- 1Department of Food Science and Engineering, Moutai Institute, Renhuai, Guizhou, China
- 2Department of Biology and Chemistry, College of Sciences, National University of Defense Technology, Changsha, Hunan, China
The precise role of lncRNAs in skeletal muscle development and atrophy remain elusive. We conducted a bioinformatic analysis of 26 GEO datasets from mouse studies, encompassing embryonic development, postnatal growth, regeneration, cell proliferation, and differentiation, using R and relevant packages (limma et al.). LncRNA-miRNA relationships were predicted using miRcode and lncBaseV2, with miRNA-mRNA pairs identified via miRcode, miRDB, and Targetscan7. Based on the ceRNA theory, we constructed and visualized the lncRNA-miRNA-mRNA regulatory network using ggalluvial among other R packages. GO, Reactome, KEGG, and GSEA explored interactions in muscle development and regeneration. We identified five candidate lncRNAs (Xist, Gas5, Pvt1, Airn, and Meg3) as potential mediators in these processes and microgravity-induced muscle wasting. Additionally, we created a detailed lncRNA-miRNA-mRNA regulatory network, including interactions such as lncRNA Xist/miR-126/IRS1, lncRNA Xist/miR-486-5p/GAB2, lncRNA Pvt1/miR-148/RAB34, and lncRNA Gas5/miR-455-5p/SOCS3. Significant signaling pathway changes (PI3K/Akt, MAPK, NF-κB, cell cycle, AMPK, Hippo, and cAMP) were observed during muscle development, regeneration, and atrophy. Despite bioinformatics challenges, our research underscores the significant roles of lncRNAs in muscle protein synthesis, degradation, cell proliferation, differentiation, function, and metabolism under both normal and microgravity conditions. This study offers new insights into the molecular mechanisms governing skeletal muscle development and regeneration.
1 Introduction
Skeletal muscle, constituting approximately 40% of body mass in mammals, is essential for posture and movement (Liu et al., 2019a; Chen et al., 2018a) and functions as an endocrine, thermogenic, and metabolic organ (Pant et al., 2016; Egan and Zierath, 2013; Pedersen and Febbraio, 2012; Lee et al., 1985). Muscle development (Zhu et al., 2021), including embryonic and postnatal growth as well as regeneration after injury (Pant et al., 2016; Egan and Zierath, 2013; Pedersen and Febbraio, 2012), plays a crucial for muscle formation and maintenance (Figure 1B), significantly impacting embryonic development. Despite its importance, the molecular mechanisms behind it remain unclear.
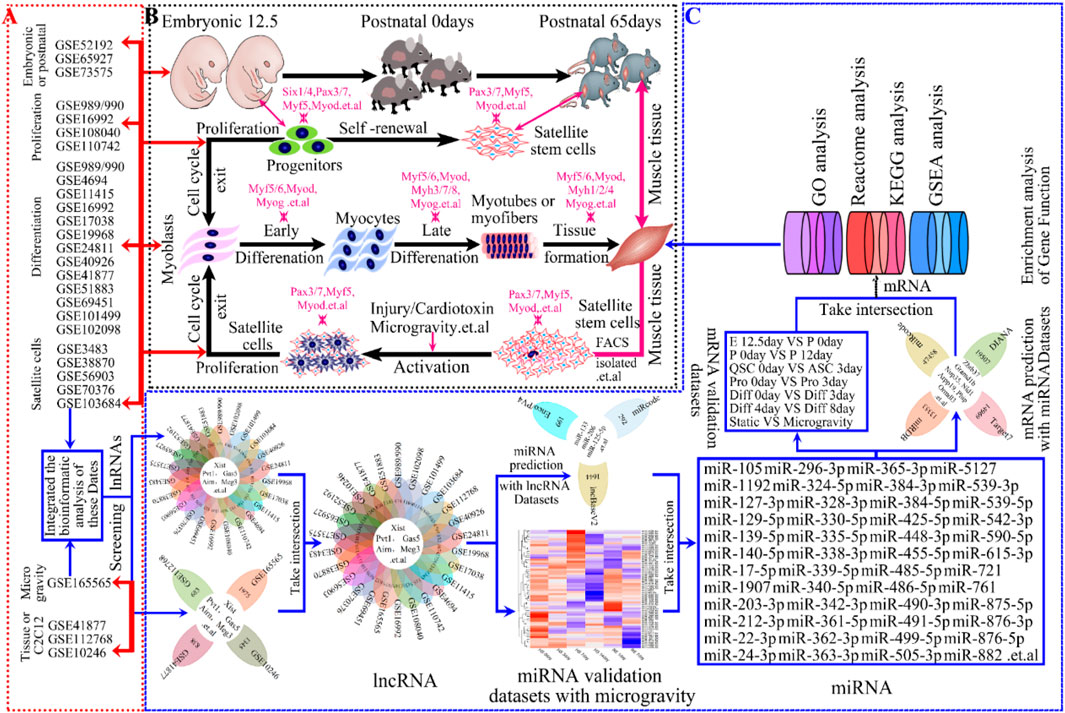
Figure 1. The progression of skeletal muscle development and Schematic workflow of screening lncRNAs and bioinformatics analyses in skeletal muscle. (A) Study flow diagram showing GEO datasets used for analyses in skeletal muscle development. (B) Hierarchy of progression of skeletal muscle development contained embryonic development, postnatal growth, cell proliferation, cell differentiation, muscle regeneration and so on. For each step, markers for the early and late differentiation are indicated. The genes indicated in magenta encode transcription factors or sarcomeric and associated proteins. (C) Schematic illustration of an integrative computational analysis to map, annotate and reconstruct, to screen, validate and gene enrichment analysis lncRNAs in skeletal muscle development, such as KEGG, GO, Reactome, GSEA enrichment analysis and so on.
Skeletal muscle development spans from mouse embryonic stages to maturity, involving myotome progenitor proliferation, differentiation, and stem cell formation during embryogenesis (Lander, 2011; Plikus et al., 2021; Hemberger et al., 2020; Gupta et al., 2021; Bentzinger et al., 2012; Chal and Pourquie, 2017; Dumont et al., 2015; Hnia et al., 2019) (Lander, 2011; Plikus et al., 2021; Hemberger et al., 2020; Gupta et al., 2021; Bentzinger et al., 2012; Chal and Pourquie, 2017; Dumont et al., 2015; Hnia et al., 2019). Postnatally, muscle cells enhance function through improved endurance, energy metabolism, and cytokine secretion (Bentzinger et al., 2012; Chal and Pourquie, 2017; Dumont et al., 2015; Hnia et al., 2019; Pillon et al., 2020; Zhang et al., 2018a). In contrast, skeletal muscle regeneration (Liu et al., 2017b; Goel et al., 2017) involves the activation, proliferation, and differentiation of quiescent satellite cells, in response to environmental stresses like microgravity. Recent studies show that skeletal muscle development and regeneration are regulated by cell cycle proteins, myogenic transcription factors, cytokines, and noncoding RNAs (Pedersen and Febbraio, 2012; Ciemerych and Sicinski, 2005; Braun and Gautel, 2011; Butchart et al., 2016; Ballarino et al., 2016).
Long noncoding RNAs (LncRNAs), over 200 nucleotides long and non-protein-coding, play roles in epigenetics, gene transcription, and protein expression by sponging miRNAs or interacting with mRNAs (Signal et al., 2016; Ponting et al., 2009; Wang et al., 2021; Yao et al., 2019; Chen, 2016; Kallen et al., 2013; Sui et al., 2019). Accumulating evidence indicates that (Butchart et al., 2016; Li et al., 2018; Goncalves and Armand, 2017; Zhao et al., 2019) lncRNAs influence skeletal muscle development and atrophy by acting as ceRNAs. Examples include LINC00961 (Matsumoto et al., 2017), LncMyoD (Gong et al., 2015), lncRNA Neat1 (Wang et al., 2019a), lncRNA YY1 (Zhou et al., 2015) and lncRNA Malat1 (Han et al., 2015; Chen et al., 2017),. Additionally, lncRNAs like lncRNA MAR1 (Zhang et al., 2018b), Linc-RAM (Yu et al., 2017), lincRNA-MD1 (Legnini et al., 2014), H19 (Kallen et al., 2013), LncRNA-mg (Zhu et al., 2017), and lincRNA Yam-1 (Lu et al., 2013) regulate both muscle proliferation and differentiation. And lncRNAs regulate muscle atrophy by influencing mitochondrial function, cell autophagy, apoptosis, and myofiber size, with examples including lncMUMA (Zhang et al., 2018c), LncIRS1 (Li et al., 2019), lncRNA SMN-AS1 (d'Ydewalle et al., 2017), lncRNA Pvt1 (Alessio et al., 2019). Dysregulated lncRNA expression is linked to skeletal muscle development and related diseases, such as DMD and muscle wasting. Therefore, studying lncRNA functions in muscle development is crucial.
Technological advancements have facilitated the use of GEO Datasets to study lncRNA in skeletal muscle development and environmental responses. Despite over 26 published GEO datasets on mouse muscle development (Figure 1B), comprehensive analysis remains limited. Our global R-analysis of these datasets identified key roles for lncRNAs Xist, Gas5, Pvt1, Airn, and Meg3 in muscle development and microgravity-induced damage (Figure 1). For understanding the mechanisms and signaling pathways involved in skeletal muscle development could shed light on muscle-related diseases and advance personalized treatments.
2 Methods and materials
2.1 Collection and processing of publicly available GEO datasets
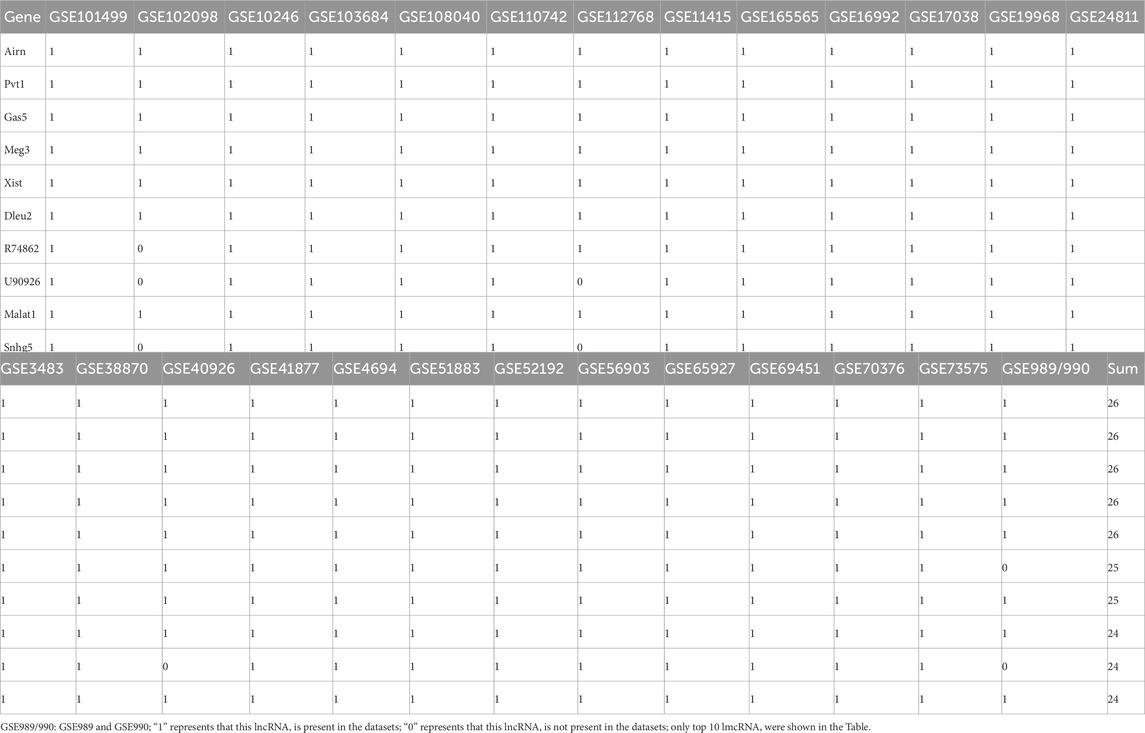
Publicly available GEO microarray datasets (Table 1) were downloaded from NCBI and re-annotated using R and Perl software. These datasets encompassed topics such as embryonic development, postnatal growth, muscle regeneration, cell proliferation, and cell differentiation. Gene symbols for lncRNA and protein-coding mRNA were annotated and extracted using R and Perl from platforms and GRCm39 (https://www.gencodegenes.org/mouse/). Final statistical analyses involved 26 studies from various microarray data (Table 1). We intersected these 26 GEO databases to identify key lncRNAs. Datasets for different phases (e.g., embryonic development, postnatal growth, muscle regeneration) were integrated by time-points (Table 1). The “limma” R package (Ritchie et al., 2015) was used for differential gene expression analysis in R. Results were visualized using heatmaps, volcano plots, bar graphs, and Venn diagrams with “pheatmap” (Metsalu and Vilo, 2015) and “ggplot2” (Ito and Murphy, 2013) R packages. More details are provided in Table 1, and the workflow is shown in Figure 1A.
2.2 Prediction of targeting relationship and construction of the lncRNA-miRNA-mRNA ceRNA regulatory network
To investigate post-transcriptional regulation in skeletal muscle development, we constructed a lncRNA-miRNA-mRNA ceRNA network. The workflow included predicting lncRNA-miRNA interactions using the miRcode (Jeggari et al., 2012), lncBaseV2 (Karagkouni et al., 2020), and Enco/PV4 (Teng et al., 2020; Li et al., 2014) (Integration database with ENCORI and NPInterv4) databases, followed by intersecting these predictions. Predicted miRNAs were filtered based on expression datasets from hindlimb suspension simulated microgravity. Subsequently, miRNA-mRNA interactions were identified through intersecting results from miRcode (Jeggari et al., 2012), DIANA (Karagkouni et al., 2018), miRDB (Chen and Wang, 2020), and TarBase7 (Agarwal et al., 2015) databases. Finally, a ceRNA regulatory network comprising 5 lncRNAs, 36 miRNAs, and 108 mRNAs was built using R software, with visualization facilitated by the “ggalluvial” R package (Rosvall and Bergstrom, 2010; Liu et al., 2017a). This comprehensive approach allowed us to elucidate the intricate regulatory relationships within the network.
2.3 Enrichment analysis
To investigate the functional ceRNA regulatory network of lncRNA-miRNA-mRNA, we performed an enrichment analysis on predicted mRNAs. This involves identifying mRNAs by intersecting predicted mRNAs with various stages of skeletal muscle development, including comparisons between embryonic and postnatal phases, quiescent and activated satellite cells, and different differentiation stages. Secondly, we applied GO (Lei et al., 2019; Sun et al., 2019) (Gene Ontology) analysis to the primary screened mRNAs for enrichment of biological processes, cellular components, and molecular functions. The Reactome (Jassal et al., 2020) knowledge base was used to further validate their molecular functions. KEGG (Lei et al., 2019; Sun et al., 2019) analysis identified potential mRNA pathways, while GSEA (Cheng et al., 2021) assessed gene distribution trends to determine their phenotypic contributions. GO and KEGG enrichment were conducted with the “clusterProfiler” R package (Wu et al., 2021), while Reactome and GSEA analyses were conducted using the “ReactomePA” (Yu and He, 2016) and “GSEA” (Reimand et al., 2019) R packages, respectively. Visualization of the GO, KEGG, Reactome, and GSEA analysis results was achieved using the “ggplot2″ R package.
2.4 Statistical analyses
Data is presented as mean ± SEM. All statistical analyses were conducted with R software, version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). The R software ggpubr, reshape2, rstatix package et al. was used to perform statistical analyses. Analysis results were plotted using the R packages ggplot2. Student’s t-test was utilized to compare the differences between the two groups. Comparisons were considered statistically significant at p ≤ 0.05.
2.5 Data availability
Further information on research design is available in the GEO datasets linked to this article (Table 1). All data were freely accessible online.
