
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioinform., 23 February 2024
Sec. Protein Bioinformatics
Volume 4 - 2024 | https://doi.org/10.3389/fbinf.2024.1295972
This article is part of the Research TopicWomen in BioinformaticsView all 6 articles
 Rang Li1
Rang Li1 Sabrina Wilderotter1
Sabrina Wilderotter1 Madison Stoddard2
Madison Stoddard2 Debra Van Egeren3
Debra Van Egeren3 Arijit Chakravarty2
Arijit Chakravarty2 Diane Joseph-McCarthy1*
Diane Joseph-McCarthy1*Introduction: A fundamental challenge in computational vaccinology is that most B-cell epitopes are conformational and therefore hard to predict from sequence alone. Another significant challenge is that a great deal of the amino acid sequence of a viral surface protein might not in fact be antigenic. Thus, identifying the regions of a protein that are most promising for vaccine design based on the degree of surface exposure may not lead to a clinically relevant immune response.
Methods: Linear peptides selected by phage display experiments that have high affinity to the monoclonal antibody of interest (“mimotopes”) usually have similar physicochemical properties to the antigen epitope corresponding to that antibody. The sequences of these linear peptides can be used to find possible epitopes on the surface of the antigen structure or a homology model of the antigen in the absence of an antigen-antibody complex structure.
Results and Discussion: Herein we describe two novel methods for mapping mimotopes to epitopes. The first is a novel algorithm named MimoTree that allows for gaps in the mimotopes and epitopes on the antigen. More specifically, a mimotope may have a gap that does not match to the epitope to allow it to adopt a conformation relevant for binding to an antibody, and residues may similarly be discontinuous in conformational epitopes. MimoTree is a fully automated epitope detection algorithm suitable for the identification of conformational as well as linear epitopes. The second is an ensemble approach, which combines the prediction results from MimoTree and two existing methods.
Antibodies are produced by B cells during the immune response to the presence of foreign matter within the body (“antigens”). Antibodies recognize and bind to specific regions of target antigens to perform their functions, and the regions of antigen molecules to which antibodies attach are defined as epitopes (Sela-Culang et al., 2013). B cell epitopes can be classified into linear and conformational epitopes; in the latter case, epitopes consist of patches of residues that lie close to each other in three-dimensional space but are separated in amino acid sequence (Potocnakova et al., 2016; Sanchez-Trincado et al., 2017). Conformational epitopes generally account for about 90% of overall antibody binding to an antigen (Barlow et al., 1986; Van Regenmortel, 1996). In addition, conformational epitopes may offer functional advantages over linear epitopes that confer enhanced viral neutralization (Vinion-Dubiel et al., 2001) and longer lasting immunity (Steimer et al., 1991). For example, conformational epitope-targeting antibodies to the HIV gp120 glycoprotein are more effective at neutralizing viral isolates than linear epitope-targeting antibodies to the same protein, and they are also responsible for the majority of gp120-specific CD4-blocking activity in HIV-1-infected human sera.
A deeper knowledge of the structure and immunogenic properties of conformational epitopes may be beneficial for designing interventions for many therapeutic areas, including viral infections and cancer (Tegoni et al., 1999; Cho et al., 2003; Adams and Weiner, 2005; Riemer et al., 2005b; Li et al., 2005; Nybakken et al., 2005; Saxena et al., 2006). Immune responses elicited by partial antigens may sometimes be sufficient to provide competent protection since most of the surface structure of the antigen molecules are not antigenic (Sanchez-Trincado et al., 2017). Therefore, identifying immunogenic conformational epitopes and developing strategies to generate an effective immune response against the identified epitopes are important problems in computational vaccinology.
A mimotope is a peptide that mimics the structure of an epitope, and which, in its most strict definition, causes an antibody response that is similar to the one elicited by the epitope (Geysen et al., 1986). An antibody for a given antigen is expected to recognize mimotopes which mimic the corresponding epitope. Mimotopes are commonly obtained from phage display libraries through bio-panning experiments (Bazan et al., 2012). Bacteriophages displaying potential mimotope peptides are incubated with the target antibody which is immobilized on a solid support. As such, specific phages in the library bind to the target antibody. Unbound phages are washed out, while bound phages are selected, eluted, and amplified. Multiple rounds of evolution may be performed. This method is intended to select peptides with high binding affinity to the chosen antibodies. A portion of the selected mimotopes will have some homology to antigen epitopes and may trigger the anticipated immune response. As a result, mimotopes can potentially be employed in and used as a starting point for vaccine design (Bakhshinejad et al., 2016; Goulart and de S. Santos, 2016).
Studies, however, suggest that peptide antigens may not always be capable of generating a sufficient immune response (Chen et al., 2009; Irving et al., 2010; Van Regenmortel, 2016). That is, antipeptide antibodies, raised against peptide antigens, may fail to bind the cognate protein antigens which are in a folded conformational state. Peptides likely exist in an ensemble of conformational states; they may predominantly exist in a linear or disordered state but be capable of adopting distinct conformations a significant percentage of the time. Those peptides that can mimic conformational epitopes found in protein antigens are expected to be able to bind antibodies generated against said protein antigens. Mimotopes, therefore, may be useful in the design of antigens for use in antibody detection in immunodiagnostic tests. Specifically, design of a set of mimotopes that mimic conformational epitopes as the antigens is of high utility in the development of immunodiagnostics to detect antibodies that recognize the corresponding conformational epitopes. Furthermore, mimotopes obtained by panning a phage-displayed random peptide library against a monoclonal antibody or specific sera may produce “target-unrelated peptides” (Huang et al., 2014) in addition to true high affinity binders; computational methods that can map the resulting peptide hits to an antigen structure may, therefore, also be useful for eliminating non-specific peptide binders since those peptides would be less likely to map to the antigen surface.
The algorithmic task of the mimotope-to-epitope mapping is to map the mimotope to the part of the antigen surface that binds the antibody used to create the mimotope. This epitope should have similar physicochemical properties to the mimotope, facilitating antibody binding to both. Since the mimotope is usually not identical but similar to its corresponding epitope at the sequence and structural level, the algorithm considers residues with a physicochemical property distance within a certain range as identical, which is a major challenge in the mapping process. The regions of the antigen surface that are aligned with the mimotopes have a higher probability of being antigenic. Similar to phage-display experiments, molecular docking has been used to enrich potential target regions of the antigen. Protein-protein docking methods have been used to identify the antibody binding site (the epitope) on an antigen protein (Desta et al., 2022); this approach requires a structure or model of both the antigen and antibody structure. Here we are mapping the mimotope to the surface of the antigen to identify the epitopes that the antibody is expected to be capable of binding. As such, only the structure or model of the antigen protein is required. That is, the process of mapping mimotopes (peptides with high binding affinity to the antibody) to the surface of the antigen identifies antibody binding sites on the antigen.
Several computational methods for mimotope mapping based on the antigen structure alone exist (Moreau et al., 2006; Bublil et al., 2007; Mayrose et al., 2007; Negi and Braun, 2009; Chen et al., 2011; Sun et al., 2016), all of which have been validated on a similar set of mimotopes and corresponding protein epitopes based on the existence of the corresponding antigen-antibody complex structures. In general, the overall sensitivity is lower than desired, meaning that residues that lie within the true epitopes are left out of the epitopes predicted by these algorithms. Thus, the existing methods are useful but imperfect.
