriman, 2014; Bitton and Dawson, 2017). Additionally, females in higher body condition may produce offspring with higher body condition that are better able to convert resources into traits that enhance fitness (i.e., silver-spoon condition-transfer effects; Bonduriansky and Crean, 2018). Therefore, we predicted that local recruits reared by mothers in higher body condition would achieve higher LRSa. Additionally, we predicted that benefits of maternal effects provided to offspring may depend on the quality of the natal environment (maternal body condition*environment interactions): (1) lower LRSa for local recruits may be expected if reared by mothers in poor body condition even when environmental conditions are good; or (2) local recruits may only achieve high LRSa if reared by mothers in better body condition in years with poor environmental conditions.
Hatching date is a nest-level variable reflecting the date within a season local recruits hatched relative to all nests. Mean (± SD) hatching date for each site and year are reported in Supplementary Table S4. To standardize hatching dates across years, we subtracted the annual date when 5% of all nests hatched from the hatching date of each nest (Clark et al., 2014). Hatching dates were standardized for each site and year separately. At the BC site, the 5% hatching date was calculated for all three sub-sites combined. At temperate latitudes, offspring hatched early in the season generally have greater fitness prospects (Svensson, 1997), being more likely to recruit (Shutler et al., 2006; Lombardo et al., 2020) and/or having higher LRSa as adults (Saino et al., 2012), either because they are reared by higher quality parents or experience more abundant food (reviewed in Verhulst and Nilsson, 2008). Therefore, we predicted that locally recruited offspring hatching from early-season nests at both sites would have higher LRSa. We also predicted this pattern may depend on environmental conditions (hatching date*environment interactions): (1) lower LRSa for local recruits may be expected if hatched later in the season even when environmental conditions are good; or (2) local recruits may achieve higher LRSa if hatched early in the season when environmental conditions are poor.
Individual quality
The quality of local recruits was estimated using their body mass as nestlings prior to fledging, which ranged from 17.0 to 28.0 g (SK) and 16.0 to 26.1 g (BC), after controlling for age-corrected structural size (i.e., wing length [SK] or ninth primary length [BC]; Supplementary Table S5). Pre-fledging body mass reflects the culmination of environmental and maternal effects of the developmental environment, where heavier offspring are indicative of good natal conditions (van de Pol et al., 2006; Spagopoulou et al., 2020). In some cases, effects of natal conditions on fitness are mediated indirectly via effects on natal body mass (Plard et al., 2015; Poli et al., 2022). We predicted that local recruits fledging at heavier masses, presumably as higher quality individuals, would thus attain greater LRSa as adults, especially if hatched earlier in the season or reared by mothers in good body condition (pre-fledging body mass*maternal effects interactions).
Statistical analysis
All statistical analyses were performed using R (version 4.2.2.; R Core Team, 2022). The sample sizes of local recruits that we used to examine silver-spoon effects of natal conditions on LRSa were 494 in SK (n = 207 females, n = 287 males) and 214 in BC (n = 117 females, n = 97 males) after we excluded those that were: (1) reared by a foster mother after being moved to a different nest (e.g., Shutler et al., 2006; SK: n = 49; BC: n = 21); (2) involved in experiments as a nestling that altered the microclimate of their nest, their hatching date within a season and/or resource competition in the nest (e.g., Dawson et al., 2005; Fairhurst et al., 2012; Berzins and Dawson, 2016; Harriman et al., 2017; Griebel et al., 2020; SK: n = 73; BC: n = 22), as such manipulations can create/prevent “silver-spoon” rearing conditions (Spagopoulou et al., 2020); (3) involved in experiments as adults that could affect their return or survival rates (e.g., geolocators, Gómez et al., 2014; SK = 15); or were (4) missing information on their hatching date (SK: n = 12) and pre-fledging body mass (SK: n = 13) or the body condition of their mother (SK: n = 22; BC: n = 21). Sample sizes of local recruits in each cohort with complete information for all variables are provided in Supplementary Table S6.
To examine whether natal conditions influenced LRSa of locally recruited tree swallows, we used generalized linear models (GLMs) fit with a binomial error distribution and a probit link function. The dependent variable was LRSa (binary response variable: 0 = produced no recruit; 1 = produced at least one recruit), and we constructed an a priori candidate set of 11 models (see Supplementary Table S7) to disentangle the relative contributions of natal conditions on LRSa of local recruits at each site separately: environmental factors, maternal effects and/or individual quality. Natal year was not included as a random or fixed effect since it was confounded with cohort-level variables of pond abundance/PDSI, and pre-fledging body mass (e.g., Berzins et al., 2021). Additionally, although 27% (SK) and 25% (BC) of local recruits shared a common mother, and 15% (SK) and 17% (BC) were siblings from the same nest, likelihood ratio tests indicated that models containing random effects of mother identity or natal nest identity were not a better fit to the data than GLMs with no random effects (p > 0.90).
For all models, continuous variables (e.g., Tmax, pre-fledging body mass) were z-score transformed (mean = 0, SD = 1) so standardized parameter estimates (relative effect sizes) could be directly compared, even in the presence of interactions (Schielzeth, 2010). Extreme values of variables, such as hatching date, were truncated by pooling with less extreme values to improve the fit of data to models (Clark et al., 2014). We assessed the fit of competing models via Q-Q and residual plots produced using the “DHARMa” package (Hartig, 2022). Generalized variance inflation factor values computed using the “car” package (Fox and Weisberg, 2019) were <2 indicating low collinearity among variables in models. Model selection was performed by generating Akaike’s information criterion corrected for sample size (AICc) values using the package “AICcmodavg” (Mazerolle, 2023). We considered the model with the lowest AICc value to be the most parsimonious, although models within 2 AICc units were considered equivalent (Burnham and Anderson, 2002). Standardized parameter estimates (β) for variables in best-supported model(s) are reported with the lower and upper 95% confidence intervals (CI), and were considered informative when 95% CI did not overlap zero (Arnold, 2010). Model predictions from the best-supported model(s) were calculated using the package “ggeffects” (Lüdecke, 2018).
Results
Apparent lifetime reproductive success of local recruits
In SK, 66% of local recruits produced no recruit themselves as breeders, while 34% produced at least one recruit (n = 494). The best-supported model explaining variation in LRSa of locally recruited offspring was the environmental model (Table 2). The maternal effects and individual quality models, as well as models containing interactions, had AICc > 2 relative to the model containing only environmental variables that local recruits experienced as nestlings (Table 2). Parameter estimates indicated that the likelihood of local recruits producing a recruit increased with warmer temperatures during development (βTmax = 0.16 ± 0.06, 95% CI = 0.04, 0.27; odds ratio [OR] = 1.17, 95% CI = 1.04, 1.32; Figure 2A). Additionally, local recruits were more likely to produce a recruit themselves when pond abundance in the natal year was intermediate compared to relatively higher (βpond abundance: high = -0.36 ± 0.15, 95% CI = -0.66, -0.05; OR = 0.70, 95% CI = 0.52, 0.95) and possibly lower abundance of ponds (βpond abundance: low = -0.31 ± 0.18, 95% CI = -0.66, 0.03; OR = 0.73, 95% CI = 0.52, 1.03; Figure 3A), although the 95% CI slightly overlapped 0.