3 Results
3.1 Screening of candidate lncRNAs from public GEO datasets
To identify lncRNAs involved in skeletal muscle development, we re-annotated and analyzed 26 publicly available GEO datasets using R and Perl software (Figures 1A, C; Table 1). These datasets, sourced from NCBI, encompass embryonic and postnatal stages (3 datasets), muscle regeneration (5 datasets), proliferation (5 datasets), differentiation (14 datasets), muscle tissue or C2C12 expression (3 datasets), and muscle wasting expression (1 datasets). Our study workflow is depicted in Figure 1C, with detailed information provided in Table 1. Through the analysis of these GEO databases (Table 2), We identified the lncRNAs—Xist, Gas, Pvt1, Airn, and Meg3 (Figure 1C; Table 1) — as having regulatory potential in skeletal muscle development (Figure 1B). These specific lncRNAs were selected based on their differential expression patterns observed across various stages and conditions of muscle development and function.
Muscle atrophy in humans and mammals exposed to microgravity leads to muscle function loss or pathological changes (Vico and Hargens, 2018; Garrett-Bakelman et al., 2019). To compare lncRNA expression between normal and atrophied muscle, we re-annotated and analyzed GEO datasets from three studies on normal skeletal muscle (GSE112768, GSE877, GSE10246) and one on microgravity-induced muscle (GSE165565) using R and Perl software (Figure 1C). By setting the logFC threshold to greater than 0.1, we identified 683, 838, 1,345, and 1975 lncRNAs retained in GSE112768, GSE877, GSE10246, and GSE165565, respectively (Figure 2A). We then re-screened and validated lncRNAs Xist, Gas5, Pvt1, Airn, and Meg3 across all four datasets (Figure 2A). In muscle tissue (SOL or EDL), lncRNA Xist and Meg3 showed significant downregulation, while lncRNAs Gas5, Pvt1, and Airn were upregulated (Figures 2B, C). In C2C12 cells versus muscle tissue (SOL or EDL), lncRNAs Xist, Gas5, Pvt1, and Airn were significantly downregulated, with Xist decreasing over 5-fold, while Meg3 was upregulated about 5-fold (Figures 2D, E). In GSE10246, Xist, Gas5, and Pvt1 were downregulated, while Meg3 and Airn were upregulated (Figure 2F). In GSE165565, Xist expression increased 1.7 times in microgravity compared to static conditions, while Meg3, Gas5, Pvt1, and Airn showed no significant change (Figure 2G). These results indicate that the lncRNAs Xist, Gas5, Pvt1, Airn, and Meg3 exhibit differential expression and diverse functions across various contexts, potentially regulating skeletal muscle development. Notably, Xist stands out for its significant changes across different conditions, suggesting a crucial role in skeletal muscle regulation.
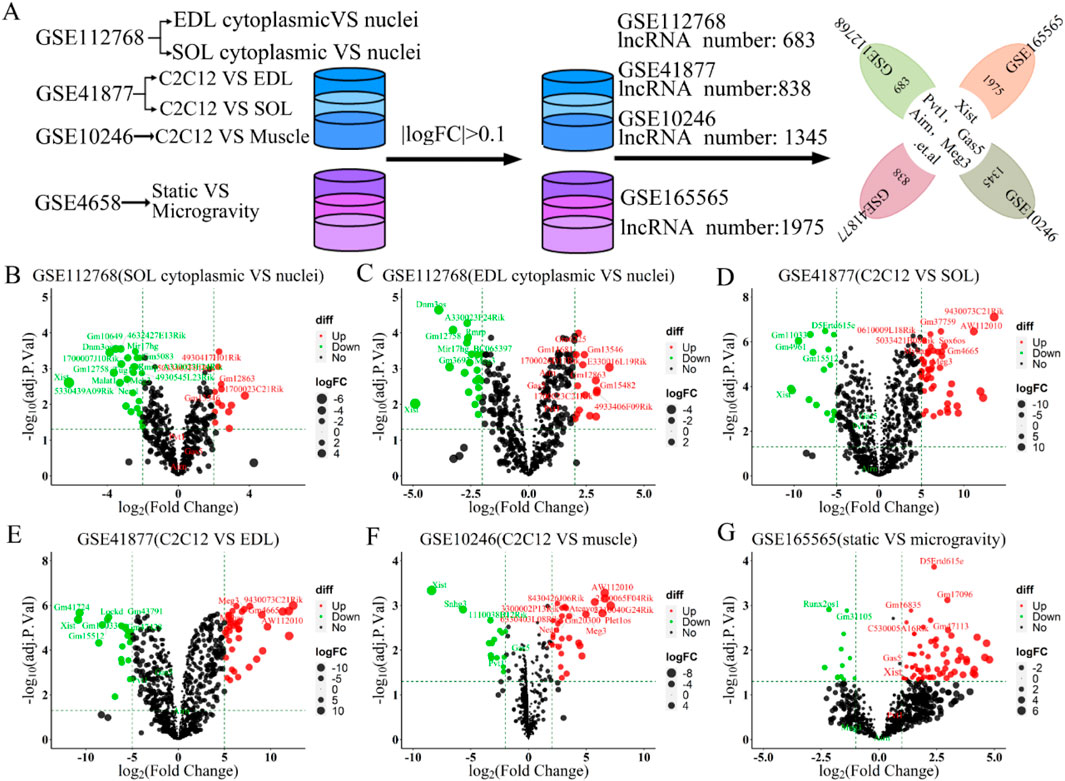
Figure 2. The 5 candidate lncRNAs (lncRNA Xist, lncRNA Gas5, lncRNA Pvt1, lncRNA Airn and lncRNA Meg3) were aberrantly expressed in GSE112768, GSE41877, GSE10246, and GSE165565 datasets. (A) Schematic workflow for statistical analysis process. A volcano plot was used to show the differentially expressed lncRNAs. The negative Log2-adjusted p-values (y-axis) are plotted against the Log2 fold changes in expression (x-axis). The horizontal dashed line indicates the threshold for significance (p ≤ 0.05) and the vertical dashed line indicates the upregulated (right side) and downregulated (left side) lncRNAs. (B) Volcano plot showing the differentially expressed lncRNAs between soleus myofibers nucleus versus cytoplasm in GSE112768. (C) Volcano plot showing the differentially expressed lncRNAs between EDL myofibers nucleus versus cytoplasm in GSE112768. (D) Volcano plot presenting the differentially expressed lncRNAs between C2C12 versus soleus in GSE41877. (E) Volcano plot presenting the differentially expressed lncRNAs between C2C12 versus EDL in GSE41877. (F) Volcano plot illustrating the differentially expressed lncRNAs between C2C12 versus muscle in GSE10246. (G) Volcano plot displaying the differentially expressed lncRNAs between static vs. simulated microgravity in GSE165565. EDL: extensor digitorum longus, SOL: Soleus.
3.2 The role of lncRNAs in skeletal muscle development: A focus on Xist and its regulatory significance
Understanding skeletal muscle development (Zhu et al., 2021), which includes embryonic formation, postnatal growth, and injury recovery, is essential for maintaining physiological functions (Hemberger et al., 2020; Gupta et al., 2021; Chal and Pourquie, 2017; Hnia et al., 2019). This process is driven by myogenesis, involving muscle cell proliferation, differentiation, and fusion (Bentzinger et al., 2012; Dumont et al., 2015; Liu et al., 2017a; Goel et al., 2017). LncRNAs (Gupta et al., 2021; Ciemerych and Sicinski, 2005; Spittau et al., 2020; Chini and Hanganu-Opatz, 2021) significantly influence skeletal muscle development during embryonic and postnatal growth in mice, contributing to muscle maturation (Figure 1B). By analyzing 23 GEO datasets (Figures 1A, C), we explore the molecular roles of specific lncRNAs—Xist, Gas5, Pvt1, Airn, and Meg3—at different developmental stage (Figure 1; Table1).
To examine the roles and expression of these lncRNAs (Figure 3A), we re-annotated and analyzed GEO datasets GSE52192, GSE73575, and GSE65927 using R and Perl software (Figure 1C; Figure 3A). Our results showed that the expression of these lncRNAs’ increased from embryonic day 12.5 to postnatal day 0, decreased until postnatal day 28, and then rose again by postnatal day 65, following a similar pattern for all five lncRNAs (Figure 3B). During embryonic development and postnatal growth, lncRNAs are regulated to ensure proper cell proliferation, differentiation, and apoptosis. For instance, levels of cyclins (Cdk1, Cdk2, Cdk4, Ki67, Pcna, Mcm2, Mcm6) decreased and inhibitors (p21, p27, p33, p53) increased over time (Figure 3C), highlighting their roles in growth. Specific myogenic markers, including Six1/4, Pax3/7, Myf5, Myod, Myog, Myf6, and Myh1/2/3/4/7/8, define each stage of skeletal muscle development. Analysis revealed that Pax3, Myod, Myog, Myf6, and Myh1/2/4/7 levels rise during development, while Myf5, Myh3/8, Pax7, and Six1/4 levels decline (Figure 3H), indicating muscle cell proliferation and differentiation during embryonic and postnatal growth. Volcano plot results further highlighted significant changes in lncRNA Xist expression: it significantly decreased from embryonic day 12.5 to postnatal day 0 (logFC = −6.22, P≤ 0.001, Figure 3D), increased from postnatal day 0 to day 12 (logFC = 5.08, P≤ 0.001, Figure 3E), remained stable from day 12 to day 28 (logFC = −0.01, P≤ 0.98, Figure 3F), and significantly decreased again from day 28 to day 65 (logFC = −6.12, P≤ 0.001, Figure 3G). In contrast, lncRNAs (Pvt1, Gas5, Airn, and Meg3) showed no significant changes (|logFC|≥3, Pvalue≤0.5) during both embryonic development and postnatal growth (Figures 3D–G). These findings suggest that while lncRNAs Pvt1, Gas5, Airn, and Meg3 may play a role in regulating embryonic development and postnatal growth, lncRNA Xist is particularly significant in this regulatory process.
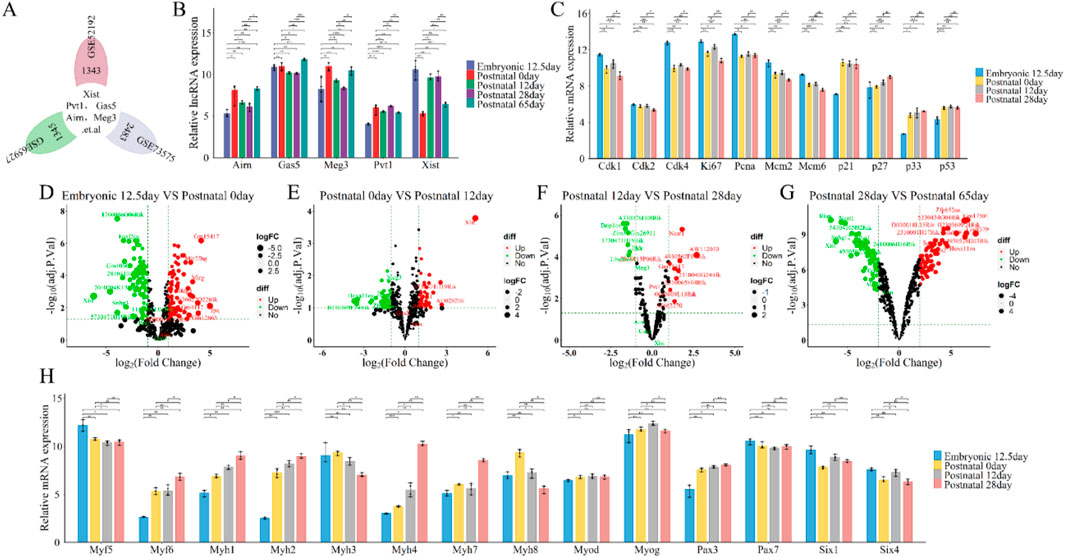
Figure 3. Functional analysis of differentially expressed lncRNAs (lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3) in stages of embryonic development and postnatal growth. (A) Flower plot diagram presents the overlap of common lncRNAs in GSE52192, GSE73575 and GSE65927 datasets. (B) The expression of lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3 during Embryonic 12.5 days, Postnatal 0day, Postnatal 12day, Postnatal 28 days, Postnatal 65 days. (C) The expression of cell cycle related protein and inhibitor at Embryonic 12.5 days, Postnatal 0 day, Postnatal 12 days, Postnatal 28 days (D) Volcano plot showing the differentially expressed lncRNAs between Embryonic 12.5 days versus Postnatal 0 day. (E) Volcano plot presenting the differentially expressed lncRNAs between Postnatal 0day versus Postnatal 12days (F) Volcano plot illustrating the differentially expressed lncRNAs between Postnatal 12 days versus Postnatal 28 days. (G) Volcano plot displaying the differentially expressed lncRNAs between Postnatal 28 days versus Postnatal 65 days (H) The expression of key myogenic regulators during Embryonic 12.5 days, Postnatal 0 days, Postnatal 12 days, Postnatal 28 days. A volcano plot was used to show the differentially expressed lncRNAs. The negative Log2-adjusted P-values (y-axis) are plotted against the Log2 fold changes in expression (x-axis). The horizontal dashed line indicates the threshold for significance (P ≤ 0.05) and the vertical dashed line indicates the upregulated (right side) and downregulated (left side) lncRNAs. The data are presented as the means ± S.D. of the samples from all different samples. The p-values were calculated using Student’s t-test. NS, non-significant; *p < 0.05; **p < 0.01; ***p < 0.0001. (E) Embryonic; P: Postnatal.
Long non-coding RNAs (lncRNAs) play a pivotal role in muscle injury and are instrumental in skeletal muscle regeneration, a process heavily reliant on satellite cells located beneath the myofiber’s basement membrane (Peault et al., 2007; Tumpel and Rudolph, 2019; Cho et al., 2019). These quiescent progenitors are essential for muscle maintenance, growth, repair, and regeneration. Upon activation, satellite cells (Peault et al., 2007; Tumpel and Rudolph, 2019; Cho et al., 2019; Sirabella et al., 2013) proliferate, differentiate into myoblasts, and fuse to form myotubes. Muscle recovery typically takes 30–90 days, with fundamental repair occurring within 14–30 days. Cell proliferation peaks at 3–4 days, while cell differentiation peaks at 7–10 days (Dumont et al., 2015; Ogawa et al., 2015; Aguilar et al., 2016; Lukjanenko et al., 2013). This timeline remains consistent regardless of the nature or cause of the damage, including that induced by microgravity (Burzyn et al., 2013).
We study the roles and expression patterns of lncRNAs Xist, Pvt1, Gas5, Airn, and Meg3 in skeletal muscle regeneration, we re-annotated and integrated data from GEO datasets GSE3483, GSE38870, GSE56903, GSE70376, and GSE103684 using R and Perl (Figures 1C; Figures 4A). Our analysis showed regular changes in lncRNA expression during muscle repair (Figure 4C). Specifically, lncRNA expression decreased from QSC 0 day to ASC 12 h, then increased from ASC 12 h to ASC 3 days, followed by a gradual decline until ASC 8 days, returning to baseline levels (Figures 4C, D). Notably, lncRNA Meg3 surged (logFC≥3) at ASC 8 days, while lncRNA Xist dropped significantly (logFC ≤ −3) at the same time. Other lncRNAs followed a similar trend (Figures 4C, D).