Specific mimotope-to-epitope mapping algorithms display unique strengths and weaknesses. For example, Mapitope (Bublil et al., 2007) is an algorithm that breaks each mimotope in the dataset into overlapping residue pairs (each residue pair contains two consecutive amino acids in the mimotope sequence), and then computationally pools these residue pairs and ranks the occurrence of each type. Next, it uses the high-frequency occurrences to search on the antigen surface to find the antigen surface residues mapped by these pairs. However, this algorithm requires many statistically relevant parameters customized by the user, and the length of the residue pairs it considers is so short that different parameter settings can have a very large impact on the prediction.
Pupko and co-workers also developed PepSurf (Mayrose et al., 2007), which uses a color-coding algorithm to find all possible simple paths on the antigen surface, matches these paths with mimotopes based on amino acid similarity, and finally clusters the paths with high similarities to get the final prediction. However, the run time of PepSurf depends linearly on the length of the mimotopes because the step of searching for every possible simple path on the antigen surface limits the rate to a great extent. Therefore, PepSurf is only able to process mimotopes with up to 15 amino acids. Subsequently, EpiSearch (Negi and Braun, 2009) solved the run time issue, being able to complete all calculations in less than a minute. However, EpiSearch’s method of dividing regions on the antigen surface is fast but imprecise, and it is also unable to process more than 30 mimotopes at a time.
The overall goal of this study is to develop mimotope-to-epitope mapping prediction methods that are more robust in terms of providing predictions with improved sensitivity. While the mimotope is similar to but usually not identical to the true epitope, most mimotope-to-epitope alignments produce some gaps in mimotope sequence (stretches of the mimotope sequence that do not map to the epitope but rather may simply allow the mimotope to adopt a conformation relevant for antibody binding) when the mimotopes are mapped to the antigen surface. In addition, most contiguous mimotope residues correspond to discontinuous binding sites on the antigen surface that bind to the antibody (Cho et al., 2003). To address this feature, we developed a prediction algorithm that takes into account these sequence gaps while also referring back to the three-dimensional (3D) structure of the antigen in the final mimotope mapping step to achieve more sensitive and accurate epitope prediction. We then explored ensemble approaches combining the new approach with existing methods.
To assess the performance of the mimotope-to-epitope mapping methods, we collated ten test cases from similar publications (Huang et al., 2008). The criteria for selecting test cases are i) the 3D crystallography structure of the antigen-antibody is available; ii) the complex contains only the antigen and antibody; iii) a set of mimotopes was derived from bio-panning experiments with the given antibody. The test set is presented in Table 1, and the sequences for the mimotope sets associated with each test case are given in Supplementary Table S1. Two of the test cases, 1AVZ and 1HX1, are for protein-protein interactions where one protein is considered as the “antigen” here since phage-display libraries of peptides were screened for binding to the other protein considered as the “antibody” in the pair for our purposes. For example, for 1AVZ, a peptide library was screening using phage display to identify peptides that bind to the Fyn SH3 domain (the “antibody”) to block its interactions with negative regulatory factor (Nef) (Rickles et al., 1994; Greenway et al., 1996). The Nef dimer, the biologically relevant form of Nef (Arold et al., 1997; Wu et al., 2018), was taken as the “antigen”.
Since mimotope-to-epitope methods are designed to predict the epitope on an antigen surface, as a more stringent test, we also compiled a set of unbound antigen structures when available that correspond to the test set antigen-antibody structures. This is a more rigorous test since the antigen may undergo conformational changes upon binding to the antibody. The antigen-only test set is given in Table 2. For two of the test cases in Table 1, 1N8Z and 1JRH, the corresponding unbound antigen structure does not exist.
Chimera (Pettersen et al., 2004) was used to define the true epitope for each antigen in the test set. An antigen residue is considered to be part of the epitope if the difference between its solvent-accessible surface area (SASA) in the antigen structure (taken from the complex) and in the antigen-antibody complex is greater than 10 Å2 (Hu and Yan, 2009).
Since the amino acids in a mimotope are not typically identical to those in the true epitope, the ability of a mimotope to bind with high affinity to a target antibody and possibly achieve the same function as the true epitope is likely due to the fact that at least a portion of the amino acids in the mimotope share similarity rather than identity with the epitope residues. Similarities between amino acids determine whether they can substitute for each other in a sequence while maintaining similar peptide/protein properties and function. Several methods exist for assessing amino acid similarity, based on the physicochemical properties of the individual amino acids, the role they play in the protein structure, or more subtle contributions (Stephenson and Freeland, 2013). We used quantitative descriptors of the properties of amino acids published by Braun and co-workers when performing mimotope to antigen surface mapping (Venkatarajan and Braun, 2001). The calculated similarity is as follows:
where PD (A, B) represents the property distance between amino acid A and amino acid B, and λi are the eigenvalues corresponding to the eigenvectors Ei (i = 1–5) representing the physicochemical properties of each amino acid. The lower the PD between two amino acid types, the more similar they are. According to Braun and co-workers, the finest grouping of amino acids based on the physicochemical properties is defined by a distance cutoff of 9.5 (Venkatarajan and Braun, 2001). In practice, we chose a distance cutoff of 8 based on the run time of the algorithm and accuracy of the output, which means that two amino acids with a PD of 8 or less are considered identical.
In order to perform mimotope mapping only on the antigen surface, we defined antigen surface residues as those having a SASA greater than 5% of their maximal SASA (Miller et al., 1987). The SASA value per residue in the antigen structure was calculated using Chimera with a default probe radius of 1.4 Å (Pettersen et al., 2004). The maximal SASA of each standard amino acid was defined as the total SASA of the residue in an extended Gly-X-Gly peptide where X represents the residue of interest (Miller et al., 1987).
Mimotopes may only partially mimic the structure and function of the true epitopes that they mimic; most mimotopes are similar but not identical to the true epitope both at the level of sequence and structure. Moreover, during the process of the mimotopes binding to the antibody, it is likely that some residues of the mimotopes will not be bound to the antibody residues, but rather will simply exist in the mimotope sequence to allow the mimotope to adopt a conformation relevant for binding to the antibody. As a result, there may be gaps in the mapping of the mimotope sequence to the antigen surface. To address this issue, some existing methods use a gap penalty parameter added to the similarity score of the mimotope and the antigen surface area or to the weighted score of the predicted path, such that the weight of a mimotope that forms gaps is reduced. Introducing a gap penalty, however, reduces the probability that a mimotope with a gap will be highly scored even if it does match a true epitope (Mayrose et al., 2007; Negi and Braun, 2009).
In fact, mimotopes that form gaps during binding should be scored equally well when mapped onto an epitope region. A unique feature of our algorithm is that it allows for these gaps and does not penalize mappings with such gaps. These gaps in the mimotope sequence binding to the antibody are expected to translate into gaps in the mapping of the mimotope onto the antigen surface. Another unique feature of MimoTree is that the 3D structure of the antigen is considered when determining if a gap in the putative epitope mapping on the antigen surface is allowed. That is, if the distance on the antigen surface is within the linear length possible for the number of residues of the mimotope gap, then the mimotope can be mapped at the corresponding position on the surface antigen. The MimoTree code is available on GitHub.
