
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Biomater. Sci., 08 November 2023
Sec. Biomaterials Manufacturing and Technology
Volume 2 - 2023 | https://doi.org/10.3389/fbiom.2023.1184662
The integrated approach in the development of nanotechnology is allowing its introduction into multiple fields, including pharmaceutical research, in which there are now several medicines containing nanomaterials or at least making nano-based claims. As a result of increasing research in nanotechnology, pre-existing medicines have been reformulated, and new medicines have been developed. This has brought challenges to the current regulatory frameworks in Europe and the United States. These regulatory agencies are known to be stringent because they have both the human capacity and skills and conducive policies and the landscape to manage new technology, unlike the agencies in most African countries. Because the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have embraced regulatory science (RS) as a means of proactive analysis of regulatory principles, those agencies will be able to address nanomedicine challenges in a straightforward manner. African countries currently do not have a harmonized regulatory framework because different national regulatory authorities are at different levels of development. The pharmaceutical sector in Africa is facing many challenges, including the non-existence of research and development partnerships between industry, universities, and research institutions that foster nanomedicine development. Now that the African Medicine Agency (AMA) is in place, Africa should see the rapid implementation of the AU Model Law on Medical Products Regulation that will assist in putting in place capacity-building programs in nanomedicine RS.
The advancement of nanotechnology research has significantly enhanced the development of new medicines and the modification of existing medicines. Lowering toxicity, increasing dissolution, and enhancing bio-availability are only a few of the many benefits nanotechnology can bring to the pharmaceutical industry. The development of new drug substances and products will continue to benefit significantly from the introduction of nanotechnology. Through a worldwide integrated approach, the technology is being introduced into multiple fields, including pharmaceutical research, in which there are now many uses and products comprising nanomaterials. According to (Soares et al., 2018), the potential for nanotechnology to offer novel and effective medical solutions to unattended healthcare challenges around the world is now acknowledged, making it a key enabling technology. Africa has abundant unmet medical needs. This paper attempts to unpack regulatory science challenges faced in Africa as nanotechnology, in general, and nanomedicine, in particular, continue to advance in development.
In 2008, the International Organization for Standardization (ISO) defined a nanoparticle as a “discrete object where all three Cartesian dimensions—the three dimensions—are less than 100 nm.” The ISO standard comparably defined two-dimensional nano-objects and one-dimensional nano-objects. The size overlaps considerably with that previously assigned to the field of colloid science—from 1 to 1,000 nm (also known as the mesoscale). Consequently, the terms nanoparticles and colloidal particles tend to be used interchangeably. The difference is essentially semantic for particles below 100 nm in size. In 2011, the Commission of the European Union endorsed a more technical but wider-ranging definition:
A natural, incidental, or manufactured material containing particles in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1–100 nm.
This statement defines a nanomaterial with regard to legislation and policy in the European Union. Derived from this definition, the regulatory authorities in Europe formulated their local guidance to enhance drug product development in their own countries. However, the marketing authorization of any nanomedical product will be granted by the European Medicines Agency (EMA). The Food and Drug Administration (FDA) in the United States currently lacks a defined legal framework for nanotechnology. Nonetheless, scientists commonly use the term to denote the manipulation of materials at an extremely small scale, often equivalent to those employed within the EU. The FDA engages in numerous initiatives aimed at advancing its regulatory science expertise, fostering partnerships, and adopting an adaptable, science-driven approach to regulating products that employ nanotechnology or incorporate nanomaterials across various domains (FDA, 2018; FDA, 2022). Despite these efforts, Soares et al. (2018) suggest that effective regulation of nanomedicine remains a considerable challenge in both Europe and the United States.
The working group within the European agency suggested that nanomedicines are intentionally crafted systems intended for clinical use, incorporating at least one nanoscale component to attain reproducible characteristics and features unique to the specific nanotechnology application and intended use (including dosage and route of administration), thereby yielding clinical benefits of nano-engineering, such as targeted distribution to specific organs or tissues (Ossa, 2014). According to this definition, a particle is considered a nanoparticle if any one of its dimensions falls within the range of 1–100 nm, regardless of whether its other dimensions exceed this range. The 1 nm lower limit is employed because atomic bond lengths are typically reached at this scale. Given the regulatory environment in Africa, it would be fair to say most national regulatory authorities will go either the EMA or FDA route, depending on the level of expertise in nanotechnology.
Nanoparticles are ultrafine units with dimensions typically measured in nanometers (nm; where 1 nm = 10−9 m). Due to their ultra-small size, they possess distinctive material properties, and synthesized nanoparticles may be employed in various practical domains, such as medicine, engineering, and environmental cleanup.