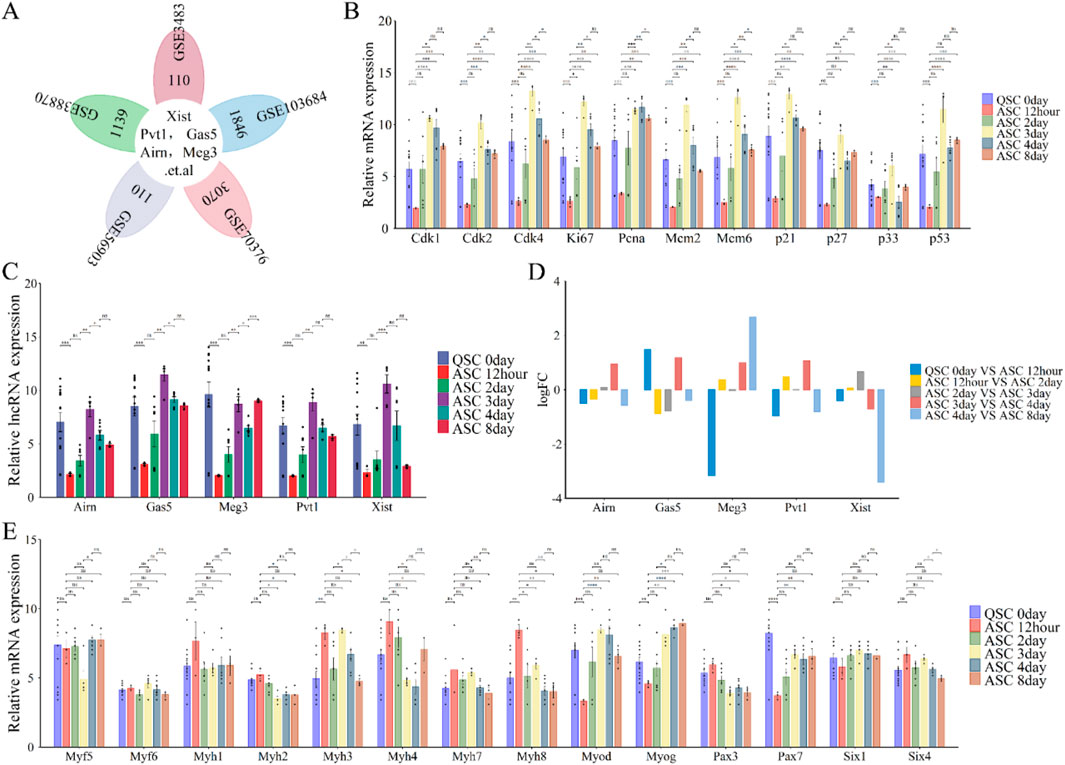
Figure 4. Functional analysis of differentially expressed lncRNAs (lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3) in phase of skeletal muscle regeneration. (A) Flower plot diagram shows the overlap of lncRNAs in GSE3483, GSE38870, GSE56903, GSE70376 and GSE103684 datasets. (B) The expression of cell cycle related protein and inhibitor at skeletal muscle regeneration. (C) The expression of lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3 during E skeletal muscle regeneration. (D) Bar graphs presenting the differentially expressed lncRNAs (lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3) in skeletal muscle development. Bar graphs was used to show the differentially expressed lncRNAs. The y-axis is Log2 fold changes in expression and x-axis is lncRNAs. (E) The expression of key myogenic regulators during skeletal muscle regeneration. The data are presented as the means ± S.D. of the samples from all different samples. The p-values were calculated using Student’s t-test. NS, non-significant; *p < 0.05; **p < 0.01; ***p < 0.0001. QSC, Quiescent satellite cells; ASC, Activated satellite cells.
Cyclins, cell cycle inhibitors, and myogenic markers are crucial for muscle regeneration. Cyclin levels (Cdk1, Cdk2, Cdk4, Ki67, Pcna, Mcm2, and Mcm6)increased significantly from 12 h to 3 days after satellite cell activation (ASC), then gradually declined until 8 days (Figure 4B). Similarly, cell cycle checkpoint proteins p21 and p33 rose sharply from 12 h to 3 days before decreasing by 8 days (Figure 4B). In contrast, p27 and p53 showed a steady increase from 12 h to 8 days, except at the 3-day mark (Figure 4B). Myogenic markers indicated that Pax3, Six4, and Myh2/4/7/8 levels declined during regeneration (Figure 4E), while Pax7, Six1, and Myog increased. Myod and Myh3 levels rose significantly from 12 h to 3 days post-ASC, then decreased from 3 to 8 days (Figure 4E). Myf5/6 and Myh1 expression was absent during regeneration (Figure 4E). These findings suggest that lncRNAs, including Xist, Pvt1, Gas5, Airn, and Meg3, regulate skeletal muscle regeneration. Notably, lncRNA Xist plays a particularly significant role.
LncRNAs such as Xist, Pvt1, Gas5, Airn, and Meg3 (Figure 5A) play a crucial role in regulating cell proliferation during skeletal muscle development and regeneration (Zhao et al., 2019; Luo et al., 2021). This process is essential for muscle growth and repair and precedes cell differentiation. To investigate their roles, we re-annotated and integrated analysis of GEO datasets (GSE989/990, GSE110742, GSE108040, and GSE16992) using R and Perl software (Figures 1C; Figures 5A).
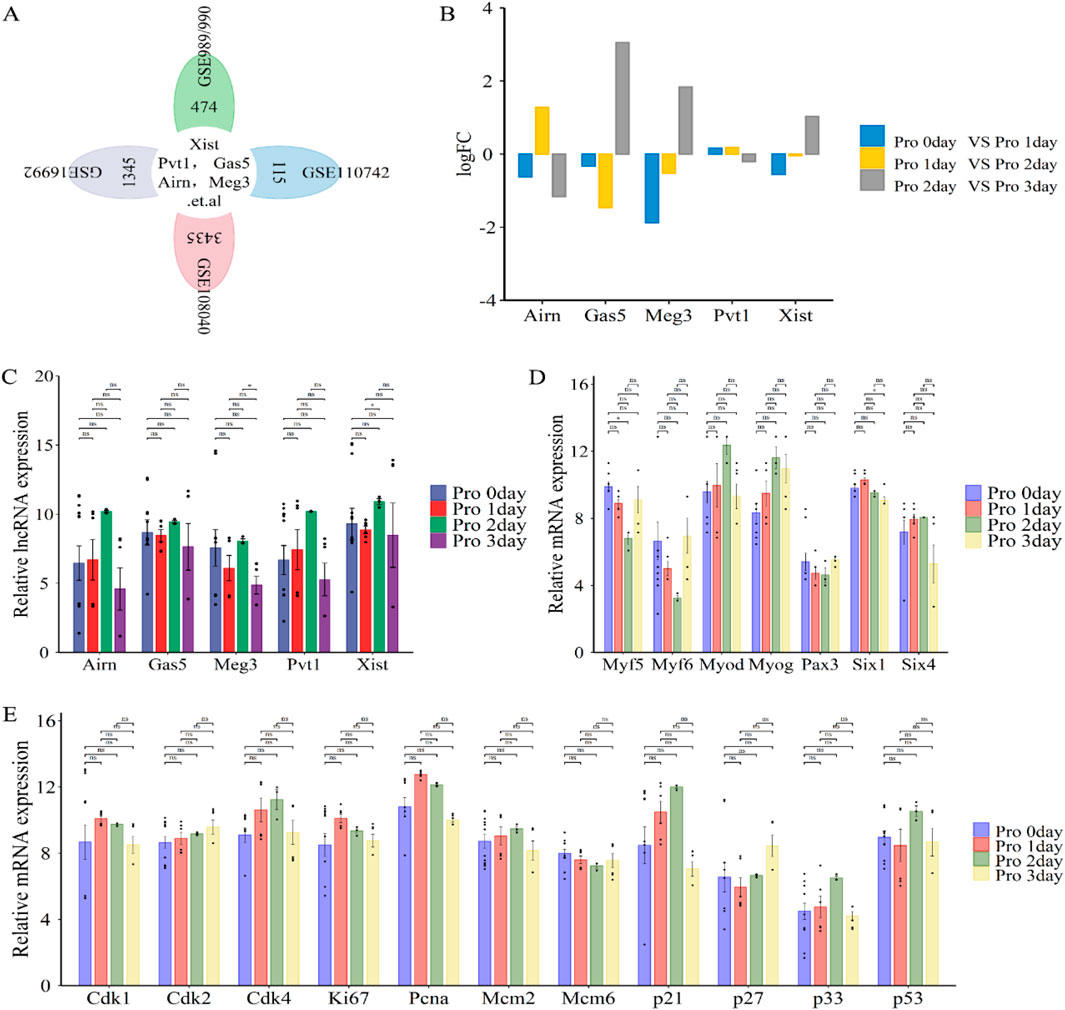
Figure 5. Functional analysis of differentially expressed lncRNAs (lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3) in phase of cell proliferation. (A) Flower plot diagram displays the overlap of lncRNAs in GSE989/990, GSE110742, GSE108040 and GSE16992 datasets. (B) Bar graphs presenting the differentially expressed lncRNAs (lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3) in cell proliferation. Bar graphs was used to illustrate the differentially expressed lncRNAs. The y-axis is Log2 fold changes in expression and x-axis is lncRNAs. (C) The expression of lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3 during cell proliferation. (D) The expression of key myogenic regulators during cell proliferation. (E) The expression of cell cycle related protein and inhibitor during cell proliferation. The data are presented as the means ± S.D. of the samples from all different samples. The p-values were calculated using Student’s t-test. NS, non-significant; *p < 0.05; **p < 0.01; ***p < 0.0001. Pro, proliferation; GSE989/990, GSE989 and GSE990.
Our analysis revealed that lncRNAs like Airn and Pvt1 were significantly upregulated from day 0 to day 2 of proliferation, then downregulated from day 2 to day 3 (Figures 5B, C). Conversely, lncRNAs such as Gas5, Meg3, and Xist showed a decrease from day 0 to day 1, followed by a progressive increase from day 1 to day 2, and were upregulated again from day 2 to day 3 (Figures 5B, C). These fluctuations align with the regulation of cell cycle progression by various proteins, including cyclins, CDKs, and inhibitors like CDK1, CDK2, CDK4, Mcm2, Mcm6, P21, P27, P57, Ki67, and PCNA (Ponnusamy et al., 2017; Mahdessian et al., 2021; Huang et al., 2017). Specifically, Cdk2 and p27 levels increased during the proliferation cycle, while Mcm6 expression notably decreased from day 0 to day 3 of proliferation (Figure 5E). Additionally, Cdk1, Ki67, and Pcna expression peaked at proliferation day 1 and decrease by day 3, while Cdk4, Mcm2, p21, p33, and p53 increased from day 0 to day 2 and then decline by day 3 (Figure 5E). These patterns suggest that muscle cells begin differentiating around day 3. During skeletal muscle cell proliferation, markers such as Six1/4, Pax3, Myf5, Myf6, Myod, and Myog are expressed (Figure 5D). Our analysis showed that Myod, Myog, and Six4 levels increased from day 0 to day 2, and decreased from day 2 to day 3. Six1 peaked at day 1 and declined thereafter. Pax3 and Myf5/6 decreased from day 0 to day 2 and increased from day 2 to day 3 (Figure 5D). This confirmed the transition from proliferation to differentiation. Overall, our findings indicate that lncRNAs, including Xist, Pvt1, Gas5, Airn, and Meg3, may play critical roles in promoting cell proliferation during muscle development or regeneration.
Long non-coding RNAs (lncRNAs) such as Xist, Pvt1, Gas5, Airn, and Meg3 play crucial roles in regulating cell differentiation, which is essential for skeletal muscle development and regeneration (Bentzinger et al., 2012; Hnia et al., 2019; Tomczak et al., 2004; Rajan et al., 2012). To investigate the function and expression of lncRNAs during this process, we re-annotated and analyzed GEO datasets (e.g., GSE989/990, GSE102098, GSE101499) using R and Perl (Figure 1C; Figure 6A). The analysis revealed varying changes in lncRNAs expression throughout cell differentiation (Figures 6C, D).
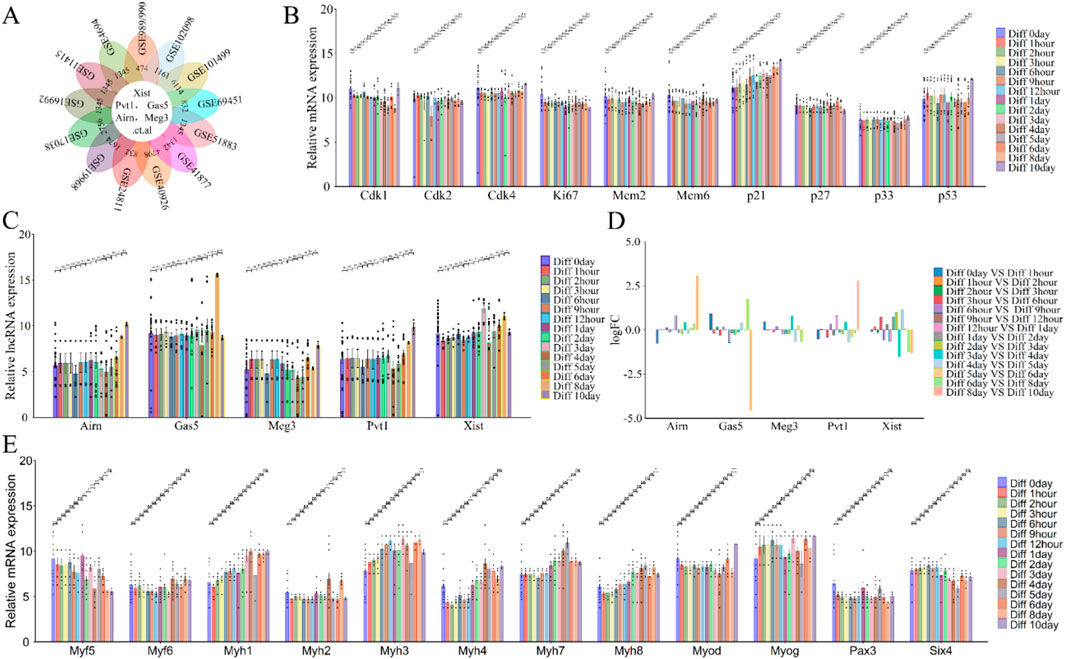
Figure 6. Functional analysis of differentially expressed lncRNAs (lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3) in phase of cell differentiation. (A) Flower plot diagram illustrates the intersection of the 13 datasets analyzed (GSE989/990, GSE102098, GSE101499 and so on). (B) The expression of cell cycle related protein and inhibitor during cell differentiation. (C) The expression of lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3 during cell differentiation. (D) Bar graphs showing the differentially expressed lncRNAs (lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3) in cell differentiation. Bar graphs was used to illustrate the differentially expressed lncRNAs. The y-axis is Log2 fold changes in expression and x-axis is lncRNAs. (E) The expression of key myogenic regulators during cell differentiation. The data are presented as the means ± S.D. of the samples from all different samples. The p-values were calculated using Student’s t-test. NS, non-significant; *p < 0.05; **p < 0.01; ***p < 0.0001. Diff, Differentiation; GSE989/990, GSE989 and GSE990.
From day 0 to day 3 of differentiation, lncRNA Airn and Meg3 expressions decreased, while lncRNA Xist increased; lncRNA Pvt1 and Gas5 showed minimal changes (Figures 6C, D). From day 4 to day 10, lncRNA Xist, Pvt1, Airn, and Meg3 continued to rise, although the increase in Gas5 was less pronounced (Figures 6C, D). Cell differentiation (Ponnusamy et al., 2017; Mahdessian et al., 2021; Huang et al., 2017) is closely linked to cell-cycle exit and is regulated by factors such as cyclins, CDKs, and proliferation markers (CDK1, CDK2, CDK4, Mcm2, Mcm6, P21, P27, P57, Ki67, and Pcna). Our analysis showed that the expression of Cdk1, Ki67, Mcm2, Mcm6, Cdk2, Cdk4, p27, p33, and p53 remained stable during differentiation, while p21 exhibited a consistent upward trend (Figure 6B). Cyclin and differentiation marker analysis confirmed muscle cell differentiation with increased Myf6, Myog, and Myh1/3/4/7/8 levels, and decreased levels of Myf5 and Six4. Myod, Pax3, and Myh2 showed fewer clear trends (Figure 6E). These results indicate that lncRNAs Xist, Pvt1, Gas5, Airn, and Meg3 play roles in muscle cell differentiation, supporting our hypothesis on skeletal muscle development or regeneration.
3.3 LncRNA–miRNA–mRNA ceRNA network construction and visualization
Our research suggests that lncRNAs Xist, Pvt1, Gas5, Airn, and Meg3 may influence various stages of skeletal muscle development, including cell proliferation and differentiation. To explore their roles, we constructed a ceRNA network based on predicted lncRNA-miRNA and miRNA-mRNA interactions from relevant datasets (Figure 7A). We identified 108 miRNAs by intersecting data from miRcode, lncBaseV2, and Enco/PV4 (Figure 7A; Supplementary Table S1). These miRNAs were validated using expression datasets from hindlimb suspension (HS) simulating microgravity. The results showed that miRNA levels, including miR-212-3p, miR-485-3p, miR-342-3p, miR-539-3p, miR-486-5p, and miR-24-3p, decreased gradually from HS 0 to HS 14 days and then quickly returned to baseline during recovery exercise (RE) from RE 1 to RE 7 days. Conversely, miRNAs such as miR-203-3p, miR-539-5p, miR-129-5p, miR-361-5p, and miR-489-5p exhibited opposite trends during HS and RE (Figure 7B; Supplementary Figure S1). These findings suggest that the predicted miRNAs have regulatory potential in skeletal muscle development.