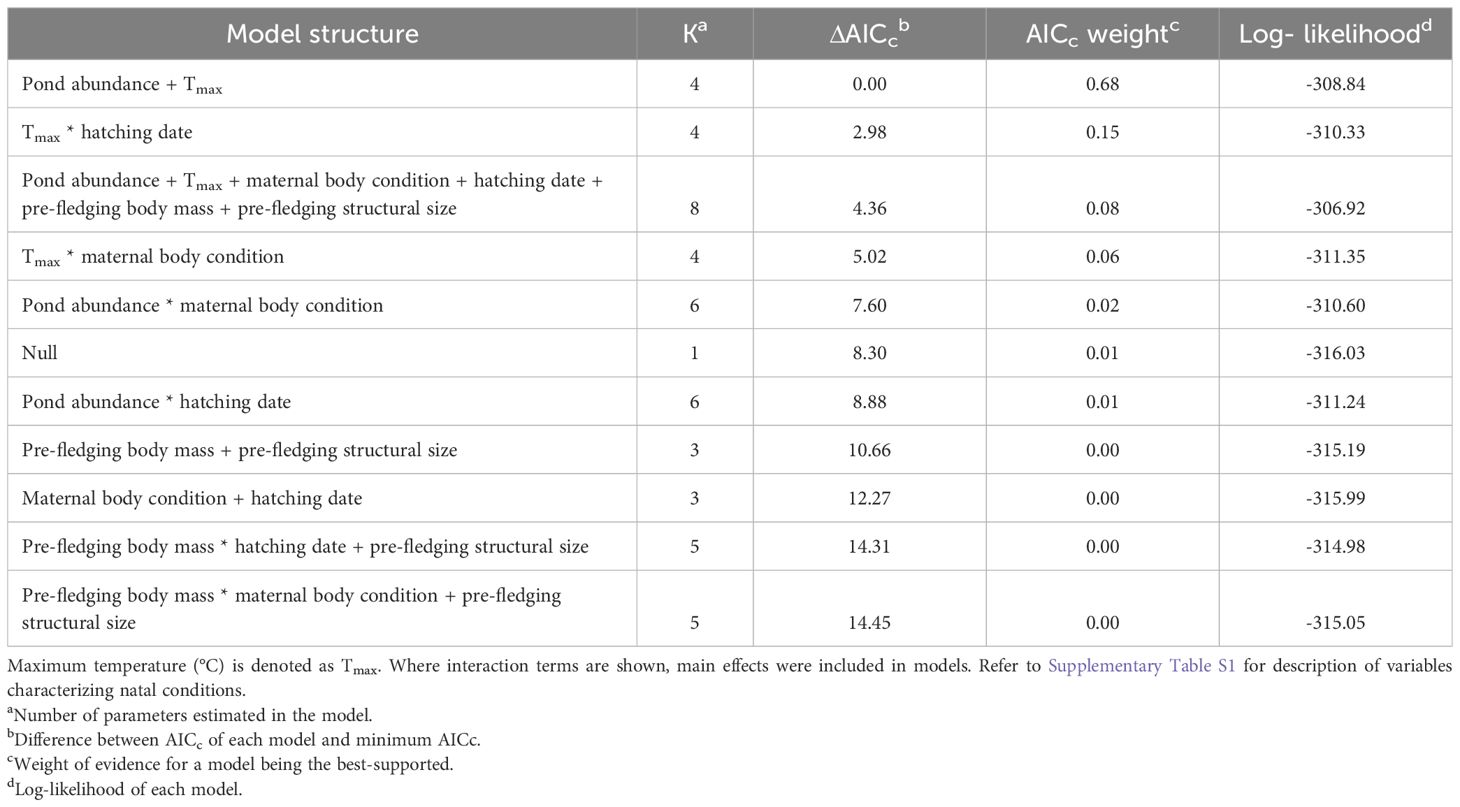
Table 2 Summary of model selection results of generalized linear models examining the effect of natal conditions on apparent lifetime reproductive success (LRSa; binomial = produced at least one recruit, produced no recruits) of locally recruited tree swallows (Tachycineta bicolor) hatched in Saskatchewan (1991–2014; n = 494), Canada.
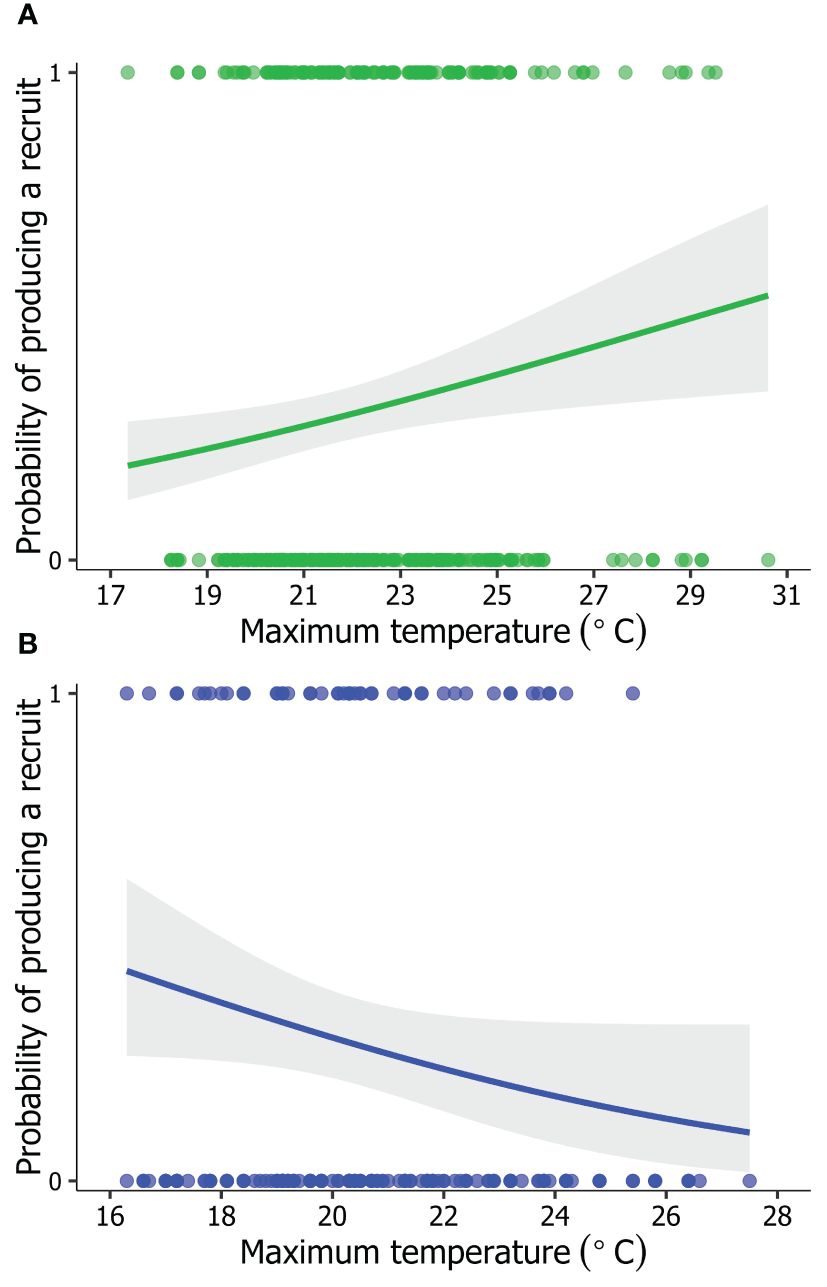
Figure 2 Model-predicted probability that locally recruited tree swallows (Tachycineta bicolor) hatched in (A) Saskatchewan (1991–2014; n = 494; green circles) and (B) British Columbia (2001–2014; n = 214; blue circles), Canada, would produce a recruit themselves as breeders in relation to maximum temperature, averaged from days 2 to 12 of nestling development. Model predicted values (line) and 95% confidence intervals (grey shaded area) are based on the top-supported model in Tables 2, 3.