Nanoparticles can be categorized into various types based on material properties, size, and shape. Several classifications differentiate between organic and inorganic nanoparticles. The former group encompasses polymeric nanoparticles, dendrimers, and liposomes, while the latter includes quantum dots, fullerenes, and gold nanoparticles. Figures 1, 2 show the different types of nanoparticles.
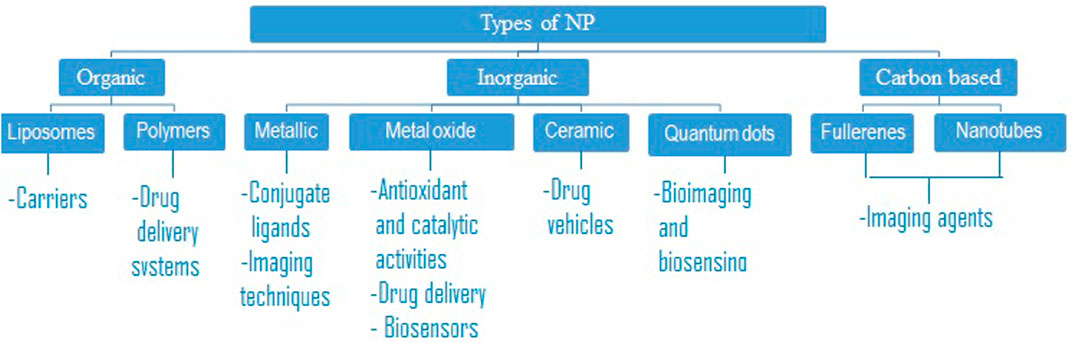
FIGURE 1. Classification of the different types of nanoparticles that have been developed for nanomedicine (Deng et al., 2020).
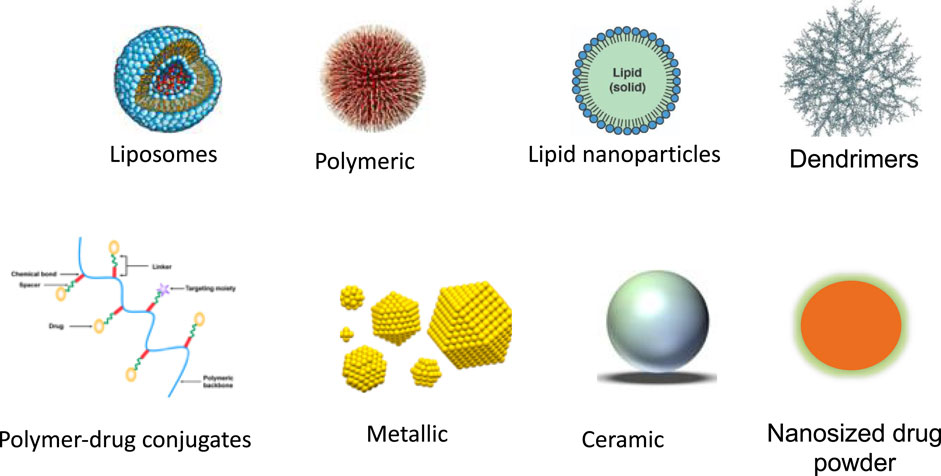
FIGURE 2. Different types of nanoparticles that have been developed for nanomedicine (Yang et al., 2021).
Other categories group nanoparticles according to their nature, such as being carbon-based, ceramic, semiconducting, or polymeric. Furthermore, nanoparticles can be categorized and described as either hard, such as metallic particles, or soft, such as liposomes, vesicles, and nanodroplets.
Nanoparticles possess three critical properties, which are: 1) high mobility in the free state, 2) large specific surface areas, and 3) exhibition of quantum effects. The intended use of the nanoparticle-composed product determines its composition.
Langer (2019) suggests that currently, the process of identifying suitable treatments for most diseases and illnesses involves a trial-and-error approach. Typically, patients receive prescribed treatments based on their efficacy for the majority, as determined by pharmacoepidemiology. If these treatments prove ineffective, the process of discovering an effective alternative has historically been lengthy and costly. Tinkle et al. (2014) termed the application of nanotechnology for medical purposes as nanomedicine. It is defined as the utilization of nanomaterials for the purposes of diagnosing, monitoring, managing, preventing, and treating disease. These materials have led to an increasing interest in and promotion of making medicine more personal. In this approach, there is no such thing as the usual dose, one drug fits all, or trial and error.