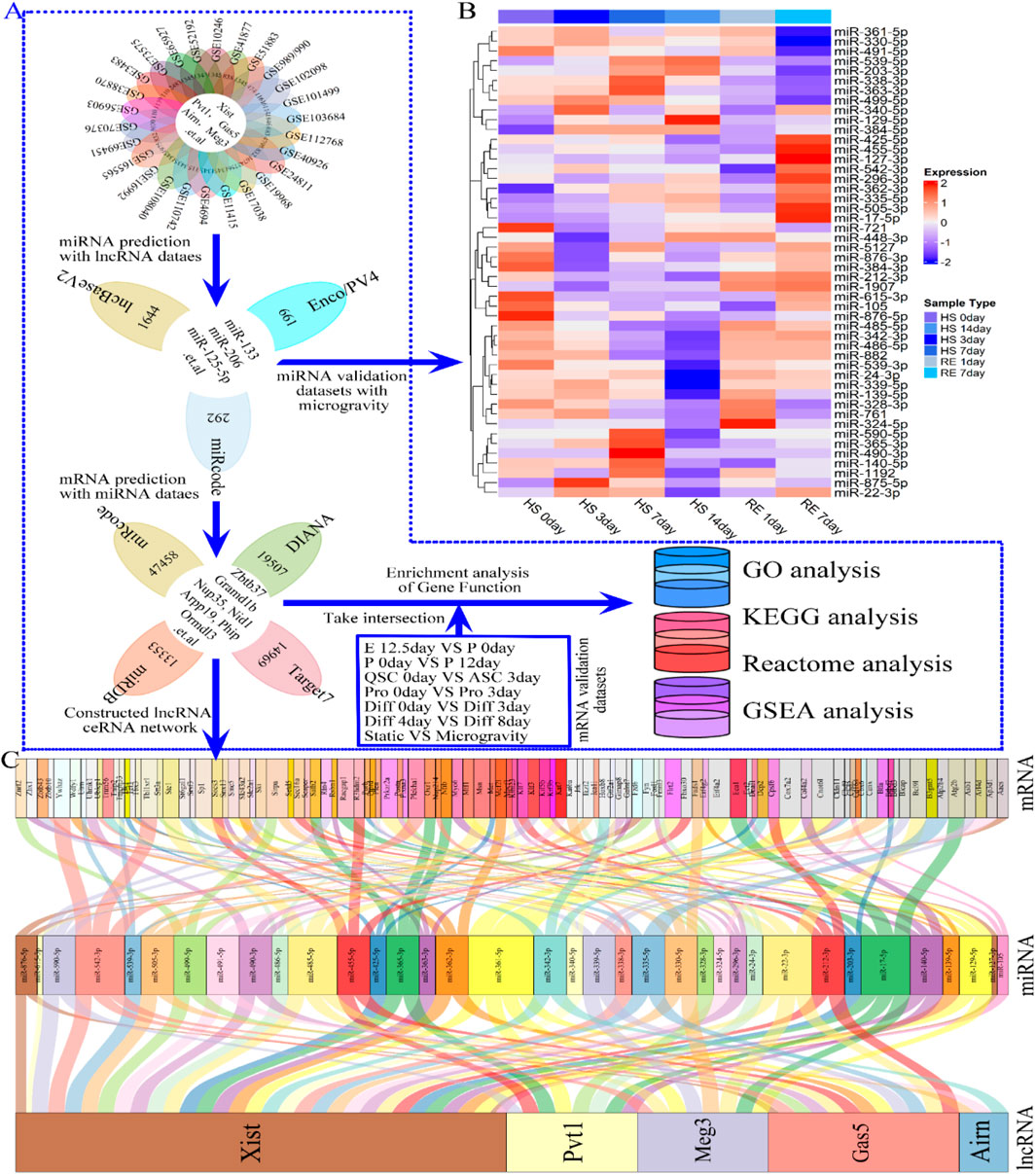
Figure 7. Flowchart indicating the downstream analysis of the lncRNAs (lncRNA Xist, lncRNA Pvt1, lncRNA Gas5, lncRNA Airn and lncRNA Meg3) and Verification of miRNA differential expression. (A) Flow chart of the ceRNA network construction. Three independent lncRNA target databases (miRcode, lncBaseV2 and Enco/PV4) were used to predict the potential miRNAs, and screening for miRNAs highly related to microgravity-induced muscle atrophy or regeneration. Subsequently, four independent miRNAs target databases (miRcode, DIANA, miRDB and Target7) were used to predict the potential mRNAs, and using to constructing lncRNA–miRNA–mRNA ceRNA regulatory network. (B) The heat map showing the differentially expressed miRNA during HS 0day, HS 3 dayS, HS 7 dayS HS 14 dayS, RE 1day and RE 14days (C) Alluvial diagram demonstrates the relationships of lncRNA-miRNA-mRNA ceRNA regulatory network. The interaction network was constructed with there were 5 lncRNAs, 36 miRNAs, and approximately 100 mRNAs. Enco/PV4, ENCORI and NPInterv4; Target7, Targetscan7; GSE989/990, GSE989 and GSE990; HS, hindlimb suspension; RE, Recovery exercise.
By analyzing four datasets (miRcode, DIANA, miRDB, and Target7), we identified almost 12,000 mRNAs (Figure 7A; Supplementary Table S1). We constructed a ceRNA regulatory network with 5 lncRNAs, 36 miRNAs, and 108 mRNAs (Figure 7C), highlighting additional targeting relationships in Supplementary Table S1. Notably, lncRNA Xist (Figure 7C) negatively regulates most miRNAs to boost downstream mRNA expression, influencing skeletal muscle development (e.g., lncRNA Xist/miR-485-5p/Msn and lncRNA Xist/miR-342-3p/Hoxb8). Similar interactions were observed for lncRNAs Airn, Gas5, Meg3, and Pvt1, with specific interactions such as lncRNA Pvt1/miR-455-5p/Fbxo30 and lncRNA Meg3/miR-330-5p/Fads1 (Figure 7C). To investigate how the ceRNA network regulates downstream biological functions and signaling pathways, we conducted GO, Reactome, KEGG, and GSAEA enrichment analyses on the intersection of 12,000 predicted mRNAs and those from each phase of skeletal muscle development using R software (Figure 1C; Figure 7A). Gene network analysis proved to be a valuable tool for studying lncRNA functional changes and understanding selective activation of signaling pathways in skeletal muscle development and regeneration.
3.4 Gene functional enrichment analysis during skeletal muscle development and regeneration
To investigate the interaction and the roles of lncRNAs, we conducted a GO enrichment analysis was conducted on a 5-lncRNA signature (Xist, Pvt1, Gas5, Airn, and Meg3) during skeletal muscle development (Figure 8A). This analysis revealed that these lncRNAs are significantly involved in various biological processes (BP), including muscle development, organ growth, cell proliferation, differentiation, cycle progression, migration, apoptosis, and adaptation. The top twenty BPs are depicted in Figure 8B; Supplementary Figure S2. We further calculated the GeneRatio (enrichment gene count/total gene count) and ranked these ratios to highlight the most significant associations. The bubble chart in Figure 8B; Supplementary Figure S2A shows that the five-lncRNA signature (Figure 8B; Supplementary Figure S2A; Supplementary Figure S8D-I) is strongly associated with metabolic processes, protein secretion, kinase activities, cytokine production, wound healing, DNA repair, mRNA processing, and negative regulation of the cell cycle. Additionally, the cellular component (CC) analysis indicated that these lncRNAs are enriched in several cell membranes, extracellular matrix, transport vesicles, myofibrils, actin cytoskeleton, and microtubules. The top 20 CCs are displayed in Figure 8C; Supplementary Figure S2. The bubble chart in Figure 8C; Supplementary Figure S2B illustrates notable associations with cell-cell junctions, secretory granules, transporter complexes, receptor complexes, nuclear envelopes, transcription regulator complexes, and lysosomes (Figure 8C; Supplementary Figure S2B; Supplementary Figure S2D-I). Finally, molecular function (MF) results showed enrichment in various enzyme activities, receptor ligand activities, transcription coregulator activities, ion channel activities, and transmembrane transporter activities. The top 20 MFs are shown in Figure 8D; Supplementary Figure S2. The bubble chart in Figure 8D; Supplementary Figure S2C highlights significant bindings involving actin-tubulin, Ras GTPase, phospholipids, GTP-ribonucleosides, amides, and nucleosides (Figure 8D; Supplementary Figure S2C; Supplementary Figure S2D-I). Under simulated microgravity, GO enrichment analysis showed a noticeable decrease or disappearance of BP, CC, or MF in muscle cells, as shown in Figure 8; Supplementary Figure S2. These findings indicate dynamic changes in GO functions during skeletal muscle development, underscoring the crucial role of lncRNAs in both muscle development and atrophy.
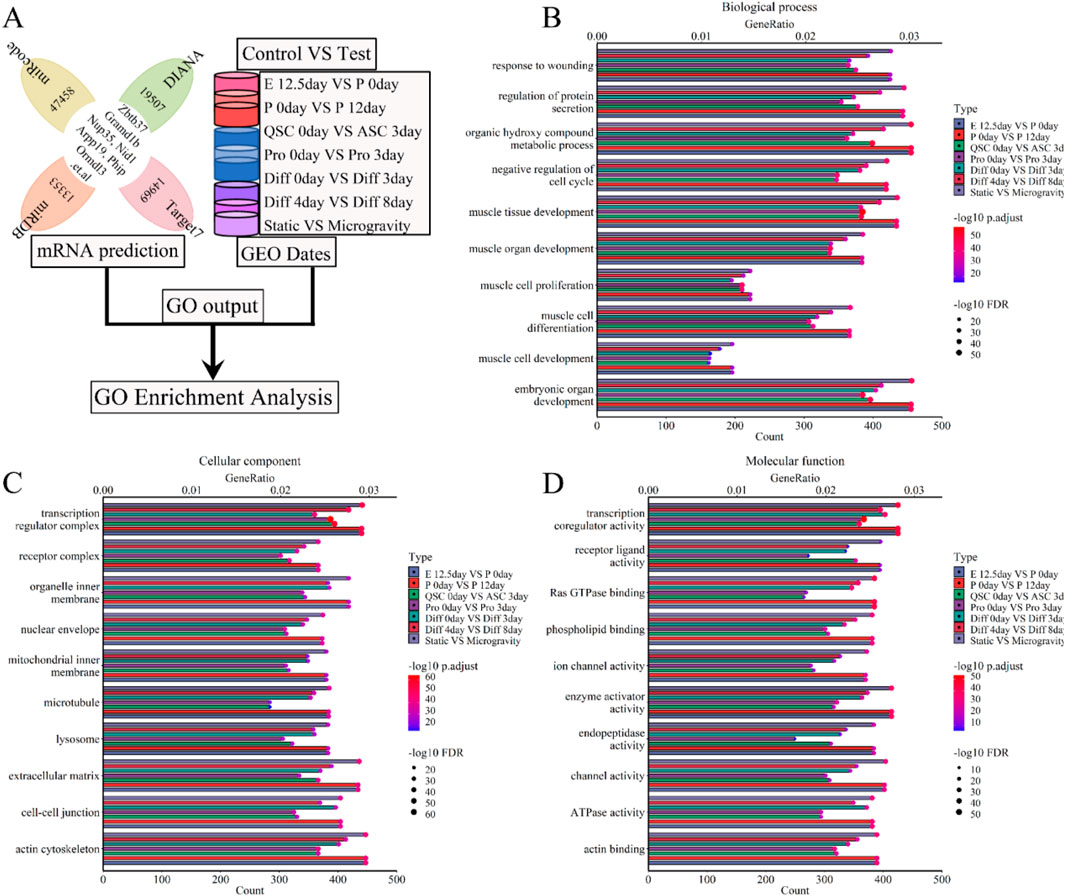
Figure 8. GO enrichment analysis for the lncRNA-miRNA-mRNA ceRNA regulatory network of various stages of skeletal muscle development. (A) The workflow showed that the mRNA expression of various stages of skeletal muscle development were enriched with GO enrichment analysis. (B) The bubble pattern and bar chart show the 10 randomly chosen biological process with GeneRatio and gene count. The skeletal muscle development, wounding respond and protein secretion et al. correlated with gene enrichment. (C) The bubble pattern and bar chart display the 10 randomly chosen cellular component with GeneRatio and gene count. The cell membrane, microtubule, lysosome and mitochondrial et al. associated with gene enrichment. (D) The bubble pattern and bar chart display the 10 randomly chosen molecular function with GeneRatio and gene count. The various enzymes (ubiquitin-protein transferase, ATPase, Ras GTPase and so on), transcription coregulator, transmembrane transporter, cell cycle et,al interrelated with gene enrichment. E, Embryonic; P, Postnatal; QSC, Quiescent satellite cells; ASC, Activated satellite cells; Pro, Proliferation; Diff, Differentiation.
We conducted a Reactome enrichment analysis to elucidate the molecular and biological functions of five lncRNAs (Xist, Pvt1, Gas5, Airn, and Meg3) skeletal muscle development. This analysis intersected predicted mRNAs with those from each phase of muscle development (Figure 9A). The results indicated that these lncRNAs are involved in muscle contraction, cell development, metabolism, and immune response. Thirty selected Reactome pathways are shown in Figures 9B; Supplementary Figure S3. We calculated and ranked the GeneRatio (enrichment gene count/total gene count) and gene count. A bubble chart illustrated the 5-lncRNA signature, showing its significant association with gene expression, small molecule transport, GPCR signaling, stress responses, Rho GTPase signaling, MAPK cascades, RAF/MAP kinase cascade, PIP3-AKT signaling, cell cycle checkpoints, and deubiquitination (Figure 9B; Supplementary Figure S3). Our used Reactome enrichment analysis revealed that cell cycle regulation, including checkpoints and mitotic phases, significantly supports muscle development (P. adjust < 0.05 and FDR < 0.1) (Figure 9B; Supplementary Figure S3). Enzymes related to RHO GTPase, RAF/MAP kinase cascade, MAPK signaling, PIP3-activated AKT signaling, and GPCR signaling also appear to be involved (P.adjust < 0.05 and FDR < 0.1) (Figure 9B; Supplementary Figure S3). Other molecular functions related to skeletal muscle development include the Adaptive Immune System, Deubiquitination, Cytokine Signaling (P.adjust < 0.05 and FDR < 0.1), and Cellular Stress Responses (P.adjust < 0.05 and FDR < 0.05). Reactome enrichment analysis (Figure 9B; Supplementary Figure S3) indicates that simulated microgravity significantly reduced or eliminated the functions and signatures of lncRNAs in muscle cells. LncRNAs Xist, Pvt1, Gas5, Airn, and Meg3 play key roles in cell cycle, metabolism, and transcription. Dysregulation in signal transduction and transcription contributes to muscle atrophy and hinders regeneration. This study highlights that these five lncRNAs significantly influence skeletal muscle development, providing valuable insights for preventing microgravity-induced+ muscle atrophy.