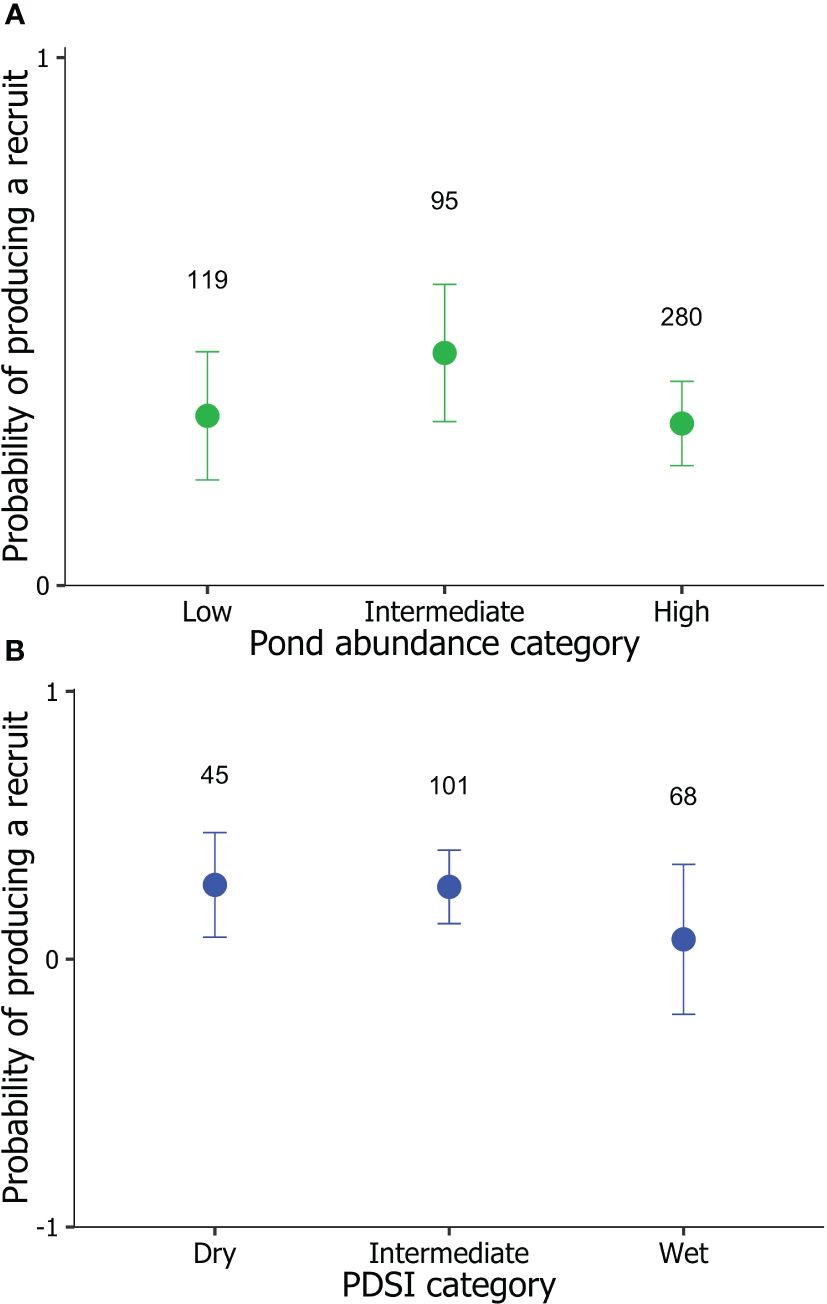
Figure 3 Model-predicted mean (± SE) probability that locally recruited tree swallows (Tachycineta bicolor) hatched in Saskatchewan (1991–2014, green circles) and British Columbia (2001–2014; blue circles), Canada, would produce a recruit themselves as breeders in relation to (A) pond abundance and (B) Palmer drought severity index (PDSI) conditions they experienced as nestlings during development. Model-predicted mean ± SE are based on the top-supported model in Tables 2, 3. Sample sizes for the number of local recruits are given above error bars.
In BC, 77% of local recruits produced no recruit themselves as breeding adults, while 23% produced at least one recruit in their lifetime (n = 214). The best-supported model explaining variation in LRSa of local recruits was the environmental model (Table 3). There was some suggestion that the likelihood of local recruits producing a recruit themselves decreased with warmer temperatures during development (βTmax = -0.24 ± 0.13, 95% CI -0.51, 0.01; OR = 0.78, 95% CI = 0.60, 1.01; Figure 2B), although the 95% CI slightly overlapped 0. Additionally, local recruits hatched in years with intermediate and dry PDSI conditions were as breeders equally likely to produce a recruit themselves (βPDSI: dry = 0.02 ± 0.25, 95% CI = -0.47, 0.52; OR = 1.02, 95% CI = 0.63, 1.68); however, compared to local recruits hatched in years with intermediate PDSI conditions, those that hatched in wetter years were less likely to produce a recruit (βPDSI: wet= -0.83 ± 0.30, 95% CI = -1.44, -0.27; OR = 0.43, 95% CI = 0.24, 0.77; Figure 3B).
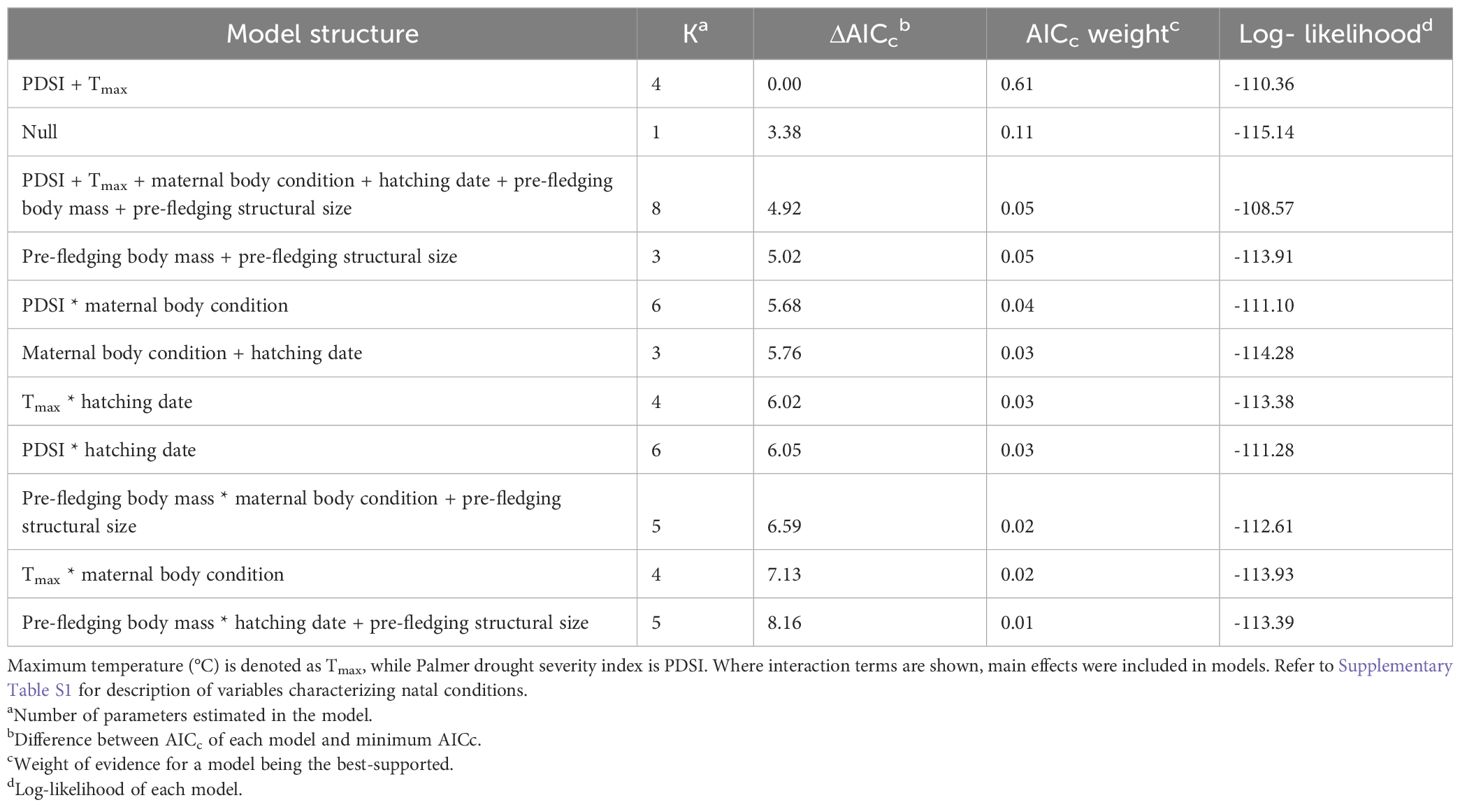
Table 3 Summary of model selection results of generalized linear models examining the effect of natal conditions on apparent lifetime reproductive success (LRSa; binomial = produced at least one recruit, produced no recruits) of locally recruited tree swallows (Tachycineta bicolor) hatched in British Columbia (2001–2014; n = 214), Canada.