There is now more funding for personalized treatment in developed countries where people can afford it. This includes tailored cancer vaccines that utilize messenger RNA in the design of vaccines against a person’s own particular cancer. Such strategies gear up a patient’s individual cells to combat illness. Virtually all RNA therapies are delivered by nanoparticles, highlighting the importance of technological advancements for site-specific, adequate delivery of pharmaceuticals. In fact, according to Verma et al. (2023), lipid nanoparticles (LNPs) have recently risen to prominence as the technology platform that enables the delivery of mRNA, the key component of Moderna and BioNTech/Pfizer COVID-19 vaccines (Dube et al., 2021).
Worldwide, medicine has moved away from the one-size-fits-all approach; unfortunately, the lack of nanotechnology research and development in Africa makes this not the case here. There are, however, current efforts to integrate African countries into the international consortium for personalized medicine action. The concept is to foster joint personalized medicine (PM) projects and programs and to strengthen international science, technology, and innovation (STI) in the areas of health. The intent is to improve access to targeted therapeutic intervention for the African population, most of whom rely on donations of drugs whose clinical trials have invariably been carried out on groups that under-represent the African population.
There is no doubt that the use of nanomaterials for diagnostics and treatment has been successful in other parts of the world. However, there have been some problems related to nanotoxicity. For example, in magnetic resonance imaging (MRI), contrast agent-induced complications range from acute kidney injury, symptoms associated with gadolinium exposure (SAGE)/gadolinium deposition disease, potentially fatal gadolinium encephalopathy, and irreversible systemic fibrosis. Gadolinium is the active ingredient of these contrast agents, a non-physiologic lanthanide metal. Regulatory agencies are now aware that nanotoxicity must be addressed as nanomedicine is being embraced. In fact, components of nanomedicines that have typically been regarded as excipients may now be considered part of the active ingredient. If that is the case, clinical translation of the innovation in Africa must be carried out cautiously, in spite of healthcare challenges such as antimicrobial resistance.
In other words, many large-scale population studies in genetics performed so far have not included Africans, even though Africa has a large genetic diversity with varying profiles from north to south and from east to west. Therefore, driving real personalized health progress in Africa requires moving toward data-driven health systems if Africa is to benefit from nanotechnology. Ultimately, this technology will be applied to prevent, diagnose, and treat both infectious diseases and non-communicable diseases such as cancer and immune diseases that utilize PM strategies.
Clearly, the practice of medicine is changing. The advent of technological advancement in, for example, molecular biology, genomics, transcriptomics, and omics (proteomics, metabolomics, etc.) research is increasingly shifting the paradigm of modern medicine. This inevitably is leading to an omics-integrated approach to diagnosis and identification of molecular targets for therapy as part of precision medicine (Karczewski and Snyder, 2018). Therefore, 21st-century technology like nanomedicine gives us the means to enable precise healthcare. Health systems in Africa must evolve to create a person-centric approach to address our disease burden, bearing in mind the legal challenges in precision medicine (McGrath et al., 2021).
There is no doubt that with the level of development of many African national regulatory agencies (Ncube et al., 2021), diagnostic tests and therapeutic products that will emerge through an omics-integrated approach will pose a greater regulatory challenge. Gaps in the areas of standardization of every stage in the omics approach, preclinical assessment procedure, reviewing clinical utility and validity, and pharmacovigilance need regulatory science input (Adamo et al., 2018).
Regulatory science (RS) refers to the scientific and technical principles that serve as the basis for regulations in various industries, especially those related to safety or health. RS should not be confused with “regulatory affairs,” which involves applying existing regulatory principles in a responsive manner to a drug’s development or lifecycle. Unlike regulatory affairs, which are reactive in nature, regulatory science takes a proactive approach by analyzing regulatory principles and seeking to advance them in line with scientific advancements, such as in the field of nanomedicine.
According to Callréus and Schneider (2013), the most all-inclusive definition was put forward by the Institute of Medicine. The Institute defines RS as “the intellectual and practical activity encompassing the systematic study of the structure and behavior of the regulatory world through observation and experiment to determine the impact of the rules, principles, and laws governing FDA-regulated research,” (Institute of Medicine, 2012; Dinda, 2021), including nanotechnological research. This could be perceived as encompassing not only the examination of the results and effectiveness of regulatory frameworks but also the scientific and non-scientific influences that shape them, as shown in Figure 3.