MimoTree is a Depth First Search (DFS)-based algorithm. The inputs to MimoTree are a protein data bank (PDB) file of the structure of the antigen of interest and a mimotope set. MimoTree initially creates a surface map of the input antigen structure. For each individual mimotope in the dataset, MimoTree performs a DFS tree-based search of the mimotope sequence to identify surface segments or seeds on the antigen surface that match to the mimotope. MimoTree then connects seeds on the antigen surface that are within a physically reasonable distance based on the 3D structure of the antigen as described above. The final output prediction for the mimotope set is the union of i) all seed connections (the concatenation of seeds matched to the antigen surface) with average PD scores of 0 (perfect matches) and ii) seed connections of the longest length for the mimotope set overall with average PD score >0 and ≤2. A detailed description of the method is outlined in Figure 1.
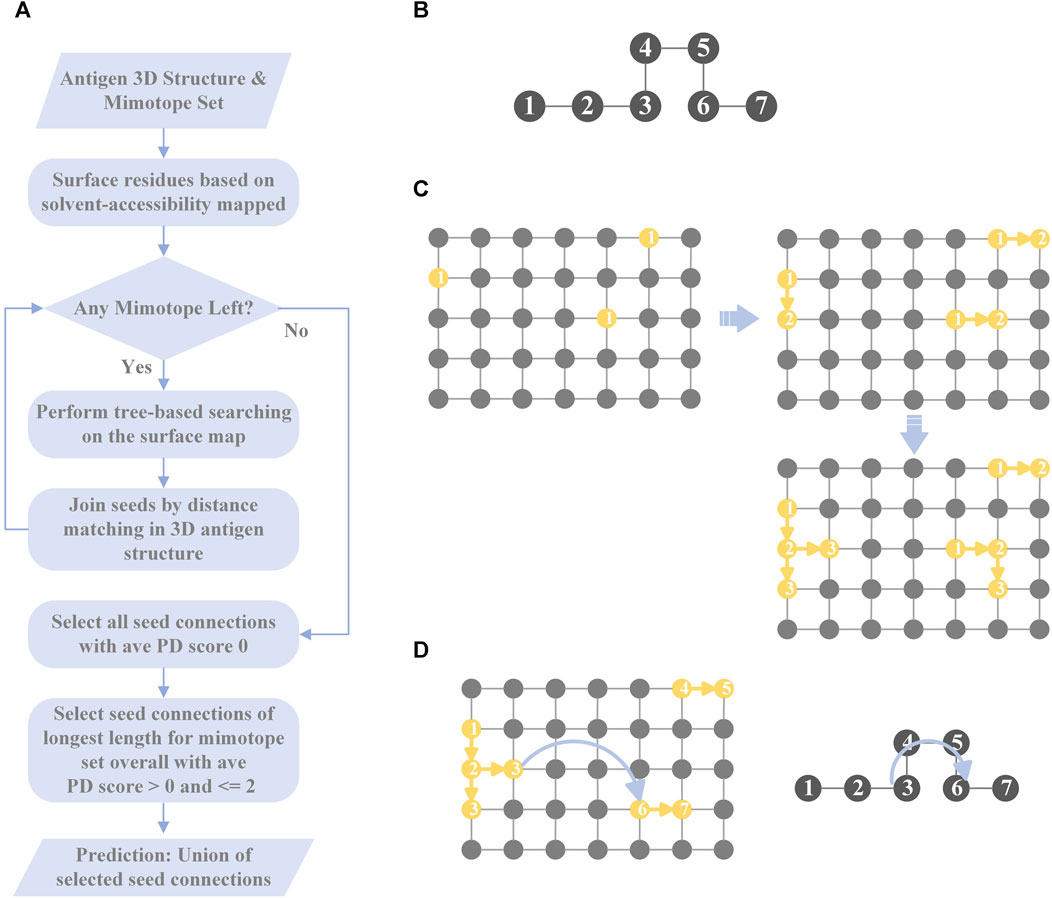
FIGURE 1. Overview of MimoTree algorithm. A flowchart of MimoTree algorithm is shown in (A) and an example of the tree-based search starting from residue 1 of the mimotope sequence given in (B) is shown in (C). An example of the seed connection process is shown in (D).
For the antigen, MimoTree creates a dictionary where each surface residue is a key, and a list containing the adjacent surface residues of each key is represented as the corresponding value. The maximum alpha-carbon to alpha-carbon distance at which two residues are treated as adjacent to each other is 4 Å.
For each mimotope in the set, starting from the first amino acid, MimoTree identifies all of the residues on the protein surface that match. If the property distance between two amino acids is within the pre-defined range (described above), the amino acids are considered as matching in this algorithm. For each surface residue that matches the first residue in the mimotope, MimoTree checks the surface map to see if any adjacent residues match the second residue in the mimotope. If so, it continues searching for the next residue in the mimotope sequence. Otherwise, it terminates that search and starts searching in a different direction from that residue. If that fails, the algorithm starts searching from the next antigen surface residue that matches the first mimotope residue. After finding all possible mimotope matching segments on the antigen surface starting from the first residue of the query mimotope, MimoTree starts a new round of surface searching from the second residue of the mimotope. It continues and completes the surface searching starting from each residue in the mimotope. Finally, the output is all the seeds (matching segments) on the antigen surface that match any part of the query mimotope sequence.
This tree-based searching is based on the DFS algorithm. It starts with a matching surface residue, searches from one direction to the end of that path, and then returns to the previous level to start the search from another direction until the end. Each surface search starting from a residue stops only after all possible paths have been searched, ensuring a comprehensive surface search.
After finding all of the seeds (the residues on the antigen surface that partially match the mimotope), MimoTree tries to join the seeds based on the sequence order of the mimotopes to form “seed connections.” The algorithm connects seeds if the mimotope gap in an extended linear conformation is sufficiently long to extend from the one seed to the other seed. That is, the length of the gap in the mimotope sequence, assuming an extended linear conformation, is compared to the distance in the 3D antigen structure from the last residue of the first seed to the first residue of the next seed. If the maximum length of the mimotope gap is greater than or equal to the distance on the 3D antigen structure between the ends of seeds that are being connected, then this mimotope can be mapped at that position (to that epitope) on the antigen surface. In the hypothetical example of a 7-residue mimotope, where residues 1-3 and 6-7 map to the epitope (Figures 1B, D), the mimotope gap of residues 4-5 is allowed if the length of residues 4-5 plus one residue in an extended linear conformation is greater than or equal to the distance between the residues in the 3D antigen structure that map to mimotope residues 3 and 6. More specifically, the algorithm considers the N-to-N distance between two adjacent residues in the mimotope as 3.5 Å (Bhagavan and Ha, 2015), so in the example above the maximum distance would be 10.5 Å (3 * 3.5 Å/residue). The N-to-N distance between the corresponding residues on the surface of the antigen (those that match to mimotope residues 3 and 6) is then calculated from the coordinates of the residues in the input PDB file and must be less than the linear mimotope gap length (10.5 Å in the example) for a connection to be made. Seeds of the longest length overall are automatically passed on as seed connections as well.
Since MimoTree searches for possible seed connections on the antigen surface based on the sequence of mimotope, the longer the seed connection is, the greater the portion of the mimotope that is matched and the more likely it is that a true epitope has been identified. The length of a seed connection excludes gaps (so is length 5 for the example above) and can extend to the limit of the length of the mimotope (7 for the example above). MimoTree balances the length of the seed connection with the exactness of the match based on the average PD score for the seed connection as described above in Section 2.5.1 to determine the final prediction for the mimotope set.