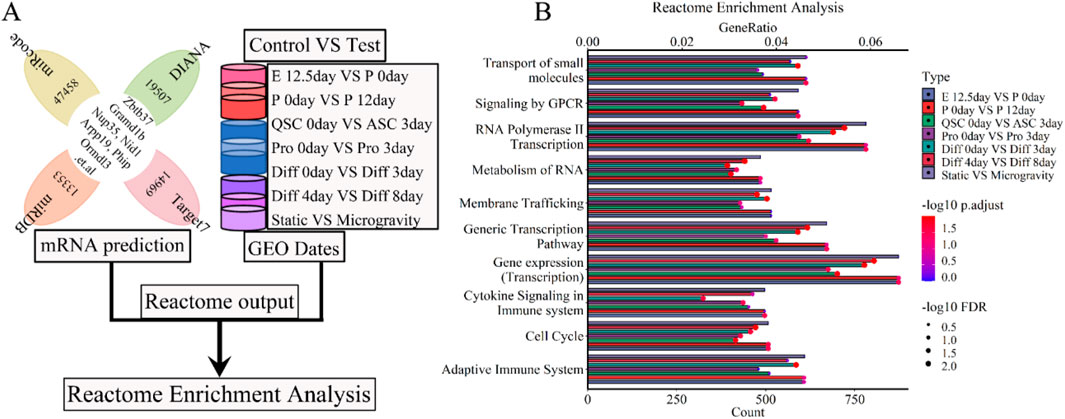
Figure 9. Reactome enrichment analysis for the lncRNA-miRNA-mRNA ceRNA regulatory network of various stages of skeletal muscle development. (A) The workflow showed that the mRNA expression of various stages of skeletal muscle development were enriched with Reactome enrichment analysis. (B) The bubble pattern and bar chart display the 10 randomly chosen molecular function with GeneRatio and gene count. The various enzymes (Rho GTPases, MAPK family signaling cascades, RAF/MAP kinase cascade, PIP3 activates AKT signaling, deubiquitination and so on), cell cycle, cell cycle checkpoints, metabolism, immune system et,al interrelated with gene enrichment. E, Embryonic; P, Postnatal; QSC, Quiescent satellite cells; ASC, Activated satellite cells; Pro, Proliferation; Diff, Differentiation.
Our analysis revealed the interdependence of molecular functions and signaling pathways in biological processes, where elements like lncRNA, miRNA, mRNA, proteins, and enzymes function as signaling molecules or regulators of downstream signaling pathways (Caffaratti et al., 2021). We conducted a KEGG enrichment analysis to clarify the roles of five lncRNAs (Xist, Pvt1, Gas5, Airn, Meg3) in skeletal muscle development, utilizing both predicted mRNAs and mRNAs from each developmental stage (Figure 10A). The analysis revealed enrichment in pathways including Carbon metabolism and the Cell cycle. Figure 10B; Supplementary Figure S4 depict thirty selective KEGG pathways. GeneRatio was calculated as the ratio of enriched gene count to total gene count, followed by ranking of these counts. The bubble chart (Figure 10B; Supplementary Figure S4) highlights significant associations of the 5-lncRNA signature with several signaling pathways including PI3K-Akt, MAPK, Calcium, Ras, Rap, mTOR, FoxO, TNF, TGF-beta, Hippo, and AMPK. Simulated microgravity conditions led to a notable reduction or loss of lncRNA functions and signatures in muscle cells, as evidenced by KEGG enrichment analysis (Figure 10B; Supplementary Figure S4). These results indicate that lncRNAs are instrumental in regulating skeletal muscle development and atrophy processes, including cell proliferation, differentiation, and regeneration, as differentially modulated by KEGG pathway analyses.
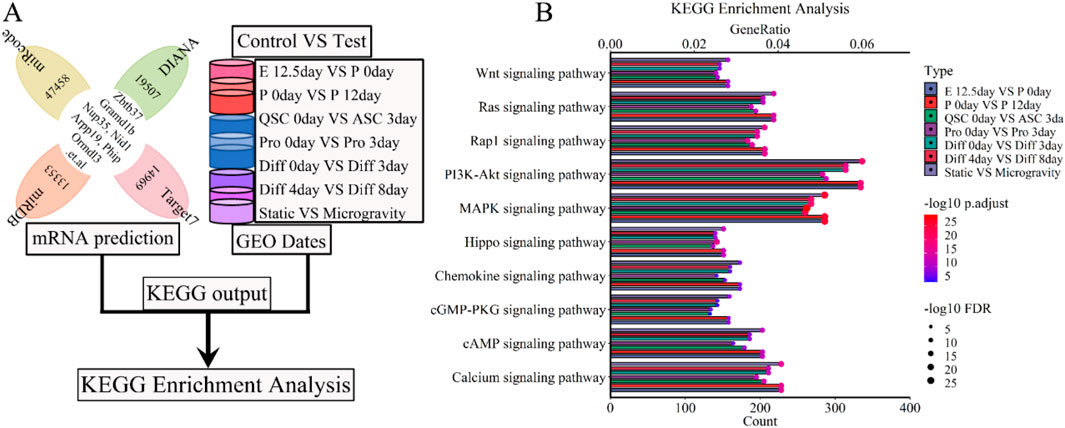
Figure 10. KEGG enrichment analysis for the lncRNA-miRNA-mRNA ceRNA regulatory network of various stages of skeletal muscle development. (A) The workflow indicated that the mRNA expression of various stages of skeletal muscle development were enriched with KEGG enrichment analysis. (B) The bubble pattern and bar chart show the 10 randomly chosen signaling pathway with GeneRatio and gene count. The PI3K-Akt, MAPK, Calcium, Ras, Rap signaling pathway et al. involved in regulating various stages of skeletal muscle development and muscle atrophy (waste). E, Embryonic; P, Postnatal; QSC, Quiescent satellite cells; ASC, Activated satellite cells; Pro, Proliferation; Diff, Differentiation.
To further validate the association of signaling pathways with the five lncRNAs (Xist, Pvt1, Gas5, Airn, and Meg3), we conducted Gene Set Enrichment Analysis (GSEA), a standard tool for assessing gene set enrichment (Wilson et al., 2010). This analysis involved the intersection of predicted mRNA from each phase of skeletal muscle development, as illustrated in the flow chart (Figure 11A). GSEA results indicated that the lncRNA signature was enriched in pathways related to muscle atrophy, focal adhesion, autophagy, actin cytoskeleton, apoptosis, ubiquitin, cell growth, carbon metabolism, and lysosome, among others. Figure 11B; Supplementary Figure S5 display 25 randomly selected pathways. We calculated and ranked the GeneRatio and gene count, then used a bubble chart to visualize the 5-lncRNA signature based on these metrics. The bubble chart shows that the 5-lncRNA signature (Figure 11B; Supplementary Figure S5) is strongly linked to various signaling pathways, including Wnt, Hippo, and others. We aimed to assess whether variations in these pathways occur at different stages of skeletal muscle development and further analyzed their association with muscle development using GSEA. We found that the Wnt, Hippo, and AMPK signaling pathways (P.adjust ≤ 0.05 and FDR ≤ 0.05) regulate differentiation from day 0 to day 3 (Figures 11B, C, H; Supplementary Figure S5A, F, W). Chemokine and Apelin pathways (P.adjust ≤ 0.005 and FDR ≤ 0.005) show regulatory functions at embryonic day 12.5 versus postnatal day 0, postnatal day 0 versus day 12, and from differentiation day 0 to day 3 (Figures 11B, I; Supplementary Figure S5A, B, D, F, V). Similar results were observed in each stage of skeletal muscle development. For instance, the cAMP (P.adjust ≤ 0.005 and FDR ≤ 0.005) and Calcium (P.adjust ≤ 0.05 and FDR ≤ 0.05) signaling pathways (Figures 10B, K, L; Supplementary Figure S5B, E) mediate cell proliferation and embryonic development. The Insulin (P.adjust ≤ 0.05 and FDR ≤ 0.05) signaling pathway was involved in cell proliferation and early differentiation (Supplementary Figure S5A, E, F, O). Additionally, the NOD-like receptor, JAK-STAT and NF-kappa B (P.adjust ≤ 0.005 and FDR ≤ 0.005) signaling pathway (Figures 11B, E, G; Supplementary Figure S5A, B, D, E, M) contributed to embryonic development, muscle regeneration and cell proliferation. Classical pathways such as PI3K-Akt and FoxO (P.adjust ≤ 0.05 and FDR ≤ 0.05) participated in postnatal growth, cell proliferation and differentiation (Figures 11B, D; Supplementary Figures S5A, B, D, E, F, P). Furthermore, the MAPK or cGMP-PKG (P.adjust ≤ 0.05 and FDR ≤ 0.05) signaling pathway was involved in embryonic development, cell proliferation and differentiation (Figures 11B, F, J; Supplementary Figure SB, E, F). Additionally, the TNF signaling pathway (P. adjust ≤ 0.005 and FDR ≤ 0.005) and Focal adhesion pathway (P. adjust ≤ 0.05 and FDR ≤ 0.05) facilitated embryonic development and postnatal growth, and were subsequently employed in cell regeneration and proliferation, differentiation respectively (Supplementary Figure S5A-C, E-G, I, Q2). The Cytokine-cytokine receptor interaction pathway (P. adjust ≤ 0.05 and FDR ≤ 0.05) influenced all processes except postnatal growth (Supplementary Figure S5A, B, D-H, R), while the Cell Cycle pathway (P. adjust ≤ 0.05 and FDR ≤ 0.05) governed the entire skeletal muscle development phase (Supplementary Figure S5A-H, T). Additional GSEA results are in Figure 11; Supplementary Figure S5. Under simulated microgravity, GSEA results showed a significant reduction or loss in the molecular functions of several signal transduction pathways involved in skeletal muscle development, including Wnt, Hippo, Chemokine, Calcium, JAK-STAT, PI3K-Akt, FoxO, MAPK, Focal adhesion, Cytokine-cytokine receptor interaction, and Cell Cycle (P. adjust ≥ 0.1 and FDR ≥ 0.1) (Figure 11B; Supplementary Figure S5). These findings demonstrate that the signaling pathway plays a role in skeletal muscle development and offers new insights and prevention strategies for microgravity-induced muscle atrophy.
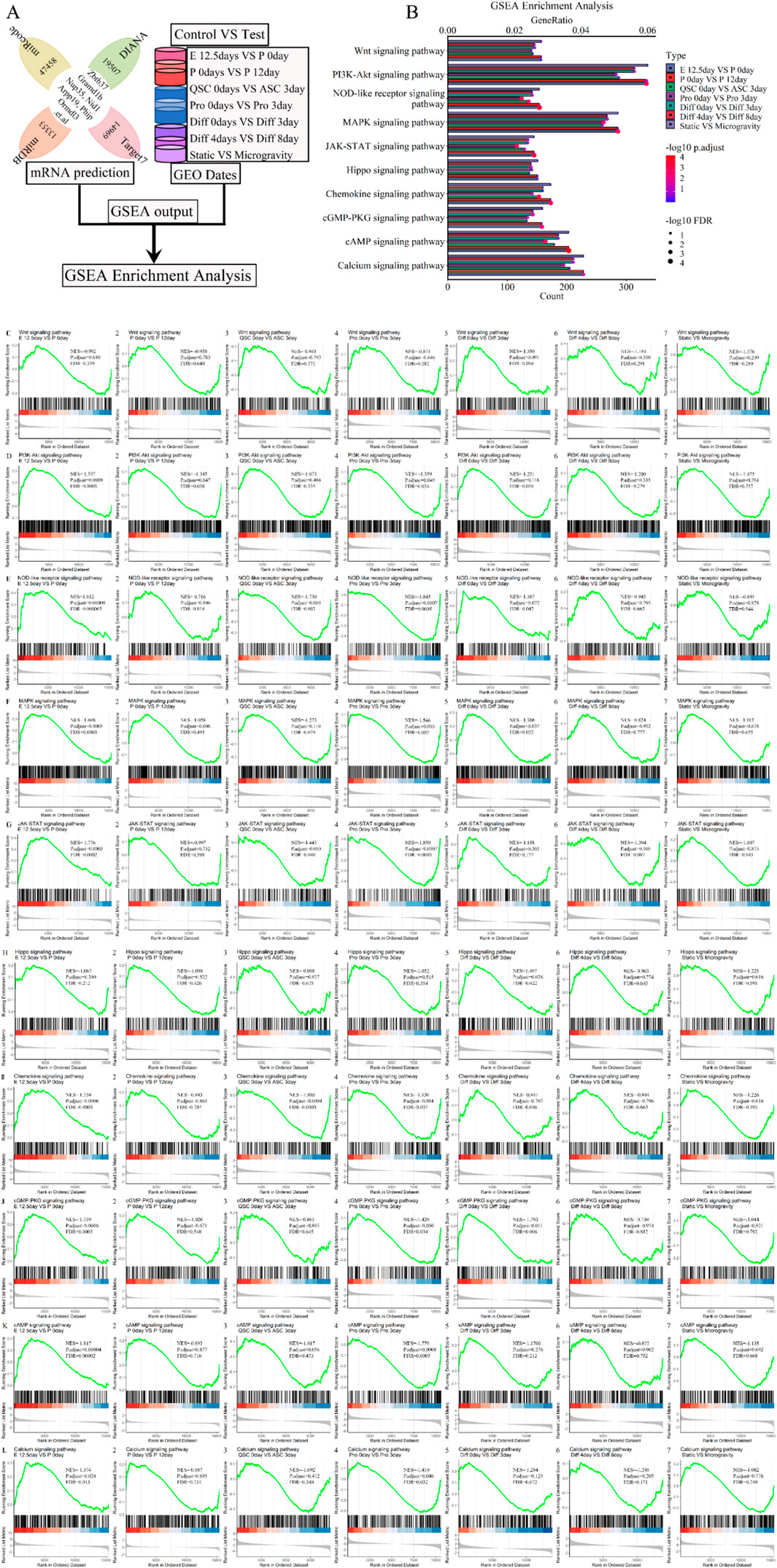
Figure 11. GSEA enrichment analysis for the lncRNA-miRNA-mRNA ceRNA regulatory network of various stages of skeletal muscle development. (A) The workflow indicated that the mRNA expression of various stages of skeletal muscle development were enriched with GSEA enrichment analysis. (B) The bubble pattern and bar chart show the 10 randomly chosen signaling pathway with GeneRatio and gene count. The Wnt, Hippo, Ampk, Chemokine, Apelin, PI3K-Akt, MAPK, Calcium, Ras, Rap signaling pathway et al. involved in regulating various stages of skeletal muscle development and muscle atrophy (waste). GSEA is a method that determines whether a set of genes shows differences between two biological states. The normalized enrichment score (NES) reflects the degree to which a gene set is upregulated (positive NES) or downregulated (negative NES). Corresponding p values are indicated. (C) GSEA plots showing enrichment of Wnt signaling pathway during skeletal muscle development and microgravity-induced muscle atrophy (waste). (D) GSEA plots showing enrichment of PI3K-Akt signaling pathway during skeletal muscle development and microgravity-induced muscle atrophy (waste). (E) GSEA plots showing enrichment of NOD-like receptor signaling pathway during skeletal muscle development and microgravity-induced muscle atrophy (waste). (F) GSEA plots showing enrichment of MAPK signaling pathway during skeletal muscle development and microgravity-induced muscle atrophy (waste). (G) GSEA plots showing enrichment of JAK-STAT signaling pathway during skeletal muscle development and microgravity-induced muscle atrophy (waste). (H) GSEA plots showing enrichment of Hippo signaling pathway during skeletal muscle development and microgravity-induced muscle atrophy (waste). (I) GSEA plots showing enrichment of Chemokine signaling pathway during skeletal muscle development and microgravity-induced muscle atrophy (waste). (J) GSEA plots showing enrichment of cGMP-PKG signaling pathway during skeletal muscle development and microgravity-induced muscle atrophy (waste). (K) GSEA plots showing enrichment of cAMP signaling pathway during skeletal muscle development and microgravity-induced muscle atrophy (waste). (L) GSEA plots showing enrichment of Calcium signaling pathway during skeletal muscle development and microgravity-induced muscle atrophy (waste). Normalized enrichment scores (NESs), FDR and nominal p values (Nom p value), as calculated by GSEA, are provided. skeletal muscle development stages contain E12.5 days VS P 0days, P 0days VS P 12 days, QSC 0days VS ASC 3 days, Pro 0days VS Pro 3 days, Diff 0 days VS Diff 3 days and Diff 4 days VS Diff 8 days. Microgravity-induced muscle atrophy (waste) is Static VS Microgravity. E, Embryonic; P, Postnatal; QSC, Quiescent satellite cells; ASC, Activated satellite cells; Pro, Proliferation; Diff, Differentiation.
4 Discussion
Skeletal muscle development (Salazar et al., 2016) is crucial for normal mouse growth and motor function. As the body’s largest organ, skeletal muscle regulates metabolism and energy balance under various stress conditions like microgravity and inflammation (Pant et al., 2016; Egan and Zierath, 2013). Our study integrates existing datasets to uncover novel lncRNAs related to skeletal muscle development, including Xist, Pvt1, Gas5, Airn, and Meg3, thereby fostering new hypotheses and enhancing our understanding of their molecular functions.