Discussion
The quality of natal conditions experienced by offspring is predicted to have carry-over effects on adult lifetime fitness. In a short-lived aerial insectivore, we showed that the best-supported models explaining LRSa were those containing environmental conditions. Therefore, environmental conditions, specifically Tmax and/or pond abundance or PDSI, that local recruits experienced as nestlings during development, appear to have long-term effects on LRSa in tree swallows. These results demonstrating consequences of variation in the natal environment on adult fitness are consistent with silver-spoon effects reported previously in diverse taxa (Reid et al., 2003; van de Pol et al., 2006; Plard et al., 2015). However, while evaluating two geographically distinct populations with contrasting environments on the breeding grounds, we also show that silver-spoon effects were context-specific depending on the populations’ response to the local environment. Maternal effects and pre-fledging quality models, including those containing interactions with environmental conditions, were not supported. As a consequence, we found no evidence that the maternal effects variables we studied, i.e., mother body condition or timing of breeding, or pre-fledging quality of local recruits influenced LRSa. This suggests that the benefits to offspring of maternal effects or heavier pre-fledging body mass previously detected in this species (Harriman, 2014; Berzins et al., 2021) may (1) not extend beyond initial recruitment to the breeding population (Nevoux et al., 2010; Mumme et al., 2015) and therefore provide only short-term benefits, and/or (2) be less influential, or outweighed by, the stronger effect of the environmental conditions (Verhulst et al., 1995; Pärt et al., 2017) that we observed on LRSa. Overall, results indicate that variation in LRSa of locally recruited adult tree swallows is shaped, at least in part, by the local environment they experienced as developing nestlings.
Maternal traits and environmental factors we measured to characterize natal conditions can be important determinants of nestling quality and recruitment in tree swallows, yet not all variables were related to LRSa. For instance, while maternal decisions on when to breed within a season are strongly related to the fitness prospects of nestlings (Verhulst et al., 1995; Verhulst and Nilsson, 2008), we did not find that an earlier hatching date within a season enhanced LRSa. This finding contrasts with a study in another aerial insectivore, the barn swallow (Hirundo rustica; Saino et al., 2012), where recruits from nests hatched early had higher LRS than recruits hatched later in the season. It is possible that these contrasting results are due to the differing proxies of lifetime reproductive success, i.e., probability of producing a recruit (sexes pooled; this study) versus total eggs (males only; Saino et al., 2012), which may differ in their ability to reliably predict fitness (Alif et al., 2022). Indeed, a previous study in song sparrows (Melospiza melodia) using a proxy similar to ours, i.e., number of offspring surviving to independence, also reported no effect of maternal laying date on LRS of local recruits (Tarwater et al., 2018). However, in some species, it is the first-hatched nestlings within a brood that achieve higher LRS than their later hatched siblings (Martínez-Padilla et al., 2017; Song et al., 2019). We recognize that tree swallow clutches hatch asynchronously (Bitton et al., 2006), but do not have long-term data on hatching sequence to explore this possibility further.
Previous studies in tree swallows have shown that nestlings hatched from early-season nests and/or those that are heavier prior to fledging are more likely to recruit locally (Shutler et al., 2006; Harriman, 2014; Lombardo et al., 2020). Thus, it is possible that we did not detect carry-over effects of these variables on LRSa because our sample of birds are mainly those early hatched and/or heavier nestlings, as reported in Shutler et al. (2006) and Harriman (2014), and are of relatively higher quality compared to non-recruiting offspring. In other species, higher quality individuals attain better breeding territories (van de Pol et al., 2006), and so lower quality individuals may have been outcompeted (e.g., Mumme et al., 2015), at least in SK where competition for nest boxes is high (Shutler et al., 2012). Alternatively, this pattern may also arise because of the selective disappearance of lower quality offspring prior to becoming a breeder (Nevoux et al., 2010; Payo-Payo et al., 2016). While the underlying mechanism is unknown, that local recruits may be relatively high quality is supported by two lines of evidence. First, a decrease in the proportion of local recruits with advancing hatching date suggests that more high-quality individuals were hatched earlier than later in the season (Supplementary Figure S5). Second, the reported negative effects of later hatching date (SK/BC), lower pond abundance (SK), and lower temperature (SK/BC) on pre-fledging body mass of nestlings (Berzins et al., 2021) are no longer detected on body mass of local recruits as adults (Supplementary Tables S8, S9; Supplementary Figures S6, S7). While a positive association between maternal body condition and nestling body mass at the SK site (Harriman et al., 2017) persisted in adulthood (Supplementary Table S8; Supplementary Figure S6), models including these variables were not supported. Such a finding, however, does align with previous work in female tree swallows (Berzins et al., 2020), great tits (Parus major, Verhulst et al., 1995), and northern wheatears (Oenanthe Oenanthe, Pärt et al., 2017) showing that individual traits are less influential for achieving high LRSa than environmental factors.
Effects of the natal environment on LRSa were context-specific, depending largely on the locally varying temperature and moisture conditions. At the SK site, local recruits that developed in warmer temperatures were heavier prior to fledging (Berzins et al., 2021) and achieved higher LRSa as adults, consistent with positive silver-spoon effects. Contrary to our hypothesis, however, we found a negative trend of warmer temperature on LRSa at the BC site. Dawson et al. (2005) demonstrated that nestlings may have benefited from being reared in experimentally heated nests at the BC site as they were heavier prior to fledging. The contrasting results between studies in the same study population may be explained by the magnitude in temperature increase that nestlings were exposed to during development. For instance, Dawson et al. (2005) experimental study elevated the temperature of nests within the range of normal variation, whereas in our study, the highest temperatures experienced by local recruits during development overlapped with record-breaking heat waves (The Canadian Press, 2013). The temperatures local recruits experienced while growing during the heat waves in June of 2004 and 2013, were ~1.5 to 3.5°C greater than the mean Tmax for the month of June and during the period of nestling growth for all nests in those years (Supplementary Figure S8). It is therefore possible that at the BC site, where temperature is generally cooler during the breeding season than the more arid SK site in the prairies (Table 1), high temperature extremes may instead reflect unfavorable environmental conditions. A positive relationship between the pre-fledging body mass of local recruits and temperature during development (Supplementary Table S8; Supplementary Figure S7) may suggest that only local recruits that were relatively high-quality (i.e., heavier as nestlings) were able to buffer the effects of developing in such high temperatures in the short-term but still incurred fitness costs as adults, consistent with the silver-spoon hypothesis. Although suggestive, our results add to the increasing number of studies reporting negative effects of extreme heat-related events on fitness prospects of offspring (Eastwood et al., 2022; Lv et al., 2023).
Moisture conditions, such as pond abundance and PDSI, are driven largely by precipitation and have contrasting effects on the local population dynamics of tree swallows across their range (Weegman et al., 2017). At the more arid SK site, wetter conditions are generally favorable, reflecting abundant ponds (Weegman et al., 2017; Berzins et al., 2020), and by association increased abundance of aerial insects with aquatic larval stages (Berzins et al., 2022). Since ponds in the prairies of North America unequivocally benefit breeding birds (Bartzen et al., 2010; Zhao et al., 2019; Devries et al., 2023), including tree swallows (Michelson et al., 2018; Berzins et al., 2021), it is unclear why local recruits reared when pond abundance was high did not achieve the highest LRSa as adults. One possibility is that the nutritional environment under higher pond abundance may actually be inferior to intermediate pond conditions due to extensive flooding and lower productivity of ponds. For instance, larger ponds can become deeper, permanent, lake-like systems during extensive flooding events (Cressey et al., 2016; Hayashi et al., 2016), which have less diverse invertebrate communities and lower nutrient turnover than smaller seasonal and temporary ponds (McLean et al., 2020; Fritz and Whiles, 2021). A study in tree swallows recently invoked this reasoning, i.e., sites with more pond area, but containing a few large lake-like ponds, may provide less aquatic insect food during growth, to explain why the observed increase in nestling quality and predicted survival leveled off when the surface area of ponds in a region was at intermediate levels (Berzins et al., 2022). While the SK site experienced an extended wet period from 2011 to 2017, with expansion of several larger ponds, ponds of varying sizes, depths, and permanence still existed within this wetland complex. We do not, however, have additional data on pond area and aquatic insect availability to further explore these relationships over the SK time series. Nevertheless, how the type and surface area of ponds in the landscape influence the nutritional environment experienced by aerial insectivores on the breeding grounds warrants further investigation.