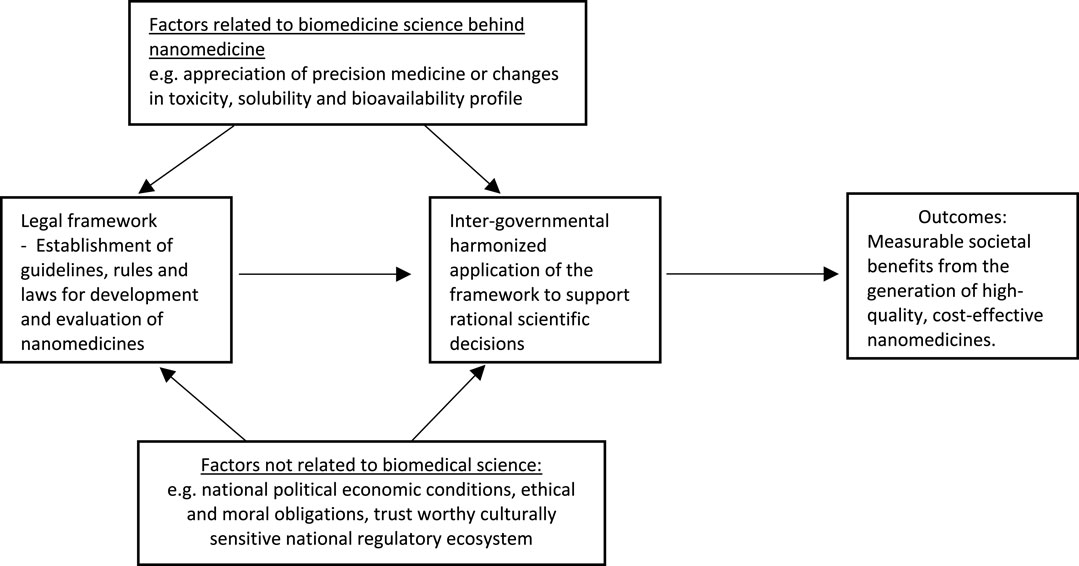
FIGURE 3. Intellectual and practical activities involved in regulatory science study and the expected outcome.
Irwin et al. (1997) developed the idea of a sociological framework that made RS be considered both interdisciplinary and multidisciplinary.
Given that the objective of a nanotechnology development initiative is to exhibit the enhanced effectiveness and safety of a product, it appears reasonable to discuss subjects like non-clinical safety assessments, the creation of biomarkers, the use of pharmacokinetic–pharmacodynamic modeling, and the identification of surrogate or composite endpoints in clinical trials related to nanotechnology. This objective calls for a multidisciplinary approach with experts to evaluate and validate, in the shortest possible time, all modes of translational research (Rouse et al., 2018). If it was possible with COVID-19 vaccine development at a global level, African countries at a regional level can certainly do so now that the African Medicines Agency exists (Ncube et al., 2021). However, some challenges, albeit surmountable, must still be faced.
The continent of Africa is home to several national regulatory agencies (NRAs) that operate at different levels (Ncube et al., 2021) of development while driving a science-based agenda. However, they have skills and human capacity gaps to apply RS in general and RS pertaining to nanomedicine in particular.
Currently, different regulatory assessment pathways are followed, as shown by examples in Table 1 that can be seen as representative of most African countries (Semete-Makokotlela et al., 2022).
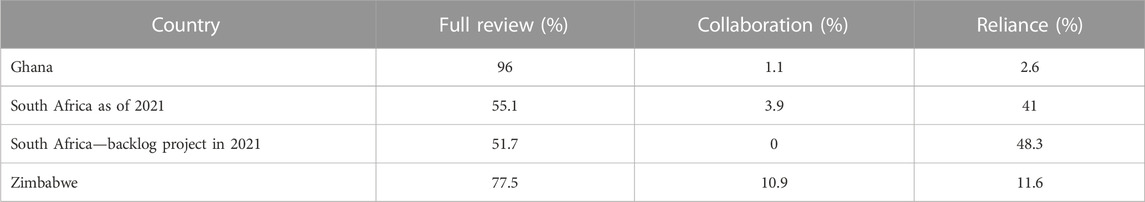
TABLE 1. Regulatory assessment pathways used by Ghana, South Africa, and Zimbabwe (Semete-Makokotlela et al., 2022).
Table 1 shows that, on average, these three countries rely on the review processes conducted by other stringent regulatory agencies to ensure conformity with formerly authorized specifications for approximately 25% of product reviews. This percentage will probably be higher for nanomedicines.
This is a challenge if the integrated omics approach mentioned previously is to be adopted for nanomedicines, especially when sponsors of a medicinal product extrapolate data wherein an African population is underrepresented (Mia, 2021). Rolled out in 2007 and endorsed in 2012, the AU’s Pharmaceutical Manufacturing Plan for Africa (PMPA) seeks increased local African pharmaceutical production. The adoption of the plan was driven by a clear objective of enhancing the continuous availability of superior medical products, with the aim of improving public health and boosting economic and industrial development across the continent.