By analyzing 26 GEO datasets using R and Perl, we identified lncRNAs Xist, Pvt1, Gas5, Airn, and Meg3, likely due to the early publication dates (2004) and low-depth sequencing of the datasets. Notably, lncRNAs Airn, Gas5, and Xist have been implicated in skeletal muscle development and regeneration. Tiffany et al. (Dill et al., 2021) reported that lncRNA Meg3 regulates EMT to support myoblast differentiation, while Liu et al. (Liu et al., 2019b) found it promotes bovine skeletal muscle differentiation through the miR-135-MEF2C pathway. Enrico et al. (Alessio et al., 2019) suggested that lncRNA Pvt1 accelerates early muscle atrophy by affecting mitochondrial respiration, morphology, and influencing mito/autophagy, apoptosis, and myofiber size in vivo. Our findings, derived from analyzing 26 datasets, align with previous studies and confirm the reliability of our results. Despite the risk of filtering out many lncRNAs and potential artefacts, our integrated analysis overcomes these limitations by pooling samples, re-annotating, analyzing all datasets, and removing batch effects. This approach minimizes single-analysis risk and human interference, allowing us to map the global response of lncRNAs Xist, Pvt1, Gas5, Airn, and Meg3 to various skeletal muscle development phases.
Studies on lncRNAs primarily focus on their ceRNA networks with miRNAs and mRNAs, influencing cell growth, development, and diseases such as muscle atrophy and cancer (Winkle et al., 2021; Thomson and Dinger, 2016; Qi et al., 2020; Matsui and Corey, 2017). Under microgravity, significant changes in lncRNAs provide new insights into skeletal muscle development. We identified relevant lncRNAs and constructed a ceRNA network by predicting their interactions with miRNAs and mRNAs. The predicted miRNAs, known to regulate muscle function (Horak et al., 2016), were also observed in a microgravity-induced muscle regeneration model, including miR-206, miR-133, miR-499, and miR-23-5p (Supplementary Figure S1). We examined several ceRNA regulatory networks in muscle tissue or cells, including lncRNA Xist/miR-499-5p/znrf2, lncRNA Pvt1/miR-140-5p/Co14a2, lncRNA Meg3/miR-330-5p/Fads1, lncRNA Gas5/miR-362-3p/Mcf21, and lncRNA Airn/miR-342-3p/Hoxb8 (Figure 7C; Supplementary Table S1). Cheng et al. (Cheng et al., 2020) and Wang et al. (Wang et al., 2021). showed that lncRNA Xist/miR-126 enhances cell proliferation and glucose metabolism via the IRS1/PI3K/Akt pathway in glioma. Liao et al. (Liao et al., 2019) and Wang et al. (Wang et al., 2021) found that lncRNA Xist/miR-17-5P/AHNAK inhibits OPLL by activating the BMP2 signaling pathway in primary human ligament fibroblast cells. Xiong et al. (Xiong et al., 2021) and Wang et al. (Wang et al., 2021) indicated that lncRNA Xist/miR-486-5p/GAB2 promotes cerebral ischemia-reperfusion injury in SH-SY5Y cells. Similar studies have been conducted on other lncRNAs. Wu et al. (Wu et al., 2019) found that lncRNA Gas5/miR-335/ROCK1 alleviates myocardial ischemia/reperfusion injury via the AKT/GSK-3β axis. Chi et al. (Chi et al., 2019) demonstrated that lncRNA Gas5/miR-455-5p/SOCS3 promotes M1 macrophage polarization by activating the JAK2/STAT3 pathway in childhood pneumonia. Xi et al. (Xi et al., 2020) showed that lncRNA Pvt1/miR-148/RAB34 enhances cell proliferation and migration in NSCLC progression. Our findings align with previous research (Figure 7C; Supplementary Table S1) on constructing a ceRNA regulatory network using R and Perl, validating our analysis. Our results suggest that lncRNAs likely collaborate with miRNAs, mRNAs, or other cytokines, rather than acting independently, in regulating physiological and biochemical functions.
The ceRNA regulatory network of lncRNA-miRNA-mRNA underpins its cellular regulatory role. Our analysis shows it primarily engages in biological processes like muscle and organ development, cell proliferation and differentiation, wound response, cell cycle, and protein secretion regulation (see Figures 8, 9; Supplementary Figure S2, 3). This activated various enzymes, proteins, transcription pathways, and cell cytokines, including ATPase, GTPase, PIP3 kinase, MAPK kinase, GPCR kinase, ion channel proteins, and cell cycle proteins, across different organelle membranes, endoplasmic reticulum, cytoskeleton, cell adhesion sites, and both inside and outside the nucleus (Figure 8; Figure 9; Supplementary Figure S2; Supplementary Figure S3). Additionally, studies (Pedersen and Febbraio, 2012; Korotkova and Lundberg, 2014; von Haehling et al., 2017; Cohen et al., 2015) indicate that muscle cells secrete cytokines and proteases that influence skeletal muscle development by activating downstream signaling molecules. Research by Chen et al. (Chen et al., 2020a; Chen et al., 2020b), Wang et al. (Wang et al., 2019b), and Luo et al. (Luo et al., 2021) further shows that the ceRNA regulatory network of lncRNA-miRNA-mRNA supports muscle development processes such as cell proliferation, differentiation, regeneration, and atrophy by regulating downstream signaling pathways. Our findings closely matched current data, validating our GO and reactome analysis. Ultimately, we identified the ceRNA regulatory network of lncRNA-miRNA-mRNA as a key facilitator of skeletal muscle development in terms of biological processes, cellular components, and molecular functions.
Signaling pathways within the ceRNA regulatory network of lncRNA-miRNA-mRNA are vital for controlling cell growth, proliferation, differentiation, and energy metabolism. Our analysis identified several key pathways—cell cycle, PI3K/Akt, TNF, Wnt, Insulin, IGF1, MAPK, NF-κB, Apoptosis, Focal adhesion, Calcium, TGF-beta, cytokine-cytokine receptor interaction, neuroactive ligand-receptor interaction, chemokine, actin cytoskeleton regulation, and protein processing in the endoplasmic reticulum—that may impact muscle diseases and skeletal muscle development (Figures 10, 11; Supplementary Figure S4, 5). Previous research has shown that pathways such as PI3K/Akt, TNF, Wnt, Insulin, IGF1, MAPK, and NF-κB regulate skeletal muscle development (Egan and Zierath, 2013; Braun and Gautel, 2011; Egan et al., 2016). Misregulation of these pathways can lead to muscle-related conditions like sarcopenia and atrophy, often caused by cancer, diabetes, chronic nephritis, and microgravity (von Haehling et al., 2017; Cohen et al., 2015; Wang and Mitch, 2014; Sharma et al., 2015; Han and Mitch, 2011). Our study supports these findings, revealing that these pathways exhibit contrasting roles at different developmental stages (Figures 10, 11; Supplementary Figure S4, 5). For example, the PI3K/Akt pathway is active during embryonic development, postnatal growth, and muscle cell proliferation but inactive at other phases. Conversely, the MAPK pathway is upregulated during embryonic development, cell proliferation, and differentiation but downregulated at other times.
The Hippo, AMPK, NF-κB, and cAMP signaling pathways show varied activation during different stages of muscle development, while the cell cycle pathway consistently supports the entire process (Figures 10, 11; Supplementary Figure S4, 5). The AMPK pathway’s (Thomson, 2018) role in muscle development is hindered under microgravity, potentially causing muscle atrophy (Figures 10, 11; Supplementary Figure S4, 5). Studies have shown that this pathway negatively affects muscle size, hypertrophy, and regeneration (Thomson, 2018). The Hippo signaling network is crucial for myogenesis, regeneration, muscular dystrophy, and rhabdomyosarcoma (Wackerhage et al., 2014; Watt et al., 2018), (Wackerhage et al., 2014; Watt et al., 2018). Additionally, NF-κB and cAMP signaling pathways regulate muscle development through cell proliferation and differentiation (Peterson et al., 2011; Ravnskjaer et al., 2016) (Peterson et al., 2011; Ravnskjaer et al., 2016), while PI3K/Akt and MAPK also play significant regulatory roles (Cohen et al., 2015; Segales et al., 2016), (Cohen et al., 2015; Segales et al., 2016). The cell cycle pathway regulates cell growth, muscle development, proliferation, differentiation, and pathology (Adhikari et al., 2019; Bischoff, 1990; Ciemerych et al., 2011; Halevy et al., 1995). Our KEGG and GSEA enrichment analysis confirmed these findings, enhancing the reliability of our results.
We identified distinct roles for lncRNAs Xist, Pvt1, Gas5, Airn, and Meg3 in muscle development and related pathologies. Demonstrating that lncRNAs can protect against muscle atrophy caused by simulated weightlessness opens new research possibilities. More data and clinical experiments are needed to test our hypothesis, along with comprehensive technical analyses using multi-omics technologies and single-cell RNA sequencing to uncover functional changes in noncoding RNA, proteins, proteases, cytokinesis, and kinase in muscle development. Our multilevel data-integration approach, using extensive mouse studies and Affymetrix chip datasets analyzed with R or Perl, will advance noncoding RNA research and develop new lncRNA-based therapies.
5 Conclusion
We demonstrated that skeletal muscle development and atrophy can be precisely controlled using a 5-lncRNA signature (Xist, Pvt1, Gas5, Airn, Meg3). Our findings suggest that well-regulated signaling pathways are essential for stable muscle development. However, further validation in larger independent studies is necessary to confirm our results.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
WW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. YC: Investigation, Methodology, Project administration, Software, Validation, Writing–review and editing. HY: Formal Analysis, Investigation, Methodology, Supervision, Validation, Writing–review and editing. LW: Investigation, Software, Validation, Visualization, Writing–review and editing. ZN: Investigation, Methodology, Project administration, Writing–review and editing. HQ: Software, Supervision, Validation, Visualization, Writing–review and editing. WS: Formal Analysis, Investigation, Methodology, Validation, Writing–review and editing. YQ: Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing–review and editing. YS: Funding acquisition, Software, Supervision, Writing–review and editing. ZF: Resources, Writing–review and editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration. ZL: Conceptualization, Formal Analysis, Investigation, Methodology, Resources, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Guizhou Provincial Basic Research Program (Natural Science), [No. QiankeHeJiChu-ZK (2024)-YiBan-659], The Youth Science and Technology Talent Growth Project of Guizhou Province Education Department [No. (2024)262], The Special Funds for Local Scientific and Technological Development Guided by the Central Government from Guizhou Science, grant number: [(2019)4006], The Engineering Research Center supported by Guizhou Provincial Education Department, grant number: [KY(2020)022], The Moutai Institute high-level talents research fund project, grant number: [mygccrc (2022)087], The Natural Science Foundation of Hunan Province, grant number: (CX20190003), the Research Foundation for Scientific Scholars of Moutai Institute, grant number: [mygccrc (2023)011].
Acknowledgments
The authors would like to thank all the members of the research groups of Zhu Lingyun. We apologize to colleagues whose work unable to cite or discuses due to space limitations. Finally, we thank all the authors who made their GEO datasets freely available, and would like to thank all authors of references.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbinf.2024.1494717/full#supplementary-material
Abbreviations
LncRNAs, long noncoding RNAs; GEO, Gene Expression Omnibus; DEG, differentially expressed gene; DMD, Duchenne Muscular Dystrophy; GO, Gene Ontology; BP, biological processes; CC, cellular component; MF, molecular function; KEGG, Kyoto Encyclopedia of Genes and Genomes; GeneRatio, enrichment gene count/total gene count; ceRNA, Competing Endogenous RNA; Xist, X-inactive specific transcript.