MimoTree contains several tunable parameters: the cutoff value for the PD (the degree of amino acid similarity), the N-to-N distance for length considered for gaps, and the average PD for seed connection selection. As described above, we calculate the PD between any two amino acids using the values of the physicochemical descriptors of each standard amino acid and the eigenvalues indicating the weights of different descriptors. When the PD between two amino acids is less than a preset cutoff value, MimoTree considers the two amino acids to be identical. Thus, in the process of mapping mimotopes to the antigen surface, if the property distance between a residue in the mimotope and a residue on the antigen surface is less than the cutoff value, the algorithm aligns the mimotope residue to that position on the antigen surface.
If the cutoff value is decreased, the algorithm will match fewer but more similar mimotope residues to antigen residues. As a result, the output will be possible epitopes that are more similar in property space to the aligned mimotopes. Thus, if the input mimotopes are very similar to the true epitope, reducing the cutoff will improve both sensitivity and precision of the algorithm, but if the input mimotopes are not similar enough to the true epitope, lowering the cutoff will reduce both sensitivity and precision. In other words, lowering the cutoff makes the algorithm more dependent on the similarity or identity between the mimotope and the true epitope, which reduces the robustness of the algorithm. Since lowering the threshold would consider fewer residues, it would also shorten the run time of the program. In practice, we found that a cutoff value of 8 yielded the best results across the test set based on the accuracy and run time.
The N-to-N distance was also varied from 3.4 to 3.8 and set to 3.5 Å; a larger distance will allow more matches but may not be physically realistic. The average PD used when selecting the longest seed connection was varied from 2 to 3. All results obtained by MimoTree herein utilize a PD cutoff of 8, an N-to-N distance of 3.5 Å for gaps, and an average PD of 2 for seed connection selection.
In this study, we compare MimoTree to two of the other methods that are available online and test several ensemble approaches. The two methods are PepSurf (Mayrose et al., 2007; Negi and Braun, 2009) and EpiSearch (Mayrose et al., 2007; Negi and Braun, 2009) which have complementary search algorithms (described below). More specifically, ensemble approaches combining PepSurf and EpiSearch as well as combining PepSurf and EpiSearch with MimoTree were examined. The PepSurf and EpiSearch calculations were performed with default parameter settings using their servers (http://pepitope.tau.ac.il/index.html and http://curie.utmb.edu/episearch.html, respectively).
PepSurf first unfolds the antigen surface onto a surface map containing all surface residues, where a pair of neighboring residues in the map is defined as any two residues within a distance of 4 Å. For each individual mimotope, PepSurf looks for all possible simple paths on the surface map that are the same residue length as the given mimotope. With its color-coding technique, PepSurf is able to find every linear path on the surface map without duplication. Afterward, PepSurf calculates the weight of each path by comparing its similarity to the query mimotope based on a modified BLOSUM62 matrix (Henikoff and Henikoff, 1992; Mayrose et al., 2007). The modified substitution score representing the similarity between amino acids i and j, h (i, j), is calculated as follows:
where q(i, j) is the observed probability of occurrence for each i, j pair in the original BLOSUM62 matrix; p(i) and f(j) are the probabilities of occurrence for i and j in the phage display library and in the original BLOSUM62 matrix, respectively (Mayrose et al., 2007). For each mimotope, the surface path with the highest weight is selected. Finally, PepSurf clusters all the selected surface paths and scores them with respect to their similarity to their corresponding mimotopes to obtain the final prediction results for the mimotope set (Mayrose et al., 2007).
EpiSearch first generates surface patches centered on each antigen surface residue using a preset radius value. These overlapping patches can cover the entire antigen surface. The type and number of amino acids contained in each surface patch and in each mimotope sequence are stored in separate matrices. Then, EpiSearch calculates the number of residues in each surface patch that are “identical” to any amino acid in the mimotope, where a pair of residues with a property distance (as defined above) less than or equal to a preset value is defined as a pair of identical residues. For each individual mimotope, EpiSearch scores all surface patches based on the degree of matching of each patch with the mimotope. The highest scoring patch is selected as the predicted patch corresponding to the query mimotope. After obtaining the predicted patches for all the input mimotopes in the set, the individual residues in the predicted patches that match to the corresponding mimotope are predicted to be part of the conformational epitope of the antigen (Negi and Braun, 2009).
To assess the complementarity of PepSurf and EpiSearch approaches in practice, we examined the performance of taking either the union or the intersection of the top-scoring predictive epitopes from each. First, PepSurf and EpiSearch were used to make predictions for each mimotope set-antigen pair independently using default parameters. For PepSurf, the BLOSUM62 substitution matrix and a gap penalty of −0.5 were used. For EpiSearch, a patch size which of 12 Å (which represents the radius value of the surface patches), a PD cutoff of 10, and an accuracy cutoff of 3 (which indicates the maximum number of mismatches allowed in each surface patch) were used. For EpiSearch, all residues in the top solution were considered. The intersection method takes the intersection of the top-scored solutions from EpiSearch and PepSurf. The union significantly improved the sensitivity of the existing methods while intersection decreased performance, so only the results of the union were analyzed further as described below.
In an effort to enhance the performance of MimoTree, two different ensemble approaches for combining the results of MimoTree, PepSurf, and EpiSearch were evaluated. For both, MimoTree, PepSurf and EpiSearch were run individually for each input mimotope set to predict the corresponding epitope on the antigen structure using default parameters as described above. First, MimoTree, PepSurf, and EpiSearch results were combined by majority vote; that is if a residue was in the prediction from at least two of the three methods, it was retained in the final ensemble prediction. A second ensemble approach involved taking the union of the predictions from Pepsurf and the intersection of the results from MimoTree and EpiSearch. PepSearch was chosen as the base prediction in this ensemble because it is the method with the highest average precision over the test set. The goal was to balance improved sensitivity and precision, while limiting the overall size and “density” of the prediction, where the density is the size of the prediction (in terms of number of residues) divided by the size of the antigen. Overall, 95% of all epitopes in the PDB have a solvent-accessible surface area of no more than 2000 Å2, and an epitope typically contains no more than 40 amino acids (Mayrose et al., 2007; Huang et al., 2008). See Figure 2 for an overview of the second approach.
To statistically evaluate the performance of the proposed methods, we use sensitivity, precision, Matthews Correlation Coefficient (MCC), and p-value on the predictions produced by the various methods.
Sensitivity is defined as the degree of coverage of the true epitope by the prediction. It is the number of residues correctly predicted divided by the number of residues in the true epitope or:
where TP is the number of true positives in the prediction and FN is the number of residues in the true epitope that are not in the prediction.
Precision is the number of correctly predicted residues divided by the number of residues in the prediction. It is calculated as follows:
where FP is the number of residues in the prediction that are not in the true epitope.
The Matthews Correlation Coefficient (MCC) is a coefficient of correlation between observed and predicted binary classifications. It is calculated as follows:
where TN is the number of residues in the antigen that are not part of the epitope.
The p-value is defined as the probability that a random prediction for a given antigen can perform as well as or better than the prediction obtained by the various methods. The hypergeometric (HG) distribution describes the number of times that n objects of a specified type are successfully drawn (without replacement) from a finite number of N objects (containing M objects of the specified type). The probability of drawing k objects of the specified type is represented as follows:
where k∈{1, 2, … , min (n, M)} and
In evaluating the performance of the various methods across the test set, the p-value calculated based on the HG distribution represents the probability of randomly drawing n residues from an antigen containing N residues, of which M residues are in the prediction, and k or more residues are in the true epitope. A prediction with a p-value less than 0.05 is considered to be statistically significant. p-values are calculated as follows:
A one-sided Wilcoxon signed-rank tests, a non-parametric statistical hypothesis test, was also performed to test the hypothesis that MimoTree has greater sensitivity or precision than PepSurf or EpiSearch (Conover, 1999).