Our study focused on testing for silver-spoon effects of natal conditions on LRSa, which predict fitness benefits to individuals developing in a good environment, independent of the adult environment (Monaghan, 2008). However, we recognize that LRSa may also be influenced by current environmental conditions experienced by local recruits during breeding. Previous studies at the SK site have shown that LRSa and apparent survival of tree swallows were higher when breeders experienced relatively high pond abundance (Clark et al., 2018; Berzins et al., 2020). Therefore, our results may also be explained by local recruits reared when pond abundance was highest being more likely to encounter poorer pond conditions as breeders due to cycling in pond abundance during wet-dry periods (Supplementary Figure S9). This reasoning would also explain the fewer breeding attempts made by local recruits reared in high versus intermediate pond abundance (Supplementary Table S10; Supplementary Figure S10). In other species, such as tawny owls (Strix aluco), the phase of the vole cycle (i.e., increasing or decreasing) that local recruits experience as first-time breeders was more influential for LRS than natal conditions (Millon et al., 2010). For tree swallows at the SK site, females generally begin breeding at 2 years old or older (Berzins et al., 2020), in contrast to males (Supplementary Figure S1). Age of first breeding does not appear to vary with natal pond abundance for females, but it does for males (Supplementary Figure S10). This points to the possibility that males hatched in years of differing natal pond abundance may encounter different competitive environments at recruitment that affects their opportunity for breeding (i.e., mismatched natal and adult environments; Monaghan, 2008). When natal pond numbers are relatively low and few males recruit to the breeding population, competition for nest boxes may be reduced which allows males to breed at younger ages and have more lifetime breeding attempts (Supplementary Figure S10). In contrast, when natal ponds are abundant and more males recruit, competition for breeding sites, particularly in SK where occupancy of nest boxes is high, becomes more intense, resulting in a concomitant delay in age at first breeding and a decrease in the number of lifetime breeding attempts (Supplementary Figure S10). While outside the scope of our present study, that LRSa of local recruits could potentially be influenced by complex interactions with pond conditions across life stages, depending on whether ponds are increasing or decreasing during wet-dry periods, is an idea that warrants further investigation.
In contrast to the more arid SK site, wetter conditions in BC are generally unfavorable (Berzins et al., 2020), as is also evident in other parts of the range of tree swallows (Weegman et al., 2017). For instance, in Ontario and Quebec, Canada, prolonged precipitation reduces the availability of insects, and so negatively affects the quality and fledging success of nestlings (Cox et al., 2019; Garrett et al., 2022). Our results at the BC site indicate that the negative effects for nestlings reared in wetter natal conditions may also carry-over to affect their fitness as adults, in accordance with the silver-spoon hypothesis. Breeding at earlier ages may enhance fitness by increasing the number of lifetime breeding attempts (Blums and Clark, 2004), especially in short-lived species (Gustafsson and Pärt, 1990). We suspect that local recruits experiencing wetter PDSI conditions had lower LRSa because they delayed breeding to 2 years old or older, and tended to breed fewer times than recruits hatched when PDSI conditions were intermediate (Supplementary Table S10; Supplementary Figure S11).
Our results have important conservation implications as climate change is predicted to increase local weather variability, and the frequency and severity of temperature extremes and drought. In the interior of BC, wetter and warmer natal conditions that resulted in reduced LRSa suggest that potential increases in precipitation and heat waves with climate change (IPCC, 2021) could have adverse demographic consequences in the future. Indeed, the number of extreme heat-related events is already increasing (Parisien et al., 2023), with the highest ever recorded temperatures in 2021 exceeding >35°C during nestling development. While warmer temperatures at the SK site had positive carry-over effects on LRSa, suggesting tree swallows may benefit from increasingly warm temperatures associated with climate change (Mantyka-Pringle et al., 2019), results from the BC population caution that higher than average temperatures as predicted for the prairies (IPCC, 2021) could led to reductions in fitness of tree swallows in the future. For instance, higher temperatures may not only increase the evaporation of ponded water, but also the susceptibility to drought (Douville et al., 2021). Recent evidence at the SK site highlights the importance of abundant ponds for promoting the population stability of tree swallows (Clark et al., 2018; Berzins et al., 2020, 2021). However, ongoing losses of ponds to agricultural drainage and infilling (Bartzen et al., 2010), combined with the predicted drying of ponds in parts of the prairies (Johnson et al., 2010; Londe et al., 2023) may lead to population declines via lower recruitment and LRSa. Conserving and restoring prairie ponds may therefore be critical for sustaining tree swallow populations in a changing climate.
Aerial insectivores have experienced steep population declines in North America (Rosenberg et al., 2019), but within species there is high spatiotemporal variability in population trends across their ranges (Michel et al., 2016). By evaluating two distinct populations of tree swallows, we show locally varying natal environments had carry-over effects on adult LRSa. Consequently, natal environmental conditions that generate differences in fitness may explain trends in population-level demographics. Unveiling population responses to environmental variability on the breeding grounds is important as offspring will be increasingly exposed to modified natal environments as climate and land use changes intensify across the globe.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by University Animal Care Committee, University of Saskatchewan; Animal Care and Use Committee, University of Northern British Columbia. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LB: Conceptualization, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. RD: Conceptualization, Funding acquisition, Investigation, Writing – review & editing, Resources. RC: Conceptualization, Funding acquisition, Investigation, Writing – review & editing, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) through grants to RC and RD. Additional funding was provided by Environment and Climate Change Canada (RC), and the University of Northern British Columbia, Canada Foundation for Innovation, and British Columbia Knowledge Development Fund (RD).
Acknowledgments
We thank all the volunteer and student field assistants, and Christy Morrissey, for contributing to the collection of data used in this paper, and Steve Leach for logistical support. We are grateful for the landowners who allowed us to work on their property. We appreciate the thorough and constructive comments on previous versions of the manuscript provided by reviewers.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbirs.2024.1348114/full#supplementary-material
References
Alif Ž., Dunning J., Chik H. Y. J., Burke T., Schroeder J. (2022). What is the best fitness measure in wild populations? A case study on the power of short-term fitness proxies to predict reproductive value. PloS One 17, e0260905. doi: 10.1371/journal.pone.0260905
Ancona S., Drummond H. (2013). Life history plasticity of a tropical seabird in response to El Niño anomalies during early life. PloS One 8, e72665. doi: 10.1371/journal.pone.0072665
Ancona S., Zúñiga-Vega J. J., Rodríguez C., Drummond H. (2018). Experiencing El Niño conditions during early life reduces recruiting probabilities but not adult survival. R. Soc Open Sci. 5, 170076. doi: 10.1098/rsos.170076
Arnold T. W. (2010). Uninformative parameters and model selection using Akaike’s information criterion. J. Wildl. Manage. 74, 1175–1178. doi: 10.1111/j.1937–2817.2010.tb01236.x
Bartzen B. A., Dufour K. W., Clark R. G., Caswell F. D. (2010). Trends in agricultural impact and recovery of wetlands in prairie Canada. Ecol. Appl. 20, 525–538. doi: 10.1890/08–1650.1
Berzins L. L., Dawson R. D. (2016). Experimentally altered plumage brightness of female tree swallows: a test of the differential allocation hypothesis. Behaviour 153, 525–550. doi: 10.1163/1568539X-00003354
Berzins L. L., Dawson R. D. (2020). Does experimentally altered plumage brightness influence extra-pair mating success in female Tree Swallows (Tachycineta bicolor)? Can. J. Zool. 98, 55–61. doi: 10.1139/CJZ-2019–0142
Berzins L. L., Dawson R. D., Morrissey C. A., Clark R. G. (2020). The relative contribution of individual quality and changing climate as drivers of lifetime reproductive success in a short-lived avian species. Sci. Rep. 10, 19766. doi: 10.1038/s41598–020-75557-w
Berzins L. L., Mazer A. K., Morrissey C. A., Clark R. G. (2021). Pre-fledging quality and recruitment in an aerial insectivore reflect dynamics of insects, wetlands and climate. Oecologia 196, 89–100. doi: 10.1007/S00442–021-04918–7
Berzins L. L., Morrissey C. A., Howerter D. W., Clark R. G. (2022). Conserving wetlands in agroecosystems can sustain aerial insectivore productivity and survival. Can. J. Zool. 100, 617–629. doi: 10.1139/cjz-2021–0204
Bitton P.-P., Dawson R. D. (2017). Age-related prenatal maternal effects and postnatal breeding experience have different influences on nestling development in an altricial passerine. J. Avian Biol. 48, 660–668. doi: 10.1111/jav.01202
Bitton P.-P., Dawson R. D., O’Brien E. L. (2006). Influence of intraclutch egg-mass variation and hatching asynchrony on relative offspring performance within broods of an altricial bird. Can. J. Zool. 84, 1721–1726. doi: 10.1139/z06–179
Blums P., Clark R. G. (2004). Correlates of lifetime reproductive success in three species of European ducks. Oecologia 140, 61–67. doi: 10.1007/s00442–004-1573–8
Blums P., Clark R. G., Mednis A. (2002). Patterns of reproductive effort and success in birds: path analyses of long-term data from European ducks. J. Anim. Ecol. 71, 280–295. doi: 10.1046/j.1365–2656.2002.00598.x
Bonduriansky R., Crean A. J. (2018). What are parental condition-transfer effects and how can they be detected? Methods Ecol. Evol. 9, 450–456. doi: 10.1111/2041–210X.12848
Bouwhuis S., Charmantier A., Verhulst S., Sheldon B. C. (2010). Trans-generational effects on ageing in a wild bird population. J. Evol. Biol. 23, 636–642. doi: 10.1111/j.1420–9101.2009.01929.x
Brown T. J., Dugdale H. L., Hammers M., Komdeur J., Richardson D. S. (2022). Seychelles warblers with silver spoons: juvenile body mass is a lifelong predictor of annual survival, but not annual reproduction or senescence. Ecol. Evol. 12, e9049. doi: 10.1002/ece3.9049
Burnham K. P., Anderson D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (New York: Springer).