Almost ten years ago, the PMPA proposed a package of technical solutions to the many challenges facing the pharmaceutical industry in terms of the human resource capacity related to regulatory issues. Unfortunately, not much has happened to address the issue concerning access to technology and technical knowledge. General nanotechnology development and nanomedicine will require specialized skills in various fields such as pharmacy, all categories of chemistry, biological sciences, the life sciences (medicine, pharmacology, and toxicology), and information and communication technology (ICT). The current landscape of education and training in these disciplines includes a number of stakeholders, with traditional universities being the most notable. Unfortunately, the training and education currently available lack industry applicability and practicality, and there is also under-utilization of people with relevant skills (Pharmaceutical Manufacturing, 2007; Semete-Makokotlela et al., 2022). The COVID-19 pandemic exposed the vulnerability in ensuring access to vital medicines, vaccines, and health technologies. However, the most advanced healthcare systems in Europe and the Americas were also overwhelmed by the impact of COVID-19 (Bright et al., 2021).
There is a need for deliberate efforts by training institutions to actively involve local and international industry (Big Pharma), local and international regulatory authorities (e.g., EMA and FDA), UN agencies (e.g., WHO and UNCTAD), and philanthropic organizations such as the Bill Gates Foundation in the development of capacity-building programs while leveraging advances in nanotechnology. Capacity-building lessons can be learned from the COVID-19 pandemic and the use of nanotechnology-based mRNA vaccines where all or some of the aforementioned organizations were involved.
Resource constraints on the African continent can be handled by the establishment of regional centers of excellence in nanotechnology with agreed governance structures that are financed by the private sector, the public sector, and international organizations. Such regional centers of excellence could also be involved in regulatory science education. Regulatory science is multidisciplinary, and the field of nanomedicine is no exception. Therefore, developing quality teaching, learning, and research programs would require collaborative efforts in developing a seamless education program. The education programs must also address the issue of the employability of experts in the field of nanotechnology in general and nanomedicine in particular.
Inventions in nanotechnology are multidisciplinary and multi-industrial in nature. Such a unique invention, by its very nature, will have a range of applications often accompanied by broader patentability claims (Khan et al., 2019). According to Keswani (2021), there is a need to take an integrated approach for sustained innovation in various areas of nanotechnology, for example, in omics-based research (genomics, transcriptomics, proteomics, and metabolomics research), paying particular attention to ethical and intellectual standards. The products of this type of research will be important in nanomedicine, and there is much to be known about nanoparticles (Khan et al., 2019). Conversely, the use of nanotechnology in developing nanomedicines presents novel challenges for the existing regulatory framework (Soares et al., 2018).
Correct policy and legislative landscape must be in place. This, unfortunately, appears not to be the case in many African countries if the study on the domestication of the African Union Model Law on Medical Products Regulation is anything to go by. The study found many challenges. Some of the factors that contributed to the challenges, in general, were competing priorities at the national level, the overlapping roles of government institutions, and the lengthy process of amending or repealing laws that have an impact on the field (Ncube et al., 2023). The study also showed that African countries do, nonetheless, have policy and legislation tools that would impact pharmaceutical research and development in general and nanotechnology in particular. Historically, the various government ministries and departments in these countries have had different focuses, which has led to clashing perspectives on the pharmaceutical industry. Certainly, the manufacture of nanomaterials, such as a smart pH-responsive nano-polymer drug against the intestinal pathogen Vibrio cholerae or plant-based nanomedicinal products, etc., would not be spared in the conflicts.
African governments utilize several policy and incentive mechanisms to achieve their goals. However, the use of these tools often leads to policy disjointedness in the pharmaceutical sector. This has been a substantial challenge due to a lack of knowledge among technocrats who may be asked to deal with intellectual property rights (IPR) and access-to-medicines issues (Pharmaceutical Manufacturing, 2007). As a result, very few countries in Africa have incorporated Trade-Related Aspects of Intellectual Property Rights (TRIPS) flexibilities in their IPR legislation. In fact, countries of the WHO African Region function under a multi-dimensional IPR and governance perspective that can cause problems with nanomedicine development (Motari et al., 2021).
According to Africanews, the continent imports nearly 95% of all medicines it uses, even though roughly 400 drug makers operate in Africa. Only 3% of global medicine manufacturing is done in Africa, and there is no medicine manufacturing in approximately 36 sub-Saharan African countries (Kato, 2022). As things currently stand in relation to access to safe and effective antibacterial nanodiamond-supported nanoparticles (Chang et al., 2019), Africa will need to find other means of dealing with antimicrobial resistance.