MimoTree was first validated on three test cases from the set (1EJ6, 1AVZ, and 1JRH) by giving it a “near exact” match to the epitope as the input “mimotope” (see Supplementary Table S2). Specifically, for a nearly contiguous stretch of the epitope the linear sequence of the antigen was given as the “mimotope”; as such gaps were included in the input mimotope. The resulting sensitivities all agreed approximately with the percentage of the epitope that was given as the input mimotope, indicating that the algorithm was working as expected.
The performance of MimoTree was then assessed over the full test set using the antigen structures from antigen-antibody complexes (Table 3), as well using antigen-alone structures as input (Table 4). As expected, the sensitivity is somewhat diminished when using antigen-alone compared to antigen-antibody complex structures. The average sensitivity over the test set dropped from 0.52 to 0.33 and the average precision from 0.17 to 0.12. None of the other published methods have been evaluated on antigen-alone structures, a more stringent test.
The average sensitivity of MimoTree is improved relative to Pepsurf and EpiSearch over the test set (0.52 vs 0.32 and 0.31, respectively; see Table 5). Statistical significance tests comparing MimoTree to PepSurf and EpiSearch indicate that the sensitivity is significantly improved (p-values <0.05) while the precision is largely maintained (Table 6; Figure 3).
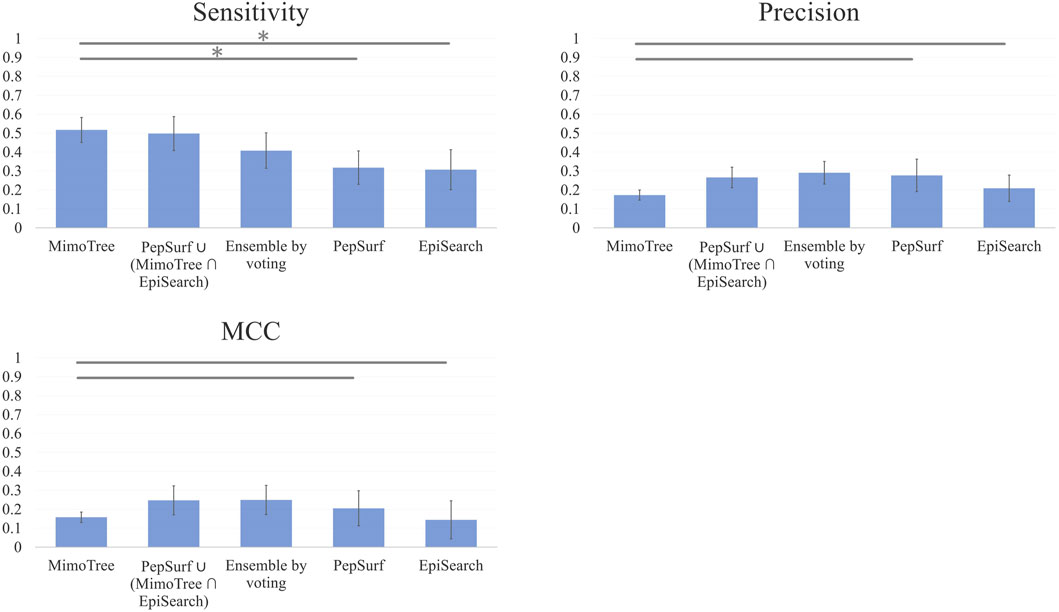
FIGURE 3. Bar plots of Mean Sensitivity, Precision, and MCC over ten case mimotope-to-epitope test set by approach with Standard Deviation indicated by the error bars. A * indicates difference is significant.
The union of PepSurf and EpiSearch also improved the average sensitivity (0.56 vs 0.32 and 0.31, see Table 5) while maintaining the average precision across the test set relative to the individual methods alone. This result indicates that in fact the two methods are complementary. However, the average size of the prediction and density (number of residues in the prediction/number of residues in the antigen) increased as well.
Taking the ensemble of MimoTree, PepSurf, and EpiSearch by majority voting gives the best average precision overall (0.29) and a low prediction size and density (20 and 13.5%). The average sensitivity, while still higher than that for PepSurf or EpiSearch, is only 0.41. Next, we chose PepSurf which has relatively high precision (0.28) and low prediction size/density (19/12.7%), as the base method to which we added the intersection of the prediction from MimoTree and EpiSearch. This yielded an average sensitivity of 0.50 (similar to MimoTree) but with a higher precision (0.27 vs 0.17) and a much smaller prediction size/density (29/18.9%) (Table 7).
Thus, this ensemble approach, PepSurf ∪ (MimoTree ∩ EpiSearch), yielded the best set of performance metrics overall (see Table 8). Images showing the prediction vs the true epitope on the antigen structure are given in Figures 4, 5 for 1N8Z and 2ADF and in the Supplementary Material for all other test cases (Supplementary Figures S1-S8). These figures serve as a guide for what the statistics reflect in terms of the mapping of the mimotope onto the antigen structure and have not generally been provided for other published methods.
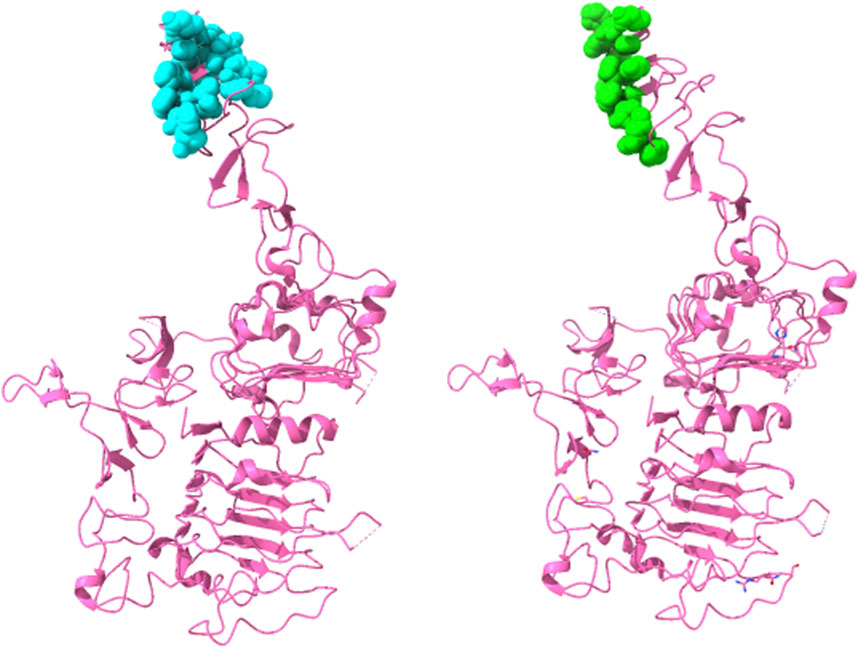
FIGURE 4. Structure of Herceptin Fab-Her-2 complex (1N8Z) showing the epitope predicted by the ensemble approach versus the true epitope. The antigen is shown in pink ribbon with residues in the prediction on the left in cyan in CPK spheres and the true epitope on the right in green.