Cam E., Aubry L. (2011). Early development, recruitment and life history trajectory in long-lived birds. J. Ornithol. 152, 187–201. doi: 10.1007/s10336–011-0707–0
Clark R. G., Pöysä H., Runko P., Paasivaara A. (2014). Spring phenology and timing of breeding in short-distance migrant birds: phenotypic responses and offspring recruitment patterns in common goldeneyes. J. Avian Biol. 45, 457–465. doi: 10.1111/jav.00290
Clark R. G., Winkler D. W., Dawson R. D., Shutler D., Hussell D. J. T., Lombardo M. P., et al. (2018). Geographic variation and environmental correlates of apparent survival rates in adult tree swallows Tachycineta bicolor. J. Avian Biol. 49, 012514. doi: 10.1111/jav.01659
Cox A. R., Robertson R. J., Lendvai Á.Z., Everitt K., Bonier F. (2019). Rainy springs linked to poor nestling growth in a declining avian aerial insectivore (Tachycineta bicolor). Proc. R. Soc B.: Biol. Sci. 286, 20190018. doi: 10.1098/rspb.2019.0018
Cressey R. L., Austin J. E., Stafford J. D. (2016). Three responses of wetland conditions to climatic extremes in the prairie pothole region. Wetlands 36, 357–370. doi: 10.1007/s13157–016-0818–8
Dawson R. D. (2008). Timing of breeding and environmental factors as determinants of reproductive performance of tree swallows. Can J. Zool. 86, 843–850. doi: 10.1139/Z08-065
Dawson R. D., Lawrie C. C., O’Brien E. L. (2005). The importance of microclimate variation in determining size, growth and survival of avian offspring: experimental evidence from a cavity nesting passerine. Oecologia 144, 499–507. doi: 10.1007/s00442–005-0075–7
Devries J. H., Armstrong L. M., Howerter D. W., Emery R. B. (2023). Waterfowl distribution and productivity in the Prairie Pothole Region of Canada: tools for conservation planning. Wildl. Monogr. 211, e1074. doi: 10.1002/wmon.1074
Douville H., Raghavan K., Renwick J., Allan R. P., Arias P. A., Barlow M., et al. (2021). “Water Cycle Changes,” in Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Eds. Masson-Delmotte V., Zhai P., Pirani A., Connors S. L., Péan C., Berger S., Caud N., Chen Y., Goldfarb L., Gomis M. I., Huang M., Leitzell K., Lonnoy E., Matthews J. B. R., Maycock T. K., Waterfield T., Yelekçi O., Yu R., Zhou B. (Cambridge University Press, New York, NY), 1055–1210. doi: 10.1017/9781009157896.010
Eastwood J. R., Connallon T., Delhey K., Hall M. L., Teunissen N., Kingma S. A., et al. (2022). Hot and dry conditions predict shorter nestling telomeres in an endangered songbird: implications for population persistence. Proc. Natl. Acad. Sci. U.S.A. 119, e2122944119. doi: 10.1073/pnas.2122944119
Engqvist L., Reinhold K. (2016). Adaptive trans-generational phenotypic plasticity and the lack of an experimental control in reciprocal match/mismatch experiments. Methods Ecol. Evol. 7, 1482–1488. doi: 10.1111/2041–210X.12618
Fairhurst G. D., Treen G. D., Clark R. G., Bortolotti G. R. (2012). Nestling corticosterone response to microclimate in an altricial bird. Can. J. Zool. 90, 1422–1430. doi: 10.1139/CJZ-2012–0096
Fox J., Weisberg S. (2019). An R Companion to Applied Regression (Thousand Oaks CA: Sage). Available at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Fritz K. A., Whiles M. R. (2021). Reciprocal subsidies between temporary ponds and riparian forests. Limnol. Oceanogr. 66, 3149–3161. doi: 10.1002/lno.11868
Garrett D. R., Pelletier F., Garant D., Bélisle M. (2022). Interacting effects of cold snaps, rain, and agriculture on the fledging success of a declining aerial insectivore. Ecol. Appl. 32, e2645. doi: 10.1002/eap.2645
Gómez J., Michelson C. I., Bradley D. W., Norris D. R., Berzins L. L., Dawson R. D., et al. (2014). Effects of geolocators on reproductive performance and annual return rates of a migratory songbird. J. Ornithol. 155, 37–44. doi: 10.1007/s10336–013-0984-x
Grafen A. (1988). “On the uses of data on lifetime reproductive success,” in Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems. Ed. Clutton-Brock T. H. (The University of Chicago Press, Chicago), 454–471.
Griebel I., Dawson R. D. (2019). Predictors of nestling survival during harsh weather events in an aerial insectivore, the Tree Swallow (Tachycineta bicolor). Can. J. Zool. 97, 81–90. doi: 10.1139/cjz-2018–0070
Griebel I. A., Dawson R. D., Clark R. G. (2020). Cavity type influences abundance of nest-dwelling avian blow flies: an experiment with tree swallows. Ecol. Entomol. 45, 434–443. doi: 10.1111/EEN.12811
Griebel I. A., Fairhurst G. D., Marchant T. A., Clark R. G. (2019). Effects of parental and nest-site characteristics on nestling quality in the Tree Swallow (Tachycineta bicolor). Can. J. Zool. 97, 63–71. doi: 10.1139/CJZ-2018–0109
Gustafsson L., Pärt T. (1990). Acceleration of senescence in the collared flycatcher Ficedula albicollis by reproductive costs. Nature 347, 279–281. doi: 10.1038/347279a0
Halliwell C., Ketcher M., Proud A., Westerberg S., Douglas D. J. T., Burgess M. D. (2023). Early life conditions influence fledging success and subsequent local recruitment rates in a declining migratory songbird, the Whinchat Saxicola rubetra. Ecol. Evol. 13, e10346. doi: 10.1002/ece3.10346
Halupka L., Arlt D., Tolvanen J., Millon A., Bize P., Adamík P., et al. (2023). The effect of climate change on avian offspring production: a global meta-analysis. Proc. Natl. Acad. Sci. U.S.A. 120, e2208389120. doi: 10.1073/pnas.2208389120
Hamel S., Gaillard J.-M., Festa-Bianchet M., Côté S. D. (2009). Individual quality, early-life conditions, and reproductive success in contrasted populations of large herbivores. Ecology 90, 1981–1995. doi: 10.1890/08–0596.1
Harriman V. B. (2014). Seasonal variation in quality and survival of nestlings tree swallows (Tachycineta bicolor): tests of alternative hypotheses (Saskatoon, SK: University of Sasaktchewan).