The review paper entitled “Needs-driven talent and competency development for the next generation of regulatory scientists in Africa” by Semete-Makokotlela et al. (2022) and the South Africa Health Products Regulatory Authority (SAHPRA) report on Innovation in RS Capacity Development in Africa (SAHPRA, 2020) clearly state what needs to be done. A conceptual framework for an African regulatory education and training system was proposed, and implementation tactics were suggested to address RS for all health products. On-the-job education and training should continue to allow experiential learning, and training content should be added to undergraduate programs (Ncube et al., 2022).
Nanotechnology and nanomedicine will require training in specialized skills in pharmacy, chemistry, biological sciences, bio-engineering, the life sciences, and ICT. Such empowerment must occur in an iterative manner, as skill in developing new tools, standards, and approaches for assessing the safety, efficacy, quality, and performance of nanotechnology products is only acquired through engagement in the thought process. The education and training must be targeted to medical professionals, engineers, task managers in various industries, and other researchers in various fields.
Depending on the partnership involved in the education and training, which should include research and training institutions, there is also a need to involve established Regional Centres of Regulatory Excellence (RCOREs). The AMA, in collaboration with national regulatory authorities, aims to monitor the quality of training and education. The courses covered include:
- The fundamental characterization for nanotechnology/the wider context of nanotechnology
- Introduction to bio-nanotechnology
- The fundamental science of nanotechnology
- Nanomedicine/science and application
- Clinical translation and commercialization of nanomedicine
There is no need to reinvent the wheel in dealing with the challenges of building African expertise. AMA can learn from other institutions, such as the USFDA, the EMA, and the Center for RS at the University of California, and involve underutilized African expertise.
In summary, empowering regulators and policymakers in Africa with knowledge of nanotechnology/nanomedicine will enable them to embrace its potential economic benefits, expedite the translation of fundamental scientific concepts from the lab to clinical applications, and reinforce the evidence-based practices of regulatory science.
There is no doubt that the environmental and public health impact of nanomaterials must be considered, along with the advancements in nanomedicines and nanotechnology as a whole burden. Much work awaits, as various nanomaterials to be used in nanomedicine have different kinds of risks associated with them. Policy development and regulation of medical products, in general, and nanomedical products, in particular, that guarantee public health protection is a complex undertaking in Africa. However, now that the African Continental Free Trade Area (AfCFTA) has been ratified by most African countries, it remains to be seen how the AMA will encourage the adoption of the AU Model Law on Medical Products Regulation on a local level. The AU Model Law on Medical Products Regulation can be effectively implemented at the local level by addressing concerns highlighted in the paper by Ncube et al. (2021). Resolving the challenges outlined by Ncube et al. (2021) will lead to a more uniform legal framework for regulating nanomedicine in Africa and enhance the performance of African NRAs, ultimately resulting in regulatory congruity. A harmonized framework will be possible with the creation of the AMA, which will need sustainable financing to deal with many other challenges. These challenges include the processing of domestication and implementing the law, which require adequate and competent human resources to handle competing priorities at the national level, and the rapid advancements in the pharmaceutical sector in general and nanotechnology in particular. It is hoped that a more uniform legal framework will result in new drug regulatory paradigms such as the “adaptive licensing” of nanotechnology products. There are still many unknowns about nanomaterials.
Harmonization of consumer laws and the development of patient charters will be important to deal with the ethical issues related to the rise of precision/personalized medicine, which is not a new concept. Medicine dosing has always been individualized and emphasized during undergraduate and in-service training of healthcare professionals. It is to be hoped that ethical considerations will be considered when amending/repealing laws to align them with the AU Model Law on Medical Products Regulation. Unfortunately, this might be a slow and lengthy process while nanotechnology continues to advance. According to Ravinetto et al. (2018), this process will require ethical guidance because most African countries have limited resources.
The establishment of the African Continental Free Trade Area agreement and other AU organs such as AMA, Africa-CDC, and NEPAD must be used to enable collaborative knowledge creation and sharing and transfer of technology in the field of nanomedicine. This will also involve regional organizations such as the East African Community, SADC, and ECOWAS. These organizations will be responsible for the regional centers of excellence. Currently, almost half of the AU countries are members of the African Regional Intellectual Property Organization (ARIPO), whose mandate is to promote the development of the intellectual property (IP) system in Africa. This is being done through the development of human capital in the field of IP. It is hoped that such a framework will encourage the aggressive promotion of research and development in the pharmaceutical sector as nanotechnology advances. Given that ARIPO is key to protecting not only patents, trademarks, etc. but also African traditional knowledge and expressions of folklore, advances in nanotechnology present an opportunity to improve the acceptability and treatment outcomes of African traditional medicines. The creation of knowledge and sharing through collaborative research will be part of the monitoring and evaluation of the benefits and risks of nanomedicines, that is, the pharmacovigilance of nanomedicines.