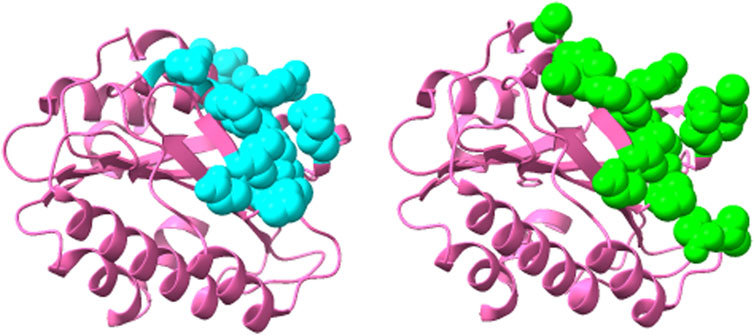
FIGURE 5. Structure of 82D6A3 IgG-Von Willebrand factor (2ADF) showing the epitope predicted by the ensemble approach versus the true epitope. The antigen is shown in pink ribbon with the prediction on the left in cyan in CPK spheres and the true epitope on the right in green.
In this study, we developed the novel method MimoTree as well as an ensemble approach combining MimoTree with PepSurf and EpiSearch for predicting the epitope on the antigen surface from mimotope data. MimoTree and the PepSurf ∪ (MimoTree ∩ EpiSearch) ensemble approach outperform PepSurf and EpiSearch by improving sensitivity while maintaining precision.
By examining the union of PepSurf and EpiSearch, we showed that PepSurf and EpiSearch use different but complementary algorithms for the process of mapping mimotopes to an antigen structure that include differences in analyzing, scoring, and clustering the locations. EpiSearch first divides the surface of the antigen into overlapping surface patches. These patches are circular regions centered on each of the surface residues. EpiSearch then calculates the similarity between the surface residues contained in these circular patches and each input mimotope and obtains an initial epitope prediction. The manner by which EpiSearch divides the antigen surfaces tends to make this algorithm more suitable for predicting compact epitopes; if the true epitope is loosely distributed in multiple patches, EpiSearch is less likely to predict it correctly as observed in the test set (e.g., for 1G9M, 1YY9, and 2ADF). Meanwhile, PepSurf uses color-coding techniques to find all possible linear paths on the antigen surface. These linear paths of different shapes may be able to traverse the entire antigen in one direction. Thus, these two methods are somewhat complementary in their surface searching approach. EpiSearch accurately locates specific residues in the epitope when it finds the right patches, but it also sometimes locates the wrong patches and therefore misses the correct epitope entirely. Conversely, PepSurf is better at finding the correct approximate epitope location but is less likely to find the specific residues within it.
MimoTree algorithm is novel in that it addresses the possibility of gaps in mimotope-epitope mapping and it considers the 3D structure of the antigen in its final epitope connection step. If a mimotope cannot be continuously mapped on the antigen surface, several existing methods, including PepSurf, reflect this feature in the scoring of this path, i.e., a gap penalty with a negative value is added to the score. However, gaps in the mimotope sequences matched to the epitope (as well as in the epitope itself if it is conformational) are often seen since mimotopes mimic the structure and sequence of epitopes, but are usually not identical to the epitope. To solve this problem, MimoTree first unfolds the antigen surface into a surface map. For each input mimotope, MimoTree looks for each of its sequence fragments (seeds) on the antigen surface and tries to join these seeds in the sequence order of the original mimotope with a distance restraint based on the 3D structure of the antigen and the maximal length of the mimotope (if in an extended linear conformation). If the distance between the ends of the two seeds on the 3D antigen structure is less than or equal to the distance between corresponding residues in the mimotope (assuming a linear extended conformation), then the seeds are connected. MimoTree predicts the mimotope to map to that position on the antigen surface.
Because MimoTree uses a depth-first search method during the searching process, the longer the length of the seed connection (matching the mimotope to the antigen surface), the greater the degree of match overall. Gaps in the seed connections of longest length that were incorporated into the MimoTree predictions for each case in the test set were analyzed. In 3/10 test cases, there were two gaps each in the longest seed connections, while in 4/10 cases there was one gap in the seed connections. The average gap length was 3.1 residues and the most common amino acids found in the gaps were Arg, Lys, Pro, Leu, Asn, and Glu. In future work these trends could be analyzed over a larger sample size.
While evaluating the performance of the various methods, we found that EpiSearch and PepSurf were unable to accurately predict any of the residues in the epitope of the 1YY9 antigen (epidermal growth factor receptor (EGFR)). MimoTree does find some matches between the mimotopes and the epitope for this test case, however, the prediction is loosely dispersed over the two main domains of the structure (Domain III, which interacts with the antibody, and Domain I, which would interact with EGF), and has low sensitivity and precision (0.30 and 0.08). This is a particularly difficult test case, in that the residues in the epitope are loosely distributed in that the true epitope consists of 20 residues but the longest contiguous stretch is of four residues, which greatly increases the difficulty of algorithmically predicting the location of the epitope. 1BJ1 is also a difficult case in that the antigen (vascular endothelial growth factor (VEGF)) is secreted as a dimer of two identical monomers and in the structure an antibody is bound at each of the identical dimer poles. MimoTree does locate both poles on the monomer of the dimer but the prediction size and density are relatively high (see Supplementary Figure S9).
For both MimoTree and the PepSurf ∪ (MimoTree ∩ EpiSearch) ensemble approach the sensitivity is highest for 1EJ6 (0.93), but with MimoTree the precision is relatively low (0.14). With the ensemble approach the sensitivity remains high and the precision increases. The true epitope is identified with high precision but a second patch on the antigen is also highlighted (Supplementary Figure S4). Two of the best cases based on the ensemble approach are 1N8Z and 2ADF both of which balance sensitivity and precision and have relatively low densities (see Figures 4, 5).
In general, our results indicate that sensitivity, precision, and density must all be considered when evaluating a given prediction and a mimotope mapping approach overall. In particular, predictions that balance sensitivity and precision tend to map most closely to the true epitope. The results also suggest that ensemble approaches likely are more effective at achieving that balance assuming that the methods included employ complementary strategies. The current limitations of MimoTree and the PepSurf ∪ (MimoTree ∩ EpiSearch) ensemble approach, however, still lie in the relatively high false positive rate. In future work, adding a machine learning component to the MimoTree combination step and/or the ensemble formation may improve the results further by reducing false positives. Mimotope methods like MimoTree and the PepSurf ∪ (MimoTree ∩ EpiSearch) ensemble approach will further our understanding of the characteristics of antigen-antibody binding interactions, may for certain pathogens lead to the design of improved vaccine with limited potential for resistance, and can aid in the rapid design of more specific and sensitive diagnostic immunoassays.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
RL: Conceptualization, Investigation, Formal Analysis, Methodology, Software, Writing–original draft. SW: Data curation, Validation, Writing–review and editing. MS: Writing–review and editing, Conceptualization. DV: Writing–review and editing, Formal Analysis. AC: Writing–review and editing, Conceptualization, Supervision. DJ-M: Conceptualization, Supervision, Writing–review and editing, Investigation, Project administration.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Authors MS and AC were employed by Fractal Therapeutics Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbinf.2024.1295972/full#supplementary-material
Adams, G. P., and Weiner, L. M. (2005). Monoclonal antibody therapy of cancer. Nat. Biotechnol. 23, 1147–1157. doi:10.1038/nbt1137
Arold, S., Franken, P., Strub, M.-P., Hoh, F., Benichou, S., Benarous, R., et al. (1997). The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure 5, 1361–1372. doi:10.1016/s0969-2126(97)00286-4
Bakhshinejad, L. A.-M., Baradaran, B., Motallebnezhad, M., Aghebati-Maleki, A., Nickho, H., Yousefi, M., et al. (2016). Phage display as a promising approach for vaccine development. J. Biomed. Sci. 23, 66. doi:10.1186/s12929-016-0285-9
Barlow, D., Edwards, M., and Thornton, J. (1986). Continuous and discontinuous protein antigenic determinants. Nature 322, 747–748. doi:10.1038/322747a0
Bazan, J., Całkosiński, I., and Gamian, A. (2012). Phage display--a powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum. Vaccin Immunother. 8, 1817–1828. doi:10.4161/hv.21703
Berkopec, A. (2007). HyperQuick algorithm for discrete hypergeometric distribution. J. Discrete Algorithms 5, 341–347. doi:10.1016/j.jda.2006.01.001
Bhagavan, N., and Ha, C. (2015). “Chapter 4—three-dimensional structure of proteins and disorders of protein misfolding,” in Essentials of medical biochemistry. Second Edition (San Diego: Academic Press), 33.