Harriman V. B., Dawson R. D., Bortolotti L. E., Clark R. G. (2017). Seasonal patterns in reproductive success of temperate-breeding birds: experimental tests of the date and quality hypotheses. Ecol. Evol. 7, 2122–2132. doi: 10.1002/ece3.2815
Hartig F. (2022). DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.6. Available at: https://cran.r-project.org/web/packages/DHARMa
Hayashi M., van der Kemp G., Rosenberry D. O. (2016). Hydrology of prairie wetlands: Understanding the integrated surface-water and groundwater processes. Wetlands 36, 237–254. doi: 10.1007/s13157–016-0797–9
Herfindal I., van de Pol M., Nielsen J. T., Saether B.-E., Møller A. P. (2015). Climatic conditions cause complex patterns of covariation between demographic traits in a long-lived raptor. J. Anim. Ecol. 84, 702–711. doi: 10.1111/1365–2656.12318
Hussell D. J. T. (2003). Climate change, spring temperatures, and timing of breeding of tree swallows (Tachycineta Bicolor) in Southern Ontario. Auk 120, 607–618. doi: 10.1093/auk/120.3.607
IPCC (2021). “Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change,” in Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Eds. Masson-Delmotte V., Zhai P., Pirani A., Connors S. L., Péan C., Berger S., Caud N., Chen Y., Goldfarb L., Gomis M. I., Huang M., Leitzell K., Lonnoy E., Matthews J. B. R., Maycock T. K., Waterfield T., Yelekçi O., Yu R., Zhou B. (Cambridge, United Kingdom: Cambridge University Press). doi: 10.1017/9781009157896
Johnson W. C., Boettcher S. E., Poiani K. A., Guntenspergen G. (2004). Influence of weather extremes on the water levels of glaciated prairie wetlands. Wetlands 24, 385–398. doi: 10.1672/0277–5212(2004)024[0385:ioweot]2.0.co;2
Johnson W. C., Werner B., Guntenspergen G. R., Voldseth R. A., Millett B., Naugle D. E., et al. (2010). Prairie wetland complexes as landscape functional units in a changing climate. BioScience 60, 128–140. doi: 10.1525/bio.2010.60.2.7
Knight S. M., Bradley D. W., Clark R. G., Gow E. A., Bélisle M., Berzins L. L., et al. (2018). Constructing and evaluating a continent-wide migratory songbird network across the annual cycle. Ecol. Monogr. 88, 445–460. doi: 10.1002/ecm.1298
Le Boeuf B., Condit R., Reiter J. (2019). Lifetime reproductive success of northern elephant seals (Mirounga angustirostris). Can. J. Zool. 97, 1203–1217. doi: 10.1139/cjz-2019–0104
Lindström J. (1999). Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. doi: 10.1016/S0169–5347(99)01639–0
Lombardo M. P., Thorpe P. A. (2010). Local breeding experience and the reproductive performance of Tree Swallows. J. Field Ornithol. 81, 294–301. doi: 10.1111/j.1557–9263.2010.00285.x
Lombardo M. P., Thorpe P. A., Otieno S., Hawker A., Welgarz D., Andrews D., et al. (2020). Yearly variation in factors associated with local recruitment of Tree Swallows. J. Ornithol. 91, 199–213. doi: 10.1111/jofo.12335
Londe D. W., Davis C. A., Loss S. R., Robertson E. P., Haukos D. A., Hovick T. J. (2023). Climate change causes declines and greater extremes in wetland inundation in a region important for wetland birds. Ecol. Appl. 34, e2930. doi: 10.1002/eap.2930
Lüdecke D. (2018). ggeffects: tidy data frames of marginal effects from regression models. J. Open Source Software 3, 772. doi: 10.21105/joss.00772
Lv L., van de Pol M., Osmond H. L., Liu Y., Cockburn A., Kruuk L. E. B. (2023). Winter mortality of a passerine bird increases following hotter summers and during winters with higher maximum temperatures. Sci. Adv. 9, eabm0197. doi: 10.1126/sciadv.abm0197
Mantyka-Pringle C., Leston L., Messmer D., Asong E., Bayne E. M., Bortolotti L. E., et al. (2019). Antagonistic, synergistic and direct effects of land use and climate on Prairie wetland ecosystems: ghosts of the past or present? Divers. Distrib. 25, 1924–1940. doi: 10.1111/ddi.12990
Martínez-Padilla J., Vergara P., Fargallo J. A. (2017). Increased lifetime reproductive success of first-hatched siblings in Common Kestrels Falco tinnunculus. Ibis 159, 803–811. doi: 10.1111/ibi.12494
Mazerolle M. J. (2023). AICcmodavg: model selection and multimodel inference based on (Q) AIC(c). R package version 2. 2–3. Available at: https://cran.r-project.org/package=AICcmodavg
McLean K. I., Mushet D. M., Sweetman J. N., Anteau M. J., Wiltermuth M. T. (2020). Invertebrate communities of Prairie-Pothole wetlands in the age of the aquatic Homogenocene. Hydrobiologia 847, 3773–3793. doi: 10.1007/s10750–019-04154–4
Michel N. L., Smith A. C., Clark R. G., Morrissey C. A., Hobson K. A. (2016). Differences in spatial synchrony and interspecific concordance inform guild-level population trends for aerial insectivorous birds. Ecography 39, 774–786. doi: 10.1111/ecog.01798
Michelson C. I., Clark R. G., Morrissey C. A. (2018). Agricultural land cover does not affect the diet of Tree Swallows in wetland-dominated habitats. Condor 120, 751–764. doi: 10.1650/CONDOR-18–16.1
Millon A., Petty S. J., Lambin X. (2010). Pulsed resources affect the timing of first breeding and lifetime reproductive success of tawny owls. J. Anim. Ecol. 79, 426–435. doi: 10.1111/j.1365–2656.2009.01637.x
Monaghan P. (2008). Early growth conditions, phenotypic development and environmental change. Philos. Trans. R. Soc B.: Biol. Sci. 363, 1635–1645. doi: 10.1098/rstb.2007.0011
Mumme R. L., Bowman R., Pruett M. S., Fitzpatrick J. W. (2015). Natal territory size, group size, and body mass affect lifetime fitness in the cooperatively breeding Florida Scrub-Jay. Auk 132, 634–646. doi: 10.1642/AUK-14–258.1
Nevoux M., Weimerskirch H., Barbraud C. (2010). Long- and short-term influence of environment on recruitment in a species with highly delayed maturity. Oecologia 162, 383–392. doi: 10.1007/s00442–009-1482-y
Newbold T. (2018). Future effects of climate and land-use change on terrestrial vertebrate community diversity under different scenarios. Proc. R. Soc B.: Biol. Sci. 285, 20180792. doi: 10.1098/rspb.2018.0792
Northrup J. M., Rivers J. W., Yang Z., Betts M. G. (2019). Synergistic effects of climate and land-use change influence broad-scale avian population declines. Glob. Change Biol. 25, 1561–1575. doi: 10.1111/gcb.14571
O’Brien E. L., Dawson R. D. (2013). Experimental dissociation of individual quality, food and timing of breeding effects on double-brooding in a migratory songbird. Oecologia 172, 689–699. doi: 10.1007/s00442–012-2544–0
Parisien M.-A., Barber Q. E., Bourbonnais M. L., Daniels L. D., Flannigan M. D., Gray R. W., et al. (2023). Abrupt, climate-induced increase in wildfires in British Columbia since the mid-2000s. Commun. Earth Environ. 4, 1–11. doi: 10.1038/s43247–023-00977–1
Pärt T., Knape J., Low M., Öberg M., Arlt D. (2017). Disentangling the effects of date, individual, and territory quality on the seasonal decline in fitness. Ecology 98, 2102–2110. doi: 10.1002/ecy.1891
Payo-Payo A., Genovart M., Bertolero A., Pradel R., Oro D. (2016). Consecutive cohort effects driven by density-dependence and climate influence early-life survival in a long-lived bird. Proc. R. Soc B.: Biol. Sci. 283, 20153042. doi: 10.1098/rspb.2015.3042
Payo-Payo A., Sanz-Aguilar A., Oro D. (2023). Long-lasting effects of harsh early-life conditions on adult survival of a long-lived vertebrate. Oikos 2023, e09371. doi: 10.1111/oik.09371
Plard F., Gaillard J.-M., Coulson T., Hewison A. J. M., Douhard M., Klein F., et al. (2015). The influence of birth date via body mass on individual fitness in a long-lived mammal. Ecology 96, 1516–1528. doi: 10.1890/14–0106.1
Poli C., Robertson E. P., Martin J., Powell A. N., Fletcher R. J. (2022). An invasive prey provides long-lasting silver spoon effects for an endangered predator. Proc. R. Soc B.: Biol. Sci. 289, 20220820. doi: 10.1098/rspb.2022.0820
Powers R. P., Jetz W. (2019). Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Change 9, 323–329. doi: 10.1038/s41558–019-0406-z
Pyle P. (1997). Identification guide to North American birds. Part I (California: Slate Creek Press).