Research at the biotechnology and material science interface has led to revolutionary innovation in biomaterials and medicine. In other parts of the world, research focused on creating polymers that can provide consistent and controlled delivery of drugs over an extended duration continues unabated. Nanomaterials and processes for everything from tissue regeneration, anticancer drugs, gene therapy, and vaccines have been developed. They are now also used for diagnosis. Because of nanotechnology, diagnosing, treating, and managing diseases will become even more personal. This practice is now referred to as precision/personalized medicine, and it has attendant ethical, legal, and regulatory issues. In Africa, these issues will require the application of harmonized medical, scientific, social, and technical competency in regulatory science, an ever-expanding field. As the AU African Model Law on Medical Products Regulation is ratified by the AMA and becomes further integrated into local systems, there should be a concerted effort to connect educational and research opportunities with career development within regulatory science and nanomedicine capacity-building programs.
NN put the concept together and drafted the first manuscript, which JC reviewed. NN and JC then equally wrote the final draft of the manuscript, which was reviewed by PK before submission. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adamo, J. E., Bienvenu, R. V., Fields, F. O., Ghosh, S., Jones, C. M., Liebman, M., et al. (2018). The integration of emerging omics approaches to advance precision medicine: how can regulatory science help? J. Clin. Transl. Sci. 2 (5), 295–300. doi:10.1017/cts.2018.330
Bright, B., Babalola, C. P., Sam-Agudu, N. A., Onyeaghala, A. A., Olatunji, A., Aduh, U., et al. (2021). COVID-19 preparedness: capacity to manufacture vaccines, therapeutics and diagnostics in sub-Saharan Africa. Glob. health 17, 24–14. doi:10.1186/s12992-021-00668-6
Callréus, T., and Schneider, C. K. (2013). The emergence of regulatory science in pharmaceutical medicine. Pharm. Med. 27, 345–351. doi:10.1007/s40290-013-0039-x
Chang, B. M., Pan, L., Lin, H. H., and Chang, H. C. (2019). Nanodiamond-supported silver nanoparticles as potent and safe antibacterial agents. Sci. Rep. 9 (1), 13164. doi:10.1038/s41598-019-49675-z
Deng, Y., Zhang, X., Shen, H., He, Q., Wu, Z., Liao, W., et al. (2020). Application of the nano-drug delivery system in treatment of cardiovascular diseases. Front. Bioeng. Biotechnol. 7, 489. doi:10.3389/fbioe.2019.00489
Dinda, A. K. (2021). Regulatory science: the need for empowering Indian innovation. Indian J. Med. Res. 154 (6), 770. doi:10.4103/ijmr.ijmr_1665_19
Dube, A., Egieyeh, S., and Balogun, M. (2021). A perspective on nanotechnology and COVID-19 vaccine research and production in South Africa. Viruses 13 (10), 2095. doi:10.3390/v13102095
FDA (2018). FDA’s approach to regulation of nanotechnology products. Available at: https://www.fda.gov/science-research/nanotechnology-programs-fda/fdas-approach-regulation-nanotechnology-products (Accessed February 7, 2023).
FDA (2022). Drug products, including biological products, that contain nanomaterials. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-products-including-bilogical-products-contain-nanomaterials-guidance-industary (Accessed February 7, 2023).
Institute of Medicine (2012). Strengthening a workforce for innovative regulatory science in therapeutics development: workshop summary. Washington, DC: The National Academies Press.
Irwin, A., Rothstein, H., Yearley, S., and McCarthy, E. (1997). Regulatory science—towards a sociological framework. Futures 29 (1), 17–31. doi:10.1016/s0016-3287(96)00063-8
Karczewski, K. J., and Snyder, M. P. (2018). Integrative omics for health and disease. Nat. Rev. Genet. 19 (5), 299–310. doi:10.1038/nrg.2018.4
Kato, R. L. (2022). Can Africa make the medicines it needs (business Africa). Available at: https://www.africanews.com.
Khan, I., Saeed, K., and Khan, I. (2019). Nanoparticles: properties, applications and toxicities. Arabian J. Chem. 12 (7), 908–931. doi:10.1016/j.arabjc.2017.05.011
Langer, R. (2019). How nanotechnology powers precision medicine. Observations: Scientific American. https://blogs.scientificamerican.com/observations/how-nanotechnology-powers-precision-medicine/.