Bienkowska, J., Cruz, M., Atiemo, A., Handin, R., and Liddington, R. (1997). The von Willebrand factor A3 domain does not contain a metal ion-dependent adhesion site motif. J. Biol. Chem. 272, 25162–25167. doi:10.1074/jbc.272.40.25162
Briknarová, K., Takayama, S., Brive, L., Havert, M. L., Knee, D. A., Velasco, J., et al. (2001). Structural analysis of BAG1 cochaperone and its interactions with Hsc70 heat shock protein. Nat. Struct. Biol. 8, 349–352. doi:10.1038/86236
Bublil, E. M., Freund, N. T., Mayrose, I., Penn, O., Roitburd-Berman, A., Rubinstein, N. D., et al. (2007). Stepwise prediction of conformational discontinuous B-cell epitopes using the Mapitope algorithm. Proteins Struct. Funct. Bioinforma. 68, 294–304. doi:10.1002/prot.21387
Chen, S.-W. W., Van Regenmortel, M. H., and Pellequer, J.-L. (2009). Structure-activity relationships in peptide-antibody complexes: implications for epitope prediction and development of synthetic peptide vaccines. Curr. Med. Chem. 16, 953–964. doi:10.2174/092986709787581914
Chen, W. H., Sun, P. P., Lu, Y., Guo, W. W., Huang, Y. X., and Ma, Z. Q. (2011). MimoPro: a more efficient Web-based tool for epitope prediction using phage display libraries. BMC Bioinforma. 12, 199–213. doi:10.1186/1471-2105-12-199
Chen, Y., Wiesmann, C., Fuh, G., Li, B., Christinger, H. W., Mckay, P., et al. (1999). Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured fab in complex with antigen 1 1Edited by I. A. Wilson. J. Mol. Biol. 293, 865–881. doi:10.1006/jmbi.1999.3192
Cho, H.-S., Mason, K., Ramyar, K. X., Stanley, A. M., Gabelli, S. B., Denney, D. W., et al. (2003). Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–760. doi:10.1038/nature01392
Desta, I. T., Kotelnikov, S., Jones, G., Ghani, U., Abyzov, M., Kholodov, Y., et al. (2022). Mapping of antibody epitopes based on docking and homology modeling. Proteins Struct. Funct. Bioinforma. 91, 171–182. doi:10.1002/prot.26420
Enshell-Seijffers, D., Denisov, D., Groisman, B., Smelyanski, L., Meyuhas, R., Gross, G., et al. (2003). The mapping and reconstitution of a conformational discontinuous B-cell epitope of HIV-1. J. Mol. Biol. 334, 87–101. doi:10.1016/j.jmb.2003.09.002
Ferguson, K. M., Berger, M. B., Mendrola, J. M., Cho, H.-S., Leahy, D. J., and Lemmon, M. A. (2003). EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol. Cell 11, 507–517. doi:10.1016/s1097-2765(03)00047-9
Gamble, T. R., Yoo, S., Vajdos, F. F., Von Schwedler, U. K., Worthylake, D. K., Wang, H., et al. (1997). Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science 278, 849–853. doi:10.1126/science.278.5339.849
Geysen, H. M., Rodda, S. J., and Mason, T. J. (1986). A priori delineation of a peptide which mimics a discontinuous antigenic determinant. Mol. Immunol. 23, 709–715. doi:10.1016/0161-5890(86)90081-7
Goulart, L. R., and De S. Santos, P. (2016). Strategies for vaccine design using phage display-derived peptides. Methods Protoc. 2, 423–435. doi:10.1007/978-1-4939-3389-1_28
Greenway, A., Azad, A., Mills, J., and Mcphee, D. (1996). Human immunodeficiency virus type 1 Nef binds directly to Lck and mitogen-activated protein kinase, inhibiting kinase activity. J. Virology 70, 6701–6708. doi:10.1128/jvi.70.10.6701-6708.1996
Henikoff, S., and Henikoff, J. G. (1992). Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. U. S. A. 89, 10915–10919. doi:10.1073/pnas.89.22.10915
Huang, Y. X., Bao, Y. L., Guo, S. Y., Wang, Y., Zhou, C. G., and Li, Y. X. (2008). Pep-3D-Search: a method for B-cell epitope prediction based on mimotope analysis. BMC Bioinforma. 9, 538. doi:10.1186/1471-2105-9-538
Huang, J., He, B., and Zhou, P. (2014). Mimotope-based prediction of B-cell epitopes. Immunoinformatics 1184, 237–243. doi:10.1007/978-1-4939-1115-8_13
Hu, J., and Yan, C. (2009). A tool for calculating binding-site residues on proteins from PDB structures. BMC Struct. Biol. 9, 52. doi:10.1186/1472-6807-9-52
Irving, M. B., Craig, L., Menendez, A., Gangadhar, B. P., Montero, M., Van Houten, N. E., et al. (2010). Exploring peptide mimics for the production of antibodies against discontinuous protein epitopes. Mol. Immunol. 47, 1137–1148. doi:10.1016/j.molimm.2009.10.015
Lang, S., Xu, J., Stuart, F., Thomas, R. M., Vrijbloed, J. W., and Robinson, J. A. (2000). Analysis of antibody A6 binding to the extracellular interferon γ receptor α-chain by alanine-scanning mutagenesis and random mutagenesis with phage display. Biochemistry 39, 15674–15685. doi:10.1021/bi000838z
Li, S., Schmitz, K. R., Jeffrey, P. D., Wiltzius, J. J., Kussie, P., and Ferguson, K. M. (2005). Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer cell 7, 301–311. doi:10.1016/j.ccr.2005.03.003
Liu, J., Bartesaghi, A., Borgnia, M. J., Sapiro, G., and Subramaniam, S. (2008). Molecular architecture of native HIV-1 gp120 trimers. Nature 455, 109–113. doi:10.1038/nature07159
Mayrose, I., Shlomi, T., Rubinstein, N. D., Gershoni, J. M., Ruppin, E., Sharan, R., et al. (2007). Epitope mapping using combinatorial phage-display libraries: a graph-based algorithm. Nucleic acids Res. 35, 69–78. doi:10.1093/nar/gkl975
Miller, S., Janin, J., Lesk, A. M., and Chothia, C. (1987). Interior and surface of monomeric proteins. J. Mol. Biol. 196, 641–656. doi:10.1016/0022-2836(87)90038-6
Moreau, V., Granier, C., Villard, S., Laune, D., and Molina, F. (2006). Discontinuous epitope prediction based on mimotope analysis. Bioinformatics 22, 1088–1095. doi:10.1093/bioinformatics/btl012
Muller, Y. A., Li, B., Christinger, H. W., Wells, J. A., Cunningham, B. C., and De Vos, A. M. (1997). Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc. Natl. Acad. Sci. 94, 7192–7197. doi:10.1073/pnas.94.14.7192