QGIS.org (2024). QGIS Georgraphic Information System. Open Source Geospatial Foundation Project. Available at: http://qgis.org.
R Core Team (2022). R: a language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Reid J. M., Bignal E. M., Bignal S., McCracken D. I., Monaghan P. (2003). Environmental variability, life-history covariation and cohort effects in the red-billed chough Pyrrhocorax pyrrhocorax. J. Anim. Ecol. 72, 36–46. doi: 10.1046/j.1365–2656.2003.00673.x
Rödel H. G., Von Holst D., Kraus C. (2009). Family legacies: short- and long-term fitness consequences of early-life conditions in female European rabbits. J. Anim. Ecol. 78, 789–797. doi: 10.1111/J.1365–2656.2009.01537.X
Rosenberg K. V., Dokter A. M., Blancher P. J., Sauer J. R., Smith A. C., Smith P. A., et al. (2019). Decline of the North American avifauna. Science 366, 120–124. doi: 10.1126/science.aaw1313
Rotella J. J., Clark R. G., Afton A. D. (2003). Survival of female lesser scaup: effects of body size, age, and reproductive effort. Condor 105, 336–347. doi: 10.1093/condor/105.2.336
Saino N., Romano M., Ambrosini R., Rubolini D., Boncoraglio G., Caprioli M., et al. (2012). Longevity and lifetime reproductive success of barn swallow offspring are predicted by their hatching date and phenotypic quality. J. Anim. Ecol. 81, 1004–1012. doi: 10.1111/j.1365–2656.2012.01989.x
Schielzeth H. (2010). Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113. doi: 10.1111/j.2041–210X.2010.00012.x
Shipley J. R., Twining C. W., Taff C. C., Vitousek M. N., Flack A., Winkler D. W. (2020). Birds advancing lay dates with warming springs face greater risk of chick mortality. Proc. Natl. Acad. Sci. U.S.A. 117, 25590–25594. doi: 10.1073/pnas.2009864117
Shutler D., Clark R. G. (2003). Causes and consequences of tree swallow (Tachycineta bicolor) dispersal in Saskatchewan. Auk 120, 619–631. doi: 10.1642/0004–8038(2003)120[0619:cacots]2.0.co;2
Shutler D., Clark R. G., Fehr C., Diamond A. W. (2006). Time and recruitment costs as currencies in manipulation studies on the costs of reproduction. Ecology 87, 2938–2946. doi: 10.1890/0012–9658(2006)87[2938:TARCAC]2.0.CO;2
Shutler D., Hussell D. J. T., Norris D. R., Winkler D. W., Robertson R. J., Bonier F., et al. (2012). Spatiotemporal patterns in nest box occupancy by Tree Swallows across North America. Avian Conserv. Ecol. 7, 3. doi: 10.5751/ACE-00517–070103
Song Z., Zou Y., Hu C., Ye Y., Wang C., Qing B., et al. (2019). Silver spoon effects of hatching order in an asynchronous hatching bird. Behav. Ecol. 30, 509–517. doi: 10.1093/beheco/ary191
Spagopoulou F., Teplitsky C., Chantepie S., Lind M. I., Gustafsson L., Maklakov A. A. (2020). Silver-spoon upbringing improves early-life fitness but promotes reproductive ageing in a wild bird. Ecol. Lett. 23, 994–1002. doi: 10.1111/ele.13501
Spooner F. E. B., Pearson R. G., Freeman R. (2018). Rapid warming is associated with population decline among terrestrial birds and mammals globally. Glob. Change Biol. 24, 4521–4531. doi: 10.1111/gcb.14361
Svensson E. (1997). Natural selection on avian breeding time: causality, fecundity-dependent, and fecundity-independent selection. Evolution 51, 1276–1283. doi: 10.1111/j.1558–5646.1997.tb03974.x
Tarwater C. E., Germain R. R., Arcese P. (2018). Examination of context-dependent effects of natal traits on lifetime reproductive success using a long-term study of a temperate songbird. Auk 135, 609–621. doi: 10.1642/AUK-17–177.1
The Canadian Press (2013) Heat wave shatters temperature records across British Columbia, U.S. with Death Valley reaching 53 degrees (National Post). Available online at: https://nationalpost.com/news/Canada/heat-wave-shatters-temperature-records-across-british-columbia-u-s-with-death-valley-reaching-53-degrees (Accessed September 1, 2023).
van de Pol M., Bruinzeel L. W., Heg D., van der Jeugd H. P., Verhulst S. (2006). A silver spoon for a golden future: long-term effects of natal origin on fitness prospects of oystercatchers (Haematopus ostralegus). J. Anim. Ecol. 75, 616–626. doi: 10.1111/j.1365–2656.2006.01079.x
Verhulst S., Nilsson J.-Å. (2008). The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Philos. Trans. R. Soc B.: Biol. Sci. 363, 399–410. doi: 10.1098/rstb.2007.2146
Verhulst S., van Balen J. H., Tinbergen J. M. (1995). Seasonal decline in reproductive success of the great tit: variation in time or quality? Ecology 76, 2392–2403. doi: 10.2307/2265815
Visconti P., Bakkenes M., Baisero D., Brooks T., Butchart S. H. M., Joppa L., et al. (2016). Projecting global biodiversity indicators under future development scenarios. Conserv. Lett. 9, 5–13. doi: 10.1111/conl.12159
Weegman M. D., Arnold T. W., Dawson R. D., Winkler D. W., Clark R. G. (2017). Integrated population models reveal local weather conditions are the key drivers of population dynamics in an aerial insectivore. Oecologia 185, 119–130. doi: 10.1007/s00442–017-3890–8
Winkler D. W., Hallinger K. K., Ardia D. R., Robertson B. J., Stuchbury B. J., Cohen R. R. (2020). “Tree swallow (Tachycineta bicolor), version 1.0,” in Birds of North America. Ed. Poole A. (Cornell Lab of Ornithology, Ithaca, NY). Retrieved from Birds of the World. doi: 10.2173/bow.treswa.01
Winkler D. W., Luo M. K., Rakhimberdiev E. (2013). Temperature effects on food supply and chick mortality in tree swallows (Tachycineta bicolor). Oecologia 173, 129–138. doi: 10.1007/s00442–013-2605-z
Keywords: body mass, cohort, early life effect, individual quality, lifetime reproductive success, maternal effect, natal environment, pond abundance
Citation: Berzins LL, Dawson RD and Clark RG (2024) Context-specific “silver-spoon” effects of wetlands, climate, and temperature on lifetime fitness in a short-lived temperate-breeding migratory songbird. Front. Bird Sci. 3:1348114. doi: 10.3389/fbirs.2024.1348114
Received: 01 December 2023; Accepted: 27 May 2024;
Published: 12 June 2024.
Edited by:
David Koons, Colorado State University, United StatesReviewed by:
Sophie Dupont, UMR5175 Centre d’Ecologie Fonctionnelle et Evolutive (CEFE), FranceStefano Filacorda, University of Udine, Italy
Copyright © 2024 Berzins, Dawson and Clark. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisha L. Berzins, bGlzaGEuYmVyemluc0B1c2Fzay5jYQ==
 Lisha L. Berzins
Lisha L. Berzins Russell D. Dawson2
Russell D. Dawson2