References
Adhikari, A., Mainali, P., and Davie, J. K. (2019). JARID2 and the PRC2 complex regulate the cell cycle in skeletal muscle. J. Biol. Chem. 294 (51), 19451–19464. doi:10.1074/jbc.ra119.010060
Agarwal, V., Bell, G. W., Nam, J. W., and Bartel, D. P. (2015). Predicting effective microRNA target sites in mammalian mRNAs. Elife 4, e05005. doi:10.7554/elife.05005
Aguilar, C. A., Pop, R., Shcherbina, A., Watts, A., Matheny, R., Cacchiarelli, D., et al. (2016). Transcriptional and chromatin dynamics of muscle regeneration after severe trauma. Stem Cell Rep. 7 (5), 983–997. doi:10.1016/j.stemcr.2016.09.009
Alessio, E., Buson, L., Chemello, F., Peggion, C., Grespi, F., Martini, P., et al. (2019). Single cell analysis reveals the involvement of the long non-coding RNA Pvt1 in the modulation of muscle atrophy and mitochondrial network. Nucleic Acids Res. 47 (4), 1653–1670. doi:10.1093/nar/gkz007
Ballarino, M., Morlando, M., Fatica, A., and Bozzoni, I. (2016). Non-coding RNAs in muscle differentiation and musculoskeletal disease. J. Clin. Invest 126 (6), 2021–2030. doi:10.1172/jci84419
Bentzinger, C. F., Wang, Y. X., and Rudnicki, M. A. (2012). Building muscle: molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 4 (2), a008342. doi:10.1101/cshperspect.a008342
Bischoff, R. (1990). Cell cycle commitment of rat muscle satellite cells. J. Cell Biol. 111 (1), 201–207. doi:10.1083/jcb.111.1.201
Braun, T., and Gautel, M. (2011). Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 12 (6), 349–361. doi:10.1038/nrm3118
Bricchi, I., Bertea, C. M., Occhipinti, A., Paponov, I. A., and Maffei, M. E. (2012). Dynamics of membrane potential variation and gene expression induced by Spodoptera littoralis, Myzus persicae, and Pseudomonas syringae in Arabidopsis. PLoS One 7 (10), e46673. doi:10.1371/journal.pone.0046673
Burzyn, D., Kuswanto, W., Kolodin, D., Shadrach, J., Cerletti, M., Jang, Y., et al. (2013). A special population of regulatory T cells potentiates muscle repair. Cell 155 (6), 1282–1295. doi:10.1016/j.cell.2013.10.054
Butchart, L. C., Fox, A., Shavlakadze, T., and Grounds, M. D. (2016). The long and short of non-coding RNAs during post-natal growth and differentiation of skeletal muscles: focus on lncRNA and miRNAs. Differentiation 92 (5), 237–248. doi:10.1016/j.diff.2016.05.003
Caffaratti, C., Plazy, C., Mery, G., Tidjani, A. R., Fiorini, F., Thiroux, S., et al. (2021). What we know so far about the metabolite-mediated microbiota-intestinal immunity dialogue and how to hear the sound of this crosstalk. Metabolites 11 (6), 406. doi:10.3390/metabo11060406
Chal, J., and Pourquie, O. (2017). Making muscle: skeletal myogenesis in vivo and in vitro. Development 144 (12), 2104–2122. doi:10.1242/dev.151035
Chen, I. H., Huber, M., Guan, T., Bubeck, A., and Gerace, L. (2006). Nuclear envelope transmembrane proteins (NETs) that are up-regulated during myogenesis. BMC Cell Biol. 7 (38), 38–16. doi:10.1186/1471-2121-7-38
Chen, L. L. (2016). Linking long noncoding RNA localization and function. Trends Biochem. Sci. 41 (9), 761–772. doi:10.1016/j.tibs.2016.07.003
Chen, R., Jiang, T., She, Y., Xie, S., Zhou, S., Li, C., et al. (2018a). Comprehensive analysis of lncRNAs and mRNAs with associated co-expression and ceRNA networks in C2C12 myoblasts and myotubes. Gene 647, 164–173. doi:10.1016/j.gene.2018.01.039
Chen, R., Jiang, T., She, Y., Xie, S., Zhou, S., Li, C., et al. (2018b). Comprehensive analysis of lncRNAs and mRNAs with associated co-expression and ceRNA networks in C2C12 myoblasts and myotubes. Gene 647 (39), 164–173. doi:10.1016/j.gene.2018.01.039
Chen, R., Lei, S., Jiang, T., She, Y., and Shi, H. (2020a). Regulation of skeletal muscle atrophy in cachexia by MicroRNAs and long non-coding RNAs. Front. Cell Dev. Biol. 8 (2020), 577010–577022. doi:10.3389/fcell.2020.577010
Chen, R., Lei, S., Jiang, T., Zeng, J., Zhou, S., and She, Y. (2020b). Roles of lncRNAs and circRNAs in regulating skeletal muscle development. Acta Physiol. (Oxf) 228 (2), e13356. doi:10.1111/apha.13356
Chen, X., He, L., Zhao, Y., Li, Y., Zhang, S., Sun, K., et al. (2017). Malat1 regulates myogenic differentiation and muscle regeneration through modulating MyoD transcriptional activity. Cell Discov. 3 (2017), 17002–17024. doi:10.1038/celldisc.2017.2
Chen, Y., and Wang, X. (2020). miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 48 (D1), D127–D131. doi:10.1093/nar/gkz757
Cheng, Q., Chen, X., Wu, H., and Du, Y. (2021). Three hematologic/immune system-specific expressed genes are considered as the potential biomarkers for the diagnosis of early rheumatoid arthritis through bioinformatics analysis. J. Transl. Med. 19 (1), 18–32. doi:10.1186/s12967-020-02689-y
Cheng, Z., Luo, C., and Guo, Z. (2020). LncRNA-XIST/microRNA-126 sponge mediates cell proliferation and glucose metabolism through the IRS1/PI3K/Akt pathway in glioma. J. Cell Biochem. 121 (3), 2170–2183. doi:10.1002/jcb.29440
Chi, X., Ding, B., Zhang, L., Zhang, J., Wang, J., and Zhang, W. (2019). lncRNA GAS5 promotes M1 macrophage polarization via miR-455-5p/SOCS3 pathway in childhood pneumonia. J. Cell Physiol. 234 (8), 13242–13251. doi:10.1002/jcp.27996
Chini, M., and Hanganu-Opatz, I. L. (2021). Prefrontal cortex development in health and disease: lessons from rodents and humans. Trends Neurosci. 44 (3), 227–240. doi:10.1016/j.tins.2020.10.017
Cho, I. J., Lui, P. P., Obajdin, J., Riccio, F., Stroukov, W., Willis, T. L., et al. (2019). Mechanisms, hallmarks, and implications of stem cell quiescence. Stem Cell Rep. 12 (6), 1190–1200. doi:10.1016/j.stemcr.2019.05.012
Ciemerych, M. A., Archacka, K., Grabowska, I., and Przewoźniak, M. (2011). Cell cycle regulation during proliferation and differentiation of mammalian muscle precursor cells. Results Probl. Cell Differ. 53, 473–527. doi:10.1007/978-3-642-19065-0_20
Ciemerych, M. A., and Sicinski, P. (2005). Cell cycle in mouse development. Oncogene 24 (17), 2877–2898. doi:10.1038/sj.onc.1208608
Cohen, S., Nathan, J. A., and Goldberg, A. L. (2015). Muscle wasting in disease: molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 14 (1), 58–74. doi:10.1038/nrd4467
Dill, T. L., Carroll, A., Pinheiro, A., Gao, J., and Naya, F. J. (2021). The long noncoding RNA Meg3 regulates myoblast plasticity and muscle regeneration through epithelial-mesenchymal transition. Development 148 (2), dev194027. doi:10.1242/dev.194027
Dumont, N. A., Bentzinger, C. F., Sincennes, M. C., and Rudnicki, M. A. (2015). Satellite cells and skeletal muscle regeneration. Compr. Physiol. 5 (3), 1027–1059. doi:10.1002/cphy.c140068
d'Ydewalle, C., Ramos, D. M., Pyles, N. J., Ng, S. Y., Gorz, M., Pilato, C. M., et al. (2017). The antisense transcript SMN-AS1 regulates SMN expression and is a novel therapeutic target for spinal muscular atrophy. Neuron 93 (1), 66–79. doi:10.1016/j.neuron.2016.11.033
Egan, B., Hawley, J. A., and Zierath, J. R. (2016). SnapShot: exercise metabolism. Cell Metab. 24 (2), 342–342.e1. doi:10.1016/j.cmet.2016.07.013
Egan, B., and Zierath, J. R. (2013). Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 17 (2), 162–184. doi:10.1016/j.cmet.2012.12.012
Farina, N. H., Hausburg, M., Betta, N. D., Pulliam, C., Srivastava, D., Cornelison, D., et al. (2012). A role for RNA post-transcriptional regulation in satellite cell activation. Skelet. Muscle 2 (1), 21. doi:10.1186/2044-5040-2-21
Frangini, M., Franzolin, E., Chemello, F., Laveder, P., Romualdi, C., Bianchi, V., et al. (2013). Synthesis of mitochondrial DNA precursors during myogenesis, an analysis in purified C2C12 myotubes. J. Biol. Chem. 288 (8), 5624–5635. doi:10.1074/jbc.m112.441147
Fukada, S., Uezumi, A., Ikemoto, M., Masuda, S., Segawa, M., Tanimura, N., et al. (2007). Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 25 (10), 2448–2459. doi:10.1634/stemcells.2007-0019
Garcia-Prat, L., Martinez-Vicente, M., Perdiguero, E., Ortet, L., Rodríguez-Ubreva, J., Rebollo, E., et al. (2016). Autophagy maintains stemness by preventing senescence. Nature 529 (7584), 37–42. doi:10.1038/nature16187
Garrett-Bakelman, F. E., Darshi, M., Green, S. J., Gur, R. C., Lin, L., Macias, B. R., et al. (2019). The NASA Twins Study: a multidimensional analysis of a year-long human spaceflight. Science 364 (6436), eaau8650. doi:10.1126/science.aau8650
Genchi, G. G., Degl'Innocenti, A., Martinelli, C., Battaglini, M., De Pasquale, D., Prato, M., et al. (2021). Cerium oxide nanoparticle administration to skeletal muscle cells under different gravity and radiation conditions. ACS Appl. Mater Interfaces 13 (34), 40200–40213. doi:10.1021/acsami.1c14176
Goel, A. J., Rieder, M. K., Arnold, H. H., Radice, G. L., and Krauss, R. S. (2017). Niche cadherins control the quiescence-to-activation transition in muscle stem cells. Cell Rep. 21 (8), 2236–2250. doi:10.1016/j.celrep.2017.10.102
Goncalves, T. J. M., and Armand, A. S. (2017). Non-coding RNAs in skeletal muscle regeneration. Noncoding RNA Res. 2 (1), 56–67. doi:10.1016/j.ncrna.2017.03.003
Gong, C., Li, Z., Ramanujan, K., Clay, I., Zhang, Y., Lemire-Brachat, S., et al. (2015). A long non-coding RNA, LncMyoD, regulates skeletal muscle differentiation by blocking IMP2-mediated mRNA translation. Dev. Cell 34 (2), 181–191. doi:10.1016/j.devcel.2015.05.009
Gupta, A., Lutolf, M. P., Hughes, A. J., and Sonnen, K. F. (2021). Bioengineering in vitro models of embryonic development. Stem Cell Rep. 16 (5), 1104–1116. doi:10.1016/j.stemcr.2021.04.005
Halevy, O., Novitch, B. G., Spicer, D. B., Skapek, S. X., Rhee, J., Hannon, G. J., et al. (1995). Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 267 (5200), 1018–1021. doi:10.1126/science.7863327
Han, H. Q., and Mitch, W. E. (2011). Targeting the myostatin signaling pathway to treat muscle wasting diseases. Curr. Opin. Support Palliat. Care 5 (4), 334–341. doi:10.1097/spc.0b013e32834bddf9
Han, X., Yang, F., Cao, H., and Liang, Z. (2015). Malat1 regulates serum response factor through miR-133 as a competing endogenous RNA in myogenesis. FASEB J. 29 (7), 3054–3064. doi:10.1096/fj.14-259952
Hemberger, M., Hanna, C. W., and Dean, W. (2020). Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 21 (1), 27–43. doi:10.1038/s41576-019-0169-4
Hnia, K., Clausen, T., and Moog-Lutz, C. (2019). Shaping striated muscles with ubiquitin proteasome system in health and disease. Trends Mol. Med. 25 (9), 760–774. doi:10.1016/j.molmed.2019.05.008
Horak, M., Novak, J., and Bienertova-Vasku, J. (2016). Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 410 (1), 1–13. doi:10.1016/j.ydbio.2015.12.013
Huang, C., Wu, S., Ji, H., Yan, X., Xie, Y., Murai, S., et al. (2017). Identification of XBP1-u as a novel regulator of the MDM2/p53 axis using an shRNA library. Sci. Adv. 3 (10), e1701383. doi:10.1126/sciadv.1701383
Hupkes, M., Sotoca, A. M., Hendriks, J. M., van Zoelen, E. J., and Dechering, K. J. (2014). MicroRNA miR-378 promotes BMP2-induced osteogenic differentiation of mesenchymal progenitor cells. BMC Mol. Biol. 15 (1), 1–15. doi:10.1186/1471-2199-15-1
Ito, K., and Murphy, D. (2013). Application of ggplot2 to pharmacometric graphics. CPT Pharmacometrics Syst. Pharmacol. 2 (2013), 1–16. doi:10.1038/psp.2013.56
Jassal, B., Matthews, L., Viteri, G., Gong, C., Lorente, P., Fabregat, A., et al. (2020). The reactome pathway knowledgebase. Nucleic Acids Res. 48 (D1), D498-D503–D503. doi:10.1093/nar/gkz1031
Jeggari, A., Marks, D. S., and Larsson, E. (2012). miRcode: a map of putative microRNA target sites in the long non-coding transcriptome. Bioinformatics 28 (15), 2062–2063. doi:10.1093/bioinformatics/bts344
Jin, J. J., Lv, W., Xia, P., Xu, Z. Y., Zheng, A. D., Wang, X. J., et al. (2018). Long noncoding RNA SYISL regulates myogenesis by interacting with polycomb repressive complex 2. Proc. Natl. Acad. Sci. U. S. A. 115 (42), E9802-E9811–E9811. doi:10.1073/pnas.1801471115
Kallen, A. N., Zhou, X. B., Xu, J., Qiao, C., Ma, J., Yan, L., et al. (2013). The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell 52 (1), 101–112. doi:10.1016/j.molcel.2013.08.027
Karagkouni, D., Paraskevopoulou, M. D., Chatzopoulos, S., Vlachos, I. S., Tastsoglou, S., Kanellos, I., et al. (2018). DIANA-TarBase v8: a decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 46 (D1), D239–D245. doi:10.1093/nar/gkx1141
Karagkouni, D., Paraskevopoulou, M. D., Tastsoglou, S., Skoufos, G., Karavangeli, A., Pierros, V., et al. (2020). DIANA-LncBase v3: indexing experimentally supported miRNA targets on non-coding transcripts. Nucleic Acids Res. 48 (D1), D101-D110–D110. doi:10.1093/nar/gkz1036
Korotkova, M., and Lundberg, I. E. (2014). The skeletal muscle arachidonic acid cascade in health and inflammatory disease. Nat. Rev. Rheumatol. 10 (5), 295–303. doi:10.1038/nrrheum.2014.2
Kostallari, E., Baba-Amer, Y., Alonso-Martin, S., Ngoh, P., Relaix, F., Lafuste, P., et al. (2015). Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development 142 (7), 1242–1253. doi:10.1242/dev.115386
Lander, A. D. (2011). Pattern, growth, and control. Cell 144 (6), 955–969. doi:10.1016/j.cell.2011.03.009
Latroche, C., Weiss-Gayet, M., Muller, L., Gitiaux, C., Leblanc, P., Liot, S., et al. (2017). Coupling between myogenesis and angiogenesis during skeletal muscle regeneration is stimulated by restorative macrophages. Stem Cell Rep. 9 (6), 2018–2033. doi:10.1016/j.stemcr.2017.10.027
Lattin, J. E., Schroder, K., Su, A. I., Walker, J. R., Zhang, J., Wiltshire, T., et al. (2008). Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 4 (5), 5–13. doi:10.1186/1745-7580-4-5
Lee, Y. S., Huynh, T. V., and Lee, S. J. (1985)2016). Paracrine and endocrine modes of myostatin action. J. Appl. Physiol. 120 (6), 592–598. doi:10.1152/japplphysiol.00874.2015
Legnini, I., Morlando, M., Mangiavacchi, A., Fatica, A., and Bozzoni, I. (2014). A feedforward regulatory loop between HuR and the long noncoding RNA linc-MD1 controls early phases of myogenesis. Mol. Cell 53 (3), 506–514. doi:10.1016/j.molcel.2013.12.012
Lei, F., Zhang, H., and Xie, X. (2019). Comprehensive analysis of an lncRNA-miRNA-mRNA competing endogenous RNA network in pulpitis. PeerJ 7 (2019), e7135. doi:10.7717/peerj.7135
Li, J. H., Liu, S., Zhou, H., Qu, L. H., and Yang, J. H. (2014). starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 42 (Database issue), D92–D97. doi:10.1093/nar/gkt1248
Li, Y., Chen, X., Sun, H., and Wang, H. (2018). Long non-coding RNAs in the regulation of skeletal myogenesis and muscle diseases. Cancer Lett. 417 (2018), 58–64. doi:10.1016/j.canlet.2017.12.015
Li, Z., Cai, B., Abdalla, B. A., Zhu, X., Zheng, M., Han, P., et al. (2019). LncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway. J. Cachexia Sarcopenia Muscle 10 (2), 391–410. doi:10.1002/jcsm.12374
Liao, X., Tang, D., Yang, H., Chen, Y., Chen, D., Jia, L., et al. (2019). Long non-coding RNA XIST may influence cervical ossification of the posterior longitudinal ligament through regulation of miR-17-5P/AHNAK/BMP2 signaling pathway. Calcif. Tissue Int. 105 (6), 670–680. doi:10.1007/s00223-019-00608-y