McGrath, S. P., Peabody, A. E., Walton, D., and Walton, N. (2021). Legal challenges in precision medicine: what duties arising from genetic and genomic testing does a physician owe to patients? Front. Med. 8, 663014. doi:10.3389/fmed.2021.663014
Mia, R. (2021). Challenges and opportunities of personalized medicine for Africa. Tenth EDCPT forum EU-africa PerMed. Building links between Europe and Africa in personalized medicine. EU-africa PerMed project YouTube channel 2021. Available at: https://www.youtube.com/watch?v=nE18oQZ_JYc.
Motari, M., Nikiema, J. B., Kasilo, O. M., Kniazkov, S., Loua, A., Sougou, A., et al. (2021). The role of intellectual property rights on access to medicines in the WHO African region: 25 years after the TRIPS agreement. BMC Public Health 21 (1), 490–519. doi:10.1186/s12889-021-10374-y
Ncube, B. M., Dube, A., and Ward, K. (2021). Establishment of the African Medicines Agency: progress, challenges and regulatory readiness. J. Pharm. policy Pract. 14, 29–12. doi:10.1186/s40545-020-00281-9
Ncube, B. M., Dube, A., and Ward, K. (2022). Medicines regulatory science expertise in Africa: workforce capacity development and harmonisation activities towards the establishment of the African Medicines Agency. Pharm. Med. 36, 83–97. doi:10.1007/s40290-022-00425-z
Ncube, B. M., Dube, A., and Ward, K. (2023). The domestication of the African Union model law on medical products regulation: perceived benefits, enabling factors, and challenges. Front. Med. 10, 1117439. doi:10.3389/fmed.2023.1117439
Ossa, D. (2014). Quality Aspects of nano-based medicines SME workshop: focus on quality for medicines containing chemical entities london. Amsterdam: European Medicines Agency.
Pharmaceutical Manufacturing (2007). Pharmaceutical manufacturing plan for Africa. Pharm. Manuf. Africa-CAHM_MIN 8, 111.
Ravinetto, R., Pinxten, W., and Rägo, L. (2018). Quality of medicines in resource-limited settings: need for ethical guidance. Glob. Bioeth. 29 (1), 81–94. doi:10.1080/11287462.2018.1522991
Rouse, R., Kruhlak, N., Weaver, J., Burkhart, K., Patel, V., and Strauss, D. G. (2018). Translating new science into the drug review process: the US FDA’s Division of Applied Regulatory Science. Ther. innovation Regul. Sci. 52, 244–255. doi:10.1177/2168479017720249
SAHPRA (2020). The innovation in regulatory sciences capacity development in Africa meeting (issue 30 june 2020). Available at: https://www.sahpra.org.za/wp-content/uploads/2020/10/The-innovation-meeting-report-30June-2020.pdf.
Semete-Makokotlela, B., Mahlangu, G. N., Mukanga, D., Darko, D. M., Stonier, P., Gwaza, L., et al. (2022). Needs-driven talent and competency development for the next generation of regulatory scientists in Africa. Br. J. Clin. Pharmacol. 88 (2), 579–586. doi:10.1111/bcp.15020
Soares, S., Sousa, J., Pais, A., and Vitorino, C. (2018). Nanomedicine: principles, properties, and regulatory issues. Front. Chem. 6, 360. doi:10.3389/fchem.2018.00360
Tinkle, S., McNeil, S. E., Mühlebach, S., Bawa, R., Borchard, G., Barenholz, Y., et al. (2014). Nanomedicines: addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 1313 (1), 35–56. doi:10.1111/nyas.12403
Verma, M., Ozer, I., Xie, W., Gallagher, R., Teixeira, A., and Choy, M. (2023). The landscape for lipid-nanoparticle-based genomic medicines. Nat. Rev. Drug Discov. 22, 349–350. doi:10.1038/d41573-023-00002-2
Keywords: nanomedicine, regulatory science, African Medicines Agency, nanotechnology and health, pharmaceutical sector in Africa
Citation: Nyazema NZ, Chanyandura JT and Kumar PO (2023) Nanomedicine and regulatory science: the challenges in Africa. Front. Front. Biomater. Sci. 2:1184662. doi: 10.3389/fbiom.2023.1184662
Received: 12 March 2023; Accepted: 18 October 2023;
Published: 08 November 2023.
Edited by:
Rúben Pereira, University of Porto, PortugalReviewed by:
Sharanabasava V. Ganachari, KLE Technological University, IndiaCopyright © 2023 Nyazema, Chanyandura and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: N. Z. Nyazema, cm90c2luaXJpQGdtYWlsLmNvbQ==; J. T. Chanyandura, anRjaGFueWFuZHVyYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.