Negi, S. S., and Braun, W. (2009). Automated detection of conformational epitopes using phage display peptide sequences, Bioinforma. Biol. insights 3, S2745. doi:10.4137/bbi.s2745
Nybakken, G. E., Oliphant, T., Johnson, S., Burke, S., Diamond, M. S., and Fremont, D. H. (2005). Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature 437, 764–769. doi:10.1038/nature03956
Pettersen, E. F., Goddard, T. D., Huang, C. C., Couch, G. S., Greenblatt, D. M., Meng, E. C., et al. (2004). UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612. doi:10.1002/jcc.20084
Potocnakova, L., Bhide, M., and Pulzova, L. B. (2016). An introduction to B-cell epitope mapping and in silico epitope prediction. J. Immunol. Res. 2016, 1–11. doi:10.1155/2016/6760830
Pratt, K. P., Shen, B. W., Takeshima, K., Davie, E. W., Fujikawa, K., and Stoddard, B. L. (1999). Structure of the C2 domain of human factor VIII at 1.5 Å resolution. Nature 402, 439–442. doi:10.1038/46601
Rickles, R., Botfield, M., Weng, Z., Taylor, J., Green, O., Brugge, J., et al. (1994). Identification of Src, Fyn, Lyn, PI3K and Abl SH3 domain ligands using phage display libraries. EMBO J. 13, 5598–5604. doi:10.1002/j.1460-2075.1994.tb06897.x
Riemer, A. B., Kraml, G., Scheiner, O., Zielinski, C. C., and Jensen-Jarolim, E. (2005a). Matching of trastuzumab (Herceptin®) epitope mimics onto the surface of Her-2/neu – a new method of epitope definition. Mol. Immunol. 42, 1121–1124. doi:10.1016/j.molimm.2004.11.003
Riemer, A. B., Kurz, H., Klinger, M., Scheiner, O., Zielinski, C. C., and Jensen-Jarolim, E. (2005b). Vaccination with cetuximab mimotopes and biological properties of induced anti–epidermal growth factor receptor antibodies. J. Natl. Cancer Inst. 97, 1663–1670. doi:10.1093/jnci/dji373
Sanchez-Trincado, J. L., Gomez-Perosanz, M., and Reche, P. A. (2017). Fundamentals and methods for T- and B-cell epitope prediction. J. Immunol. Res. 2017, 1–14. doi:10.1155/2017/2680160
Saxena, A. K., Singh, K., Su, H.-P., Klein, M. M., Stowers, A. W., Saul, A. J., et al. (2006). The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms. Nat. Struct. Mol. Biol. 13, 90–91. doi:10.1038/nsmb1024
Sela-Culang, I., Kunik, V., and Ofran, Y. (2013). The structural basis of antibody-antigen recognition. Front. Immunol. 4, 302. doi:10.3389/fimmu.2013.00302
Steimer, K. S., Scandella, C. J., Skiles, P. V., and Haigwood, N. L. (1991). Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to Gp120. Science 254, 105–108. doi:10.1126/science.1718036
Stephenson, J. D., and Freeland, S. J. (2013). Unearthing the root of amino acid similarity. J. Mol. Evol. 77, 159–169. doi:10.1007/s00239-013-9565-0
Sun, P., Qi, J., Zhao, Y., Huang, Y., Yang, G., Ma, Z., et al. (2016). A novel conformational B-cell epitope prediction method based on mimotope and patch analysis. J. Theor. Biol. 394, 102–108. doi:10.1016/j.jtbi.2016.01.021
Takenaka, I. M., Leung, S.-M., Mcandrew, S. J., Brown, J. P., and Hightower, L. E. (1995). Hsc70-binding peptides selected from a phage display peptide library that resemble organellar targeting sequences (∗). J. Biol. Chem. 270, 19839–19844. doi:10.1074/jbc.270.34.19839
Tegoni, M., Spinelli, S., Verhoeyen, M., Davis, P., and Cambillau, C. (1999). Crystal structure of a ternary complex between human chorionic gonadotropin (hCG) and two Fv fragments specific for the α and β-subunits 1 1Edited by I. A. Wilson. J. Mol. Biol. 289, 1375–1385. doi:10.1006/jmbi.1999.2845
Vanhoorelbeke, K., Depraetere, H., Romijn, R. A., Huizinga, E. G., De Maeyer, M., and Deckmyn, H. (2003). A consensus tetrapeptide selected by phage display adopts the conformation of a dominant discontinuous epitope of a monoclonal anti-VWF antibody that inhibits the von Willebrand factor-collagen interaction. J. Biol. Chem. 278, 37815–37821. doi:10.1074/jbc.m304289200
Van Regenmortel, M. H. (1996). Mapping epitope structure and activity: from one-dimensional prediction to four-dimensional description of antigenic specificity. Methods 9, 465–472. doi:10.1006/meth.1996.0054
Van Regenmortel, M. H. (2016). Structure-based reverse vaccinology failed in the case of HIV because it disregarded accepted immunological theory. Int. J. Mol. Sci. 17, 1591. doi:10.3390/ijms17091591
Venkatarajan, M. S., and Braun, W. (2001). New quantitative descriptors of amino acids based on multidimensional scaling of a large number of physical–chemical properties. Mol. Model. Annu. 7, 445–453. doi:10.1007/s00894-001-0058-5
Villard, S., Lacroix-Desmazes, S., Kieber-Emmons, T., Piquer, D., Grailly, S., Benhida, A., et al. (2003). Peptide decoys selected by phage display block in vitro and in vivo activity of a human anti-FVIII inhibitor. Blood 102, 949–952. doi:10.1182/blood-2002-06-1886
Vinion-Dubiel, A. D., Mcclain, M. S., Cao, P., Mernaugh, R. L., and Cover, T. L. (2001). Antigenic diversity among Helicobacter pylori vacuolating toxins. Infect. Immun. 69, 4329–4336. doi:10.1128/iai.69.7.4329-4336.2001
Keywords: conformational epitope, mimotopes, structure-based mimotope mapping, antigen-antibody binding-site prediction, immunodiagnostics design
Citation: Li R, Wilderotter S, Stoddard M, Van Egeren D, Chakravarty A and Joseph-McCarthy D (2024) Computational identification of antibody-binding epitopes from mimotope datasets. Front. Bioinform. 4:1295972. doi: 10.3389/fbinf.2024.1295972
Received: 17 September 2023; Accepted: 24 January 2024;
Published: 23 February 2024.
Edited by:
Constanza Cárdenas Carvajal, Pontificia Universidad Católica de Valparaíso, ChileReviewed by:
Chinh Tran-To Su, Bioinformatics Institute (A∗STAR), SingaporeCopyright © 2024 Li, Wilderotter, Stoddard, Van Egeren, Chakravarty and Joseph-McCarthy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diane Joseph-McCarthy, ZGpvc2VwaG1AYnUuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.