Liu, J., Yan, Y., Xi, B., Huang, G., and Mi, J. (2019a). Skeletal muscle reference for Chinese children and adolescents. J. Cachexia Sarcopenia Muscle 10 (1), 155–164. doi:10.1002/jcsm.12361
Liu, M., Li, B., Peng, W., Ma, Y., Huang, Y., Lan, X., et al. (2019b). LncRNA-MEG3 promotes bovine myoblast differentiation by sponging miR-135. J. Cell Physiol. 234 (10), 18361–18370. doi:10.1002/jcp.28469
Liu, N., Garry, G. A., Li, S., Bezprozvannaya, S., Sanchez-Ortiz, E., Chen, B., et al. (2017a). A Twist2-dependent progenitor cell contributes to adult skeletal muscle. Nat. Cell Biol. 19 (3), 202–213. doi:10.1038/ncb3477
Liu, W., Nanetti, A., and Cheong, S. A. (2017b). Knowledge evolution in physics research: an analysis of bibliographic coupling networks. PLoS One 12 (9), e0184821. doi:10.1371/journal.pone.0184821
Liu, Y., Chu, A., Chakroun, I., Islam, U., and Blais, A. (2010). Cooperation between myogenic regulatory factors and SIX family transcription factors is important for myoblast differentiation. Nucleic Acids Res. 38 (20), 6857–6871. doi:10.1093/nar/gkq585
Lu, L., Sun, K., Chen, X., Zhao, Y., Wang, L., Zhou, L., et al. (2013). Genome-wide survey by ChIP-seq reveals YY1 regulation of lincRNAs in skeletal myogenesis. EMBO J. 32 (19), 2575–2588. doi:10.1038/emboj.2013.182
Lukjanenko, L., Brachat, S., Pierrel, E., Lach-Trifilieff, E., and Feige, J. N. (2013). Genomic profiling reveals that transient adipogenic activation is a hallmark of mouse models of skeletal muscle regeneration. PLoS One 8 (8), e71084. doi:10.1371/journal.pone.0071084
Luo, H., Lv, W., Tong, Q., Jin, J., Xu, Z., and Zuo, B. (2021). Functional non-coding RNA during embryonic myogenesis and postnatal muscle development and disease. Front. Cell Dev. Biol. 9 (2021), 628339–628343. doi:10.3389/fcell.2021.628339
Ma, Q., Chirn, G. W., Szustakowski, J. D., Bakhtiarova, A., Kosinski, P. A., Kemp, D., et al. (2008). Uncovering mechanisms of transcriptional regulations by systematic mining of cis regulatory elements with gene expression profiles. BioData Min. 1 (1), 4–10. doi:10.1186/1756-0381-1-4
Mahdessian, D., Cesnik, A. J., Gnann, C., Danielsson, F., Stenström, L., Arif, M., et al. (2021). Spatiotemporal dissection of the cell cycle with single-cell proteogenomics. Nature 590 (7847), 649–654. doi:10.1038/s41586-021-03232-9
Matsui, M., and Corey, D. R. (2017). Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 16 (3), 167–179. doi:10.1038/nrd.2016.117
Matsumoto, A., Pasut, A., Matsumoto, M., Yamashita, R., Fung, J., Monteleone, E., et al. (2017). mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature 541 (7636), 228–232. doi:10.1038/nature21034
Metsalu, T., and Vilo, J. (2015). ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 43 (W1), W566–W570. doi:10.1093/nar/gkv468
Militello, G., Hosen, M. R., Ponomareva, Y., Gellert, P., Weirick, T., John, D., et al. (2018). A novel long non-coding RNA Myolinc regulates myogenesis through TDP-43 and Filip1. J. Mol. Cell Biol. 10 (2), 102–117. doi:10.1093/jmcb/mjy025
Mourikis, P., Gopalakrishnan, S., Sambasivan, R., and Tajbakhsh, S. (2012). Cell-autonomous Notch activity maintains the temporal specification potential of skeletal muscle stem cells. Development 139 (24), 4536–4548. doi:10.1242/dev.084756
Ogawa, R., Ma, Y., Yamaguchi, M., Ito, T., Watanabe, Y., Ohtani, T., et al. (2015). Doublecortin marks a new population of transiently amplifying muscle progenitor cells and is required for myofiber maturation during skeletal muscle regeneration. Development 142 (1), 810–861. doi:10.1242/dev.122317
Pant, M., Bal, N. C., and Periasamy, M. (2016). Sarcolipin: a key thermogenic and metabolic regulator in skeletal muscle. Trends Endocrinol. Metab. 27 (12), 881–892. doi:10.1016/j.tem.2016.08.006
Peault, B., Rudnicki, M., Torrente, Y., Cossu, G., Tremblay, J. P., Partridge, T., et al. (2007). Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol. Ther. 15 (5), 867–877. doi:10.1038/mt.sj.6300145
Pedersen, B. K., and Febbraio, M. A. (2012). Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8 (8), 457–465. doi:10.1038/nrendo.2012.49
Peterson, J. M., Bakkar, N., and Guttridge, D. C. (2011). NF-κB signaling in skeletal muscle health and disease. Curr. Top. Dev. Biol. 96 (2011), 85–119. doi:10.1016/B978-0-12-385940-2.00004-8
Pillon, N. J., Gabriel, B. M., Dollet, L., Smith, J. A. B., Sardón Puig, L., Botella, J., et al. (2020). Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat. Commun. 11 (1), 470–484. doi:10.1038/s41467-019-13869-w
Plikus, M. V., Wang, X., Sinha, S., Forte, E., Thompson, S. M., Herzog, E. L., et al. (2021). Fibroblasts: origins, definitions, and functions in health and disease. Cell 184 (15), 3852–3872. doi:10.1016/j.cell.2021.06.024
Ponnusamy, M., Li, P. F., and Wang, K. (2017). Understanding cardiomyocyte proliferation: an insight into cell cycle activity. Cell Mol. Life Sci. 74 (6), 1019–1034. doi:10.1007/s00018-016-2375-y
Ponting, C. P., Oliver, P. L., and Reik, W. (2009). Evolution and functions of long noncoding RNAs. Cell 136 (4), 629–641. doi:10.1016/j.cell.2009.02.006
Qi, X., Lin, Y., Chen, J., and Shen, B. (2020). Decoding competing endogenous RNA networks for cancer biomarker discovery. Brief. Bioinform 21 (2), 441–457. doi:10.1093/bib/bbz006
Rajan, S., Chu Pham Dang, H., Djambazian, H., Zuzan, H., Fedyshyn, Y., Ketela, T., et al. (2012). Analysis of early C2C12 myogenesis identifies stably and differentially expressed transcriptional regulators whose knock-down inhibits myoblast differentiation. Physiol. Genomics 44 (2), 183–197. doi:10.1152/physiolgenomics.00093.2011
Ravnskjaer, K., Madiraju, A., and Montminy, M. (2016). Role of the cAMP pathway in glucose and lipid metabolism. Handb. Exp. Pharmacol. 233 (2021), 29–49. doi:10.1007/164_2015_32
Reimand, J., Isserlin, R., Voisin, V., Kucera, M., Tannus-Lopes, C., Rostamianfar, A., et al. (2019). Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 14 (2), 482–517. doi:10.1038/s41596-018-0103-9
Ritchie, M. E., Phipson, B., Wu, D., Hu, Y., Law, C. W., Shi, W., et al. (2015). Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43 (7), e47. doi:10.1093/nar/gkv007
Rosvall, M., and Bergstrom, C. T. (2010). Mapping change in large networks. PLoS One 5 (1), e8694. doi:10.1371/journal.pone.0008694
Salazar, V. S., Gamer, L. W., and Rosen, V. (2016). BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 12 (4), 203–221. doi:10.1038/nrendo.2016.12
Segales, J., Perdiguero, E., and Munoz-Canoves, P. (2016). Regulation of muscle stem cell functions: a focus on the p38 MAPK signaling pathway. Front. Cell Dev. Biol. 4 (2016), 91–105. doi:10.3389/fcell.2016.00091
Sharma, M., McFarlane, C., Kambadur, R., Kukreti, H., Bonala, S., and Srinivasan, S. (2015). Myostatin: expanding horizons. IUBMB Life 67 (8), 589–600. doi:10.1002/iub.1392
Signal, B., Gloss, B. S., and Dinger, M. E. (2016). Computational approaches for functional prediction and characterisation of long noncoding RNAs. Trends Genet. 32 (10), 620–637. doi:10.1016/j.tig.2016.08.004
Sirabella, D., De Angelis, L., and Berghella, L. (2013). Sources for skeletal muscle repair: from satellite cells to reprogramming. J. Cachexia Sarcopenia Muscle 4 (2), 125–136. doi:10.1007/s13539-012-0098-y
Soleimani, V. D., Yin, H., Jahani-Asl, A., Ming, H., Kockx, C., van Ijcken, W., et al. (2012). Snail regulates MyoD binding-site occupancy to direct enhancer switching and differentiation-specific transcription in myogenesis. Mol. Cell 47 (3), 457–468. doi:10.1016/j.molcel.2012.05.046
Spittau, B., Dokalis, N., and Prinz, M. (2020). The role of TGFβ signaling in microglia maturation and activation. Trends Immunol. 41 (9), 836–848. doi:10.1016/j.it.2020.07.003
Sui, Y., Han, Y., Zhao, X., Li, D., and Li, G. (2019). Long non-coding RNA Irm enhances myogenic differentiation by interacting with MEF2D. Cell Death Dis. 10 (3), 181–193. doi:10.1038/s41419-019-1399-2
Sun, F., Liang, W., Tang, K., Hong, M., and Qian, J. (2019). Profiling the lncRNA-miRNA-mRNA ceRNA network to reveal potential crosstalk between inflammatory bowel disease and colorectal cancer. PeerJ 7, e7451. doi:10.7717/peerj.7451
Teng, X., Chen, X., Xue, H., Tang, Y., Zhang, P., Kang, Q., et al. (2020). NPInter v4.0: an integrated database of ncRNA interactions. Nucleic Acids Res. 48 (D1), D160-D165–D165. doi:10.1093/nar/gkz969
Thomson, D. M. (2018). The role of AMPK in the regulation of skeletal muscle size, hypertrophy, and regeneration. Int. J. Mol. Sci. 19 (10), 3125–3144. doi:10.3390/ijms19103125
Thomson, D. W., and Dinger, M. E. (2016). Endogenous microRNA sponges: evidence and controversy. Nat. Rev. Genet. 17 (5), 272–283. doi:10.1038/nrg.2016.20
Tomczak, K. K., Marinescu, V. D., Ramoni, M. F., Sanoudou, D., Montanaro, F., Han, M., et al. (2004). Expression profiling and identification of novel genes involved in myogenic differentiation. FASEB J. 18 (2), 1–23. doi:10.1096/fj.03-0568fje
Tumpel, S., and Rudolph, K. L. (2019). Quiescence: good and bad of stem cell aging. Trends Cell Biol. 29 (8), 672–685. doi:10.1016/j.tcb.2019.05.002
Vico, L., and Hargens, A. (2018). Skeletal changes during and after spaceflight. Nat. Rev. Rheumatol. 14 (4), 229–245. doi:10.1038/nrrheum.2018.37
von Haehling, S., Ebner, N., Dos Santos, M. R., Springer, J., and Anker, S. D. (2017). Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat. Rev. Cardiol. 14 (6), 323–341. doi:10.1038/nrcardio.2017.51
Wackerhage, H., Del Re, D. P., Judson, R. N., Sudol, M., and Sadoshima, J. (2014). The Hippo signal transduction network in skeletal and cardiac muscle. Sci. Signal 7 (337), re4. doi:10.1126/scisignal.2005096
Wang, S., Jin, J., Xu, Z., and Zuo, B. (2019b). Functions and regulatory mechanisms of lncRNAs in skeletal myogenesis, muscle disease and meat production. Cells 8 (9), 1107–1125. doi:10.3390/cells8091107
Wang, S., Zuo, H., Jin, J., Lv, W., Xu, Z., Fan, Y., et al. (2019a). Long noncoding RNA Neat1 modulates myogenesis by recruiting Ezh2. Cell Death Dis. 10 (7), 505–519. doi:10.1038/s41419-019-1742-7
Wang, W., Min, L., Qiu, X., Wu, X., Liu, C., Ma, J., et al. (2021). Biological function of long non-coding RNA (LncRNA) xist. Front. Cell Dev. Biol. 9, 645647–645673. doi:10.3389/fcell.2021.645647
Wang, X. H., and Mitch, W. E. (2014). Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 10 (9), 504–516. doi:10.1038/nrneph.2014.112
Watt, K. I., Goodman, C. A., Hornberger, T. A., and Gregorevic, P. (2018). The Hippo signaling pathway in the regulation of skeletal muscle mass and function. Exerc Sport Sci. Rev. 46 (2), 92–96. doi:10.1249/jes.0000000000000142
Wilson, B. G., Wang, X., Shen, X., McKenna, E. S., Lemieux, M. E., Cho, Y. J., et al. (2010). Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell 18 (4), 316–328. doi:10.1016/j.ccr.2010.09.006
Winkle, M., El-Daly, S. M., Fabbri, M., and Calin, G. A. (2021). Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov. 20 (8), 629–651. doi:10.1038/s41573-021-00219-z
Wu, N., Zhang, X., Bao, Y., Yu, H., Jia, D., and Ma, C. (2019). Down-regulation of GAS5 ameliorates myocardial ischaemia/reperfusion injury via the miR-335/ROCK1/AKT/GSK-3β axis. J. Cell Mol. Med. 23 (12), 8420–8431. doi:10.1111/jcmm.14724
Wu, T., Hu, E., Xu, S., Chen, M., Guo, P., Dai, Z., et al. (2021). clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innov. (N Y) 2 (3), 100141. doi:10.1016/j.xinn.2021.100141
Wust, S., Drose, S., Heidler, J., Wittig, I., Klockner, I., Franko, A., et al. (2018). Metabolic maturation during muscle stem cell differentiation is achieved by miR-1/133a-mediated inhibition of the dlk1-dio3 mega gene cluster. Cell Metab. 27 (5), 1026–1039.e6. doi:10.1016/j.cmet.2018.02.022
Xi, Y., Shen, W., Jin, C., Wang, L., and Yu, B. (2020). PVT1 promotes the proliferation and migration of non-small cell lung cancer via regulating miR-148/RAB34 signal Axis. Onco Targets Ther. 13, 1819–1832. doi:10.2147/ott.s222898
Xiong, F., Wei, W. P., Liu, Y. B., Wang, Y., Zhang, H. Y., and Liu, R. (2021). Long noncoding RNA XIST enhances cerebral ischemia-reperfusion injury by regulating miR-486-5p and GAB2. Eur. Rev. Med. Pharmacol. Sci. 25 (4), 2013–2020. doi:10.26355/eurrev_202102_25103
Yao, R. W., Wang, Y., and Chen, L. L. (2019). Cellular functions of long noncoding RNAs. Nat. Cell Biol. 21 (5), 542–551. doi:10.1038/s41556-019-0311-8
Yu, G., and He, Q. Y. (2016). ReactomePA: an R/Bioconductor package for reactome pathway analysis and visualization. Mol. Biosyst. 12 (2), 477–479. doi:10.1039/c5mb00663e
Yu, X., Zhang, Y., Li, T., Ma, Z., Jia, H., Chen, Q., et al. (2017). Long non-coding RNA Linc-RAM enhances myogenic differentiation by interacting with MyoD. Nat. Commun. 8, 14016–14027. doi:10.1038/ncomms14016
Zhang, J., Zhao, J., Dahan, P., Lu, V., Zhang, C., Li, H., et al. (2018a). Metabolism in pluripotent stem cells and early mammalian development. Cell Metab. 27 (2), 332–338. doi:10.1016/j.cmet.2018.01.008
Zhang, Z. K., Li, J., Guan, D., Liang, C., Zhuo, Z., Liu, J., et al. (2018b). A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration. J. Cachexia Sarcopenia Muscle 9 (3), 613–626. doi:10.1002/jcsm.12281
Zhang, Z. K., Li, J., Guan, D., Liang, C., Zhuo, Z., Liu, J., et al. (2018c). Long noncoding RNA lncMUMA reverses established skeletal muscle atrophy following mechanical unloading. Mol. Ther. 26 (11), 2669–2680. doi:10.1016/j.ymthe.2018.09.014
Zhao, Y., Chen, M., Lian, D., Li, Y., Li, Y., Wang, J., et al. (2019). Non-coding RNA regulates the myogenesis of skeletal muscle satellite cells, injury repair and diseases. Cells 8 (9), 988–993. doi:10.3390/cells8090988
Zhou, L., Sun, K., Zhao, Y., Zhang, S., Wang, X., Li, Y., et al. (2015). Linc-YY1 promotes myogenic differentiation and muscle regeneration through an interaction with the transcription factor YY1. Nat. Commun. 6 (11), 10026. doi:10.1038/ncomms10026
Zhu, M., Liu, J., Xiao, J., Yang, L., Cai, M., Shen, H., et al. (2017). Lnc-mg is a long non-coding RNA that promotes myogenesis. Nat. Commun. 8, 14718–14728. doi:10.1038/ncomms14718
Keywords: lncRNA, muscle development and regeneration, cell proliferation and differentiation, muscle atrophy, ceRNA network, signaling pathway
Citation: Wenlun W, Chaohang Y, Yan H, Wenbin L, Nanqing Z, Qianmin H, Shengcai W, Qing Y, Shirui Y, Feng Z and Lingyun Z (2025) Developing a ceRNA-based lncRNA-miRNA-mRNA regulatory network to uncover roles in skeletal muscle development. Front. Bioinform. 4:1494717. doi: 10.3389/fbinf.2024.1494717
Received: 11 September 2024; Accepted: 23 December 2024;
Published: 15 January 2025.
Edited by:
Zhi-Ping Liu, Shandong University, ChinaReviewed by:
Yang Zhang, Carnegie Mellon University, United StatesJiashun Mao, Yonsei University, Republic of Korea
Copyright © 2025 Wang, Chaohang, Yan, Wenbin, Nanqing, Qianmin, Shengcai, Qing, Shirui, Feng and Lingyun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Wenlun, MTAxOTE1OTMzNUBxcS5jb20=; Zhang Feng, bmt6aGFuZ2ZlbmdAMTYzLmNvbQ== Zhu Lingyun, bGluZ3l1bnpodUBudWR0LmVkdS5jbg==
†These authors have contributed equally to this work
 Wang Wenlun
Wang Wenlun Yu Chaohang1†
Yu Chaohang1† Zhang Feng
Zhang Feng Zhu Lingyun
Zhu Lingyun